Enhancement of Sulfur Oxide Capture Capacity by Deposition of Iron Oxide Particles on Graphene Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Iron Oxide/Graphene Oxide Composite Characterization
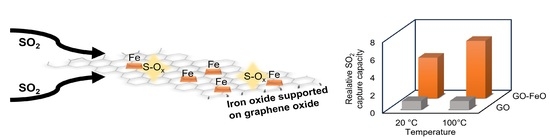
2.2. SO2 Capture Studies
2.2.1. Breakthrough Curves
2.2.2. Capacity for SO2
2.3. Interaction of the Materials with Sulfur
3. Materials and Methods
3.1. Materials
3.2. Iron Oxide Graphene Oxide Preparation
3.3. SO2 Capture Studies
3.4. Calculation of Capture Capacities
3.5. Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, R.K.; Cui, Z.; Domen, J.K. (Eds.) Chapter 4—Environmental Impacts of Mining. In Environmental Impact of Mining and Mineral Processing; Butterworth-Heinemann: Boston, MA, USA, 2016; pp. 53–157. ISBN 978-0-12-804040-9. [Google Scholar]
- USEPA. Effects of Acid Rain. Available online: https://www.epa.gov/acidrain/effects-acid-rain (accessed on 1 July 2023).
- Reducing Sulphur Dioxide Emissions from Ontario’s Petroleum Facilities|Environmental Registry of Ontario. Available online: https://ero.ontario.ca/notice/019-3443 (accessed on 5 June 2023).
- USEPA. Applying or Implementing Sulfur Dioxide Standards. Available online: https://www.epa.gov/so2-pollution/applying-or-implementing-sulfur-dioxide-standards (accessed on 5 June 2023).
- Liu, X.; Lin, B.; Zhang, Y. Sulfur Dioxide Emission Reduction of Power Plants in China: Current Policies and Implications. J. Clean. Prod. 2016, 113, 133–143. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Canadian Environmental Sustainability Indicators: Air Pollutant Emissions; Environment and Climate Change Canada: Ottawa, ON, Canada, 2021. [Google Scholar]
- Rezaei, F.; Rownaghi, A.A.; Monjezi, S.; Lively, R.P.; Jones, C.W. SOx/NOx Removal from Flue Gas Streams by Solid Adsorbents: A Review of Current Challenges and Future Directions. Energy Fuels 2015, 29, 5467–5486. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jozewicz, W. Flue Gas Desulfurization: The State of the Art. J. Air Waste Manag. Assoc. 2001, 51, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Poullikkas, A. Review of Design, Operating, and Financial Considerations in Flue Gas Desulfurization Systems. Energy Technol. Policy 2015, 2, 92–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, G.; Sun, Z.; Yang, J. Simultaneous Removal of SO2 and NO2 on α-Al2O3 Absorbents Loaded with Sodium Humate and Ammonia Water. Energy Fuels 2011, 25, 2927–2931. [Google Scholar] [CrossRef]
- Alver, B.E. A Comparative Adsorption Study of C2H4 and SO2 on Clinoptilolite-Rich Tuff: Effect of Acid Treatment. J. Hazard. Mater. 2013, 262, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Savage, M.; Cheng, Y.; Easun, T.L.; Eyley, J.E.; Argent, S.P.; Warren, M.R.; Lewis, W.; Murray, C.; Tang, C.C.; Frogley, M.D.; et al. Selective Adsorption of Sulfur Dioxide in a Robust Metal–Organic Framework Material. Adv. Mater. 2016, 28, 8705–8711. [Google Scholar] [CrossRef] [PubMed]
- Kopaç, T. Non-Isobaric Adsorption Analysis of SO2 on Molecular Sieve 13X and Activated Carbon by Dynamic Technique. Chem. Eng. Process. Process Intensif. 1999, 38, 45–53. [Google Scholar] [CrossRef]
