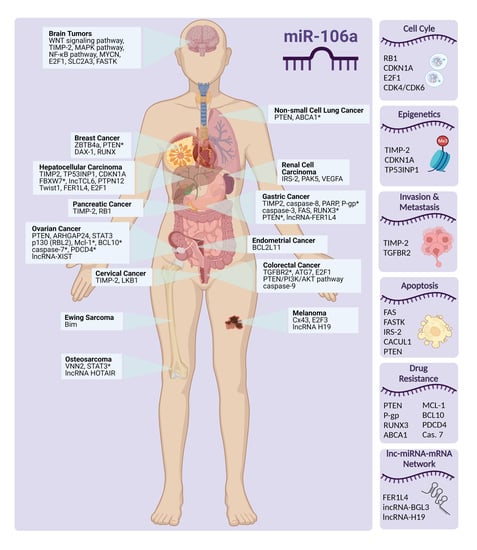
Overview of miR-106a Regulatory Roles: from Cancer to Aging
Abstract
:1. Introduction
2. mir-106a: Dysregulation in Cancers
2.1. Colorectal Cancer

2.2. Cholangiocarcinoma
2.3. Ewing Sarcoma
2.4. Gastric Cancer
2.5. Esophageal Carcinoma
2.6. Renal Carcinoma
2.7. Lung Cancer
2.8. Pancreatic Cancer
2.9. Ovarian Cancer
2.10. Brain Tumors
2.11. Breast Cancer
2.12. Endometrial Adenocarcinoma
2.13. Cervical Cancer
2.14. Hepatocellular Carcinoma
2.15. Melanoma
2.16. Osteosarcoma
3. Cancer Drug Resistance: The Role of miR-106a
4. mir-106a: Dysregulation in Non-Cancer Diseases
4.1. Hepatitis B
4.2. Multiple Sclerosis
4.3. Myasthenia Gravis
4.4. Cardiac Hypertrophy
5. The Role in Spermatogenesis
6. mir-106a and Human Aging
7. Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | Adenosine triphosphatase-binding cassette A1 |
| ABCG2 | Adenosine triphosphatase-binding cassette G2 |
| AKT (PKB) | Serine/threonine protein kinase B |
| AMPK-mTOR | AMP-activated protein kinase-mammalian target of the rapamycin |
| AP-1 | Activator protein 1 |
| Apaf-1 | Apoptotic peptidase activating factor 1 |
| APP | Amyloid precursor protein |
| ARHGAP24 | Rho GTPase-activating protein 24 |
| ATG7 | Autophagy-related gene 7 |
| Bak | Bcl-2-antagonist killer |
| Bax | Bcl-2-associated x protein |
| BC | Breast cancer |
| Bcl-1 | B-cell lymphoma 1 |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-x | B-cell lymphoma-extra-large |
| BCL2L11 | Bcl-2-like protein 11 |
| Bid | BH3 interacting-domain death agonist |
| Bim | Bcl-2 interacting mediator of cell death |
| BMP2 | Bone morphogenetic protein 2 |
| CC | Cervical cancer |
| CCA | Cholangiocarcinoma |
| Ccnd1 | Cyclin D1 |
| CDK | Cyclin-dependent kinase |
| CDKN1A | Cyclin dependent kinase inhibitor 1A |
| CDX2 | Caudal-type homeobox transcription factor 2 |
| ceRNA | Competing endogenous RNA |
| CHB | Chronic hepatitis B |
| CRC | Colorectal cancer |
| Cx43 | Connexin 43 |
| CYP19A1 | Cytochrome P450 family 19 subfamily A |
| DAX-1 | Dosage sensitive sex reversal, adrenal hypoplasia, critical region on the X chromosome, gene 1 |
| DDP | Diamminedichloroplatinum |
| DISC | Death inducing signaling complex |
| E2F1 | E2 promoter binding factor 1 (Transcription factor E2F1) |
| eIF4A | Eukaryotic initiation factor 4A-I |
| EMC | Endometrial cancer |
| EMT | Epithelial–mesenchymal transition |
| ERCC1 | Excision repair cross complementation group 1 |
| ESCC | Esophageal squamous cell carcinoma |
| EWS | Ewing sarcoma |
| EZH2 | Enhancer of zeste homolog 2 |
| FADD | Fas-associated death domain |
| FAF-1 | FAS-associated factor 1 |
| FASL | FAS ligand |
| FASTK | Fas-activated serine/threonine kinase |
| FBXW7 | F-box and WD repeat domain containing 7 |
| FER1L4 | Long non-coding RNA Fer-1 protein 4 |
| FER1L4 | Fer-1 like family member 4 |
| GBMs | Glioblastoma multiforms |
| GC | Gastric cancer |
| GMG | Generalized myasthenia gravis |
| GR | Gemcitabine-resistant |
| GST-π | Glutathione S-transferase π |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HEMn | Human epidermal melanocytes |
| HGSOC | High-grade serous ovarian carcinomas |
| HIPK3 | Homeodomain-interacting protein kinase 3 |
| HOTAIR | HOX antisense intergenic RNA |
| HPV | Human papillomavirus |
| IGF-1 | Insulin-like growth factor-1 |
| IL | Interleukin |
| IncTCL6 | Long noncoding RNA T cell leukemia/lymphoma 6 |
| IRS | Insulin receptor substrates |
| lncRNA | Long noncoding RNA |
| lncRNA | Long noncoding RNA |
| lncRNA XIST, XIST | Long noncoding RNA X inactive specific transcript |
| MAPK | Mitogen-activated protein kinase |
| Mcl-1 | Myeloid cell leukemia 1 |
| MDR | Multidrug resistance |
| MDR1, MRP1 | Multidrug resistance protein 1 |
| MET | Mesenchymal-epithelial transition |
| mfn2 | Mitofusin 2 |
| MG | Myasthenia gravis |
| miRNA | Microrna |
| MMP | Matrix metalloproteinase |
| MMP2 | Mmp2 |
| MRE | Mirna response element |
| MS | Multiple sclerosis |
| MYCN | MYC expressed in neuroblastoma |
| NF-ᴋB | Nuclear factor kappa B |
| NMJ | Neuromuscular junction |
| NSCLC | Non-small cell lung cancer |
| OC | Ovarian cancer |
| 4-Oct | Octamer-binding transcription factor 4 |
| OMG | Ocular myasthenia gravis |
| OS | Osteosarcoma |
| PA | Pilocytic astrocytomas |
| PAK7 | P21-activated kinase 7 |
| PARP | Poly-ADP ribose polymerase |
| PC | Pancreatic cancer |
| PDCD4 | Programmed cell death 4 |
| PDGF-D | Platelet-derived growth factor-D |
| PDK1 | Phosphoinositide-dependent protein kinase 1 |
| P-gp | P-glycoprotein |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol diphosphate |
| PIP3 | Phosphatidylinositol trisphosphate |
| PNET | Primitive neuroectodermal tumor |
| PPMS | Primary progressive multiple sclerosis |
| PRMS | Progressive relapsing multiple sclerosis |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| PTPN12 | Protein tyrosine phosphatase non-receptor type 12 |
| RB1 | Retinoblastoma protein 1 |
| RBL2 | Retinoblastoma 2 |
| RCC | Renal cell carcinoma |
| RNA | Ribonucleic acid |
| RRMS | Relapsing-remitting multiple sclerosis |
| RT-PCR | Real-time reverse transcriptase PCR |
| RUNX1 | Runt-related transcription factor 1 |
| RUNX2 | Runt-related transcription factor 2 |
| RUNX3 | Runt-related transcription factor 3 |
| SLC2A3, GLUT3 | Solute carrier family 2 member 3 |
| SOX9 | Sry-box transcription factor 9 |
| SPMS | Secondary progressive multiple sclerosis |
| SSCs | Spermatogonial stem cells |
| STAT3 | Signal transducers and activators of transcription 3 |
| TGFBR2 | Transforming growth factor-b receptor 2 |
| TGF-β | Transforming growth factor-β |
| TIMP2 | Tissue inhibitors of metalloproteinase 2 |
| TME | Tumor microenvironment |
| TNF | Tumor necrosis factor |
| Topo-II | DNA-topoisomerase II |
| TP53INP1 | Tumor protein p53-inducible nuclear protein 1 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| VEGF | Vascular endothelial growth factor |
| VEGFA | Endogenous vascular endothelial growth factor-a |
| VNN2 | Vascular non-inflammatory molecule 2 |
| WHO | World Health Organization |
| WNT | Wingless-related integration site |
References
