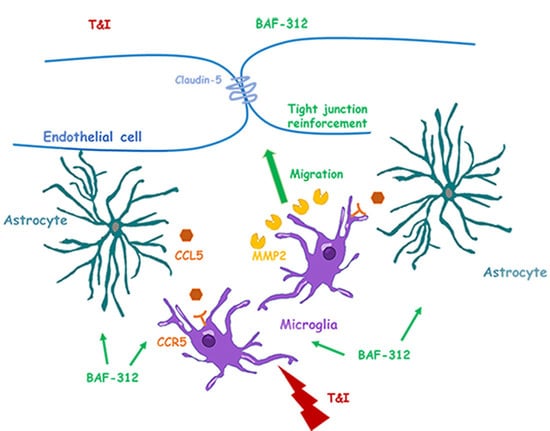
Microglia Contributes to BAF-312 Effects on Blood–Brain Barrier Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Cell Cultures
2.3. FITC–Dextran Permeability Assay
2.4. Scratch Wound Assay
2.5. Transwell Migration Assay
2.6. Western Blot
2.7. Gelatin Zymography Assay
2.8. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.9. Statistical Analysis
3. Results
3.1. BAF-312 Induces Microglia Migration
3.2. HMC3 Mobility Is Mediated by MMP2
3.3. HMC3 Cells’ Mobility towards Endothelial/Astrocyte Co-Cultures Is Facilitated by BAF-312
3.4. HMC3 Cells Exposed to BAF-312 Modulate BBB Properties
3.5. BAF-312 Modulates the Expression and Activity of MMP9 in Microglia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging. Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef]
- Merlo, S.; Caruso, G.I.; Bonfili, L.; Spampinato, S.F.; Costantino, G.; Eleuteri, A.M.; Sortino, M.A. Microglial polarization differentially affects neuronal vulnerability to the beta-amyloid protein: Modulation by melatonin. Biochem. Pharmacol. 2022, 202, 115151. [Google Scholar] [CrossRef]
- Caruso, G.I.; Spampinato, S.F.; Costantino, G.; Merlo, S.; Sortino, M.A. SIRT1-Dependent Upregulation of BDNF in Human Microglia Challenged with Abeta: An Early but Transient Response Rescued by Melatonin. Biomedicines 2021, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Spampinato, S.F.; Caruso, G.I.; Sortino, M.A. The Ambiguous Role of Microglia in Abeta Toxicity: Chances for Therapeutic Intervention. Curr. Neuropharmacol. 2020, 18, 446–455. [Google Scholar] [CrossRef]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef]
- Joost, E.; Jordão, M.J.; Mages, B.; Prinz, M.; Bechmann, I.; Krueger, M. Microglia contribute to the glia limitans around arteries, capillaries and veins under physiological conditions, in a model of neuroinflammation and in human brain tissue. Brain Struct. Funct. 2019, 224, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity. Front. Pharmaco.l 2019, 10, 1346. [Google Scholar] [CrossRef] [Green Version]
- Mehrabadi, A.R.; Korolainen, M.A.; Odero, G.; Miller, D.W.; Kauppinen, T.M. Poly(ADP-ribose) polymerase-1 regulates microglia mediated decrease of endothelial tight junction integrity. Neurochem. Int. 2017, 108, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Milner, R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc. Natl. Acad. Sci. USA 2019, 116, 26029–26037. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef]
- Yang, Y.; Rosenberg, G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef]
- Akundi, R.S.; Candelario-Jalil, E.; Hess, S.; Hüll, M.; Lieb, K.; Gebicke-Haerter, P.J.; Fiebich, B.L. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005, 51, 199–208. [Google Scholar] [CrossRef]
- Lassmann, H.; Van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Sortino, M.A.; Salomone, S. Sphingosine-1-phosphate and Sphingosine-1-phosphate receptors in the cardiovascular system: Pharmacology and clinical implications. Adv. Pharmacol. 2022, 94, 95–139. [Google Scholar]
- Rosen, H.; Germana Sanna, M.; Gonzalez-Cabrera, P.J.; Roberts, E. The organization of the sphingosine 1-phosphate signaling system. Curr. Top. Microbiol. Immunol. 2014, 378, 1–21. [Google Scholar]
- Montarolo, F.; Perga, S.; Martire, S.; Brescia, F.; Caldano, M.; Lo Re, M.; Panzica, G.; Bertolotto, A. Study of the NR4A family gene expression in patients with multiple sclerosis treated with Fingolimod. Eur. J. Neurol. 2019, 26, 667–672. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Merlo, S.; Costantino, G.; Sano, Y.; Kanda, T.; Sortino, M.A. Decreased Astrocytic CCL2 Accounts for BAF-312 Effect on PBMCs Transendothelial Migration Through a Blood Brain Barrier in Vitro Model. J. Neuroimmune. Pharmacol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Merlo, S.; Sano, Y.; Kanda, T.; Sortino, M.A. Protective effect of the sphingosine-1 phosphate receptor agonist siponimod on disrupted blood brain barrier function. Biochem. Pharmacol. 2021, 186, 114465. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Obermeier, B.; Cotleur, A.; Love, A.; Takeshita, Y.; Sano, Y.; Kanda, T.; Ransohoff, R.M. Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli? PLoS ONE 2015, 10, e0133392. [Google Scholar] [CrossRef] [PubMed]
- Bigaud, M.; Rudolph, B.; Briard, E.; Beerli, C.; Hofmann, A.; Hermes, E.; Muellershausen, F.; Schubart, A.; Gardin, A. Siponimod (BAF312) penetrates, distributes, and acts in the central nervous system: Preclinical insights. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211049168. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Takeshita, Y.; Obermeier, B. An In Vitro Model of the Blood-Brain Barrier to Study Alzheimer’s Disease: The Role of beta-Amyloid and Its Influence on PBMC Infiltration. Methods Mol. Biol. 2022, 2492, 333–352. [Google Scholar] [PubMed]
