- 1School of Physical Education, Xuzhou Kindergarten Teachers College, Xuzhou, China
- 2Institute of Motor Quotient, Southwest University, Chongqing, China
- 3Qingdao Mental Health Center, Qingdao University, Qingdao, China
- 4Ministry of Sports, Shandong Technology and Business University, Yantai, China
- 5Financial Department, Shandong Sports University, Jinan, China
This review aims to systematically review the effects of physical exercise on the cognitive performance of patients with Alzheimer's disease (AD) and its mechanisms of action. Databases such as Web of Science, PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched until December 2021. A randomized controlled trial (RCT) to assess the effect of an exercise intervention (compared with no exercise) on patients with AD. The measures included cognitive function [Mini-Mental State Examination (MMSE), Alzheimer's Disease assessment scale-cognitive (ADAS-Cog), Montreal cognitive assessment scale (MoCA) and Executive Function (EF)]. The methodological quality of the included literature was assessed using the Physiotherapy Evidence Database (PEDro) scale. Twenty-two studies (n = 1647, mean age: 77.1 ± 6.3 years) were included in the systematic review, sixteen of which were included in the meta-analysis. A systematic review and meta-analysis revealed that physical exercise positively affects cognitive performance in older patients with AD. However, the positive effects of the intervention should be interpreted with caution considering the differences in methodological quality, type, frequency, and duration of exercise in the included studies. Future studies should consider the design rigor and specification of RCT protocols.
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disease that has no known treatable cure (1). As the disease gradually destroys brain structures (e.g., hippocampus and internal olfactory cortex) (2), it leads to loss of cognitive mental functions, including memory, language, attention, and perception, reduced activities of daily living, and diminished quality of life (3).
The global increase in the prevalence of AD is closely linked to the aging of the population (4). According to the World Alzheimer's Disease Report 2021, more than 55 million people worldwide are living with cognitive impairment, which is expected to reach 78 million by 2030 (5). The direct cost to American society of caring for patients with AD was estimated at $305 billion in 2020 and was expected to exceed $1 trillion by 2050 (6). The high cost of treatment prevents 75% of people with dementia worldwide from being effectively diagnosed and treated (5).
Although not all studies support it, there is growing evidence that physical exercise can prevent cognitive decline and dementia (7–10). Physical exercise, such as aerobic (11–13), stretching (14), resistance (15), or combined exercises (16–20), may delay and prevent cognitive decline in older adults with AD. Therefore, physical exercise emerges as one of the most promising, effective, and least expensive strategies to prevent and delay cognitive decline in patients with AD (21, 22).
Numerous studies have shown that physical exercise is associated with positive effects on brain health (23–25). High levels of aerobic exercise have been associated with improved brain volume and factors of cognitive decline (26, 27). Some studies have shown that aerobic exercise can boost brain plasticity (28), reduce hippocampal atrophy (29), and even increase the hippocampus (30). Physical exercise appears to affect brain atrophy positively in older adults with AD (31). Cognitive impairment is one of the forms of brain atrophy presentation, which results in difficulty in controlling physical mobility in patients with AD (32). Regular exercise at an appropriate intensity and physically demanding level may stimulate some cognitive functions in older adults with AD (33). Therefore, physical exercise appears to be one of the active strategies to resist brain atrophy in older adults with AD.
Several reviews have been published on the effects of physical exercise on the cognitive performance of older adults with AD (19, 34–37). Some reviews concluded that the intervention was beneficial for global cognitive impairment (19, 35). Others concluded that the intervention effect was not beneficial (36); some meta-analyses concluded that physical exercise improved cognition in the AD group with an effect comparable to donepezil (38). By way of example, physical exercise can improve cognition. Previous studies have confirmed that physical activity can improve cognition in AD patients, but the dose-effect relationship between physical activity and AD is not clear. Moreover, systematic reviews are lacking for analyzing the type, intensity, frequency, and duration of physical exercise interventions in patients with AD.
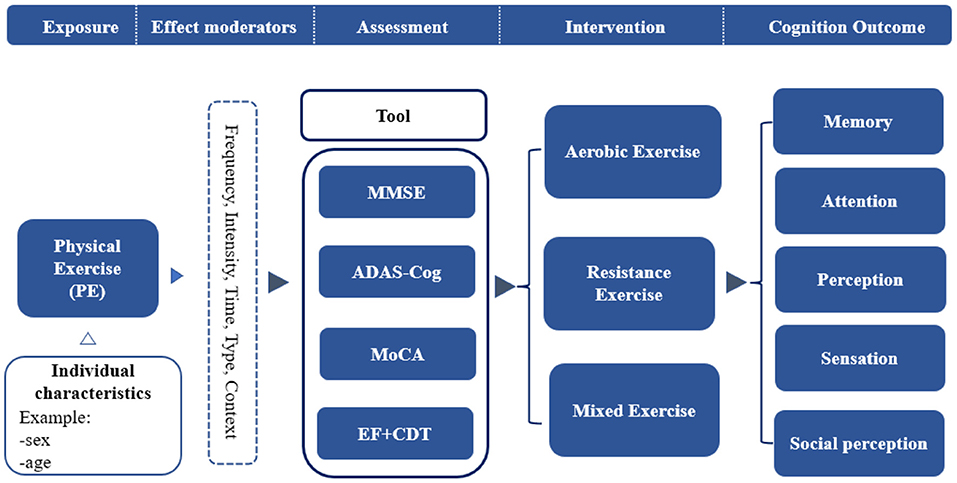
Therefore, the purposes of this review were (1) to systematically review the effects of physical exercise on the cognitive performance of patients with AD; (2) to determine the dose-effect of physical exercise on patients with AD, and (3) to explore the mechanisms underlying the effects of physical exercise on the cognitive performance of patients with AD. To this end, we developed a conceptual model of physical exercise interventions for the cognitive performance of AD patients (Figure 1).
