- 1MOE Key Laboratory of Plant-Soil Interactions, Department of Plant Nutrition, China Agricultural University, Beijing, China
- 2Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
- 3Department of Crop Sciences, Shanxi Agricultural University, Taigu, China
- 4College of Life Sciences, Shanxi Agricultural University, Taigu, China
Foxtail millet [Setaria italica (L.) Beauv.], a widely cultivated food and fodder crop, develops a smaller root system while enlarges the root diameter facilitating nutrient transport under nitrogen limitation. How foxtail millet responds to phosphate limitation (LP) remains unaddressed. LP seedlings of the sequenced variety Yugu1 had significantly lower P concentrations in both shoots and roots and displayed higher levels of anthocyanin accumulation in leaves, indicating that the seedlings suffered from P limitation under hydroponic culture. One obvious and adaptive phenotype of LP plants was the larger root system mostly as the result of stimulation of lateral root proliferation in terms of the number, density, and length. Preferential biomass accumulation in the root under LP ensured carbon provision for root expansion and resulted in significant increases in the total and specific root length, which substantially extended the absorptive surface of P in the growth medium. Elevation of auxin and gibberellin concentrations might serve as an internal boost underpinning root architectural re-patterning under LP. Not just morphological adaptation, up-regulation of expression of SiPHT1;1 and SiPHT1;4 in roots and that of SiPHT1;2 in roots and shoots preconditioned adaptive enhancement of P uptake and translocation under LP. Interestingly, internal nitrogen surpluses occurred as indicated by dramatic increases in free amino acids in LP shoots and roots and higher concentrations of nitrogen in roots. Such nitrogen surplus ‘signals’ tended to switch down expression of nitrate transporters SiNRT2.1 and SiNAR2.1 in the root and that of SiNRT1.11 and SiNRT1.12 in the shoot to reduce nitrate mobilization toward or within the shoot. Together, our work provided new insights into adaption of a critical cereal crop to LP and its innate connection with nitrogen nutrition.
Introduction
For a typical plant, the phosphorus (P) concentration is about 1 μM in the soil, 400 μM in the xylem, and 10,000 μM in the cytoplasm (Fang et al., 2009). P is utilized by plants for numerous functions including photosynthesis, respiration, energy generation, nucleic acid synthesis, glycolysis, redox reactions, membrane synthesis and stability, and nitrogen fixation (Abel et al., 2002; Vance et al., 2003). Despite its importance for plants, phosphate bio-availability in soil solution is very low due to its tendency to bind strongly to soil surfaces or form pH-dependent insoluble complexes with cations (Shen et al., 2011). P deficiency is one of the greatest limitations in agricultural production (Schachtman et al., 1998; Lynch and Brown, 2008). It has been estimated that 5.7 billion hectares of agricultural land is deficient in phosphorus worldwide (Cordell et al., 2009). Continuous provision of P fertilizers is required to sustain high productivity levels (Schachtman et al., 1998). Phosphate rock, the primary source of inorganic phosphorus fertilizers, is minable in only a few areas in the world and likely becomes more costly when approaching toward peak phosphorus demand around 2030 (Cordell et al., 2009).
Plants have evolved a complex array of strictly regulated mechanisms to maintain P homeostasis under P deficient conditions. First, the modification of root architecture is a powerful tool of plants for high P acquisition (Lynch, 1995). Typical morphological responses to P limitation (LP) include a highly branched root system with more and longer root hairs and/or associations of mycorrhizal fungi, which enlarges the total surface area for soil exploration and P acquisition (Raghothama, 1999; Vance et al., 2003; Lambers et al., 2006). Upon LP, white lupin develops densely branched cluster roots (Gardner et al., 1982); Phosphorus uptake 1 (Pup1), a major QTL conferring rice tolerance to P deficiency in the soil, promotes crown root proliferation in rice (Wissuwa et al., 1998, 2002; Gamuyao et al., 2012). In rice and Brassica oleracea, P uptake is correlated with the lateral root number, lateral root length, and root growth rate under LP (Li et al., 2007; Hammond et al., 2009); or plants increase number and/or length of lateral roots and root hairs to enhance P capture (Raghothama, 1999; Lynch, 2011). Second, hormone synthesis, transport, and signaling may be affected by nutrient limitations, which in turn cause morphological, physiological, and molecular alterations in the root (Torrey, 1976; Malamy, 2005; Osmont et al., 2007; Kiba et al., 2010). Auxin plays a fundamental role in regulating root development (Sabatini et al., 1999), and blockage of auxin synthesis or signaling causes severe developmental defects in roots (Went and Thimann, 1937). P limitation alters auxin transport, distribution, or sensitivity to promote lateral root development in Arabidopsis (López-Bucio et al., 2005; Nacry et al., 2005). Gibberellin regulates organ differentiation (Yamaguchi, 2008). Third, phosphate transporters, i.e., PHT1, PHT2, PHT3, and PHT4 families, play vital roles in P acquisition and translocation (Rausch and Bucher, 2002). Among these four transporter families, the PHT1 family is most widely studied and most PHT1 members are specifically or preferentially expressed in root epidermis cells primarily under the regulation of the cellular P concentration (Mudge et al., 2002; Rae et al., 2003).
Foxtail millet [Setaria italica (L.) Beauv.] was domesticated from Setaria viridis in northern China between 8700 and 5900 years ago (Barton et al., 2009). At present, foxtail millet is cultivated in 26 countries and ranks second in world’s millet production (Li and Wu, 1996; Yang et al., 2012). Release of the complete genome facilitates functional and evolutionary investigation of foxtail millet as a model crop species (Li and Brutnell, 2011; Bennetzen et al., 2012; Zhang et al., 2012; Daverdin et al., 2015). Foxtail millet develops a smaller root system under nitrogen limitation, enlarges the root diameter, and up-regulates expression of nitrogen transporters for enhanced nitrogen uptake and translocation (Nadeem et al., 2018). It remained an intriguing question how foxtail millet responds to LP? Our hydroponic experiments provided insights into adaptations of root architecture, hormone and metabolite accumulation, and expression of transporters to LP and their connection with nitrogen nutrition in foxtail millet seedlings.
Materials and Methods
Seeds of foxtail millet (the sequenced variety Yugu1) (Cheng and Dong, 2010) were washed three times with deionized water, sterilized for half an hour with 10% H2O2, imbibed in saturated CaSO4 solution for 5 h, and germinated on moist filter paper. Seedlings with 2-cm roots were wrapped in moist filter paper and placed vertically in the growth holder saturated by deionized water and covered with black plastic until leaf emergence. Uniform seedlings having fully expanded leaves were grown in the greenhouse of China Agricultural University, Beijing, P. R. China (temperature 26/20°C; photoperiod 14/10 h day/night; relative humidity 45–55%). The whole nutrient solution as control (CK) consisted of 2 mM NH4NO3, 0.25 mM KH2PO4, 0.75 mM K2SO4, 0.1 mM KCl, 2 mM CaCl2, 0.65 mM MgSO4, 0.2 mM Fe-EDTA, 1 × 10-3 mM MnSO4, 1 × 10-3 mM ZnSO4, 1 × 10-4 mM CuSO4, 5 × 10-6 mM (NH4)6Mo7O24, 1 × 10-3 mM H3BO3. 25% nutrient solution was applied for the first three days, 50% nutrient solution for next four days and 100% nutrient solution for 1 week. Seedlings were subjected to LP during the third week of millet growth. To make the LP medium, 0.25 mM KH2PO4 was reduced to 1% CK, while 0.1 mM KCl was used to replenish potassium concentration in the LP nutrient solution while other nutrients remained unchanged. The pH was maintained at 6.0. Every 3.4-L continuously aerated pot containing four seedlings represented one biological replicate. Each treatment had six biological replicates and the nutrient solution was changed every 2 days.
SPAD values were measured before harvest with a Chlorophyll Meter (SPAD-502, Konica Minolta Sensing Inc., Japan). The 4th leaf (the youngest fully expanded leaf) of all four plants in each pot was analyzed three times with the SPAD meter. Average of three SPAD values from one leaf represented one read and average of four reads from one pot was taken as one biological replicate. Root and shoot samples were harvested on the 21st day after transfer to the nutrient solution. Samples were carefully washed three times with deionized water, gently wiped with blot paper, immediately frozen in liquid N2, and stored at -80°C for physiological measurements. Samples were harvested and washed three times, oven-dried at 105°C for 30 min, then dried at 70°C until constant weight for dry weight (DW) and other related analysis.
Analysis of P and N Concentrations and the C/N Ratio
Oven-dried shoot and root tissues were ground into fine powder and digested with H2SO4-H2O2 followed by P analysis using spectrophotometer at 440 nm by the modified vanadomolybdate method (Johnson and Ulrich, 1959), and total N analysis using a modified Kjeldahl digestion method (Baker and Thompson, 1992). The C/N ratio was analyzed by loading ∼50 mg fine shoot or root powder into the Elementar vario Macro CN analyzer (Elementar Technologies, Hanau, Germany).
Biochemical Analysis
Fresh leaves were weighed and ground in liquid nitrogen, dissolved in the anthocyanin isolation solution (methanol: concentrated hydrochloric acid, 99:1v/v). Spectrophotometer was used to determine OD530 and OD657 values (Rabino and Mancinelli, 1986). The concentration of soluble sugars was determined using a commercially available kit (Boehringer Mannheim, Germany). The concentration of total free amino acids was measured according to the Rosen ninhydrin colorimetric method by using leucine as a standard (Rosen, 1957). A standard kit (Coomassie Protein assay reagent; Bio-Rad, Hercules, CA, United States) was used as a reference to extract and analyze soluble proteins, with bovine serum albumin as the reference.
