- Division of Neuroscience, Laboratory Department of Neuropsychiatry, Graduate School of Medicine, Mie University, Tsu, Japan
Several mood-stabilizing atypical antipsychotics and antidepressants weakly block serotonin (5-HT) receptor type-7 (5-HT7R); however, the contributions of 5-HT7R antagonism to clinical efficacy and pathophysiology are yet to be clarified. A novel mood-stabilizing antipsychotic agent, lurasidone exhibits predominant binding affinity to 5-HT7R when compared with other monoamine receptors. To date, we have failed to discover the superior clinical efficacy of lurasidone on schizophrenia, mood, or anxiety disorders when compared with conventional mood-stabilizing atypical antipsychotics; however, numerous preclinical findings have indicated the possible potential of 5-HT7R antagonism against several neuropsychiatric disorders, as well as the generation of novel therapeutic options that could not be expected with conventional atypical antipsychotics. Traditional experimental techniques, electrophysiology, and microdialysis have demonstrated that the effects of 5-HT receptor type-1A (5-HT1AR) and 5-HT7R on neurotransmission are in contrast, but the effect of 5-HT1AR is more predominant than that of 5-HT7R, resulting in an insufficient understanding of the 5-HT7R function in the field of psychopharmacology. Accumulating knowledge regarding the pharmacodynamic profiles of 5-HT7R suggests that 5-HT7R is one of the key players in the establishment and remodeling of neural development and cytoarchitecture during the early developmental stage to the mature brain, and dysfunction or modulation of 5-HT7R is linked to the pathogenesis/pathophysiology of neuropsychiatric and neurodevelopmental disorders. In this review, to explore candidate novel applications for the treatment of several neuropsychiatric disorders, including mood disorders, schizophrenia, and other cognitive disturbance disorders, we discuss perspectives of psychopharmacology regarding the effects of 5-HT7R antagonism on transmission and intracellular signaling systems, based on preclinical findings.
Introduction
In the body, 95% of tryptophan is metabolized by the kynurenine pathway and 5% by the serotonin (5-HT) pathway (1–4). Only 5% of 5-HT is distributed in the central nervous system (CNS), and the remainder is synthesized and degraded in peripheral tissues (3). In the CNS, serotonergic transmission plays a fundamental role in the pathophysiology of mood disorders and schizophrenia (5, 6). 5-HT receptor type 7 (5-HT7R) is one of the most recently (1993) identified members of the 5-HT receptor family (7–10) and is highly expressed in functionally relevant regions of the brain (11, 12). In the mammalian CNS, 5-HT7R is most predominantly expressed in the thalamus, and in the hypothalamus, hippocampus, prefrontal cortex, striatal complex, amygdala, and dorsal raphe nucleus (DRN) (13–19). Over the last two decades, preclinical studies have accumulated various findings highlighting that 5-HT7R is one of the key players in the regulation of mood, memory processing, cognition, and emotional perception, as demonstrated by various experiments using selective 5-HT7R antagonist and 5-HT7R knockout mice model (20–25). Moreover, the predominant expression of 5-HT7R in the limbic regions supports the hypothesis that 5-HT7R contributes to the regulation of memory processing, cognition, and emotional perception in association with several types of cognitive domains (14, 16, 18, 19, 25).
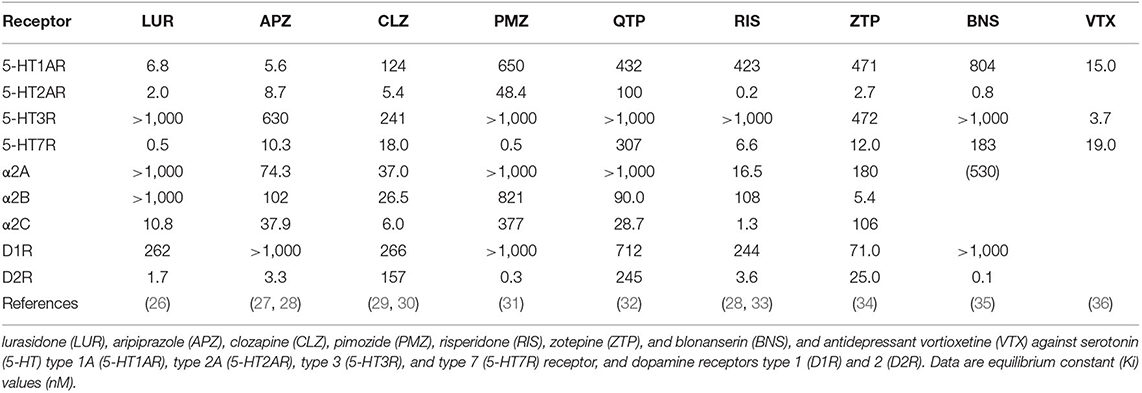
It has been postulated that 5-HT7R antagonism probably plays an important role in the clinical efficacy of several mood-stabilizing antipsychotics, as aripiprazole, clozapine, quetiapine, risperidone, and zotepine are known to antagonize 5-HT7R (Table 1) (18, 26, 27, 29–34, 37–39). Additionally, a novel antidepressant, vortioxetine, which is categorized as a 5-HT partial agonist reuptake inhibitor (SPARI), inhibits 5-HT7R (Table 1) (36, 39). However, the clinical potential of 5-HT7R antagonism is yet to be comprehensively elucidated as binding affinities of these conventional antipsychotics and vortioxetine are more highly sensitive to other 5-HT receptor subtypes than to 5-HT7R (Table 1). In contrast, a novel mood-stabilizing antipsychotic agent, lurasidone, is the only antipsychotic agent with the highest binding affinity to 5-HT7R when compared with other monoamine receptors (26) (Table 1). Therefore, to clarify crucial clinical targets of 5-HT7R antagonism for the treatment of neuropsychiatric disorders, including schizophrenia, mood disorders, and other cognitive disturbance disorders, in this review, we discuss the psychopharmacological perspectives of 5-HT7R antagonism, based on the preclinical findings of 5-HT7R antagonism and clinical evaluation of lurasidone to date.
Clinical Evaluation of Lurasidone and Preclinical Potentials of 5-HT7R Antagonism
Lurasidone has been approved for the treatment of schizophrenia by the United States Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Japanese Pharmaceuticals and Medical Devices (PMDA) (40). Additionally, lurasidone is has been approved by the FDA and PMDA, but not by the EMA, for the treatment of bipolar depression in monotherapy and combined with lithium or valproate in adults, and as monotherapy in children and adolescents (41).
Schizophrenia
A network meta-analysis of placebo-controlled and head-to-head randomized controlled trials indicated that lurasidone significantly improves positive, negative, and depressive symptoms, and improves the quality of life and social functioning when compared with placebo (42). Therefore, the general efficacy of lurasidone for the treatment of schizophrenia seems to be comparable to other atypical antipsychotics; however, the crucial superiority and specific targets responsible for the clinical efficacy of 5-HT7R antagonism remain to be detected.
Moreover, the optimal dose outcomes of lurasidone remain unknown as this drug is being assessed further in ongoing clinical trials. Indeed, a dose-response meta-analysis demonstrated that the 95% effective dose (ED95) of lurasidone for acute schizophrenia symptoms was achieved at 147 mg/day, which was calculated by six dose-finding studies. However, the dose-response curve suggested that higher doses could be more efficacious than the highest dose tested so far (160 mg/day) (43) Therefore, in combination with these findings, the clinical evaluation of a higher dose of lurasidone is possibly more efficacious than that of the conventional rating scale.
It has been well-established that dopamine D2 receptor (D2R) antagonism with 5-HT receptor type 2A (5-HT2AR) antagonism or 5-HT1AR partial agonism, with well-known receptor binding profiles in atypical antipsychotics, contribute to the clinical efficacy of antipsychotics against positive and negative symptoms of schizophrenia (44, 45). Although each antipsychotic has subtly distinct receptor binding profiles, lurasidone reportedly exhibits a receptor binding profile like an atypical antipsychotic agent, because lurasidone is a potent partial agonist of 5-HT1A, as well as a D2R and 5-HT2AR antagonist (44, 45). A positron emission tomography (PET) study demonstrated that clozapine, olanzapine, risperidone, and ziprasidone display high 5-HT2AR occupancy even at a low dose (~70–80%), but dose-dependently increase D2R occupancy (46–48), whereas lurasidone preferentially displayed D2R, rather than 5-HT2AR, occupancy (48, 49). D2R and 5-HT2AR occupancy levels of lurasidone (80 mg) were ~70–80% and lower than 40%, respectively (48, 50). Consistent with the in vitro receptor binding profile of lurasidone to 5-HT1AR (6.8 nM), 5-HT2AR (2.0 nM), 5-HT7R (0.5 nM), and D2R (1.7 nM) (Table 1) (26), the approved dosage of lurasidone probably displays predominant binding to D2R and 5-HT7R over binding to 5-HT1AR and 5-HT2AR. Therefore, the clinical efficacy of relatively low doses of lurasidone can be evaluated as 5-HT7R antagonism, whereas the clinical effects of a relatively high dose of lurasidone are probably affected by additional effects associated with 5-HT2A antagonism, along with 5-HT1AR partial agonism.
Several clinical studies have reported that clinical targets of 5-HT7R antagonism might differ from those of conventional atypical antipsychotics (51–59). Notably, 5-HT7R variants are not associated with response to atypical antipsychotics in schizophrenia (60, 61). Furthermore, a recent meta-analysis study revealed a significant association between responses to positive and negative symptoms with lurasidone and functional polymorphism of 5-HT receptor type 1A (5-HT1AR), but not those of 5-HT7R (62). The candidate superiorities of lurasidone are an improvement of atypical antipsychotic-resistant cognitive impairments (55, 57, 63) and prevention of relapse/recurrence, resulting in an improvement in the quality of life (51, 52, 56, 58, 59, 64–66).
For schizophrenia, improvements in cognitive impairment by atypical antipsychotics are limited (67). It is well-known that the executive function cognitive domain is a critical antipsychotic-resistant cognitive domain (68). Both atypical antipsychotics, clozapine and olanzapine, slightly improved the executive function in schizophrenia (67, 69), but only lurasidone has been confirmed to improve executive function in patients with atypical antipsychotics-resistant schizophrenia (57). The overall effectiveness of lurasidone against treatment-resistant schizophrenia is reportedly considered to be almost equal to that observed with clozapine, olanzapine, and risperidone. However, a recent clinical study demonstrated that lurasidone improved several cognitive domains, including executive function in patients with atypical antipsychotics-resistant schizophrenia (especially clozapine-resistant schizophrenia) (57). Interestingly, the approved dose of lurasidone (80 mg/day) improved the executive functions in atypical antipsychotic-resistant schizophrenia rather than higher doses (57). In particular, the improvement of executive function by lurasidone was independent of improvements in the positive and negative syndrome scales (57). The improvement of executive functions (atypical antipsychotic-resistant cognitive domains) in atypical antipsychotic-resistant schizophrenia suggested that 5-HT7R antagonism plays an important role in the cognitive promoting effects of lurasidone during atypical antipsychotic-resistant cognitive impairment, rather than 5-HT2A antagonism or 5-HT1AR partial agonism. Therefore, the discrepancy between the therapeutic dose ranges of lurasidone for cognitive promoting action (55, 57, 63) and acute schizophrenia symptoms (43) suggests that relatively low doses contribute to the cognitive promoting effects via predominantly 5-HT7R antagonism, but the improvement of acute schizophrenia symptoms requires a relatively high dose via 5-HT2AR antagonism with 5-HT1AR partial agonism. Collectively, relatively low doses of lurasidone improved executive functions in a significant proportion of atypical antipsychotic-resistant schizophrenia via different pharmacological mechanisms with conventional atypical antipsychotics (possibly 5-HT7R antagonism).
