- Department of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Savolitinib, a small-molecule inhibitor of the receptor tyrosine kinase mesenchymal-epithelial transition (MET) factor, was approved for the treatment of non-small cell lung cancer (NSCLC) by the China National Medical Products Administration in June 2021. Its safety for NSCLC treatment has been confirmed in several prospective cohort studies. Herein, we report a rare case of shock, a serious adverse event, after treatment with savolitinib in an HIV-1–positive patient with advanced NSCLC. A 38-year-old man with an 8-year history of HIV-1 positivity was diagnosed with NSCLC 5 years ago; the lung cancer recurred after surgical resection. Despite chemotherapy, immunotherapy, and targeted therapy, tumor progression continued. He received savolitinib because of MET amplification. In the first 2 weeks of savolitinib use, he developed a mild rash on his trunk. In the following month, he was hospitalized for fever and circulatory shock thrice after taking savolitinib 400 mg. He had no urticaria or eosinophilia. During the three hospitalizations, he was negative for pathogens. His condition gradually improved after treatment with antibiotics, steroids, and vasopressors. Attention should be paid to the occurrence of septic shock-like presentations when using savolitinib in HIV-1 patients with NSCLC.
Introduction
Mesenchymal-epithelial transition (MET) or hepatocyte growth factor receptor (HGFR) is a member of the receptor tyrosine kinase family, which combines with its ligand HGF to regulate basic cell functions (Tovar and Graveel, 2017; Wood, 2021). However, abnormal MET gene amplification can lead to a wide range of human cancers (Grundy and Narendran, 2022). Thus, the HGF/MET axis is a feasible target for the development of cancer drugs. In recent years, MET inhibitors have been evaluated in preclinical investigations and clinical trials, and against various malignancies, and found to be capable of prolonging survival (Forde and Rudin, 2012; D'Arcangelo and Cappuzzo, 2013; Woo, 2015). The oral formulation of savolitinib, a highly selective type Ib MET inhibitor, has been approved by the China National Medical Products Administration for the treatment of patients with advanced non-small cell lung cancer (NSCLC) (Markham, 2021).
We found that savolitinib did appear to be associated with a series of adverse events (AEs) in relevant clinical trials in which its safety was investigated. Among the drug-associated AEs, nausea, fatigue, peripheral edema, and abnormal hepatic function were the most common (Lu, 2021; Wang, 2022). However, the incidence of severe allergic reactions (drug hypersensitivity, anaphylactic shock and hypersensitivity) was rare (Sequist, 2020). Herein, we report a rare case of shock, a serious adverse event, after treatment with savolitinib in an HIV-1–positive patient with advanced NSCLC.
Case description
The patient was a 38-year-old man with an 8-year history of HIV-1 positivity. His initial combination antiviral therapy (cART) regimen was lamivudine plus efavirenz plus tenofovir, and the CD4+ cell count remained >500/µL after the regimen. The cART regimen was eventually adjusted to bictegravir in January 2022, with good compliance. The patient was diagnosed with NSCLC 5 years ago and underwent right middle lung lobectomy and mediastinal lymph node resection under video-assisted thoracoscopic surgery in the year of diagnosis. Postoperative pathology confirmed poorly-to-moderately differentiated adenocarcinoma with lymph node metastasis, with a size of 3.0 cm × 2.0 cm, and tumor infiltration involved the lung membrane. Immunohistochemical staining was positive for PD-L1 (membrane +10%), TTF-1 (+), Ki-67 (+20%), Napsin A (+), and CK7 (+), and negative for ALK (−). Gene testing (next-generation sequencing, NGS) of tumor tissue sections showed an epidermal growth factor receptor (EGFR) exon 21 L858R mutation. The patient started taking icotinib. One and a half years after operation, pulmonary computed tomography (CT) reexamination revealed tumor recurrence in the right nodule and new lesions of the left lung, which were considered metastatic tumors. Although blood NGS revealed the T790M mutation was negative, the patient perferred to switch molecular targeted drug to osimertinib after completely discussion with his oncological doctor. Because the tumor unfortunately progressed repeatedly, he received various treatments, including immunotherapy, targeted therapy, and chemotherapy (Figure 1). Positron emission tomography–computed tomography (PET–CT) performed approximately 3 months ago (1 August 2022) suggested that the disease had progressed again. NGS of tumor tissues suggested EGFR L858R mutation (abundance: 54%) and MET amplification (copy number: 5.9), and the former was detected with 5.4% abundance in the blood sample. Based on the recommendations of oncologists, the patient began to take the third-generation EGFR-tyrosine kinase inhibitor (TKI) almonertinib (110 mg per day) and the MET inhibitor savolitinib (400 mg per day) from 1 August 2022. He habitually takes almonertinib in the morning and savolitinib at night.
