Regional differences in the temporal expression of non-apoptotic caspase-3-positive Bergmann glial cells in the developing rat cerebellum
1
Department of Cell Physiology and Molecular Biophysics, Texas Tech University Health Sciences Center, Lubbock, TX, USA
2
Department of Pharmacology and Neurosciences, Texas Tech University Health Sciences Center, Lubbock, TX, USA
Although caspases have been intimately linked to apoptotic events, some of the pro-apoptotic caspases also may regulate differentiation. We previously demonstrated that active caspase-3 is expressed and has an apparent non-apoptotic function during the development of cerebellar Bergmann glia. The current study seeks to further correlate active/cleaved caspase-3 expression with the developmental phenotype of Bergmann glia by examining regional differences in the temporal pattern of expression of cleaved caspase-3 immunoreactivity in lobules of the cerebellar vermis. In general, we found that the expression pattern of cleaved caspase-3 corresponds to the reported developmental temporal profile of the lobes and that its levels peak at 15 days and declines thereafter. Compared to intermediate or late maturing lobules, early maturing lobules had higher levels of active caspase-3 at earlier postnatal times. This period of postnatal development is precisely the time during which Bergmann glia initiate differentiation.
Although caspase-3 is one of the key executioners of apoptosis (Hirata et al., 1998
), our lab serendipitously revealed, utilizing morphological and biochemical techniques, a non-apoptotic role for the active form of caspase-3 in differentiation of cerebellar cells (Oomman et al., 2004
). In support of our findings, there are a few additional reports that suggest caspases may influence proliferation and differentiation of cells (Fischer et al., 2003
; Los et al., 2001
). Proliferation of T cells is inhibited by caspase inhibitors (Alam et al., 1999
; Kennedy et al., 1999
; Woo et al., 2003
). In this regard, Wee1, an inhibitor of the G2/M cell cycle checkpoint machinery leading to cell cycle arrest and p21 and p27, two cyclin-dependent kinases that serve as negative regulators of G1-phase cell cycle progression, are substrates of caspases. Thus, cleavage by caspase of any of the three pro-differentiation proteins promotes cell progression/proliferation (Fischer et al., 2003
). Caspase activation has been reported to abort or promote incomplete apoptosis in various blood cells (DeBotton et al., 2002
; Zermati et al., 2001
), keratinocytes (Weil et al., 1999
), macrophages (Sordet et al., 2002
), lens fiber cells (Ishizaki et al., 1998
) and skeletal muscle cells (Fernando et al., 2002
). The enucleation observed in keratinocytes and lens fiber cells during development appear to represent such an incomplete apoptotic process (Ishizaki et al., 1998
; Weil et al., 1999
), whereas in the case of erythrocyte formation, caspase inhibitors suppressed the nuclear extrusion process and erythrocyte formation (De Maria et al., 1999
; Zermati et al., 2001
). Caspase activation has been associated with thrombopoiesis, fragmentation of proplatelets from megakaryocytes (DeBotton et al., 2002
), and the differentiation of macrophages (Sordet et al., 2002
) and muscle cells (Fernando et al., 2002
; Murray et al., 2008
). Additional involvement of caspase-3 in differentiation was observed in forebrain neurons (Yan et al., 2001
), trophoblast differentiation, syncytial fusion, and trophoblast turnover in human placenta (Huppertz et al., 1999
). In each of these processes, expression of caspase-3 was transient and only portions of the apoptotic machinery were utilized during the differentiation process. The involvement of caspases in differentiation may relate to a subset of apoptotic-like morphological changes in cellular state that involve cytoskeletal rearrangements, changes in cell adhesion and differential transcriptional regulation (Fischer et al., 2003
). In further support of a role of cleaved caspase-3 in differentiation, neurosphere cultures generated from striatum of CD1 murine embryos were treated with the peptide inhibitor z-DEVD-fmk causing failure to differentiate into neurons, glia or oligodendrocytes. Moreover, similar neurospheres obtained from caspase-3 null mice also failed to differentiate and continued to express the early neural progenitor marker nestin (Fernando et al., 2005
). Caspases also have been reported to function in synaptic plasticity (Dash et al., 2000
; Mattson and Duan, 1999
). It is significant that many cytoskeletal and membrane proteins are substrates for caspase-3, including actin (Kayalar et al., 1996
; Mashima et al., 1997
), spectrin (Wang et al., 1998
), fodrin (Martin et al., 1995
), and vimentin (Hashimoto et al., 1998
; van Engeland et al., 1997
). Proteolysis of these various proteins could be responsible for cytoskeletal changes associated with differentiation and may constitute the basis for the involvement of caspases during development.