This hypothesis is supported by preclinical behavioral findings (70). Social withdrawal, which is a core negative symptom of schizophrenia, can be modeled in the social interaction test using N-methyl-D-aspartate receptor (NMDA)/glutamate antagonists in rodents (70, 71). Acute administration of SB269970 (a 5-HT7R antagonist) ameliorated ketamine-induced social withdrawal, whereas sulpiride was ineffective (72). Interestingly, the co-administration of an inactive low dose of SB269970 displayed the prosocial effects of sulpiride (72). Another behavioral study demonstrated that lurasidone ameliorated the deficits of novel object recognition induced by phencyclidine and was antagonized by AS-19 (5-HT7R agonist) (22). Therefore, D2R antagonism with 5-HT7R antagonism may be a novel candidate pharmacological profile of an atypical antipsychotic class. In particular, the D2R, 5-HT1AR, 5-HT2AR, and 5-HT7R occupancies mediated by the approved dose of lurasidone (80 mg) should be determined by employing PET.
Mood and Anxiety Disorders
A Bayesian network meta-analysis reported that lurasidone was more efficacious than aripiprazole and ziprasidone, and demonstrated comparable efficacy to quetiapine and olanzapine monotherapies for the management of bipolar depression (73). The efficacy of lurasidone in the acute treatment of bipolar depression, as both monotherapy and adjunctive therapy to lithium/valproate, has been reported in clinical trials (74, 75). Therefore, the general efficacy of lurasidone for the treatment of bipolar depression seems to be comparable with other mood-stabilizing atypical antipsychotics, whereas neither the superiority nor specific targets of the clinical efficacy of 5-HT7R antagonism have been demonstrated.
Although 5-HT7R variants are not associated with response to 5-HT7R antagonistic atypical antipsychotics in schizophrenia (see section Schizophrenia), a promoter single-nucleotide polymorphism in 5-HTR7 gene, rs7905446, which increases 5-HT7R expression (76), was positively associated with response to SSRI in individuals with mood disorders (76). These clinical findings indicates that 5-HT7R antagonism probably provides antidepressant-like action.
Major depression with mixed-features is regarded as conventional antidepressant-resistance and is associated with suicide ideation, manic switching agitation, and impulsivity; however, the standard medication for the mixed-features variant is yet to be clarified (77, 78). The specific “mixed-features variant” was incorporated in the Diagnostic Statistical Manual of Mental Disorders 5th edition. Therefore, the clinical efficacy of conventional mood-stabilizing atypical antipsychotics and antidepressants, except for lurasidone, remain to be clarified (78). A randomized double-blind, placebo-controlled study and its post-hoc studies demonstrated that lurasidone improved symptoms of depression/mania, anxiety, and irritability in post-menopausal women (79–82). Furthermore, a double-blind placebo control study revealed that lurasidone improved depressive symptoms without affecting manic symptoms in mixed-feature variants (83). Several studies have compared mood-stabilizing atypical antipsychotics (including olanzapine, lurasidone, brexpiprazole, ziprasidone, and cariprazine) with placebo, assessing the efficacy in the acute phase of presumptive mixed-feature variants (40, 84, 85). The available studies support the expected benefits of mixed-feature variants, whereas the overall effect may be similar in these mood-stabilizing atypical antipsychotics (40, 84, 85). To explore the efficacy of 5-HT7R antagonism against mixed-feature variants, the detailed clinical effects of conventional mood-stabilizing atypical antipsychotics and antidepressants on mixed-features variants need to be clinically investigated.
Preclinical findings using selective 5-HT7R antagonist and 5-HT7R knockout mice suggest that 5-HT7R inhibits both antidepressant-like and anxiolytic-like effects (21, 24, 86, 87). A selective 5-HT7R antagonist, SB269970, exhibited anti-immobility-like and antidepressant-like effects in the forced swim and tail suspension tests (21, 24). Furthermore, 5-HT7R knockout mice displayed tolerability to depression-like behavior in these tests (21, 88). These preclinical behavioral findings suggest that activation of 5-HT7R contributes to the pathomechanisms of depression. 5-HT7R antagonism mediated by SB269970 or JNJ18038683 produced antidepressant-like effects and promoted the antidepressant effects of several antidepressants, including citalopram, imipramine, desipramine, and moclobemide (24, 89, 90). Notably, SB269970 displayed rapid-acting antidepressant effects (91).
Based on the fast-acting antidepressant-like effects of 5-HT7R antagonists (91), several psychiatrists anticipated the development of a novel rapid-onset antidepressant class when compared with conventional antidepressants prescribed in clinical settings. Available medications using monoamine transporters that inhibit antidepressants and psycho-behavioral therapies require more than several weeks for the onset of beneficial effects (92). The delay of conventional monoaminergic antidepressants and psycho-behavioral therapies remains one of the major drawbacks of current treatments for depressive disorder, and faster-acting antidepressants are needed for patients with suicidal tendencies (93). Unfortunately, the onset of the antidepressant effect of lurasidone seems to require a duration equivalent to those of conventional antidepressants (73, 80, 82, 94, 95). However, a recent clinical trial demonstrated that both intravenous and oral administrations of vortioxetine displayed significant improvements in depression (Montgomery Åsberg Depression Rating Scale and Hospital Depression Scale) and anxiety (Hospital Anxiety Scale) after 3 days (95). Vortioxetine is a high-affinity inhibitor of human 5-HT transporter (Ki = 1.6 nM), 5-HT3R (Ki = 3.7 nM), 5-HT7R (Ki = 19 nM), and an agonist of 5-HT1AR (Ki = 15 nM) (Table 1) (36). Although the affinity of vortioxetine to rat 5-HT7R (Ki = 200 nM) is lower compared to human 5-HT7R (96), subacute administration (within 3 days) of effective dose of vortioxetine rapidly downregulates rat 5-HT7R (97). These preclinical demonstrations suggest that vortioxetine is a relatively low affinity to 5-HT7R compared to other 5-HT receptor subtypes, but suppresses 5-HT7R function with rapid 5-HT7R downregulation as an inverse agonist, similar to other 5-HT7R inhibiting mood-stabilizing atypical antipsychotics, clozapine, lurasidone, and olanzapine (97, 98). In other words, the rapid-acting antidepressant and anxiolytic actions of 5-HT7R antagonism have not been completely refuted and are worth reassessing after future clinical findings have accumulated.
Local hippocampal administration of SB269970 produced antidepressant-like activity in the rat forced swim test (87). Thus, blockade of 5-HT7R in the hippocampus might be beneficial in depression. Enhanced serotonergic transmission (activation of 5-HT1AR) plays an important role in the anti-depressive mechanisms of selective 5-HT reuptake inhibitors, but hippocampal 5-HT7R suppression is probably required for anti-depressive action. Acute stress increased 5-HT7R mRNA expression in the hippocampus (99). The anxiolytic-like effects of SB269970 were revealed in the Vogel drinking test, the elevated plus-maze test, and the four-plate test in mice (24). Local administration of SB269970 exhibited anxiety-like effects in the Vogel conflict test (87). SB269970 decreased the number of marbles buried in the marble-burying test (86). These findings suggest the effectiveness of 5-HT7R antagonists in the treatment of obsessive-compulsive disorder and anxiety disorders. 5-HT7R knockout mice exhibited similar behaviors in mice treated with SB269970 and antidepressants (21, 88); however, 5-HT7R knockout mice showed decreased immobility in forced swim and tail suspension tests and decreased both the duration and frequency in the rapid-eye-movement sleep phase (21, 88).
Neurodevelopmental and Neurodegeneration Disorders
The majority of approved agents for the treatment of tic disorders and Tourette syndrome, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, and pimozide, which reportedly reduce the severity of tic disorders and Tourette syndrome, are weak 5-HT7R antagonists (Table 1) (100–102). It is generally known that α2/α2A adrenoceptor agonism reduces the severity of tic disorders and Tourette syndrome (100, 102), whereas these agents are insensitive or α2 adrenoceptor antagonists (Table 1). Taken together with the in vitro binding profiles, 5-HT7R antagonism is a candidate target for the improvement of neurocognition.
Activation of 5-HT7R during adolescence induced persistent upregulation of 5-HT7R (17), and LP211 (selective 5-HT7R agonist) enhanced learning and memory in Fmr1 knockout mice, a genetic fragile-X syndrome mouse model (20). During adolescence, SB266970 enhanced impulsive behavior but attenuated the methylphenidate-induced reduction of impulsivity (103). These preclinical findings suggest the clinical potential of 5-HT7R agonists for the treatment of autism spectrum disorder and fragile-X syndrome (congenital X-linked disease associated with autistic traits and cognitive deficits) (20, 25). Conversely, chronic exposure to methylphenidate during postnatal and adolescence probably provides persistent structural rearrangements of the brain reward pathways associated with 5-HT7R (103). Therefore, the effects of 5-HT7R activation on neuronal plasticity during early development are not limited to embryonic and early postnatal development, but can also persist during adolescence and adulthood.
Although the 5-HT7R density decreased in correlation with age-dependent spatial memory deficits, which is possibly compensated by the 5-HT7R agonist (104), both 5-HT7R antagonists lurasidone and vortioxetine could clinically improve global cognitive performance (94, 105). Indeed, lurasidone is also significantly efficacious in older adults (≥55 years old) with bipolar depression (94), and bipolar depression comorbid with attention-deficit hyperactivity disorder (ADHD) in children and adolescents (106). The clinical improvement in cognitive disturbances in the elderly (94) and neurodevelopmental disorders (100, 102, 106) of 5-HT7R antagonism are in line with the preclinical findings on the effects of 5-HT7R antagonists on transmitter release. However, preclinical studies have previously reported more potentially important mechanisms associated with 5-HT7R antagonism. It is well-known that neurodegenerative processes and neurite retraction are considered to play important roles in the pathomechanisms of dementia and neurodevelopmental disorders (107). Preclinical findings support the clinical advantages of these 5-HT7R antagonistic agents owing to protection via inhibition of active polymerization and neurite retraction.
Cognition
Both 5-HT1AR partial agonism and 5-HT7R antagonism improved executive functions in a mouse model, but 5-HT7R agonism failed to demonstrate this effect (108). Lurasidone improved executive function, but the selective 5-HT1AR antagonist, WAY100635, blocked the ability of lurasidone (108). These results indicate that the combination of 5-HT7R antagonism and 5-HT1AR partial agonism plays an important role in executive functioning. The cognitive promoting action of 5-HT7R antagonist is constructed by 5-HT1AR sensitive hippocampal-dependent and other hippocampal-independent neural circuits (25, 109, 110). Executive functions have been long known to involve the frontal cortex, and two projections from the hippocampus and thalamus (111). Mediodorsal thalamic nucleus (MDTN), which regulates outputs to the frontal cortex via integration of sensory and emotional inputs, is an essential partner of the frontal cortex in mediating executive functions (71, 112–115). Along with findings on 5-HT7R expression regions (14, 16, 18, 19, 25), the thalamocortical pathway is a candidate cognitive bottom-up regulation system (113, 115) associated with 5-HT7R antagonism.