Methods
Data Sources and Search Strategies
The study of this systematic review and meta-analysis followed the PRISMA guidelines (39). Three databases, including Web of Science, PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials, were searched for literature written in English only. The search terms used include “Alzheimer's” or “Alzheimer's disease” or “AD” or “dementia” and “exercise” or “physical exercise” or “aerobic exercise” or “resistance training” and “cognitive function” or “executive function.” The search strategy was determined by three investigators, with two investigators working independently on the search task and the third involved in resolving any search disputes. The search covered the period from creating the database to December 31, 2021.
Study Selection
The criteria for inclusion in the study were (1) design: randomized controlled trial (RCT); (2) sample: at least one group of participants with a diagnosis of Alzheimer's disease type dementia in older adults (mean age 65 years or older), excluding other diagnoses of dementia or MCI; (3) intervention: aerobic, resistance, or stretching type of physical exercise was performed; (4) outcome: at least one executive function or cognitive function was measured. The following studies were excluded from systematic evaluation: (1) non-interventional studies; (2) non-exercise type studies of intervention modalities; (3) theoretical studies, descriptions of treatments, or methodological protocols; (4) review articles; and (5) non-English language articles.
Data Extraction
Two investigators (WL and JL) retrieved and collected data, and potential disagreements were resolved by joint discussion with two other investigators (JC and QL). Data collected from each study included subject characteristics (mean age, number of genders), intervention protocol (exercise type, frequency, duration, intensity), indicators of cognitive outcomes, intervention effects, and pre- and post-intervention outcomes (expressed as mean ± standard deviation). The conversion was performed using the formula for studies that provided standard error of the mean (SEM) for outcomes. When the outcomes data were expressed as mean and confidence interval (CI), the formula for conversion of CI to SD was (40). If some studies only displayed graphs containing means and standard deviations, the GetData Graph Digitizer was used for digitizing and extracting the data (41). All studies included in the meta-analysis were used for data synthesis, regardless of their methodological quality.
Qualitative Analysis
The methodological quality assessment of each study was conducted independently by two reviewers (JZ and YW) using the Physiotherapy Evidence Database (PEDro) Scale (42). The PEDro scale was developed to assess the quality of a treatment or intervention study design, including assessment of randomization, blinding, attrition, design, and statistics. According to the PEDro scale scoring rules, each item was scored independently, with “yes” being scored as “1” and “no” or “unclear” as “0,” and the maximum score for the ten criteria was 10. The possible risk of bias was determined from the extracted information, with <5/10 being rated as “high” risk and more than 5/10 as “low” risk (43). If the details in the article were unclear, we judged the risk of bias as “unclear” and contacted the corresponding author for more information. If the corresponding author did not provide clarification within ten working days, the item was scored as “0.”
Statistical Analysis
Review Manager Software V.5.3 was used for statistical analysis of the combined data. Statistical significance was defined for bilateral p <0.05. The combined data effects were presented using the mean difference (MD) and the corresponding 95%CI of the continuous effects. If data were available and no significant heterogeneity was detected, a fixed-effects model was used to calculate the combined effect. Otherwise, a random-effects model was applied. Statistical heterogeneity was assessed using the I2 statistic. However, when heterogeneity between studies was high (I2 > 75%), overall pooled analysis was considered inappropriate; clinical or methodological heterogeneity was considered a potential cause. Heterogeneity between studies was explored using the χ2 test and Higgins I2 values (44). Studies with different intervention types were divided into subgroups for analysis based on different factors, given the potential heterogeneity between studies.
Results
Study Selection
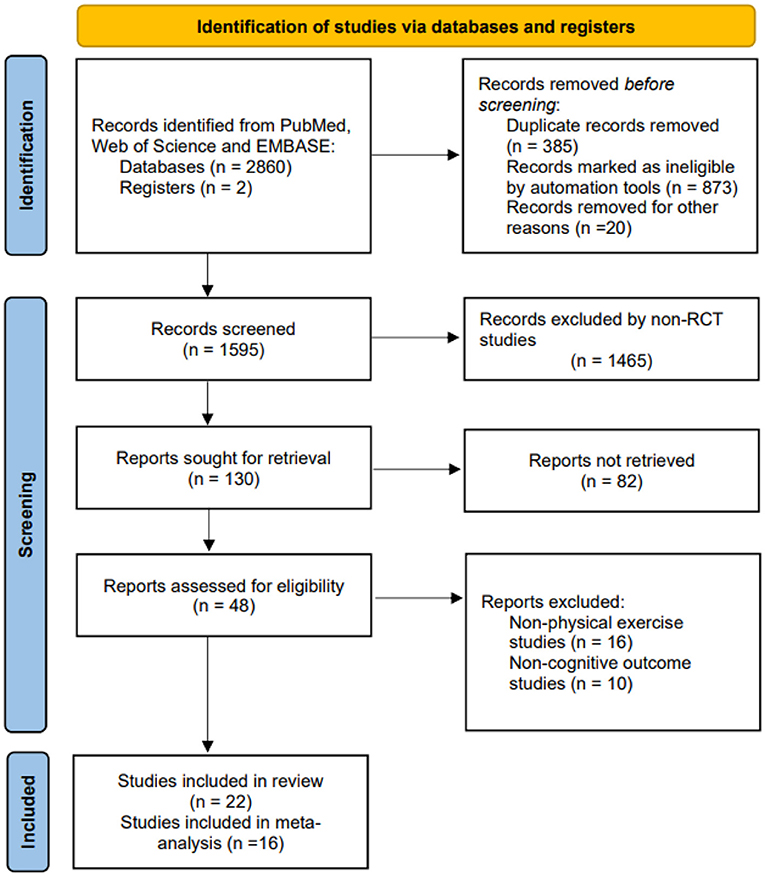
A total of 2,862 literature records were initially identified according to the proposed search strategy. Two investigators (WL, JL) screened by abstract and title, and apparently irrelevant records were excluded. A total of forty-eight potential studies were included for further evaluation. Of these, sixteen studies that did not provide physical exercise and ten studies that did not involve cognitive outcomes were excluded. Finally, twenty-two studies were eligible for inclusion in the systematic review, and the data from sixteen studies were extracted for meta-analysis. The detailed literature selection and screening process are described in Figure 2.
