- 1Department of Surgery, Clinic of Surgical Oncology and Neuroendocrine Tumors, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
- 2Department of Endocrinology, Medical Centre of Postgraduate Education, Bielański Hospital, Warsaw, Poland
- 3Department of Surgical and Transplantation Nursing and Extracorporeal Therapies, Medical University of Warsaw, Warsaw, Poland
- 4Department of General and Transplant Surgery , Medical University of Warsaw, Warsaw, Poland
- 5Department of Pathology, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland
Purpose: Adrenal gland is a common site of metastasis and on the other hand, metastases are the most frequent malignant adrenal tumors. The aim of this study was to estimate the risk of malignancy in suspicious adrenal mass in patients with a history of cancer.
Methods: This is a single-center retrospective analysis of patients with adrenal tumors treated previously for different types of cancers. Between 2004 and 2021 a hundred and six such patients were identified. Mean age of patients was 62.6 years (30-78), and mean time from oncologic treatment was 55.8 months (0-274). The most common primary cancer was kidney (RCC): 29 (27.4%), colon/rectum (CRC): 20 (18.9%) and lung (NSCLC): 20 (18.9%).
Results: Of 106 patients, 12 had hormonally active (HA) (11,3%) and 94 (88,7%) non active (HNA) tumors In group of patients with HA tumours 4 had hypercortisolaemia and 8 had elevation of urinary metanephrines. In the first group of HA patients pathology confirmed preoperative diagnosis of adrenocortical cancer and no metastasis was found. In all patients from the second group pheochromocytomas were confirmed. Primary (PM) and secondary (SM) malignancies were found in 50 patients (47.2%). In hormone inactive group only SM - 46/94 (48.9%) were diagnosed. The odds that adrenal lesion was a metastasis were higher if primary cancer was RCC (OR 4.29) and NSCLC (OR 12.3). Metastases were also more likely with high native tumor density, and bigger size in CT. The cut-off values for tumor size and native density calculated from receiver operating characteristic (ROC) curves were 37mm and 24, respectively.
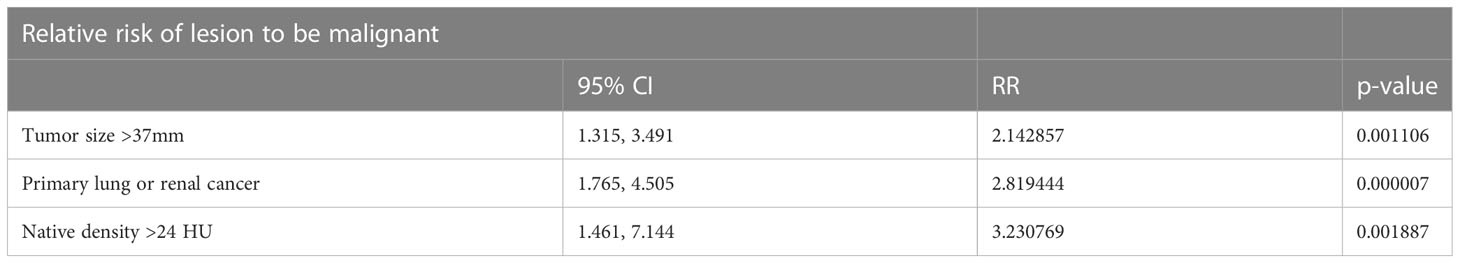
Conclusion: Risk of malignancy of adrenal mass in a patient with a history of cancer is high (47,2%), regardless of hormonal status. 47,2% risk of malignancy. In preoperative assessment type of primary cancer, adrenal tumour size and native density on CT should be taken into consideration as predictive factors of malignancy. Native density exceeding 24 HU was the strongest risk factor of adrenal malignancy (RR 3.23), followed by history of lung or renal cancer (RR 2.82) and maximum tumor diameter over 37 mm (RR 2.14).
Introduction
Incidentally detected adrenal masses in patients with no known malignancy occur in 1-5% of all abdominal computed tomography (CT) examinations (1, 2), and prevalence of such findings increases with age (3, 4). Majority of adrenal masses in patients with no known malignancy have been shown to be benign (1). In the absence of primary malignancy the risk of the adrenal lesion to be malignant is approximately one in a thousand (5).
