Enterobactin Deficiency in a Coliform Mastitis Isolate Decreases Its Fitness in a Murine Model: A Preliminary Host–Pathogen Interaction Study
- 1Laboratory of Biochemistry, Department of Pharmacology, Toxicology and Biochemistry, Faculty of Veterinary Medicine, Ghent University, Merelbeke, Belgium
- 2Molecular Neurogenetics Unit, Department of Neurology and Center for Molecular Imaging Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
- 3Centre of Excellence for Bovine TB, Institute of Biology, Environmental and Rural Sciences, Aberystwyth University, Aberystwyth, United Kingdom
- 4Department of Pathobiology and Population Sciences, Royal Veterinary College, Hatfield, United Kingdom
- 5School of Engineering and Physical Sciences, Institute of Biological Chemistry, Biophysics and Bioengineering, Heriot-Watt University, Edinburgh, United Kingdom
- 6INRAE, UMR ISP, Université François Rabelais de Tours, Nouzilly, France
Iron is an essential nutrient for bacterial growth. Therefore, bacteria have evolved chelation mechanisms to acquire iron for their survival. Enterobactin, a chelator with high affinity for ferric iron, is secreted by Escherichia coli and contributes to its improved bacterial fitness. In this preliminary study, we evaluated enterobactin deficiency both in vitro and in vivo in the context of E. coli mastitis. Firstly, we showed that expression of lipocalin 2, a protein produced by the host that is able to both bind and deplete enterobactin, is increased upon E. coli infection in the cow's mastitic mammary gland. Secondly, we demonstrated in vitro that enterobactin deficiency does not alter interleukin (IL)-8 expression in bovine mammary epithelial cells and its associated neutrophil recruitment. However, a significantly increased reactive oxygen species production of these neutrophils was observed. Thirdly, we showed there was no significant difference in bacterial in vitro growth between the enterobactin-deficient mutant and its wild-type counterpart. However, when further explored in a murine model for bovine mastitis, the enterobactin-deficient mutant vs. the wild-type strain revealed a significant reduction of the bacterial load and, consequently, a decrease in pro-inflammatory cytokines (IL-1α,−1β,−4,−6, and−8). A reduced neutrophilic influx was also observed immunohistochemically. These findings therefore identify interference of the enterobactin iron-scavenging mechanism as a potential measure to decrease the fitness of E. coli in the mastitic mammary gland.
Introduction
Iron (Fe) is an essential micronutrient and co-factor in several oxidation–reduction reactions required for basic cell metabolism, e.g., DNA stability and cell cycle control (1, 2). In mammals, iron availability is tightly regulated by host iron-binding proteins such as transferrin, ferritin, and lactoferrin. The latter is specifically secreted in milk in order to limit bacterial growth (3, 4). However, bacteria secrete high affinity iron-binding siderophores to circumvent iron restriction by the host. Enterobactin (Ent) is a prototypic siderophore that is highly conserved in Escherichia coli, being optimally structured for binding ferric iron (5–7). For its synthesis, Ent relies on the conversion of amino acid precursors by the non-ribosomal peptide synthetase EntA which catalyzes an early step in Ent biosynthesis (6, 8, 9). The production of siderophores like Ent is particularly induced under low iron level conditions, aiding bacteria in their competition with the host for scarcely available iron (1, 10). The role of Ent is especially well-studied in the inflamed gut in which iron-bound Ent from intestinal E. coli clearly contributes to pathogen growth and immune resistance during disease development (11–13). Therefore, Ent and other siderophores have been identified as potential preventive or therapeutic targets in general and have shown some applicability, also to bovine mastitis pathogens (14, 15). Bovine mastitis, an inflammation of the cow's udder, is currently the most prevalent production disease in the dairy industry and is frequently caused by E. coli (16, 17). Escherichia coli is an environmental pathogen that takes advantage of poor hygiene conditions and is best controlled with appropriate farm and animal management measures (18). However, full eradication of E. coli mastitis even with these appropriate measures implemented proves impossible (19).
Bacterial siderophores have been associated with lipocalin 2 (LCN2) released by host immune cells, primarily polymorphonuclear neutrophilic granulocytes (PMN), as part of their immune response against propagating pathogens (11–13, 20). LCN2 is able to bind and deplete both iron-bound and iron-lacking bacterial siderophores, thus further preventing iron uptake by bacteria (12, 21, 22). These LCN2-producing PMN are attracted to the infected mammary gland by the chemotactic molecule interleukin (IL)-8 that is secreted by the bovine mammary epithelium to enhance phagocytosis and reactive oxygen species (ROS) production of PMN to clear the infection present (23, 24).
However, the interplay between bacterial Ent and the host's immune system remains poorly studied in the context of E. coli mastitis (25). For this purpose, limited in vitro and in vivo studies were combined to preliminarily investigate the involvement of iron availability, innate immunity, and a local bacterial infection.
Materials and Methods
Selection of E. coli Strains
Three E. coli strains were selected: P4, 1303, and MG1655. The P4 strain consisted of a wild-type bovine mastitis isolate (P4) as well as its genetically modified EntA-deficient counterpart (P4 ΔentA) unable to produce enterobactin. To demonstrate a strain-independent effect, E. coli strains 1303 and MG1665 were included additionally. Escherichia coli 1303 is bovine mastitis-derived, and E. coli MG1655 is a non-pathogenic E. coli K-12 derivative (26, 27). All E. coli strains were grown in Dulbecco's modified Eagle's medium (DMEM)/F12 (Thermo Fisher Scientific) at 37°C and 5% CO2.
Quantitative Polymerase Chain Reaction of LCN2 mRNA in Udder Tissue of Experimentally E. coli-Infected Dairy Cows
The experimental procedures on the cows were executed at the Clinic for Ruminants in Munich under ethical approval by the regional government ethics committee of upper Bavaria (approval number 55.2-1-54-2531-108-05). Five mid-lactating Holstein Friesian cows were experimentally infected in one quarter with either 500 colony-forming units (CFU) of E. coli mastitis isolate 1303 or were sham-inoculated (28). Prior to the infection, milk samples of all udder quarters confirmed normal somatic cell counts (50,000 somatic cells/ml) and the absence of mastitis pathogens. Twelve hours post-infection (h.p.i.), all inoculated animals displayed clinical mastitis in the affected quarters. Animals were humanely euthanized 24 h.p.i. following the recommended guidelines of the American Veterinary Medical Association (“AMVA Guidelines for the Euthanasia of Animals”), and ~100 mg of lobulo-alveolar udder tissue was snap frozen in liquid nitrogen immediately after euthanasia. RNA was extracted from the udder tissue of the E. coli-infected cows using TRIzol (Invitrogen). Reverse transcription of 100 ng total RNA into cDNA was performed using iScript Reverse Transcription Supermix (Bio-Rad). Subsequently, iQ SYBR Green Supermix (Bio-Rad) was used for quantitative polymerase chain reaction (qPCR) with the primers described in Supplementary Table 1. Expression of two reference genes PPIA and 18S, according to MIQE guidelines, was used for normalization, and Genex macro (Bio-Rad) then calculated the final gene expression in infected vs. uninfected udder quarters from the same cow (29).