Study Characteristics
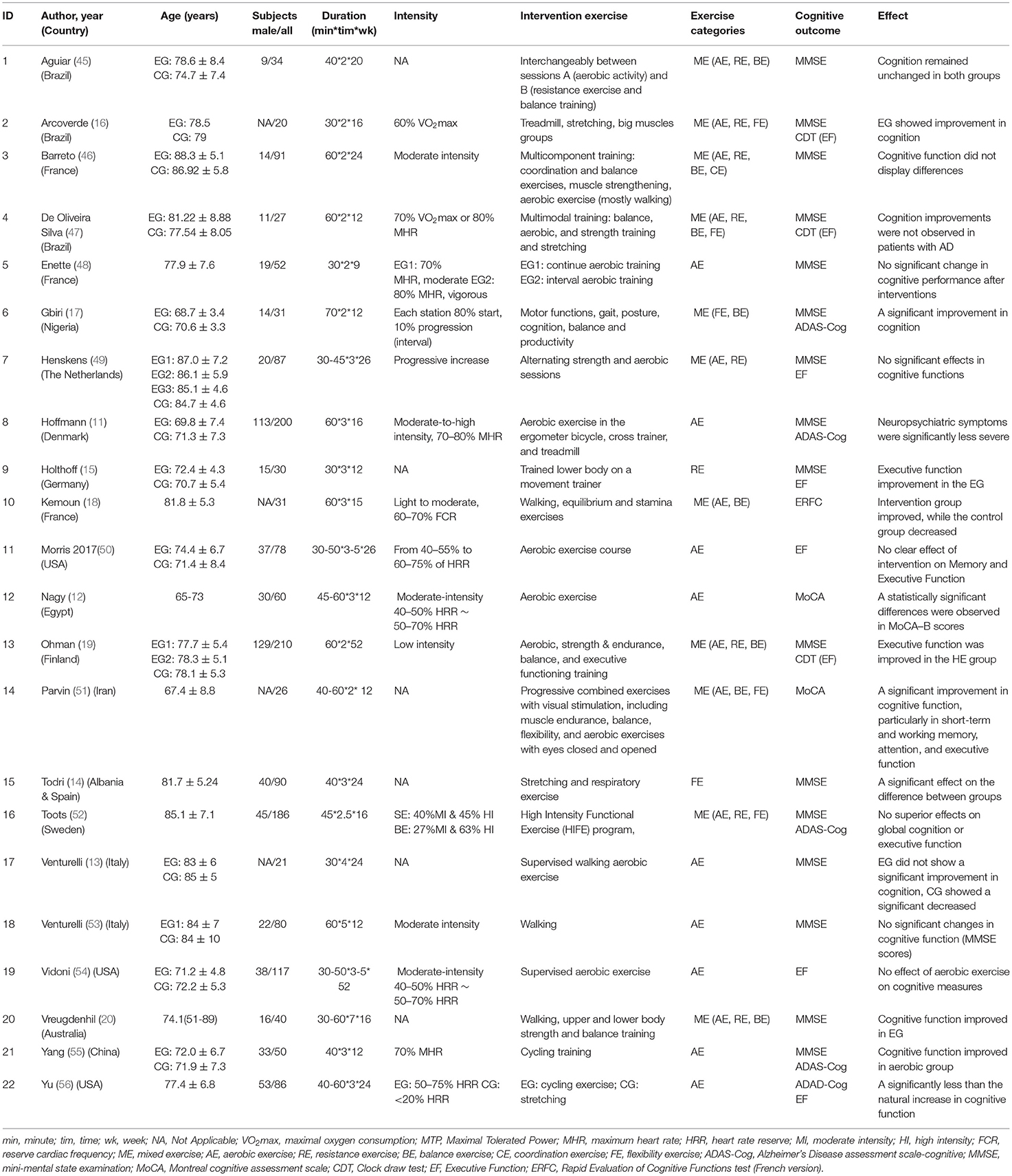
The characteristics of selected studies are detailed in Table 1. A total of twenty-two RCTs involving 1,647 AD participants (age 77.1 ± 6.3 years) were included in the study for review. The included studies were from sixteen countries, namely Brazil (16, 45, 47), France (18, 46, 48), USA (50, 54, 56), Italy (13, 53), Nigeria (17), Netherlands (49), Denmark (11), Germany (15), Egypt (12), Finland (19), Iran (51), Albania & Spain (14), Sweden (52), Australia (20), and China (55). Participants were from the community, social centers, social clubs or individuals, memory clinics, or hospitals. All studies reported identified inclusion and exclusion criteria for the diagnosis of AD participants. The study interventions contained aerobic, resistance, stretching, or mixed exercise (aerobic, resistance, balance, and stretching exercises). Nine studies performed a single aerobic training session, including supervised aerobic, walking, or cycling training (11–13, 48, 50, 53–56). Eleven studies used mixed exercise modalities, combining aerobic, resistance, balance, stretching, or cognitive training (16–20, 45–47, 49, 51, 52). Besides, two studies were conducted on resistance exercise (15) and stretching exercise (14), respectively.
The frequency of interventions ranged from 2 to 5 times per week for 30–70 min each. The duration of the interventions ranged from 9 to 52 weeks. Participants had heart rate reserve (HRR) from 40 to 80%, maximum oxygen uptake (VO2max) from 60 to 70%, and maximum heart rate (MHR) from 60 to 80% in the inclusion studies. Furthermore, six studies did not describe the intensity of intervention exercise (13–15, 20, 45, 47). The control group interventions generally utilized low-intensity activities/exercises such as social activities, stretching exercises, or health education.