Root Architecture
The whole root was well spread and scanned with a scanner (Epson 1680, Indonesia). The scanned images were analyzed using the WinRHIZO software (version 5.0) (Regent Instruments Inc., Quebec City, QC, Canada) to get the total root length following the previously described method (Peng et al., 2010). Each type of root was counted manually. The crown root length was measured with a ruler. The lateral root density was defined as the number of lateral roots per unit of the crown root length.
Hormone Extraction and Quantification by Enzyme Linked Immunosorbent Assay (ELISA)
Fresh shoot and root samples (0.5 g) were homogenized individually in 2 mL of 80% methanol (containing 40 mg butylated hydroxytoluene as antioxidant). This mixture was incubated at 4°C for 48 h, and then centrifuged at 1900 ×g at 4°C for 15 min. The supernatant was passed through C18 Sep-Pak cartridges (Waters Corp), and the hormone fraction was eluted with 10 mL of 100% (v/v) methanol and then 10 mL ether. The elute was N2-dried at 20°C. The N2-dried extracts were dissolved in 2.0 mL phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween-20 and 0.1% (w/v) gelatin (pH 7.5) to analyze the concentration of free indole-3-acetic acid (IAA), zeatin riboside (ZR), gibberellin (GA3), and abscisic acid (ABA) by ELISA following a well-established protocol (Weiler et al., 1981).
RNA Isolation and Quantitative Real-Time PCR
Total RNA was extracted from root and shoot samples by using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). RNA samples (4–5 g) were digested by DNase I (Takara Biomedicals, Kyoto, Japan) to remove any potential DNA contamination, then reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen). Gene expression levels were determined by quantitative real-time PCR in a Bio-Rad iCycler iQ5 system (Bio-Rad, Hercules, CA, United States) using the SYBR Premix Ex TaqTM (Takara) and gene specific primers (Supplementary Table S1). The program was 10 min pre-incubation at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The relative gene expression levels were calculated following the standard comparative method (Livak and Schmittgen, 2001). Each treatment had three biological replicates, with three technical replicates for every biological replicate.
Statistical Analysis
Data were analyzed using the one-way ANOVA in Statistix 8.1 (Analytical Software, 2005). Means of different treatments were compared using the least significant difference at a 0.05 level of probability.
Results
Physiological Responses of Foxtail Millet to LP
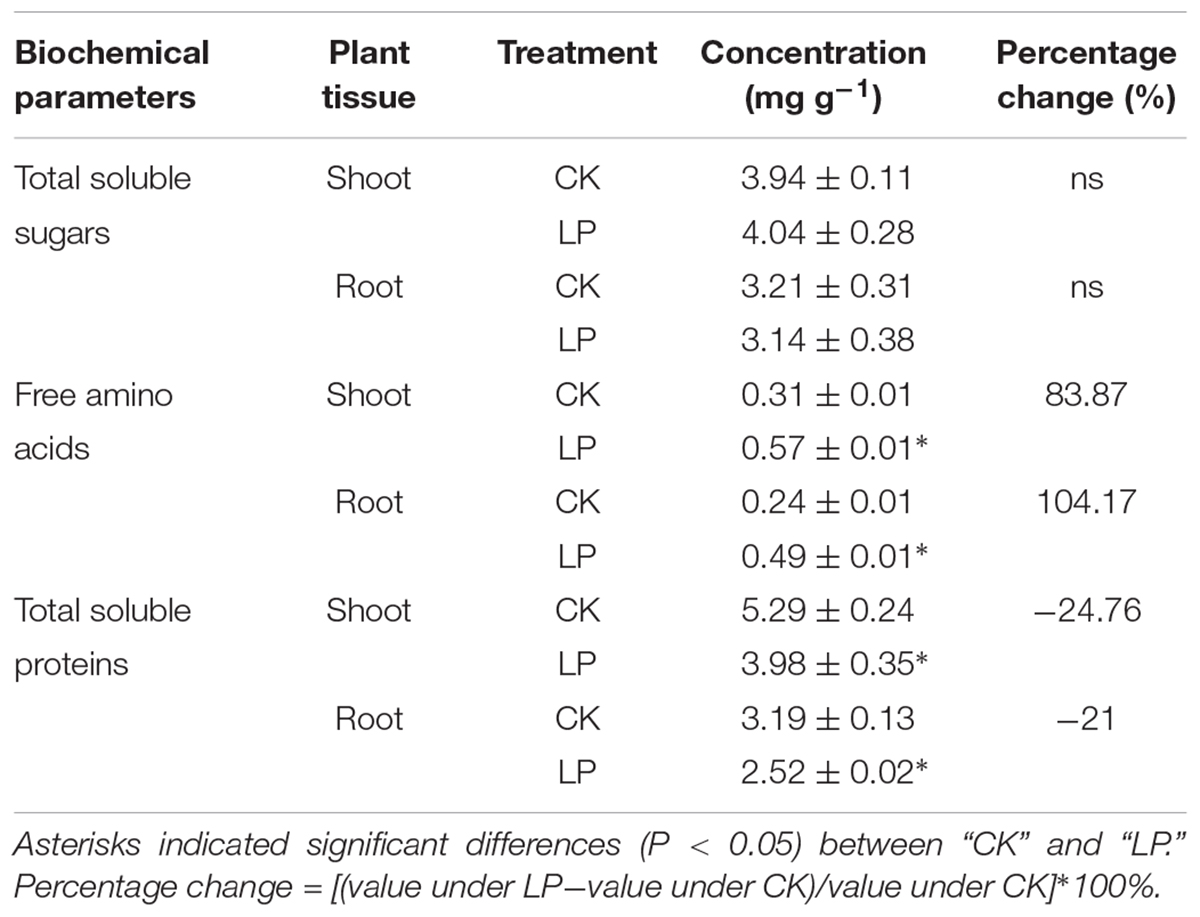
Seedlings of foxtail millet were exposed to P limitation (LP) condition for 1 week. Compared to control (CK) plants, LP plants had a relatively larger root system (Figure 1A). P deficiency caused a 37.5% increase in the root dry weight while significantly reduced biomass accumulation in the shoot as compared to the control. As a result, the root/shoot ratio increased by 77.78% (Figures 1B,C). LP reduced P accumulation in the shoot and root, with a larger decrease in the P concentration in the root than in the shoot (Figures 1D,E). We defined P utilization efficiency (PUtE) as cumulative biomass per unit of P (g DW g-1) and found 30.46% higher PUtE in the shoot and 63.27% higher in the root under LP (Figure 1F).
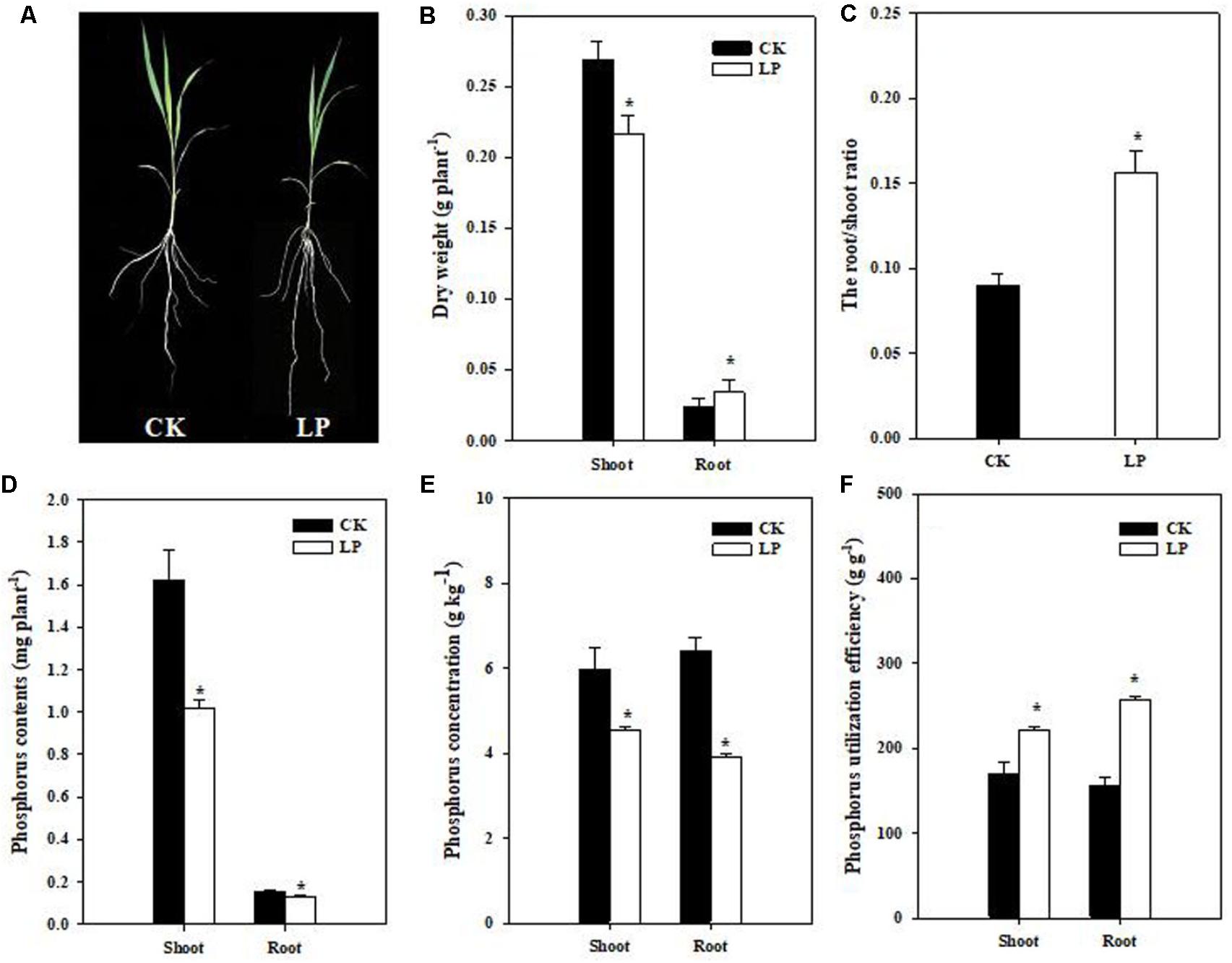
FIGURE 1. Shoot and root responses of foxtail millet [Setaria italica (L.) Beauv.] to LP. Plant growth with intact root and shoot (A), shoot and root dry weight (g plant-1) (B), the root/shoot ratio (C), P accumulation (D), phosphorus concentration (E), P utilization efficiency (F). Each bar represented SE of six biological replicates. ∗indicated significant difference between treatments (P < 0.05).