IL-8 Expression of E. coli-Exposed Bovine Mammary Epithelial Cells
Two bovine mammary epithelial cell lines (boMECs), MAC-T and PS, were exposed to either E. coli P4, P4 ΔentA, 1303, or MG1665 as described (30). Briefly, boMECs were seeded to flat microtiter plates to contain 105 cells/well in assay medium DMEM/F12 (Thermo Fisher Scientific) and grown to confluence at 37°C and 5% CO2 for 72 h (31). Prior to infection of boMECs, E. coli strains were inoculated into DMEM/F12 and grown standing at 37°C for 2–4 h to reach OD600 = 0.4 before diluting to 106 CFU/ml. After co-incubation of each E. coli with boMECs for 3 h, supernatants were collected. Escherichia coli were removed by washing with Hanks' balanced salt solution (HBSS) (Gibco) and media replenished supplemented with 50 μg/ml gentamycin (Sigma) for a further 21 h. Supernatants were additionally collected at 24 h. Secreted IL-8 levels were then measured in the supernatant of both exposed boMECs by enzyme-linked immunosorbent assay (ELISA) as described (32).
Isolation and Migration of and Reactive Oxygen Species Production by PMN After Co-culture With E. coli-Exposed boMECs
Migration of PMN was evaluated by a transwell assay. PMN were isolated from peripheral bovine blood by density centrifugation and flash lysis as described (33). PMN purity was assessed by microscopy after Diff-Quik (Reagena) staining. Isolations that were at least 90% pure were subsequently used for migration assays. The latter were performed using transwell inserts for 24-well tissue culture plates containing a pore size of 2 μm (Greiner). BoMECs were introduced into 24-well plates at a concentration of 105 cells/well and allowed to adhere overnight. Cells were washed and media replaced with E. coli isolates at a multiplicity of infection (MOI) of 105 CFU/well, arbitrarily set as 1 (MOI = 1). Media alone and recombinant bovine IL-8 (Kingfisher Biotech) at 5 μg/ml served as the negative and positive control, respectively. PMN were immediately added to the inner transwell at 106 cells/well and subsequently incubated during 24 h. Migration was assessed 3 and 24 h after E. coli exposure using modified counting chambers (Immune Systems) as described (34). Reactive oxygen species production of PMN was investigated by culturing isolated PMN in HBSS either in the presence of P4 or P4 ΔentA heat-killed E. coli. Reactive oxygen species was measured as described (35). Briefly, production of ROS by PMN was measured by oxidation of 2,7-dichlorofluorescein diacetate (DCFH-DA) (Sigma) to fluorescent 2,7-dichlorofluorescein (DCFH) at 2 h post-exposure in black microtiter plates. Zymosan-stimulated and unstimulated PMN served as positive and negative control, respectively. Relative fluorescence units were measured using a spectrophotometer (Tecan M200) at 485 nm excitation and 530 nm emission wavelengths, while cells were incubated at 37°C and 5% CO2.
In vitro Growth Curves of E. coli P4 and E. coli P4 ΔentA
An overnight bacterial culture was diluted 1:100 in DMEM/F12 (Thermo Fisher Scientific) and vortexed. Subsequently, 200 μl thereof was added to wells in a 96-well plate. This 96-well plate was thereafter incubated at 37°C and the optical density was measured at 600 nm (OD600) every hour during 8 h.
Mouse Model for Bovine E. coli Mastitis and Processing of the Harvested E. coli-Infected Mammary Glands
All experimental procedures on mice were executed at the Faculty of Veterinary Medicine of Ghent University (Merelbeke, Belgium) and with approval of the ethics committee (Approval Number 2015/127). In brief, female C57BL/6 and CD-1 mice (purchased from Envigo) were coupled with male mice. Ten days post-partum, the lactating dams were intraductally inoculated with a 32-gauge pediatric needle in the fourth abdominal mammary gland pair after properly disinfecting the teat. Intraductal inoculations were performed with an inoculum dose of ~250 CFU at 1 h post-weaning of either P4 or P4 ΔentA in 100 μl phosphate buffered saline (PBS). This inoculation was performed under general gas anesthesia, using a mixture of oxygen and isoflurane at 2–3% for induction and 1–1.5% for maintenance. The long-acting analgesic buprenorphine (Vetergesic, Patheon UK Ltd., Swindon, UK) was administered at 10 μg/kg intraperitoneally for post-surgical pain relief. At 24 h.p.i., the inoculated mice were sedated with a cocktail of 100 mg/kg ketamine (Ketamidor, Ecuphar nv/sa, Belgium) and 10 mg/kg xylazine (Xylazini Hydrochloridum, Val d'Hony-Verdifarm, Belgium) and subsequently euthanized by cervical dislocation to harvest the mammary glands. In order to quantify the bacterial loads (expressed as CFU), the isolated mammary glands were homogenized using TissueRuptor (Qiagen) and serially diluted in PBS, followed by plating on tryptic soy agar (Oxoid). To compensate for the different volumes of the harvested tissues, mammary glands were weighed and bacterial load was expressed as CFU/g tissue.
The host response to the experimental mammary gland infection was investigated by determining the levels of LCN2 and selected inflammatory cytokines in mammary gland lysates obtained by mixing the homogenates with 300 μl lysis buffer containing protease inhibitors. Quantification of LCN2 was performed by ELISA (Bio-Techne, Minneapolis, MN, USA). Levels of the selected cytokines IL-1α,−1β,−4,−6, and−8 were determined by multiplex assay (Luminex, Thermo Fisher Scientific). Neutrophil influx was evaluated by immunohistochemical staining for the neutrophil marker Ly6G (anti-Ly6G-APC, clone 1A8, Miltenyi Biotec) on paraffin sections of the infected mouse mammary glands at 24 h.p.i. Deparaffinized and hydrated tissue slides were incubated in citrated buffer at pH 6.0 (10 mM tri-sodium citrate and 0.05% Tween-20) in a decloacking chamber under pressure at 95°C for 30 min. Following peroxidase blocking [3% H2O2 in methanol for 10 min at room temperature (RT)], sections were incubated with primary rat anti-mouse Ly6G antibody for 1 h at RT. Rat-on-mouse HRP-Polymer (Biocare Medical) served as secondary antibody on the tissue sections for 30 min at RT. Detection of the staining was performed by applying a buffer with 3,3′-diaminobenzidine on the tissue sections for 10 min at RT. For microscopic evaluation of this immunohistochemical staining, the tissue sections were rehydrated and mounted with a cover glass.