Meta-analysis was used to analyze cognitive outcomes, including cognitive functions, mental health, and executive functions. Various measurement tools were used to assess studies in the same or similar cognitive domains, including the MMSE, ADAS-Cog, MoCA, CAMCOG, CDT, and others. Of the studies included in the meta-analysis, thirteen studies measured the MMSE (11, 13–20, 45–47, 49, 52, 53, 55), four measured the ADAS-Cog (20, 52, 55, 56), six measured the executive function (EF+CDT) (16, 19, 49, 50, 54, 56), and two measured the MoCA (12, 51).
Quality Assessment
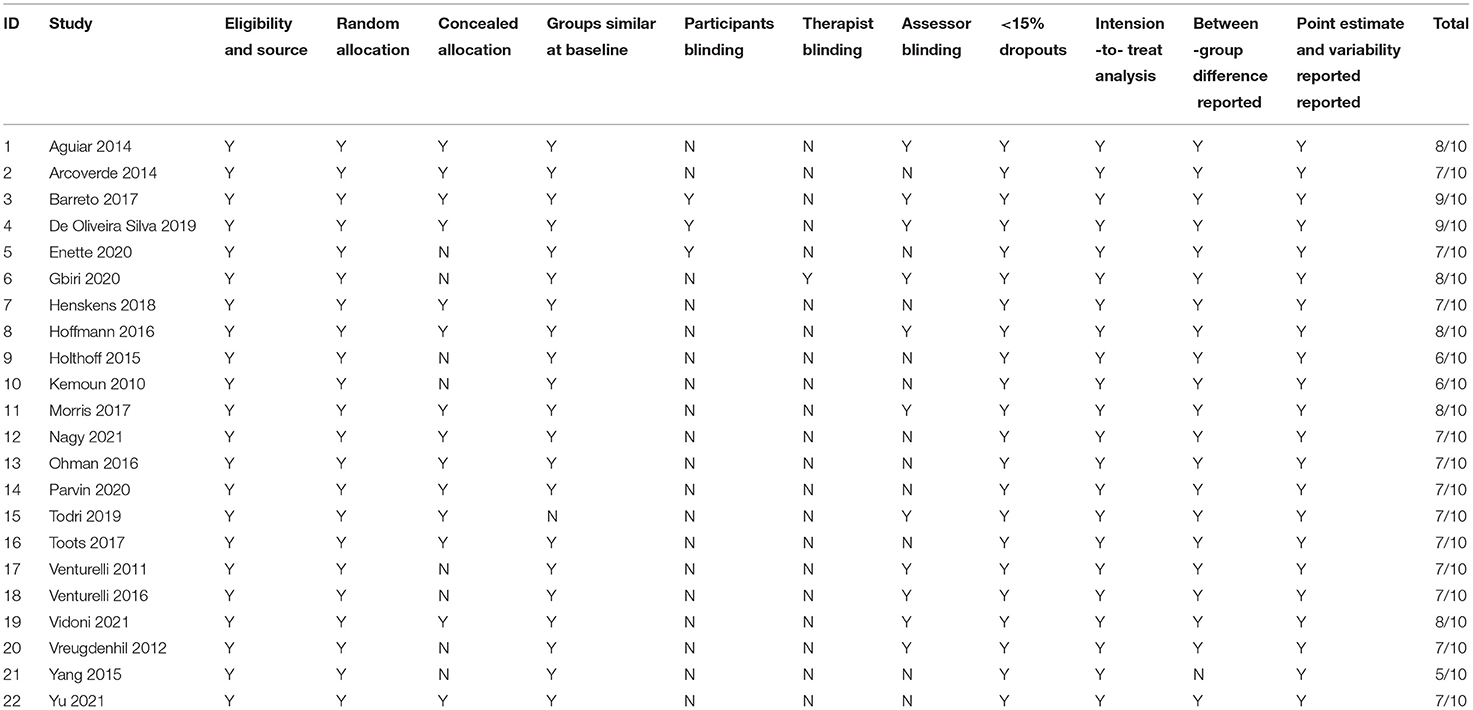
Twenty-two studies included in the systematic review were identified with the PEDro score to assess methodological quality. Only one study had a PEDro score of 5, and the other twenty-one studies had a PEDro score of ≥6, indicating good quality. Nevertheless, we also found fewer studies on participant and therapist blinding in the PEDro quality assessment. Only three studies blinded participants, and only one study blinded therapists. Details of the raw records are shown in Table 2.
Effects of Interventions
A total of thirteen of the included studies showed significant cognitive and executive functions changes (11–17, 19, 20, 51, 53, 55, 56), and nine studies showed no significant differences in cognitive and executive functions (45–50, 52–54).
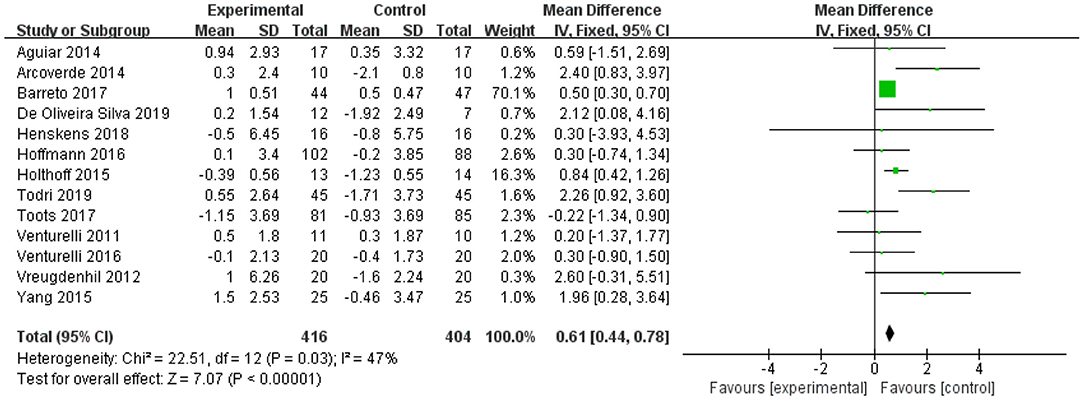
Thirteen studies involving 820 participants measured the effect of physical exercise on cognitive function in patients with AD using the MMSE (11–20, 45–47, 49, 52, 53, 55). The meta-analyses showed a significant improvement in cognitive function with physical exercise (n = 820, MD = 0.83, 95% CI = 0.43 to 1.23, p <0.00001; I2 = 47%, fixed-effect model; Figure 3).