Adrenal gland is one of the most prevalent sites for metastases from various malignant tumors. The most common type of adrenal tumors are benign cortical adenomas (6), however metastases are the most frequent malignant tumors of the adrenal gland and second cause of an adrenal mass (7). Presence of adrenal metastases in patients with a history of cancer varies in different series between 10 and 27% (8, 9). Rich sinusoidal blood supply of adrenal glands may favor this localization (9). Adrenal glands are the fourth most common site of metastases in malignant diseases (10).
Surveillance protocols in cancer treated patients have increased identification of incidental adrenal lesions. Good proportion of them are metastases. Sensitive and reliable methods of diagnostic imaging lead to earlier detection of adrenal metastases. In majority of cases it’s a manifestation of disseminated disease, however detection and surgical resection of isolated adrenal metastasis can improve prognosis in selected patients (11, 12). When adrenal mass is found in a patient with a history of extra-adrenal malignancy it is important to try to distinguish primary adrenal tumor from metastasis. Determination of malignant potential is usually a function of radiographic characteristics and size of an adrenal mass.
The aim of the study was to assess the risk of malignancy in adrenal tumors found in patients with a history of treatment for primary extra-adrenal cancer.
Retrospective chart review of all patients with a history of malignancy who underwent resection of an adrenal mass was performed.
Patients and methods
Medical records of patients referred to our center for adrenal mass with a history of cancer were reviewed. Pre-operative clinical, radiological and biochemical data as well as detailed operation and histology reports retrieved. Radiology and pathology reports were intentionally not re-evaluated, to keep the data entirely observational. All patients were initially evaluated and followed outside our hospital with adrenal CT protocol. Native, enhanced (after 1 min) and delayed (after 15 min) density was measured and an absolute contrast washout and relative contrast washout were calculated according to standard methods. Adrenal mass size was defined as the largest diameter on axial plane CT, attenuation on unenhanced CT was given in Hounsfield units (HU) and measured as a region of interest (ROI). A ROI was placed at the center of the lesion and its size and shape were adjusted to avoid calcification, cystic or necrotic area. Neither MRI nor PET-scan were used in the routine decision tree for the study purpose.
From January 2004 till December 2021, six hundred thirty patients with various adrenal tumors were referred to our center for surgical treatment. Initial diagnostic procedures (hormonal status, imaging) were most often performed in referring centers. As for functional status, at least levels of urinary catecholamines and plasma cortisol level with dexamethasone suppression test were done in all patients as well as others routine hormonal tests.
According to current guidelines, adrenal biopsies were not performed. Indications for surgery were determined on clinical history, cross-sectional imaging and/or hormonal activity. Of these, a hundred and six (16,8%) patients had a history of treatment for solid cancer. Patients with a history of hematological malignancies (n=2) were not included in this analysis. At adrenalectomy, the disease was clinically and radiographically limited to the adrenal gland in all patients. Extra-adrenal malignancy in every case was excluded with abdominal, pelvic and chest CT.
General anesthesia and appropriate antibiotic prophylaxis with second generation cephalosporin (cefuroxime) were used according to hospital protocol. All patients received low molecular weight heparins perioperatively as venous thromboembolism prophylaxis. Student’s t test was used to analyze variables with normal distribution. In the presence of non-normally distributed data, nonparametric tests (Mann-Whitney U test/χ2 test) were used. A multivariate logistic regression was used to identify predictive factors for metastasis in patients with a history of extra-adrenal malignancy having solitary adrenal mass. A receiver-operating characteristic (ROC) curve was used to identify the cut-off value of size, precontrast density and absolute contrast washout between adrenal metastases and benign lesions on preoperative CT. Analysis was performed with STATISTICA 13.3 software (TIBCO Software Inc, Tulsa, Oklahoma, United States). P value less than.05 was considered significant.
