- 1Clinical Applied Physiology Lab, College of Health Sciences, The University of Texas at El Paso, El Paso, TX, United States
- 2Doctor of Physical Therapy Program, Rehabilitation Sciences Department, College of Health Sciences, The University of Texas at El Paso, El Paso, TX, United States
Endothelial dysfunction is the first pathophysiological step of atherosclerosis, which is responsible for 90% of strokes. Exercise programs aim to reduce the risk of developing stroke; however, the majority of the beneficial factors of exercise are still unknown. Endothelial shear stress (ESS) is associated with endothelial homeostasis. Unfortunately, ESS has not been characterized during different exercise modalities and intensities in the carotid artery. Therefore, the purpose of this study was to determine exercise-induced blood flow patterns in the carotid artery. Fourteen apparently healthy young adults (males = 7, females = 7) were recruited for this repeated measures study design. Participants completed maximal oxygen consumption (VO2max) tests on a Treadmill, Cycle-ergometer, and Arm-ergometer, and 1-repetition maximum (1RM) tests of the Squat, Bench Press (Bench), and Biceps Curl (Biceps) on separate days. Thereafter, participants performed each exercise at 3 different exercise intensities (low, moderate, high) while a real-time ultrasound image and blood flow of the carotid artery was obtained. Blood flow patterns were assessed by estimating ESS via Womersley’s estimation and turbulence via Reynold’s number (Re). Data were analyzed using a linear mixed-effects model. Pairwise comparisons with Holm-Bonferroni correction were conducted with Hedge’s g effect size to determine the magnitude of the difference. There was a main effect of intensity, exercise modality, and intensity * exercise modality interaction on both ESS (p < 0.001). Treadmill at a high intensity yielded the greatest ESS when compared to the other exercise modalities and intensities, while Bench Press and Biceps curls yielded the least ESS. All exercise intensities across all modalities resulted in turbulent blood flow. Clinicians must take into consideration how different exercise modalities and intensities affect ESS and Re of the carotid artery.
Introduction
Cardiovascular (CV) diseases, including coronary artery disease and stroke, are the leading cause of death worldwide. One in every 19 deaths are produced by a stroke, and there are more than 610,000 new cases of stroke per year. The total direct and indirect costs of CV for the USA are estimated to be around 43.6 billion dollars (Urbich et al., 2020). Atherosclerosis is responsible for 9 in every 10 cases of Stroke (Qaja and Bhimji, 2017); in addition, CV comorbidities are common features among stroke survivors (Tang et al., 2009).
Endothelial dysfunction is recognized as the first step for the development of 90% of all CV diseases (Benjamin et al., 2017), is a pathological condition characterized by an unbalance between vasodilatory and vasoconstrictory mechanisms (Flammer et al., 2012), and is generally defined as the decrease in nitric oxide (NO) bio-availability within the endothelium (Harris et al., 2010). The primary physiological mechanism that regulates endothelial function in endothelial shear stress (ESS), which is the frictional force produced between blood flow and endothelial cells (Sriram et al., 2016); where increments of ESS (e.g., during exercise) are known to improve endothelial nitric oxide synthase (eNOS) gene expression (Ishibazawa et al., 2011) and NO bioavailability (Rodríguez and González, 2014). Exercise programs are one of the best-suited approaches to prevent CV comorbidities and a subsequent stroke (Tang et al., 2009; Jurczak et al., 2014; Kirk et al., 2014; Marzolini et al., 2014; Prior et al., 2017), however, different exercise modalities and intensities, such as endurance versus resistance and low versus high intensities, could elicit different CV outcomes. Moreover, and to the best of our knowledge, there are no studies regarding carotid ESS during different modalities of exercises and intensities.
The purpose of this study was to determine exercise-induced blood flow patterns across different exercise modalities at three different intensities in the carotid artery. It was hypothesized that ESS and turbulent flow in the carotid artery would increase in an intensity-dependent manner and that exercises involving larger and more muscle groups would have larger ESS and more turbulent flow.
Methods
Experimental Design
Twenty participants were recruited for a repeated-measures study design. Participation within the study involved 2 sessions for maximal testing and 2 sessions of submaximal testing with 24–48 h between sessions. A priori power analysis was conducted in Rstudio using R statistical programing language and the “pwr” library; a total of 14 subjects with stratification by sex (7 per group) at an alpha level (α) of 0.05 with a large effect size (f) of 0.4, was determined to be enough to obtain power (β) of 0.80. All study protocols were in accordance with the Declaration of Helsinki and were approved by the Institution Review Board at the University of Texas at El Paso (Reference number: 1250657). All participants signed an informed consent form before engaging in their first testing session. Females were tested within an 8-day period, spanned from 4 days before to 4 days after the start of menses, to reduce any hormonal influence on vascular response (Adkisson et al., 2010; Mattu et al., 2020).
Study Protocol
All testing was performed in a temperature-controlled room (24°C–26°C) and participants were asked to refrain of food, alcohol, and smoking for at least 8 h before any testing session. Participants completed demographic and screening questionnaires to determine eligibility. Height and mass were taken using a calibrated stadiometer and scale, respectively (Detecto PHR, Detecto, Webb, MO, United States). Then, resting blood pressure was obtained using an automated brachial blood pressure cuff (BP760, Omnron Healthcare, Inc., Lake Forest, IL, United States). In addition, and at the beginning of every visit, hematocrit (HemataStat II Hematocrit Analyzer, Separation Technology Inc., Sanford, FL, United States) and resting blood lactate (BLa) levels (Lactate Plus, Nova Inc., Boston, MA, United States) were obtained from the lower end of the earlobe as previously described (Rascon et al., 2020; Gurovich et al., 2021a). Then, for session 1, subjects completed 3 maximal strength tests (Squat, Bench Press, and Biceps curls), then subjects rested for at least 30 min (Tagesson and Kvist, 2007; Neto et al., 2015; García-Ramos et al., 2019) and performed a graded exercise test on the treadmill (Trackmaster TMX58, Newton, KS, United States) to determine maximal oxygen consumption (VO2max) and lactate threshold. In addition, and to confirm recovery, BLa levels were obtained after the 30 min resting period and participants were not allowed to perform the next exercise testing if BLa leveles were not back to baseline levels. In session 2, participants performed two other graded exercise tests in the cycle-ergometer (Corival, Lode, Groningen, Netherlands) and arm-ergometer (Angio, Lode, Groningen, Netherlands) with at least 30 min between tests. All three VO2max tests included blood draws from the earlobe to determine BLa at the end of each exercise stage. The 6 sub-maximal exercises (i.e., Squat, Bench Press, Biceps curls, treadmill, cycling, and arm-ergometer) were randomly assigned to sessions 3 and 4, and performed each exercise at three different exercise intensities. Participants performed three repetitions of Squat, Bench Press, and Biceps curls at low (45% 1-RM), moderate (65% 1-RM), and high intensity (85% 1-RM) each and 3-min steady-state exercise stages of treadmill, cycling, and arm-ergometer at low (BLa < 2 mmol/L), moderate (BLa 2–4 mmol/L), and high intensity (BLa > 4 mmol/L) (Rascon et al., 2020) (Figure 1). At least 30 min between sub-maximal exercise sets were provided to recovery.