- Meimand, M.M.; Jafari, A.J.; Nasiri, A.; Malakootian, M. Sulfur Dioxide Adsorption by Iron Oxide Nanoparticles@clinoptilolite/HCl. J. Air Pollut. Health 2020, 5, 107–120. [Google Scholar] [CrossRef]
- Hanif, M.A.; Ibrahim, N.; Abdul Jalil, A. Sulfur Dioxide Removal: An Overview of Regenerative Flue Gas Desulfurization and Factors Affecting Desulfurization Capacity and Sorbent Regeneration. Environ. Sci. Pollut. Res. 2020, 27, 27515–27540. [Google Scholar] [CrossRef]
- Babu, D.J.; Puthusseri, D.; Kühl, F.G.; Okeil, S.; Bruns, M.; Hampe, M.; Schneider, J.J. SO2 Gas Adsorption on Carbon Nanomaterials: A Comparative Study. Beilstein J. Nanotechnol. 2018, 9, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Lee, C.-H. Sorption Kinetics of Eight Gases on a Carbon Molecular Sieve at Elevated Pressure. Carbon 2005, 43, 95–107. [Google Scholar] [CrossRef]
- Kühl, F.G.; Kazdal, T.J.; Lang, S.; Hampe, M.J. Adsorption of Sulfur Dioxide and Mixtures with Nitrogen at Carbon Nanotubes and Graphene: Molecular Dynamics Simulation and Gravimetric Adsorption Experiments. Adsorption 2017, 23, 293–301. [Google Scholar] [CrossRef]
- Babu, D.J.; Kühl, F.G.; Yadav, S.; Markert, D.; Bruns, M.; Hampe, M.J.; Schneider, J.J. Adsorption of Pure SO2 on Nanoscaled Graphene Oxide. RSC Adv. 2016, 6, 36834–36839. [Google Scholar] [CrossRef]
- Stirling, D. The Sulfur Problem; The Royal Society of Chemistry: London, UK, 2000; ISBN 978-0-85404-541-9. [Google Scholar]
- Otsuka, N. Fireside Corrosion. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Cham, Swizterland, 2010; Volume 1, ISBN 978-0-444-52787-5. [Google Scholar]
- Li, B.; Ma, C. Study on the Mechanism of SO2 Removal by Activated Carbon. Energy Procedia 2018, 153, 471–477. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, L.; Li, B.; Wang, W.; Ma, C. Experimental Study of SO2 Removal by Powder Activated Carbon in Fluidized Bed Reactor. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010; pp. 591–594. [Google Scholar]
- Lizzio, A.A.; DeBarr, J.A. Mechanism of SO2 Removal by Carbon. Energy Fuels 1997, 11, 284–291. [Google Scholar] [CrossRef]
- Gaur, V.; Asthana, R.; Verma, N. Removal of SO2 by Activated Carbon Fibers in the Presence of O2 and H2O. Carbon 2006, 44, 46–60. [Google Scholar] [CrossRef]
- Liu, X.-L.; Guo, J.-X.; Chu, Y.-H.; Luo, D.-M.; Yin, H.-Q.; Sun, M.-C.; Yavuz, R. Desulfurization Performance of Iron Supported on Activated Carbon. Fuel 2014, 123, 93–100. [Google Scholar] [CrossRef]
- Davini, P. The Effect of Certain Metallic Derivatives on the Adsorption of Sulphur Dioxide on Active Carbon. Carbon 2001, 39, 419–424. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Wu, H.; Yin, Y.; Chen, J. Heterogeneous Uptake and Oxidation of SO2 on Iron Oxides. J. Phys. Chem. C 2007, 111, 6077–6085. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.; Zhu, Y.; Chen, G.; Wu, S.; Qin, Y. Adsorption of SO2 by Typical Carbonaceous Material: A Comparative Study of Carbon Nanotubes and Activated Carbons. Adsorption 2013, 19, 959–966. [Google Scholar] [CrossRef]
- Mechanism and Application of Carbon Nanotube Sensors in SF6 Decomposed Production Detection: A Review|Nanoscale Research Letters|Full Text. Available online: https://nanoscalereslett.springeropen.com/articles/10.1186/s11671-017-1945-8 (accessed on 19 April 2022).