Results
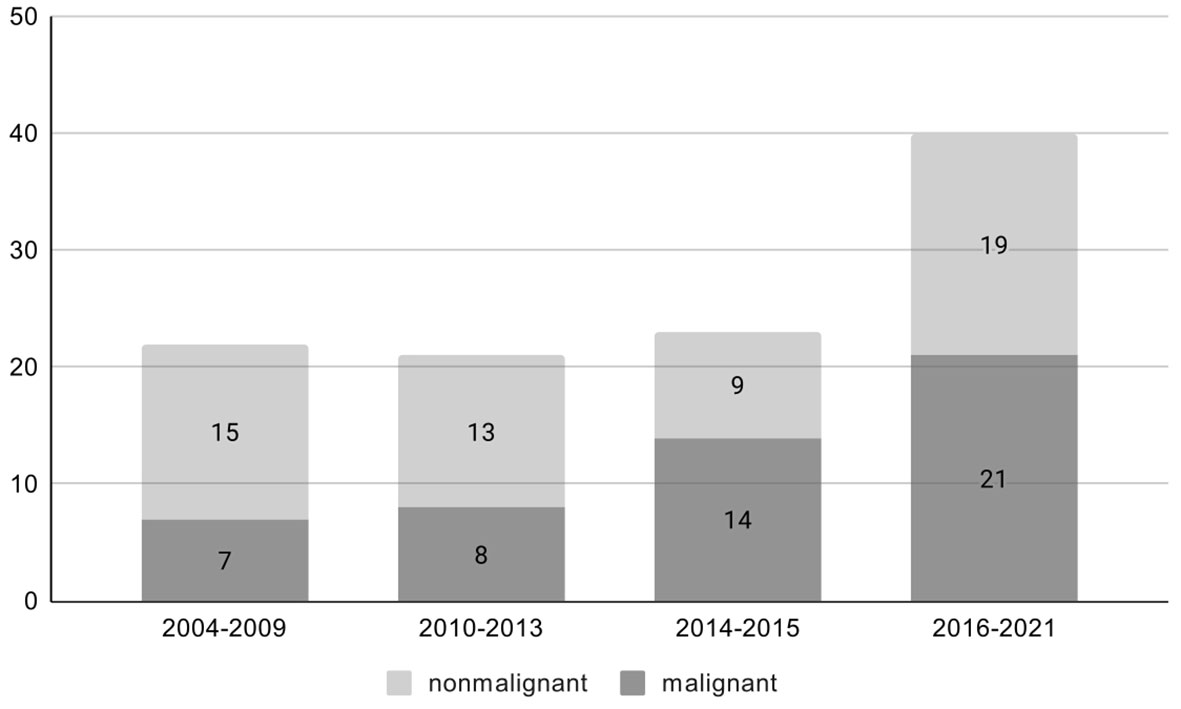
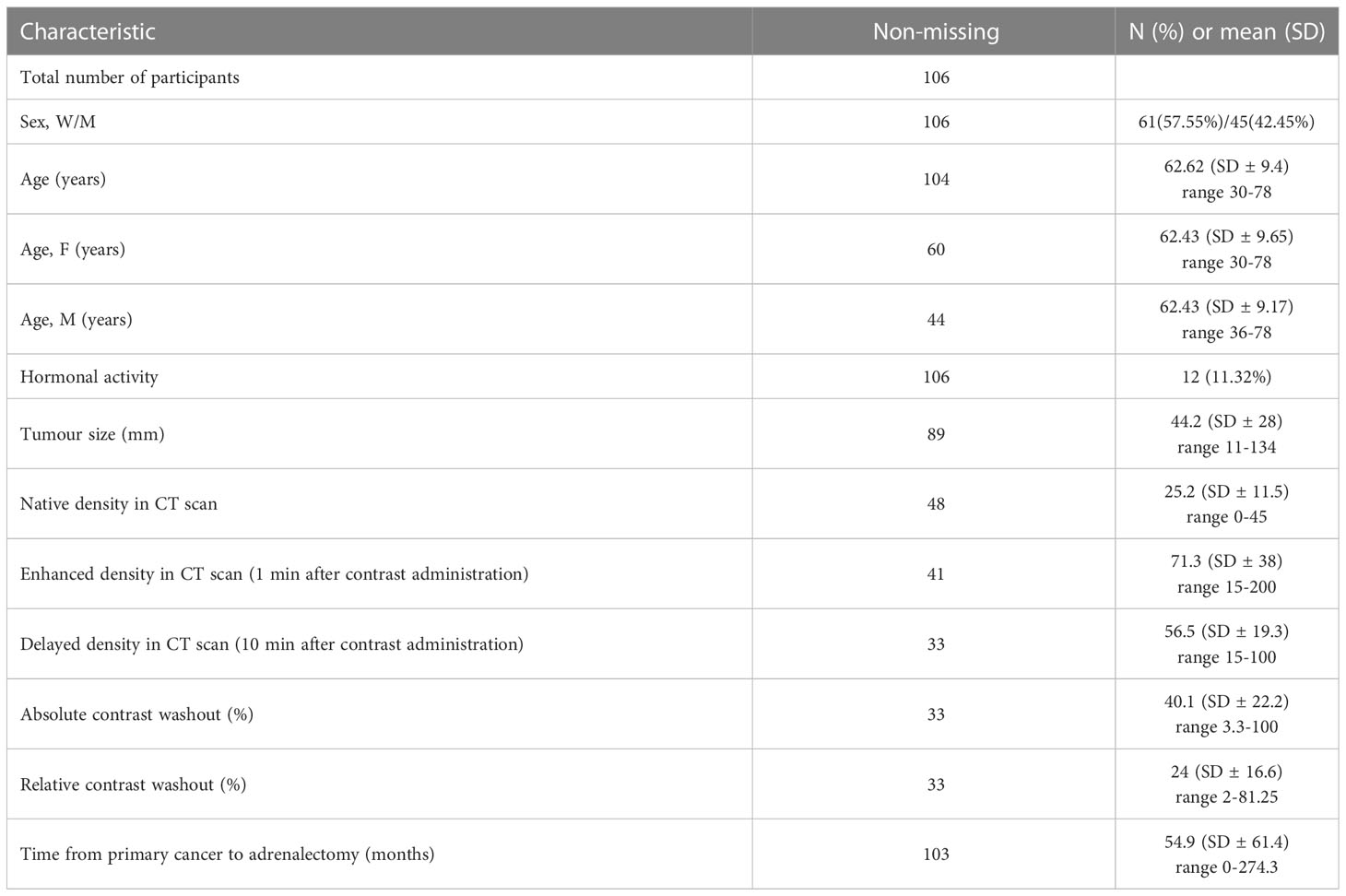
There were 106 patients with an adrenal mass in the setting of a prior or concurrent diagnosis of at least one extra-adrenal malignancy who were treated with adrenalectomy. The number of treated patients increased over time, accompanied by not significantly higher number of malignant lesions (p=0.17) Figure 1. Sixty-one patients (57,5%) were women. Mean age at the time of operation was 62.6 ± 9.4 years (range 30-78), and it was similar for men 62.4 ± 9.2 and women 62.4 ± 9.6. Baseline characteristics of the study population are presented in Table 1.
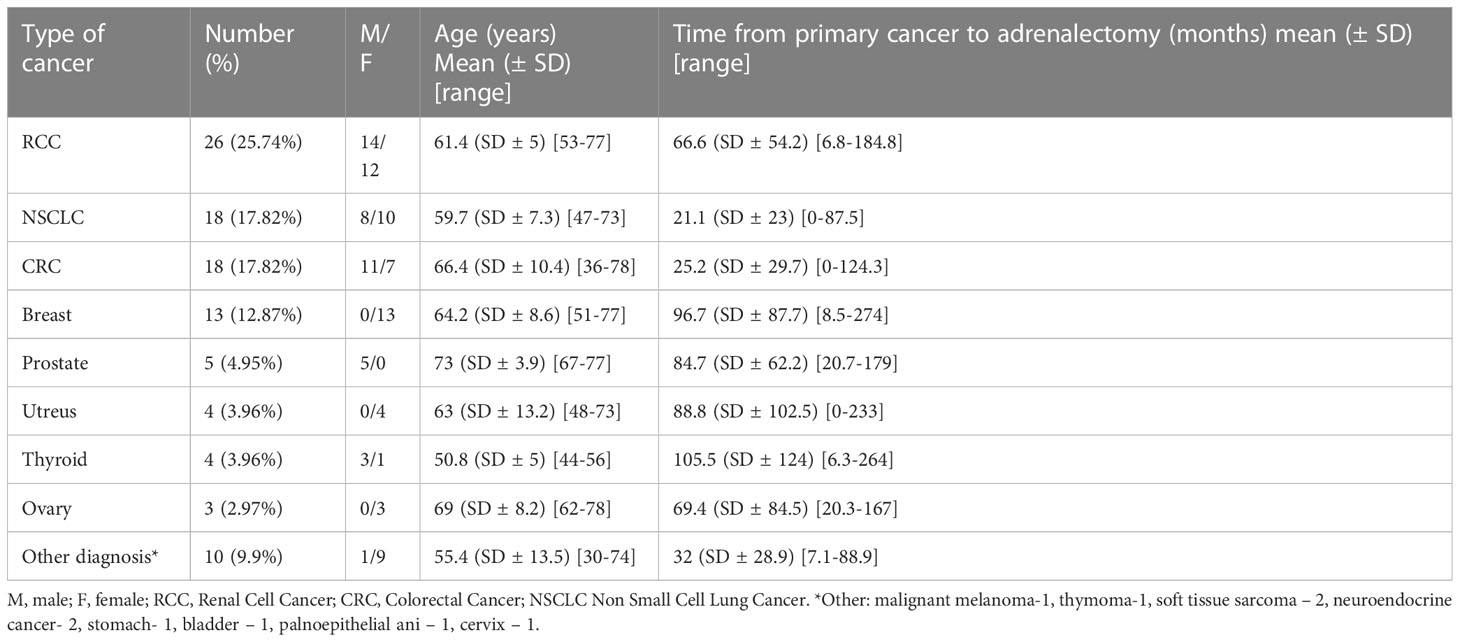
In a hundred and six patients, there were 112 cancers diagnosed: 4 patients were diagnosed with 2 cancers (RCC+NSCLC, RCC+melanoma, CRC+breast, CRC+ NSCLC) and one patient had history of 3 different