Promoting Effect of Cu on Pd Applied to the Hydrazine Electro-Oxidation and Direct Hydrazine Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
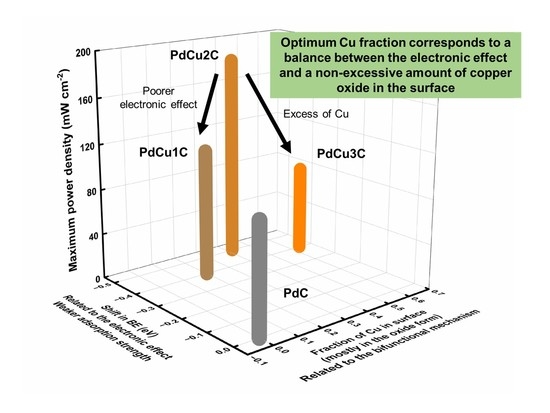
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct Liquid Fuel Cells: A Review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Liquid Fuel Cells. Beilstein J. Nanotechnol. 2014, 5, 1399–1418. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, V. Review on Challenges of Direct Liquid Fuel Cells for Portable Application. World J. Eng. 2015, 12, 591–606. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Imran Jafri, R.; Dhathathreyan, K.S. Research Advancements in Low-Temperature Fuel Cells. In Electrocatalysts for Low Temperature Fuel Cells; Maiyalagan, T., Saji, V.S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 35–74. ISBN 9783527803873. [Google Scholar]
- Corti, H.R.; Gonzalez, E.R. Introduction to Direct Alcohol Fuel Cells. In Direct Alcohol Fuel Cells: Materials, Performance, Durability and Applications; Corti, H.R., Gonzalez, E.R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–32. ISBN 978-94-007-7708-8. [Google Scholar]
- Gonzalez, E.R.; Mota-Lima, A. Catalysts for Methanol Oxidation. In Direct Alcohol Fuel Cells: Materials, Performance, Durability and Applications; Corti, H.R., Gonzalez, E.R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 33–62. ISBN 978-94-007-7708-8. [Google Scholar]
- Solorza-Feria, O.; Varela, F.J.R. Pt and Pd-Based Electrocatalysts for Ethanol and Ethylene Glycol Fuel Cells. In Direct Alcohol Fuel Cells: Materials, Performance, Durability and Applications; Corti, H.R., Gonzalez, E.R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 63–78. ISBN 978-94-007-7708-8. [Google Scholar]
- Gomes, J.F.; Pratta, P.M.P.; Tremiliosi-Filho, G. Electro-Oxidation of 3-Carbon Alcohols and Its Viability for Fuel Cell Application. In Direct Alcohol Fuel Cells. Materials, Performance, Durability and Applications; Corti, H.R., Gonzalez, E.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 79–98. ISBN 978-94-007-7708-8. [Google Scholar]
- Hayashi, H. Hydrazine Synthesis: Commercial Routes, Catalysis and Intermediates. Res. Chem. Intermed. 1998, 24, 183–196. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct Hydrazine Fuel Cells: A Review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Halim, E.M.; Chemchoub, S.; El Attar, A.; Salih, F.E.; Oularbi, L.; EL RHAZI, M. Recent Advances in Anode Metallic Catalysts Supported on Conducting Polymer-Based Materials for Direct Alcohol Fuel Cells. Front. Energy Res. 2022, 10, 1–29. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct Liquid-Feed Fuel Cells: Thermodynamic and Environmental Concerns. J. Power Sources 2007, 169, 239–246. [Google Scholar] [CrossRef]