- Zhang, X.; Meng, F.; Yang, B. Use of Hydroxyl-Modified Carbon Nanotubes for Detecting SF6 Decomposition Products under Partial Discharge in Gas Insulated Switchgear. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2246–2253. [Google Scholar] [CrossRef]
- An, L.; Jia, X.; Liu, Y. Adsorption of SO2 Molecules on Fe-Doped Carbon Nanotubes: The First Principles Study. Adsorption 2019, 25, 217–224. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.-J.; Liu, P.; Pan, Z.-L.; He, Q.-Y. First-Principles Study of the Adsorption Sensitivity of Ni-Doped Single-Walled Zigzag (n,0)CNTs (N=4,5,6) toward SO2 Molecules. Appl. Surf. Sci. 2015, 335, 17–22. [Google Scholar] [CrossRef]
- Yoosefian, M.; Zahedi, M.; Mola, A.; Naserian, S. A DFT Comparative Study of Single and Double SO2 Adsorption on Pt-Doped and Au-Doped Single-Walled Carbon Nanotube. Appl. Surf. Sci. 2015, 349, 864–869. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, H. Recent Advances in the Catalytic Applications of GO/rGO for Green Organic Synthesis. Green Process. Synth. 2020, 9, 515–537. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, C.; Wang, X.; Gao, J.; Wang, W.; Liu, Y. Oxidation of SO2 to SO3 Catalyzed by Graphene Oxide Foams. J. Mater. Chem. 2011, 21, 13934–13941. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, W.; Liu, J.; Guo, J.; Yin, H.; Ning, P. Adsorption and Oxidation of SO2 by Graphene Oxides: A van Der Waals Density Functional Theory Study. Appl. Surf. Sci. 2015, 324, 61–67. [Google Scholar] [CrossRef]
- Shabestari, M.E.; Martín, O.; Díaz-García, D.; Gómez-Ruiz, S.; Gonzalez, V.J.; Baselga, J. Facile and Rapid Decoration of Graphene Oxide with Copper Double Salt, Oxides and Metallic Copper as Catalysts in Oxidation and Coupling Reactions. Carbon 2020, 161, 7–16. [Google Scholar] [CrossRef]
- Rodríguez-García, S.; Santiago, R.; López-Díaz, D.; Merchán, M.D.; Velázquez, M.M.; Fierro, J.L.G.; Palomar, J. Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4-Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 12464–12473. [Google Scholar] [CrossRef]
- Soni, J.; Sethiya, A.; Sahiba, N.; Agarwal, S. Recent Advancements in Organic Synthesis Catalyzed by Graphene Oxide Metal Composites as Heterogeneous Nanocatalysts. Appl. Organomet. Chem. 2021, 35, e6162. [Google Scholar] [CrossRef]
- Rodríguez, C.; Tapia, C.; Leiva-Aravena, E.; Leiva, E. Graphene Oxide–ZnO Nanocomposites for Removal of Aluminum and Copper Ions from Acid Mine Drainage Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 6911. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ye, Z.; Hmidi, N. High-Performance Iron Oxide-Graphene Oxide Nanocomposite Adsorbents for Arsenic Removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 161–172. [Google Scholar] [CrossRef]
- Lowell, P.S.; Schwitzgebel, K.; Parsons, T.B.; Sladek, K.J. Selection of Metal Oxides for Removing SO2 from Flue Gas. Ind. Eng. Chem. Proc. Des. Dev. 1971, 10, 384–390. [Google Scholar] [CrossRef]
- Deberry, D.W.; Sladek, K.J. Rates of Reaction of SO2 with Metal Oxides. Can. J. Chem. Eng. 1971, 49, 781–785. [Google Scholar] [CrossRef]