Visible accumulation of anthocyanin pigmentation is a characteristic response of plants to P starvation. We observed enhanced accumulation of anthocyanin (1.35 μg g-1) in leaves of LP-seedlings (Figure 2A). Interestingly, P deficiency caused significant decreases in the SPAD value of the fourth leaf at harvest (Figure 2B) in contrast to a significant increase in the nitrogen concentration in the root (Figure 2C) and a consequent decrease in the C/N ratio of the LP root (Figure 2D). In spite of lower SPAD values under LP, there was no significant difference in the concentration of soluble sugars in plants under LP and control conditions (Table 1). On the other hand, LP resulted in increases in the concentration of free amino acids by 83.87% in the shoot and 104.17% in the root (Table 1) and decreases in accumulation of soluble protein in the root (21%) and shoot (24.76%) (Table 1).
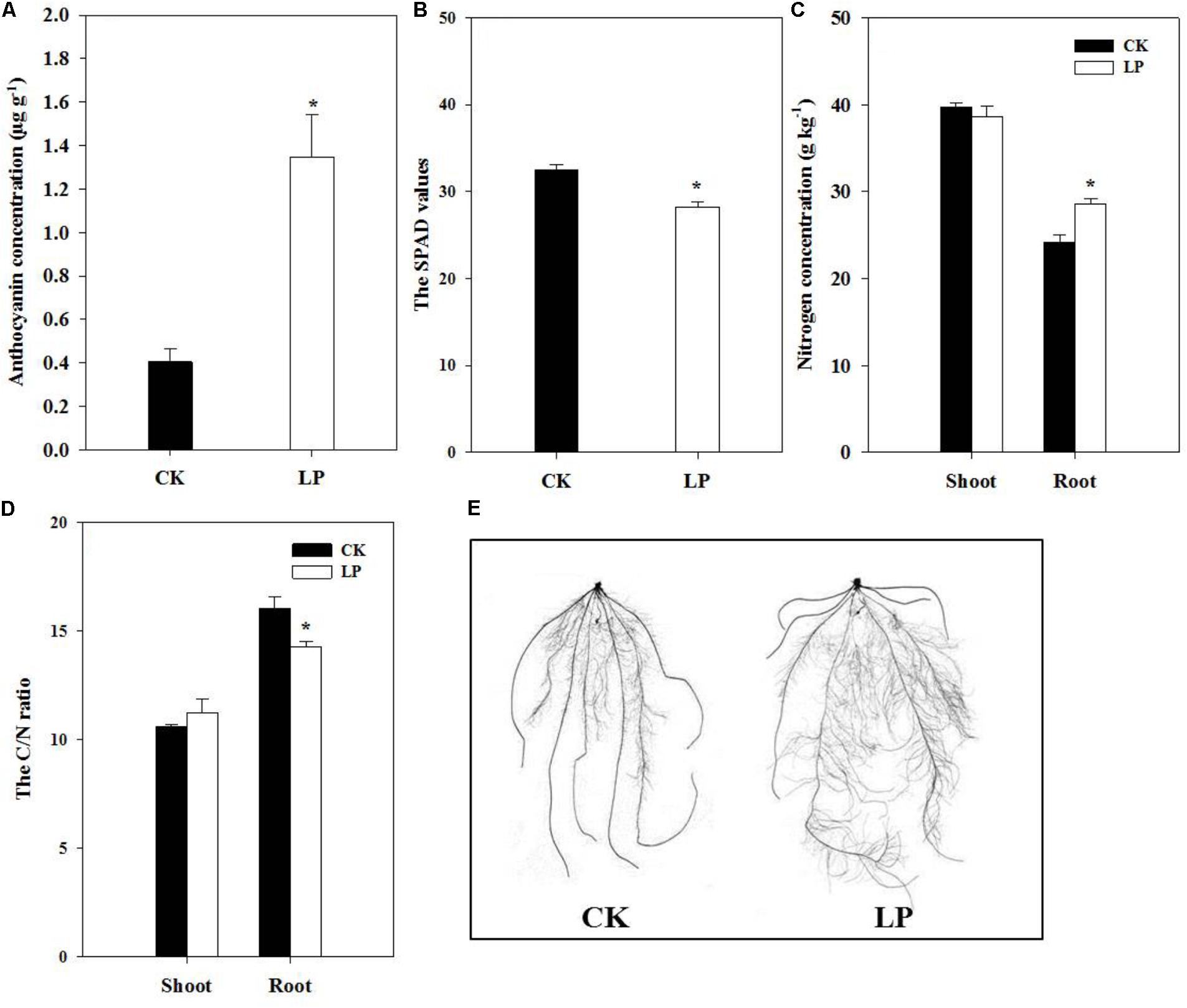
FIGURE 2. Biochemical and morphological responses of foxtail millet to LP. The anthocyanin accumulation in leaves (A), the SPAD value (B), nitrogen concentration in the shoot and root (C), the C/N ratio (D), scanned root systems (E). Each bar represented SE of six biological replicates. ∗indicated significant difference between treatments (P < 0.05).
Root Architectural Alterations Under LP
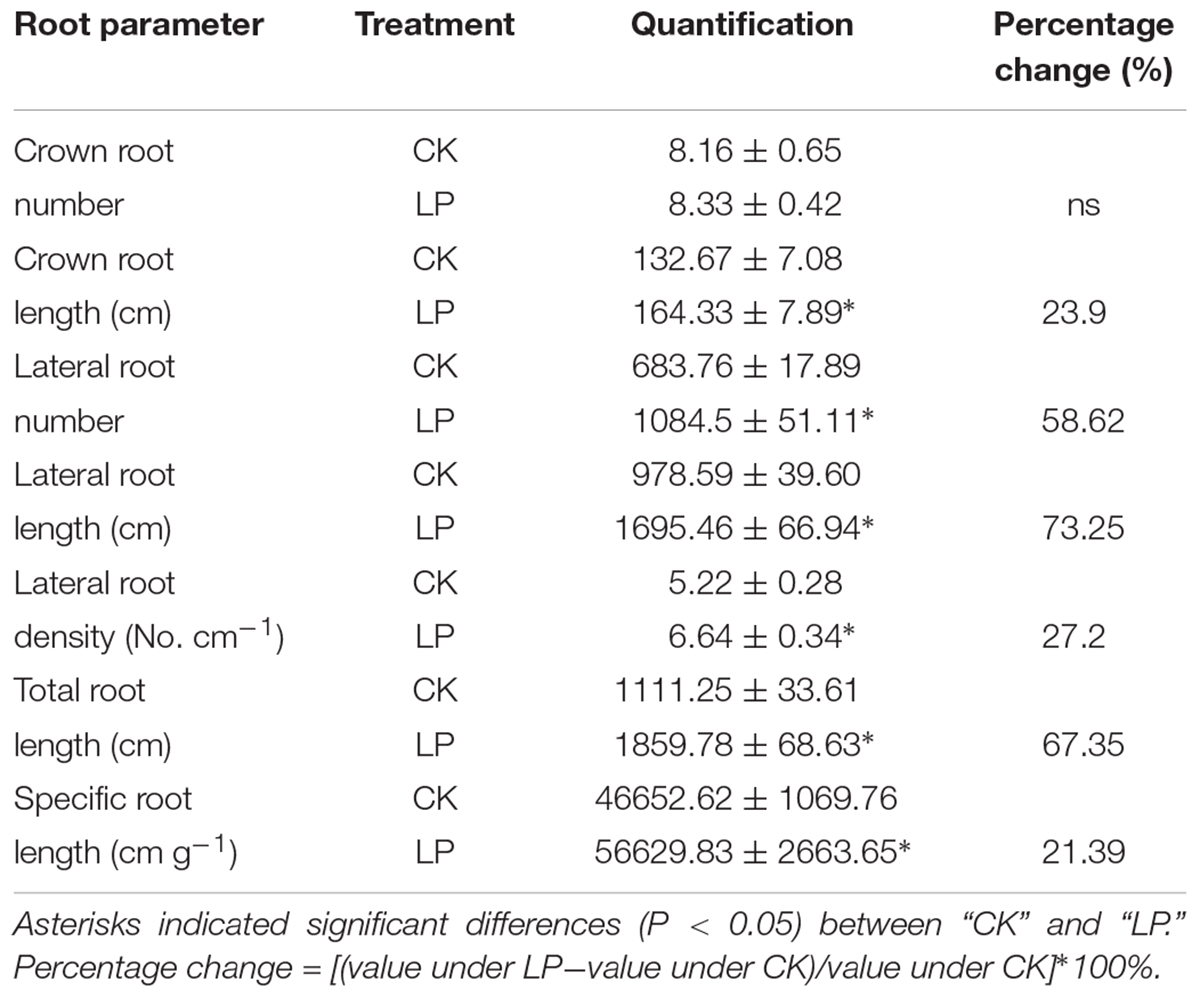
The plasticity of root architecture is a developmental advantage of plants in response to environmental stresses. Plants may undergo dramatic root morphological alterations to enhance P foraging capacity. To quantify changes in root architecture due to P deficiency, we scanned the root system of foxtail millet at harvest (Figure 2E). LP caused no difference in the number of crown roots but increased the number of lateral roots by 58.62%, crown root length by 23.9%, lateral root length by 73.25%, lateral root density (the ratio of the lateral root number to the crown root length) by 27.2%, total root length by 67.35%, and specific root length (the ratio of the total root length to root biomass) by 21.39% compared to the control (Table 2), indicating an overall stimulatory effect of LP on lateral root development and longitudinal growth of the entire root system.
