1. Introduction
Glioblastoma (GBM) is an extremely aggressive primary brain malignancy in adults. It has a close to a 100% fatality rate, largely due to an almost inevitable regrowth of the tumor after treatment [
1,
2]. The current standard of care is a combination of surgical resection, tumor treating fields, temozolomide (TMZ)-based chemotherapy, and radiation treatment. However, even with that standard of care, patients have a median survival of approximately 21 months with a significant decline in their quality of life throughout the treatment regimen [
3,
4,
5]. The incredibly low survival time is driven by the fact that the recurrent tumor is resistant to our current therapeutic strategies, necessitating the investigation of the mechanism of therapeutic resistance.
It is now well-established that GBM contains a subpopulation of cells known as glioma stem cells (GSCs) that contribute to the therapeutic resistance displayed by the recurrent tumor [
6,
7,
8,
9,
10]. We and others have demonstrated that, during therapy, expansion of the treatment-resistant GSC pool appears to play a crucial role in therapeutic resistance [
11,
12]. In order to prevent this expansion, it is necessary to understand exactly how GSC populations are enriched during and after treatment. Previous studies have suggested that GSCs expand through both symmetric and asymmetric cell division [
13,
14]. Symmetric cell division is when one GSC gives rise to two more GSCs, whereas asymmetric cell division is when a GSC gives rise to one GSC and one differentiated cell. However, it is not clear if cell division patterns play a role in post-therapy stemness in GBM. Here, we examine if anti-GBM chemotherapy TMZ influences polarized cell division to enhance the post-therapy cellular plasticity in GBM [
14].
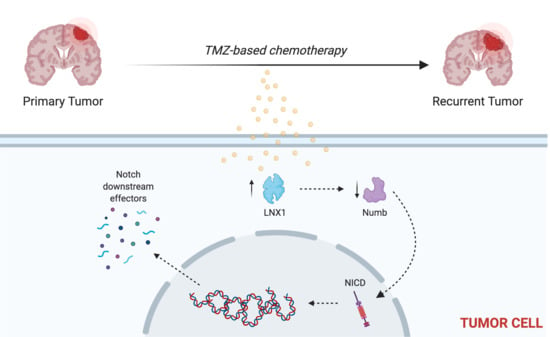
Notch1 has consistently been shown to play a role in cell division and stemness, and resulting therapeutic resistance, both in GBM and in other cancers [
15,
16,
17,
18,
19]. Unfortunately, clinical trials directly targeting Notch1 have consistently failed due to unacceptable side effects from Notch1 inhibitors [
20]. Here, we examine LNX1 as a regulator of Notch1 signaling to ultimately control the expansion of the stem cell compartment after TMZ therapy. This allows us a novel pathway through which to target the oncogenic activity of Notch1 in the GBM—a pathway that is much more targeted to tumor cells and therefore, may be much less likely to be toxic to patients.
3. Discussion
GBM is a devastating disease that currently carries a dismal prognosis for patients. As such, it is desperately in need of novel therapies that may provide improved outcomes for patients. Previously, our lab and others have shown that there is a subpopulation of cells in GBM termed GSCs that likely drive the aggressive nature of GBM by driving its therapeutic resistance and recurrence. We have further shown that treatment of tumors with TMZ results in expansion of the GSC compartment, which we believe is occurring due to plasticity-dependent mechanisms within the tumor.
Here, we have shown that cell cycle and stemness programs are significantly upregulated in GBM cells following TMZ therapy. This result has been corroborated by other studies across many different cancers, further bolstering the idea that cell division and expansion of the stem cell compartment are key for tumor progression after therapy [
8,
13,
24]. We have additionally shown that polarized cell division regulators are specifically altered after TMZ therapy in GBM, suggesting that the expansion of the stem cell compartment observed after TMZ therapy in part driven by changes in regulators of polarized cell division. Of these regulators, we were able to identify LNX1 as a novel target that is significantly upregulated after TMZ therapy.
Other studies have shown that LNX1 regulates Numb and Notch1 and may have a role in cell division programs [
26,
33]. However, no study has examined the specific role of this entire axis and how it may contribute to the expansion of the stem cell compartment, specifically in GBM post-therapy. We were able to show that LNX1 does negatively regulate Numb, which in turn negatively regulates Notch1 expression in GBM cells. In response to TMZ, LNX1 was elevated, resulting in elevations in Notch1 and corresponding gene-level activation of Notch1 downstream genes, which further results in an increased stemness phenotype in cells. This axis was validated both by overexpression studies and by knockdown studies. Furthermore, from a clinical perspective, we were able to show both that LNX1 enrichments do occur in patients and that they are associated with decreases in survival. We were also able to show that loss of LNX1 expression can result in longer survival times in our clinically relevant animal model.