- Wang, J.; Khaniya, A.; Hu, L.; Beazley, M.J.; Kaden, W.E.; Feng, X. A Bifunctional Catalyst for Efficient Dehydrogenation and Electro-Oxidation of Hydrazine. J. Mater. Chem. A 2018, 6, 18050–18056. [Google Scholar] [CrossRef]
- Das, A.K.; Kim, N.H.; Pradhan, D.; Hui, D.; Lee, J.H. Electrochemical Synthesis of Palladium (Pd) Nanorods: An Efficient Electrocatalyst for Methanol and Hydrazine Electro-Oxidation. Compos. Part B Eng. 2018, 144, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.X.-Y.; Shi, S.; Yin, H.H.-M. CuPd Alloy Oxide Nanobelts as Electrocatalyst Towards Hydrazine Oxidation. ChemElectroChem 2019, 6, 1514–1519. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Daneshvari-Esfahlan, V.; Wolf, S.; Hacker, V. Cobalt-Modified Palladium Nanocatalyst on Nitrogen-Doped Reduced Graphene Oxide for Direct Hydrazine Fuel Cell. RSC Adv. 2021, 11, 39223–39232. [Google Scholar] [CrossRef] [PubMed]
- Korovin, N.V.; Yanchuk, B.N. Hydrogen Sorption by Palladium in Hydrazine Electro-Oxidation. Electrochim. Acta 1970, 15, 569–580. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, W.; Gao, W.; Qi, L.; Zhao, J.; Xu, M.; Xu, G. Facet-Dependent Electrocatalytic Activities of Pd Nanocrystals toward the Electro-Oxidation of Hydrazine. Electrochem. Commun. 2013, 37, 57–60. [Google Scholar] [CrossRef]
- Yamada, K.; Yasuda, K.; Tanaka, H.; Miyazaki, Y.; Kobayashi, T. Effect of Anode Electrocatalyst for Direct Hydrazine Fuel Cell Using Proton Exchange Membrane. J. Power Sources 2003, 122, 132–137. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Matsunaga, T.; Hayashi, T. Electrocatalytic Activities of Metal Electrodes in Anodic Oxidation of Hydrazine in Alkaline Solution. Electrochim. Acta 1981, 26, 631–636. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Hui, D.; Lee, J.H. Surfactant-Free Synthesis of NiPd Nanoalloy/Graphene Bifunctional Nanocomposite for Fuel Cell. Compos. Part B Eng. 2017, 114, 319–327. [Google Scholar] [CrossRef]
- Wu, L.-S.; Wen, X.-P.; Wen, H.; Dai, H.-B.; Wang, P. Palladium Decorated Porous Nickel Having Enhanced Electrocatalytic Performance for Hydrazine Oxidation. J. Power Sources 2019, 412, 71–77. [Google Scholar] [CrossRef]
- Aldous, L.; Compton, R.G. The Mechanism of Hydrazine Electro-Oxidation Revealed by Platinum Microelectrodes: Role of Residual Oxides. Phys. Chem. Chem. Phys. 2011, 13, 5279–5287. [Google Scholar] [CrossRef]
- Garside, M. Global Mine Production of Palladium in 2021, by Country. Available online: https://www.statista.com/statistics/273647/global-mine-production-of-palladium/ (accessed on 28 September 2022).