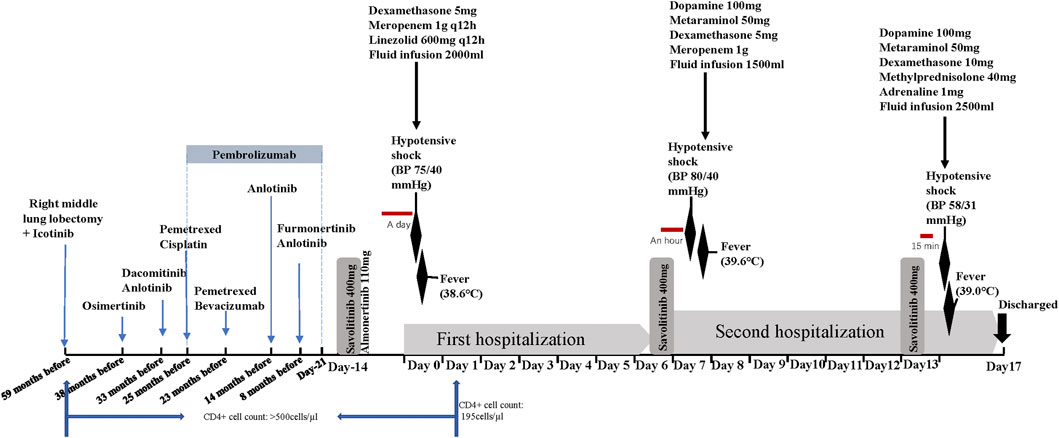
FIGURE 1. Treatment axis of the patient (time flows from left to right, day 0 is the time of the first adverse reaction).
Despite a mild rash after receiving savolitinib and almonertinib, the patient was not concerned. After 2 weeks of taking savolitinib and almonertinib, he was hospitalized with a fever. He denied a history of food and drug allergies. His CD4+ count dropped to <200 cells/μL. On admission (day 0), the patient had a temperature of 38.6°C accompanied by dizziness and chest tightness. He developed hypotensive shock, with blood pressure (BP) 75/40 mmHg and a heart rate >110 beats/min. The initial laboratory results were: C-reactive protein (CRP) level, 108.5 mg/L; white blood cell (WBC) count, 8.44×109/L (93.4% neutrophils, 0.8% eosinophils); and lactate level, 2.8 mmol/L. Lung CT and B-ultrasound of the organs showed no signs of infection. Because of the patient’s septic shock-like symptoms, we added empirical antibiotics besides glucocorticoid for antishock therapy (concrete therapy shown in Figure 1). The hypotension resolved 7 h later. Savolitinib and almonertinib were discontinued. Four days later, the patient’s indicators of infection normalized. Further tests (such as blood culture, urine culture, and pathogen NGS) did not clearly reveal infection foci to confirm the diagnosis of septic shock. Hypotension did not develop during the subsequent hospitalization. On day 6, he was discharged from the hospital and asked to continue savolitinib and almonertinib.
However, he was readmitted because of a fever (39.6°C), nausea, dizziness, and palpitations approximately 1 h after oral administration of savolitinib on the night of hospital discharge. His BP was 80/40 mmHg, and laboratory examination showed a WBC count of 13.75×109/L (97% neutrophils and 0.1% eosinophils) and lactate and CRP levels of 3.2 mmol/L and 108.6 mg/L, respectively. He received treatment similar to that administered on the previous hospital admission, and his clinical symptoms and inflammatory indicators resolved within a short time. We wondered if these were adverse reactions caused by savolitinib. Therefore, we asked the patient to take savolitinib on a trial basis after 1 week of continuous antibiotic therapy (i.e., on day 13). Just 15 min after taking savolitinib, he complained of chest tightness, shortness of breath, dizziness, and cold limbs. His BP was 58/31 mmHg, heart rate 142 beats/min, and temperature 39°C. He promptly received 10 mg dexamethasone, 40 mg methylprednisolone, and 1 mg adrenaline infusion. With continuous antishock treatment, the patient improved 1 h later. His BP gradually recovered to 127/55 mmHg, and his heart rate to 100 beats/min. Based on these three similar occurrences, we confirmed that these phenomena were savolitinib-related AEs. Although the lung CT indicated that tumor was shrinking and according to RECIST1.1, the patient was in partial response condition after taking savolitinib. In order to avoid the recurrence of life-threatening shock, crizotinib, instead of savolitinib, combined with almonertinib was the final targeted regimen according to oncologists’ recommendations. The patient did not experience similar adverse reactions over the next 2 months.