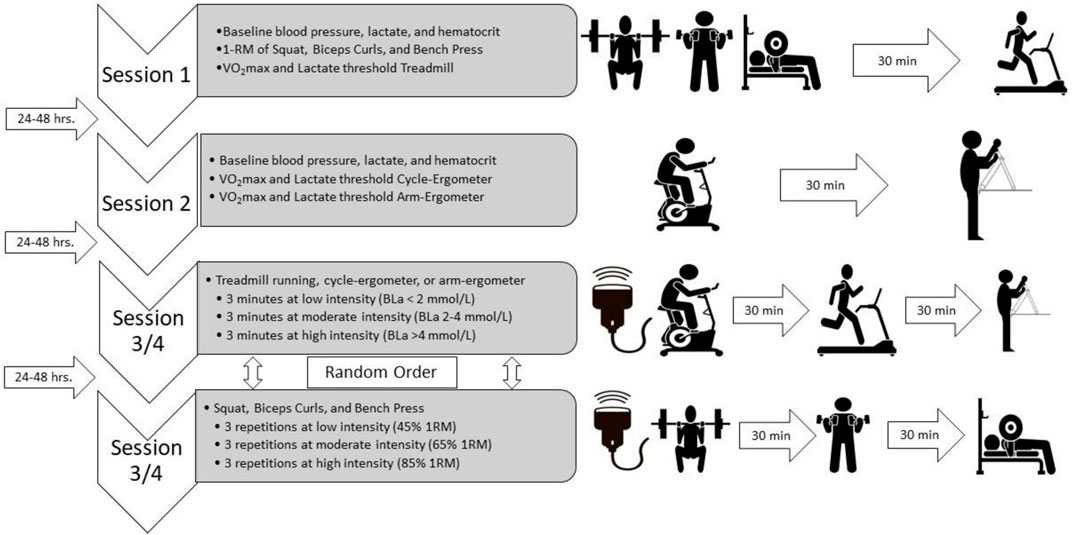
FIGURE 1. Study design. Four exercise sessions, 2 for maximal tests and 2 for submaximal tests, with 24–48 h between sessions. Ultrasound assessment during both submaximal sessions and exercise modalities sets were randomly assigned to either session 3 or session 4. VO2max, maximal oxygen consumption; BLa, blood lactate levels.
All three graded exercise tests used a protocol with speed/workload increased every 2-min (Beltz et al., 2016). VO2max was obtained using a metabolic cart (TrueOne 2400, Parvomedics Inc., Sandy, UT, United States). At 30 s before the end of each stage, BLa was drawn from the participant’s earlobe, to determine BLa threshold, along with reported heart rate and rate of perceived exertion. A successful trial was considered if the following criteria were met: 1) BLa > 8.0 mmol/L, respiratory exchange ratio (RER) > 1.10, heart rate was within 10 bmp of estimated maximal heart rate (220—age), and RPE > 17 (Beltz et al., 2016).
The 1-RM testing consisted of a familiarization and technique inspection of the individual’s exercise execution. Thereafter, participants were asked to predict the maximal load they could achieve. Then, participants performed 5–10 repetitions of the predicted load at a comfortable pace. The load was increased by 20% for the following set and performed for 2–3 repetitions. Then load was increased by 2.5–5 kg until participants reached failure (Seo et al., 2012; Montalvo et al., 2021). Technical execution analysis, as well as spotting, was performed by a Certified Strength and Conditioning Specialist (SM).
Blood Flow Pattern Testing
During sub-maximal exercise sets (sessions 3 and 4), real-time carotid artery longitudinal images and blood flow velocity were recorded with a 12 MHz ultrasound transducer and Doppler, (LA435, MyLab30 Gold, Esaote, Firenze, Italy), which has secure with a cervical probe holder placed on the participant’s neck as previously described (Gurovich et al., 2021b; Morales-Acuna et al., 2020). Ultrasound images and Doppler signals were obtained on the common carotid artery 2 cm below the bifurcation of the anterior and posterior carotid arteries and then analyzed with edge detection technology (Vascular Analysis Integrative System, Medical Imaging Applications, Coralville, IA, United States) and a data acquisition system (MP150WSW, BIOPAC Systems Inc., Goleta, CA, United States) (Figure 2). ESS was obtained by Womersley’s approximation and the presence of turbulent flow via Reynold’s number (Re) as previously described (Gurovich and Braith, 2012; Morales-Acuna et al., 2019; Rascon et al., 2020; Gurovich et al., 2021a). The presence of laminar or turbulent flow was defined via Re, where undisturbed laminar flow values were < 200, disturbed blood flow values between 200–1800, and turbulent flow values > 2000 (Davies, 2009). Both ESS and Re were determined within a single cardiac cycle to minimize the effects that heart rate and cardiac output have in ESS, as previousle described (Gurovich and Braith, 2012; Morales-Acuna et al., 2019; Rascon et al., 2020; Gurovich et al., 2021b).
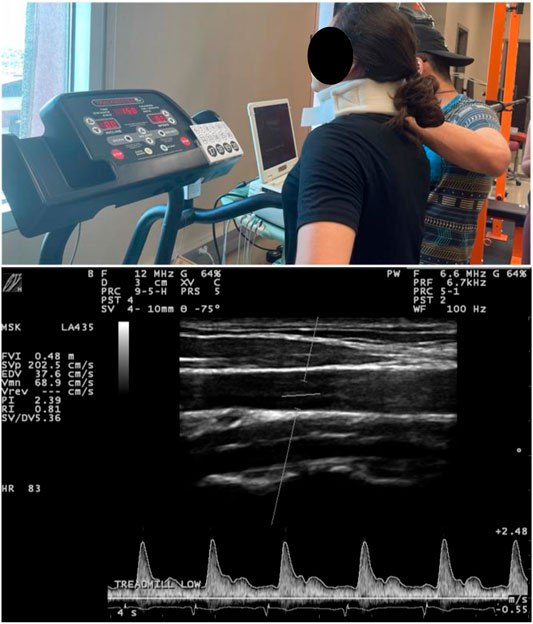
FIGURE 2. Typical ultrasound testing setup with neck probe holder utilized during all exercise testing and representative ultrasound image.
