Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Treatment with Cold Plasma (CP) and Electromagnetic Field (EMF)
2.3. Seed Germination Tests
2.4. Plant Cultivation in Field and Morphometric Analysis
2.5. Measurement of Photosynthetic Indices
2.6. Measurement of Radical Scavenging Activity and Total Amount of Phenolic Compounds
2.7. Detection of Seed Mineral Content
2.8. Analysis of Flavonoid and Fagomine Amount
2.9. Statistical Analysis
3. Results
3.1. Effects on Germination and Seedling Emergence
3.2. Changes Induced in Growth Dynamics in the Field
3.3. Changes Induced in Photosynthetic Efficiency
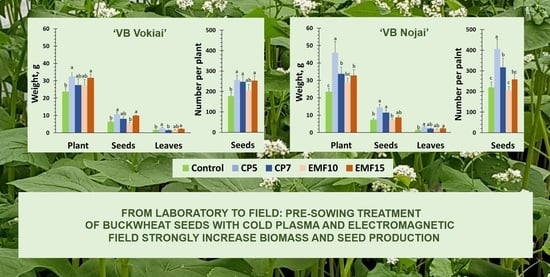
3.4. Changes in the Harvest Yield
3.5. Changes in Antioxidant Activity, Content of Secondary Metabolites and Minerals in Harvested Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Tang, Y.; Deng, X.; Ruan, C.; Wu, Y. Classification and nomenclature of buckwheat plants. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press: London, UK, 2018; pp. 9–20. [Google Scholar]
- Kreft, I.; Zhou, M.-L.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Li, H. Buckwheat. In Bioactive Factors and Processing Technology for Cereal Foods; Wang, J., Sun, B., Cao, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 137–150. [Google Scholar]
- Kim, S.-L.; Kim, S.-K.; Park, C.-H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the versatile buckwheat: Reinvigorating genetic gains through integrated breeding and genomics approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Huda, N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Chitarrini, G.; Nobili, C.; Pinzari, F.; Antonini, A.; De Rossi, P.; Del Fiore, A.; Procacci, S.; Tolaini, V.; Scala, V.; Scarpari, M.; et al. Buckwheat achenes antioxidant profile modulates Aspergillus flavus growth and aflatoxin production. Int. J. Food Microbiol. 2014, 189, 1–10. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.; Hu, C.; Jiang, Y.; Qin, L.; Zheng, C. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Technol. 2015, 44, 58–65. [Google Scholar] [CrossRef]
- Benković, E.T.; Kreft, S. Fagopyrins and protofagopyrins: Detection, analysis, and potential phototoxicity in buckwheat. J. Agric. Food Chem. 2015, 63, 5715–5724. [Google Scholar] [CrossRef]
- Mendler-Drienyovszki, N.; Cal, A.J.; Dobránszki, J. Progress and prospects for interspecific hybridization in buckwheat and the genus Fagopyrum. Biotechnol. Adv. 2013, 31, 1768–1775. [Google Scholar] [CrossRef]
- Woo, S.-H.; Roy, S.K.; Kwon, S.J.; Cho, S.-W.; Sarker, K.; Lee, M.-S.; Chung, K.-Y.; Kim, H.-H. Concepts, prospects, and potentiality in buckwheat (Fagopyrum esculentum Moench): A research perspective. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, UK, 2016; pp. 21–49. [Google Scholar]
- Cawoy, V.; Ledent, J.F.; Kinet, J.M.; Jacquemart, A.L. Floral biology of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Plant Sci. Biotechnol. 2009, 3, 1–9. [Google Scholar]
- Katagiri, C.; Morishita, T.; Suzuki, T.; Mukasa, Y. Growth and yield of self-compatible and hybrid common buckwheat lines pollinated with and without flies. Plant Prod. Sci. 2017, 20, 384–388. [Google Scholar] [CrossRef]
- Matsui, K.; Yasui, Y. Genetic and genomic research for the development of an efficient breeding system in heterostylous self-incompatible common buckwheat (Fagopyrum esculentum). Theor. Appl. Genet. 2020, 133, 1641–1653. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: NewYork, NY, USA, 2014; pp. 79–161. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Frederiks, C.; Wesseler, J.H.H. A comparison of the EU and US regulatory frameworks for the active substance registration of microbial biological control agents. Pest Manag. Sci. 2019, 75, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Mafei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant. Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Pietruszewski, S.; Martínez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophys. 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef] [Green Version]
- Staric, P.; Vogel-Mikuš, K.; Mozetic, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Holubová, L.; Kyzek, S.; Durovcová, I.; Fabová, J.; Horváthová, E.; Ševcovicová, A.; Gálová, E. Non-thermal plasma—A new green priming agent for plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Nauciene, Z.; Zukiene, R.; Malakauskiene, A.; Norkeviciene, E.; Stukonis, V.; Slepetiene, A.; Olsauskaite, V.; Padarauskas, A.; et al. Effect of seed treatment with cold plasma and electromagnetic field on red clover germination, growth and content of major isoflavones. J. Phys. D Appl. Phys. 2020, 53, 26. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdovâ, I.; Černák, M.; Gavril, B.; Hnatiuc, E.; Kováčik, D.; Kříha, V.; Sláma, J.; Šerý, M.; Špatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 2012 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; pp. 1365–1370.