Discussion
Herein, we present a case of a patient who developed resembling septic shock-like symptoms after taking savolitinib. Systemic examinations and the patient’s responses to treatment showed that savolitinib was the most likely culprit. To our knowledge, this is the first report of MET inhibitor-induced resembling septic shock-like presentations.
Lung cancer remains the leading cause of cancer-related deaths worldwide, with NSCLC accounting for 80% of cases (Servetto et al., 2022). Although the development of immunotherapy has greatly improved therapeutic effectiveness against NSCLC, its role in driving mutant NSCLC remains to be determined. Targeted therapy is currently considered to be one of the most promising options for managing the manifestations of mutations in carcinogenic drivers (López-Castro, 2022). Savolitinib, a type of MET-TKI, was approved for the first time in China in 2021 for the treatment of metastatic NSCLC (Markham, 2021). A multicenter phase II trial (NCT02897479) demonstrated that savolitinib monotherapy affords a good disease control rate and overall survival in patients with advanced NSCLC (Lu, 2021).
Regarding the known adverse effects of savolitinib, to date, severe systemic reactions resembling septic shock have never been reported. The main reason may be that most savolitinib-related studies included HIV-negative patients. Studies have established that HIV-positive individuals are more likely to develop drug reactions compared with the general population. Multiple heterogeneous factors are responsible for the substantial risk in HIV-infected patients, including polypharmacy, slow acetylator phenotype, glutathione deficiency, a CD4+ T Cell count of <200 cells/mm3 or >25 cells/mm3, latent cytomegalovirus and Epstein-Barr virus infections (Carr and Cooper, 2000). It was reported that the incidence of serious adverse drug reactions (sADRs) of trimethoprim-sulfamethoxazole in HIV-infected persons correlated with the HIV load (Coopman, 1993; Liu et al., 2018). Moreover, Rabaud et al. studied 592 HIV-infected patients who were first treated with cotrimoxazole during the Delta trial (a randomized, double-blind, controlled trial). They found that a low CD4+ cell count at the time of cotrimoxazole introduction was the only factor associated with the onset of sADRs (Rabaud, 2001). Our patient had an 8-year history of HIV-1 infection. Laboratory tests showed that his recent CD4+ count was significantly lower than that in previous measurements. HIV-infected patients with poor immune status may be highly sensitive to drug therapy (Greenberger, 2019). Therefore, we wondered whether the low CD4+ cell count was related to the circulatory shock induced by savolitinib.
Other possible causes of shock in this patient, such as sepsis, anaphylaxis, and severe immune-related AEs (irAEs), were also considered. The shock in this case had some similarities to septic shock (e.g., hypotension, fever, and elevated inflammatory markers). However, no causative infectious agent has been identified. Anaphylaxis is a rapidly evolving multisystem process involving the epidermis, lungs, and gastrointestinal and cardiovascular systems, which can lead to fatal airway obstruction, and ultimately cause hypoxemia and even shock (Simons, 2014; LoVerde, 2018). The classic anaphylaxis pathway is mediated via the production of IgE by T and B Cells and subsequent cross-linking of IgE with high-affinity receptors on mast cells and basophils (Nguyen, 2021). The serum IgE level of the patient did not increase. Other symptoms of anaphylaxis include airway edema, bronchospasm, eosinophilia, and vascular edema. However, these clinical events did not occur in this patient. Of course, other types of allergic reactions might occur through non-IgE–mediated pathways.