Given that we know GSCs are a major driver of GBM’s aggressiveness, this increased stemness phenotype associated with LNX1 activation suggests that LNX1 may be a promising therapeutic target to block Notch signaling in GBM. Targeting LNX1 may allow for more nuanced modulation of Notch1, which may result in significantly improved outcomes for patients. Notch1 is a well-known modulator of GBM and many clinical trials have been attempted to target Notch1. This is a gene known to be significantly elevated across all GBM tumors and known to be involved in promoting cellular plasticity as it has a multitude of functions in determining cell fate, differentiation, and proliferation. As such, it is able to promote more aggressive cell states. Unfortunately, all therapies developed to target Notch1 have failed due to the unbearable toxicities associated with these inhibitors and the involved dosing schedules required of patients. LNX1 therefore could be a way to target Notch1 more simply. Furthermore, since LNX1 is only elevated in GBM cells, it also provides a more nuanced and targeted approach to reducing Notch1 activity.
4. Materials and Methods
4.1. Gene Expression Analysis
RNA was extracted from samples using the RNEasy kit (Qiagen, Hilden, Germany), as per manufacturer’s instructions. For each analysis, 1ug of RNA was utilized with Human HT12 (48,000 probes, RefSeq + Expressed Sequence Tag (EST)), again as per the manufacturer’s directions (Illumina, San Diego, CA, USA). All microarrays were performed in triplicate to ensure appropriate replicates for the experiment.
These data were analyzed for gene expression pre- and post-TMZ, looking at genes involved in cell division and stemness pathways. This work was performed by a trained biostatistician using an established gene set enrichment analysis algorithm [
34]. Briefly, this algorithm identifies biological pathways as a whole and assesses their enrichment over different conditions as well as the significance of that enrichment (FDR value). It also generates fold change for expression for specific genes based on the microarray data.
4.2. RNA-Seq Analysis
Cells were treated for four days with 50 µM TMZ or equimolar DMSO in triplicate. Cell pellets were then harvested and are sent to Novogene’s sequencing lab (UC Davis, CA, USA). Novogene performed extractions and sequencing and returned results of sequencing.
These data were analyzed for gene expression pre- and post-TMZ, looking at genes involved in polarized cell division, as identified in Drosophila studies [
14]. This work was performed by applying the DESeq2 algorithm as previously described across our genes of interest [
35]. Briefly, this algorithm estimates the dispersion of gene counts as compared to an overall mean and thus generates a fold change and a
p-value for each gene of interest.
4.3. Cell Lines and Culture
U251, a human glioma cell line, was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; HyClone, Thermo Fisher Scientific, San Jose, CA, USA), with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA) and 1% penicillin-streptomycin antibiotic mixture (Cellgro, Herndon, VA, USA; Mediatech, Herndon, VA, USA).
Our patient-derived xenograft (PDX) glioma cells (GBM43, GBM5, and GBM6) were obtained from Dr. C. David James at Northwestern University and maintained according to published protocols [
36]. They were cultured in DMEM, supplemented with 1% FBS and 1% penicillin-streptomycin. Cells were used for a maximum of 4 passages before being replenished from frozen stock. Frozen cells were maintained in pure FBS supplemented with 10% dimethyl sulfoxide (DMSO) in liquid nitrogen at −180 °C.
4.4. Immunofluorescence Staining
For in vitro experiments, cells were cultured as described above. In preparation for staining, they were re-plated in Lab-Tek II 8-well chambers (Thermo Fisher Scientific, Waltham, MA, USA) and then treated with the appropriate drug (DMSO or TMZ 50 µM) 24 h after being plated. After treatment duration was complete, cells were washed once with PBS and then fixed for 10 min at room temperature with cold 4% PFA (Thermo Fisher Scientific). They were washed after fixation and then blocked in 10% BSA + Triton-X (Thermo Fisher Scientific) for 2 h. Cells were then maintained in the appropriate primary antibodies—LNX1 1:200 (Invitrogen, Carlsbad, CA, USA) or Numb 1:200 (Thermo Fisher Scientific)—an diluted in 1% BSA overnight at 4 °C. The next day, cells were washed 3 times for 8 min each and then placed in the secondary antibodies conjugated to either Cy3 or FITC (Thermo Fisher Scientific) at a dilution of 1:1000 in 1% BSA for 2 h. Following secondary incubation, cells were washed 3 times for 10 min and then mounted with DAPI-containing mounting media (Invitrogen).