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef]
- Paužaitė, G.; Malakauskienė, A.; Naučienė, Z.; Žūkienė, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildažienė, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. DBD plasma treatment induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. Changes in growth and production of non-psychotropic cannabinoids induced by pre-sowing treatment of hemp seeds with cold plasma, vacuum and electromagnetic field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Richards, F.J.A. Flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Hara, Y. Calculation of population parameters using Richards function and application of indices of growth and seed vigor to rice plants. Plant Prod. Sci. 1999, 2, 129–135. [Google Scholar] [CrossRef]
- Buivydaite, V.V. Soil Survey and Available Soil Data in Lithuania 2005, ESB-RR9. pp. 211–223. Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/esb_rr/n09_soilresources_of_europe/Lithuania.pdf (accessed on 11 May 2021).
- Rasineni, G.K.; Guha, A.; Reddy, A.R. Elevated atmospheric CO2 mitigated photoinhibition in a tropical tree species, Gmelina arborea. J. Photochem. Photobiol. B Biol. 2011, 103, 159–165. [Google Scholar] [CrossRef]
- Harbinson, J. Chlorophyll fluorescence as a tool for describing the operation and regulation of photosynthesis in vivo. In Light Harvesting in Photosynthesis, 1st ed.; Croce, R., van Grondelle, R., van Amerongen, H., van Stokkum, I., Eds.; CRC Press: London, UK, 2018; pp. 539–572. [Google Scholar]
- Strasser, R.J.; Srivatava, A.; Tsimilli-Michael, M. Screening the vitality and photosynthetic activity of plants by fluorescence transient. In Crop Improvement for Food Security, 1st ed.; Behl, R.K., Punia, M.S., Lather, B.P.S., Eds.; SSARM: Hisar, India, 1999; pp. 72–115. [Google Scholar]
- Zielinski, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Malakauskiene, A.; Žūkienė, R.; Januškaitienė, I.; Jakštas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2018, 14, 1700059. [Google Scholar] [CrossRef]
- Žvikas, V.; Pukelevicienė, V.; Ivanauskas, L.; Pukalskas, A.; Ražukas, A.; Jakštas, V. Variety-based research on the phenolic content in the aerial parts of organically and conventionally grown buckwheat. Food Chem. 2016, 213, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Amelioration of photosynthesis and quality of wheat under non-thermal radio frequency plasma treatment. Sci. Rep. 2018, 8, 11655. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C. Quality and safety of fresh fruits and vegetables at harvest. Sci. Hortic. 2018, 239, 78–79. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and microorganisms boost the nutritional composition of buckwheat (Fagopyrum esculentum Moench) sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Witkowicz, R.; Biel, W.; Skrzypek, E.; Chłopicka, J.; Gleń-Karolczyk, K.; Krupa, M.; Prochownik, E.; Galanty, A. Microorganisms and biostimulants impact on the antioxidant activity of buckwheat (Fagopyrum esculentum Moench) Sprouts. Antioxidants 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Michno, K.; Dubert, F.; Dziurka, M.; Kopeć, P.; Płażek, A. Embryological background of low seed set in distylous common buckwheat (Fagopyrum esculentum Moench) with biased morph ratios, and biostimulant-induced improvement of it. Crop Pasture Sci. 2017, 68, 680. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Park, B.S.; Adnan, M.; Germ, M.; Kreft, I.; Woo, S.H.; Park, C.H. Silicon biostimulant enhances the growth characteristics and fortifies the bioactive compounds in common and Tartary buckwheat plant. J. Crop Sci. Biotechnol. 2021, 24, 51–59. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radio frequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 12, 741. [Google Scholar] [CrossRef]