Statistical Analysis
Data were analyzed using GraphPad Prism (version 5.00.288) to calculate P-values and determine statistically significant differences (P < 0.05). D'Agostino-Pearson was used to check for normally distributed data. If needed, data underwent a log10 transformation, leading to a normal distribution of the data. Two groups were compared with unpaired t-tests, and multiple groups with analysis of the variance (ANOVA) and Tukey's post hoc-test.
Results
Expression of Host LCN2 Is Increased in the E. coli-Infected Mammary Gland
To confirm that iron is sequestered in the cow's mastitic mammary gland by the host's immune system, Holstein Friesian cows were experimentally infected with E. coli 1303 and LCN2 expression was determined. qPCR analysis revealed that LCN2 expression in the E. coli-infected bovine mammary gland tissue was indeed significantly increased compared with sham-inoculated quarters (P < 0.01; Figure 1).
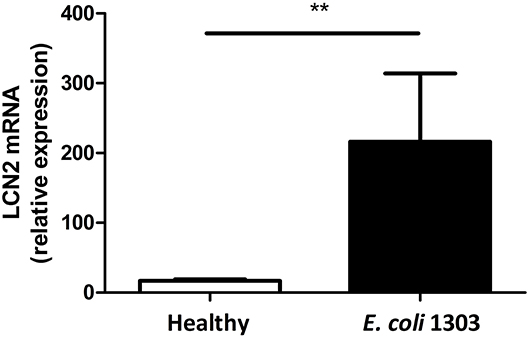
Figure 1. mRNA lipocalin 2 (LCN2) relative expression calculated by the ΔΔCt method taking as reference genes PPIA and 18S in udder quarters 24 h.p.i. with 500 colony-forming units (CFU) E. coli 1303 compared with sham-inoculated udder quarters (nquarters = 5 per condition). Data are presented as means ± standard error of the mean. **P < 0.01.
Since our group previously observed the Ent expression system is commonly present in mastitis-causing E. coli (36) and the role of LCN2 includes sequestration of bacterial Ent, this observation in the target species raises a subsequent question: How is the host's immune response, i.e., chemokines produced by boMECs, PMN migration, and activation, affected in the presence and absence of bacteria producing Ent? Therefore, a bovine mastitis-derived E. coli strain, i.e., P4, was made deficient in the EntA gene, and Ent deficiency was preliminarily determined in vitro on the previously mentioned cell types.
IL-8 Secretion Is Not Altered in BoMECs by Bacterial Ent Depletion
IL-8 secretion, a key inflammatory marker of mastitis, was determined as a first parameter in two boMEC lines [i.e., MAC-T (Figure 2A) and PS (Figure 2B)] upon incubation with E. coli P4 and its enterobactin-deficient counterpart: P4 ΔentA. BoMECs were incubated either with or without bacteria for 3 or 24 h. Untreated BoMECs showed very low levels of IL-8 secretion after 3 h and no increase in their IL-8 secretion over time at 24 h (Figure 2). The IL-8 secretion of boMECs incubated with E. coli P4 ΔentA vs. E. coli P4 did not differ significantly (P > 0.05; Figure 2). To confirm that this single observation was P4 strain-independent, boMECs were additionally incubated with two other E. coli strains, i.e., the bovine mastitis-derived E. coli 1303 and the non-pathogenic, human-derived E. coli MG1655. Both strains induced secretion of similar amounts of IL-8 compared with P4 and P4 ΔentA (Figure 2). It was also observed that increased incubation times from 3 to 24 h resulted in higher concentrations of secreted IL-8 in both boMEC lines and with all E. coli tested, but no differential secretion was observed upon Ent depletion.
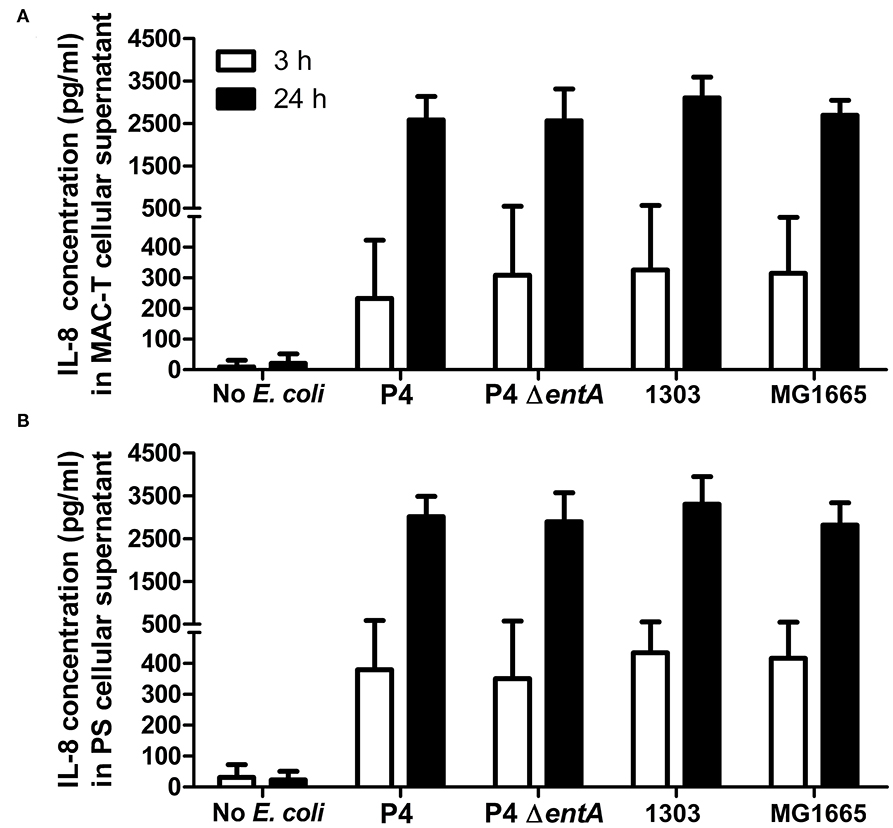
Figure 2. Secreted interleukin (IL)-8 concentrations by bovine mammary epithelial cell lines (boMECs) as measured in the supernatant of bovine MAC-T (A) and PS (B) cells at both 3 and 24 h after co-incubation with E. coli P4 ΔentA vs. P4 and two additional E. coli strains, i.e., 1303 and MG1655. Each cell type and incubation time had six replicates (n = 6). Data are presented as means ± standard error of the mean.
Bacterial Ent Depletion Does Not Alter PMN Migration but Influences ROS Production
Secondly, to investigate whether the unaltered IL-8 expression levels did not affect PMN functionality, PMN migration and ROS production of PMN were determined under the same experimental conditions.