For in vivo experiments, mice were perfused with ice-cold PBS (Gibco, Waltham, MA, USA) following euthanasia. They were then frozen in cryoprotectant on ethanol and dry ice and were subsequently stored at −80 °C until required for experimentation. For staining, samples were sectioned at a thickness of 8 μM and then stained as per standard immunohistochemistry protocols [
37]. In brief, sections were thawed for 30 min at 37 °C. They were washed with PBS 3 times for 5 min each following thawing to remove additional cryoprotectant. The cells were then fixed in cold 4% PFA (Thermo Fisher Scientific) at room temperature for 15 minutes and were once again washed with PBS 3 times for 5 min each. Sections were then incubated in 10% BSA + Triton-X for 2 h to block and were then incubated in the appropriate primary antibodies, as above, overnight at 4 °C. Following this, sections were washed in PBS 3 times for 10 min each and then incubated in secondary antibodies as above at 1:1000 for 2 h at room temperature. Finally, samples are thoroughly washed (3–4 times for 10 min each) and mounted with DAPI-containing mounting media.
All slides were imaged using a Leica confocal and images were analyzed using ImageJ software [
38]. Where relevant, counts of cell division were performed by calculating relative intensity values in ImageJ. In addition, where relevant, colocalization correlation coefficients (i.e., Pearson’s correlation coefficient) were calculated by using the in-built Coloc2 package in ImageJ [
39].
4.5. Immunohistochemistry of Human Samples
Human glioma samples (primary and recurrent) were obtained from Northwestern University’s Nervous System Tumor Bank. All patients gave consent as per the defined Institutional Review Board (IRB) policies prior to obtaining samples. Staining was performed as per standard immunohistochemistry protocols, previously established in that lab [
37]. Briefly, samples were formalin-fixed and paraffin-embedded (FFPE). They were then sectioned at a thickness of 4 μM, after being heated at 60 °C for at least 1 h. Antigen retrieval was performed with a BioCare Medical Decloaking Chamber using high (LC3) or low pH antigen retrieval buffer from Dako (Agilent, Santa Clara, CA, USA). Primary antibodies were incubated for 1 hour at room temperature followed with horseradish peroxidase (HRP)-tagged secondary antibodies as appropriate. Slides were scored for LNX1 expression on a scale of 1 (lowest) to 3 (highest) by a board-certified neuropathologist (CMH), and scores were plotted alongside survival data.
4.6. Quantitative PCR
Cells were harvested and RNA extraction was performed using Qiagen RNA extraction kits (Qiagen), as per the manufacturer’s protocol. cDNA was generated from RNA samples using iScript kits (BioRad, Hercules, CA, USA), as per the manufacturer’s protocol. Once generated, cDNA was diluted 1:10 to use for downstream qPCR. PCR reactions were set up with a standard amount of cDNA, SyberGreen, forward and reverse primers, and ddH2O. All primers were generated from Primer-BLAST using the native settings. Reactions were all performed in biological triplicate and technical duplicate.
4.7. Western Blot
Cells were harvested and protein was extracted using mPER lysis buffer (Thermo Fisher Scientific) with protease and phosphatase inhibitors (Cell Signaling Technologies, Danvers, MA, USA). Cells were then sonicated 3 times for 30 s each with 10 s intervals in between. Next, they were incubated on ice for 10 min and then centrifuged for 10 min at 13,000 rpm. The clear supernatant was recovered and used for protein assays. Samples were equalized using the Pierce BSA Protein Assay Kit (Thermo Fisher Scientific), as per the manufacturer’s instructions. Prepared samples were incubated at 95 °C for 10 min and were then cooled to room temperature and loaded into a pre-poured 10% SDS-PAGE gel. Gels were run for 30 min at 40 V and then 95 V until samples had run all the way through. Gels were then transferred to nitrocellulose membranes for 1 h at 14 V, blocked for 1 h in 5% milk (Thermo Fisher Scientific), and incubated overnight in primary antibody diluted in 5% BSA (Thermo Fisher Scientific). Primaries used include LNX1 1:1000 (LSBio, Seattle, WA, USA) and Numb, Notch1, Hes1, p21, MAML, and beta-Actin, all used at a dilution of 1:1000 (Cell Signaling Technologies). The next morning, membranes were washed 3 times for 10 min in TBS-T buffer and then incubated in secondary antibodies made against mouse and rabbit as appropriate in milk at a dilution of 1:4000 (Cell Signaling Technologies). Membranes were washed again 3 times for 20 min and then developed using the BioRad imaging system (BioRad).