cancers (RCC, thymoma and prostate). Four patients with multiple cancer diagnosis had adrenal metastases and 1 patient with rectal and breast cancer (16 and 6 years prior to adrenalectomy, respectively) was diagnosed with 50 mm non-functioning adenoma. Detailed characteristics of patients with single cancer diagnosis are presented in Table 2.
Fifty-seven left, 41 right and 6 bilateral adrenalectomies were performed. In two patients tumors were unresectable and only biopsy was carried out. Ninety open and 16 laparoscopic procedures were executed. Typical surgical approach was transabdominal lateral flank incision. Laparoscopic technique was introduced in our center in 2013, and since then majority of adrenalectomies have been performed with transabdominal approach. There were no fatalities or major complications associated with surgery. Three patients (2.8%) had mild complications, grade I and II in the Clavien-Dindo Classification (1 postoperative pneumonia, 1 wound hematoma without the need for surgical intervention, and 1 prolonged postoperative pain). Average postoperative hospital stay was 6.5 days (range 2-30). One prolonged hospitalization (30 days) was related to severe wound pain requiring prolonged opioids.
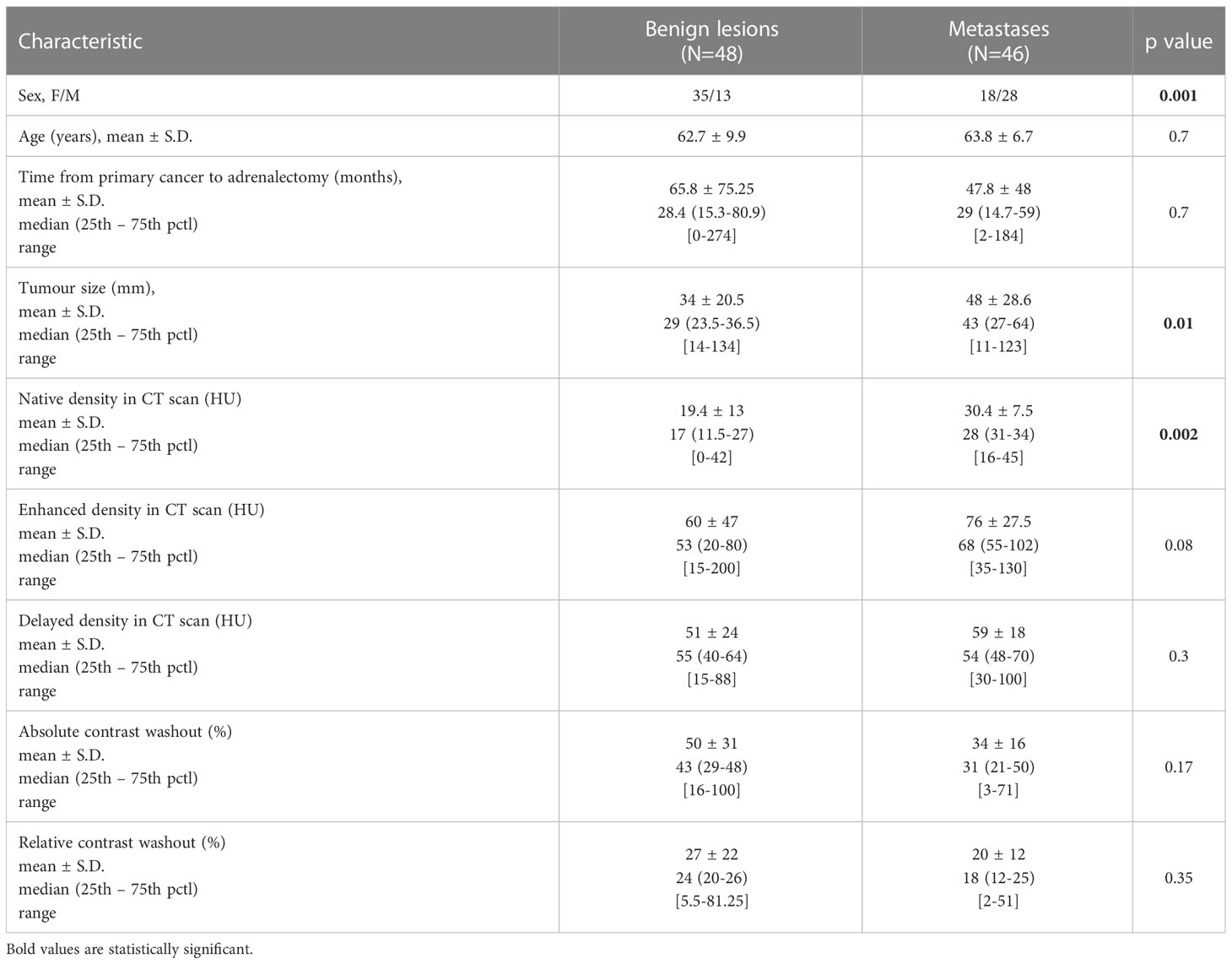
Primary or secondary malignancy was found in 50 of 106 (47.2%) patients. Risk of primary malignancy was relatively low, 4/106 (3,8%). There were 12 patients (11.3%) with hormonally active (HA) tumours of whom 4 had elevated cortisol levels unresponsive to dexamethasone suppression and 8 had elevations of urinary catecholamine levels. None of the patients had hyperaldosteronism or other type of hormonal abnormalities. In the first group of HA patients pathology confirmed preoperative diagnosis of adrenocortical cancer and no metastasis was found. In all patients from the second group pheochromocytoma was confirmed. Adrenocortical carcinoma was recognized only in females, after treatment of breast (n=2) and endometrial (n=2) cancers. In hormonally nonactive tumor group (HNA, n=94) all malignancies proved to be metastases (n=46, 48.9%). In HNA patients with benign lesions histopathological examination revealed 33 adenomas, 8 ACTH-independent macronodular adrenal hyperplasia (AIMAH), 3 post-hemorrhage changes, 2 cystic lesions and 2 cases of pheochromocytoma. Tumor characteristic in patients without hormonal activity is presented in Table 3.
In multivariable regression analyses of potential risk factors for metastases before adrenalectomy, tumor size, native density, and primary RCC and NSCLC were significant predictive factors for adrenal metastasis – Tables 4, 5.