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent Advances in Palladium-Based Electrocatalysts for Fuel Cell Reactions and Hydrogen Evolution Reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Bianchini, C. Palladium-Based Electrocatalysts for Alcohol Oxidation in Direct Alcohol Fuel Cells. In Interfacial Phenomena in Electrocatalysis; Vayenas, C.G., Ed.; Springer: New York, NY, USA, 2011; pp. 203–253. ISBN 978-1-4419-5580-7. [Google Scholar]
- Crisafulli, R.; de Barros, V.V.S.; Rodrigues de Oliveira, F.E.; de Araújo Rocha, T.; Zignani, S.; Spadaro, L.; Palella, A.; Dias, J.A.; Linares, J.J. On the Promotional Effect of Cu on Pt for Hydrazine Electrooxidation in Alkaline Medium. Appl. Catal. B Environ. 2018, 236, 36–44. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, L.; Qin, Q.; Pei, F.; Hu, C.; Zheng, N. Self-Supported 3D PdCu Alloy Nanosheets as a Bifunctional Catalyst for Electrochemical Reforming of Ethanol. Small 2017, 13, 1602970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Y.-Y.; Zhao, Z.-G. Surface Voltammetric Dealloying Investigation on PdCu/C Electrocatalysts toward Ethanol Oxidation in Alkaline Media. J. Nanoparticle Res. 2018, 20, 314. [Google Scholar] [CrossRef]
- Babu, S.P.; Elumalai, P. Tunable Compositions of Pd100-xCux Catalysts towards the Electrooxidation of Ethanol and Ethylene Glycol. New J. Chem. 2017, 41, 13812–13822. [Google Scholar] [CrossRef]
- Mirza, S.; Chen, H.; Gu, Z.-G.; Zhang, J. Electrooxidation of Pd–Cu NP Loaded Porous Carbon Derived from a Cu-MOF. RSC Adv. 2018, 8, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Xu, H.; Jin, L.; Chen, C.; Shang, H.; Liu, Q.; Du, Y. A Novel Catalyst for Efficient Electrooxidation of Ethanol Enabled by 3D Open-Structured PdCu Nanocages. J. Colloid Interface Sci. 2019, 555, 195–202. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, N.; Yin, Y.; Xue, J.; Dong, C.; Guo, P. Synthesis of PdCu Nanowire Assembly and Their Catalytic Activity toward Ethanol Oxidation. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123909. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, J.-J.; Jiang, K.; Wang, J.-Q.; Cai, W.-B. Pd–Cu/C Electrocatalysts Synthesized by One-Pot Polyol Reduction toward Formic Acid Oxidation: Structural Characterization and Electrocatalytic Performance. Int. J. Hydrogen Energy 2015, 40, 1726–1734. [Google Scholar] [CrossRef]
- Sakong, S.; Mosch, C.; Groß, A. CO Adsorption on Cu–Pd Alloy Surfaces: Ligand versus Ensemble Effects. Phys. Chem. Chem. Phys. 2007, 9, 2216–2225. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, W.; Chen, W.; Wu, Y.; Xu, Q.; Yan, Y.; Zhang, H.; Wu, J.; Yang, D. PdCu Alloy Nanodendrites with Tunable Composition as Highly Active Electrocatalysts for Methanol Oxidation. RSC Adv. 2017, 7, 5800–5806. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Inam, M.A.; Zam Zam, S.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter Under Different PH Values. Sustainability 2019, 11, 2825. [Google Scholar] [CrossRef]
- Mukundan, V.; Yin, J.; Joseph, P.; Luo, J.; Shan, S.; Zakharov, D.N.; Zhong, C.-J.; Malis, O. Nanoalloying and Phase Transformations during Thermal Treatment of Physical Mixtures of Pd and Cu Nanoparticles. Sci. Technol. Adv. Mater. 2014, 15, 25002–250017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.R.; Ghasemi, S.; Ghasemi, S.A. Fabrication and Performance Evaluation of Pd-Cu Nanoparticles for Hydrogen Evolution Reaction. ChemistrySelect 2019, 4, 6854–6861. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Shan, S.; Xie, Z.-H.; Kang, N.; Park, K.; Hopkins, E.; Yan, S.; Sharma, A.; Luo, J.; Wang, J.; et al. Revealing the Role of Phase Structures of Bimetallic Nanocatalysts in the Oxygen Reduction Reaction. ACS Catal. 2018, 8, 11302–11313. [Google Scholar] [CrossRef]