Changes in Hormone Accumulation Under LP
Hormones are crucial regulators of plant growth and development under frequently changing environmental conditions (Marsch-Martinez and de Folter, 2016). Our results showed distinct effects of LP on hormone accumulation in the root and shoot (Figure 3). LP led to increases in the concentration of auxin and gibberellin (GA3) in the root (Figures 3A–C). P withdrawal from the nutrient solution did not change IAA and GA3 concentrations in the shoot as well as the zeatin riboside concentration in the shoot and root (Figures 3A–C). The ABA concentration increased by 88.3% in the root while decreased by 64% in the shoot under P deficient conditions (Figure 3D).
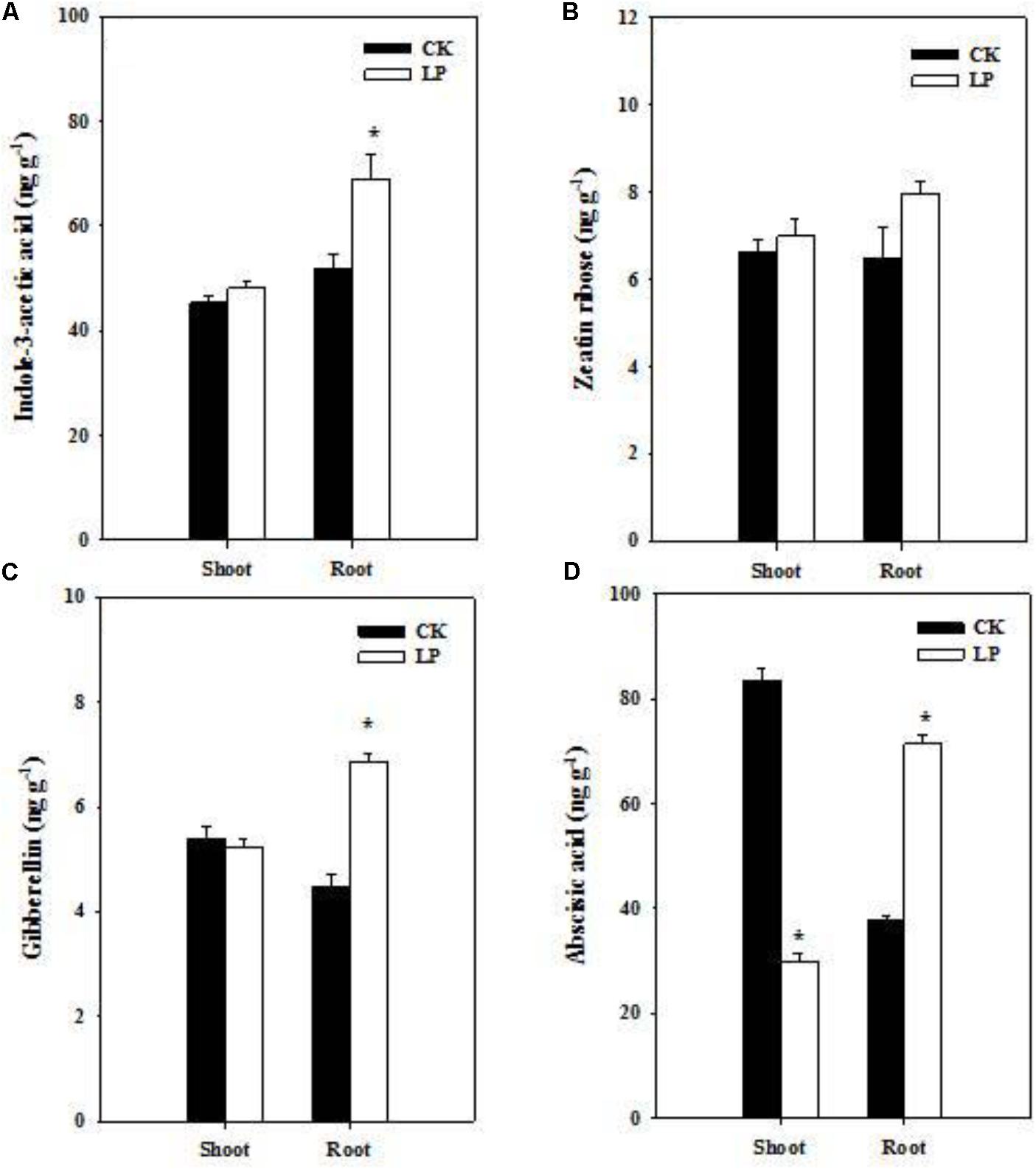
FIGURE 3. Hormonal responses of foxtail millet to LP. Indole-3-acetic acid (A), zeatin ribose (B), gibberellin (C), abscisic acid (D) in the shoot and root. Each bar represented SE of six biological replicates. ∗indicated significant differences between treatments (P < 0.05).
Expression Analysis of Phosphate and Nitrogen Transporters by Quantitative Real Time RT-PCR
Enhanced nutrient uptake or translocation is another crucial mechanism for plants to encounter nutrient limitation in addition to root morphological adaption. Phosphate transporters mediate P uptake from the growth medium and translocation within the plant and expression of a series of phosphate transporters is up-regulated under P limitation (Leggewie et al., 1997). We identified a subset of phosphate transporters in foxtail millet (gene IDs were listed in Supplementary Table S1) and analyzed their expression levels in the root and shoot by quantitative real time PCR. SiPHT1;1 expression was up-regulated in roots (Figure 4A). SiPHT1;2 showed a dramatic upregulation of expression in shoots (>20 fold) and roots (>8 fold) (Figure 4B) while SiPHT1;3 expression was down-regulated in shoots (Figure 4C), whereas, SiPHT1;4 expression was also up-regulated in roots (∼7 fold) (Figure 4D). No change was observed in expression patterns of SiPHT1;8 and SiPHT1;12 under LP (Figures 4E,F).
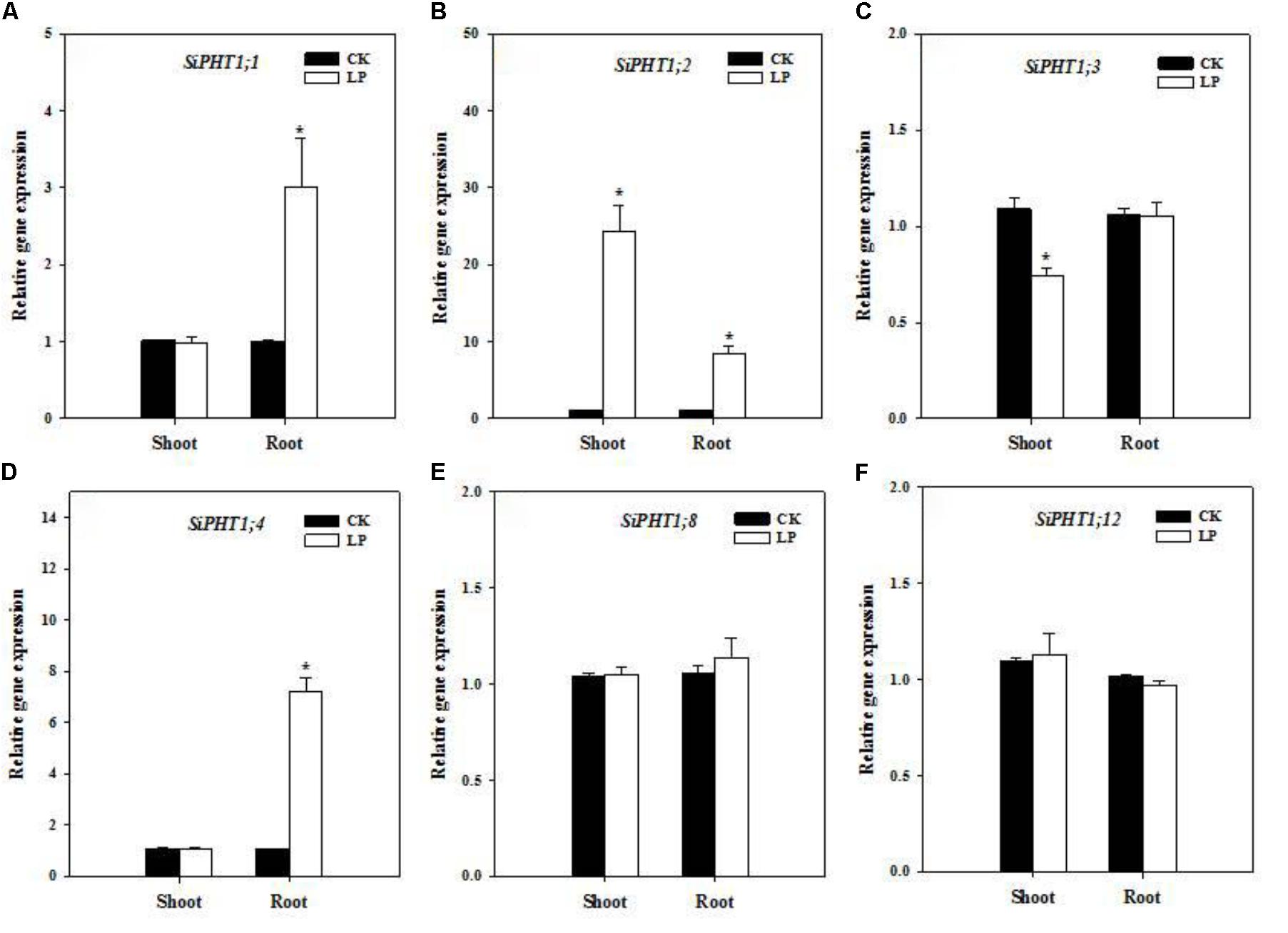
FIGURE 4. Transcript abundance of phosphate transporters. Relative expression of SiPHT1;1 (A), SiPHT1;2 (B), SiPHT1;3 (C), SiPHT1;4 (D), SiPHT1;8 (E), SiPHT1;12 (F) in the shoot and root under CK and LP. Each bar represented SE of three biological replicates. ∗indicated significant differences between treatments (P < 0.05).