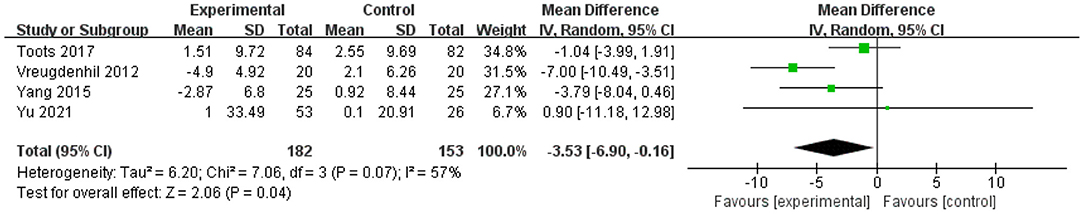
Four studies assessed the effect of physical exercise on cognitive function using the ADAS-Cog (20, 52, 55, 56). The meta-analysis showed a significant improvement in MD scores of cognitive functions with physical exercise (n = 335, MD = −3.533, 95% CI = −6.9 to −0.16, p = 0.04; I2 = 57%, random-effect model; Figure 4).
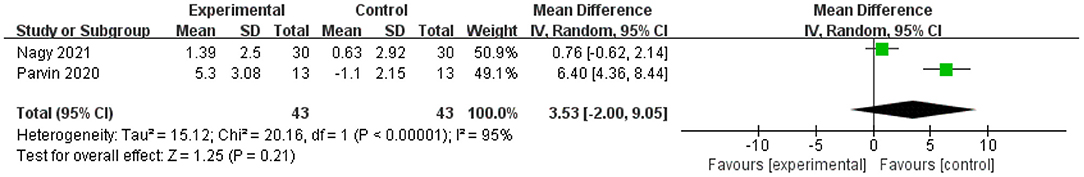
Two studies were performed to assess the effect of physical activity on cognitive function using MoCA (12, 51). Both studies showed a positive effect of physical exercise on cognitive function. Nevertheless, the meta-analysis showed no significant improvement in MD scores of cognitive functions (n = 86, MD = 3.53, 95% CI = −2.00 to 9.05, p = 0.21; I2 = 95%, random-effects model; Figure 5).
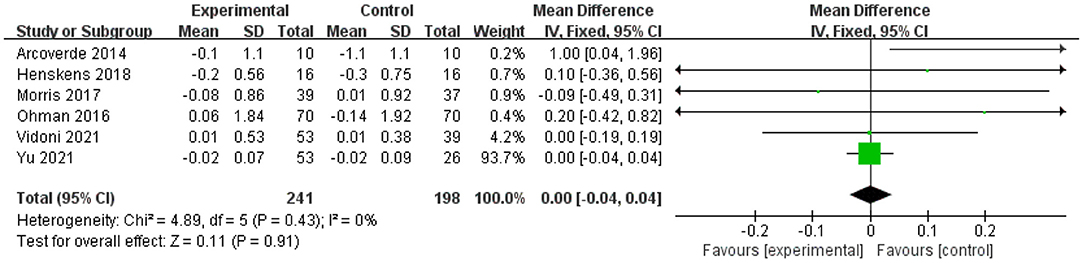
Six studies analyzed the effects of physical exercise on executive function (16, 19, 49, 50, 54, 56). The meta-analysis showed no significant improvement in MD scores of cognitive functions with physical exercise (n = 439, MD = 0, 95% CI = −0.04 to 0.04, p = 0.91; I2 = 0%, fixed-effects model; Figure 6).
Discussion
This review examined the effects of physical activity on the cognitive function of older patients with AD. Although 41% of the studies in the systematic review did not show a positive intervention effect, there was a significant improvement in the cognitive performance of patients in 59% of the studies. In the meta-analysis, the studies using the MMSE (p <0.0001) and ADAS-Cog (p = 0.04) measures of cognitive performance both showed significant improvements, while the studies using the MoCA (p = 0.21) and EF (p = 0.91) tests did not show significant improvements. Thus, we identified that physical exercise interventions are beneficial for improving cognitive function in AD patients.
Several previous meta-analyses reported a significant positive effect of physical exercise interventions on attenuating a cognitive decline in patients with AD (57–59). The present study was consistent with using the MMSE test for significant positive effects of exercise on cognitive performance interventions. In this study, the percentage of significant studies was higher when analyzing aerobic exercise separately than mixed exercise. For example, aerobic exercise interventions were significant in 67% of the included studies; mixed exercise was 55%. Notably, the benefits of aerobic exercise on cognitive performance in patients with AD appear to be at least similar to the minimal clinically significant differences (MCID) reported in previous studies (60).