- Leitao, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Non-Coding RNA 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J.; Zhuang, Y.; Zheng, J.N.; Pei, D.S. MiR-106a: Promising biomarker for cancer. Bioorganic Med. Chem. Lett. 2016, 26, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Kennel, P.J.; Schulze, P.C. A Review on the Evolving Roles of MiRNA-Based Technologies in Diagnosing and Treating Heart Failure. Cells 2021, 10, 3191. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Azmi, A.S.; Moore, A. microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip. Rev. RNA 2021, 12, e1662. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Daneshpour, M.; Omidfar, K.; Ghanbarian, H. A novel electrochemical nanobiosensor for the ultrasensitive and specific detection of femtomolar-level gastric cancer biomarker miRNA-106a. Beilstein J. Nanotechnol. 2016, 7, 2023–2036. [Google Scholar] [CrossRef] [Green Version]
- Gruszka, R.; Zakrzewska, M. The Oncogenic Relevance of miR-17-92 Cluster and Its Paralogous miR-106b-25 and miR-106a-363 Clusters in Brain Tumors. Int. J. Mol. Sci. 2018, 19, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Kaur, P.; Kotru, S.; Singh, S.; Munshi, A. Role of miRNAs in diabetic neuropathy: Mechanisms and possible interventions. Mol. Neurobiol. 2022, 59, 1836–1849. [Google Scholar] [CrossRef]
- Kudelova, E.; Holubekova, V.; Grendar, M.; Kolkova, Z.; Samec, M.; Vanova, B.; Mikolajcik, P.; Smolar, M.; Kudela, E.; Laca, L.; et al. Circulating miRNA expression over the course of colorectal cancer treatment. Oncol. Lett. 2022, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Ak, S.; Tunca, B.; Tezcan, G.; Cecener, G.; Egeli, U.; Yilmazlar, T.; Ozturk, E.; Yerci, O. MicroRNA expression patterns of tumors in early-onset colorectal cancer patients. J. Surg. Res. 2014, 191, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Yang, W.; Duan, L.; Wang, X.; Li, Y.; Xu, C.; Liu, C.; Zhang, Y.; Zhou, W.; Liu, J.; et al. Biological Implications and Clinical Potential of Metastasis-Related miRNA in Colorectal Cancer. Mol. Ther. Nucleic Acids 2021, 23, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Xia, G.; Wang, C.; Zhong, F.; Liu, L.; Zhang, D. miR-106a suppresses tumor cells death in colorectal cancer through targeting ATG7. Med. Mol. Morphol. 2017, 50, 76–85. [Google Scholar] [CrossRef]
- He, Y.; Wang, G.; Zhang, L.; Zhai, C.; Zhang, J.; Zhao, X.; Jiang, X.; Zhao, Z. Biological effects and clinical characteristics of microRNA-106a in human colorectal cancer. Oncol. Lett. 2017, 14, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, P.; Yang, J.; Liu, Z.; Yang, Z.; Qin, H. Candidate microRNA biomarkers in human colorectal cancer: Systematic review profiling studies and experimental validation. Int. J. Cancer 2012, 130, 2077–2087. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.F.; Xu, Y.W.; Li, J.; Niu, H.L.; Ma, W.X.; Xu, J.; Zhou, P.R.; Liu, X.; Ye, D.L.; Liu, X.R.; et al. Mir20a/106a-WTX axis regulates RhoGDIa/CDC42 signaling and colon cancer progression. Nat. Commun. 2019, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Dong, T.T.; Wang, L.L.; Zhou, H.M.; Zhao, H.C.; Dong, F.; Zheng, M.H. Colorectal cancer migration and invasion initiated by microRNA-106a. PLoS ONE 2012, 7, e43452. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Wang, H.; Wu, D. MiR-106a-5p promotes 5-FU resistance and the metastasis of colorectal cancer by targeting TGFbetaR2. Int. J. Clin. Exp. Pathol. 2018, 11, 5622–5634. [Google Scholar]
- Qin, Y.; Huo, Z.; Song, X.; Chen, X.; Tian, X.; Wang, X. mir-106a regulates cell proliferation and apoptosis of colon cancer cells through targeting the PTEN/PI3K/AKT signaling pathway. Oncol. Lett. 2018, 15, 3197–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermudez Brito, M.; Goulielmaki, E.; Papakonstanti, E.A. Focus on PTEN Regulation. Front. Oncol. 2015, 5, 166. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Zheng, W.; Li, N.; Su, Z.; Zhao, L.; Zhou, H.; Jia, L. MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci. Rep. 2017, 7, 41942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ke, F.; Chen, T.; Zhou, Q.; Weng, L.; Tan, J.; Shen, W.; Li, L.; Zhou, J.; Xu, C.; et al. MicroRNAs that regulate PTEN as potential biomarkers in colorectal cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ma, Q. MicroRNA-106a inhibits cell proliferation and induces apoptosis in colorectal cancer cells. Oncol. Lett. 2018, 15, 8941–8944. [Google Scholar] [CrossRef] [Green Version]
- Phin, S.; Moore, M.W.; Cotter, P.D. Genomic Rearrangements of PTEN in Prostate Cancer. Front. Oncol. 2013, 3, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q.; Feng, F.; Zhu, L.; Zheng, Y.; Luo, X.; Liu, C.; Yi, B.; Jiang, X. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci. Rep. 2015, 5, 16103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbato, A.; Piscopo, F.; Salati, M.; Reggiani-Bonetti, L.; Franco, B.; Carotenuto, P. Micro-RNA in Cholangiocarcinoma: Implications for Diagnosis, Prognosis, and Therapy. J. Mol. Pathol. 2022, 3, 88–103. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, J.; Wu, B.; Chen, J.; Zhu, Z.; Cai, P.; Guo, W.; Gu, Z.; Wang, J.; Huang, S. Diagnostic and prognostic value of microRNAs in cholangiocarcinoma: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 2125–2139. [Google Scholar] [CrossRef] [Green Version]
- Dylla, L.; Moore, C.; Jedlicka, P. MicroRNAs in Ewing Sarcoma. Front. Oncol. 2013, 3, 65. [Google Scholar] [CrossRef] [Green Version]
- Drobna, M.; Szarzynska, B.; Jaksik, R.; Sedek, L.; Kuchmiy, A.; Taghon, T.; Van Vlierberghe, P.; Szczepanski, T.; Witt, M.; Dawidowska, M. hsa-miR-20b-5p and hsa-miR-363-3p Affect Expression of PTEN and BIM Tumor Suppressor Genes and Modulate Survival of T-ALL Cells In Vitro. Cells 2020, 9, 1137. [Google Scholar] [CrossRef]
- Dylla, L.; Jedlicka, P. Growth-promoting role of the miR-106a~363 cluster in Ewing sarcoma. PLoS ONE 2013, 8, e63032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Cheng, T.T.; He, Q.J.; Lei, Z.Y.; Chi, J.; Tang, Z.; Liao, Q.X.; Zhang, H.; Zeng, L.S.; Cui, S.Z. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/beta-catenin pathway. Mol. Cancer 2018, 17, 126. [Google Scholar] [CrossRef] [Green Version]