Cytokine release syndrome (CRS) is a rare and life-threatening immune checkpoint inhibitor (ICI)-related adverse reaction, which is caused by systemic immune dysregulation, immune cell overactivation, and cytokine release (Li, 2021). The clinical manifestations of CRS are fever, hemodynamic instability, and organ failure (Rotz, 2017). In most reported cases, CRS occurred within 2 weeks after the last ICI treatment (Ciner, 2021). Our patient exhibited fever and hypotensive shock 21 days after pembrolizumab discontinuation. The levels of cytokines such as interleukin (IL)-4 (4.42 pg/mL; normal <3.20 pg/mL), IL-6 (7.66 pg/mL; normal <2.90 pg/mL), and IL-10 (6.17 pg/mL; normal <5.00 pg/mL) were higher than the corresponding normal ranges in this case. These findings are common in patients with CRS. Nevertheless, the serum levels of these cytokines were very low compared with those previously reported for cases of CRS (Rotz, 2017; Dimitriou, 2019), and the serum IFN-γ and TNF-α levels were normal. The possibility of savolitinib triggering delayed and atypical CRS after the switch between immunotherapy and targeted therapy is interesting. The latency period for the three episodes progressively shortened and the symptoms continued to worsen. The pathogenesis of the circulatory shock was unclear; however, it was, to different extents, different from those of septic shock, anaphylactic shock, and CRS. Studies have established that HIV-positive individuals are a hundred times more susceptible to drug reactions than the general population, and advanced immunodeficiency portends an even greater risk (Hoosen, 2019). The symptoms clearly indicated the patient’s decreased immunity, whereby his CD4+ cell count dropped from >500 to <200 cells/μL. At present, it is unclear whether this phenomenon represents immune activation, drug toxicity, or a non-specific immune response, or appears independently in HIV-infected patients.
Conclusion
To date, severe systemic reactions resembling septic shock caused by savolitinib have not been reported. To our knowledge, this is the first such case reported in an HIV-1–positive patient with advanced NSCLC. Savolitinib-induced symptoms that resemble septic shock are not a widely recognized phenomenon. However, they are worth considering in patients with immunodeficiency accompanied by circulatory shock who have recently taken savolitinib. It is critical to consider adding savolitinib to the allergy list of these patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of the Medical College of Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors participated in the diagnosis and treatment process. YX and QC wrote the original draft. YG, XL and BZ diagnosed the patient and developed the treatment plan. XZ and BD performed the data collection. BZ designed and revised the original draft. All authors have reviewed and approved its publication.
Funding
This work was supported by the National Key R&D Program of China (grant numbers 2021YFC2301900, 2021YFC2301901).
Acknowledgments
We thank the patient who participated in this study for his cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Carr, A., and Cooper, D. A., Adverse effects of antiretroviral therapy. Lancet, 2000. 356(9239): p. 1423–1430. doi:10.1016/S0140-6736(00)02854-3
Ciner, A. T., Hochster, H. S., August, D. A., Carpizo, D. R., and Spencer, K. R. Delayed cytokine release syndrome after neoadjuvant nivolumab: A case report and literature review. Immunotherapy, 2021. 13(13): p. 1071–1078. doi:10.2217/imt-2020-0329
Coopman, S. A., Johnson, R. A., Platt, R., and Stern, R. S. Cutaneous disease and drug reactions in HIV infection. N. Engl. J. Med., 1993. 328(23): p. 1670–1674. doi:10.1056/NEJM199306103282304
D'Arcangelo, M., and Cappuzzo, F., Focus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancer. Biologics, 2013. 7: p. 61–68. doi:10.2147/BTT.S28908
Dimitriou, F., Matter, A. V., Mangana, J., Urosevic-Maiwald, M., Micaletto, S., Braun, R. P., et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J. Immunother., 2019. 42(1): p. 29–32. doi:10.1097/CJI.0000000000000236
Forde, P. M., and Rudin, C. M., Crizotinib in the treatment of non-small-cell lung cancer. Expert Opin. Pharmacother., 2012. 13(8): p. 1195–1201. doi:10.1517/14656566.2012.688029
Greenberger, P. A., Drug allergy. Allergy Asthma Proc., 2019. 40(6): p. 474–479. doi:10.2500/aap.2019.40.4275
Grundy, M., and Narendran, A., The hepatocyte growth factor/mesenchymal epithelial transition factor axis in high-risk pediatric solid tumors and the anti-tumor activity of targeted therapeutic agents. Front. Pediatr., 2022. 10: p. 910268, doi:10.3389/fped.2022.910268
Hoosen, K., Mosam, A., Dlova, N. C., and Grayson, W. An update on adverse cutaneous drug reactions in HIV/AIDS. Dermatopathol. (Basel), 2019. 6(2): p. 111–125. doi:10.1159/000496389
Li, X., Shao, M., Zeng, X., Qian, P., and Huang, H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct. Target Ther., 2021. 6(1): p. 367, doi:10.1038/s41392-021-00764-4
Liu, P., Ranches, G. P., and Gold, J. A., Trimethoprim-sulfamethoxazole induced circulatory shock in a human immunodeficiency virus uninfected patient: A case report and review. BMC Pharmacol. Toxicol., 2018. 19(1): p. 76, doi:10.1186/s40360-018-0269-3
López-Castro, R., Garcia-Pena, T., Mielgo-Rubio, X., Riudavets, M., Teixido, C., Vilarino, N., et al. Targeting molecular alterations in non-small-cell lung cancer: what's next? Per Med., 2022. 19(4): p. 341–359. doi:10.2217/pme-2021-0059
LoVerde, D., Iweala, O. I., Eginli, A., and Krishnaswamy, G. Anaphylaxis. Chest, 2018. 153(2): p. 528–543. doi:10.1016/j.chest.2017.07.033
Lu, S., Fang, J., Li, X., Cao, L., Zhou, J., Guo, Q., et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med., 2021. 9(10): p. 1154–1164. doi:10.1016/S2213-2600(21)00084-9
Markham, A., Savolitinib: First approval. Drugs, 2021. 81(14): p. 1665–1670. doi:10.1007/s40265-021-01584-0
Nguyen, S. M. T., Rupprecht, C. P., Haque, A., Pattanaik, D., Yusin, J., and Krishnaswamy, G. Mechanisms governing anaphylaxis: Inflammatory cells, mediators, endothelial gap junctions and beyond. Int. J. Mol. Sci., 2021. 22, 7785, doi:10.3390/ijms2215778515).