Tonic hyperactivation of thalamocortical glutamatergic transmission has been observed in patients and experimental animal models of schizophrenia, ADHD, and autism (39, 112, 116–121). Although enhancement of serotonergic transmission plays an important role in the clinical efficacy of several atypical antipsychotics and conventional antidepressants, activation of serotonergic transmission to the MDTN, at least partially, negatively affects executive functions associated with the thalamocortical pathway via activation of excitatory postsynaptic 5-HT7R in the MDTN (18, 38, 122). A potent 5-HT1AR and 5-HT7R agonist, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) (9) suppresses several types of learning (25, 110). 8-OH-DPAT administration suppressed retention performance in passive avoidance training; however, SB269970 facilitated the negative effects of 8-OH-DPAT on the passive avoidance task for emotional learning (110). 5-HT1AR and 5-HT7R are expressed in hippocampal CA3 dendritic and neuronal cell body regions, respectively (123, 124). Administration of a selective 5-HT1AR antagonist and selective 5-HT7R agonist enhanced emotional memory in the passive avoidance behavior test (125). These findings indicate that 5-HT7R enhances emotional memory via hippocampal serotonergic transmission independent of 5-HT1AR.
Both aripiprazole and clozapine suppressed the tonic activation of thalamocortical glutamatergic transmission via activation of group II and III metabotropic glutamate receptors in the frontal cortex, respectively (116, 117). Contrary to aripiprazole and clozapine, lurasidone suppressed tonic hyperactivation of thalamocortical glutamatergic transmission through inhibition of postsynaptic 5-HT7R in the MDTN (18, 38). The different pharmacodynamic suppressive mechanisms of lurasidone and aripiprazole/clozapine on tonic hyperactivation of thalamocortical glutamatergic transmission suggest that the complementary effects of lurasidone on the rational integration of signaling inputs to the thalamocortical pathway regarding thalamic executive function are more effective than those of clozapine. Therefore, the cognitive (executive function) promoting effects of adjuvant lurasidone on atypical antipsychotic-resistant schizophrenia are probably demonstrated when tonic hyperactivation of thalamocortical glutamatergic transmission cannot be sufficiently improved by other atypical antipsychotics, via additional inhibition of excitatory 5-HT7R in the MDTN. The 5-HT7R antagonist facilitated the consolidation and reconsolidation of contextual fear conditioning memory (23). Local administration of SB269970 into the basolateral amygdala, but not the hippocampal CA1 region, facilitated the extinction of contextual fear conditioning memory (109). 5-HT7R inhibition appears to facilitate memory processes in broader cortico-limbic circuits, but not the hippocampus alone (109). Thus, 5-HT7R inhibition probably improves contextual learning and memory through a mechanism independent of the hippocampus (25). 5-HT7R may contribute to effective switching between hippocampus-dependent and hippocampus-independent learning strategies (126).
Others
Recent findings have elucidated the role of 5-HT7R in a wide range of physiological functions in the mammalian CNS and peripheral organs (127–131). Noticeable modulation of peripheral 5-HT7R is expected to progress to the development of clinical applications in the medical field for autoimmune diseases and carcinoma. In peripheral tissues, the majority of 5-HT is produced by enterochromaffin cells of the gut mucosa and various immune cells. Peripheral 5-HT, which is produced in T lymphocytes and mast cells, is one immune system modulator that affects various immune cells via 5-HT receptors (3). A clinical study reported that several psychiatric disorders, including bipolar disorder, anxiety disorder, and major depression, are highly comorbid with inflammatory bowel diseases (around 50%) (132). Functional abnormalities of the serotonergic system contribute to the pathogenesis/pathophysiology of inflammatory bowel diseases (133). Silencing 5-HT7R, which is predominantly expressed in the immune system (134), reduced the severity of inflammation in an ulcerative colitis experimental mouse model (135). These findings suggest that functional abnormalities in 5-HT7R signaling in the brain, gut, and immune cells are involved in several inflammation- and immune-induced psychiatric disorders in the gut and brain.
A comprehensive in vivo screen test demonstrated that 297 psychoactive agents were 18-fold more likely to exert antiproliferative effects when compared with a random molecule population (136). Numerous clinical and meta-analysis studies reported that despite most patients with schizophrenia smoking heavily, the pooled overall cancer incidence rates for these patients were lower than their cancer risk factor exposure (136). Based on this clinical evidence, several antipsychotics are attracting attention as candidates for new treatment options for brain cancers owing to their ability to cross the blood-brain barrier (31). 5-HT7R antagonists have been proposed to block the growth of glioblastomas via inhibition of extracellular-regulated kinase (Erk) and interleukin 6 activities (137).
5-HT7R and Signal Transduction
Effects of 5-HT7R on Organizing and Remodeling of Neural Circuits
The effects of 5-HT7R on intracellular signal transduction are summarized in Figure 1. 5-HT7R expresses several functional splice variants, distinct in their carboxyl terminals due to introns in the 5-HT7R gene (11, 138–140). Splice variants of 5-HT7R (5-HT7Ra, 5-HT7Rb, and 5-HT7Rc in rodents, and 5-HT7Ra, 5-HT7Rb, and 5-HT7d in humans) have been established (11, 98, 138–141). Functional differences among the splice variants include the 5-HT7Ra isoform activating types 1 and 8 of adenylyl cyclase (AC) Gs-independently (Figure 1) (142), and the internalization pattern of 5-HT7Rd differs from those presented by other isoforms (98, 141).
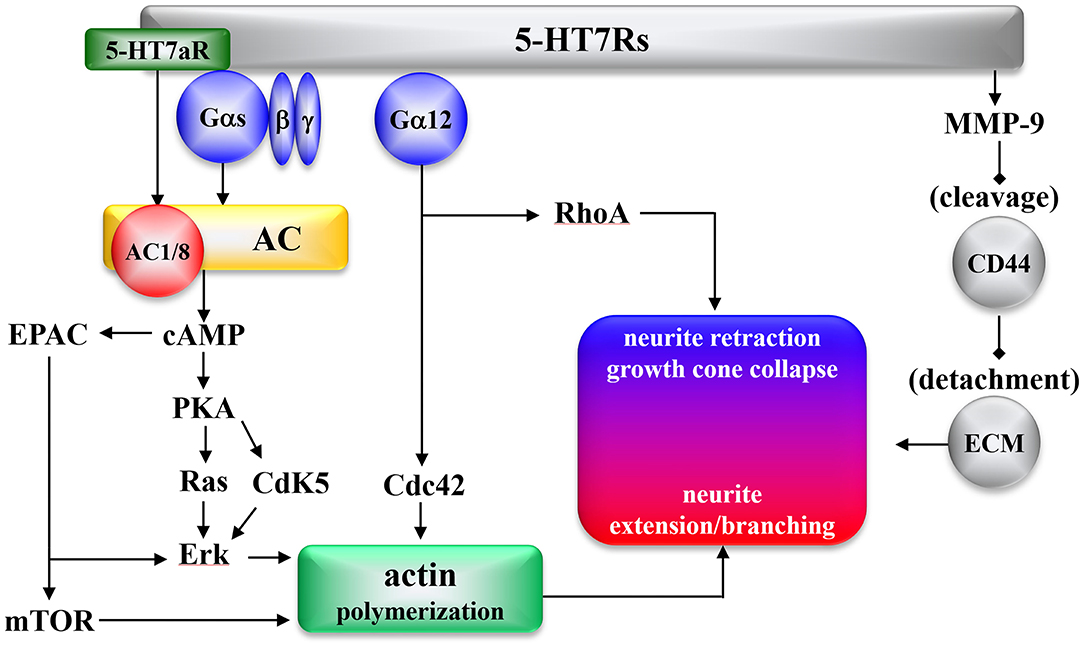
Figure 1. Morphogenic signaling mediated by the 5-HT7R. AC, Adenylyl cyclase; cAMP, cyclic adenosine monophosphate; Cdc42, cell division cycle protein 42; Cdk5, cyclin-dependent kinase 5; EPAC, exchange protein directly activated by cAMP; Erk, extracellular-regulated kinase; mTOR, mammalian target of rapamycin; PKA, protein kinase A; RhoA, Ras homolog gene family member A; CD44, hyaluronic acid receptor; MMP9, metallo proteinase 9; ECM, extracellular matrix.
To clarify the mechanism of 5-HT7R on neuronal plasticity and cognitive and mood regulation, psychopharmacological studies have focused on the impact of 5-HT7R on neurodevelopmental processes, including migration, axon guidance, dendrite development, synapse formation, and nerve wiring during the early developmental stage.
5-HT7R was found to activate several signaling pathways involved in molecular mechanisms, such as Gαs and Gα12 proteins, which underlie neural remodeling (Figure 1). 5-HT7R activates AC via activation of Gαs activity (10). Apart from Gαs, the 5-HT7Ra isoform activates AC1 and AC8 via Ca2+/calmodulin-dependent and Gs-independent signaling (142). Increased cyclic adenosine monophosphate (cAMP) stimulates both protein kinase A (PKA) and subsequent activation of cyclin-dependent kinase 5 (Cdk5) (19, 143), Ras (144, 145), and exchange protein directly activated by cAMP (EPAC) (146), resulting in Erk signaling activation (144, 147). Furthermore, 5-HT7R-induced Ras and EPAC signaling promote the activation of mammalian target of rapamycin (mTOR) (Figure 1) (148).
Additionally, 5-HT7R activates several signaling pathways associated with Gα12 (143). 5-HT7R/Gα12 activates both Ras homolog gene family member A (RhoA) and cell division cycle protein 42 (Cdc42) (143, 149). Another 5-HT7R pathway was recently reported: in the detachment from the extracellular matrix (ECM), 5-HT7R cleaves the extracellular domain of the hyaluronic acid receptor (CD44) via activation of metalloproteinase-9 (MMP9) (150). The detachment from ECM via CD44/MMP9 plays an initial role in both dendritic spine remodeling and synaptic pruning, followed by neurite retraction by RhoA and neurite extension/branching by mTOR, Erk, and Cdc42 (Figure 1).
The serotonergic system, which is one of the initial organizing systems in development, generates neurogenesis, cell migration, axon guidance, dendritogenesis, synaptogenesis, and brain wiring throughout life (151). The impact of 5-HT7R on neuronal morphology in early developmental stages plays a fundamental role in the establishment and maintenance of neural connectivity and synaptic plasticity. During the embryonic stage, 5-HT7R induces neurite outgrowth of cortical, striatal, and hippocampal neurons via activation of Cdk5 and mTOR (145, 152). 5-HT7R is thought to contribute to the modulation of synaptic plasticity and neuronal connectivity during the developing and mature brain (128). Chronic 5-HT7R activation generates dendritic spine formation and increases the number of structurally intact synapses and expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/glutamate receptor in hippocampal neurons (143). Neural circuits can be remodeled, induced by reactions to physiological and pathological inputs well into adulthood, continuing to exhibit robust plasticity throughout the entire lifespan of individuals (153). During the pre- and postnatal periods, exposure to selective serotonin reuptake inhibitors generates long-term anxiety in adulthood without affecting the morphological alterations of the brain (104, 154). Therefore, reorganization of dendritic morphology induced by 5-HT7R signaling provides new synapse growth and initial neuronal network formation, which is the target of event-related structural and functional plasticity in the early developmental stage (Figure 1) (155, 156).