- Gavaskar, V.S.; Abbasian, J. Dry Regenerable Metal Oxide Sorbents for SO2 Removal from Flue Gases. 1. Development and Evaluation of Copper Oxide Sorbents. Ind. Eng. Chem. Res. 2006, 45, 5859–5869. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, S.; Pei, J.; Li, X.; Yu, R.; Zhao, J.; Martyniuk, C.J. Adsorption Performance of SO2 over ZnAl2O4 Nanospheres. J. Ind. Eng. Chem. 2016, 41, 151–157. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Qu, Z.; Zhao, Q.; Liu, S.; Hu, X. The NiAl Mixed Oxides: The Relation between Basicity and SO2 Removal Capacity. Sep. Purif. Technol. 2011, 80, 345–350. [Google Scholar] [CrossRef]
- Koballa, T.E.; Dudukovic, M.P. Sulfur Dioxide Adsorption on Metal Oxides Supported on Alumina. AIChE Symp. Ser. 1977, 73, 199–228. [Google Scholar]
- Brandt, P.; Nuhnen, A.; Lange, M.; Möllmer, J.; Weingart, O.; Janiak, C. Metal–Organic Frameworks with Potential Application for SO2 Separation and Flue Gas Desulfurization. ACS Appl. Mater. Interfaces 2019, 11, 17350–17358. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.-G.; Walton, K.S. Stability and Degradation Mechanisms of Metal–Organic Frameworks Containing the Zr6O4(OH)4 Secondary Building Unit. J. Mater. Chem. A 2013, 1, 5642–5650. [Google Scholar] [CrossRef]
- Li, L.; da Silva, I.; Kolokolov, D.I.; Han, X.; Li, J.; Smith, G.; Cheng, Y.; Daemen, L.L.; Morris, C.G.; Godfrey, H.G.W.; et al. Post-Synthetic Modulation of the Charge Distribution in a Metal–Organic Framework for Optimal Binding of Carbon Dioxide and Sulfur Dioxide. Chem. Sci. 2019, 10, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Panaritis, C.; Zgheib, J.; Ebrahim, S.A.H.; Couillard, M.; Baranova, E.A. Electrochemical In-Situ Activation of Fe-Oxide Nanowires for the Reverse Water Gas Shift Reaction. Appl. Catal. B Environ. 2020, 269, 118826. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, H.; Kukkar, D. Synthesis and Characterization of Graphene Oxide Using Modified Hummer’s Method. AIP Conf. Proc. 2018, 1953, 030180. [Google Scholar] [CrossRef]
- Dave, K.; Park, K.H.; Dhayal, M. Two-Step Process for Programmable Removal of Oxygen Functionalities of Graphene Oxide: Functional, Structural and Electrical Characteristics. RSC Adv. 2015, 5, 95657–95665. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. X-ray Photoelectron Spectroscopy Database XPS, NIST Standard Reference Database. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 1 March 2023).
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the Determination of Chemical Environment and Oxidation State of Iron and Sulfur Samples: Constitution of a Data Basis in Binding Energies for Fe and S Reference Compounds and Applications to the Evidence of Surface Species of an Oxidized Pyrite in a Carbonate Medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar] [CrossRef]
- Hollinger, G.; Kumurdjian, P.; Mackowski, J.M.; Pertosa, P.; Porte, L.; Duc, T.M. ESCA Study of Molecular GeS3−x Tex As2 Glasses. J. Electron. Spectrosc. Relat. Phenom. 1974, 5, 237–245. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Lin, H.-P.; Kuo, T.-H.; Chen, S.-S.; Chang, T.-C.; Su, K.-H.; Hsi, H.-C. Simultaneous Control of Elemental Mercury/Sulfur Dioxide/Nitrogen Monoxide from Coal-Fired Flue Gases with Metal Oxide-Impregnated Activated Carbon. Aerosol Air Qual. Res. 2015, 15, 2094–2103. [Google Scholar] [CrossRef]