- Sengar, S.K.; Mehta, B.R. Govind Size and Alloying Induced Shift in Core and Valence Bands of Pd-Ag and Pd-Cu Nanoparticles. J. Appl. Phys. 2014, 115, 124301. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Zhang, Z.; Chen, B.; Huang, Y.; Chen, J.; Lai, Z.; Chen, Y.; Sindoro, M.; Wang, A.-L.; Cheng, H.; et al. Synthesis of Ultrathin PdCu Alloy Nanosheets Used as a Highly Efficient Electrocatalyst for Formic Acid Oxidation. Adv. Mater. 2017, 29, 1700769. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Cai, K.; Zhang, H.; Lu, Z.; Li, T.; Zuo, Y.; Han, H. Clean Synthesis of an Economical 3D Nanochain Network of PdCu Alloy with Enhanced Electrocatalytic Performance towards Ethanol Oxidation. Chem.—A Eur. J. 2015, 21, 17779–17785. [Google Scholar] [CrossRef]
- Zhu, L.; Song, K.; Yi, C. One-Pot Aqueous Synthesis of Porous Hollow PdCu Alloy Nanoparticles for Enhanced Ethanol Electrooxidation. Inorg. Chem. 2022, 61, 5474–5478. [Google Scholar] [CrossRef]
- Harrison, J.A.; Khan, Z.A. The Oxidation of Hydrazine in Alkaline Solution at Platinum and Mercury. J. Electroanal. Chem. Interfacial Electrochem. 1970, 26, 1–11. [Google Scholar] [CrossRef]
- Maillard, F.; Silva, W.O.; Castanheira, L.; Dubau, L.; Lima, F.H.B. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: Spectrometric Evidence for Pt-Catalysed Decarboxylation at Anode-Relevant Potentials. ChemPhysChem 2019, 20, 3106–3111. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced Analysis of Copper X-Ray Photoelectron Spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Electrochemical Study of Bulk and Monolayer Copper in Alkaline Solution. J. Electrochem. Soc. 2016, 163, H252. [Google Scholar] [CrossRef]
- Grdeń, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical Behaviour of Palladium Electrode: Oxidation, Electrodissolution and Ionic Adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]







| Catalyst | Metallic Loading | Pd:Cu Atomic Ratio | Label |
|---|---|---|---|
| Pd/C | 23 | - | PdC |
| Pd3Cu/C | 20 | 3.5 (nominal of 3) | PdCu1C |
| Pd1Cu/C | 19 | 1.13 (nominal of 1) | PdCu2C |
| Pd0.33Cu/C | 19 | 0.45 (nominal of 0.33) | PdCu3C |
| Cu/C | 13 | - | CuC |
| Catalyst | dXRD (nm) | dTEM (nm) | Lattice Parameter (nm) | Alloy Composition | Cualloyed |
|---|---|---|---|---|---|
| PdC | 4.3 | 6.4 | 0.3890 | -- | -- |
| PdCu1C | 3.0 | 5.3 | 0.3856 | Pd85Cu15 | 0.59 |
| PdCu2C | 2.7 | 5.0 | 0.3845 | Pd81Cu19 | 0.26 |
| PdCu3C | -- | 4.8 | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisafulli, R.; de Paula, D.F.; Zignani, S.C.; Spadaro, L.; Palella, A.; Boninelli, S.; Dias, J.A.; Linares, J.J. Promoting Effect of Cu on Pd Applied to the Hydrazine Electro-Oxidation and Direct Hydrazine Fuel Cells. Catalysts 2022, 12, 1639. https://doi.org/10.3390/catal12121639
Crisafulli R, de Paula DF, Zignani SC, Spadaro L, Palella A, Boninelli S, Dias JA, Linares JJ. Promoting Effect of Cu on Pd Applied to the Hydrazine Electro-Oxidation and Direct Hydrazine Fuel Cells. Catalysts. 2022; 12(12):1639. https://doi.org/10.3390/catal12121639
Chicago/Turabian StyleCrisafulli, Rudy, Dryade Ferreira de Paula, Sabrina C. Zignani, Lorenzo Spadaro, Alessandra Palella, Simona Boninelli, José A. Dias, and José J. Linares. 2022. "Promoting Effect of Cu on Pd Applied to the Hydrazine Electro-Oxidation and Direct Hydrazine Fuel Cells" Catalysts 12, no. 12: 1639. https://doi.org/10.3390/catal12121639