A recent study suggest that rs300774, which is a candidate variant of LMWPTP associated with suicide, various transmission, including 5-HT and GABA (157). It has been reported that CD44 plays as a signaling receptor for LMWPTP induction (158), whereas redox-dependent downregulation of RhoA activity is modulated by oxidative modification of low-molecular weight protein tyrosine phosphatase (159). Therefore, the interaction between downstream signaling of LMWPTP and 5-HT7R possibly provides us a novel pathophysiological hypothesis regarding mood disorder and/or suicidal ideation. Furthermore, it has been demonstrated that a heterodimer and homodimers composed of 5-HT1AR and 5-HT7R, together with monomers, coexist in the cells (160). The heterodimer suppresses and enhances the stimulatory effects of 5-HT1AR on G-protein-gated inwardly rectifying potassium channels and mitogen-activated protein kinases, respectively. Interestingly, the heterodimer enhances the internalization of 5-HT1AR (160). Considering that the highest affinity for complex formation was obtained for the 5-HT7R/5-HT7R homodimers, followed by the 5-HT7R/5-HT1AR heterodimers and 5-HT1AR/5-HT1AR homodimers, determination of the effects of vortioxetine and lurasidone on the functional interactions between the heterodimer, homodimers, and monomers of 5-HT1AR and 5-HT7R could possibly clarify the complicated action of subchronic administration of these agents.
Effects of 5-HT7R on Transmission Associated With Neuronal Plasticity
5-HT7R knockout mice displayed impaired long-term potentiation (LTP) in the hippocampus, and impairments in contextual learning, seeking behavior, and allocentric spatial memory (161, 162). Functionally, 5-HT7R activates neuronal excitability and LTP in the hippocampus of juvenile rodents without affecting those of adult individuals (163). Notably, 5-HT7R enhanced population spike amplitude and bursting frequency in the hippocampal CA1 and CA3 regions, respectively (164, 165). 5-HT7R-induced activation of cAMP/PKA signaling enhanced NMDA-evoked currents, resulting in the enhancement of the population spike amplitude and bursting frequency in hippocampal CA1 and CA3 regions, respectively (164–166). Furthermore, 5-HT7R activates hippocampal transmission postsynaptically owing to enhanced phosphorylation of the GluA1 AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/glutamate receptor, induced via cAMP/cAMP response element-binding protein (CREB) signaling (167, 168). Reportedly, 5-HT7R reverses long-term depression (LTD) associated with metabotropic glutamate receptors (20). These electrophysiological findings describe the molecular mechanisms underlying the positive effects of 5-HT7R on cognition and memory.
Activation of 5-HT7R during adolescence induces persistent upregulation of 5-HT7R (17). Chronic exposure to methylphenidate during postnatal and adolescence probably provides persistent structural rearrangements of the brain reward pathways associated with 5-HT7R (103). The molecular mechanism underlying 5-HT7R-induced remodeling increases neurite and dendritic spine elongation via matrix metalloproteinase (MMP)-9/CD44 and Cdc42 in reversal learning and neuronal regeneration (150). Therefore, the effects of 5-HT7R on neuronal plasticity during early development are not limited to embryonic and early postnatal development but can also persist in adolescence and adulthood.
Pathologically, activation of 5-HT7R during adolescence leads to increased dendritic arborization in the nucleus accumbens, which is one of the most important neural circuits associated with the pathophysiology of schizophrenia (169–171).
Effects of 5-HT7R on Transmission After Maturation of the Nervous System
Microdialysis studies demonstrated that acute administration of a therapeutically relevant lurasidone dose increased the extracellular levels of dopamine, L-glutamate, and norepinephrine without affecting those of 5-HT or γ-aminobutyric acid (GABA) in the frontal cortex (Table 2) (18, 38, 172, 184). The effects of systemic administration of therapeutically relevant doses of conventional atypical antipsychotics on transmitter release are summarized in Table 2. The profile of lurasidone on transmitter release in the frontal cortex is similar to quetiapine but not to aripiprazole, blonanserin, clozapine, risperidone, or zotepine (18, 38, 116, 117, 172–181). D2R blockade with enhanced dopamine release in the frontal cortex produces the fundamental psychopharmacological effects of atypical antipsychotics (29). The combination of D2R inhibition with 5-HT2AR inhibition or 5-HT1AR activation contributes to enhanced dopamine release in the frontal cortex (185). Additionally, GABAergic disinhibition in the frontal cortex probably provides enhanced dopamine release induced by aripiprazole and clozapine (174, 176); however, lurasidone-induced dopamine release is not modulated by regional GABAergic disinhibition, similar to blonanserin, quetiapine, risperidone, and zotepine. These discrepancies in the frontal transmitter release profiles among 5-HT7R antagonistic antipsychotics (lurasidone, aripiprazole, clozapine, quetiapine, risperidone, and zotepine) suggest that 5-HT7R antagonism probably does not play an important role in enhanced dopamine release in the frontal cortex mediated by these antipsychotics. Therefore, 5-HT7R antagonism is possibly more effective in cognitive impairment and mood disturbances than against core (positive and negative) symptoms of schizophrenia.
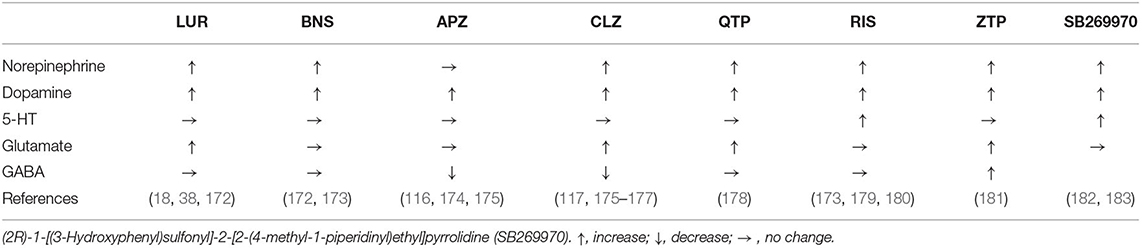
Table 2. Summary of effects of the systemic administration of therapeutic-relevant dose of antipsychotics and 5-HT7R antagonist on transmitter release in the frontal cortex.
Acute systemic administration of selective 5-HT7R antagonist, (2R)-1-[(3-hydroxyphenyl) sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine (SB269970) increased the basal release of norepinephrine and dopamine without affecting their metabolites in the medial prefrontal cortex (mPFC) (182). In contrast to catecholamines, acute systemic administration of SB269970 increased the basal release of 5-HT and 5-hydroxyindole acetic acid (5-HIAA) in the mPFC (183). Therefore, inhibition of 5-HT7R contributes to increased monoamine release in the mPFC; however, the mechanisms of catecholamine and 5-HT release induced by 5-HT7R inhibition vary, as catecholamine metabolites are not affected by 5-HT7R inhibition.
Serotonergic neurons in the DRN, which are regulated by inhibitory GABAA receptor and 5-HT1AR, project into GABAergic interneurons in the DRN, glutamatergic neurons in the frontal cortex, and the MDTN (18, 38, 39, 186). GABAergic interneurons in the DRN are regulated by both excitatory 5-HT7R and NMDA/glutamate receptors (18, 38, 39, 186). In DRN slice patch-clamp investigations, inhibition of 5-HT7R generated depolarization and increased neuronal firing frequency via attenuation of spontaneous inhibitory postsynaptic potential (sIPSP) (183, 186). A multiprobe dialysis study demonstrated that under 5-HT1AR blockade, inhibition of 5-HT7R reduced GABA release; however, during 5-HT1AR activation, the stimulatory effects of SB269970 on 5-HT release in the DRN could not be observed (18, 39, 186, 187). Therefore, enhanced 5-HT release in the DRN activates 5-HT1AR and GABAergic inhibition, resulting in the suppression of serotonergic neurons in the DRN. Collectively, these findings indicate that inhibition of 5-HT7R suppresses GABAergic negative feedback in the DRN, increasing 5-HT release in the frontal cortex (186).
In contrast to 5-HT, local administration of SB269970 into the mPFC weakly but not significantly increased regional basal release of norepinephrine and dopamine (38). Although local administration of SB269970 into the frontal cortex has not been demonstrated to increase regional catecholamine release, the regulation system in the frontal cortex is probably not the region responsible for the increased basal region catecholamine release induced by systemic SB269970 administration. In particular, the regions responsible for catecholamine release induced by systemic SB269970 administration might occur outside the cortex (182).
Clinical and preclinical studies have emphasized that disturbance of the MDTN is particularly relevant for cognitive dysfunction characterized by developmental disorders and psychoses, including autism spectrum disorder, intellectual disability, ADHD, mood disorder, epileptic psychosis, and schizophrenia (112, 118, 119, 188–193). MDTN receives several types of inputs from the cortical and subcortical regions associated with learning, memory, emotion, and perceptual integration (194–196). MDTN-lesioned monkeys continued to respond to stimuli after being satiated with associated food rewards (191). Thalamocortical glutamatergic transmission is considered to play a role in maintaining flexible stimulus–reward associations (120, 122, 197). Therefore, enhancement of the sensitivity and reliability of MDTN signaling partially regulates the promotion processes of wide range cognitive functions via the integration of emotional and sensory information.
Monoaminergic neurons in the DRN, ventral tegmental area (VTA), and locus coeruleus (LC) project selective terminals to deeper layers of the frontal cortex, whereas part of the catecholaminergic neurons in the LC project co-releasing terminals (dopamine and norepinephrine) to superficial layers (Figure 2) (178, 181). The co-releasing terminals presynaptically receive excitatory AMPA/glutamate receptors from MDTN (Figure 2) (112, 120, 122, 178, 181, 186). Continuously hyperactivated MDTN glutamatergic transmission results in the relative deterioration of sensitivity to input signaling from other regions, similar to the functional disruption of MDTN activity (193). Recent preclinical studies demonstrated that several cognitive promoting agents, guanfacine, memantine, aripiprazole, and clozapine, compensate for the hyperactivation of thalamocortical glutamatergic transmission due to modulation of α2A adrenoceptor, system Xc-, and metabotropic glutamate receptors (116, 117, 120, 121). MDTN is regulated by excitatory serotonergic and inhibitory GABAergic inputs via the 5-HT7R and GABAA receptors, respectively (39, 112, 116–121). Similarly, lurasidone compensated for hyperactivation of thalamocortical glutamatergic transmission via inhibition of 5-HT7R (18, 38). The mechanisms of 5-HT7R inhibition on complicated interactions between thalamocortical glutamatergic and mesocortical catecholaminergic pathways in the frontal cortex provided the first glimpses of the pathophysiology underlying hippocampal-independent cognitive-modulatory functions associated with 5-HT7R (Figure 2) (25, 126).

Figure 2. Proposed hypothesis for the extended complicated neural circuit connectivities involved in thalamocortical glutamatergic pathway, from the reticular thalamic nucleus (RTN), mediodorsal thalamic nucleus (MDTN) to the frontal cortex, mesothalamic serotonergic pathway, from the dorsal raphe nucleus (DRN) to the MDTN, mesocortical noradrenergic pathway, from the locus coeruleus (LC), dopaminergic pathway, from the ventral tegmental area (VTA), and serotonergic pathway from the DRN to the frontal cortex.
Remaining Challenges and Conclusion
Despite the advantages of 5-HT7R antagonism, the following challenges need to be resolved to develop effective clinical applications of 5-HT7R modulation for the treatment of numerous disorders involving cognitive impairments.
1) Although 5-HT7R antagonism possibly improves cognitive impairments, the 5-HT7R expression in the frontal cortex of patients with schizophrenia is lower than that in healthy controls (129, 130). Subchronic administration of the 5-HT7R agonist (LP211) and antagonists (SB269970) upregulated and downregulated 5-HT7R expression, respectively (17, 98). Therefore, reduced 5-HT7R expression in patients with schizophrenia cannot be determined to be directly involved in the pathogenesis of schizophrenia; these findings might have been induced by 5-HT7R antagonism of several antipsychotics. In particular, the inhibition of 5-HT7R is a candidate for the treatment of schizophrenia.