Statistical Analysis
Data were compiled into a master data spreadsheet (Excel, Microsoft 2021). Data were then exported into Rstudio Integrative Development Environment (Rstudio, 2020) and analyzed using a custom-built script in R statistical programming language (R 4.1.2). The “dplyr” package was used for grammar data manipulation, “forecats” for factor re-leveling, “ggplot2” and “ggpurb” for data visualization, “psych” for data descriptives, “cvcqv” for reliability analysis, “lm4” and “lmerTest” for linear mixed-effects models, “rstatix” for post-hoc pairwise comparisons, and effect sizes. Data distribution was assessed via Shapiro-Wilk test. Baseline demographic data were analyzed by a series of independent t-tests between males and females. Reliability of baseline ESS and Re were analyzed using a coefficient of variation (CV) and interpreted as < 10% as very good, 10%–20% as good, 20%–30% as acceptable, and >30% as poor (Campbell et al., 2010). Differences between exercise modalities and intensities were assessed using a general linear mixed-effects model for repeated measures with adjusting for individual differences as a random effect; the model was as follows: dependent variable ∼ exercise modality + exercise intensity + Sex + exercise modality*exercise intensity + (1|Participant). Pairwise differences were analyzed post-hoc with a Holm-Bonferroni p-value correction (p.adj) when appropriate; the effect size was obtained through standardized mean differences using Cohen’s D with a Hedge’s g (ESg) correction for a small sample size, and interpreted as follows: ESg < 0.2 as very small, 0.2–0.49 as small, 0.5–0.79 as moderate, and > 0.8 as large (Hopkins, 2009). Statistical significance was set priori at an alpha level of 0.05. Re was analyzed by visual analysis using a 95% confidence interval (CI) as previously described (Gurovich et al., 2021a). Data and data analysis scripts are available in a repository for data analysis replication in https://github.com/Samuelmontalvo/Modalities.
Results
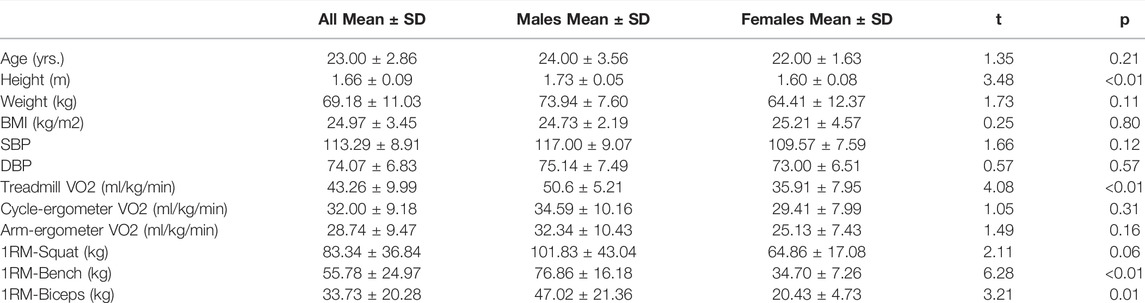
Out of the 20 participants, 6 were unable to finish all 4 visits due to the COVID-19 lockdown. Hence, only 14 participants were able to complete the study. All data analyzed was normally distributed. Demographics and descriptive data for the final participants are provided in Table 1. Males were taller, had a higher VO2max on treadmill (t = 2, p = 0.002), 1-RM Bench Press (t = 6, p < 0.01), and 1-RM Biceps curls (t = 3, p = 0.02) than females. Baseline reliability analysis showed a good inter-testing reliability on ESS [CV = 16.9 (95%CI = 12.3–21.5)] and acceptable inter-testing reliability on Re [CV = 22.7 (95%CI = 16.4–29.1)].
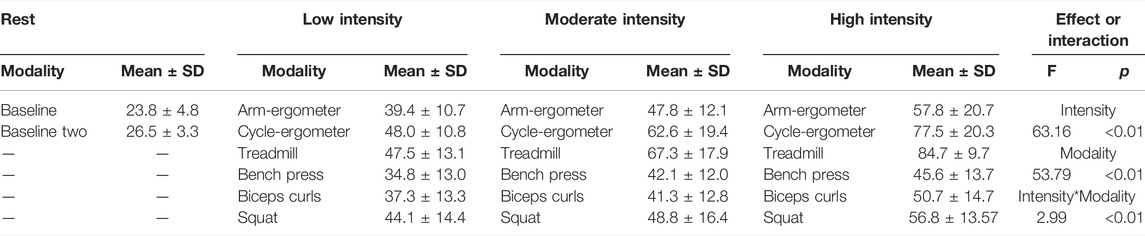
Overall, the model indicated a main effect of exercise modality (F(2,247) = 53.78, p < 0.001) and intensity (F(2,247) = 63.16, p < 0.001), and a significant intensity * modality interaction (F(10,247) = 2.99, p < 0.01) on ESS (Table 2). However, there was no main effect of sex on ESS (F(1,12) = 2.01, p = 0.18) or Re (F(1,12) = 0.12, p = 0.73). Moreover, there was a significant random effect (p < 0.001), indicating significant individual variability in ESS. Due to the no effect of sex within our model, post-hoc pairwise comparisons were performed with all individuals as one group.
Post-hoc pairwise analysis within exercise modalities showed that almost all exercise modalities were influenced by intensity (p < 0.01) with large effect size between intensities (Figure 2; Table 3). Only Squat at low intensity vs. moderate intensity [t = -1.17, p.adj = 0.26, ESg (small) = -0.29], Bench Press at low intensity vs. high intensity [t = -2.08, p.adj = 0.12, ESg(moderate) = -0.52], and Bench Press at moderate intensity vs. high intensity [t = -0.81, p.adj = 0.43, ESg(small) = -0.20] were not statistically different (Figure 3; Table 3).