- Spampinato, S.F.; Merlo, S.; Sano, Y.; Kanda, T.; Sortino, M.A. Astrocytes contribute to Abeta-induced blood-brain barrier damage through activation of endothelial MMP9. J. Neurochem. 2017, 142, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Sortino, M.A. Estrogen activates matrix metalloproteinases-2 and -9 to increase beta amyloid degradation. Mol. Cell. Neurosci. 2012, 49, 423–429. [Google Scholar] [CrossRef]
- Sinagra, T.; Merlo, S.; Spampinato, S.F.; Pasquale, R.D.; Sortino, M.A. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front. Pharmacol. 2015, 6, 126. [Google Scholar] [CrossRef]
- Munaut, C.; Noël, A.; Hougrand, O.; Foidart, J.M.; Boniver, J.; Deprez, M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int. J. Cancer 2003, 106, 848–855. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Zhou, H.; Lapointe, B.M.; Clark, S.R.; Zbytnuik, L.; Kubes, P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 2006, 177, 8103–8110. [Google Scholar] [CrossRef]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry. 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Juif, P.E.; Kraehenbuehl, S.; Dingemanse, J. Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin. Drug Metab. Toxicol. 2016, 12, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.; Spampinato, S.F.; Ransohoff, R.M. Sphingosine 1-phosphate signaling at the blood-brain barrier. Trends Mol. Med. 2015, 21, 354–363. [Google Scholar] [CrossRef]
- Gergely, P.; Nuesslein-Hildesheim, B.; Guerini, D.; Brinkmann, V.; Traebert, M.; Bruns, C.; Pan, S.; Gray, N.S.; Hinterding, K.; Cooke, N.G.; et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br. J. Pharmacol. 2012, 167, 1035–1047. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflammation 2013, 10, 75. [Google Scholar] [CrossRef]
- Lam, D.; Lively, S.; Schlichter, L.C. Responses of rat and mouse primary microglia to pro- and anti-inflammatory stimuli: Molecular profiles, K(+) channels and migration. J. Neuroinflammation 2017, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNgamma+TNFalpha) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Colombo, E.; Bassani, C.; De Angelis, A.; Ruffini, F.; Ottoboni, L.; Comi, G.; Martino, G.; Farina, C. Siponimod (BAF312) Activates Nrf2 While Hampering NFkappaB in Human Astrocytes, and Protects From Astrocyte-Induced Neurodegeneration. Front. Immunol. 2020, 11, 635. [Google Scholar] [CrossRef]
- Montarolo, F.; Martire, S.; Marnetto, F.; Valentino, P.; Valverde, S.; Capobianco, M.A.; Bertolotto, A. The Selective Agonist for Sphingosine-1-Phosphate Receptors Siponimod Increases the Expression Level of NR4A Genes in Microglia Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Lou, N.; Takano, T.; Pei, Y.; Xavier, A.L.; Goldman, S.A.; Nedergaard, M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. USA 2016, 113, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laudati, E.; Currò, D.; Navarra, P.; Lisi, L. Blockade of CCR5 receptor prevents M2 microglia phenotype in a microglia-glioma paradigm. Neurochem. Int. 2017, 108, 100–108. [Google Scholar] [CrossRef]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediators. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yamamoto, A.; Lin, Y.T.; Fong, Y.C.; Tan, T.W. Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem. Pharmacol. 2010, 79, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Li, T.; Chen, D.; Jia, S.; Yang, X.; Liang, L.; Chai, J.; Cheng, X.; Yang, X.; Sun, M. The CCL5/CCR5 axis contributes to the perineural invasion of human salivary adenoid cystic carcinoma. Oncol. Rep. 2014, 31, 800–806. [Google Scholar] [CrossRef]
- Yu-Ju Wu, C.; Chen, C.H.; Lin, C.Y.; Feng, L.Y.; Lin, Y.C.; Wei, K.C.; Huang, C.Y.; Fang, J.Y.; Chen, P.Y. CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase 2. Neuro. Oncol. 2020, 22, 253–266. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spampinato, S.F.; Costantino, G.; Merlo, S.; Canonico, P.L.; Sortino, M.A. Microglia Contributes to BAF-312 Effects on Blood–Brain Barrier Stability. Biomolecules 2022, 12, 1174. https://doi.org/10.3390/biom12091174
Spampinato SF, Costantino G, Merlo S, Canonico PL, Sortino MA. Microglia Contributes to BAF-312 Effects on Blood–Brain Barrier Stability. Biomolecules. 2022; 12(9):1174. https://doi.org/10.3390/biom12091174
Chicago/Turabian StyleSpampinato, Simona Federica, Giuseppe Costantino, Sara Merlo, Pier Luigi Canonico, and Maria Angela Sortino. 2022. "Microglia Contributes to BAF-312 Effects on Blood–Brain Barrier Stability" Biomolecules 12, no. 9: 1174. https://doi.org/10.3390/biom12091174