BoMECs were incubated either with or without the different E. coli strains, and PMN migration was subsequently evaluated using a transwell assay. Similarly as for IL-8 secretion, PMN migration did not differ significantly between P4 ΔentA and P4 (P > 0.05; Figures 3A,B). Longer incubation times resulted in a higher number of migrated PMN, but again no differential effect of Ent depletion was observed. The lack of differences was not due to a functional impairment of PMN, as PMN migrated strongly in the presence of added bovine IL-8 (positive control). After 24 h, this positive control had the highest number of migrated PMN. Furthermore, all wells containing either treated boMEC or IL-8 contained more migrated PMN compared with the negative control (untreated boMEC), although no statistically significant difference was observed either between the E. coli strains tested and the controls on both boMEC cell lines and as a result of increased incubation times (P > 0.05; Figures 3A,B). Finally, PMN migrated in general less when the wells contained only the bacterial isolates compared to incubation with E. coli-exposed boMECs (data not shown).
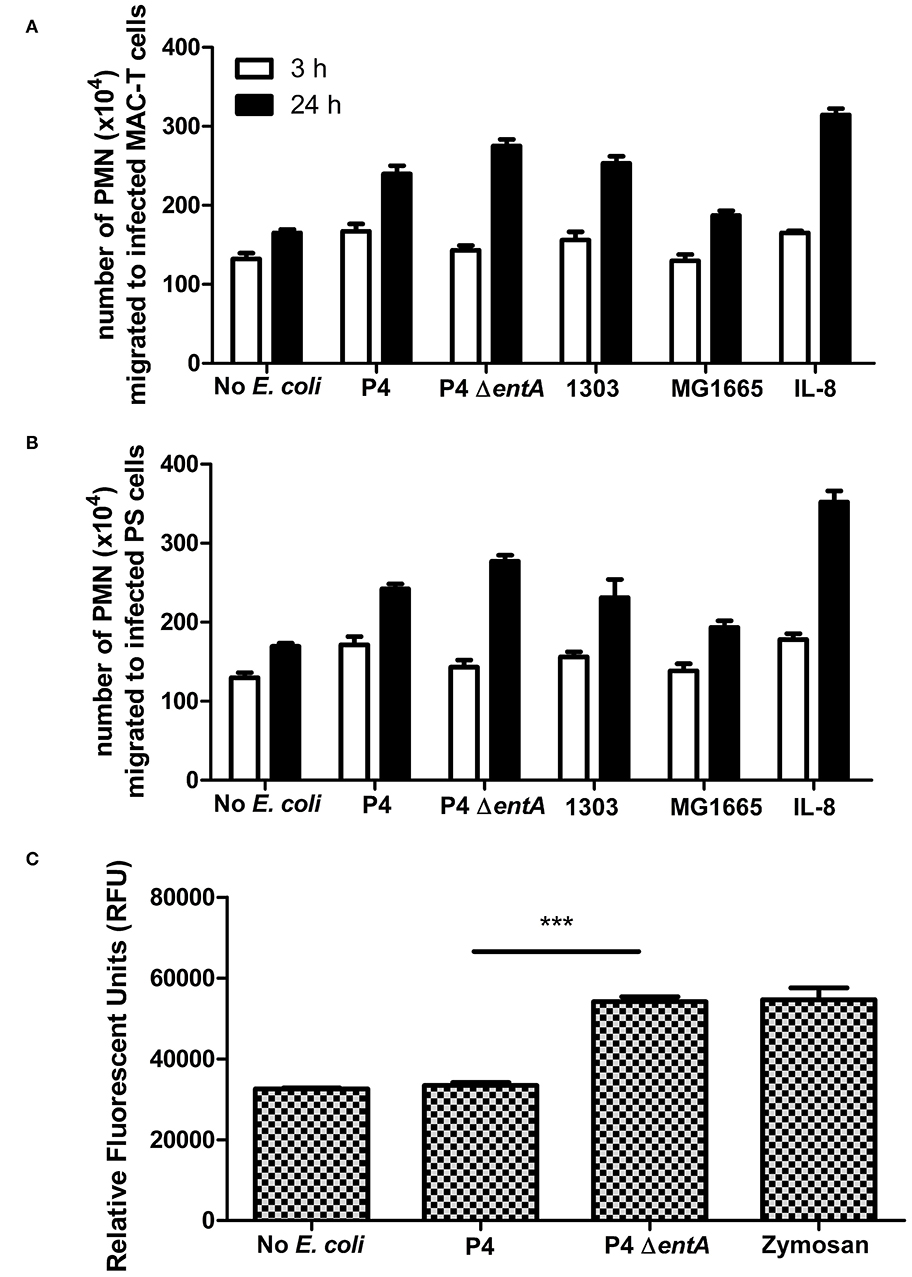
Figure 3. (A,B) Transwell migration of bovine blood-derived polymorphonuclear neutrophilic granulocytes (PMN) to boMEC lines MAC-T and PS incubated either 3 or 24 h with E. coli P4 ΔentA vs. P4 and two additional E. coli strains: 1303 and MG1655. Recombinant IL-8 and the absence of bacteria were used as positive and negative control, respectively. (C) Reactive oxygen species (ROS) production in relative fluorescence units (RFU) by bovine blood PMN measured at 485 nm excitation and 530 nm emission wavelengths for 120 min following incubation with E. coli P4 ΔentA vs. P4. Zymosan-stimulated PMN served as a positive control and were compared with unstimulated PMN as a negative control. Each cell type and incubation time had at least three replicates (n ≥ 3). Data are presented as means ± standard error of the mean. ***P < 0.001.
In order to determine if Ent affected oxidative antimicrobial responses of PMN, ROS production in response to P4 and P4 ΔentA was measured. Interestingly, measuring fluorescence intensity as marker for ROS production was significantly increased in bovine PMN incubation with P4 ΔentA compared with P4 (P < 0.001; Figure 3C). The level of ROS production of PMN in response to P4 ΔentA was similar to the ROS production measured in zymosan-stimulated PMN, which served as a positive control. Unstimulated and P4-stimulated PMN produced similar amounts of ROS, indicating that wild-type P4 might interfere with ROS production (Figure 3C).
E. coli P4 and P4 ΔentA Show a Similar in vitro Growth
Demonstrating that there is no negative impact of enterobactin depletion on IL-8 expression, PMN migration, and activation in an in vitro setting, it was subsequently investigated whether the fitness of E. coli was hindered by Ent deficiency in vitro. Escherichia coli P4 and P4 ΔentA were separately grown in DMEM/F12 under the same experimental conditions, and the OD600 was measured every hour during 8 h. Both bacteria showed a similar growth curve and no significant differences were observed (Figure 4). This observation therefore indicates E. coli P4 ΔentA has no reduced fitness relative to E. coli P4 under the determined experimental conditions.