- Koga, K.; Attri, P.; Kamataki, K.; Itakagi, N.; Shiratani, M.; Mildažienė, V. Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, M. Impact of seed color and storage time on the radish seed germination and sprout growth in plasma agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Degutytė-Fomins, L.; Paužaitė, G.; Žukienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Mildažienė, V.; Ivankov, A.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Degutytė-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyuskevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Proc. Polym. 2020, 18, 2000160. [Google Scholar] [CrossRef]
- Suriyasak, S.; Hatanaka, K.; Tanaka, H.; Okumura, T.; Yamashita, D.; Attri, P.; Koga, K.; Shiratani, M.; Hamaoka, N.; Ishibashi, Y. Alterations of DNA Methylation Caused by Cold Plasma Treatment Restore Delayed Germination of Heat-Stressed Rice (Oryza sativa L.) Seeds. ACS Agric. Sci. Technol. 2021, 1, 5–10. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Tamošiūnė, I.; Gelvonauskienė, D.; Haimi, P.; Mildažienė, V.; Koga, K.; Shiratani, M.; Baniulis, D. Cold plasma treatment of sunflower seeds modulates plant-associated microbiome and stimulates root and lateral organ growth. Front. Plant Sci. 2020, 11, 568924. [Google Scholar] [CrossRef] [PubMed]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Molaei, H.; Oraghi Ardebili, N.; Amini, M. Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in hemp (Cannabis sativa L.). Plasma Chem. Plasma Proc. 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewel, J.J.; Schreeg, L.A.; Sinclair, T.R. Resources for crop production: Accessing the unavailable. Trends Plant Sci. 2019, 24, 121–129. [Google Scholar] [CrossRef] [PubMed]







| Treatment | ‘VB Vokiai’ | ‘VB Nojai’ | ||
|---|---|---|---|---|
| Vi, % | Me, Hours | Vi, % | Me, Hours | |
| Control | 97.3 ± 2.7 a | 42.6 ± 0.6 a | 98.7 ± 0.7 a | 43.6 ± 1.2 a |
| CP5 | 96.6 ± 0.7 a | 43.1 ± 1.7 a | 99.3 ± 0.7 a | 41.0 ± 0.2 ab |
| CP7 | 96.7 ± 2.4 a | 41.0 ± 0.4 a | 98.7 ± 0.7 a | 40.6 ± 0.3 b |
| EMF10 | 99.3 ± 0.7 a | 41.1 ± 0.4 a | 98.3 ± 0.3 a | 42.4 ± 0.6 a |
| EMF15 | 100.0 ± 0.0 a | 41.7 ± 0.2 a | 100.0 ± 0.0 a | 42.2 ± 0.6 a |
| Treatment | ‘VB Vokiai’ | ‘VB Nojai’ |
|---|---|---|
| Control | 79.5 ± 3.9 a | 71.5 ± 6.0 a |
| CP5 | 66.7 ± 4.8 b | 51.3 ± 5.2 b |
| CP7 | 69.0 ± 1.0 a,b | 72.7 ± 4.4 a |
| EMF10 | 72.5 ± 2.5 a,b | 56.8 ± 3.8 b |
| EMF15 | 68.5 ± 2.1 b | 63.0 ± 3.7 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A.; et al. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Appl. Sci. 2021, 11, 4391. https://doi.org/10.3390/app11104391
Ivankov A, Naučienė Z, Degutytė-Fomins L, Žūkienė R, Januškaitienė I, Malakauskienė A, Jakštas V, Ivanauskas L, Romanovskaja D, Šlepetienė A, et al. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Applied Sciences. 2021; 11(10):4391. https://doi.org/10.3390/app11104391
Chicago/Turabian StyleIvankov, Anatolii, Zita Naučienė, Laima Degutytė-Fomins, Rasa Žūkienė, Irena Januškaitienė, Asta Malakauskienė, Valdas Jakštas, Liudas Ivanauskas, Danuta Romanovskaja, Alvyra Šlepetienė, and et al. 2021. "Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field" Applied Sciences 11, no. 10: 4391. https://doi.org/10.3390/app11104391