We previously demonstrated that active caspase-3 expression was not limited to neuronal cells but also was located in Bergmann glia of the postnatal cerebellum (Oomman et al., 2005
, 2006
). Bergmann glia function as developmental organizers of the cerebellar cortex to orchestrate the normal anatomical arrangement of Purkinje neurons and their dendrites and to ensure the survival of granule cells. We demonstrated that cleaved caspase-3 expression in Bergmann glia is highest when glia are undergoing maximum differentiation/transformation (Oomman et al., 2005
). Concerning Bergmann glia development, it has known that Bergmann glial precursors take the form of radial cells at E14 (Yamada and Watanabe, 2002
). These glia precursor cells show active migration along with the Purkinje cells from E16–E19. In the primitive cerebellum, premature Bergmann glia are evenly arranged within the Purkinje cell layer by the first postnatal week. At the beginning of the second postnatal week, glial production shifts from the ventricular zone to local proliferation (Yamada and Watanabe, 2002
) and in addition, cells by this time acquire a unipolar shape. From P7–P30 Bergmann glia undergo a transformation mainly affecting the fibers, where the simple glial palisade acquires lateral appendages that resemble a reticular meshwork (Altman, 1975
; Das, 1976
; Rakic, 1971
; Shiga et al., 1983
). Glial fiber arborization coincides with Purkinje cell dendritogenesis, resulting in ensheathing of the parallel fiber-Purkinje cell synaptic cleft and also in ensheathing of the non-synaptic neuronal excrescences to form glial caps (Grosche et al., 2002
).
Through immunolabeling for glial markers using glutamate/aspartate transporters (Glast), 3-phosphoglycerate dehydrogenase, brain lipid binding protein (BLBP) and glutamine synthetase, it is known that the lobules within the cerebellum have different developmental temporal profiles (Altman, 1969
) and that this profile correlates with the development of Bergmann glia (Shiga et al., 1983
). The purpose of the present study was to evaluate regional differences in the temporal pattern of expression of cleaved caspase-3 immunoreactivity in the various lobules of the vermis in order to further correlate cleaved caspase-3 expression with the developmental phenotype of Bergmann glia and ultimately to its function.
Materials
Antibodies specific for the active cleaved form of caspase-3 (rabbit polyclonal antibody, catalog No. 9661L, Cell Signaling Technology, Beverly, MA, USA), S-100b (mouse monoclonal antibody, catalog No. S-2532, Sigma-Aldrich, St. Louis, MO, USA), GFAP (mouse monoclonal antibody, catalog No. G3893, Sigma-Aldrich), Glast (rabbit polyclonal antibody, catalog No. ab416-200, AbCam, Cambridge, MA, USA), and calbindin D28K (N-18) (goat polyclonal antibody, catalog No. SC-7692, Santa Cruz, Santa Cruz, CA, USA) were used. Donkey anti-rabbit, anti-mouse and anti-goat secondary antibodies linked to fluorescent tags, Alexa 488, Alexa 546 and Alexa 633, and Prolong Antifade mounting medium were obtained from Molecular Probes (Invitogen, Eugene, OR, USA).