2) Although sleep disturbance (reduced duration and frequency of rapid-eye-movement sleep phase) was observed in 5-HT7R knockout mice (21), a clinical study failed to detect any sleep disturbance (sleep onset, rapid-eye-movement, or slow-wave sleep) in individuals receiving lurasidone (198). Additionally, another clinical study reported that lurasidone did not affect the sleep phase, whereas 5-HT7R knockout mice exhibited sleep phase disruption (21). Along with 5-HT7R downregulation induced by long-term administration of lurasidone, sleep disturbance caused by lurasidone remains one of the side effects necessitating attention.
3) Cognitive impairment in the elderly positively is known to correlate with an age-dependent reduction of 5-HT7R expression (104); however, both vortioxetine and lurasidone can improve cognitive decline and in the elderly (94, 105). Preclinical findings support the clinical advantages of these 5-HT7R antagonistic agents owing to their inhibitory action on active polymerization and neurite retraction. We speculated the clinical advantage of 5-HT7R antagonism in elderly individuals; however, long-term exposure to 5-HT7R antagonists could facilitate the fragility of transmission associated with 5-HT7R in elderly individuals.
4) Reportedly, approved agents for the treatment of behavioral manifestations of autism spectrum disorder, including pimozide, aripiprazole, and risperidone, exhibit a 5-HT7R antagonistic profile (28); however, the effectiveness of 5-HT7R agonists for the treatment of autism spectrum disorder and fragile-X syndrome was demonstrated in experimental models (20, 25). These discrepancies suggest the fundamental pathomechanisms of neurodevelopmental disorders, as these have been considered a dysfunction of both development and/or collapse of the neural circuit system. Neurodevelopmental dysfunction can be compensated by 5-HT7R-induced enhancement of the remodeling of neuronal connectivity (possibly collapse and branching). In contrast, after the maturation of neural circuits, the disruption of neuronal connectivity can be prevented by 5-HT7R inhibition. In particular, a possible crucial therapeutic time-window exists based on the pathomechanism of each disorder for the 5-HT7R modulating agent. In this review, we postulate that 5-HT7R inhibition might contribute to the stability of neuronal connectivity and transmission; however, rational enhancement of remodeling through 5-HT7R modulation may provide us with a novel strategy for various other neuropsychiatric disorders.
5) In this review, we hypothesized that the clinical efficacy of the approved lurasidone dose (80 mg) is mediated predominantly via D2R and 5-HT7R antagonism, rather than 5-HT2AR antagonism with 5-HT1AR partial agonism according to the PET and receptor binding studies. It is unlikely that our assumptions will be surpassed as the positive relationship between receptor occupancy and the binding affinity of most antipsychotics has been well-demonstrated. The 5-HT7R occupancy level of the approved lurasidone dosage should be clarified.
Author Contributions
MO: conceptualization, writing—original draft preparation, project administration, and funding acquisition. RO, TH, and KF: validation. RO, TS, and MO: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Japan Society for the Promotion of Science (19K08073).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fukuyama K, Tanahashi S, Hoshikawa M, Shinagawa R, Okada M. Zonisamide regulates basal ganglia transmission via astroglial kynurenine pathway. Neuropharmacology. (2014) 76:137–45. doi: 10.1016/j.neuropharm.2013.08.002
2. Fukuyama K, Okada M. Effects of levetiracetam on astroglial release of kynurenine-pathway metabolites. Br J Pharmacol. (2018) 175:4253–65. doi: 10.1111/bph.14491
3. Platten M E, Nollen AA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. (2019) 18:379–401. doi: 10.1038/s41573-019-0016-5
4. Yamamura S, Hoshikawa M, Dai K, Saito H, Suzuki N, Niwa O, et al. ONO-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation. Br J Pharmacol. (2013) 168:1088–100. doi: 10.1111/j.1476-5381.2012.02132.x
5. Meltzer HY. New trends in the treatment of schizophrenia. CNS Neurol Disord Drug Targets. (2017) 16:900–6. doi: 10.2174/1871527316666170728165355
6. Zmudzka E, Salaciak K, Sapa J, Pytka K. Serotonin receptors in depression and anxiety: insights from animal studies. Life Sci. (2018) 210:106–24. doi: 10.1016/j.lfs.2018.08.050
7. Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. (1993) 268:23422–6. doi: 10.1016/S0021-9258(19)49479-9
8. Lovenberg TW, Baron BM, De Lecea L, Miller JD, Prosser RA, Rea MA, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. (1993) 11:449–58. doi: 10.1016/0896-6273(93)90149-L
9. Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, et al. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. (1993) 90:8547–51. doi: 10.1073/pnas.90.18.8547
10. Shen Y, Monsma F, Metcalf M, Jose P, Hamblin M, Sibley D. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chemy. (1993) 268:18200–4. doi: 10.1016/S0021-9258(17)46830-X
11. Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, et al. The serotonin 5-HT 7 receptors: two decades of research. Exp Brain Res. (2013) 230:555–68. doi: 10.1007/s00221-013-3694-y
12. Matthys A, Haegeman G, Van Craenenbroeck K, Vanhoenacker P. Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol Neurobiol. (2011) 43:228–53. doi: 10.1007/s12035-011-8175-3
13. Aubert Y, Allers KA, Sommer B, De Kloet ER, Abbott DH, Datson NA. Brain regionspecific transcriptomic markers of serotonin1a receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. J Sexual Med. (2013) 10:1461–75. doi: 10.1111/jsm.12131
14. Gocho Y, Sakai A, Yanagawa Y, Suzuki H, Saitow F. Electrophysiological and pharmacological properties of GABAergic cells in the dorsal raphe nucleus. J Physiol Sci. (2013) 63:147–54. doi: 10.1007/s12576-012-0250-7
15. L'estrade ET, Erlandsson M, Edgar FG, Ohlsson T, Knudsen GM, Herth MM. Towards selective CNS PET imaging of the 5-HT7 receptor system: past, present and future. Neuropharmacology. (2020) 172:107830. doi: 10.1016/j.neuropharm.2019.107830
16. Lippiello P, Hoxha E, Speranza L, Volpicelli F, Ferraro A, Leopoldo M, et al. The 5-HT7 receptor triggers cerebellar long-term synaptic depression via PKC-MAPK. Neuropharmacology. (2016) 101:426–438. doi: 10.1016/j.neuropharm.2015.10.019
17. Nativio P, Zoratto F, Romano E, Lacivita E, Leopoldo M, Pascale E, et al. Stimulation of 5-HT 7 receptor during adolescence determines its persistent upregulation in adult rat forebrain areas. Synapse. (2015) 69:533–42. doi: 10.1002/syn.21846
18. Okada M, Fukuyama K, Okubo R, Shiroyama T, Ueda Y. Lurasidone sub-chronically activates serotonergic transmission via desensitization of 5-HT1A and 5-HT7 receptors in dorsal raphe nucleus. Pharmaceuticals. (2019) 12:149. doi: 10.3390/ph12040149
19. Volpicelli F, Speranza L, Di Porzio U, Crispino M, Perrone-Capano C. The serotonin receptor 7 and the structural plasticity of brain circuits. Front Behav Neurosci. (2014) 8:318. doi: 10.3389/fnbeh.2014.00318
20. Costa L, Spatuzza M, D'antoni S, Bonaccorso CM, Trovato C, Musumeci SA, et al. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry. (2012) 72:924–33. doi: 10.1016/j.biopsych.2012.06.008
21. Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. (2005) 58:831–7. doi: 10.1016/j.biopsych.2005.05.012
22. Horiguchi M, Huang M, Meltzer HY. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J Pharmacol Exp Ther. (2011) 338:605–14. doi: 10.1124/jpet.111.180638
23. Schmidt S, Furini C, Zinn C, Cavalcante L, Ferreira F, Behling J, et al. Modulation of the consolidation and reconsolidation of fear memory by three different serotonin receptors in hippocampus. Neurobiol Learn Memory. (2017) 142:48–54. doi: 10.1016/j.nlm.2016.12.017
24. Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. (2006) 51:578–86. doi: 10.1016/j.neuropharm.2006.04.017
25. Zareifopoulos N, Papatheodoropoulos C. Effects of 5-HT-7 receptor ligands on memory and cognition. Neurobiol Learn Mem. (2016) 136:204–9. doi: 10.1016/j.nlm.2016.10.011
26. Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. (2010) 334:171–81. doi: 10.1124/jpet.110.167346
27. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. (2003) 28:1400–11. doi: 10.1038/sj.npp.1300203
28. Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. (2014) 45:185–92. doi: 10.1007/s10578-013-0390-x
29. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. (1991) 17:263–87. doi: 10.1093/schbul/17.2.263
30. Su TP, Malhotra AK, Hadd K, Breier A, Pickar D. D2 dopamine receptor occupancy: a crossover comparison of risperidone with clozapine therapy in schizophrenic patients. Arch Gen Psychiatry. (1997) 54:972–3. doi: 10.1001/archpsyc.1997.01830220102017
31. Elmaci I, Altinoz MA. Targeting the cellular schizophrenia. likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit Rev Oncol Hematol. (2018) 128:96–109. doi: 10.1016/j.critrevonc.2018.06.004
32. Lopez-Munoz F, Alamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. (2013) 4:102. doi: 10.3389/fpsyt.2013.00102
33. Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol. (2006) 70:1264–70. doi: 10.1124/mol.106.024612
34. Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. (1996) 124:57–73. doi: 10.1007/BF02245606
35. Tenjin T, Miyamoto S, Ninomiya Y, Kitajima R, Ogino S, Miyake N, et al. Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat. (2013) 9:587–94. doi: 10.2147/NDT.S34433
36. Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. (2011) 54:3206–21. doi: 10.1021/jm101459g