- Ahadi, A. A systematic review of microRNAs as potential biomarkers for diagnosis and prognosis of gastric cancer. Immunogenetics 2021, 73, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, N.; He, S.; Lui, Y.; Lu, G.; Zhao, L. MicroRNA-106a targets TIMP2 to regulate invasion and metastasis of gastric cancer. FEBS Lett. 2014, 588, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshpour, M.; Karimi, B.; Omidfar, K. Simultaneous detection of gastric cancer-involved miR-106a and let-7a through a dual-signal-marked electrochemical nanobiosensor. Biosens. Bioelectron. 2018, 109, 197–205. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.M.; Lou, Y.R.; Zhang, X.J.; Zhong, F.D.; Jiang, Z.; Cheng, J.; Xiao, B.X. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J. Mol. Med. 2010, 88, 709–717. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Zhu, H.; Zhang, W.; He, S.; Hu, C.; Quan, L.; Bai, J.; Xu, N. miR-106a is frequently upregulated in gastric cancer and inhibits the extrinsic apoptotic pathway by targeting FAS. Mol. Carcinog. 2013, 52, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, R.; Yi, B.; Riker, A.I.; Xi, Y. MicroRNAs are involved in the development and progression of gastric cancer. Acta Pharmacol. Sin. 2021, 42, 1018–1026. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C. Identification and validation of potential mRNA- microRNA- long-noncoding RNA (mRNA-miRNA-lncRNA) prognostic signature for cervical cancer. Bioengineered 2021, 12, 898–913. [Google Scholar] [CrossRef]
- Xia, T.; Chen, S.; Jiang, Z.; Shao, Y.; Jiang, X.; Li, P.; Xiao, B.; Guo, J. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci. Rep. 2015, 5, 13445. [Google Scholar] [CrossRef] [Green Version]
- Rhode, P.; Mehdorn, M.; Lyros, O.; Kahlert, C.; Kurth, T.; Venus, T.; Schierle, K.; Estrela-Lopis, I.; Jansen-Winkeln, B.; Lordick, F.; et al. Characterization of Total RNA, CD44, FASN, and PTEN mRNAs from Extracellular Vesicles as Biomarkers in Gastric Cancer Patients. Cancers 2021, 13, 5975. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; Cai, X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett. 2013, 587, 3069–3075. [Google Scholar] [CrossRef]
- Guo, C.; Ding, J.; Yao, L.; Sun, L.; Lin, T.; Song, Y.; Sun, L.; Fan, D. Tumor suppressor gene Runx3 sensitizes gastric cancer cells to chemotherapeutic drugs by downregulating Bcl-2, MDR-1 and MRP-1. Int. J. Cancer 2005, 116, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wen, W.; Zhu, J.; Huang, Z.; Zhang, L.; Zhang, H.; Qi, L.W.; Shan, X.; Wang, T.; Cheng, W.; et al. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget 2017, 8, 34468–34480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyama, T.; Yoshihara, M.; Tanaka, S.; Chayama, K. Genetic polymorphisms and esophageal cancer risk. Int. J. Cancer 2007, 121, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Jamali, L.; Tofigh, R.; Tutunchi, S.; Panahi, G.; Borhani, F.; Akhavan, S.; Nourmohammadi, P.; Ghaderian, S.M.H.; Rasouli, M.; Mirzaei, H. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J. Cell Physiol. 2018, 233, 8538–8550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Chen, X.; Yang, C.; Li, K.; Wang, J.; Dai, J.; Hu, Z.; Zhou, X.; Chen, L.; et al. Expression profile of microRNAs in serum: A fingerprint for esophageal squamous cell carcinoma. Clin. Chem. 2010, 56, 1871–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, R.; Hussey, D.J.; Michael, M.Z.; Haier, J.; Bruewer, M.; Senninger, N.; Watson, D.I. MiRNAs and their association with locoregional staging and survival following surgery for esophageal carcinoma. Ann. Surg. Oncol. 2011, 18, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J.; Wei, L.L.; Wu, X.J.; Huo, F.C.; Mou, J.; Pei, D.S. MiR-106a-5p inhibits the cell migration and invasion of renal cell carcinoma through targeting PAK5. Cell Death Dis. 2017, 8, e3155. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Majidpoor, J.; Taheri, M. The emerging role of non-coding RNAs in the regulation of PI3K/AKT pathway in the carcinogenesis process. Biomed. Pharmacother. 2021, 137, 111279. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; He, X.; Song, H.; Qiang, Y.; Li, Y.; Gao, J.; Wang, Z. miR-106a* inhibits the proliferation of renal carcinoma cells by targeting IRS-2. Tumour Biol. 2015, 36, 8389–8398. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Dhe-Paganon, S.; Melendez, P.A.; Lee, J.; Shoelson, S.E. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003, 278, 25323–25330. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; White, M.F. Insulin receptor substrate proteins and diabetes. Arch. Pharm. Res. 2004, 27, 361–370. [Google Scholar] [CrossRef]
- Huo, F.C.; Zhu, Z.M.; Du, Q.Y.; Pei, D.S. PAK5-stabilized Smuc confers renal cell carcinoma metastasis. Clin. Transl. Med. 2021, 11, e559. [Google Scholar] [CrossRef]
- Li, Y.K.; Zou, J.; Ye, D.M.; Zeng, Y.; Chen, C.Y.; Luo, G.F.; Zeng, X. Human p21-activated kinase 5 (PAK5) expression and potential mechanisms in relevant cancers: Basic and clinical perspectives for molecular cancer therapeutics. Life Sci. 2020, 241, 117113. [Google Scholar] [CrossRef]
- Yang, L.; Zou, X.; Zou, J.; Zhang, G. A Review of Recent Research on the Role of MicroRNAs in Renal Cancer. Med. Sci. Monit. 2021, 27, e930639. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Azhati, B.; Wang, Y.; Tusong, H. miR-106a-5p Functions as a Tumor Suppressor by Targeting VEGFA in Renal Cell Carcinoma. Dis. Markers 2020, 2020, 8837941. [Google Scholar] [CrossRef]