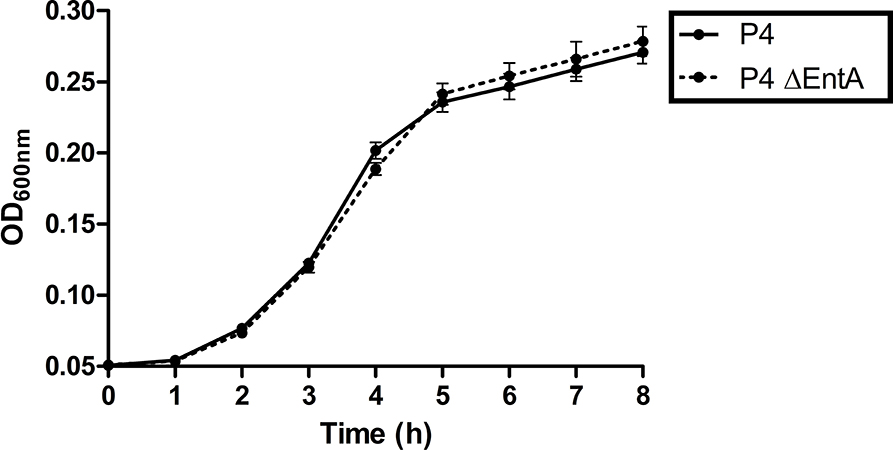
Figure 4. Growth curves of E. coli P4 and P4 ΔentA in DMEM/F12. Overnight bacterial cells were diluted 1:100 and 200 μl of the diluent was added to 12 (n = 12) wells in a 96-well plate. Optical density was measured at 600 nm (OD600) every hour during 8 h while incubating the plate at 37°C. Data are presented as means ± standard deviation. No significant statistical differences were observed (P > 0.05).
P4 ΔentA-Infected Murine Mammary Glands Show Lower Bacterial Loads and a Decreased Inflammatory Response Compared With P4-Infected Glands
To investigate the influence of Ent depletion on E. coli growth in vivo, a situation in which iron availability is more tightly regulated, murine mammary glands were intraductally inoculated with E. coli P4 and P4 ΔentA. Bacterial loads in P4 ΔentA-infected mammary glands were significantly decreased compared with P4-infected glands at 24 h.p.i. (P < 0.001; Figure 5). This observation was mouse strain-independent as consistent observations were obtained in both C57BL/6 and CD-1 mouse strains (Figure 5). Concomitant with the reduced IL-8 levels analyzed in mammary gland homogenates, Ly6G staining of mammary gland tissue showed a significantly decreased influx of PMN in the alveolar lumen upon mammary gland infection with P4 ΔentA compared with that seen in P4-infected mammary glands (P < 0.001; Figure 6). Similar to the reduced levels of IL-8, levels of pro-inflammatory cytokines IL-1α,−1β,−4, and−6 as well as LCN2 were also reduced upon P4 ΔentA infection in mammary gland homogenates of both mouse strains compared with their P4-infected counterparts (P ≤ 0.08; Figure 7).
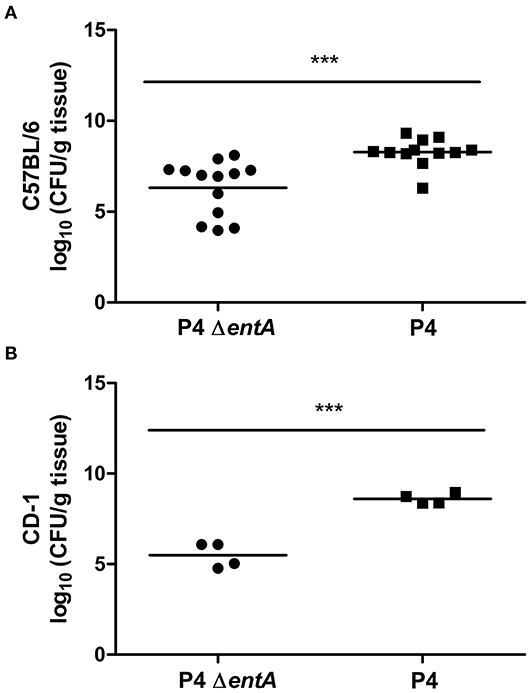
Figure 5. Bacterial load expressed as CFU/g mammary gland in C57BL/6 (A) (n ≥ 12) and CD-1 (B) (n = 4) mice at 24 h.p.i. with E. coli P4 ΔentA (dots) vs. P4 (squares). Data are presented as single sample values. The bar indicates the mean. ***P < 0.001.
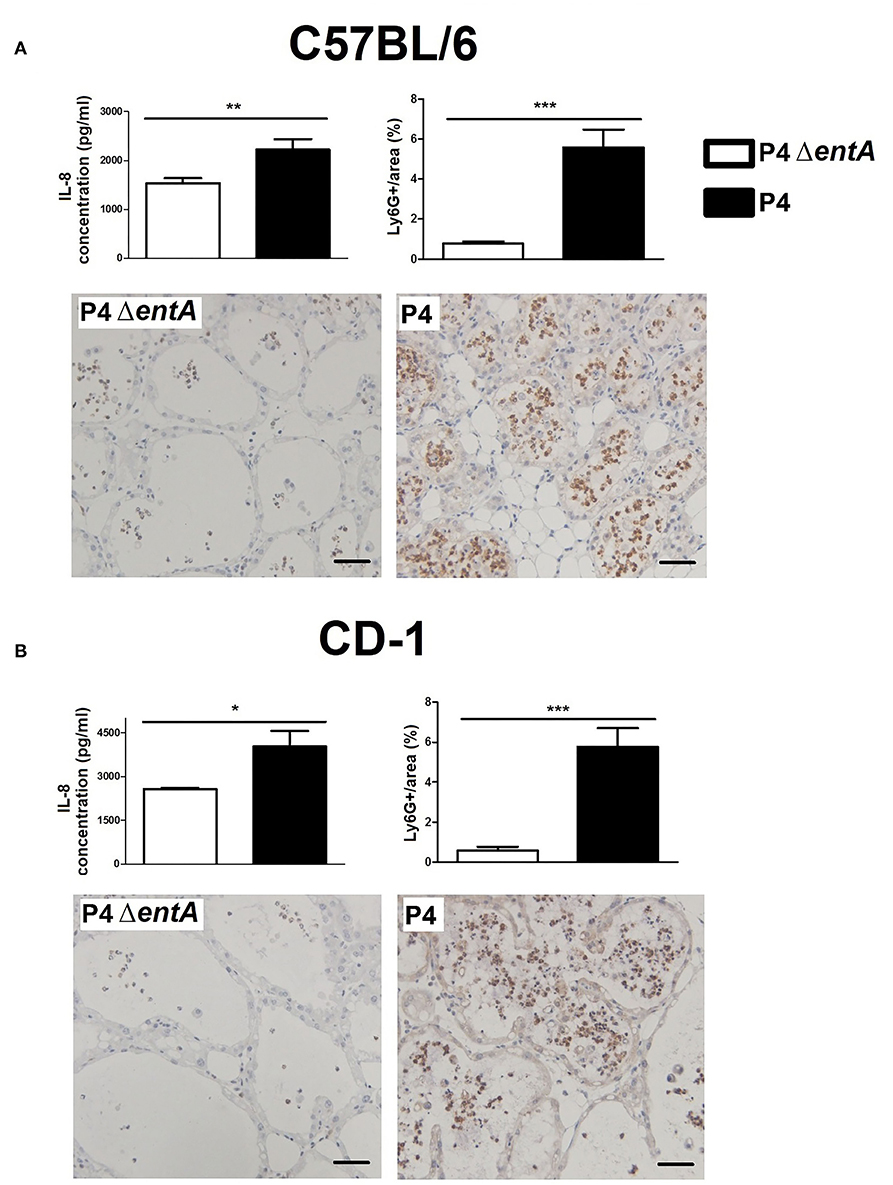
Figure 6. Concentration of the PMN chemoattractant IL-8 and immunohistochemical staining of PMN (Ly6G) in mammary glands of C57BL/6 (A) (n ≥ 12) and CD-1 (B) (n = 4) mice at 24 h.p.i. with E. coli P4 ΔentA vs. P4. Scale bar = 50 μm. Data are presented as means ± standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001.