37. Fernandez J, Alonso JM, Andres JI, Cid JM, Diaz A, Iturrino L, et al. Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J Med Chem. (2005) 48:1709–12. doi: 10.1021/jm049632c
38. Okada M, Fukuyama K, Ueda Y. Lurasidone inhibits NMDA/glutamate antagonist-induced functional abnormality of thalamocortical glutamatergic transmission via 5-HT7 receptor blockade. Br J Pharmacol. (2019) 176:4002–18. doi: 10.1111/bph.14804
39. Okada M, Okubo R, Fukuyama K. Vortioxetine subchronically activates serotonergic transmission via desensitization of serotonin 5-HT1A receptor with 5-ht3 receptor inhibition in rats. Int J Mol Sci. (2019) 20:6235. doi: 10.3390/ijms20246235
40. Corponi F, Fabbri C, Bitter I, Montgomery S, Vieta E, Kasper S, et al. Novel antipsychotics specificity profile: a clinically oriented review of lurasidone, brexpiprazole, cariprazine and lumateperone. Eur Neuropsychopharmacol. (2019) 29:971–85. doi: 10.1016/j.euroneuro.2019.06.008
41. Ali Z, Tegin C, El-Mallakh RS. Evaluating lurasidone as a treatment option for bipolar disorder. Expert Opin Pharmacother. (2020) 21:253–60. doi: 10.1080/14656566.2019.1695777
42. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/S0140-6736(19)31135-3
43. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. (2020) 177:342–53. doi: 10.1176/appi.ajp.2019.19010034
44. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2003) 27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010
45. Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. (2011) 11:59–67. doi: 10.1016/j.coph.2011.02.007
46. Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry. (2001) 158:360–9. doi: 10.1176/appi.ajp.158.3.360
47. Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, et al. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. (2004) 161:818–25. doi: 10.1176/appi.ajp.161.5.818
48. Nakazawa S, Yokoyama C, Nishimura N, Horisawa T, Kawasaki A, Mizuma H, et al. Evaluation of dopamine D(2)/D(3) and serotonin 5-HT(2)A receptor occupancy for a novel antipsychotic, lurasidone, in conscious common marmosets using small-animal positron emission tomography. Psychopharmacology. (2013) 225:329–39. doi: 10.1007/s00213-012-2815-9
49. Potkin SG, Keator DB, Kesler-West ML, Nguyen DD, Van Erp TG, Mukherjee J, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. (2014) 19:176–81. doi: 10.1017/S109285291300059X
50. Wong DF, Kuwabara H, Brasic JR, Stock T, Maini A, Gean EG, et al. Determination of dopamine D(2) receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology. (2013) 229:245–52. doi: 10.1007/s00213-013-3103-z
51. Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. (2012) 27:165–76. doi: 10.1097/YIC.0b013e32835281ef
52. Citrome L, Weiden PJ, Mcevoy JP, Correll CU, Cucchiaro J, Hsu J, et al. Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr. (2014) 19:330–9. doi: 10.1017/S109285291300093X
53. Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res. (2011) 127:188–94. doi: 10.1016/j.schres.2011.01.004
54. Harvey PD, Siu CO, Hsu J, Cucchiaro J, Maruff P, Loebel A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. (2013) 23:1373–82. doi: 10.1016/j.euroneuro.2013.08.003
55. Harvey PD, Siu CO, Ogasa M, Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: post-hoc analysis of a long-term, double-blind continuation study. Schizophr Res. (2015) 166:334–8. doi: 10.1016/j.schres.2015.06.008
56. Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. (2013) 147:95–102. doi: 10.1016/j.schres.2013.03.013
57. Meltzer HY, Share DB, Jayathilake K, Salomon RM, Lee MA. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J Clin Psychopharmacol. (2020) 40:240–9. doi: 10.1097/JCP.0000000000001205
58. Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. (2013) 74:507–15. doi: 10.4088/JCP.12m08084
59. Tandon R, Cucchiaro J, Phillips D, Hernandez D, Mao Y, Pikalov A, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. (2016) 30:69–77. doi: 10.1177/0269881115620460
60. Takekita Y, Fabbri C, Kato M, Nonen S, Sakai S, Sunada N, et al. Serotonin 7 receptor variants are not associated with response to second-generation antipsychotics in japanese schizophrenia patients. Neuropsychobiology. (2015) 72:118–25. doi: 10.1159/000441629
61. Takekita Y, Fabbri C, Kato M, Koshikawa Y, Tajika A, Kinoshita T, et al. HTR1A polymorphisms and clinical efficacy of antipsychotic drug treatment in schizophrenia: a meta-analysis. Int J Neuropsychopharmacol. (2016) 19:pyv125. doi: 10.1093/ijnp/pyv125
62. Yoshikawa A, Li J, Meltzer HY. A functional HTR1A polymorphism, rs6295, predicts short-term response to lurasidone: confirmation with meta-analysis of other antipsychotic drugs. Pharmacogenom J. (2020) 20:260–70. doi: 10.1038/s41397-019-0101-5
63. Potkin SG, Ogasa M, Cucchiaro J, Loebel A. Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr Res. (2011) 132:101–7. doi: 10.1016/j.schres.2011.04.008
64. Rajagopalan K, O'day K, Meyer K, Pikalov A, Loebel A. Annual cost of relapses and relapse-related hospitalizations in adults with schizophrenia: results from a 12-month, double-blind, comparative study of lurasidone vs quetiapine extended-release. J Med Econ. (2013) 16:987–96. doi: 10.3111/13696998.2013.809353
65. Rajagopalan K, Meyer K, O'day K, Denno M, Loebel A. Cost-effectiveness of lurasidone vs quetiapine extended-release (XR) in patients with bipolar depression. J Med Econ. (2015) 18:821–7. doi: 10.3111/13696998.2015.1052462
66. Restelli U, Garcia-Goni M, Lew-Starowicz M, Mierzejewski P, Silvola S, Mayoral-Van Son J, et al. Cost of relapse management in patients with schizophrenia in Italy Spain: comparison between lurasidone quetiapine XR. Clin Drug Investig. (2020) 40:861–71. doi: 10.1007/s40261-020-00944-0
67. Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, et al. Placebo response and practice effects in schizophrenia cognition trials. JAMA Psychiatry. (2017) 74:807–14. doi: 10.1001/jamapsychiatry.2017.1574
68. Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. (2005) 8:457–72. doi: 10.1017/S146114570500516X
69. Bender S, Dittmann-Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, et al. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs. clozapine. Int J Neuropsychopharmacol. (2006) 9:135–45. doi: 10.1017/S1461145705005924
70. Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. (2013) 16:2181–94. doi: 10.1017/S1461145713000928
71. Okada M, Kawano Y, Fukuyama K, Motomura E, Shiroyama T. Candidate strategies for development of a rapid-acting antidepressant class without neuropsychiatric adverse effects: prevention of ketamine-Induced neuropsychiatric adverse reactions. Int J Mol Sci. (2020) 21:7951. doi: 10.3390/ijms21217951
72. Holuj M, Popik P, Nikiforuk A. Improvement of ketamine-induced social withdrawal in rats: the role of 5-HT7 receptors. Behav Pharmacol. (2015) 26:766–75. doi: 10.1097/FBP.0000000000000132
73. Ostacher M, Ng-Mak D, Patel P, Ntais D, Schlueter M, Loebel A. Lurasidone compared to other atypical antipsychotic monotherapies for bipolar depression: a systematic review and network meta-analysis. World J Biol Psychiatry. (2018) 19:586–601. doi: 10.1080/15622975.2017.1285050
74. Loebel A, Cucchiaro J, Silva R, Kroger H, Hsu J, Sarma K, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. (2014) 171:160–8. doi: 10.1176/appi.ajp.2013.13070984
75. Loebel A, Cucchiaro J, Silva R, Kroger H, Sarma K, Xu J, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. (2014) 171:169–77. doi: 10.1176/appi.ajp.2013.13070985
76. Wei YB, Mccarthy M, Ren H, Carrillo-Roa T, Shekhtman T, Demodena A, et al. A functional variant in the serotonin receptor 7 gene (HTR7), rs7905446, is associated with good response to SSRIs in bipolar and unipolar depression. Mol Psychiatry. (2020) 25:1312–22. doi: 10.1038/s41380-019-0397-1
77. De Carlo V, Calati R, Serretti A. Socio-demographic and clinical predictors of non-response/non-remission in treatment resistant depressed patients: a systematic review. Psychiatry Res. (2016) 240:421–30. doi: 10.1016/j.psychres.2016.04.034
78. Verdolini N, Hidalgo-Mazzei D, Murru A, Pacchiarotti I, Samalin L, Young A, et al. Mixed states in bipolar and major depressive disorders: systematic review and quality appraisal of guidelines. Acta Psychiatrica Scand. (2018) 138:196–222. doi: 10.1111/acps.12896
79. Sramek J, Loebel A, Murphy M, Mao Y, Pikalov A, Cutler NR. Lurasidone in post-menopausal females with major depressive disorder with mixed features: post-hoc analysis of a placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 78:12–7. doi: 10.1016/j.pnpbp.2017.05.002
80. Suppes T, Silva R, Cucchiaro J, Mao Y, Targum S, Streicher C, et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. (2016) 173:400–7. doi: 10.1176/appi.ajp.2015.15060770
81. Swann AC, Fava M, Tsai J, Mao Y, Pikalov A, Loebel A. Lurasidone for major depressive disorder with mixed features and irritability: a post-hoc analysis. CNS Spectr. (2017) 22:228–35. doi: 10.1017/S1092852917000232
82. Tsai J, Thase ME, Mao Y, Ng-Mak D, Pikalov A, Loebel A. Lurasidone for major depressive disorder with mixed features and anxiety: a post-hoc analysis of a randomized, placebo-controlled study. CNS Spectr. (2017) 22:236–45. doi: 10.1017/S1092852917000074
83. Mcintyre RS, Cucchiaro J, Pikalov A, Kroger H, Loebel A. Lurasidone in the treatment of bipolar depression with mixed (subsyndromal hypomanic) features: post hoc analysis of a randomized placebo-controlled trial. J Clin Psychiatry. (2015) 76:398–405. doi: 10.4088/JCP.14m09410
84. Hammett S, Youssef NA. Systematic review of recent guidelines for pharmacological treatments of bipolar disorders in adults. Ann Clin Psychiatry. (2017) 29:266–82.