One interesting question is whether over-accumulation of amino acids and a large root system under LP affect expression of nitrogen transporters involved in nitrogen uptake and allocation? To this respect, we analyzed transcript abundance of nitrogen transporters involved in nitrogen uptake, transport or remobilization (gene IDs were listed in Supplementary Table S1; Nadeem et al., 2018) under control and LP conditions. The expression level of SiAMT1.1 and SiAMT1.3 did not change in roots (Figures 5A,B). SiNRT1.1 expression was dramatically up-regulated in the root grown under LP compared to the control (Figure 5C). Transcript abundance of SiNRT2.1 and SiNAR2.1 in the roots and that of SiNRT1.11 and SiNRT1.12 in shoots was significantly reduced under LP (Figures 5D–G).
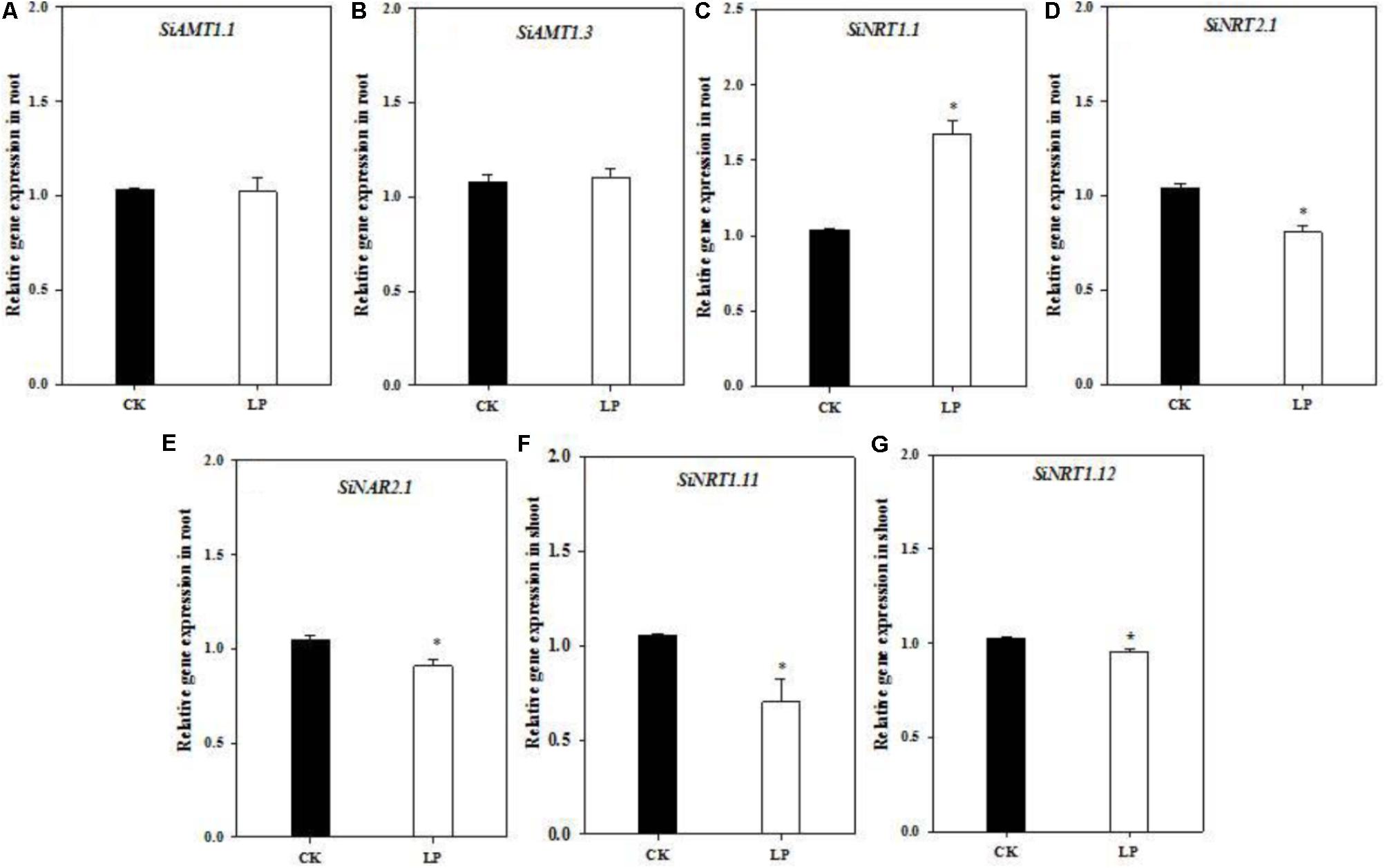
FIGURE 5. Quantitative real-time PCR analysis of nitrogen transporters. Relative expression of SiAMT1.1 (A), SiAMT1.3 (B), SiNRT1.1 (C), SiNRT2.1 (D), SiNAR2.1 (E), in the root and SiNRT1.11 (F), SiNRT1.12 (G) in the shoot under CK and LP. Each bar represented SE of three biological replicates. ∗indicated significant differences between treatments (P < 0.05).
Discussion
Phosphate is easily fixed in the soil across different eco-zones worldwide, which makes P a low bio-available macronutrient (Hinsinger, 2001; Shen et al., 2011). To promote P acquisition, plants reshape root architecture to favor P capture, modulate transporter systems to facilitate P influx and remobilization, release carboxylates to activate fixed phosphate, and/or allow colonization of mycorrhizal fungi to enhance P forage (Jakobsen et al., 1992; Bates and Lynch, 1996; Jeschke et al., 1997; Hinsinger, 2001; Williamson et al., 2001; Vance et al., 2003; Ticconi and Abel, 2004; Bucher, 2007). Foxtail millet is a large-root crop and responds to nitrogen limitation by reducing its total root length and increasing the root diameter presumably for higher transport efficiencies (Nadeem et al., 2018). How foxtail millet physiologically responds to LP becomes an interesting question to be addressed.
P Limitation Stimulated Root Growth in Foxtail Millet
The LP treatment significantly reduced P accumulation in the shoot and root, and the shoot displayed discernible LP symptoms (higher levels of anthocyanins; Figure 2A), indicating that seedlings suffered from P limitation. One obvious and adaptive phenotype of LP plants was the larger root system (Figure 2E and Table 2). Root architecture generally refers to the shape and structure of a root system programmed by intrinsic developmental regulators and external environmental cues in a well coordinative manner (Hodge et al., 2009; Wei and Li, 2016). P uptake by plants heavily depends on the total length and surface area of the root in a given soil volume (Richardson et al., 2009; Balemi and Negisho, 2012; Lambers et al., 2015). In contrast to overall inhibitory effects of N limitation on root growth (Nadeem et al., 2018), LP promoted lateral root proliferation in terms of number, density, and length in foxtail millet (Table 2) in contr. Such multilevel stimulation of lateral root growth generated a highly branched root structure, and more importantly enlarged the absorptive surface area (Bates and Lynch, 1996; Williamson et al., 2001; López-Bucio et al., 2002; Pérez -Torres et al., 2008; Péret et al., 2011). The length of crown roots increased under LP although their number remained a similar level to the control (Table 2). As a result, the total root length increased by 67.35% (Table 2), which substantially improved capabilities of P capture in the growth medium at the morphological level. Supporting root growth, preferential biomass accumulation in the root occurred under LP at the expense of shoot growth (Figures 1B,C), which ensured carbon provision for further root expansion and resulted in an ultimate significant increase in the specific root length (Table 2). On the other hand, root patterning is orchestrated by internal regulators in the LP-triggered signaling cascade. Auxin and gibberellin signaling plays critical roles in root genesis and patterning (Went and Thimann, 1937; Sabatini et al., 1999; Yamaguchi, 2008). P availability-dependent hormone functioning in crop growth and development has been frequently documented (Rubio et al., 2009); however, many observations are not so conclusive at physiological levels (Borch et al., 1999; Kumar et al., 2014). Notably, concentrations of auxin and gibberellin increased in roots rather than in shoots (Figures 3A,C). Over-accumulation of these two hormones in roots boosted lateral root proliferation and elongation of the whole root system and gave rise to many other profound biological consequences to be further investigated. ABA could function as a promoter in lateral root development under nutrient limitation (De Smet et al., 2003). Higher levels of ABA under LP might have a synergistically stimulatory role in root development (Figure 3D) in addition to its primary role in the stress response.