Although the findings of this study meta-analysis support the effect of physical exercise on cognitive improvement in patients with AD, it is difficult to determine whether the cognitive improvement is due to the type of exercise (aerobic, resistance, stretching, or mixed), the amount of exercise, or the intensity of exercise. This review includes three exercise types: aerobic exercise, resistance exercise, stretching exercise, and mixed exercise. Both resistance and stretching exercises separately showed a positive effect of the intervention. However, 45% of the studies in mixed exercise did not show a positive effect of the intervention. Moreover, the least amount of exercise in all studies was 540 min (48), and the most were 7,800 min (54), and neither study found a significant cognitive improvement effect. Therefore, this means that the relationship between the amount of exercise and the effect of exercise is not clear. As for exercise intensity, of the fifteen studies in the included literature that involved moderate exercise intensity, 47% had positive intervention effects, while 53% had insignificant effects (Table 1). It remains to be verified that moderate exercise intensity is the recommended criterion, as mentioned in previous studies (61, 62).
The strength of this systematic review is the methodological design, in which the construction of a conceptual model is methodologically focused on the study of the mechanisms of physical exercise (63). First, we propose a “conceptual model of physical exercise interventions for the cognitive performance of patients with AD.” Second, the present study focused on different types of physical exercise, including the intensity, duration, and categories of exercise. In addition, the meta-analysis included randomized controlled trials to ensure the quality of the study literature. The current study also has some limitations. First, the included studies used different measurement instruments and had methodological compatibility issues, which may affect our interpretation of data integration and findings. Second, heterogeneity exists across intervention characteristics, including type, intensity, frequency, and duration of exercise. The type of included studies varied, such as aerobic exercise, resistance exercise, stretching exercise, or mixed exercise; the duration of each exercise session ranged from 30 to 70 min, and the duration ranged from 9 to 52 weeks. Therefore, the optimal design of intervention studies remains unclear, and further research is necessary. Third, the different levels of quality of the included studies and the methodological heterogeneity may lead to our interpretation of the results. Fourth, based on the current systematic review and meta-analysis of studies, we found few studies reporting analysis of the effects of combined interventions (only two), such as physical exercise with cognitive training interventions and physical exercise with pharmacological interventions. We will focus on the above issues in future studies. Additionally, since it is not possible to blind participants in physical exercise intervention experiments; therefore, such bias in the study design may exist.
Conclusion
Physical exercise interventions effectively improve cognitive performance in older patients with AD, which may indicate the potential value of physical exercise in improving cognitive performance and preventing conversion to severe dementia in patients with AD. However, considering the differences in methodological quality, type, frequency, and duration of exercise in the included studies, the positive effects of the intervention should be interpreted with caution. More rigorous designs and standardized RCT protocols will be considered for future studies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
WL and JL: data collection. JZ and YW: data analysis, conception, and design. WL, JL, JC, and QJ: research design, writing the manuscript, and revision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Humanities and Social Sciences Project of the Ministry of Education of China (No. 17YJC890020), the Ability Promotion Program of the Fundamental Research Funds for the Central Universities of Southwest University (No. SWU2209230), the Humanities and Social Sciences Planning Project of Chongqing Municipal Education Commission (No. 20SKGH046), and the Teaching Reform Project of Southwest University (No. 2021JY071).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jia LF, Du YF, Chu L, Zhang Z, Li F, Lyu D., et al Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 12:E661–E71. doi: 10.1016/S2468-2667(20)30185-7
2. Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. (2018) 1:59–70. doi: 10.1111/ene.13439
3. McGough EL, Lin SY, Belza B, Becofsky KM, Jones DL, Liu M, et al. A Scoping review of physical performance outcome measures used in exercise interventions for older adults with alzheimer disease and related dementias. J Geriatr Phys Ther. (2019) 1:28–47. doi: 10.1519/JPT.0000000000000159
4. Šerý O, Povová J, Balcar VJ. Perspectives in genetic prediction of Alzheimer's disease. Neuro Endocrinol Lett. (2014) 35:359–66.
5. Alzheimer's Disease International McGill University. World Alzheimer Report 2021. Available online at: https://www.alzint.org/resource/world-alzheimer-report-2021
6. Available, online at: https://www.chinadaily.com.cn/a/202112/28/WS61cab732a310cdd39bc7de87.html
7. Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. (1999) 6743:418-9. doi: 10.1038/22682
8. Maass A, Duzel S, Goerke M, Banich MT, McAuley E, Harrison CR, et al.Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. (2015) 5:585–93. doi: 10.1038/mp.2014.114
9. Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, et al. Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS ONE. (2015) 7:e0131647. doi: 10.1371/journal.pone.0131647
10. Chang J, Chen Y, Liu C, Yong L, Yang M, Zhu W, et al. Effect of square dance exercise on older women with mild mental disorders. Front Psych. (2021) 4:699778. doi: 10.3389/fpsyt.2021.699778
11. Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, et al. Moderate-to-high intensity physical exercise in patients with alzheimer's disease: a randomized controlled trial. J Alzheimers Dis. (2016) 2:443–53. doi: 10.3233/Jad-150817
12. Nagy EN, Ali AY, Behiry ME, Naguib MM, Elsayed MM. Impact of combined photo-biomodulation and aerobic exercise on cognitive function and quality-of-life in elderly Alzheimer patients with anemia: a randomized clinical trial. Int J Gen Med. (2021) 141–52. doi: 10.2147/Ijgm.S280559
13. Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis. (2011) 5:381–8. doi: 10.1177/1533317511418956
14. Todri J, Todri A, Lena O. Why Not a Global postural reeducation as an alternative therapy applied to alzheimer's patients in nursing homes? a pioneer randomized controlled trial. Dement Geriatr Cogn. (2019) 3-4:172–9. doi: 10.1159/000505397
15. Holthoff VA, Marschner K, Scharf M, et al. Effects of physical activity training in patients with Alzheimer's Dementia: results of a pilot RCT study. Plos ONE. (2015) 4. doi: 10.1371/journal.pone.0121478
16. Arcoverde C, Deslandes A, Moraes H, Steding J, Meyer S, Koch R, et al. Treadmill training as an augmentation treatment for Alzheimer's disease: a pilot randomized controlled study. Arq Neuropsiquiatr. (2014) 3:190–6. doi: 10.1590/0004-282X20130231
17. Gbiri CAO, Amusa BF. Progressivetask-orientedcircuit training for cognition, physical functioning and societal participation in individuals with dementia. Physiother Res Int. (2020) 4:1866. doi: 10.1002/pri.1866
18. Kemoun G, Thibaud M, Roumagne N, Carette P, Albinet C, Toussaint L, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn. (2010) 2:109–14. doi: 10.1159/000272435
19. Ohman H, Savikko N, Strandberg TE, Kautiainen H, Raivio MM, Laakkonen ML, et al. Effects of exercise on cognition: the finnish alzheimer disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc. (2016) 4:731–8. doi: 10.1111/jgs.14059
20. Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer's disease: a randomized controlled trial. Scand J Caring Sci. (2012) 1:12–9. doi: 10.1111/j.1471-6712.2011.00895.x
21. Gaitán JM, Moon HY, Stremlau M, Dubal DB, Cook DB, Okonkwo OC, et al. Effects of Aerobic exercise training on systemic biomarkers and cognition in late middle-aged adults at risk for Alzheimer's disease. Front Endocrinol. (2021)12:562. doi: 10.3389/fendo.2021.660181
22. Rosenberg A, Ngandu T, Rusanen M, Antikainen R, Bäckman L, Havulinna S, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics. The FINGER trial. Alzheimers Dement. (2018) 3:263–70. doi: 10.1016/j.jalz.2017.09.006
23. Sutkowy P, Wozniak A, Mila-Kierzenkowska C, Szewczyk-Golec K, Wesołowski R, Pawłowska M, et al. Physical activity vs. redox balance in the brain: brain health, aging and diseases. Antioxidants. (2021) 1:95. doi: 10.3390/antiox11010095
24. Bray NW, Pieruccini-Faria F, Bartha R, Doherty TJ, Nagamatsu LS, Montero-Odasso M. The effect of physical exercise on functional brain network connectivity in older adults with and without cognitive impairment. A systematic review. Mech Ageing Dev. (2021) 196:111493. doi: 10.1016/j.mad.2021.111493
25. Consorti A, Di Marco I, Sansevero G. Physical exercise modulates brain physiology through a network of long- and short-range cellular interactions. Front Mol Neurosci. (2021) 14:710303. doi: 10.3389/fnmol.2021.710303
26. Johansson ME, Cameron IG, Van der Kolk NM, de Vries NM, Klimars E, Toni I, et al. Aerobic exercise alters brain function and structure in parkinson's disease: a randomized controlled trial. Annals Neurol. (2022) 2:203–16. doi: 10.1002/ana.26291
27. Begde A, Jain M, Hogervorst E, Wilcockson T. Does physical exercise improve the capacity for independent living in people with dementia or mild cognitive impairment: an overview of systematic reviews and meta-analyses. Aging Ment Health. (2021) 5:1–11. doi: 10.1080/13607863.2021.2019192
28. Zhang K, Jan YK, Liu Y, Zhao T, Zhang L, Liu R, et al. Exercise intensity and brain plasticity: what's the difference of brain structural and functional plasticity characteristics between elite aerobic and anaerobic athletes? Front Hum Neurosci. (2022) 16:757522. doi: 10.3389/fnhum.2022.757522
29. Tarumi T, Rossetti H, Thomas BP, Harris T, Tseng BY, Turner M, et al. Exercise training in amnestic mild cognitive impairment: a one-year randomized controlled trial. J Alzheimers Dis. (2019) 2:421–33. doi: 10.3233/JAD-181175
30. Hendrikse J, Chye Y, Thompson S, Rogasch NC, Suo C, Coxon JP, et al. Regular aerobic exercise is positively associated with hippocampal structure and function in young and middle-aged adults. Hippocampus. (2022) 3:137–52. doi: 10.1002/hipo.23397
31. Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. (2006) 2:73–81. doi: 10.7326/0003-4819-144-2-200601170-0000
32. Kondo R, Miyano I, Lee S, Shimada H, Kitaoka H. Association between self-reported night sleep duration and cognitive function among older adults with intact global cognition. Int J Geriatr Psychiatry. (2021)5: 766-74. doi: 10.1002/gps.5476
33. Valenzuela PL, Castillo-García A, Morales JS, de la Villa P, Hampel H, Emanuele E, et al. Exercise benefits on Alzheimer's disease: state-of-the-science. Ageing Res Rev. (2020) 62:101108. doi: 10.1016/j.arr.2020.101108
34. Lopez-Ortiz S, Valenzuela PL, Seisdedos MM, Morales JS, Vega T, Castillo-García A, et al. Exercise interventions in Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Ageing Res Rev. (2021) 101479. doi: 10.1016/j.arr.2021.101479
35. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. (2015) 4:CD006489. doi: 10.1002/14651858.CD006489.pub4
36. Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Arch Med Res. (2012) 8:615-21. doi: 10.1016/j.arcmed.2012.09.008
37. Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. (2010) 3:239-52. doi: 10.1097/PSY.0b013e3181d14633
38. Pisani S, Mueller C, Huntley J, Aarsland D, Kempton MJ. A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer's disease and mild cognitive impairment with a comparison to donepezil. Int J Geriatr Psychiatry. (2021) 10:1471–87. doi: 10.1002/gps.5581
39. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 3:n71. doi: 10.1136/bmj.n71
40. Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions. (2019) 56:143-176. doi: 10.1002/9781119536604.ch6
41. Taova S. GetData Digitizing Program Code: Description, Testing, Training. Sarov: Russian Federal Nuclear Center (2013).
42. Moseley AM, Herbert RD, Sherrington C, Maher CG.Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. (2002) 1:43-9. doi: 10.1016/s0004-9514(14)60281-6
43. Verhagen AP, De Vet HCW, De Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. (1998) 12:1235-1241. doi: 10.1016/s0895-4356(98)00131-0
44. Borenstein M, Hedges L V, Higgins J P T, Rothstein HR. Introduction to Meta-Analysis. New York, NY: John Wiley & Sons (2021).
