Construction of Benzo-Fused Polycyclic Heteroaromatic Compounds through Palladium-Catalyzed Intramolecular C-H/C-H Biaryl Coupling
Abstract
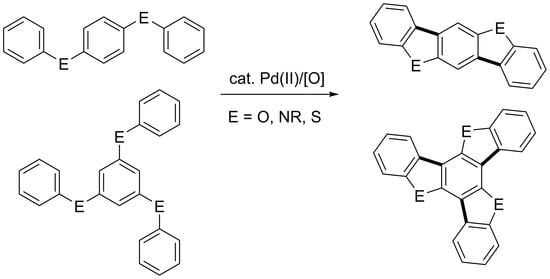
:1. Introduction
2. Dibenzofuran Derivatives and Related Compounds
3. Carbazole Derivatives and Related Compounds
4. Dibenzothiophene Derivatives and Related Compounds
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, T.; Zhao, J.; Asao, N.; Yamamoto, Y. Metal-Catalyzed Annulation Reactions for π-Conjugated Polycycles. Chem. Eur. J. 2014, 20, 3554–3576. [Google Scholar] [CrossRef]
- Segawa, Y.; Maekawa, T.; Itami, K. Synthesis of Extended π-Systems through C–H Activation. Angew. Chem. Int. Ed. 2015, 54, 66–81. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Grzybowski, M.; Sadowski, B.; Butenschön, H.; Gryko, D.T. Synthetic Applications of Oxidative Aromatic Coupling—From Biphenols to Nanographenes. Angew. Chem. Int. Ed. 2020, 59, 2998–3027. [Google Scholar] [CrossRef] [Green Version]
- Schatz, J.; Brendgen, T.; Schühle, D. Comprehensive Heterocyclic Chemistry IV, Vol. 3, Five-Membered Rings with One Heteroatom Together with Their Benzo- and other Carbocyclic-Fused Derivatives; Wong, N.H.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts; Wiley: Chichester, UK, 2004. [Google Scholar]
- Colletto, C.; Panigrahi, A.; Fernández-Casado, J.; Larrosa, I. Ag(I)–C–H Activation Enables Near-Room-Temperature Direct α-Arylation of Benzo[b]thiophenes. J. Am. Chem. Soc. 2018, 140, 9638–9643. [Google Scholar] [CrossRef] [Green Version]
- Tlahuext-Aca, A.; Lee, S.Y.; Sakamoto, S.; Hartwig, J.F. Direct Arylation of Simple Arenes with Aryl Bromides by Synergistic Silver and Palladium Catalysis. ACS Catal. 2021, 11, 1430–1434. [Google Scholar] [CrossRef]
- Mudarra, A.L.; de Salinas, S.M.; Pérez-Temprano, M.H. Beyond the traditional roles of Ag in catalysis: The transmetalating ability of organosilver(I) species in Pd-catalysed reactions. Org. Biomol. Chem. 2019, 17, 1655–1667. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Itatani, H. Palladium-Catalyzed Coupling Reaction of Aromatic Compounds. Bull. Chem. Soc. Jpn. 1973, 46, 2490–2492. [Google Scholar] [CrossRef] [Green Version]
- Shiotani, A.; Itatani, H. Dibenzofurans by Intramolecular Ring Closire Reactions. Angew. Chem. Int. Ed. Engl. 1974, 13, 471–472. [Google Scholar] [CrossRef]
- Åkermark, B.; Eberson, L.; Jonsson, E.; Pettersson, E. Palladium-Promoted Cyclization of Diphenyl Ether, Diphenylamine, and Related Compounds. J. Org. Chem. 1975, 40, 1365–1367. [Google Scholar] [CrossRef]
- Hagelin, H.; Oslob, D.J.; Åkermark, B. Oxygen as Oxidant in Palladium-Catalyzed Inter- and Intramolecular Coupling Reactions. Chem. Eur. J. 1999, 5, 2413–2416. [Google Scholar] [CrossRef]
- Liégault, B.; Lee, D.; Huestis, M.P.; Stuart, D.R.; Fagnou, K. Intramolecular Pd(II)-Catalyzed Oxidative Biaryl Synthesis Under Air: Reaction Development and Scope. J. Org. Chem. 2008, 73, 5022–5028. [Google Scholar] [CrossRef]