P Limitation Caused Up-Regulation of Expression of Phosphate Transporters and P Utilization Efficiency
Root morphological adaptation was a primary strategy of foxtail millet to cope with P limitation (Figure 2E and Table 2), by which the root grew substantially larger in terms of the absorptive surface area. Ultimate physiological outcomes of root enlargement heavily relied on the functionality of phosphate transporters in the root and shoot. Expression of many PHT1 members is stimulated by P limitation (Mudge et al., 2002; Rae et al., 2003). OsPHT1;2 expression is strongly induced in roots (especially the stele and lateral roots) by LP presumably to enhance P uptake through the root and translocation to the shoot although OsPHT1;1 expression is independent of P levels (Shrawat and Lörz, 2006; Ye et al., 2015). OsPHT1;4, primarily expressed in the root and embryo, shows up-regulation in the shoot under P deficient conditions (Ye et al., 2015; Zhang et al., 2015). Phosphate transporters are broadly conserved across cereal crops (Rakshit and Ganapathy, 2014). According to phylogenetic analysis, SiPHT1;2 is homologous to high affinity transporter OsPHT1;8 that is involved in source-to-sink P mobilization (Jia et al., 2011; Ceasar et al., 2014), with SiPHT1;3 homologous to OsPHT1;4 and OsPHT1;5 and SiPHT1;4 homologous to OsPHT1;1 and OsPHT1;2 (Ceasar et al., 2014). Our results showed significant up-regulation of expression of SiPHT1;1 and SiPHT1;4 in root tissues (Figures 4A,D), presumably preconditioning enhanced P uptake from the root.
Interestingly, SiPHT1;3 expression was down-regulated in the shoot under LP (Figure 4C). Down-regulation of SiPHT1;3 expression probably favored P retention in the shoot or allocation toward nascent leaves. Although LP-plants had larger root systems and higher levels of expression of P transporters, phosphate in the nutrient solution was so limited that stressed seedlings inevitably took advantage of the internal P reserve, which resulted in decreases in total P accumulation and P concentration, and higher PUtE (Figures 1D–F), in agreement with previous studies with different crops (Barker and Pilbeam, 2007; Rose et al., 2011).
P Limitation Altered Accumulation of Nitrogen and Nitrogen Metabolites and Expression of Nitrogen Transporters
Beyond root morphological alterations and differential expression of phosphate transporters, LP plants had higher nitrogen concentrations and lower C/N ratios in the root (Figures 2C,D), indicating that P limitation caused elevation of nitrogen accumulation in the root. Further, the concentration of free amino acids approximately doubled in the shoot and root of LP-seedlings probably due to enhanced protein degradation, as indicated by decreases in concentrations of soluble proteins in the shoot and root (Table 1). Our results were consistent with previous reports on increases in accumulation of amino acids under P deficiency (Rufty et al., 1990, 1993; Huang et al., 2008). Indeed, protein degradation is stimulated while photosynthesis and protein synthesis are down-regulated at the transcription level in P deficient Arabidopsis (Wu et al., 2003; Misson et al., 2005). In this context, lower levels of chlorophylls (indicated by SPAD values) in LP-leaves (Figure 2B) contributed to reduction of overall photosynthesis, thus biomass accumulation in the shoot decreased (Brahim et al., 1996).
The crucial function of a root system is to absorb nutrients from the growth environment for photosynthesis, growth and development, and biomass accumulation, which requires a large quantity of nitrogen influx and subsequent metabolism and accumulation (Miller et al., 2007, 2009; Tsay et al., 2007; Forde and Walch-Liu, 2009; Gojon et al., 2009; Krouk et al., 2010). However, nitrate uptake in the root is regulated by shoot-derived HY5 and CLE peptides (Jonassen et al., 2008; Tabata et al., 2014; Chen et al., 2016). Likewise, nitrogen capture of LP-seedlings was also modulated by shoot-derived signals. Nitrogen surplus ‘signals’ putatively generated by higher concentrations of free amino acids in the shoot tended to down-regulate nitrogen uptake. Nitrate uptake mediated by a sophisticated sensing and transport system is a critical step in nitrogen acquisition under frequently changing external environments (Dechorgnat et al., 2011; Wang et al., 2012). Among nitrate transporters, NRT1.1 functions as a nitrate sensor and dual-affinity nitrate transporter (Liu et al., 1999; Krouk et al., 2006; Ho et al., 2009). NRT2.1 serves as a high-affinity nitrate transporter whereas NAR2.1 is an assessory protein closely related to NRT2.1 functioning (Okamoto et al., 2006; Orsel et al., 2006; Tsay et al., 2007; Miller et al., 2009). As expected, expression of both SiNRT2.1 and SiNAR2.1 was significantly down-regulated in the root of LP-plants (Figures 5D,E). Alternatively, P and nitrogen signals are integrated by nitrate inducible GARP-type transcriptional repressor 1 (NIGT1) in Arabidopsis, and PHR1 promotes expression of NIGT1-clade genes under LP which in turn down-regulate NRT2.1 expression (Maeda et al., 2018). Further, the expression of SiNRT1.11 and SiNRT1.12 was also down-regulated in the shoot of LP-plants (Figures 5F,G), likely reducing xylem-to-phloem transfer and within-shoot remobilization of nitrate (Hsu and Tsay, 2013). As a result, more nitrogen retained in the LP-root (Figure 2C). On the other hand, a larger root under LP somehow favored up-regulation of expression of SiNRT1.1 (probably as a low-affinity transporter in this context) in the root (Figure 5C).
Conclusion
Under P deficiency, a larger root developed primarily via stimulation of lateral root proliferation in terms of the number, density, and length in foxtail millet [Setaria italica (L.) Beauv.]. Preferential carbon provision and elevation of auxin and GA3 accumulation in the LP-root might serve as an internal boost for root morphological enlargement. Up-regulation of expression of SiPHT1;1, SiPHT1;2, and SiPHT1;4 favored the functionality of the LP-root for P uptake and translocation. Interestingly, LP enhanced accumulation of amino acids and caused down-regulation of expression of nitrate transporters SiNRT2.1, SiNAR2.1, SiNRT1.11, and SiNRT1.12, indicating complicated connections between P and nitrogen nutrition. Exploration of underlying molecular mechanisms of P and nitrogen interaction holds the promise for breeding dual-nutrient efficient crop varieties in the future.
Author Contributions
XL, ZA, and XD designed the research. ZA, FN, and RW performed the research. XL, ZA, YH, and XW analyzed the data. ZA and XL wrote the paper. FN, XD, YH, and XW revised the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the NSFC grant (31471928 and 31772385).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01367/full#supplementary-material
References
Abel, S., Ticconi, C. A., and Delatorre, C. A. (2002). Phosphate sensing in higher plants. Physiol. Plant. 115, 1–8. doi: 10.1034/j.1399-3054.2002.1150101.x
Baker, W., and Thompson, T. (1992). “Determination of total nitrogen in plant samples by Kjeldahl,” in Plant Analysis Reference Procedures for the Southern Region of the United States, Vol. 368, ed. C. O. Plank (Athens: The Georgia Agricultural Experiment), 13–16.
Balemi, T., and Negisho, K. (2012). Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J. Soil Sci. Plant Nutr. 12, 547–562. doi: 10.4067/S0718-95162012005000015
Barton, L., Newsome, S. D., Chen, F. H., Wang, H., Guilderson, T. P., and Bettinger, R. L. (2009). Agricultural origins and the isotopic identity of domestication in northern China. Proc. Natl. Acad. Sci. U.S.A. 106, 5523–5528. doi: 10.1073/pnas.0809960106
Bates, T. R., and Lynch, J. P. (1996). Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 19, 529–538. doi: 10.1111/j.1365-3040.1996.tb00386.x
Bennetzen, J. L., Schmutz, J., Wang, H., Percifield, R., Hawkins, J., Pontaroli, A. C., et al. (2012). Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30, 555–561. doi: 10.1038/nbt.2196
Borch, K., Bouma, T. J., Lynch, J. P., and Brown, K. M. (1999). Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 22, 425–431. doi: 10.1046/j.1365-3040.1999.00405.x
Brahim, M. B., Loustau, D., Gaudillère, J. P., and Saur, E. (1996). Effects of phosphate deficiency on photosynthesis and accumulation of starch and soluble sugars in one-year-old seedlings of maritime pine (Pinus pinaster). Ann. Sci. For. 53, 801–810. doi: 10.1051/forest:19960401
Bucher, M. (2007). Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 173, 11–26. doi: 10.1111/j.1469-8137.2006.01935.x
Ceasar, S. A., Hodge, A., Baker, A., and Baldwin, S. A. (2014). Phosphate concentration and arbuscular mycorrhizal colonisation influence the growth, yield and expression of twelve PHT1 family phosphate transporters in foxtail millet (Setaria italica). PLoS One 9:e108459. doi: 10.1371/journal.pone.0108459
Chen, X., Yao, Q., Gao, X., Jiang, C., Harberd, N. P., and Fu, X. (2016). Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 26, 640–646. doi: 10.1016/j.cub.2015.12.066
Cheng, R., and Dong, Z. (2010). “Breeding and production of foxtail millet in China,” in Cereals in China, eds Z. H. He and A. P. A. Bonjean (Chile: Limagrain and CIMMYT).