- Situ, Y.; Xu, Q.; Deng, L.; Zhu, Y.; Gao, R.; Lei, L.; Shao, Z. System analysis of VEGFA in renal cell carcinoma: The expression, prognosis, gene regulation network and regulation targets. Int. J. Biol. Markers 2022, 37, 90–101. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, C.; Zhang, L.; Pan, Y. Clinical significance of miRNA-106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy. Cancer Biol. Med. 2018, 15, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Heegaard, N.H.; Schetter, A.J.; Welsh, J.A.; Yoneda, M.; Bowman, E.D.; Harris, C.C. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int. J. Cancer 2012, 130, 1378–1386. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Liu, H.T.; Mei, J.; Ding, F.B.; Xiao, H.B.; Hu, F.Q.; Hu, R.; Wang, M.S. miR-106a promotes growth and metastasis of non-small cell lung cancer by targeting PTEN. Int. J. Clin. Exp. Pathol. 2015, 8, 3827–3834. [Google Scholar]
- Lee, B.H.; Taylor, M.G.; Robinet, P.; Smith, J.D.; Schweitzer, J.; Sehayek, E.; Falzarano, S.M.; Magi-Galluzzi, C.; Klein, E.A.; Ting, A.H. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013, 73, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.; Land, H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012, 2, 580–590. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, X.; Cheng, S.; Wei, W.; Li, Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol. Med. Rep. 2015, 11, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, R.; Saberi, S.; Zali, M.; Sadeghi, A.; Ghafouri-Fard, S.; Aghdaei, H.A. Identification of potential microRNA panels for pancreatic cancer diagnosis using microarray datasets and bioinformatics methods. Sci. Rep. 2020, 10, 7559. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Q.; Zhang, D.; Li, X.; Han, L.; Lei, J.; Duan, W.; Ma, Q.; Wu, Z.; Wang, Z. Upregulated miR-106a plays an oncogenic role in pancreatic cancer. FEBS Lett. 2014, 588, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.; Zhang, M.; Hou, H.; Zhang, Z.; Li, N. HOTAIRM1 suppresses cell proliferation and invasion in ovarian cancer through facilitating ARHGAP24 expression by sponging miR-106a-5p. Life Sci. 2020, 243, 117296. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, F.; Sheng, X.G.; Zhang, S.Q.; Chen, Y.T.; Liu, B.W. MicroRNA-106a regulates phosphatase and tensin homologue expression and promotes the proliferation and invasion of ovarian cancer cells. Oncol. Rep. 2016, 36, 2135–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gersbach, E.; Zhang, X.; Xu, X.; Dong, R.; Lee, P.; Liu, J.; Kong, B.; Shao, C.; Wei, J.J. miR-106a represses the Rb tumor suppressor p130 to regulate cellular proliferation and differentiation in high-grade serous ovarian carcinoma. Mol. Cancer Res. 2013, 11, 1314–1325. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.M.; Shi, H.R.; Ji, M.; Chen, C.H. MiR-106a targets Mcl-1 to suppress cisplatin resistance of ovarian cancer A2780 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 567–572. [Google Scholar] [CrossRef]
- Huh, J.H.; Kim, T.H.; Kim, K.; Song, J.A.; Jung, Y.J.; Jeong, J.Y.; Lee, M.J.; Kim, Y.K.; Lee, D.H.; An, H.J. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br. J. Cancer 2013, 109, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; Kim, E.Y.; Jeon, D.; Liu, J.L.; Kim, H.S.; Choi, J.W.; Ahn, W.S. Differential microRNA expression signatures and cell type-specific association with Taxol resistance in ovarian cancer cells. Drug Des. Dev. Ther. 2014, 8, 293–314. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, H.; Shen, H.; Li, H. microRNA-106a modulates cisplatin sensitivity by targeting PDCD4 in human ovarian cancer cells. Oncol. Lett. 2014, 7, 183–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Yuan, D.; Zhang, W.; Zhu, D.; Xiao, A.; Mao, G.; Jiang, W.; Lin, M.; Wang, J. Upregulation of long noncoding RNA XIST has anticancer effects on ovarian cancer through sponging miR-106a. Hum. Cell 2021, 34, 579–587. [Google Scholar] [CrossRef]
- Buruiana, A.; Florian, S.I.; Florian, A.I.; Timis, T.L.; Mihu, C.M.; Miclaus, M.; Osan, S.; Hrapsa, I.; Cataniciu, R.C.; Farcas, M.; et al. The Roles of miRNA in Glioblastoma Tumor Cell Communication: Diplomatic and Aggressive Negotiations. Int. J. Mol. Sci. 2020, 21, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathupala, S.P.; Mittal, S.; Guthikonda, M.; Sloan, A.E. MicroRNA and brain tumors: A cause and a cure? DNA Cell Biol. 2007, 26, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Petrescu, G.E.D.; Sabo, A.A.; Torsin, L.I.; Calin, G.A.; Dragomir, M.P. MicroRNA based theranostics for brain cancer: Basic principles. J. Exp. Clin. Cancer Res. 2019, 38, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wang, Z.; Chen, Z.; Lin, L.; Wang, Y.; Sailike, D.; Luo, K.; Du, G.; Xiang, X.; Jiafu, G.D. MicroRNA-106a-5p facilitates human glioblastoma cell proliferation and invasion by targeting adenomatosis polyposis coli protein. Biochem. Biophys. Res. Commun. 2016, 481, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, B.; Shi, Y.; Xu, C.; Xiao, H.L.; Ma, L.N.; Xu, S.L.; Yang, L.; Wang, Q.L.; Dang, W.Q.; et al. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene 2015, 34, 1407–1419. [Google Scholar] [CrossRef]
- Dai, D.W.; Lu, Q.; Wang, L.X.; Zhao, W.Y.; Cao, Y.Q.; Li, Y.N.; Han, G.S.; Liu, J.M.; Yue, Z.J. Decreased miR-106a inhibits glioma cell glucose uptake and proliferation by targeting SLC2A3 in GBM. BMC Cancer 2013, 13, 478. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhang, R.; Chen, X.; Mu, Y.; Ai, J.; Shi, C.; Liu, Y.; Shi, C.; Sun, L.; Rainov, N.G.; et al. MiR-106a inhibits glioma cell growth by targeting E2F1 independent of p53 status. J. Mol. Med. 2011, 89, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, G.; Mu, Y.; Han, D.; Shi, C.; Chen, X.; Deng, Y.; Zhang, D.; Wang, L.; Liu, Y.; et al. MiR-106a is an independent prognostic marker in patients with glioblastoma. Neuro Oncol. 2013, 15, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, F.; Zhou, G.; Shao, N.; Xia, X.; Shi, Y.; Wang, Q.; Zhang, Y.; Wang, R.; Xue, L.; Wang, S.; et al. miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS ONE 2013, 8, e72390. [Google Scholar] [CrossRef]
- Jones, T.A.; Jeyapalan, J.N.; Forshew, T.; Tatevossian, R.G.; Lawson, A.R.; Patel, S.N.; Doctor, G.T.; Mumin, M.A.; Picker, S.R.; Phipps, K.P.; et al. Molecular analysis of pediatric brain tumors identifies microRNAs in pilocytic astrocytomas that target the MAPK and NF-kappaB pathways. Acta Neuropathol. Commun. 2015, 3, 86. [Google Scholar] [CrossRef] [Green Version]