45. Aguiar P, Monteiro L, Feres A, Gomes I, Melo A. Rivastigmine transdermal patch and physical exercises for alzheimer's disease: a randomized clinical trial. Curr Alzheimer Res. (2014) 6:532-7. doi: 10.2174/1567205011666140618102224
46. De Souto Barreto P, Cesari M, Denormandie P, Armaingaud D, Vellas B, Rolland Y. Exercise or Social Intervention for nursing home residents with dementia: a pilot randomized, controlled trial. J Am Geriatr Soc. (2017) 9:E123-E9. doi: 10.1111/jgs.14947
47. De Oliveira Silva F, Ferreira JV, Placido J, Sant'Anna P, Araújo J, Marinho V, et al. Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer's disease: A randomized controlled trial. Maturitas. (2019) 217:28–33. doi: 10.1016/j.maturitas.2019.04.217
48. Enette L, Vogel T, Merle S, Valard-Guiguet AG, Ozier-Lafontaine N, Neviere R, et al. Effect of 9 weeks continuous vs. interval aerobic training on plasma BDNF levels, aerobic fitness, cognitive capacity and quality of life among seniors with mild to moderate Alzheimer's disease: a randomized controlled trial. Eur Rev Aging Phys A. (2020) 1:556. doi: 10.1186/s11556-019-0234-1
49. Henskens M Nauta IM van Eekeren MCA and Scherder EJA. Effects of physical activity in nursing home residents with dementia: a randomized controlled trial. Dement Geriatr Cogn Disord. (2018) 1-2:60–80. doi: 10.1159/000491818
50. Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS ONE. (2017) 2:ARTN e0170547. doi: 10.1371/journal.pone.0170547
51. Parvin E, Mohammadian F, Amani-Shalamzari S, Bayati M, Tazesh B. Dual-task training affect cognitive and physical performances and brain oscillation ratio of patients with Alzheimer's disease: a randomized controlled trial. Front Aging Neurosci. (2020) 8:5317. doi: 10.3389/fnagi.2020.605317
52. Toots A, Littbrand H, Bostrom G, Hörnsten C, Holmberg H, Lundin-Olsson L, et al. Effects of exercise on cognitive function in older people with dementia: a randomized controlled trial. J Alzheimers Dis. (2017) 1:323–32. doi: 10.3233/Jad-170014
53. Venturelli M, Sollima A, Ce E, Limonta E, Bisconti AV, Brasioli A, et al. Effectiveness of exercise- and cognitive-based treatments on salivary cortisol levels and sundowning syndrome symptoms in patients with Alzheimer's disease. J Alzheimers Dis. (2016) 4:1631–40. doi: 10.3233/Jad-160392
54. Vidoni ED, Morris JK, Watts A, Perry M, Clutton J, Van Sciver A, et al. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer's: a 1-year randomized controlled trial. PLoS ONE. (2021) 1: ARTN e0244893. doi: 10.1371/journal.pone.0244893
55. Yang SY, Shan CL, Qing H, Wang W, Zhu Y, Yin MM, et al. The effects of aerobic exercise on cognitive function of Alzheimer's disease patients. Cns Neurol Disord-Dr. (2015) 10:1292-7. doi: 10.2174/1871527315666151111123319
56. Yu F, Vock DM, Zhang L, Salisbury D, Nelson NW, Chow LS, et al. Cognitive effects of aerobic exercise in Alzheimer's disease: a pilot randomized controlled trial. J Alzheimers Dis. (2021) 1:233-44. doi: 10.3233/Jad-201100
57. Groot C, Hooghiemstra AM, Raijmakers PG, et al. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. (2016) 5:13-23. doi: 10.1016/j.arr.2015.11.005
58. Jia RX Liang JH Xu Y and Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. (2019) 1:181. doi: 10.1186/s12877-019-1175-2
59. Zhu L, Li L, Wang L, Jin X, Zhang H. Physical activity for executive function and activities of daily living in AD patients: a systematic review and meta-analysis. Front Psychol. (2020) 5:560461. doi: 10.3389/fpsyg.2020.560461
60. Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer's disease clinical trials. Alzheimers Dement. (2019) 32:354-63. doi: 10.1016/j.trci.2019.06.005
61. Chang J, Zhu W, Zhang J, Yong L, Yang M, Wang J, et al. The effect of Chinese square dance exercise on cognitive function in older women with mild cognitive impairment: the mediating effect of mood status and quality of life. Front Psychiatry. (2021) 28:711079. doi: 10.3389/fpsyt.2021.711079
62. Yong L, Liu L, Ding T, Yang G, Su H, Wang J, et al. Evidence of effect of aerobic exercise on cognitive intervention in older adults with mild cognitive impairment. Front Psychiatry. (2021) 3:713671. doi: 10.3389/fpsyt.2021.713671
Keywords: Alzheimer's disease, cognition, physical exercise, systematic review, meta-analysis
Citation: Liu W, Zhang J, Wang Y, Li J, Chang J and Jia Q (2022) Effect of Physical Exercise on Cognitive Function of Alzheimer's Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Psychiatry 13:927128. doi: 10.3389/fpsyt.2022.927128
Received: 23 April 2022; Accepted: 19 May 2022;
Published: 16 June 2022.
Edited by:
Zezhi Li, Guangzhou Medical University, ChinaCopyright © 2022 Liu, Zhang, Wang, Li, Chang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfeng Li, 01513386@sdtbu.edu.cn; Jindong Chang, changlai@163.com; Qingyin Jia, jiaqingyin@sdpei.edu.cn
 Wei Liu
Wei Liu Jia Zhang3
Jia Zhang3 Jindong Chang
Jindong Chang