Cordell, D., Drangert, J. O., and White, S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Change 19, 292–305. doi: 10.1016/j.gloenvcha.2008.10.009
Daverdin, G., Bahri, B. A., Wu, X., Serba, D. D., Tobias, C., Saha, M. C., et al. (2015). Comparative relationships and chromosome evolution in switchgrass (Panicum virgatum) and its genomic model, foxtail millet (Setaria italica). Bioenergy Res. 8, 137–151. doi: 10.1371/journal.pone.0064594
De Smet, I., Signora, L., Beeckman, T., Inze, D., Foyer, C. H., and Zhang, H. M. (2003). An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 33, 543–555. doi: 10.1046/j.1365-313X.2003.01652.x
Dechorgnat, J., Nguyen, C. T., Armengaud, P., Jossier, M., Diatloff, E., Filleur, S., et al. (2011). From the soil to the seeds: the long journey of nitrate in plants. J. Exp. Bot. 62, 1349–1359. doi: 10.1093/jxb/erq409
Fang, Z., Shao, C., Meng, Y., Wu, P., and Chen, M. (2009). Phosphate signaling in Arabidopsis and Oryza sativa. Plant Sci. 176, 170–180. doi: 10.1016/j.plantsci.2008.09.007
Forde, B. G., and Walch-Liu, P. (2009). Nitrate and glutamate as environmental cues for behavioral responses in plant roots. Plant Cell Environ. 32, 682–693. doi: 10.1111/j.1365-3040.2008.01927.x
Gamuyao, R., Chin, J. H., Pariasca-Tanaka, J., Pesaresi, P., Catausan, S., Dalid, C., et al. (2012). The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488, 535–539. doi: 10.1038/nature11346
Gardner, W. K., Parbery, D. G., and Barber, D. A. (1982). The acquisition of phosphorus by Lupinus albus L. I. Some characteristics of the soil/root interface. Plant Soil 68, 19–32. doi: 10.1007/BF02374724
Gojon, A., Nacry, P., and Davidian, J. C. (2009). Root uptake regulation: a central process for NPS homeostasis in plants. Curr. Opin. Plant Biol. 12, 328–338. doi: 10.1016/j.pbi.2009.04.015
Hammond, J. P., Broadley, M. R., White, P. J., King, G. J., Bowen, H. C., Hayden, R., et al. (2009). Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 60, 1953–1968. doi: 10.1093/jxb/erp083
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi: 10.1023/A:1013351617532
Ho, C. H., Lin, S. H., Hu, H. C., and Tsay, Y. F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. doi: 10.1016/j.cell.2009.07.004
Hodge, A., Berta, G., Doussan, C., Merchan, F., and Crespi, M. (2009). Plant root growth, architecture and function. Plant Soil 321, 153–187. doi: 10.1007/s11104-009-9929-9
Hsu, P. K., and Tsay, Y. F. (2013). Two phloem nitrate transporters, NRT1.11 and NRT1.12 are important for redistributing xylem born nitrate to enhance plant growth. Plant Physiol. 163, 844–856. doi: 10.1104/pp.113.226563
Huang, C. Y., Roessner, U., Eickmeier, I., Genc, Y., Callahan, D. L., Shirley, N., et al. (2008). Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 49, 691–703. doi: 10.1093/pcp/pcn044
Jakobsen, I., Abbott, L. K., and Robson, A. D. (1992). External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium-subterraneum L.1. Spread of hyphae and phosphorus in flow into roots. New Phytol. 120, 371–380. doi: 10.1111/j.1469-8137.1992.tb01077.x
Jeschke, W. D., Kirkby, E. A., Peuke, A. D., Pate, J. S., and Hartung, W. (1997). Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). J. Exp. Bot. 48, 75–91. doi: 10.1093/jxb/48.1.75
Jia, H., Ren, H., Gu, M., Zhao, J., Sun, S., Zhang, X., et al. (2011). The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiol. 156, 1164–1175. doi: 10.1104/pp.111.175240
Johnson, C. M., and Ulrich, A. (1959). Analytical Methods for Use in Plant Analysis. Berkeley, CA: University of California, Agricultural Experiment Station.
Jonassen, E. M., Lea, U. S., and Lillo, C. (2008). HY5 and HYH are positive regulators of nitrate reductase in seedlings and rosette stage plants. Planta 227, 559–564. doi: 10.1007/s00425-007-0638-4
Kiba, T., Kudo, T., Kojima, M., and Sakakibara, H. (2010). Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 62, 1399–1409. doi: 10.1093/jxb/erq410
Krouk, G., Crawford, N. M., Coruzzi, G. M., and Tsay, Y. F. (2010). Nitrate signaling: adaptation to fluctuating environments. Curr. Opin. Plant Biol. 13, 266–273. doi: 10.1016/j.pbi.2009.12.003
Krouk, G., Tillard, P., and Gojon, A. (2006). Regulation of the high - affinity NO3- uptake system by NRT1.1 - mediated NO3- demand signaling in Arabidopsis. Plant Physiol. 142, 1075–1086. doi: 10.1104/pp.106.087510
Kumar, R., Khurana, A., and Sharma, A. K. (2014). Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 65, 4561–4575. doi: 10.1093/jxb/eru277
Lambers, H., Clode, P., Hawkins, H., Laliberte, E., Oliveira, R., Reddell, P., et al. (2015). Metabolic Adaptations of the Non-Mycotrophic Proteaceae to Soil with a Low Phosphorus Availability: Phosphorus Metabolism in Plants in the Post-Genomic Era: from Gene to Ecosystem. Oxford: Wiley-Blackwell.
Lambers, H., Shane, M. W., Cramer, M. D., Pearse, S. J., and Veneklaas, E. J. (2006). Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot. 98, 693–713. doi: 10.1093/aob/mcl114
Leggewie, G., Wilmitzer, L., and Riesmeier, J. W. (1997). Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: identification of phosphate transporters from higher plants. Plant Cell 9,381–392. doi: 10.1105/tpc.9.3.381
Li, P., and Brutnell, T. P. (2011). Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J. Exp. Bot. 62, 3031–3037. doi: 10.1093/jxb/err096
Li, Y., Luo, A., Wei, X., and Yao, X. (2007). Genotypic variation of rice in phosphorus acquisition from iron phosphate: contributions of root morphology and phosphorus uptake kinetics. Russ. J. Plant Physiol. 54, 230–236. doi: 10.1134/S1021443707020112
Li, Y., and Wu, S. (1996). Traditional maintenance and multiplication of foxtail millet (Setariaitalica (L.) P. Beauv.) landraces in China. Euphytica 87, 33–38. doi: 10.1007/BF00022961
Liu, K. H., Huang, C. Y., and Tsay, Y. F. (1999). CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11, 865–874. doi: 10.1105/tpc.11.5.865
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real- time quantitative PCR and the 2-DDCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
López-Bucio, J., Hernández-Abreu, E., Sánchez-Calderón, L., Pérez-Torres, A., Rampey, R. A., Bartel, B., et al. (2005). An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol. 137, 681–691. doi: 10.1104/pp.104.049577
López-Bucio, J., Hernández-Abreu, E., Sańchez-Calderón, L., Nieto-Jacobo, M. F., Simpson, J., and Herrera-Estrella, L. (2002). Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 129, 244–256. doi: 10.1104/pp.010934
Lynch, J. P. (1995). Root architecture and plant productivity. Plant Physiol. 109, 7–13. doi: 10.1104/pp.109.1.7
Lynch, J. P. (2011). Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 156, 1041–1049. doi: 10.1104/pp.111.175414
Lynch, J. P., and Brown, K. M. (2008). Root strategies for phosphorus acquisition. Plant Ecophysiol. 7, 83–116. doi: 10.1007/978-1-4020-8435-5_5
Maeda, Y., Konishi, M., Kiba, T., Sakuraba, Y., Sawaki, N., Kurai, T., et al. (2018). A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 9:1376. doi: 10.1038/s41467-018-03832-6
Malamy, J. E. (2005). Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28, 67–77. doi: 10.1111/j.1365-3040.2005.01306.x
Marsch-Martinez, N., and de Folter, S. (2016). Hormonal control of the development of the gynoecium. Curr. Opin. Plant Biol. 29, 104–114. doi: 10.1016/j.pbi.2015.12.006
Miller, A. J., Fan, X. R., Orsel, M., Smith, S. J., and Wells, D. M. (2007). Nitrate transport and signalling. J. Exp. Bot. 58, 2297–2306. doi: 10.1093/jxb/erm066
Miller, A. J., Shen, Q., and Xu, G. (2009). Freeways in the plant: transporters for N, P and S and their regulation. Curr. Opin. Plant Biol. 12, 284–290. doi: 10.1016/j.pbi.2009.04.010
Misson, J., Raghothama, K. G., Jain, A., Jouhet, J., Block, M. A., Bligny, R., et al. (2005). A genome-wide transcriptional analysis using Arabidopsis thaliana affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. U.S.A. 102, 11934–11939. doi: 10.1073/pnas.0505266102