- Schulte, J.H.; Horn, S.; Otto, T.; Samans, B.; Heukamp, L.C.; Eilers, U.C.; Krause, M.; Astrahantseff, K.; Klein-Hitpass, L.; Buettner, R.; et al. MYCN regulates oncogenic MicroRNAs in neuroblastoma. Int. J. Cancer 2008, 122, 699–704. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Chu, L.; Li, X.; Kan, P.; Xin, X.; Zhu, Y.; Yang, P. The Effects and Molecular Mechanisms of MiR-106a in Multidrug Resistance Reversal in Human Glioma U87/DDP and U251/G Cell Lines. PLoS ONE 2015, 10, e0125473. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.G.; Davies, M.; Lowery, A.J.; Miller, N.; Kerin, M.J. The Role of MicroRNA as Clinical Biomarkers for Breast Cancer Surgery and Treatment. Int. J. Mol. Sci. 2021, 22, 8290. [Google Scholar] [CrossRef]
- You, F.; Luan, H.; Sun, D.; Cui, T.; Ding, P.; Tang, H.; Sun, D. miRNA-106a Promotes Breast Cancer Cell Proliferation, Clonogenicity, Migration, and Invasion Through Inhibiting Apoptosis and Chemosensitivity. DNA Cell Biol. 2019, 38, 198–207. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Xia, T.; Zhou, X.; Huang, Z.; Zhang, H.; Zhu, W.; Ding, Q.; Wang, S. Circulating microRNAs from the miR-106a-363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Res. Treat. 2018, 170, 257–270. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.H.; Mao, Y.; Wang, H.B.; Nie, G. MiRNA-106a promotes breast cancer progression by regulating DAX-1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1574–1583. [Google Scholar] [CrossRef]
- Yang, W.S.; Chadalapaka, G.; Cho, S.G.; Lee, S.O.; Jin, U.H.; Jutooru, I.; Choi, K.; Leung, Y.K.; Ho, S.M.; Safe, S.; et al. The transcriptional repressor ZBTB4 regulates EZH2 through a MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia 2014, 16, 1059–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favier, A.; Rocher, G.; Larsen, A.K.; Delangle, R.; Uzan, C.; Sabbah, M.; Castela, M.; Duval, A.; Mehats, C.; Canlorbe, G. MicroRNA as Epigenetic Modifiers in Endometrial Cancer: A Systematic Review. Cancers 2021, 13, 1137. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Gorini, F.; De Crescenzo, E.; De Leo, A.; De Biase, D.; Di Stanislao, M.; Hrelia, P.; Angelini, S.; De Iaco, P.; Perrone, A.M. Can miRNAs be useful biomarkers in improving prognostic stratification in endometrial cancer patients? An update review. Int. J. Cancer 2022, 150, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.N.; Bai, J.X.; Zhou, Q.; Yan, B.; Qin, W.W.; Jia, L.T.; Meng, Y.L.; Jin, B.Q.; Yao, L.B.; Wang, T.; et al. TSA suppresses miR-106b-93-25 cluster expression through downregulation of MYC and inhibits proliferation and induces apoptosis in human EMC. PLoS ONE 2012, 7, e45133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xu, K.; Tong, Y.; Dai, X.; Xu, T.; He, D.; Ying, J. Novel miRNA markers for the diagnosis and prognosis of endometrial cancer. J. Cell. Mol. Med. 2020, 24, 4533–4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Li, J.; Liu, H.; Zhou, F.; Liu, M. MiR-106a promotes tumor growth, migration, and invasion by targeting BCL2L11 in human endometrial adenocarcinoma. Am. J. Transl. Res. 2017, 9, 4984–4993. [Google Scholar]
- Oplawski, M.; Nowakowski, R.; Srednicka, A.; Ochnik, D.; Grabarek, B.O.; Boron, D. Molecular Landscape of the Epithelial-Mesenchymal Transition in Endometrioid Endometrial Cancer. J. Clin. Med. 2021, 10, 1520. [Google Scholar] [CrossRef]
- Lai, Y.; Zhou, B.; Tan, Q.; Xu, J.; Wan, T.; Zhang, L. LINC00116 enhances cervical cancer tumorigenesis through miR-106a/c-Jun pathway. J. Cell. Biochem. 2020, 121, 2247–2257. [Google Scholar] [CrossRef]
- Mitra, T.; Elangovan, S. Cervical cancer development, chemoresistance, and therapy: A snapshot of involvement of microRNA. Mol. Cell. Biochem. 2021, 476, 4363–4385. [Google Scholar] [CrossRef]
- Sadri Nahand, J.; Moghoofei, M.; Salmaninejad, A.; Bahmanpour, Z.; Karimzadeh, M.; Nasiri, M.; Mirzaei, H.R.; Pourhanifeh, M.H.; Bokharaei-Salim, F.; Mirzaei, H.; et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int. J. Cancer 2020, 146, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, X.; Zhou, X.; Jia, J.; Chen, H.; Zhao, W. miR-106a Regulates Cell Proliferation and Autophagy by Targeting LKB1 in HPV-16-Associated Cervical Cancer. Mol. Cancer Res. 2020, 18, 1129–1141. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Tao, L.; Yu, C. MicroRNA-106a promotes cell migration and invasion by targeting tissue inhibitor of matrix metalloproteinase 2 in cervical cancer. Oncol. Rep. 2017, 38, 1774–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.H.; Jin, M.; Wang, L.Q.; Xu, G.J.; Lin, Z.Y.; Yu, D.D.; Yang, S.L.; Ran, R.Z.; Wu, G.; Zhang, T. Long noncoding RNA TCL6 binds to miR-106a-5p to regulate hepatocellular carcinoma cells through PI3K/AKT signaling pathway. J. Cell. Physiol. 2020, 235, 6154–6166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhi, Q.; Zhao, H.; Han, Y.; Gao, L.; Wang, B.; Kou, Z.; Guo, Z.; He, S.; Xue, X.; et al. Upregulated expression of miR-106a by DNA hypomethylation plays an oncogenic role in hepatocellular carcinoma. Tumour Biol. 2015, 36, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Dong, K.; Ma, Y.; Jin, Q.; Yin, S.; Zhu, X.; Wang, S. Effects of FER1L4-miR-106a-5p/miR-372-5p-E2F1 regulatory axis on drug resistance in liver cancer chemotherapy. Mol. Ther. Nucleic Acids 2021, 24, 449–461. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, Y.; Wang, X.; Qin, L.; Hu, R. Development and validation of serum exosomal microRNAs as diagnostic and prognostic biomarkers for hepatocellular carcinoma. J. Cell. Biochem. 2019, 120, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, Z.; Qiao, G.; Tang, B.; Li, P. Visualization of endoplasmic reticulum viscosity in the liver of mice with nonalcoholic fatty liver disease by a near-infrared fluorescence probe. Chin. Chem. Lett. 2021, 32, 3641–3645. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Hou, Y.; Yang, Q.; Chen, S.; Wang, X.; Wang, Z.; Yang, Y.; Chen, C.; Wang, Z.; et al. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget 2015, 6, 7000–7010. [Google Scholar] [CrossRef] [Green Version]