Experimental Animal Preparation and Tissue Slicing
Postnatal 9- to 30-day-old rats (Sprague-Dawley strain, Sasco Labs) of either sex were used in these studies. Rats were either decapitated or anesthetized with sodium pentobarbital prior to the intracardiac perfusion of heparin/saline (1:1000 U) followed by direct fixation with 4% paraformaldehyde. Following fixation, the cerebellum was removed and placed in paraformaldehyde overnight and then placed in 30% sucrose in phosphate buffered saline (PBS, pH 7.4) to cryoprotect prior to sectioning with a cryostat. Twelve micron sagittal vermal sections were retrieved for immunohistochemical analyses. Each experiment used a minimum of three animals per age [Postnatal day (P)9, P15, P21,and P30] from which one cerebellar slice was taken at the same region of the vermis. The methods employed in this study have been approved by TTUHSC Animal Care and Use Committee (ACUC) and conform to NIH guidelines.
Immunohistochemical Assessments
Sections were immunohistochemically stained for the presence of cleaved caspase-3, calbindin D28K, GFAP, Glast, and S-100b. Sections were washed in PBS (pH 7.4), treated with acetone (−20°C) for 10 min to enhance antigenicity followed by three rinses in PBS, and permeabilized with Triton X-100 (0.5%) overnight at 4°C. The following day, sections were treated with a blocking solution (PBS containing 10% normal donkey serum and 0.5% Triton X-100) for 1 h. After five rinses in PBS, sections were exposed overnight to primary antibody solution containing 10% normal donkey serum and 0.5% Triton X-100 in PBST (PBS in 0.1% Tween 20 and 0.5 M NaCl) on a rotating stand at 4°C. Primary antibody concentrations were as follows: caspase-3 (17/20 kDa fragment, 1:50), calbindin D28k (1:100 dilution), GFAP (1:500), Glast (1:100), and S-100b (1:200). Following the removal of the primary antibodies, sections were rinsed three times in PBS and then treated with secondary antibodies (1:250 dilution of Alexa dye labeled donkey anti-mouse, anti-rabbit, anti-goat secondary antibody in PBST containing 0.1% Triton X-100 and 10% normal donkey serum). For triple labeling, slices were exposed to three primary antibodies simultaneously followed by three secondary antibodies using our standard protocol (Oomman et al., 2004
, 2005
). Sections were dehydrated with increasing concentrations of alcohol and mounted on slides with non-fluorescent antifade medium for viewing. Negative controls (omission of primary antibody) consistently were included in each experiment in order to subtract background autofluorescence.
To determine the total number of Bergmann glia for the calculation of percentage of glia that contain caspase-3 immunoreactivity, slices were triple stained with cleaved caspase-3, calbindin-28k to identify Purkinje neurons, and S-100b to identify surrounding Bergmann glia. Previously, our lab and others have reported that S-100b selectively labels the rod-like processes and reticular processes of cerebellar Bergmann glia in the cerebellum, positioned in the vicinity of Purkinje neurons (Hachem et al., 2007
; Oomman et al., 2004
, 2005
). Since Bergmann glia are known to surround Purkinje cells, we used this triple label to distinguish them and quantitate them based upon their size, location, and immunohistochemical selectivity.
Confocal Microscopy
Images were obtained using a Zeiss LSM 510 Meta Axioplot 2 laser scanning confocal imaging system equipped with Helium–Neon lasers, coupled to a Zeiss inverted microscope with X60 or X25 oil immersion objectives, using dichroic HFT UV/488/543/633 for conventional laser lines. The imaging system was controlled by LSM 510 META software (version 3.2, Carl Zeiss, Thornwood, NY, USA). Images were recorded at 488-nm excitation using a BP 510–520 IR band pass filter for Alexa Fluor 488, at 543-nm excitation using a BP 565–615 IR pass filter for Alexa 546, and at 633-nm excitation and a BP 644–676 meta filter for Alexa 633. The pinhole size was adjusted to obtain the optimal spatial resolution and high-magnification images were recorded with a 0.08 μm x/y pixel size. A rigorous lambda mode analysis using the META function provided excitation and emission spectra of each dye to optimize filter configuration. Settings of the confocal microscope were optimized for imaging each experiment and then equivalently applied to all sections. Laser power and PMT gain were adjusted to ensure that even the brightest fluorescence was below saturation (16 bit). Glial cells were chosen in similar regions within each lobule and scanned at 1024 × 1024 pixel resolution.