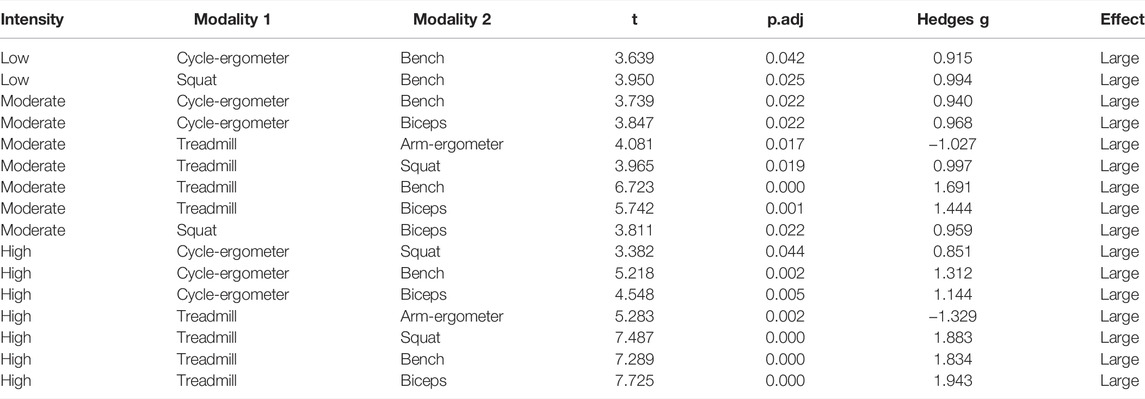
TABLE 3. Pairwise comparisons between exercise modalities by exercise intensity for endothelial shear stress (ESS).
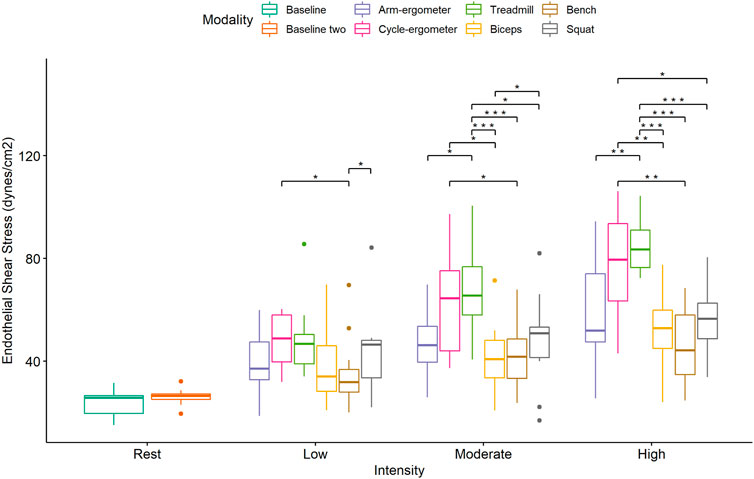
FIGURE 3. Boxplot of Endothelial Shear Stress (ESS) by exercise modality and intensity with comparisons between modalities at each intensity. *p < 0.05; **p < 0.01; ***,p < 0.001.
Pairwise comparisons for ESS during low-intensity exercise showed significant differences and large effects between cycle-ergometer vs. Bench Press and Squat vs. Bench Press (Figure 4; Table 4). Similarly, there were significant differences and large effects at moderate exercise intensity between treadmill vs. arm-ergometer, treadmill vs. Squat, treadmill vs. Bench Press, treadmill vs. Biceps curls, and Squat vs. Biceps curls (Figure 4; Table 4). Finally, there were significant differences and large effects at high exercise intensity between cycle-ergometer vs. Squat, cycle-ergometer vs. Bench Press, cycle-ergometer vs. Biceps curls, treadmill vs. arm-ergometer, treadmill vs. Squat, treadmill vs. Bench Press, and treadmill vs. Biceps curls.
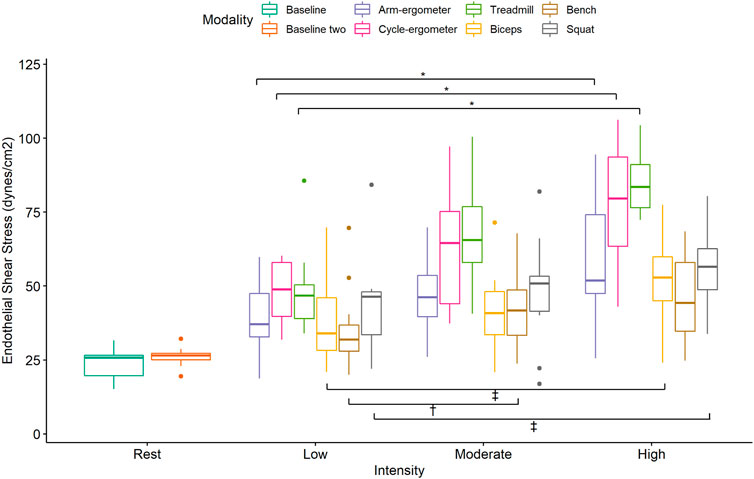
FIGURE 4. Boxplot of Endothelial Shear Stress (ESS) by exercise modality and intensity with comparisons within modalities at each intensity. *, p < 0.05 low vs. moderate, low vs. high, and moderate vs. high; †, p < 0.05 low vs. moderate Bench press; ‡, p < 0.05 low vs. high and moderate vs. high; †, p < 0.05 low vs. moderate Biceps and Squat.
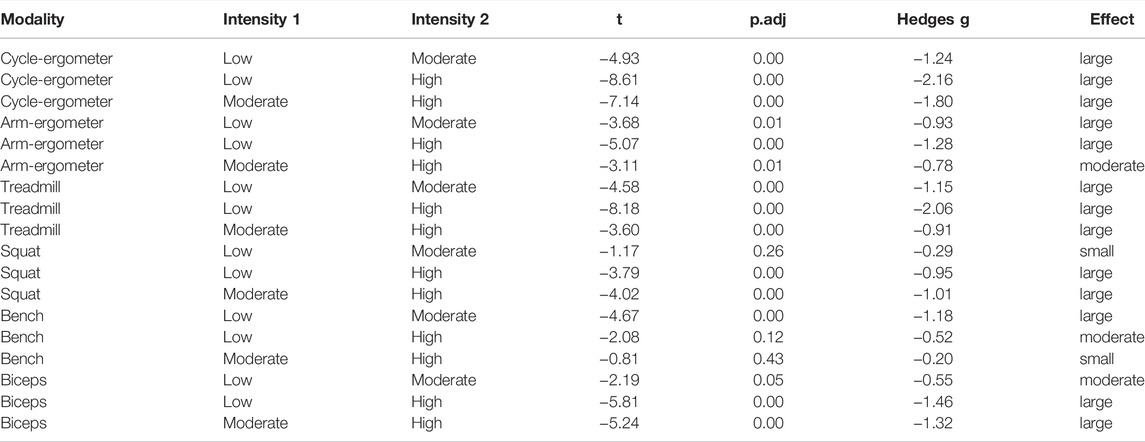
TABLE 4. Pairwise comparisons within exercise modality by intensity for endothelial shear stress (ESS).