85. Shim IH, Bahk WM, Woo YS, Yoon BH. Pharmacological treatment of major depressive episodes with mixed features: a systematic review. Clin Psychopharmacol Neurosci. (2018) 16:376–82. doi: 10.9758/cpn.2018.16.4.376
86. Hedlund PB, Sutcliffe JG. The 5-HT7 receptor influences stereotypic behavior in a model of obsessive-compulsive disorder. Neurosci Lett. (2007) 414:247–51. doi: 10.1016/j.neulet.2006.12.054
87. Wesolowska A, Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol. (2006) 553:185–90. doi: 10.1016/j.ejphar.2006.09.064
88. Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. (2005) 48:492–502. doi: 10.1016/j.neuropharm.2004.11.015
89. Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. (2007) 321:690–8. doi: 10.1124/jpet.107.119404
90. Bonaventure P, Dugovic C, Kramer M, De Boer P, Singh J, Wilson S, et al. Translational evaluation of JNJ-18038683, a 5-hydroxytryptamine type 7 receptor antagonist, on rapid eye movement sleep and in major depressive disorder. J Pharmacol Exp Ther. (2012) 342:429–40. doi: 10.1124/jpet.112.193995
91. Mnie-Filali O, Faure C, Lambas-Senas L, El Mansari M, Belblidia H, Gondard E, et al. Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology. (2011) 36:1275–88. doi: 10.1038/npp.2011.13
92. Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. (2009) 60:1439–45. doi: 10.1176/ps.2009.60.11.1439
93. Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. (2011) 475:91–5. doi: 10.1038/nature10130
94. Sajatovic M, Forester BP, Tsai J, Kroger H, Pikalov A, Cucchiaro J, et al. Efficacy of lurasidone in adults aged 55 years and older with bipolar depression: post hoc analysis of 2 double-blind, placebo-controlled studies. J Clin Psychiatry. (2016) 77:e1324–e31. doi: 10.4088/JCP.15m10261
95. Vieta E, Florea I, Schmidt SN, Areberg J, Ettrup A. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. (2019) 34:153–60. doi: 10.1097/YIC.0000000000000271
96. Mork A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y, et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav. (2013) 105:41–50. doi: 10.1016/j.pbb.2013.01.019
97. Okada M, Matsumoto R, Yamamoto Y, Fukuyama K. Effects of subchronic administrations of vortioxetine, lurasidone and escitalopram on thalamocortical glutamatergic transmission associated with serotonin 5-HT7 receptor. Int J Mol Sci. (2020) 21:1351. doi: 10.3390/ijms22031351
98. Andressen KW, Manfra O, Brevik CH, Ulsund AH, Vanhoenacker P, Levy FO, et al. The atypical antipsychotics clozapine and olanzapine promote down-regulation and display functional selectivity at human 5-HT7 receptors. Br J Pharmacol. (2015) 172:3846–60. doi: 10.1111/bph.13169
99. Yau JL, Noble J, Seckl JR. Acute restraint stress increases 5-HT7 receptor mRNA expression in the rat hippocampus. Neurosci Lett. (2001) 309:141–4. doi: 10.1016/S0304-3940(01)02054-7
100. Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment of Tourette syndrome. Drugs Today. (2014) 50:159–79. doi: 10.1358/dot.2014.50.2.2097801
101. Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. (2011) 20:173–96. doi: 10.1007/s00787-011-0163-7
102. Yang C, Hao Z, Zhu C, Guo Q, Mu D, Zhang L. Interventions for tic disorders: an overview of systematic reviews and meta analyses. Neurosci Biobehav Rev. (2016) 63:239–55. doi: 10.1016/j.neubiorev.2015.12.013
103. Leo D, Adriani W, Cavaliere C, Cirillo G, Marco E, Romano E, et al. Methylphenidate to adolescent rats drives enduring changes of accumbal Htr7 expression: implications for impulsive behavior and neuronal morphology. Genes Brain Behav. (2009) 8:356–68. doi: 10.1111/j.1601-183X.2009.00486.x
104. Beaudet G, Bouet V, Jozet-Alves C, Schumann-Bard P, Dauphin F, Paizanis E, et al. Spatial memory deficit across aging: current insights of the role of 5-HT7 receptors. Front Behav Neurosci. (2014) 8:448. doi: 10.3389/fnbeh.2014.00448
105. Lenze EJ, Stevens A, Waring JD, Pham VT, Haddad R, Shimony J, et al. Augmenting computerized cognitive training with vortioxetine for age-related cognitive decline: a randomized controlled trial. Am J Psychiatry. (2020) 177:548–555. doi: 10.1176/appi.ajp.2019.19050561
106. Delbello MP, Goldman R, Phillips D, Deng L, Cucchiaro J, Loebel A. Efficacy and safety of lurasidone in children and adolescents with bipolar i depression: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. (2017) 56:1015–25. doi: 10.1016/j.jaac.2017.10.006
107. Karameh WK, Murari G, Schweizer TA, Munoz DG, Fischer CE. Psychosis in neurodegenerative disorders: recent developments. Curr Opin Psychiatry. (2019) 32:117–22. doi: 10.1097/YCO.0000000000000476
108. Rajagopal L, Massey BW, Michael E, Meltzer HY. Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology. (2016) 233:649–60. doi: 10.1007/s00213-015-4137-1
109. De Assis Brasil ES, Guerino Furini CR F, Da Silva R, odrigues Nachtigall EG, Kielbovicz Behling JA . The blockade of the serotoninergic receptors 5-HT5A, 5-HT6 and 5-HT7 in the basolateral amygdala, but not in the hippocampus facilitate the extinction of fear memory. Behav Brain Res. (2019) 372:112055. doi: 10.1016/j.bbr.2019.112055
110. Eriksson TM, Golkar A, Ekstrom JC, Svenningsson P, Ogren SO. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT(1A) receptor stimulation on contextual learning in mice. Eur J Pharmacol. (2008) 596:107–10. doi: 10.1016/j.ejphar.2008.08.026
111. Bueno-Junior LS, Leite JP. Input convergence, synaptic plasticity and functional coupling across hippocampal-prefrontal-thalamic circuits. Front Neural Circuits. (2018) 12:40. doi: 10.3389/fncir.2018.00040
112. Fukuyama K, Fukuzawa M, Shiroyama T, Okada M. Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant alpha4 subunit of nicotinic ACh receptor. Br J Pharmacol. (2020) 177:2143–62. doi: 10.1111/bph.14974
113. Halassa MM, Sherman SM. Thalamocortical circuit motifs: a general framework. Neuron. (2019) 103:762–70. doi: 10.1016/j.neuron.2019.06.005
114. Okada M, Fukuyama K, Shiroyama T, Murata M. A working hypothesis regarding identical pathomechanisms between clinical efficacy and adverse reaction of clozapine via the activation of connexin43. Int J Mol Sci. (2020) 21:7019. doi: 10.3390/ijms21197019
115. Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry. (2018) 83:648–56. doi: 10.1016/j.biopsych.2017.11.008
116. Fukuyama K, Hasegawa T, Okada M. Cystine/Glutamate antiporter and aripiprazole compensate NMDA antagonist-induced dysfunction of thalamocortical L-Glutamatergic transmission. Int J Mol Sci. (2018) 19:3645. doi: 10.3390/ijms19113645
117. Fukuyama K, Kato R, Murata M, Shiroyama T, Okada M. Clozapine normalizes a glutamatergic transmission abnormality induced by an impaired nmda receptor in the thalamocortical pathway via the activation of a Group III metabotropic glutamate receptor. Biomolecules. (2019) 9:234. doi: 10.3390/biom9060234
118. Fukuyama K, Fukuzawa M, Okada M. Upregulated and hyperactivated thalamic Connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant alpha4 subunit of Nicotinic ACh receptor. Pharmaceuticals. (2020) 13:99. doi: 10.3390/ph13050099
119. Fukuyama K, Fukuzawa M, Okubo R, Okada M. Upregulated Connexin 43 induced by loss-of-functional S284L-Mutant alpha4 subunit of nicotinic ACh receptor contributes to pathomechanisms of autosomal dominant sleep-related hypermotor epilepsy. Pharmaceuticals. (2020) 13:58. doi: 10.3390/ph13040058
120. Okada M, Fukuyama K, Kawano Y, Shiroyama T, Suzuki D, Ueda Y. Effects of acute and sub-chronic administrations of guanfacine on catecholaminergic transmissions in the orbitofrontal cortex. Neuropharmacology. (2019) 156:107547. doi: 10.1016/j.neuropharm.2019.02.029
121. Okada M, Fukuyama K, Kawano Y, Shiroyama T, Ueda Y. Memantine protects thalamocortical hyper-glutamatergic transmission induced by NMDA receptor antagonism via activation of system xc(). Pharmacol Res Perspect. (2019) 7:e00457. doi: 10.1002/prp2.457
122. Okada M, Fukuyama K. Interaction between mesocortical and mesothalamic catecholaminergic transmissions associated with NMDA Receptor in the locus coeruleus. Biomolecules. (2020) 10:990. doi: 10.3390/biom10070990
123. Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. (1996) 14:35–46. doi: 10.1016/S0893-133X(96)80057-1
124. Bickmeyer U, Heine M, Manzke T, Richter DW. Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci. (2002) 16:209–18. doi: 10.1046/j.1460-9568.2002.02072.x
125. Eriksson TM, Holst S, Stan TL, Hager T, Sjogren B, Ogren SO, et al. 5-HT1A and 5-HT7 receptor crosstalk in the regulation of emotional memory: implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology. (2012) 63:1150–60. doi: 10.1016/j.neuropharm.2012.06.061
126. Sarkisyan G, Hedlund PB. The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav Brain Res. (2009) 202:26–31. doi: 10.1016/j.bbr.2009.03.011
127. Blattner KM, Canney DJ, Pippin DA, Blass BE. Pharmacology and therapeutic potential of the 5-HT7 receptor. ACS Chem Neurosci. (2018) 10:89–119. doi: 10.1021/acschemneuro.8b00283
128. Crispino M, Volpicelli F, Perrone-Capano C. Role of the serotonin receptor 7 in brain plasticity: from development to disease. Int J Mol Sci. (2020) 21:505. doi: 10.3390/ijms21020505
129. Dean B, Pavey G, Thomas D, Scarr E. Cortical serotonin7, 1D and 1F receptors: effects of schizophrenia, suicide and antipsychotic drug treatment. Schizophr Res. (2006) 88:265–74. doi: 10.1016/j.schres.2006.07.003
130. East S, Burnet P, Kerwin R, Harrison P. An RT-PCR study of 5-HT6 and 5-HT7 receptor mRNAs in the hippocampal formation and prefrontal cortex in schizophrenia. Schizophr Res. (2002) 57:15–26. doi: 10.1016/S0920-9964(01)00323-1
131. Ikeda M, Iwata N, Kitajima T, Suzuki T, Yamanouchi Y, Kinoshita Y, et al. Positive association of the serotonin 5-HT 7 receptor gene with schizophrenia in a Japanese population. Neuropsychopharmacology. (2006) 31:866–71. doi: 10.1038/sj.npp.1300901
132. Marafini I, Longo L, Lavasani DM, Rossi R, Salvatori S, Pianigiani F, et al. High frequency of undiagnosed psychiatric disorders in inflammatory bowel diseases. J Clin Med. (2020) 9:1387. doi: 10.3390/jcm9051387
133. Shajib MS, Baranov A, Khan WI. Diverse effects of gut-derived serotonin in intestinal inflammation. ACS Chem Neurosci. (2017) 8:920–31. doi: 10.1021/acschemneuro.6b00414
134. Polat B, Halici Z, Cadirci E, Karakus E, Bayir Y, Albayrak A, et al. Liver 5-HT7 receptors: a novel regulator target of fibrosis and inflammation-induced chronic liver injury in vivo and in vitro. Int Immunopharmacol. (2017) 43:227–35. doi: 10.1016/j.intimp.2016.12.023
135. Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. (2013) 190:4795–804. doi: 10.4049/jimmunol.1201887
136. Wiklund ED, Catts VS, Catts SV, Ng TF, Whitaker NJ, Brown AJ, et al. Cytotoxic effects of antipsychotic drugs implicate cholesterol homeostasis as a novel chemotherapeutic target. Int J Cancer. (2010) 126:28–40. doi: 10.1002/ijc.24813
137. Kast RE. Glioblastoma chemotherapy adjunct via potent serotonin receptor-7 inhibition using currently marketed high-affinity antipsychotic medicines. Br J Pharmacol. (2010) 161:481–7. doi: 10.1111/j.1476-5381.2010.00923.x
138. Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization. J Neurochem. (1997) 68:1372–81. doi: 10.1046/j.1471-4159.1997.68041372.x
139. Heidmann DE, Szot P, Kohen R, Hamblin MW. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. (1998) 37:1621–32. doi: 10.1016/S0028-3908(98)00070-7
140. Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. (2001) 363:620–32. doi: 10.1007/s002100000369
141. Guthrie CR, Murray AT, Franklin AA, Hamblin MW. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J Pharmacol Exp Ther. (2005) 313:1003–10. doi: 10.1124/jpet.104.081919