Statistical Analyses
Statistical analyses were performed using PSI-Plot (Poly Software International, Pearl River, NY, USA) or Sigma Stat (SPSS Science, Chicago, IL, USA). Data are expressed as mean (±SEM). To compare two groups, a Mann–Whitney Rank Sum Test for non-parametric data was used. P ≤ 0.05 was considered significant.
To test for the specificity of the active caspase-3 antibody, a preabsorption assay was performed with a caspase-3 blocking peptide (No. 1050; Cell Signaling Technology). The blocking peptide contains the amino acid sequence present in the carboxy-terminus of the catalytic subunit (from amino acids 165 to amino acids 175; human caspase 3). Figure 1
A (inset) demonstrates the lack of immunoreactivity in the slice when exposed to the primary antibody that had been preabsorbed with the blocking peptide. This panel shows an adjoining section labeled for active caspase-3 without the antigen preabsorption step from the same experiment. Western blots were run in cells treated with a pro-apoptotic stimulus (cytochrome C) to verify the specificity of the polyclonal antibody used in these studies (Figure 1
B). In addition, we have utilized a cleaved caspase-3 monoclonal antibody obtained from Cell Signaling (5A1E, catalog No. 9664) to compare labeling of Bergmann glia with the polyclonal derived cleaved caspase-3 antibody labeling (Figures 1
C,D).
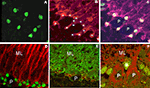
Figure 1. Panel (A) demonstrates a cerebellar section treated in the absence of blocking peptide, and the inset shows an adjacent section treated after co-incubating the antibody with the blocking peptide. As noted, the blocking peptide completely prevented the ability of the antibody raised against the cleaved form of caspase-3 to recognize cleaved caspase-3 in Bergmann cells. Faintly stained cells are presumed epithelial cells. Panel (B) is a Western blot analysis of extracts from HeLa cells treated with cytochrome c (0.25 mg/ml in vitro) using the cleaved polyclonal caspase-3 antibody to demonstrate the antibody specificity. The left hand column is molecular weight markers, and the right hand column shows authentic cleaved caspase-3 at 17 and 19 kDa. Panels (C) and (D) illustrate cultured Bergmann glia co-labeled with either a polyclonal (C) or monoclonal (D) antibody for cleaved caspase-3 (red, Alexa 594), and TU-01 (green, Alexa 488), an antitubulin antibody that selectively labels Bergmann glia. Note the consistent nuclear label for cleaved caspase-3.
Figures 2
A–C illustrate that cells immunoreactive-positive for S-100b and cleaved caspase-3 are situated as classic Bergmann glia surrounding the calbindin-positive Purkinje neurons. S-100b-positive cells also co-labeled for cleaved caspase-3 immunoreactivity in the nuclei rather than cytosol, suggesting a unique role related to nuclear function. We confirmed the apparent authenticity of the S-100b-labeled cells as Bergmann glia by performing additional immunohistochemical studies using antigens known to localize to Bergmann glia, including Glast, and GFAP (Feng et al., 1994
; Kuhar et al., 1993
). As seen with S-100b, GFAP colocalized with cleaved caspase-3 in the same cells that co-labeled with Glast and S-100b (Figures 2
D,E). Figure 2
F is a modified image (original active caspase-3 labeled as green fluorescence merged with enhanced Purkinje neuron autofluorescence labeled as red fluorescence) which clearly demonstrates the close proximity of these cells to Purkinje neurons. In Figure 2
F, to visualize Purkinje neurons, autofluorescence derived from endogenous, intrinsic fluorophores such as NADPH and flavin coenzymes present in mitochondria and lysosomes was intensified by increasing the gain of the microscope detector. Our lab previously demonstrated (Oomman et al., 2005
, 2006
) that the S-100b labeled cells failed to label with postmitotic neuronal markers TUJ1, a beta III tubulin cytosolic marker (Cameron and McKay, 2001
; Yan et al., 2001
), and NeuN, a neuron-specific neuronal marker (Hoshimaru et al., 1996
; Yan et al., 2001
), further verifying the active caspase-positive cells are not neurons.