Visual analysis of the Re plot using mean and error plot indicates that all exercise modalities from low to high intensity resulted in turbulent flow (Re > 2000) (Figure 5).
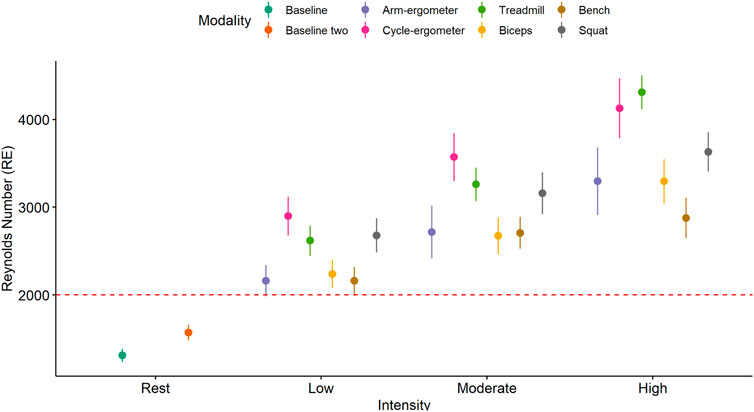
FIGURE 5. Mean and 95% CI of Reynolds number (Re) by exercise modality and intensity with a turbulent flow threshold number of 2,000 (red dash line).
Discussion
The purpose of the current study was to determine the effects of different exercise modalities at three different exercise intensities on endothelial shear stress (ESS) and blood flow patterns (presence of turbulent flow) at the carotid artery. Our primary hypothesis was confirmed as exercises involving larger and more muscle groups, like running on a treadmill, at higher intensities produced greater ESS and turbulent blood flow than other exercise modalities with less muscle recruitment, like Biceps curls, and lower intensities. However, exercise duration could be a confounding factor. In addition, the results of the present study confirmed that ESS increases in an intensity-dependent manner regardless of the exercise modality and that blood flow is mainly turbulent regardless of exercise modality or exercise intensity.
Approximately 40%–60% of the beneficial effects of exercise training in preventing/treating cardiovascular disease, including stroke, are unrelated to the reduction in traditional cardiovascular risk factors (Mora et al., 2007; Green, 2009). Several authors (Laughlin, 1995; Hambrecht et al., 2000; Green et al., 2002; Green et al., 2008; Laughlin et al., 2008; Green, 2009) have established a close relationship between exercise training and improvements in endothelial function. In addition, previous studies have shown that the lack of or low ESS can result in vascular inflammation, upregulation of matrix-degrading proteases, and arterial wall remodeling, which promotes the transition of stable to unstable plaque in atherosclerotic lesions (Koskinas et al., 2009). Moreover, it has been reported that low ESS and oscillatory shear stress can result in atherosclerotic lesions due to plaque formation (Cheng et al., 2006). Therefore, it is possible to speculate that the direct mechanical effects of exercise-induced blood flow patterns on the vascular endothelium could be a major mitigating factor in the prevention of cardiovascular disease.
The results of the current study showed that exercise-induced blood flow patterns are associated with exercise modality. For example, running on a treadmill or cycling at high intensity elicits more ESS than any of the resistance exercises at a comparable high intensity (Figure 3). This difference can be attributed to a larger muscle mass recruitment during running or cycling when compared to a single resistance exercise. In addition, ESS is intensity-dependent as almost all exercise modalities showed an increase in ESS when intensity is increased (Figure 4). This finding might be also associated with an increase in muscle recruitment as higher intensities would recruit a larger percentage of muscle mass. Moreover, and interestingly, it appears that all exercise modalities produced turbulent flow in the carotid artery, regardless of the exercise intensity (Figure 5). The presence of turbulent flow during exercise could be explained by the rather larger size of the carotid artery, compared to the brachial artery (Gurovich and Braith, 2012), and the systematic increase in blood flow velocity with the increased exercise intensity (Nichols and O'Rourke, 2005). Finally, the results of the current study showed no sex differences in any of the exercise modalities at any of the intensities. This is consistent with previous findings from our laboratory when assessing the brachial artery during cycle-ergometry at low, moderate, and high intensities (Gurovich et al., 2021b).
Even though the current study is not the first one assessing carotid blood flow during exercise (Babcock et al., 2015; Hellstrom et al., 1996; Jiang et al., 1995; Liu et al., 2015; Sato et al., 2011; Sato and Sadamoto, 2010; Wang et al., 2019), this is the first study comparing different exercise modalities and intensities. Previous studies have predominantly used walking/running on a treadmill or cycling, both upright and recumbent. For example, Jiang et al. (Jiang et al., 1995) assessed carotid blood flow velocity in eight healthy male participants during a graded exercise test on a treadmill. Unfortunately, the authors were not able to determine ESS as they found some technical difficulties with ultrasound imaging during their protocol. These technical difficulties were avoided in the current study by using a customized, patent-pending cervical probe holder placed on the participant’s neck (Gurovich et al., 2021a; Morales-Acuna et al., 2020). Nevertheless, Jiang et al. found a significant increase in carotid artery blood flow velocity, up to 52% from baseline values, during exercise. Similar results were found during cycling at submaximal intensities in healthy men (Hellstrom et al., 1996), healthy women (Sato and Sadamoto, 2010), and healthy men and women (Sato et al., 2011). Similar to the findings of the present study, all these studies showed an intensity-depend increase in carotid artery blood flow, ranging from 17% to 42%, with submaximal exercise. Interestingly, Babcock, Heffernan, et al. (Babcock et al., 2015) measured carotid blood flow before and after a short bout (e.g., 30 s) of maximal exercise in 55 healthy adults, with different exercise backgrounds. Their findings showed a 15%, 19%, and 19% increase in mean carotid blood flow velocity, mean carotid blood flow, and mean shear rate, respectively. These rather smaller increases after maximal exercise could be explained by the short bout of exercise as 30 s might not be enough to elicit other vascular acute adaptations. Even though shear rate might reflect ESS, the results of the current study showed an increase in ESS from 39%, during Bench Press at low intensity, to 239%, during treadmill running at a high intensity (Table 2). These findings may confirm that longer exercise bouts could elicit different acute vascular responses and that shear rate should not be considered as a surrogate for ESS (Gurovich and Braith, 2012). The ESS data shown in the current study are in agreement with previous reports (Liu et al., 2015; Wang et al., 2019). Both Wang et al. (2019) and Liu et al. (2015) reported an increased ESS, from 40% to 100%, during exercise. In addition, Wang et al. (2019) showed an intensity-dependent increase in ESS from resting to moderate and high intensity cycling exercise (∼50 dynes/cm2 vs. ∼75 dynes/cm2 vs. 100 dynes/cm2, respectively) that are comparable to the cycling ESS in the current study (resting: 26.5 ± 3.3 dynes/cm2, low: 48.0 ± 10.8 dynes/cm2, moderate: 62.6 ± 19.4 dynes/cm2, and high: 77.5 ± 20.3 dynes/cm2). Interestingly, the resting data from the current study is very similar to data obtained with echo particles imaging velocimetry (PIV) (Gates et al., 2018); however, exercise PIV data has yet to be determined.