- Deng, P.; Wu, Y. Knockdown of miR-106a suppresses migration and invasion and enhances radiosensitivity of hepatocellular carcinoma cells by upregulating FBXW7. Int. J. Clin. Exp. Pathol. 2019, 12, 1184–1193. [Google Scholar]
- Liang, Z.; Li, X.; Duan, F.; Song, L.; Wang, Z.; Li, X.; Yang, P.; Li, L. Protein tyrosine phosphatase non-receptor type 12 (PTPN12), negatively regulated by miR-106a-5p, suppresses the progression of hepatocellular carcinoma. Hum. Cell 2022, 35, 299–309. [Google Scholar] [CrossRef]
- Wang, J.L.; Li, H.; Zhang, J.B.; Zhang, C.H.; Hou, X.Q. Suppression of connexin 43 expression by miR-106a promotes melanoma cell proliferation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lai, Y.C.; Husna, A.A.; Chen, H.W.; Tanaka, Y.; Kawaguchi, H.; Miyoshi, N.; Nakagawa, T.; Fukushima, R.; Miura, N. Micro RNA Transcriptome Profile in Canine Oral Melanoma. Int. J. Mol. Sci. 2019, 20, 4832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.; Raymond, G.; Tzou, K.S.; Baxi, S.; Mani, R.R.; Kumar Govind, S.; Chandramoorthy, H.C.; Sivanandy, P.; Rajagopal, M.; Samiappan, S.; et al. Molecular Investigation of miRNA Biomarkers as Chemoresistance Regulators in Melanoma: A Protocol for Systematic Review and Meta-Analysis. Genes 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, J.P.; Lach, M.S.; Ustaszewski, A.; Kulcenty, K.; Ibbs, M.; Jagiello, I.; Suchorska, W.M.; Marszalek, A. The Potential Role of Selected miRNA in Uveal Melanoma Primary Tumors as Early Biomarkers of Disease Progression. Genes 2020, 11, 271. [Google Scholar] [CrossRef] [Green Version]
- Ming, J.; Zhou, Y.; Du, J.; Fan, S.; Pan, B.; Wang, Y.; Fan, L.; Jiang, J. miR-381 suppresses C/EBPalpha-dependent Cx43 expression in breast cancer cells. Biosci. Rep. 2015, 35, e00266. [Google Scholar] [CrossRef]
- Luan, W.; Zhou, Z.; Ni, X.; Xia, Y.; Wang, J.; Yan, Y.; Xu, B. Long non-coding RNA H19 promotes glucose metabolism and cell growth in malignant melanoma via miR-106a-5p/E2F3 axis. J. Cancer Res. Clin. Oncol. 2018, 144, 531–542. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, T.; Yang, X.; Liu, C.; Li, P.; Wang, Z.; Zhi, S. MicroRNA106a regulates the proliferation and invasion of human osteosarcoma cells by targeting VNN2. Oncol. Rep. 2018, 40, 2251–2259. [Google Scholar] [CrossRef]
- Guo, J.; Dou, D.; Zhang, T.; Wang, B. HOTAIR Promotes Cisplatin Resistance of Osteosarcoma Cells by Regulating Cell Proliferation, Invasion, and Apoptosis via miR-106a-5p/STAT3 Axis. Cell Transplant. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Nakka, M.; Allen-Rhoades, W.; Li, Y.; Kelly, A.J.; Shen, J.; Taylor, A.M.; Barkauskas, D.A.; Yustein, J.T.; Andrulis, I.L.; Wunder, J.S.; et al. Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget 2017, 8, 96738–96752. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Takeda, Y.; Watanabe, T.; Sendo, F. A sandwich ELISA for detection of soluble GPI-80, a glycosylphosphatidyl-inositol (GPI)-anchored protein on human leukocytes involved in regulation of neutrophil adherence and migration—Its release from activated neutrophils and presence in synovial fluid of rheumatoid arthritis patients. Microbiol. Immunol. 2001, 45, 467–471. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, P.; Wang, J.; Zhang, Y.; Zhang, M.; Wang, Z.; Fu, Q.; Liang, W. Clinical significance of tumor miR-21, miR-221, miR-143, and miR-106a as biomarkers in patients with osteosarcoma. Int. J. Biol. Markers 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhao, T.; Xu, Z.; Chi, W.; Chai, X.; Ai, J.; Zhang, J.; Huo, F.; Strongin, R.M.; Yin, C. Enlarging the Stokes Shift by Weakening the pi-Conjugation of Cyanines for High Signal-to-Noise Ratiometric Imaging. Adv. Sci. 2023, 10, e2205080. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, W.; Feng, W.; Cao, L.; Wang, X.; Niu, L.; Li, Y.; Zhou, W.; Zhang, Y.; Liu, J.; et al. Molecular mechanisms and clinical implications of miRNAs in drug resistance of colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12, 1–28. [Google Scholar] [CrossRef]
- Ren, X.; Liao, L.; Yang, Z.; Li, H.; Li, X.; Wang, Y.; Ye, Y.; Song, X. Rational design of a bifunctional fluorescent probe for distinguishing Hcy/Cys from GSH with ideal properties. Chin. Chem. Lett. 2021, 32, 1061–1065. [Google Scholar] [CrossRef]
- Ghasabi, M.; Mansoori, B.; Mohammadi, A.; Duijf, P.H.; Shomali, N.; Shirafkan, N.; Mokhtarzadeh, A.; Baradaran, B. MicroRNAs in cancer drug resistance: Basic evidence and clinical applications. J. Cell. Physiol. 2019, 234, 2152–2168. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, D.; Tout, I.; Narguet, S.; Benazzouz, S.M.; Mansouri, A.; Asselah, T. miRNAs as Potential Biomarkers for Viral Hepatitis B and C. Viruses 2020, 12, 1440. [Google Scholar] [CrossRef]
- Morgan, M.; Keeffe, E.B. Diagnosis and treatment of chronic hepatitis B: 2009 update. Minerva Gastroenterol. Dietol. 2009, 55, 5–22. [Google Scholar] [PubMed]
- Boltjes, A.; Groothuismink, Z.M.; van Oord, G.W.; Janssen, H.L.; Woltman, A.M.; Boonstra, A. Monocytes from chronic HBV patients react in vitro to HBsAg and TLR by producing cytokines irrespective of stage of disease. PLoS ONE 2014, 9, e97006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, Y.; Du, C.; Markowitz, G.J.; Fu, J.; Zhang, Z.; Liu, C.; Qin, W.; Wang, H.; Wang, F.; et al. Hepatitis B-Induced IL8 Promotes Hepatocellular Carcinoma Venous Metastasis and Intrahepatic Treg Accumulation. Cancer Res. 2021, 81, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Hong, H.; Liu, J.; Zheng, X.; Huang, M.; Li, C.; Xia, J. miR-106a Is Downregulated in Peripheral Blood Mononuclear Cells of Chronic Hepatitis B and Associated with Enhanced Levels of Interleukin-8. Mediat. Inflamm. 2015, 2015, 629862. [Google Scholar] [CrossRef] [Green Version]
- Basak, J.; Majsterek, I. miRNA-Dependent CD4(+) T Cell Differentiation in the Pathogenesis of Multiple Sclerosis. Mult. Scler. Int. 2021, 2021, 8825588. [Google Scholar] [CrossRef]