Figure 2. Panels (A) and (B) are fluorescently labeled images from 15-day-old cerebellum using cleaved caspase-3 and S-100b immunohistochemistry visualized with Alexa 488 and Alexa 546, respectively. It is apparent that caspase-3 labels the nuclei of Bergmann glia. Asterisks identify the Bergmann glia in which approximately 60% contain cleaved caspase-3 immunoreactivity. Panel (C) is the triple labeled fluorescent image of caspase-3 (Alexa 488), S-100b (Alexa 546) and calbindin (Alexa 633) to identify and quantitate the total number of Bergmann glia within each lobule at the different ages, surrounding the Purkinje neurons. Panel (D) is a double labeled image using cleaved caspase-3 and GFAP immunoreactivity (visualized with Alexa 488 (green) and Alexa 546 (red), respectively). In Panel (E) cells are double labeled with S-100b (red fluorescence) and Glast (green fluorescence), two markers of Bergmann glia, respectively, to further verify the presence of caspase-3 in the nuclei of Bergmann glia surrounding Purkinje neurons obtained from 15-day-old cerebellum. Panel (F) is a modified image of an original cleaved caspase-3 labeling visualized with green wavelength using a Alexa 488 fluorescent dye coupled with an auto-fluorescent label of the tissue at the red wavelength (594 nm), showing the location of immunoreactive cells within the Purkinje cell layer in a 21-day-old cerebellum. P – Purkinje cell; B – Bergmann glia; ML – molecular layer.
Bergmann glia immunopositive for S-100b and GFAP, two markers of glia maturation (Boyes et al., 1986
; Tramontina et al., 2002
), showed a strong co-labeling with active caspase-3, suggesting a link between the degree of differentiation as indexed by the marker proteins S-100b and GFAP, and the presence of active caspase-3. Furthermore, quantitation of the S-100b immunohistochemical fluorescent intensity within the processes of the Bergmann glia that were immunopositive for cleaved caspase-3 compared to the S-100b immunoreactivity in non-caspase-3 labeled Bergmann glia revealed significant differences (P ≤ 0.05) between the two groups. S-100b immunoreactivity obtained from Bergmann glia processes in caspase-3 containing glia in 9-, 15-, and 21-day-old rats were significantly higher by 54, 50 and 48%, respectively than the non-caspase-3 containing glia, suggesting the presence of nuclear active caspase-3 enhanced expression of a cytosolic marker of Bergmann glia maturation.
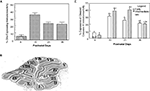
We determined the percentage of Bergmann glia that expressed cleaved caspase-3 immunoreactivity in all lobules at each of the developmental ages (P9, P15, P21, and P30). As indicated in Figure 3
A, we found that Bergmann glia in all vermal lobules expressed peak caspase-3 immunoreactivity at P15, in which approximately 35% of Bergmann glia expressed positive immunoreactivity; whereas the lowest percentage of expression was apparent at P9. On P21 and P30, fewer Bergmann glia (approximately 20%) were immunoreactive for cleaved caspase-3, apparently attaining a plateau by day P21. Although active caspase-3 immunoreactivity was observed in cells lying within the PC layer from P8 through P65, the expression decreased markedly in P45 and P65 days, representing less than 5% of the BG.
Figure 3. Caspase-3 immunoreactivity varies with age in the cerebellar vermis. Panel (A) is a graph that depicts the percentage of Bergmann glia expressing cleaved caspase-3 as a function of the developmental age of the rat. Panel (B) is an example of a vermal sagittal cerebellar slice from a 21-day-old rat stained with Toluidine blue to depict the classification used to characterize the various lobules. Panel (C) is a comparison of the expression of caspase-3 immunoreactivity in Bergmann glia in the early vs. intermediate/late maturing lobules of the cerebellar vermis.