Even though the current study was designed to assess blood flow patterns during an acute bout of exercise, these findings can be associated with adaptations such as endothelial function and atherosclerotic plaque vulnerability. For example, there are some conflicting results when comparing endothelial function before and after resistance and aerobic exercises (Iwamoto et al., 2018; Boeno et al., 2019). Boeno et al. (2019) showed no improvement in endothelial function, measured via flow-mediated dilation, with a single session of repeated knee extension exercise at a moderate and high intensity. In contrast, Iwamoto et al. (2018) showed that endothelial function improved in an intensity-dependent manner after low intensity (50%–55% of HRmax) and high intensity (75%–80% HRmax) cycle-ergometry. Similarly, Spence et al. showed that 6 months of aerobic and resistance training induced significant changes in the carotid artery size and function (Spence et al., 2013). In addition, there is some evidence that turbulent flow can improve the strength of atherosclerotic plaque (Cheng et al., 2006; Koskinas et al., 2009). Both Cheng et al. (2006) and Koskinas et al. (2009) using very elegant study designs, showed that turbulent flow in pro-atherosclerotic vascular areas can induce stable lesions by mobilizing smooth muscle cells. Altogether, the significant increase in ESS and turbulent flow at higher intensities observed in the current study, if applied chronically (i.e., exercise training), may elicit beneficial effects to treat and prevent cardiovascular diseases.
Limitations
The present study is not exempt from limitations. Our study was limited to the sample size. Our between-subjects comparison analyzes could have been compromised by the low sample size (males = 7, females = 7). However, because of inexistence differences between males and females in blood flow patterns, our overall sample size was 14 participants, which was enough to show differences in responses through the standardized mean difference as denoted by the effect size. Moreover, each of the pairwise comparisons (exercise modality by intensity) yielded a possible 42 comparisons. Thus, in order to avoid the increased chance of committing type 1 (false positive) and type 2 (false negative) errors, we utilized a Holm-Bonferroni correction (Eichstaedt et al., 2013). Another possible limitation is the difference in the exercise duration of each exercise modality. Future studies should use standardize time/volume of each exercise bout to make it more comparable.
The inferences derived from this investigation can only be extrapolated to a similar population (healthy young male and female participants), and the effects of different exercise modalities and intensities on ESS and Re for clinical populations (i.e., CV problems) remain unknown. Moreover, it is unknown if other alternative exercise modalities such as plyometrics (jumping), boxing, agility training, balance, Taichi, Yoga, etc,. would affect (short or long term) endothelial shear stress and function, and as such, researchers should investigate these. Finally, our study was cross-sectional, and only acute interaction of exercise modality and intensity and ESS or Re can be inferred. Thus, the differences between exercise modalities and intensities on ESS or Re at short and long-term exercise remains unknown.
Conclusion
Blood flow patterns during exercise in the carotid artery show that flow is mainly turbulent, independent of the exercise modality and intensity and that ESS is dependent on exercise intensity regardless of the exercise modality. In addition, activities engaging larger and more muscle groups, like running or biking, at a high intensity yield the greatest ESS. Thus, clinicians should take into consideration exercise-induced blood flow patterns at the carotid artery during the different exercise intensities and modalities.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/Samuelmontalvo/Modalities.
Ethics Statement
The studies involving human participants were reviewed and approved by Institution Review Board at The University of Texas at El Paso. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AG and FM-A contributed to conception and design of the study. SM, MG, AL, SA, and LR collected the data. SM performed the statistical analysis. SM and AG wrote the first draft of the manuscript. MG wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
Research reported in this publication was partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number SC2GM140952.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adkisson E. J., Casey D. P., Beck D. T., Gurovich A. N., Martin J. S., Braith R. W. (2010). Central, Peripheral and Resistance Arterial Reactivity: Fluctuates during the Phases of the Menstrual Cycle. Exp. Biol. Med. (Maywood) 235 (1), 111–118. doi:10.1258/ebm.2009.009186
Babcock M. C., Lefferts W. K., Hughes W. E., Fitzgerald K. L., Leyer B. K., Redmond J. G., et al. (2015). Acute Effect of High-Intensity Cycling Exercise on Carotid Artery Hemodynamic Pulsatility. Eur. J. Appl. Physiol. 115 (5), 1037–1045. doi:10.1007/s00421-014-3084-6
Beltz N. M., Gibson A. L., Janot J. M., Kravitz L., Mermier C. M., Dalleck L. C. (2016). Graded Exercise Testing Protocols for the Determination of VO2max: Historical Perspectives, Progress, and Future Considerations. J. Sports Med. 2016, 1–12. doi:10.1155/2016/3968393
Benjamin E. J., Blaha M. J., Chiuve S. E., Cushman M., Das S. R., Deo R., et al. (2017). Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 135 (10), e146–e603. doi:10.1161/CIR.0000000000000485
Boeno F. P., Farinha J. B., Ramis T. R., Macedo R. C. O., Rodrigues-Krause J., do Nascimento Queiroz J., et al. (2019). Effects of a Single Session of High- and Moderate-Intensity Resistance Exercise on Endothelial Function of Middle-Aged Sedentary Men. Front. Physiol. 10. doi:10.3389/fphys.2019.00777
Campbell M. J., Machin D., Walters S. J. (2010). “Reliability and Method Comparison Studies,” in Medical Statistics: A Textbook for the Health Sciences (John Wiley & Sons).