Rabaud, C., Charreau, I., Izard, S., Raffi, F., Meiffredy, V., Leport, C., et al. Adverse reactions to cotrimoxazole in HIV-infected patients: Predictive factors and subsequent HIV disease progression. Scand. J. Infect. Dis., 2001. 33(10): p. 759–764. doi:10.1080/003655401317074581
Rotz, S. J., Leino, D., Szabo, S., Mangino, J. L., Turpin, B. K., and Pressey, J. G. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr. Blood Cancer, 2017. 64, e26642, doi:10.1002/pbc.2664212).
Sequist, L. V., Han, J. Y., Ahn, M. J., Cho, B. C., Yu, H., Kim, S. W., et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol., 2020. 21(3): p. 373–386. doi:10.1016/S1470-2045(19)30785-5
Servetto, A., Esposito, D., Ferrara, R., Signorelli, D., Belli, S., Napolitano, F., et al. RET rearrangements in non-small cell lung cancer: Evolving treatment landscape and future challenges. Biochim. Biophys. Acta Rev. Cancer, 2022. 1877(6): p. 188810. doi:10.1016/j.bbcan.2022.188810
Simons, F. E., Ardusso, L. R., Bilo, M. B., Cardona, V., Ebisawa, M., El-Gamal, Y. M., et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J., 2014. 7(1): p. 9, doi:10.1186/1939-4551-7-9
Tovar, E. A., and Graveel, C. R., MET in human cancer: Germline and somatic mutations. Ann. Transl. Med., 2017. 5(10): p. 205, doi:10.21037/atm.2017.03.64
Wang, Y., Liu, T., Chen, G., Gong, J., Bai, Y., Zhang, T., et al. Phase ia/ib study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: Safety, efficacy, and biomarkers. Oncologist, 2022. 27(5): p. 342-e383. doi:10.1093/oncolo/oyab066
Woo, J. K., Kang, J. H., Kim, B., Park, B. H., Shin, K. J., Song, S. W., et al. Humanized anti-hepatocyte growth factor (HGF) antibody suppresses innate irinotecan (CPT-11) resistance induced by fibroblast-derived HGF. Oncotarget, 2015. 6(27): p. 24047–24060. doi:10.18632/oncotarget.4369
Keywords: shock, adverse drug rection, targeted therapy, savolitinib, HIV-1
Citation: Xiong Y, Cao Q, Guo Y, Liu X, Zhu X, Dai B and Zhu B (2023) Case report: Savolitinib induced severe adverse reactions resembling septic shock in an HIV-1–positive patient with advanced non-small cell lung cancer. Front. Pharmacol. 14:1089184. doi: 10.3389/fphar.2023.1089184
Received: 04 November 2022; Accepted: 20 January 2023;
Published: 02 February 2023.
Edited by:
Fanfan Zhou, The University of Sydney, AustraliaReviewed by:
Wen Ye, The First Affiliated Hospital of Sun Yat-sen University, ChinaHeidi Mikaela Haikala, University of Helsinki, Finland
Yi Cheng, Huazhong University of Science and Technology, China
Copyright © 2023 Xiong, Cao, Guo, Liu, Zhu, Dai and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Zhu, zhubiao1207@zju.edu.cn
†These authors have contributed equally to this work
 Ye Xiong†
Ye Xiong† Yongzheng Guo
Yongzheng Guo Xiang Liu
Xiang Liu Xueling Zhu
Xueling Zhu Biao Zhu
Biao Zhu