142. Baker LP, Nielsen MD, Impey S, Metcalf MA, Poser SW, Chan G, et al. Stimulation of type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases by the Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J Biol Chem. (1998) 273:17469–76. doi: 10.1074/jbc.273.28.17469
143. Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, et al. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. (2005) 25:7821–30. doi: 10.1523/JNEUROSCI.1790-05.2005
144. Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem. (2003) 87:1076–85. doi: 10.1046/j.1471-4159.2003.02076.x
145. Speranza L, Chambery A, Di Domenico M, Crispino M, Severino V, Volpicelli F, et al. The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology. (2013) 67:155–67. doi: 10.1016/j.neuropharm.2012.10.026
146. Grimes MT, Powell M, Gutierrez SM, Darby-King A, Harley CW, Mclean JH. Epac activation initiates associative odor preference memories in the rat pup. Learn Mem. (2015) 22:74–82. doi: 10.1101/lm.037101.114
147. Costa L, Sardone LM, Bonaccorso CM, D'antoni S, Spatuzza M, Gulisano W, et al. Activation of serotonin 5-HT7 receptors modulates hippocampal synaptic plasticity by stimulation of adenylate cyclases and rescues learning and behavior in a mouse model of fragile X syndrome. Front Mol Neurosci. (2018) 11:353. doi: 10.3389/fnmol.2018.00353
148. Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol. (2015) 114:2015–22. doi: 10.1152/jn.00374.2015
149. Ponimaskin E, Voyno-Yasenetskaya T, Richter DW, Schachner M, Dityatev A. Morphogenic signaling in neurons via neurotransmitter receptors and small GTPases. Mol Neurobiol. (2007) 35:278–87. doi: 10.1007/s12035-007-0023-0
150. Bijata M, Labus J, Guseva D, Stawarski M, Butzlaff M, Dzwonek J, et al. Synaptic remodeling depends on signaling between serotonin receptors and the extracellular matrix. Cell Rep. (2017) 19:1767–82. doi: 10.1016/j.celrep.2017.05.023
151. Wirth A, Holst K, Ponimaskin E. How serotonin receptors regulate morphogenic signalling in neurons. Prog Neurobiol. (2017) 151:35–56. doi: 10.1016/j.pneurobio.2016.03.007
152. Speranza L, Giuliano T, Volpicelli F, De Stefano ME, Lombardi L, Chambery A, et al. Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci. (2015) 9:62. doi: 10.3389/fnbeh.2015.00062
153. Hübener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. (2014) 159:727–37. doi: 10.1016/j.cell.2014.10.035
154. Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. (2008) 28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008
155. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. (2008) 33:18–41. doi: 10.1038/sj.npp.1301559
156. Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. (2008) 57:819–26. doi: 10.1016/j.neuron.2008.02.031
157. Li J, Yoshikawa A, Meltzer HY. Replication of rs300774, a genetic biomarker near ACP1, associated with suicide attempts in patients with schizophrenia: relation to brain cholesterol biosynthesis. J Psychiatr Res. (2017) 94:54–61. doi: 10.1016/j.jpsychires.2017.06.005
158. Kusuyama J, Bandow K, Ohnishi T, Hisadome M, Shima K, Semba I, et al. Osteopontin inhibits osteoblast responsiveness through the down-regulation of focal adhesion kinase mediated by the induction of low-molecular weight protein tyrosine phosphatase. Mol Biol Cell. (2017) 28:1326–36. doi: 10.1091/mbc.e16-10-0716
159. Lam CR, Tan C, Teo Z, Tay CY, Phua T, Wu YL, et al. Loss of TAK1 increases cell traction force in a ROS-dependent manner to drive epithelial-mesenchymal transition of cancer cells. Cell Death Dis. (2013) 4:e848. doi: 10.1038/cddis.2013.339
160. Renner U, Zeug A, Woehler A, Niebert M, Dityatev A, Dityateva G, et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J Cell Sci. (2012) 125:2486–99. doi: 10.1242/jcs.101337
161. Beaudet G, Jozet-Alves C, Asselot R, Schumann-Bard P, Freret T, Boulouard M, et al. Deletion of the serotonin receptor type 7 disrupts the acquisition of allocentric but not egocentric navigation strategies in mice. Behav Brain Res. (2017) 320:179–85. doi: 10.1016/j.bbr.2016.12.006
162. Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur J Neurosci. (2004) 19:1913–22. doi: 10.1111/j.1460-9568.2004.03288.x
163. Kobe F, Guseva D, Jensen TP, Wirth A, Renner U, Hess D, et al. 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J Neurosci. (2012) 32:2915–30. doi: 10.1523/JNEUROSCI.2765-11.2012
164. Bacon WL, Beck SG. 5-Hydroxytryptamine(7) receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther. (2000) 294:672–9.
165. Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. (2003) 993:230–4. doi: 10.1016/j.brainres.2003.09.015
166. Vasefi MS, Yang K, Li J, Kruk JS, Heikkila JJ, Jackson MF, et al. Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol Brain. (2013) 6:24. doi: 10.1186/1756-6606-6-24
167. Andreetta F, Carboni L, Grafton G, Jeggo R, Whyment AD, Van Den Top M, et al. Hippocampal 5-HT7 receptors signal phosphorylation of the GluA1 subunit to facilitate AMPA receptor mediated-neurotransmission in vitro and in vivo. Br J Pharmacol. (2016) 173:1438–51. doi: 10.1111/bph.13432
168. Costa L, Trovato C, Musumeci SA, Catania MV, Ciranna L. 5-HT(1A) and 5-HT(7) receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus. (2012) 22:790–801. doi: 10.1002/hipo.20940
169. Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. (2012) 22:383–8. doi: 10.1016/j.conb.2011.09.002
170. Chang JY, Parra-Bueno P, Laviv T, Szatmari EM, Lee SR, Yasuda R. CaMKII autophosphorylation is necessary for optimal integration of Ca2+ signals during LTP induction, but not maintenance. Neuron. (2017) 94:800–8. e804. doi: 10.1016/j.neuron.2017.04.041
171. Lai K.-O, Ip NY. Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta. (2013) 1832:2257–63. doi: 10.1016/j.bbadis.2013.08.012
172. Huang M, Panos JJ, Kwon S, Oyamada Y, Rajagopal L, Meltzer HY. Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: role of relative serotonin, (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J Neurochem. (2014) 128:938–49. doi: 10.1111/jnc.12512
173. Ohoyama K, Yamamura S, Hamaguchi T, Nakagawa M, Motomura E, Shiroyama T, et al. Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur J Pharmacol. (2011) 653:47–57. doi: 10.1016/j.ejphar.2010.11.023
174. Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Dopamine D2 and serotonin 5-HT1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology. (2012) 62:765–74. doi: 10.1016/j.neuropharm.2011.08.031
175. Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. (2005) 387:157–61. doi: 10.1016/j.neulet.2005.06.035
176. Bourdelais AJ, Deutch AY. The effects of haloperidol and clozapine on extracellular GABA levels in the prefrontal cortex of the rat: an in vivo microdialysis study. Cereb Cortex. (1994) 4:69–77. doi: 10.1093/cercor/4.1.69
177. Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol. (2012) 165:1543–55. doi: 10.1111/j.1476-5381.2011.01638.x
178. Yamamura S, Ohoyama K, Hamaguchi T, Kashimoto K, Nakagawa M, Kanehara S, et al. Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex. Psychopharmacology. (2009) 206:243–58. doi: 10.1007/s00213-009-1601-9
179. Huang M, He W, Rajagopal L, Kudwa A, Grigoriadis DE, Meltzer HY. Effects of NBI-98782, a selective vesicular monoamine transporter 2 (VMAT2) inhibitor, on neurotransmitter efflux and phencyclidine-induced locomotor activity: relevance to tardive dyskinesia and antipsychotic action. Pharmacol Biochem Behav. (2020) 190:172872. doi: 10.1016/j.pbb.2020.172872
180. Mork A, Witten LM, Arnt J. Effect of sertindole on extracellular dopamine, acetylcholine, and glutamate in the medial prefrontal cortex of conscious rats: a comparison with risperidone and exploration of mechanisms involved. Psychopharmacology. (2009) 206:39–49. doi: 10.1007/s00213-009-1578-4
181. Yamamura S, Ohoyama K, Hamaguchi T, Nakagawa M, Suzuki D, Matsumoto T, et al. Effects of zotepine on extracellular levels of monoamine, GABA and glutamate in rat prefrontal cortex. Br J Pharmacol. (2009) 157:656–65. doi: 10.1111/j.1476-5381.2009.00175.x
182. Wesolowska A, Kowalska M. Influence of serotonin 5-HT(7) receptor blockade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol Rep. (2008) 60:464–74.
183. Kusek M, Sowa J, Kaminska K, Golembiowska K, Tokarski K, Hess G. 5-HT7 receptor modulates GABAergic transmission in the rat dorsal raphe nucleus and controls cortical release of serotonin. Front Cell Neurosci. (2015) 9:324. doi: 10.3389/fncel.2015.00324
184. Huang M, Kwon S, He W, Meltzer HY. Neurochemical arguments for the use of dopamine D4 receptor stimulation to improve cognitive impairment associated with schizophrenia. Pharmacol Biochem Behav. (2017) 157:16–23. doi: 10.1016/j.pbb.2017.04.010
185. Meltzer HY. Serotonergic mechanisms as targets for existing and novel antipsychotics. Handb Exp Pharmacol. (2012) 2012:87–124. doi: 10.1007/978-3-642-25761-2_4
186. Okada M, Fukuyama K, Nakano T, Ueda Y. Pharmacological discrimination of effects of MK801 on thalamocortical, mesothalamic, mesocortical transmissions. Biomolecules. (2019) 9:746. doi: 10.3390/biom9110746
187. Yamamura S, Abe M, Nakagawa M, Ochi S, Ueno S, Okada M. Different actions for acute and chronic administration of mirtazapine on serotonergic transmission associated with raphe nuclei and their innervation cortical regions. Neuropharmacology. (2011) 60:550–60. doi: 10.1016/j.neuropharm.2010.12.025
188. Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry. (2011) 69:1133–9. doi: 10.1016/j.biopsych.2010.08.008
189. Karlsen AS, Korbo S, Uylings HB, Pakkenberg B. A stereological study of the mediodorsal thalamic nucleus in Down syndrome. Neuroscience. (2014) 279:253–9. doi: 10.1016/j.neuroscience.2014.08.046
190. Leeman-Markowski BA, Smart OL, Faught RE, Gross RE, Meador KJ. Cessation of gamma activity in the dorsomedial nucleus associated with loss of consciousness during focal seizures. Epilepsy Behav. (2015) 51:215–20. doi: 10.1016/j.yebeh.2015.07.027
191. Mitchell AS, Browning PG, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci. (2007) 27:11289–95. doi: 10.1523/JNEUROSCI.1914-07.2007
192. Schuetze M, Park MT, Cho IY, Macmaster FP, Chakravarty MM, Bray SL. Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology. (2016) 41:2627–37. doi: 10.1038/npp.2016.64
193. Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. (2015) 54:89–107. doi: 10.1016/j.neubiorev.2015.01.014
194. Mccormick DA, Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol. (1991) 442:235–55. doi: 10.1113/jphysiol.1991.sp018791
195. Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol. (1981) 198:121–36. doi: 10.1002/cne.901980111
196. Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol. (1987) 256:175–210. doi: 10.1002/cne.902560202
197. Sadacca BF, Wikenheiser AM, Schoenbaum G. Toward a theoretical role for tonic norepinephrine in the orbitofrontal cortex in facilitating flexible learning. Neuroscience. (2017) 345:124–9. doi: 10.1016/j.neuroscience.2016.04.017
Keywords: antipsychotics, bipolar disorder (BD), cognition, lurasidone, schizophrenia, 5-HT7, transmission
Citation: Okubo R, Hasegawa T, Fukuyama K, Shiroyama T and Okada M (2021) Current Limitations and Candidate Potential of 5-HT7 Receptor Antagonism in Psychiatric Pharmacotherapy. Front. Psychiatry 12:623684. doi: 10.3389/fpsyt.2021.623684
Received: 30 October 2020; Accepted: 29 January 2021;
Published: 18 February 2021.
Edited by:
Colin T. Dourish, P1vital Limited, United KingdomReviewed by:
James E. Barrett, Drexel University, United StatesLakshmi Rajagopal, Northwestern University, United States
Copyright © 2021 Okubo, Hasegawa, Fukuyama, Shiroyama and Okada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Motohiro Okada, okadamot@clin.medic.mie-u.ac.jp
 Ruri Okubo
Ruri Okubo Motohiro Okada
Motohiro Okada