Cheng C., Tempel D., van Haperen R., van der Baan A., Grosveld F., Daemen M. J. A. P., et al. (2006). Atherosclerotic Lesion Size and Vulnerability Are Determined by Patterns of Fluid Shear Stress. Circulation 113 (23), 2744–2753. doi:10.1161/circulationaha.105.590018
Davies P. F. (2009). Hemodynamic Shear Stress and the Endothelium in Cardiovascular Pathophysiology. Nat. Rev. Cardiol. 6 (1), 16–26. doi:10.1038/ncpcardio1397
Eichstaedt K. E., Kovatch K., Maroof D. A. (2013). A Less Conservative Method to Adjust for Familywise Error Rate in Neuropsychological Research: the Holm's Sequential Bonferroni Procedure. Nre 32 (3), 693–696. doi:10.3233/nre-130893
Flammer A. J., Anderson T., Celermajer D. S., Creager M. A., Deanfield J., Ganz P., et al. (2012). The Assessment of Endothelial Function. Circulation 126 (6), 753–767. doi:10.1161/CIRCULATIONAHA.112.093245
García-Ramos A., Barboza-González P., Ulloa-Díaz D., Rodriguez-Perea A., Martinez-Garcia D., Guede-Rojas F., et al. (2019). Reliability and Validity of Different Methods of Estimating the One-Repetition Maximum during the Free-Weight Prone Bench Pull Exercise. J. sports Sci. 37 (19), 2205–2212. doi:10.1080/02640414.2019.1626071
Gates P. E., Gurung A., Mazzaro L., Aizawa K., Elyas S., Strain W. D., et al. (2018). Measurement of Wall Shear Stress Exerted by Flowing Blood in the Human Carotid Artery: Ultrasound Doppler Velocimetry and Echo Particle Image Velocimetry. Ultrasound Med. Biol. 44 (7), 1392–1401. doi:10.1016/j.ultrasmedbio.2018.02.013
Green D., Cheetham C., Mavaddat L., Watts K., Best M., Taylor R., et al. (2002). Effect of Lower Limb Exercise on Forearm Vascular Function: Contribution of Nitric Oxide. Am. J. Physiology-Heart Circulatory Physiology 283 (3), H899–H907. doi:10.1152/ajpheart.00049.2002
Green D. J. (2009). Exercise Training as Vascular Medicine. Exerc Sport Sci. Rev. 37 (4), 196–202. doi:10.1097/jes.0b013e3181b7b6e3
Green D. J., O'Driscoll G., Joyner M. J., Cable N. T. (2008). Exercise and Cardiovascular Risk Reduction: Time to Update the Rationale for Exercise? J. Appl. Physiology 105 (2), 766–768. doi:10.1152/japplphysiol.01028.2007
Gurovich A. N., Braith R. W. (2012). Analysis of Both Pulsatile and Streamline Blood Flow Patterns during Aerobic and Resistance Exercise. Eur. J. Appl. Physiol. 112 (11), 3755–3764. doi:10.1007/s00421-012-2367-z
Gurovich A. N., Rodriguez L. A., Gómez M. M., Caraveo P., Ochoa L., Morales-Acuna F. (2021a). Imaging Ultrasound Assessment of Exercise-Induced Endothelial Shear Stress of the Brachial and Carotid Arteries. Cardiopulm. Phys. Ther. J. doi:10.1097/cpt.0000000000000167
Gurovich A. N., Rodriguez L., Morales‐Acuna F. (2021b). There Are No Differences in Brachial Artery Endothelial Shear Stress and Blood Flow Patterns between Males and Females during Exercise. Clin. Physiol. Funct. Imaging 41 (6), 471–479. doi:10.1111/cpf.12722
Hambrecht R., Wolf A., Gielen S., Linke A., Hofer J., Erbs S., et al. (2000). Effect of Exercise on Coronary Endothelial Function in Patients with Coronary Artery Disease. N. Engl. J. Med. 342 (7), 454–460. doi:10.1056/nejm200002173420702
Harris R. A., Nishiyama S. K., Wray D. W., Richardson R. S. (2010). Ultrasound Assessment of Flow-Mediated Dilation. Hypertension 55 (5), 1075–1085. doi:10.1161/HYPERTENSIONAHA.110.150821
Hellstrom G., Fischer-Colbrie W., Wahlgren N. G., Jogestrand T. (19961985). Carotid Artery Blood Flow and Middle Cerebral Artery Blood Flow Velocity during Physical Exercise. J. Appl. Physiology 81 (1), 413–418. doi:10.1152/jappl.1996.81.1.413
Hopkins W. (2009). A Scale of Magnitude for Effect Statistics. Accessed January 24th Available at: http://www.sportsci.org/resource/stats/index.html
Ishibazawa A., Nagaoka T., Takahashi T., Yamamoto K., Kamiya A., Ando J., et al. (2011). Effects of Shear Stress on the Gene Expressions of Endothelial Nitric Oxide Synthase, Endothelin-1, and Thrombomodulin in Human Retinal Microvascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 52 (11), 8496–8504. doi:10.1167/iovs.11-7686
Iwamoto E., Bock J. M., Casey D. P. (2018). High-Intensity Exercise Enhances Conduit Artery Vascular Function in Older Adults. Med. Sci. sports Exerc. 50 (1), 124–130. doi:10.1249/MSS.0000000000001405
Jiang Z.-L., Yamaguchi H., Takahashi A., Tanabe S., Utsuyama N., Ikehara T., et al. (1995). Blood Flow Velocity in the Common Carotid Artery in Humans during Graded Exercise on a Treadmill. Eur. J. Appl. Physiol. 70 (3), 234–239. doi:10.1007/bf00238569
Jurczak I., Kowalski J., Irzmański R. (2014). Impact of Cardiac Rehabilitation on Peripheral Circulation as Assessed by Impedance Plethysmography: a Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 50 (6), 609–616.