To further analyze differences in the developmental expression of cleaved caspase-3 immunoreactivity in Bergmann glia, the cerebellum was subdivided into various lobules based upon their developmental profile. We classified lobules II–V as early developing, and lobules VIa-X and I as intermediate/late developing lobules (Figure 3
B) (Altman, 1969
). As seen in Figure 3
C, significant differences in the number of Bergmann glia expressing cleaved caspase-3 immunoreactivity were apparent at all the developmental ages except P30. Specifically, the earlier developing lobules expressed a higher mean percentage of cleaved caspase-3-positive Bergmann glia at P9 compared to the intermediate/late lobules, attaining the highest expression at P15; whereas, the intermediate and later developing lobules expressed a higher percentage at P15 and P21. By P30, the percentages of Bergmann glia in both regions exhibiting expression of cleaved caspase-3 were similar.
It is well known that cleaved caspase-3 is instrumental in the execution of morphological and biochemical features associated with apoptosis. Specifically, it has been shown to act in an executive role in nuclear apoptosis by activating downstream factors that disassemble nuclei (Jänicke et al., 1998
; Samejima et al., 1999
; Woo et al., 1998
), after turning off a number of critical pathways that promote cell survival (Samejima and Earnshaw, 1998
). However, in our previous study, the potential apoptotic fate of active caspase-3-positive Bergmann glia was ruled out based on the failure to correlate the co-expression of cleaved caspase-3 immunoreactivity with markers of apoptosis that included: Annexin V (exclusion of phosphatidylserine exposure), terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL to detect DNA fragmentation), and DNA compaction (TOPRO-3) (Oomman et al., 2005
). Specifically, we reported that more than 90% of the active caspase-3-positive cells lacked co-labeling for one of these apoptotic markers, providing evidence for a role of cleaved caspase-3 independent of classical apoptosis (Oomman et al., 2005
).
In the present study, the expression pattern of cleaved caspase-3 in the anterior and posterior lobes of the cerebellar vermis corresponds to the reported developmental profile of these lobes. Specifically, the anterior lobe expressed a higher percentage of cleaved caspase-3 immunoreactive Bergmann glia compared to the posterior lobe at P9. Previous reports indicate that the anterior lobes of the vermis are an earlier developing region of the cerebellum compared to the posterior lobe that represents the intermediate and late developing lobes (Oomman et al., 2005
). If nuclear caspase-3 has a role in promoting differentiation, then the observed earlier expression of cleaved caspase-3 at P9 in the lobules in the anterior lobe could indicate that a higher percentage of Bergmann glia presumably have initiated differentiation at this time period compared to the later developing posterior lobe (with more Bergmann glia still in the stage of proliferation). At P21–P30, Bergmann glia in the anterior lobe would be near terminal stages of differentiation, and thus require less cleaved caspase-3; whereas those in the later developing posterior lobe would be near initiation of differentiation, and therefore require higher levels of caspase-3 as our data show. At P30, only 20% of Bergmann glia in both regions of the cerebellum continued to express cleaved caspase-3, suggesting that because differentiation of Bergmann glia in all lobules had plateaued compared to the earlier time periods, the functional requirement for active caspase-3 was diminished. Since cell production is thought to cease by the end of the first postnatal month, the appearance of cleaved caspase-3 in cells up to P60 was intriguing. Classic understanding of cerebellar development holds that the mammalian cerebellum does not appear to engage in persistent neurogenesis after the end of postnatal granule cell genesis. However, the cerebellum is the only CNS area where radial glia cells, the type of cell that corresponds to the embryonic neural progenitor cells (Anthony et al., 2004
), exist in the adult. Furthermore, as also seen in the hippocampus, stem cells have been identified in the adult cerebellum suggesting the continued capacity for cellular proliferation (Klein et al., 2005
; Lee et al., 2005
). Additionally, Ponti et al. (2008)
observed the presence of a proliferative subpial layer in the rabbit cerebellum that replaces the external granule cell layer between the fourth and fifth postnatal week. This layer persists from postnatal day 40 to 150 and participates in the proliferation of Bergmann glia in the Purkinje cell layer as evidenced by the presence of BrdU, Brain Lipid Binding Protein (BLBP) and Sox2-positive nuclei. Interestingly, the transcription factors Sox1, a known marker of early neural progenitors (Aubert et al., 2003
; Barraud et al., 2005
; Li et al., 2001
), Sox2 and Sox9, known to be molecular markers of adult neural stem cells (Ellis et al., 2004
; Episkopou, 2005
; Sottile et al., 2006
), were located in the adult cerebellar Bergmann glia of rodents (Sottile et al., 2006
), suggesting that Bergmann glia may represent stem cells in the adult cerebellum. Additionally, recent evidence has emerged to suggest the existence of a fifth cellular element in the brain known as NG2 glia or synantocytes (Butt et al., 2005
). These cells reside in the location of Bergmann glia and retain the capacity to proliferate and differentiate throughout adulthood (Levine et al., 1993
). Future studies need to further address the potential of glia-genesis in peripuberal and adult rats and the potential role of caspase-3 in these cells.