- Gupta, N.K.; Kim, E.J.; Baek, S.; Bae, J.; Kim, K.S. Ternary Metal Oxide Nanocomposite for Room Temperature H2S and SO2 Gas Removal in Wet Conditions. Sci. Rep. 2022, 12, 15387. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Márquez, A.; Atanes, E.; Morena, J.; Fernández-Martínez, F.; Valverde, J.L. Upgrading Waste Tires by Chemical Activation for the Capture of SO2. Fuel Process. Technol. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- Zafari, R.; Mendonça, F.G.; Tom Baker, R.; Fauteux-Lefebvre, C. Efficient SO2 Capture Using an Amine-Functionalized, Nanocrystalline Cellulose-Based Adsorbent. Sep. Purif. Technol. 2023, 308, 122917. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of Divalent Metal Ions from Aqueous Solutions Using Graphene Oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]





| Operating Conditions | Capacity Ratio for SO2 (mgSO2 on GO–FeO/mgSO2 on GO) |
|---|---|
| 20 °C and 15 mL/min | 4.6 |
| 60 °C and 10 mL/min | 8.1 |
| 100 °C and 15 mL/min | 6.5 |
| 100 °C and 5 mL/min | 2.8 |
| Sample | C (At%/Mass%) | O (At%/Mass%) | Fe (At%/Mass%) | S (At%/Mass%) | Change in S Amount after Capture (Mass%) |
|---|---|---|---|---|---|
| GO | 71.46/64.86 | 28.02/33.88 | 0.00 | 0.52/1.26 | – |
| GO–FeO | 73.05/66.75 | 26.79/32.61 | 0.15/0.64 | 0.00 | – |
| GO/SO2 | 70.47/63.75 | 28.98/34.92 | 0.00 | 0.55/1.33 | 0.07 |
| GO–FeO/SO2 | 74.11/67.65 | 25.52/31.03 | 0.23/0.98 | 0.14/0.34 | 0.34 |
| Material Description | Inlet SO2 Concentration (ppm) | Temperature (K) | Capture Capacity (mg SO2/g sorbent) | Reference |
|---|---|---|---|---|
| Modified coconut shell | 200 | 298 | 0.4–0.8 | [61] |
| Mn–Zn–Fe metal oxide nanocomposite | 100 | 298 | 31 | [62] |
| Fly ash/ KOH | 40 | 298 | 7.0 | [63] |
| Modified nanocellulose | 25 | 298 | 2.0 | [64] |
| This study | 25 | 293–393 | 0.6–1.5 | N/A |
| Sample Type | Temperature (°C) | Inlet Flow Rate 2 (mL/min) | Inlet Gas Hourly Space Velocity 2 (mL/h/gsample) |
|---|---|---|---|
| None 1 | 20/60/100 | 5/10/15 | 6000/12,000/18,000 |
| GO | 20 | 15 | 18,000 |
| GO | 100 | 5 | 6000 |
| GO | 100 | 15 | 18,000 |
| GO | 60 | 10 | 12,000 |
| GO–FeO | 20 | 15 | 18,000 |
| GO–FeO | 100 | 5 | 6000 |
| GO–FeO | 100 | 15 | 18,000 |
| GO–FeO | 60 | 10 | 12,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanyal, T.S.; Mugisha, A.I.; Sowinski, A.; Fauteux-Lefebvre, C. Enhancement of Sulfur Oxide Capture Capacity by Deposition of Iron Oxide Particles on Graphene Oxide. Catalysts 2023, 13, 1469. https://doi.org/10.3390/catal13121469
Sanyal TS, Mugisha AI, Sowinski A, Fauteux-Lefebvre C. Enhancement of Sulfur Oxide Capture Capacity by Deposition of Iron Oxide Particles on Graphene Oxide. Catalysts. 2023; 13(12):1469. https://doi.org/10.3390/catal13121469
Chicago/Turabian StyleSanyal, Tanushree Sankar, Amanda Ineza Mugisha, Andrew Sowinski, and Clémence Fauteux-Lefebvre. 2023. "Enhancement of Sulfur Oxide Capture Capacity by Deposition of Iron Oxide Particles on Graphene Oxide" Catalysts 13, no. 12: 1469. https://doi.org/10.3390/catal13121469