- Hellwinkel, D.; Kistenmacher, T. Palladium Acetate-Mediated Cyclizations of Di- and Trifunctional Triarylamines, Diaryl Ethers, and Diaryl Ketones. Liebigs Ann. Chem. 1989, 1989, 945–949. [Google Scholar] [CrossRef]
- Bergman, J.; Egestad, B. Cyclocondensation of 3(2H)-benzofuranone. Tetrahedron Lett. 1978, 19, 3143–3146. [Google Scholar] [CrossRef]
- Kaida, H.; Satoh, T.; Nishii, Y.; Hirano, K.; Miura, M. Synthesis of Benzo-bis- and Benzo-tris-benzofurans by Palladium-Catalyzed Multiple Intramolecular C-H/C-H Coupling. Chem. Lett. 2016, 45, 1069–1071. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Tsuboi, M.; Taniguchi, T.; Nishii, Y.; Tohnai, N.; Miura, M. Room Temperature Phosphorescent Crystals Consisting of Cyclized Guests and Their Uncyclized Mother Host Molecules. Chem. Lett. 2020, 49, 921–924. [Google Scholar] [CrossRef]
- Nakamura, S.; Okamoto, M.; Tohnai, M.; Nakayama, K.; Nishii, Y.; Miura, M. Synthesis and Properties of Tri-tert-Butylated Trioxa and Trithia Analogues of Truxene. Bull. Chem. Soc. Jpn. 2020, 93, 99–108. [Google Scholar] [CrossRef]
- Ogaki, T.; Ohta, E.; Oda, Y.; Sato, H.; Matsui, Y.; Komeda, M.; Ikeda, H. Intramolecular Triple Cyclization Strategy for Sila- and Oxa-Analogues of Truxene with Long-Lived Phosphorescence. Asian J. Org. Chem. 2017, 6, 290–296. [Google Scholar] [CrossRef]
- Saito, K.; Chikkade, P.K.; Kanai, M.; Kuninobu, Y. Palladium-Catalyzed Construction of Heteroatom-Containing π-Conjugated Systems by Intramolecular Oxidative C-H/C-H Coupling Reaction. Chem. Eur. J. 2015, 21, 8365–8368. [Google Scholar] [CrossRef]
- Kaida, H.; Satoh, T.; Hirano, K.; Miura, M. Synthesis of Thieno[3,2-b]benzofurans by Palladium-Catalyzed Intramolecular C-H/C-H Coupling. Chem. Lett. 2015, 44, 1125–1127. [Google Scholar] [CrossRef]
- Kaida, H.; Goya, T.; Nishii, Y.; Hirano, K.; Satoh, T.; Miura, M. Construction of Bisbenzofuro[2,3-b:3′,2′-e]pyridines by Palladium-Catalyzed Double Intramolecular Oxidative C-H/C-H Coupling. Org. Lett. 2017, 19, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Itai, Y.; Nishii, Y.; Stachelek, P.; Data, P.; Takeda, Y.; Minakata, S.; Miura, M. Syntheses of Diverse Donor-Substituted Bisbenzofuro[2,3-b:3’,2’-e]pyridines (BBZFPys) via Pd Catalysis, and Their Photophysical Properties. J. Org. Chem. 2018, 83, 10289–10302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Hori, Y.; Kobayashi, T. A New Hybrid Phosphine Ligand for Palladium-Catalyzed Amination of Aryl Halides. Adv. Synth. Catal. 2008, 350, 652–656. [Google Scholar] [CrossRef]
- Takishima, R.; Nishii, Y.; Hinoue, T.; Imai, Y.; Miura, M. Synthesis and circularly polarized luminescence properties of BINOL-derived bisbenzofuro[2,3-b:3′,2′-e]pyridines (BBZFPys). Beilstein J. Org. Chem. 2020, 16, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Knölker, H.-J.; O’Sullivan, N. Indoloquinones—3. Palladium-promoted synthesis of hydroxy-substituted 5-Cyano-5H-benzo[b]carbazole-6, 11-diones. Tetrahedron 1994, 50, 10893–10908. [Google Scholar] [CrossRef]
- Watanabe, T.; Oishi, S.; Fujii, N.; Ohno, H. Palladium-Catalyzed Direct Synthesis of Carbazoles via One-Pot N-Arylation and Oxidative Biaryl Coupling: Synthesis and Mechanistic Study. J. Org. Chem. 2009, 74, 4720–4726. [Google Scholar] [CrossRef]