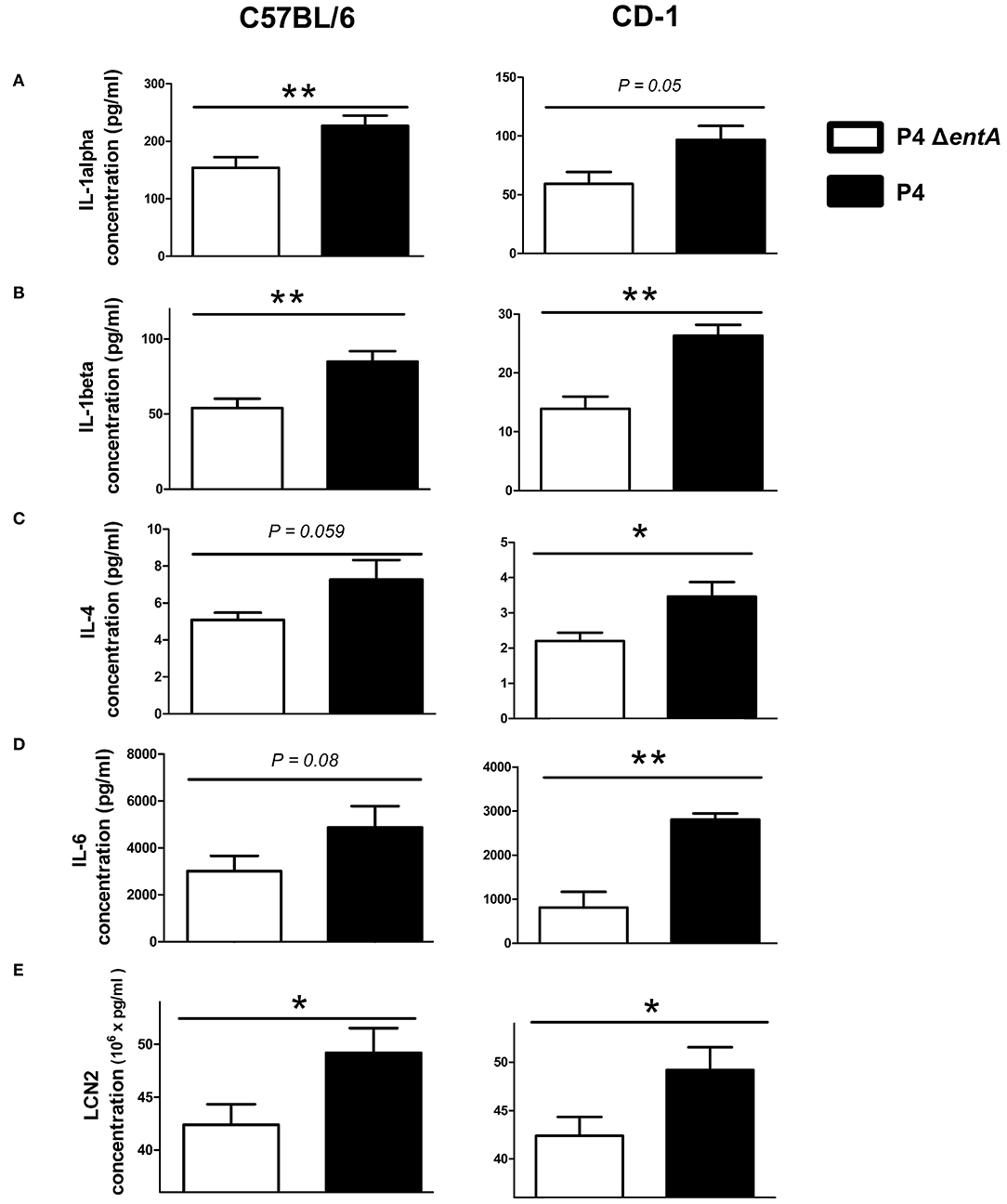
Figure 7. Levels of pro-inflammatory cytokines IL-1α (A), IL-1β (B), IL-4 (C), and IL-6 (D) and of LCN2 (E) in mammary glands of C57BL/6 (n ≥ 12) and CD-1 (n = 4) mice at 24 h.p.i. with E. coli P4 ΔentA vs. P4. Data are presented as means ± standard error of the mean. *P < 0.05 and **P < 0.01.
Discussion
Due to the high prevalence of bovine mastitis and the consecutive overuse of antibiotics in the dairy industry, novel compounds that target other determinants such as bacterial fitness factors are desired (16, 35). Limiting iron uptake of E. coli, regarded an essential bacterial growth factor, could offer such antimicrobial potential (14, 37). In this preliminary host–pathogen interaction study, we demonstrated the potential of decreasing the fitness of E. coli in the murine mammary gland if E. coli is Ent deficient. This decreased fitness was observed with significantly reduced bacterial loads in murine mammary gland lysates and, consequently, a decreased inflammatory response which was characterized by diminished chemokine levels and less infiltration of PMN.