Mudge, S. R., Rae, A. L., Diatloff, E., and Smith, F. W. (2002). Expression analysis suggests novel roles for members of the Pht 1 family of phosphate transporters in Arabidopsis. Plant J. 31, 341–353. doi: 10.1046/j.1365-313X.2002.01356.x
Nacry, P., Canivenc, G., Muller, B., Azmi, A., Van, Onckelen H, Rossignol, M., et al. (2005). A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 138, 2061–2074. doi: 10.1104/pp.105.060061
Nadeem, F., Ahmad, Z., Wang, R., Shen, Q., Chang, F., Zhang, F., et al. (2018). Foxtail millet [Setaria italica (L.) Beauv.] grown under low nitrogen shows a smaller root system, enhanced biomass accumulation and nitrate transporter expression. Front. Plant Sci. 9:205. doi: 10.3389/fpls.2018.00205
Okamoto, M., Kumar, A., Li, W., Wang, Y., Siddiqi, M. Y., Crawford, N. M., et al. (2006). High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 140, 1036–1046. doi: 10.1104/pp.105.074385
Orsel, M., Chopin, F., Leleu, O., Smith, S. J., Krapp, A., Daniel-Vedele, F., et al. (2006). Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis: physiology and protein-protein interaction. Plant Physiol. 142, 1304–1317. doi: 10.1104/pp.106.085209
Osmont, K. S., Sibout, R., and Hardtke, C. S. (2007). Hidden branches: developments in root system architecture. Annu. Rev. Plant Biol. 58, 93–113. doi: 10.1146/annurev.arplant.58.032806.104006
Peng, Y. F., Niu, J. F., Peng, Z. P., Zhang, F. S., and Li, C. J. (2010). Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Res. 115, 85–93. doi: 10.1016/j.fcr.2009.10.006
Péret, B., Clément, M., Nussaume, L., and Desnos, T. (2011). Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci. 16, 442–450. doi: 10.1016/j.tplants.2011.05.006
Pérez -Torres, C. A., López-Bucio, J., Cruz-Ramírez, A., Ibarra-Laclette, E., Dharmasiri, S., Estelle, M., et al. (2008). Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20, 3258–3272. doi: 10.1105/tpc.108.058719
Rabino, I., and Mancinelli, A. L. (1986). Light, temperature and anthocyanin production. Plant Physiol. 81, 922–924. doi: 10.1104/pp.81.3.922
Rae, A. L., Cybinski, D. H., Jarmey, J. M., and Smith, F. W. (2003). Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht 1 family. Plant Mol. Biol. 53, 27–36. doi: 10.1023/B:PLAN.0000009259.75314.15
Raghothama, K. G. (1999). Phosphate acquisition. Annu. Rev. Plant Biol. 50, 665–693. doi: 10.1146/annurev.arplant.50.1.665
Rakshit, S., and Ganapathy, K. N. (2014). “Comparative genomics of cereal crops: status and future prospects,” in Agricultural Bioinformatics, eds P. B. Kishor, R. Bandopadhyay, and P. Suravajhala (New Delhi: Springer).
Rausch, C., and Bucher, M. (2002). Molecular mechanisms of phosphate transport in plants. Planta 216, 23–37. doi: 10.1007/s00425-002-0921-3
Richardson, A. E., Hocking, P. J., Simpson, R. J., and George, T. S. (2009). Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 60, 124–143. doi: 10.1071/CP07125
Rose, T. J., Rose, M. T., Pariasca-Tanaka, J., Heuer, S., and Wissuwa, M. (2011). The frustration with utilization: why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Nutr. 2:73. doi: 10.3389/fpls.2011.00073
Rosen, H. (1957). A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 67, 10–15. doi: 10.1016/0003-9861(57)902412
Rubio, V., Bustos, R., Irigoyen, M. L., Cardona-Lopez, X., Rojas-Triana, M., and Paz-Ares, J. (2009). Plant hormones and nutrient signaling. Plant Mol. Biol. 69, 361–373. doi: 10.1007/s11103-008-9380-y
Rufty, T. W., Israel, D. W., Volk, R. J., Qiu, J., and Sa, T. (1993). Phosphate regulation of nitrate assimilation in soybean. J. Exp. Bot. 44, 879–891. doi: 10.1007/s00425-014-2165-4
Rufty, T. W., MacKown, C. T., and Israel, D. W. (1990). Phosphorus stress effects on assimilation of nitrate. Plant Physiol. 94, 328–333. doi: 10.1104/pp.94.1.328
Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., et al. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. doi: 10.1016/S0092-8674(00)81535-4
Schachtman, D. P., Reid, R. J., and Ayling, S. M. (1998). Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116, 447–453. doi: 10.1104/pp.116.2.447
Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., et al. (2011). Phosphorus dynamics: from soil to plant. Plant Physiol. 156, 997–1005. doi: 10.1104/pp.111.175232
Shrawat, A. K., and Lörz, H. (2006). Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol. J. 4, 575–603. doi: 10.1111/j.1467-7652.2006.00209.x
Tabata, R., Sumida, K., Yoshii, T., Ohyama, K., Shinohara, H., and Matsubayashi, Y. (2014). Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346. doi: 10.1126/science.1257800
Ticconi, C. A., and Abel, S. (2004). Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci. 9, 548–555. doi: 10.1016/j.tplants.2004.09.003
Torrey, J. G. (1976). Root hormones and plant growth. Ann. Rev. Plant Physiol. 27, 435–459. doi: 10.1146/annurev.pp.27.060176.002251
Tsay, Y. F., Chiu, C. C., Tsai, C. B., Ho, C. H., and Hsu, P. K. (2007). Nitrate transporters and peptide transporters. FEBS Lett. 581, 2290–2300. doi: 10.1016/j.febslet.2007.04.047
Vance, C. P., Uhde-Stone, C., and Allan, D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
Wang, Y. Y., Hsu, P. K., and Tsay, Y. F. (2012). Uptake, allocation and signaling of nitrate. Trends Plant Sci. 17, 458–467. doi: 10.1016/j.tplants.2012.04.006
Wei, Z., and Li, J. (2016). Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant 9, 86–100. doi: 10.1016/j.molp.2015.12.003
Weiler, E. W., Jourdan, P. S., and Conrad, W. (1981). Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta 153, 561–571. doi: 10.1007/BF00385542
Williamson, L. C., Ribrioux, S. P. C. P., Fitter, A. H., and Leyser, H. M. O. (2001). Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 126, 875–882. doi: 10.1104/pp.126.2.875
Wissuwa, M., Wegner, J., Ae, N., and Yano, M. (2002). Substitution mapping of Pup1: a major QTL increasing phosphorus uptake of rice from a phosphorus deficient soil. Theor. Appl. Genet. 105, 890–897. doi: 10.1007/s00122-002-1051-9
Wissuwa, M., Yano, M., and Ae, N. (1998). Mapping of QTLs for phosphorus deficiency tolerance in rice (Oryza sativa L.). Theor. Appl. Genet. 97, 777–783. doi: 10.1007/s00122-009-1235-7
Wu, P., Ma, L., Hou, X., Wang, M., Wu, Y., Liu, F., et al. (2003). Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 132, 1260–1271. doi: 10.1104/pp.103.021022
Yamaguchi, S. (2008). Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59, 225–251. doi: 10.1146/annurev.arplant.59.032607.092804
Yang, X., Wan, Z., Perry, L., Lu, H., Wang, Q., Zhao, C., et al. (2012). Early millet use in northern China. Proc. Natl. Acad. Sci. U.S.A. 109, 3726–3730. doi: 10.1073/pnas.1115430109
Ye, Y., Yuan, J., Chang, X., Yang, M., Zhang, L., Lu, K., et al. (2015). The phosphate transporter gene OsPht1; 4 is involved in phosphate homeostasis in rice. PLoS One 10:e0126186. doi: 10.1371/journal.pone.0126186
Zhang, F., Sun, Y., Pei, W., Jain, A., Sun, R., Cao, Y., et al. (2015). Involvement of OsPht1; 4 in phosphate acquisition and mobilization facilitates embryo development in rice. Plant J. 82, 556–569. doi: 10.1111/tpj.12804
Keywords: foxtail millet, phosphate limitation, root system, hormone accumulation, phosphate transporters, nitrate transporters
Citation: Ahmad Z, Nadeem F, Wang R, Diao X, Han Y, Wang X and Li X (2018) A Larger Root System Is Coupled With Contrasting Expression Patterns of Phosphate and Nitrate Transporters in Foxtail Millet [Setaria italica (L.) Beauv.] Under Phosphate Limitation. Front. Plant Sci. 9:1367. doi: 10.3389/fpls.2018.01367
Received: 27 April 2018; Accepted: 28 August 2018;
Published: 13 September 2018.
Edited by:
Stefano Cesco, Free University of Bozen-Bolzano, ItalyReviewed by:
Fangsen Xu, Huazhong Agricultural University, ChinaPetronia Carillo, Università degli Studi della Campania “Luigi Vanvitelli,” Italy
Copyright © 2018 Ahmad, Nadeem, Wang, Diao, Han, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuexian Li, steve@cau.edu.cn
 Zeeshan Ahmad
Zeeshan Ahmad Faisal Nadeem
Faisal Nadeem Ruifeng Wang
Ruifeng Wang Xianmin Diao
Xianmin Diao Yuanhuai Han
Yuanhuai Han Xingchun Wang
Xingchun Wang Xuexian Li
Xuexian Li