Our earlier report indicated that cleaved caspase-3 immunoreactivity was more frequently seen in non-proliferating (Ki67-negative) than in proliferating Bergmann glia (Ki67-positive) (Oomman et al., 2005
, 2006
). The expression trend of cleaved caspase-3 immunoreactivity in Bergmann glia and the lack of co-labeling with Ki67 suggest that caspase-3 expression does not correlate with the temporal period of proliferation. Our present data demonstrate that levels of cleaved caspase-3 expression in Bergmann glia are highest at P15–P21 when they initiate differentiation/transformation that includes the formation of lateral expansions protruding from the proximal shaft of Bergmann fibers (Shiga et al., 1983
). Additionally, we have previously shown that cleaved caspase-3 colocalizes with BLBP, which is a marker for Bergmann glia that have undergone differentiation (Oomman et al., 2005
, 2006
). Additional studies in our lab using cultured Bergmann glia have shown that the caspase-3 peptide inhibitor, DMQD-fmk (80 μM) caused an almost threefold increase in the number of proliferating precursor glial cells and decreased the number of differentiating Bergmann glia, without significantly altering the non-glial active caspase-3-negative population. That is, caspase-3 inhibition arrested Bergmann glia differentiation and caused reversion to a proliferative state (Oomman et al., 2006)
. The transformation in the developmental state of Bergmann glia occurring after suppression of caspase-3 activity strongly suggests an involvement of this enzyme in promoting differentiation of Bergmann glia, possibly by allowing cells to exit the cell cycle. Therefore in this context, active caspase-3 could be a genetic manipulator playing a key role in controlling spatially distant and dynamic events like glial morphogenesis and arborization, and maintaining the differentiated state of the cell. In this regard, numerous substrates have been linked to cleaved caspase-3 and in the case of nuclear proteins, the involvement of transcription factors and cell cycle proteins may be of relevance to the initiation of differentiation (Fischer et al., 2003
). Future studies need to be directed at identifying the cellular cascade and substrates utilized in this novel non-apoptotic function.
Research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Janet Dertien for her technical help and support in this project.
Aubert, J., Stavridis, M. P., Tweedie, S., O’Reilly, M., Vierlinger, K., Li, M., Ghazal, P., Pratt, T., Mason, J. O., Roy, D., and Smith, A. (2003). Screening for mammalian neural genes in fluorescence-activated cell sorter purification of neuronal precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl. 1), 11836–11841.
Oomman, S., Finckbone, V., Dertien, J., Attridge, J., Henne, W., Medina, M., Mansouri, B., Singh, H., Strahlendorf, H., and Strahlendorf, J. (2004). Active caspase-3 expression during postnatal development of rat cerebellum is not systematically or consistently associated with apoptosis. J. Comp. Neurol. 476, 154–173.
Woo, M., Hakem, R., Soengas, M. S., Duncan, G. S., Shahinian, A., Kägi, D., Hakem, A., McCurrach, M., Khoo, W., Kaufman, S. A., Senaldi, G., Tamara, H., Lowe, S. W., and Mak, T. W. (1998). Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12, 806–819.