If immunoprecipitation was required prior to Western blotting, cells were thoroughly washed and then protein was isolated using mPER and protease/phosphatase inhibitors as described above. However, prior to equalization and further blotting, an immunoprecipitation was performed using the antibody of interest and the Protein A/G Plus Ultralink Resin Kit (Thermo Fisher Scientific), as per the manufacturer’s instructions. Briefly, the protein extract and relevant antibody were incubated together overnight with gentle rotation at 4 °C. The next day, a coupling with UltraLink resin was performed for 2 h at room temperature, also with gentle rotation. Following that, IP buffer was added, and samples were centrifuged for 3 min at 2500× g, after which the supernatant was discarded. This step was repeated 3 times. Finally, the binding protein was eluted with 50 μL of elution buffer and Western blot samples were then prepared as above.
4.8. Neurosphere Assays and Extreme Limiting Dilution Analysis (ELDA)
Cell lines were cultured as described. They were then harvested, washed with PBS, and plated in serial dilutions, specifically 200, 150, 100, 50, 25, 12, 6 and 3 cells per well. Each dilution was performed in 12 replicates. Cells were maintained in neurobasal media (Gibco) supplemented with B27 (no Vitamin A; Invitrogen, Carlsbad, CA, USA), basic fibroblast growth factor (bFGF; 10 ng/mL; Invitrogen), epidermal growth factor (EGF; 10 ng/mL; Invitrogen), and N2 (Invitrogen). Cells were treated either with 50 μM TMZ or equimolar DMSO. A blinded experimenter examined the wells after 7 days. The number of formed neurospheres with a diameter greater than 20 cells was counted. Counts were analyzed using the Walter + Eliza Hall Institute of Medical Research platform (
http://bioinf.wehi.edu.au/software/elda/). This platform allows for the determination of stem cell frequency and quantification of significant differences between groups. In addition, the absolute number of spheres was plotted visually, and images were taken of the wells using a Leica confocal microscope (Buffalo Grove, IL, USA).
4.9. Notch Reporter Transfection
Cells were plated at approximately 60% confluency, 24 h prior to transfection. High Efficiency DNA transfection reagent (Sigma Aldrich, St Louis, MO, USA) was used to perform transfections, as per the manufacturer’s protocols. Briefly, transfection reagent and Notch reporter plasmids (#44211, 47684, 47683; Addgene, Cambridge, MA, USA) were combined in serum free OptiMEM media (Gibco). Specifically, 2 μg of plasmid was used per well for a 6-well plate and transfection reagent was added at a ratio of 1:3. The mix was incubated for 30 min with a thorough vortex every 10 min. Then, it was distributed dropwise to plated cells. Cells were maintained for 48–72 h and were then harvested for downstream analysis by flow cytometry, as described below.
4.10. Flow Cytometry
Cells were cultured as described above. At relevant time points following transfection or transduction as needed, cells were collected, and fresh surface staining was performed. Cells were first washed with sterile PBS (Gibco). Next, they were detached from the plate using 0.05% trypsin/0.53 mM EDTA (Corning, Corning, NY, USA). Trypsin was neutralized using an equal amount of culture media, and cells were collected in appropriately sized tubes. Cells were then incubated with conjugated antibodies against CD133-APC (Miltenyi Biotc, Bergisch Gladbach, Germany) for 30 min at room temperature. They were washed thoroughly with PBS (3 washes for 5 min each) and were analyzed using the flow cytometer. Further analysis and quantification of results was performed using the FlowJo software.