- Rahimirad, S.; Navaderi, M.; Alaei, S.; Sanati, M.H. Identification of hsa-miR-106a-5p as an impact agent on promotion of multiple sclerosis using multi-step data analysis. Neurol. Sci. 2021, 42, 3791–3799. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Huang, R.H.; Jones, A.A.; Luck, M.E.; Aherne, C.M.; Jedlicka, P.; de Zoeten, E.F.; Collins, C.B. miR-106a deficiency attenuates inflammation in murine IBD models. Mucosal Immunol. 2019, 12, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Zailaie, S.A.; Siddiqui, J.J.; Al Saadi, R.M.; Anbari, D.M.; Alomari, A.S.; Cupler, E.J. Serum Based miRNA as a Diagnostic Biomarker for Multiple Sclerosis: A Systematic Review and Meta-Analysis. Immunol. Investig. 2022, 51, 947–962. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, C.; Li, L.; Bian, Z.; Lv, Z.; Shi, L.; Zhang, J.; Li, D.; Gu, H.; Zhang, C.Y.; et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. J. Allergy Clin. Immunol. 2013, 132, 426–436 e8. [Google Scholar] [CrossRef] [Green Version]
- Bo, C.; Wang, J.; Zhang, H.; Cao, Y.; Lu, X.; Wang, T.; Wang, Y.; Li, S.; Kong, X.; Sun, X.; et al. Global pathway view analysis of microRNA clusters in myasthenia gravis. Mol. Med. Rep. 2019, 19, 2350–2360. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, A.; Rezaei, N. MicroRNA expression profiles of peripheral blood and mononuclear cells in myasthenia gravis: A systematic review. Int. Immunopharmacol. 2022, 112, 109205. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bao, Z.; Liang, D.; Li, M.; Wei, M.; Ge, X.; Liu, J.; Li, J. Plasma exosomal miR-106a-5p expression in myasthenia gravis. Muscle Nerve 2020, 61, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P. Non-Coding RNAs in Cardiomyopathy and Heart Failure. In Non-Coding RNAs at the Cross-Road of Cardiometabolic Diseases and Cancer; Springer International Publishing: Cham, Switzerland, 2021; pp. 119–147. [Google Scholar]
- Guan, X.; Wang, L.; Liu, Z.; Guo, X.; Jiang, Y.; Lu, Y.; Peng, Y.; Liu, T.; Yang, B.; Shan, H.; et al. miR-106a promotes cardiac hypertrophy by targeting mitofusin 2. J. Mol. Cell. Cardiol. 2016, 99, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, C.; Liu, J.; Wang, Z.; Liu, Y.; Luo, C.; Chen, Y.; Wen, S. Expression Profile of microRNAs in Hypertrophic Cardiomyopathy and Effects of microRNA-20 in Inducing Cardiomyocyte Hypertrophy Through Regulating Gene MFN2. DNA Cell Biol. 2019, 38, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Qi, Y.F.; Xiao, Z.X.; Chen, H.; Liu, S.H.; Li, Z.Z.; Zeng, Z.F.; Wu, H.F. CircHIPK3 Regulates Vascular Smooth Muscle Cell Calcification Via the miR-106a-5p/MFN2 Axis. J. Cardiovasc. Transl. Res. 2022, 15, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wu, W.; Qu, J.; Wang, X.; Lei, S.; Yuan, L.; Liu, X. Inhibition of Mitofusin-2 Promotes Cardiac Fibroblast Activation via the PERK/ATF4 Pathway and Reactive Oxygen Species. Oxidative Med. Cell Longev. 2019, 2019, 3649808. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Ikeda, G.; Tada, Y.; von Bornstadt, D.; Santoso, M.R.; Wahlquist, C.; Rhee, S.; Jeon, Y.J.; Yu, A.C.; O’Brien, C.G.; et al. miR-106a-363 cluster in extracellular vesicles promotes endogenous myocardial repair via Notch3 pathway in ischemic heart injury. Basic. Res. Cardiol. 2021, 116, 19. [Google Scholar] [CrossRef]
- Sethi, S.; Mehta, P.; Pandey, A.; Gupta, G.; Rajender, S. miRNA Profiling of Major Testicular Germ Cells Identifies Stage-Specific Regulators of Spermatogenesis. Reprod. Sci. 2022, 29, 3477–3493. [Google Scholar] [CrossRef]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef]
- Kotaja, N. MicroRNAs and spermatogenesis. Fertil. Steril. 2014, 101, 1552–1562. [Google Scholar] [CrossRef]
- He, Z.; Jiang, J.; Kokkinaki, M.; Tang, L.; Zeng, W.; Gallicano, I.; Dobrinski, I.; Dym, M. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells 2013, 31, 2205–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackl, M.; Brunner, S.; Fortschegger, K.; Schreiner, C.; Micutkova, L.; Muck, C.; Laschober, G.T.; Lepperdinger, G.; Sampson, N.; Berger, P.; et al. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell 2010, 9, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandiford, O.A.; Moore, C.A.; Du, J.; Boulad, M.; Gergues, M.; Eltouky, H.; Rameshwar, P. Human Aging and Cancer: Role of miRNA in Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1056, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Grillari, J.; Grillari-Voglauer, R. Novel modulators of senescence, aging, and longevity: Small non-coding RNAs enter the stage. Exp. Gerontol. 2010, 45, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Lin, P.; Wang, J.; Yu, H.; Lv, T.; Sun, L.; Du, G. Circular RNAs: Promising Molecular Biomarkers of Human Aging-Related Diseases via Functioning as an miRNA Sponge. Mol. Ther. Methods Clin. Dev. 2020, 18, 215–229. [Google Scholar] [CrossRef]
- Tai, L.; Huang, C.J.; Choo, K.B.; Cheong, S.K.; Kamarul, T. Oxidative Stress Down-Regulates MiR-20b-5p, MiR-106a-5p and E2F1 Expression to Suppress the G1/S Transition of the Cell Cycle in Multipotent Stromal Cells. Int. J. Med. Sci. 2020, 17, 457–470. [Google Scholar] [CrossRef] [Green Version]
- ElSharawy, A.; Keller, A.; Flachsbart, F.; Wendschlag, A.; Jacobs, G.; Kefer, N.; Brefort, T.; Leidinger, P.; Backes, C.; Meese, E.; et al. Genome-wide miRNA signatures of human longevity. Aging Cell 2012, 11, 607–616. [Google Scholar] [CrossRef]
- Tiberio, P.; Callari, M.; Angeloni, V.; Daidone, M.G.; Appierto, V. Challenges in using circulating miRNAs as cancer biomarkers. Biomed. Res. Int. 2015, 2015, 731479. [Google Scholar] [CrossRef] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ramasubramanian, B.; Kanji, S.; Chakraborty, A.R.; Haque, S.J.; Chakravarti, A. Circulating microRNAs in cancer: Hope or hype? Cancer Lett. 2016, 381, 113–121. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliminejad, K.; Khorram Khorshid, H.R.; Ghaffari, S.H. Why have microRNA biomarkers not been translated from bench to clinic? Future Oncol. 2019, 15, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Kotowska-Zimmer, A.; Pewinska, M.; Olejniczak, M. Artificial miRNAs as therapeutic tools: Challenges and opportunities. Wiley Interdiscip. Rev. RNA 2021, 12, e1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des. Dev. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef]