Contradictory to our in vivo observations, we demonstrated that bacterial Ent depletion did not impact on IL-8 secretion by the bovine mammary epithelium and its associated PMN migration in vitro. It must be noted that these experiments were all performed in DMEM/F12, implying there was most likely no significant difference in bacterial growth between E. coli P4 ΔentA and its P4 wild-type counterpart, because both bacteria showed similar growth patterns in this culture medium. It is therefore highly likely that this similar in vitro bacterial growth is responsible for the absence of differences observed in our other in vitro assays. Performing these assays again under iron-restricted conditions might reveal novel data that more closely mimic the outcomes of our murine model, and as such, this should be regarded a follow-up experiment. In fact, E. coli features more mechanisms than just Ent to acquire iron, which might have compensated for the Ent deficiency of E. coli P4 ΔentA when grown in high iron containing DMEM/F12 (1, 3, 4). To proceed with our in vitro observations, it was also noticed that PMN might have significantly improved phagocytosis and enzymatic activity in their phagosomes if E. coli is Ent deficient, although more research remains warranted to fully confirm this statement. Nonetheless, a higher ROS production by PMN in the E. coli P4 ΔentA-infected murine mammary glands is another possible factor that might have contributed to the observed differences between the E. coli P4 ΔentA vs. P4-infected mouse mammary glands. Furthermore, it should also be noticed that our in vitro research was limited to the interplay between boMECs and PMN. Our goal in this preliminary study was to evaluate the presence of Ent on the established biomarkers of mastitis, primarily IL-8 produced by boMECs and its associated PMN recruitment and activation (38). However, also other cell types such as macrophages are known to play a crucial role in the latter mentioned processes (38, 39). A previous study of our group showed that macrophages are major secretors of LCN2 upon mammary gland infection (39). Including macrophages to these in vitro assays, which can also be proposed as a follow-up experiment, could drastically change E. coli growth, IL-8 levels, and concomitant PMN recruitment and activation. Taken together, (1) the high iron availability in vitro, (2) a higher ROS production by PMN in E. coli P4 ΔentA, and (3) the presence of macrophages in the murine mammary gland are factors that most likely contributed to the contradictory observations between our in vitro and in vivo data.
In comparison, another study also reports the reduced bacterial fitness of an E. coli P4 fec deletion mutant (E. coli P4 Δfec), which is an iron-acquiring mechanism to capture ferric citrate (10). This fec-lacking E. coli P4 mutant was not capable anymore to induce mastitis in cows in comparison with the E. coli P4 wild type (10). Our E. coli P4 ΔentA was challenged at a similar CFU/ml in mice and was still able to establish infection, but significantly lower bacterial loads were observed. Our findings in mice are therefore in line with the observations made in the bovine target species, although it should be noticed that a direct comparison between these two E. coli that are deficient in different iron acquisition mechanisms and were evaluated in different species remains difficult. Therefore, a follow-up study in which cows are challenged with E. coli P4 ΔentA urges to allow a correct comparison between E. coli P4 ΔentA and E. coli P4 Δfec. Nonetheless, our preliminary results in mice together with the observations made for E. coli P4 Δfec in dairy cattle pinpoint toward the potential of decreasing E. coli viability in the mammary gland by targeting E. coli's iron acquisition mechanisms.
In conclusion, the inability of E. coli to produce Ent results in a decreased bacterial fitness of E. coli in the murine mastitic mammary gland by a reduced bacterial load and, consequently, a diminished associated inflammatory response. Our study therefore identifies Ent interference as a potential target to decrease E. coli growth in the mastitic mammary gland, although several in vitro follow-up experiments remain to fully understand the complex interaction between involvement of iron availability, immunity, and an E. coli infection. In addition, future research in dairy cattle remains necessary to assess if interference with bacterial Ent indeed has antimicrobial potential in the bovine udder.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Regional Government Ethics Committee of Upper Bavaria, Germany (Approval Number: 55.2-1-54-2531-108-05) and the ethics committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (Approval Number: 2015/127).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
AG holds a Sêr Cymru II Lectureship funded by the European Research Development Fund and Welsh Government. NV holds a fellowship by the Research Foundation of Flanders (FWO) grant number 1.S.236.20N. This study was financially supported through the KOlimastIR—ANIHWA consortium of Era-Net, funded in France by ANR (ANR-13-ANWA-0003-06) and in the UK by DEFRA and BBSRC (Reference OD1718).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge Dr. Wolfram Petzl for performing the cow experiments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.576583/full#supplementary-material
References
1. Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. (2000) 54:881–941. doi: 10.1146/annurev.micro.54.1.881
2. Zhang C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. (2014) 5:750–60. doi: 10.1007/s13238-014-0083-7
3. Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron—a metal at the host—pathogen interface. Cell Microbiol. (2010) 12:1691–702. doi: 10.1111/j.1462-5822.2010.01529.x
4. Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. (2010) 6:e1000949. doi: 10.1371/journal.ppat.1000949
5. O'Brien IG, Gibson F. The structure of enterochelin and related 2,3-dihydro-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. (1970) 215:393–402. doi: 10.1016/0304-4165(70)90038-3
6. Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA. (2003) 100:3584–8. doi: 10.1073/pnas.0630018100
7. Johnstone TC, Nolan EM. Determination of the molecular structures of ferric enterobactin and ferric enantioenterobactin using racemic crystallography. J Am Chem Soc. (2017) 139:15245–50. doi: 10.1021/jacs.7b09375
8. Sakaitani M, Rusnak F, Quinn NR, Tu C, Frigo TB, Berchtold GA, et al. Mechanistic studies on trans-2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase (Ent A) in the biosynthesis of the iron chelator enterobactin. Biochemistry. (1990) 29:6789–98. doi: 10.1021/bi00481a006
9. Pakarian P, Pawelek PD. Intracellular co-localization of the Escherichia coli enterobactin biosynthetic enzymes EntA, EntB, and EntE. Biochem Biophys Res Commun. (2016) 478:25–32. doi: 10.1016/j.bbrc.2016.07.105
10. Blum SE, Goldstone RJ, Connolly JPR, Répérant-Ferter M, Germon P, Inglis NF, et al. Postgenomics characterization of an essential genetic determinant of mammary pathogenic Escherichia coli. mBio. (2018) 9:e00423–18. doi: 10.1128/mBio.00423-18