4.11. Viability Assays
Viability assays were conducted using the MTT assay. In brief, cells were plated at a density of 3000 cells per well in a 96-well plate with 8 replicates plated for each condition. After 24 h, media was changed to media supplemented with the appropriate drug concentration. Three days later, cells were thoroughly washed with phosphate buffered saline (PBS) and incubated with the MTT reagent (Thermo Fisher Scientific) diluted 10% in regular culture media. Cells were then maintained for 4 h at 37 °C. After this period, the MTT reagent was removed and samples were thoroughly re-suspended in DMSO. The absorbance of each well was assessed using a standard plate reader and results were tabulated as per standard protocols [
40].
4.12. Generation of Viral Particles
Low passage 293T cells (ATCC, Manassas, VA, USA) were used to generate lentiviral particles. Briefly, cells were plated at 90% confluency in preparation. After 6 h, they were transfected with a mix of HP DNA Transfection Reagent (Sigma Aldrich) diluted in OptiMEM media (Gibco) as well as appropriate packaging plasmids and CRISPR-Cas9 plasmids, as per the manufacturer’s instructions. Inducible Cas9 was obtained from Addgene (Cambridge, MA, USA) and guide RNA plasmids were obtained from Genecopoeia (Rockville, MD, USA). The transfected 293T cells were maintained in culture for 48–72 h, after which the virus-containing supernatant was harvested. The supernatant was centrifuged at 1200 rpm for 5 min to remove cell debris. It was additionally filtered with a 45-micron filter to sterilize. It was then ultra-centrifuged at 288,000× g for 3 h to pellet virus particles. Particles were resuspended in 200 μL of PBS and frozen at −80 °C until use.
4.13. Transduction of Cell Lines with Lentiviral Particles
U251 and GBM43 lines were obtained and maintained in culture as detailed above. For infection, cells were harvested and resuspended in a small volume of media (~50 μL). Appropriate lentivirus amounts were added (~10–20 multiplicities of infection per sample). Polybrene (4 ug/mL) was added to the virus-cell mixture. The tubes were incubated for 30 min at room temperature and were then plated in appropriately sized flasks. Cells were maintained in culture with regular media changes for 48–72 h. Efficiency of the resulting modifications was assessed by western blotting, as previously described.
4.14. Animal Studies
The mice used in this study were athymic nude mice (nu/nu; Charles River, Skokie, IL, USA). They were housed in accordance with all Institutional Animal Care and Use Committee (IACUC) requirements and in compliance with all applicable federal and state statutes. Animals were housed in shoebox cages with food and water available and with a strict 12 h light and dark cycle.
Our lab has a previously established glioblastoma mouse model, where an intracranial implantation of glioblastoma cells was performed [
21]. Briefly, animals received an injection of buprenex and metacam by intraperitoneal (IP) injection. Next, they received a second injection of ketamine/xylazine anesthesia (Henry Schein; New York, NY, USA). Complete sedation of the mice was confirmed by pinching the foot. To protect the mice, artificial tears were then applied to each eye and the scalp was sterilized with ethanol and betadine. A small incision was made using a scalpel, exposing the skull. A drill was used to make a ~1mm burr hole above the right frontal lobe. A stereotactic rig and a Hamilton syringe loaded with cells were used to implant 5 μL of cell solution 3 mm from the dura. Injections occurred over a period of one minute. The needle was then raised slightly and left for an additional one minute to ensure release of the cell suspension. Finally, the syringe was carefully removed, and the scalp was closed with sutures (Ethicon; Cincinnati, OH, USA). Head position was maintained throughout the closing process. Animals were maintained on heat pads until awake and reactive following surgery.
Any drug treatments necessary were started one week following the implantation. Animals received IP injections of either TMZ (2.5 mg/kg) or equimolar DMSO for 5 consecutive days, once per day. Animals were monitored daily for any signs of tumor progression (i.e., weight reduction, reduced body temperature, hunching, etc.). Animals were euthanized when it was determined that they would likely not survive to the next morning by two independent researchers. These sacrifices were performed according to Northwestern University and IACUC guidelines.
4.15. Statistics
Statistical analyses were performed with GraphPad Prism v8 (GraphPad Software; San Diego, CA, USA). Data are presented as mean for continuous variables and number or percentage for categorical variables. Differences between two groups were assessed using Student’s t-test. Differences between multiple groups were assessed using analysis of variance (ANOVA) with Turkey’s post-hoc correction. Survival curves were graphed with the Kaplan-Meier method and compared by log-rank test. All tests are two-sided and a p-value of under 0.05 is considered significant for the purposes of our study.