| Product | Platform | Company | Targeted miRNA | Disease/Condition | Development Phase |
|---|---|---|---|---|---|
| ThyGeNEXT®/ThyraMIR®v2 | microRNA Pairwise Expression Profiling | Interpace Diagnostics | miR-29b-1-5p miR-31-5p miR-138-1-3p miR-139-5p miR-146b-5p miR-155 miR-204-5p miR-222-3p miR-375 miR-551b-3p | Thyroid cancer | Available-No Certification Information |
| CogniMIR™ | Blood-based assay for assessing plasma levels of specific miRNAs | DIAMIR | miRNA panel is not published | Alzheimer’s disease | Clinical Trial Assay (CTA) |
| OsteomiR™ kit | qPCR | TAmiRNA | let-7b-5p miR-127-3p miR-1336 miR-141-3p miR-143-3p miR-144-5p miR-152-3p miR-17-5p miR-188-5p miR-19b-3p miR-203a miR-214-3p miR-29b-3p miR-31-5p miR-320a miR-335-5p miR-375 miR-550a-3p miR-582-5p | Bone Quality | Research Use Only (RUO) |
| ThrombomiR® kit | qPCR | TAmiRNA | hsa-miR-126-3p hsa-miR-223-3p hsa-miR-197-3p hsa-miR-191-5p hsa-miR-24-3p hsa-miR-21-5p hsa-miR-28-3p hsa-miR-320a hsa-miR-150-5p hsa-miR-27b-3p hsa-miR-122-5p | Intrinsic and on-treatment platelet reactivity | Research Use Only (RUO) |
| HepatomiR® kit | qPCR | TAmiRNA | hsa-miR-122-5p hsa-miR-192-5p hsa-miR-151a-5p | Liver function and disease | CE-IVD (Conformité Européene- In Vitro Diagnostic) |
| miRisk | qPCR | Hummingbird Diagnostics GmbH | miRNA panel is not published. | Predicts immunotherapy response in stage IV non-small cell lung cancer | Clinical Validation |
| miLung | Early detection of lung cancer | Clinical Validation |
| Clinical Trial Number(s) * | Therapeutic Molecule | Targeted miRNA | Mode of Action | Disease | Biotechnology or Biopharmaceutical Company | Stage of Development (Clinical Trial/Preclinical Trial) | Status | Last Update Posted |
|---|---|---|---|---|---|---|---|---|
| NCT02508090 NCT02452814 NCT01200420 NCT01872936 NCT01727934 NCT01646489 | Miravirsen/SPC3649 | miR-122 | Anti-miR | Chronic hepatitis C virus | Roche/Santaris Pharma A/S | Phase II | Completed-Unknown (Study has passed its completion date and status has not been verified in more than two years) | February 2018 |
| EudraCT numbers 2015-001535-21 2015-004702-42 2016-002069-77 | RG-101 | miR-122 | Anti-miR | Chronic hepatitis C virus | Regulus Therapeutics Inc. | Phase II | Terminated (Side effects were reported) | September 2017 |
| NCT04536688 | RGLS4326 | miR-17 | Anti-miR | Polycystic kidney disease (PKD) | Regulus Therapeutics Inc. | Phase I | Completed | December 2021 |
| NCT03373786 NCT02855268 | RG-012/Lademirsen/SAR339375 | miR-21 | Anti-miR | Alport Syndrome | Genzyme (a Sanofi Company) | Phase II | Terminated (The results of the futility analysis led to the study termination. No unexpected safety findings were identified) | October 2022 |
| NCT02612662 NCT02826525 | RG-125/AZD4076 | miR-103/miR-107 | Anti-miR | Non-alcoholic Steatohepatitis (NASH) | AstraZeneca | Phase I | Completed | June 2022 |
| NCT05243017 NCT04120493 | AMT-130 | Artificial miRNA | Artificial miRNA Expression | Huntington Disease | UniQure Biopharma B.V. | Integrated Phase I/II | Ongoing | October 2022 |
| NCT02369198 | Mesomir 1 | miR-16 | miRNA mimic | Malignant Pleural Mesothelioma, Non-Small Cell Lung Cancer | Asbestos Diseases Research Foundation/EnGeneIC Limited | Phase I | Completed | April 2017 |
| NCT05507216 NCT05507203 | ABX464/Obefazimod | miR-124 | miRNA upregulation | Ulcerative Colitis | Abivax S.A. | Phase III | Ongoing | November 2022 |
| NCT05350969 NCT04045405 | CDR132L | miR-132 | Anti-miR | Heart failure | Cardior Pharmaceuticals GmbH | Phase II | Ongoing | November 2022 |
| NCT04675996 | INT-1B3 | miR-193a-3p | miRNA mimic | Solid Tumor | InteRNA | Phase Ib | Ongoing | February 2022 |
| NCT02603224 NCT03601052 | Remlarsen/MRG-201 | miR-29b | miRNA mimic | Keloid disorder | miRagen Therapeutics, Inc. | Phase II | Completed | August 2021 |
| NCT02580552 NCT03713320 NCT03837457 | Cobomarsen/MRG-106 | miR-155 | Anti-miR | Mycosis fungoides | miRagen Therapeutics, Inc. | Phase II | Terminated (The study was terminated early for business reasons, not due to concerns regarding safety or lack of efficacy) | April 2022 |
| NCT01829971 NCT02862145 | MRX34 | miR-34a | miRNA mimic | Primary Liver Cancer, SCLC, Lymphoma, Melanoma, Multiple Myeloma, Renal Cell Carcinoma, NSCLC | miRagen Therapeutics, Inc. | Phase I | Prematurely terminated/Withdrawn (5 immune related serious adverse events in Phase 1 study) | September 2016 |
| NCT03603431 | MRG-110 | miR-92a | Anti-miR | Wounds | miRagen Therapeutics, Inc. | Phase I | Completed | May 2019 |
| _ | MGN-2677 | miR-143/miR-145 | Anti-miR | Vascular Disease | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-1374 | miR-15/miR-195 | Anti-miR | Post-myocardial infection | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MRG-107 | miR-155 | Anti-miR | Amyotrophic lateral sclerosis (ALS) | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-4220 | miR-29 | Anti-miR | Cardiac fibrosis | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-4893 | miR-451 | Anti-miR | Abnormal red blood cell | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-5804 | miR-378 | Anti-miR | Cardiometabolic disease | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-6114 | miR-92/miR-92a | Anti-miR | Peripheral artery disease (PAD) | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
| _ | MGN-9103 | miR-208/miR-208a | Anti-miR | Chronic heart failure | miRagen Therapeutics, Inc. | Pre-clinical | _ | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshpour, M.; Ghadimi-Daresajini, A. Overview of miR-106a Regulatory Roles: from Cancer to Aging. Bioengineering 2023, 10, 892. https://doi.org/10.3390/bioengineering10080892
Daneshpour M, Ghadimi-Daresajini A. Overview of miR-106a Regulatory Roles: from Cancer to Aging. Bioengineering. 2023; 10(8):892. https://doi.org/10.3390/bioengineering10080892
Chicago/Turabian StyleDaneshpour, Maryam, and Ali Ghadimi-Daresajini. 2023. "Overview of miR-106a Regulatory Roles: from Cancer to Aging" Bioengineering 10, no. 8: 892. https://doi.org/10.3390/bioengineering10080892