Low-Cost Nanocarbon-Based Peroxidases from Graphite and Carbon Fibers
Abstract
:1. Introduction
2. Results and Discussion
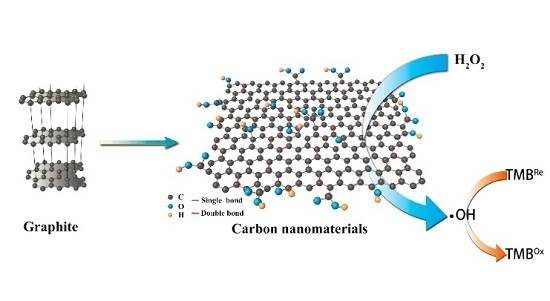
2.1. Synthesis and Characterization of Carbon Nanomaterials
2.2. The Peroxidase Catalytic Activities of Carbon Nanomaterials
2.3. H2O2 Detection Utilizing Carbon Nanomaterials
2.4. Catalytic Mechanism of Carbon Nanomaterials
2.5. Phenolic Compounds Removal Utilizing Carbon Nanomaterials
3. Materials and Methods
3.1. Synthesis of Carbon Nanomaterials-1 and Carbon Nanomaterials-2
3.2. Synthesis of Carbon Nanomaterials-3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breslow, R.; Overman, L.E. Artificial enzyme combing a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme mimics: Advances and applications. Chem. Eur. J. 2016, 22, 8404–8430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yan, X. Nanozymes: An emerging field bridging nanotechnology and biology. Sci. China Life Sci. 2016, 59, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T. Carbon nanodots as peroxidase nanozymes for biosensing. Molecules 2016, 21, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, F. Application of graphene/graphene oxide in biomedicine and biotechnology. Curr. Med. Chem. 2014, 21, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ren, J.; Qu, X. Carbon nanomaterials and DNA: From molecular recognition to applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Maas, M. Carbon nanomaterials as antibacterial colloids. Materials 2016, 9, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Muňoz, J.; Baeza, M. Customized bio-functionalization of nanocomposite carbon paste electrodes for electrochemical sensing: A mini review. Electroanalysis 2017, 29, 1660–1669. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon nanostructures in biology and medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Navalon, S.; Herance, J.R.; Alvaro, M.; García, H. Covalently modified graphenes in catalysis, electrocatalysis and photoresponsive materials. Chem. Eur. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mitra, K.; Shukla, A.; Singh, R.; Gundampati, R.K.; Misra, N.; Maiti, P.; Ray, B. Brominated graphene as mimetic peroxidase for sulfide ion recognition. Anal. Chem. 2017, 89, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Xu, C.; Ren, J.; Qu, X. Visual and quantitative detection of copper ions using magnetic silica nanoparticles clicked on multiwalled carbon nanotubes. Chem. Commun. 2010, 46, 6572–6574. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Chai, L.; Ma, J.; Qian, Z.; Chen, J.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, X.; Huang, X.; Wang, K.; Liu, Q. Low-cost preparation of photoluminescent carbon nanodots and application as peroxidase mimetics in colorimetric detection of H2O2 and glucose. J. Nanosci. Nanotechnol. 2013, 13, 6611–6616. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Ji, Z.; Li, C.; Shi, G. Aryl-modified graphene quantum dots with enhanced photoluminescence and improved pH tolerance. Nanoscale 2013, 5, 7361–7367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Shen, Y.; Wu, L.; Yu, L.; Liang, F.; Xi, J. Carbon dots promoted vanadium flow batteries for all-climate energy storage. Chem. Commun. 2017, 53, 7565–7568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, L.; Wang, J.; Liu, L.; Liang, F.; Xi, J. Rational use and reuse of Nafion 212 membrane in vanadium flow batteries. RSC Adv. 2017, 7, 19425–19433. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a nanocarbon-based artificial peroxidase: Chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of graphene quantum dots and Fe3O4 nanoparticles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, J.; Yang, J.; Xue, Q.; Miele, P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide-polyaniline and graphene-polyaniline hybrid papers. ACS Appl. Mater. Interfaces 2010, 2, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]







| Catalyst | Substrate Varied | Substrate Fixed (mM) | Km (mM) | Vmax (10−8 M·s−1) |
|---|---|---|---|---|
| Carbon nanomaterials-1 (this work) | TMB (3,3’,5,5’-Tetramethylbenzidine) | H2O2 (25) | 2.45 | 155.9 |
| Carbon nanomaterials-1 (this work) | H2O2 | TMB (0.2) | 0.01 | 10.31 |
| Carbon nanomaterials-2 (this work) | TMB | H2O2 (5) | 1.06 | 337.7 |
| Carbon nanomaterials-2 (this work) | H2O2 | TMB (0.2) | 0.001 | 55.13 |
| Carbon nanomaterials-3 (this work) | TMB | H2O2 (5) | 0.97 | 328.5 |
| Carbon nanomaterials-3 (this work) | H2O2 | TMB (0.2) | 0.0006 | 27.69 |
| N-GQDs (N-doped graphene quantum dots) (Ref. [27]) | TMB | H2O2 (3.3) | 11.2 | 0.38 |
| N-GQDs (Ref. [27]) | H2O2 | TMB (0.3) | 0.10 | 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Miao, F.; Zhao, Z.; Zhu, Y.; Liu, T.; Chen, R.; Liu, S.; Lv, Z.; Liang, F. Low-Cost Nanocarbon-Based Peroxidases from Graphite and Carbon Fibers. Appl. Sci. 2017, 7, 924. https://doi.org/10.3390/app7090924
Zeng Y, Miao F, Zhao Z, Zhu Y, Liu T, Chen R, Liu S, Lv Z, Liang F. Low-Cost Nanocarbon-Based Peroxidases from Graphite and Carbon Fibers. Applied Sciences. 2017; 7(9):924. https://doi.org/10.3390/app7090924
Chicago/Turabian StyleZeng, Yan, Feifan Miao, Zhiyong Zhao, Yuting Zhu, Tao Liu, Rongsheng Chen, Simin Liu, Zaosheng Lv, and Feng Liang. 2017. "Low-Cost Nanocarbon-Based Peroxidases from Graphite and Carbon Fibers" Applied Sciences 7, no. 9: 924. https://doi.org/10.3390/app7090924