Kirk H., Kersten P., Crawford P., Keens A., Ashburn A., Conway J. (2014). The Cardiac Model of Rehabilitation for Reducing Cardiovascular Risk Factors Post Transient Ischaemic Attack and Stroke: a Randomized Controlled Trial. Clin. Rehabil. 28 (4), 339–349. doi:10.1177/0269215513502211
Koskinas K. C., Chatzizisis Y. S., Baker A. B., Edelman E. R., Stone P. H., Feldman C. L. (2009). The Role of Low Endothelial Shear Stress in the Conversion of Atherosclerotic Lesions from Stable to Unstable Plaque. Curr. Opin. Cardiol. 24 (6), 580–590. doi:10.1097/HCO.0b013e328331630b
Laughlin M. H. (1995). Endothelium-mediated Control of Coronary Vascular Tone after Chronic Exercise Training. Med. Sci. Sports Exerc. 27 (8), 1135–1144. doi:10.1249/00005768-199508000-00006
Laughlin M. H., Newcomer S. C., Bender S. B. (2008). Importance of Hemodynamic Forces as Signals for Exercise-Induced Changes in Endothelial Cell Phenotype. J. Appl. Physiology 104 (3), 588–600. doi:10.1152/japplphysiol.01096.2007
Liu H. B., Yuan W. X., Qin K. R., Hou J. (2015). Acute Effect of Cycling Intervention on Carotid Arterial Hemodynamics: Basketball Athletes versus Sedentary Controls. Biomed. Eng. Online 14 Suppl 1 (1), S17–S13. doi:10.1186/1475-925X-14-S1-S17
Marzolini S., Tang A., McIlroy W., Oh P. I., Brooks D. (2014). Outcomes in People after Stroke Attending an Adapted Cardiac Rehabilitation Exercise Program: Does Time from Stroke Make a Difference? J. Stroke Cerebrovasc. Dis. 23 (6), 1648–1656. doi:10.1016/j.jstrokecerebrovasdis.2014.01.008
Mattu A. T., MacInnis M. J., Doyle‐Baker P. K., Murias J. M. (2020). Effects of the Menstrual and Oral Contraceptive Cycle Phases on Microvascular Reperfusion. Exp. Physiol. 105 (1), 184–191. doi:10.1113/ep088135
Montalvo S., Gruber L. D., Gonzalez M. P., Dietze-Hermosa M. S., Dorgo S. (2021). Effects of Augmented Eccentric Load Bench Press Training on One Repetition Maximum Performance and Electromyographic Activity in Trained Powerlifters. J. Strength & Cond. Res. 35 (6). doi:10.1519/jsc.0000000000004030
Mora S., Cook N., Buring J. E., Ridker P. M., Lee I.-M. (2007). Physical Activity and Reduced Risk of Cardiovascular Events. Circulation 116 (19), 2110–2118. doi:10.1161/circulationaha.107.729939
Morales‐Acuna F., Ochoa L., Valencia C., Gurovich A. N. (2019). Characterization of Blood Flow Patterns and Endothelial Shear Stress during Flow‐mediated Dilation. Clin. Physiol. Funct. Imaging 39 (4), 240–245. doi:10.1111/cpf.12564
Morales-Acuna F., Ochoa L. A., Gurovich A. N. (2020). Ultrasound Transducer Holder for Vascular Assessments during Hyperemic Blood Flow. USA Patent No. G. Patents.
Neto J. C., Cedin L., Dato C. C., Bertucci D. R., Perez S. E. A., Baldissera V. (2015). A Single Session of Testing for One Repetition Maximum (1RM) with Eight Exercises Is Trustworthy. J. Exerc. physiology, 74–80.
Prior P. L., Hachinski V., Chan R., Unsworth K., Mytka S., Harnadek M., et al. (2017). Comprehensive Cardiac Rehabilitation for Secondary Prevention after Transient Ischemic Attack or Mild Stroke. J. Cardiopulm. Rehabil. Prev. 37, 428–436. doi:10.1097/HCR.0000000000000274
Rascon J., Trujillo E., Morales-AcuÑa F., Gurovich A. N. (2020). Differences between Males and Females in Determining Exercise Intensity. Int. J. Exerc Sci. 13 (4), 1305–1316. https://pubmed.ncbi.nlm.nih.gov/33042374.
Rodrà guez I. n., González M. (2014). Physiological Mechanisms of Vascular Response Induced by Shear Stress and Effect of Exercise in Systemic and Placental Circulation. Front. Pharmacol. 5, 209. doi:10.3389/fphar.2014.00209
Rstudio T. (2020). “RStudio: Integrated Development for R,” in Rstudio PBC. http://www.rstudio.com/.
Sato K., Ogoh S., Hirasawa A., Oue A., Sadamoto T. (2011). The Distribution of Blood Flow in the Carotid and Vertebral Arteries during Dynamic Exercise in Humans. J. physiology 589 (11), 2847–2856. doi:10.1113/jphysiol.2010.204461
Sato K., Sadamoto T. (2010). Different Blood Flow Responses to Dynamic Exercise between Internal Carotid and Vertebral Arteries in Women. J. Appl. Physiology 109 (3), 864–869. doi:10.1152/japplphysiol.01359.2009
Seo D. I., Kim E., Fahs C. A., Rossow L., Young K., Ferguson S. L., et al. (2012). Reliability of the One-Repetition Maximum Test Based on Muscle Group and Gender. J. Sports Sci. Med. 11 (2), 221–225. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3737872/.
Spence A. L., Carter H. H., Naylor L. H., Green D. J. (2013). A Prospective Randomized Longitudinal Study Involving 6 Months of Endurance or Resistance Exercise. Conduit Artery Adaptation in Humans. J. physiology 591 (5), 1265–1275. doi:10.1113/jphysiol.2012.247387
Sriram K., Laughlin J. G., Rangamani P., Tartakovsky D. M. (2016). Shear-Induced Nitric Oxide Production by Endothelial Cells. Biophysical J. 111 (1), 208–221. doi:10.1016/j.bpj.2016.05.034
Tagesson S. K. B., Kvist J. (2007). Intra- and Interrater Reliability of the Establishment of One Repetition Maximum on Squat and Seated Knee Extension. J. Strength Cond. Res. 21 (3), 801–807. R-20846 [pii]. doi:10.1519/r-20846.1
Tang A., Closson V., Marzolini S., Oh P., McIlroy W., Brooks D. (2009). Cardiac Rehabilitation after Stroke-Need and Opportunity. J. Cardiopulm. Rehabil. Prev. 29 (2), 97–104. doi:10.1097/HCR.0b013e31819a00d4
Urbich M., Globe G., Pantiri K., Heisen M., Bennison C., Wirtz H. S., et al. (2020). A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020.
Keywords: atherosclerosis, blood lactate levels, aerobic exercise, resistance exercise, stroke
Citation: Montalvo S, Gomez M, Lozano A, Arias S, Rodriguez L, Morales-Acuna F and Gurovich AN (2022) Differences in Blood Flow Patterns and Endothelial Shear Stress at the Carotid Artery Using Different Exercise Modalities and Intensities. Front. Physiol. 13:857816. doi: 10.3389/fphys.2022.857816
Received: 19 January 2022; Accepted: 22 April 2022;
Published: 10 May 2022.
Edited by:
Giuseppe D’Antona, University of Pavia, ItalyReviewed by:
Olivier Gavarry, Université de Toulon, FranceErhan Tenekecioglu, University of Health Sciences, Turkey
Copyright © 2022 Montalvo, Gomez, Lozano, Arias, Rodriguez, Morales-Acuna and Gurovich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvaro N. Gurovich, agurovich@utep.edu
 Samuel Montalvo
Samuel Montalvo Manuel Gomez
Manuel Gomez Alondra Lozano
Alondra Lozano Sabrina Arias
Sabrina Arias Lisa Rodriguez
Lisa Rodriguez Francisco Morales-Acuna
Francisco Morales-Acuna Alvaro N. Gurovich
Alvaro N. Gurovich