- Kober, U.; Knölker, H.-J. Palladium-Catalyzed Approach to Malasseziazole A and Fiest Total Synthesis of Malasseziazole C. Synlett 2015, 26, 1549–1552. [Google Scholar]
- Wang, X.; Lu, W.; Gu, Z.-Y.; Weia, Z.; Zhou, H.-C. Topology-guided design of an anionic bor-network for photocatalytic [Ru(bpy)3]2+ encapsulation. Chem. Commun. 2016, 52, 1926–1929. [Google Scholar] [CrossRef]
- Jones, A.W.; Louillat-Habermeyer, M.-L.; Patureau., F.W. Strained Dehydrogenative Ring Closure of Phenylcarbazoles. Adv. Synth. Catal. 2015, 357, 945–949. [Google Scholar] [CrossRef]
- Taniguchi, T.; Itai, Y.; Nishii, Y.; Tohnai, N.; Miura, M. Construction of Nitrogen-Containing Polycyclic Aromatic Compounds by Intramolecular Oxidative C-H/C-H Coupling of Bis(9H-Carbazol-9-yl)benzenes and Their Properties. Chem. Lett. 2019, 48, 1160–1163. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Nishii, Y.; Mori, T.; Nakayama, K.; Miura, M. Synthesis, Structure, and Chiroptical Properties of Indolo- and Pyridopyrrolo- Carbazole-Based C2-Symmetric Azahelicenes. Chem. Eur. J. 2021, 27, 7356–7361. [Google Scholar] [CrossRef]
- Vicente, J.; Abad, J.A.; López-Nicolás, R.-M.; Jones, P.G. Palladated Oligophenylene Thioethers: Synthesis and Reactivity toward Isocyanides, Carbon Monoxide, and Alkynes. Organometallics 2011, 30, 4983–4998. [Google Scholar] [CrossRef]
- Che, R.; Wu, Z.; Li, Z.; Xiang, H.; Zhou, X. Synthesis of Dibenzothiophenes by Pd-Catalyzed Dual C-H Activation from Diaryl Sulfides. Chem. Eur. J. 2014, 20, 7258–7261. [Google Scholar] [CrossRef]
- Oechsle, P.; Paradies, J. Ambidextrous Catalytic Access to Dithieno[3,2-b:2’,3’-d]thiophene (DTT) Derivatives by Both Palladium-Catalyzed C-S and Oxidative Dehydro C-H Coupling. Org. Lett. 2014, 16, 4086–4089. [Google Scholar] [CrossRef]
- Masui, K.; Ikegami, H.; Mori, A. Palladium-Catalyzed C−H Homocoupling of Thiophenes: Facile Construction of Bithiophene Structure. J. Am. Chem. Soc. 2004, 126, 5074–5075. [Google Scholar] [CrossRef]
- Tsuboi, M.; Nakamura, S.; Nandi, S.; de Silva, P.; Takeda, Y.; Miura, M. Syntheses and Room Temperature Phosphorescence Properties of Dibenzobenzodithiophenes and Dibenzothiophenes. Bull. Chem. Soc. Jpn. 2021, 94, 2498–2504. [Google Scholar] [CrossRef]



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishii, Y.; Miura, M. Construction of Benzo-Fused Polycyclic Heteroaromatic Compounds through Palladium-Catalyzed Intramolecular C-H/C-H Biaryl Coupling. Catalysts 2023, 13, 12. https://doi.org/10.3390/catal13010012
Nishii Y, Miura M. Construction of Benzo-Fused Polycyclic Heteroaromatic Compounds through Palladium-Catalyzed Intramolecular C-H/C-H Biaryl Coupling. Catalysts. 2023; 13(1):12. https://doi.org/10.3390/catal13010012
Chicago/Turabian StyleNishii, Yuji, and Masahiro Miura. 2023. "Construction of Benzo-Fused Polycyclic Heteroaromatic Compounds through Palladium-Catalyzed Intramolecular C-H/C-H Biaryl Coupling" Catalysts 13, no. 1: 12. https://doi.org/10.3390/catal13010012