11. Pi H, Jones SA, Mercer LE, Meador JP, Caughron JE, Jordan L, et al. Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut. PLoS ONE. (2012) 7:e50020. doi: 10.1371/journal.pone.0050020
12. Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, et al. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. (2015) 12:7113. doi: 10.1038/ncomms8113
13. Singh V, Yeoh B, Chassaing B, Zhang B, Saha P, Xiao X, et al. Microbiota-inducible innate immune siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell Mol Gastroenterol Hepatol. (2016) 2:482–98. doi: 10.1016/j.jcmgh.2016.03.007
14. Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med. (2016) 22:1077–90. doi: 10.1016/j.molmed.2016.10.005
15. Gorden PJ, Kleinhenz MD, Ydstie JA, Brick TA, Slinden LM, Peterson MP, et al. Efficacy of vaccination with a Klebsiella pneumoniae siderophore receptor protein vaccine for reduction of Klebsiella mastitis in lactating cattle. J Dairy Sci. (2018) 101:10398–408. doi: 10.3168/jds.2017-14267
16. de Jong A, El Garch F, Simjee S, Moyaert H, Rose M, Youala M. Monitoring of antimicrobial susceptibility of udder pathogens recovered from cases of clinical mastitis in dairy cows across Europe: VetPath results. Vet Microbiol. (2018) 213:73–81. doi: 10.1016/j.vetmic.2017.11.021
17. Thomas V, de Jong A, Moyaert H, Simjee S, El Garch F, Morrissey I, et al. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int J Antimicrob Agents. (2015) 46:13–20. doi: 10.1016/j.ijantimicag.2015.03.013
18. Verbeke J, Piepers S, Supré K, De Vliegher S. Pathogen-specific incidence rate of clinical mastitis in Flemish dairy herds, severity, and association with herd hygiene. J Dairy Sci. (2014) 97:6926–34. doi: 10.3168/jds.2014-8173
19. Klaas IC, Zadoks RN. An update on environmental mastitis: challenging perceptions. Transbound Emerg Dis. (2018) 65:166–85. doi: 10.1111/tbed.12704
20. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. (2004) 432:917–21. doi: 10.1038/nature03104
21. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. (2002) 10:1033–43. doi: 10.1016/S1097-2765(02)00708-6
22. Huang X, Slavkovic S, Song E, Botta A, Mehrazma B, Lento C, et al. A unique conformational distortion mechanism drives lipocalin 2 binding to bacterial siderophores. ACS Chem Biol. (2020) 15:234–42. doi: 10.1021/acschembio.9b00820
23. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. (1994) 56:559–64. doi: 10.1002/jlb.56.5.559
24. Lahouassa H, Moussay E, Rainard P, Riollet C. Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli. Cytokine. (2007) 38:12–21. doi: 10.1016/j.cyto.2007.04.006
25. Garénaux A, Caza M, Dozois CM. The Ins and Outs of siderophore mediated iron uptake by extraintestinal pathogenic Escherichia coli. Vet Microbiol. (2011) 153:89–98. doi: 10.1016/j.vetmic.2011.05.023
26. Duda KA, Lindner B, Brade H, Leimbach A, Brzuszkiewicz E, Dobrindt U, et al. The lipopolysaccharide of the mastitis isolate Escherichia coli strain 1303 comprises a novel O-antigen and the rare K-12 core type. Microbiology. (2011) 157:1750–60. doi: 10.1099/mic.0.046912-0
27. Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. (1972) 36:525–57. doi: 10.1128/MMBR.36.4.525-557.1972
28. Mitterhuemer S, Petzl W, Krebs S, Mehne D, Klanner A, Wolf E, et al. Escherichia coli infection induces distinct local and systemic transcriptome responses in the mammary gland. BMC Genomics. (2010) 11:138. doi: 10.1186/1471-2164-11-138
29. Bougarn S, Cunha P, Gilbert FB, Meurens F, Rainard P. Technical note: validation of candidate reference genes for normalization of quantitative PCR in bovine mammary epithelial cells responding to inflammatory stimuli. J Dairy Sci. (2011) 94:2425–30. doi: 10.3168/jds.2010-3859
30. Roussel P, Porcherie A, Répérant-Ferter M, Cunha P, Gitton C, Rainard P, et al. Escherichia coli mastitis strains: in vitro phenotypes and severity of infection in vivo. PLoS ONE. (2017) 12:e0178285. doi: 10.1371/journal.pone.0178285
31. Roussel P, Cunha P, Porcherie A, Petzl W, Gilbert FB, Riollet C, et al. Investigating the contribution of IL-17A and IL-17F to the host response during Escherichia coli mastitis. Vet Res. (2015) 46:56. doi: 10.1186/s13567-015-0201-4
32. Cronin JG, Hodges R, Pedersen S, Sheldon IM. Enzyme linked immunosorbent assay for quantification of bovine interleukin-8 to study infection and immunity in the female genital tract. Am J Reprod Immunol. (2015) 73:372–82. doi: 10.1111/aji.12344
33. Conejeros I, Gibson AJ, Werling D, Muñoz-Caro T, Hermosilla C, Taubert A. Effect of the synthetic Toll-like receptor ligands LPS, Pam3CSK4, HKLM and FSL-1 in the function of bovine polymorphonuclear neutrophils. Dev Comp Immunol. (2015) 52:215–25. doi: 10.1016/j.dci.2015.05.012
34. Tombácz K, Peters LM, Chan YM, Steele M, Werling D, Gibson AJ. Short communication: pegbovigrastim treatment in vivo does not affect granulocyte ability to migrate to endometrial cells and kill bacteria in vitro in healthy cows. J Dairy Sci. (2019) 102:9389–95. doi: 10.3168/jds.2019-16563
35. Gomez F, Henriques M. Control of bovine mastitis: old and recent therapeutic approaches. Curr Microbiol. (2016) 72:377–82. doi: 10.1007/s00284-015-0958-8
36. Kempf F, Slugocki C, Blum SE, Leitner G, Germon P. Genomic comparative study of bovine mastitis Escherichia coli. PLoS ONE. (2016) 11:e0147954. doi: 10.1371/journal.pone.0147954
37. Baghal SM, Gargari SL, Rasooli I. Production and immunogenicity of recombinant ferric enterobactin protein (FepA). Int J Infect Dis. (2010) 14(Suppl. 3):e166–70. doi: 10.1016/j.ijid.2009.12.009
38. Riollet C, Rainard P, Poutrel B. Cells and cytokines in inflammatory secretions of bovine mammary gland. In: Mol JA, Clegg RA, editors. Biology of the Mammary Gland. Advances in Experimental Medicine and Biology 480. Boston, MA: Springer (2002).
Keywords: E. coli mastitis, enterobactin, iron scavenging, siderophores, lipocalin 2 (also known as NGAL)
Citation: Vander Elst N, Breyne K, Steenbrugge J, Gibson AJ, Smith DGE, Germon P, Werling D and Meyer E (2020) Enterobactin Deficiency in a Coliform Mastitis Isolate Decreases Its Fitness in a Murine Model: A Preliminary Host–Pathogen Interaction Study. Front. Vet. Sci. 7:576583. doi: 10.3389/fvets.2020.576583
Received: 26 June 2020; Accepted: 05 October 2020;
Published: 09 November 2020.
Edited by:
Massimo Amadori, Experimental Zooprophylactic Institute of Lombardy and Emilia Romagna (IZSLER), ItalyReviewed by:
Maria Filippa Addis, University of Milan, ItalySteven Lee Foley, National Center for Toxicological Research (FDA), United States
Copyright © 2020 Vander Elst, Breyne, Steenbrugge, Gibson, Smith, Germon, Werling and Meyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niels Vander Elst, niels.vanderelst@ugent.be; Evelyne Meyer, evelyne.meyer@ugent.be
†These authors share first authorship
‡These authors share last authorship
 Niels Vander Elst
Niels Vander Elst Koen Breyne1,2†
Koen Breyne1,2†  Jonas Steenbrugge
Jonas Steenbrugge Amanda Jane Gibson
Amanda Jane Gibson David George Emslie Smith
David George Emslie Smith Dirk Werling
Dirk Werling Evelyne Meyer
Evelyne Meyer