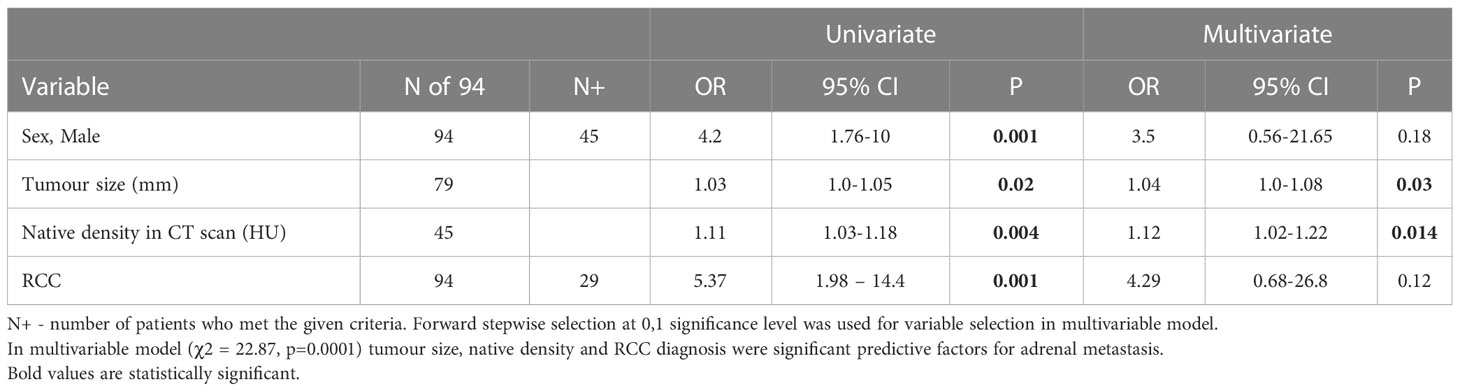
Table 4 Multivariable regression analysis of potential risk factors for metastasis before adrenalectomy (RCC included as a covariate).
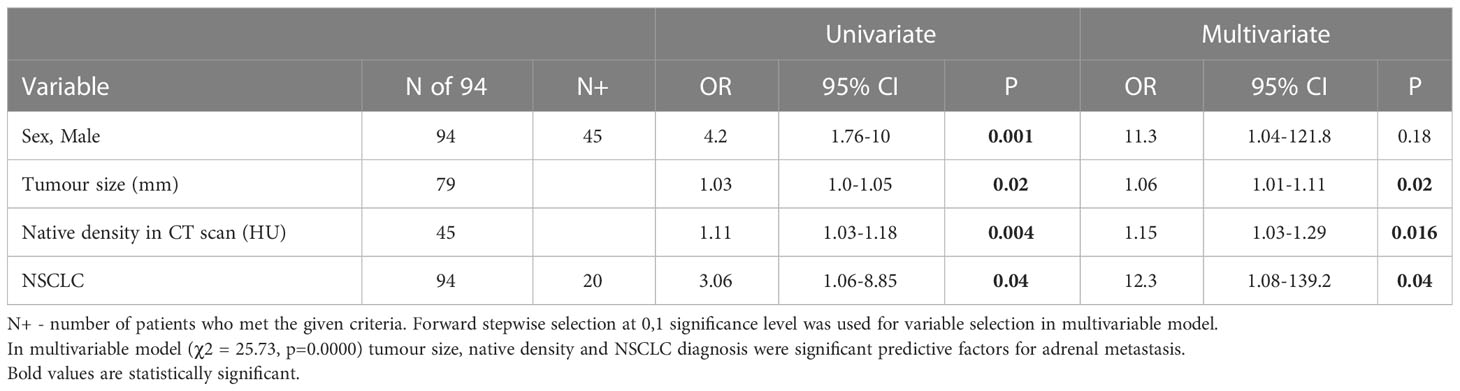
Table 5 Multivariable analysis of potential risk factors for metastasis before adrenalectomy with NSCLC diagnosis as a covariate.
Cut-off values for tumor size and native density were calculated from receiver operating characteristic (ROC) curves. Risk of malignancy is higher for tumor exceeding 37 mm (59% sensitivity and 77.5% specificity) and with native density over 24 HU (80% sensitivity and 75% specificity). I the group of 34 patients with together diameter exceeding 37 mm and native density over 24 HU, 23 had malignant lesions on pathologic report (67,6%) In the opposite of 13 patients with tumors 37mm and smaller and native density lower than 24 HU only 1 patients finally had malignant adrenal tumor (7,8%).
Risk factor was calculated and was the strongest for native density exceeding 24 HU (RR 3.23),followed by history of lung or renal cancer (RR 2.82) and maximum tumor diameter over 37 mm (RR 2.14) Table 6.
Discussion
A solitary adrenal mass is identified at the time of cancer diagnosis or during follow-up in many oncological patients. Few reports have addressed an issue of evaluation and treatment of adrenal masses in patients with a history of malignancy. Studies of adrenal incidentaloma usually exclude patients with synchronous or metachronous extra-adrenal malignancies (13) whereas reports of adrenalectomy for metastatic cancer exclude those with primary adrenal tumors (14, 15). Patients with adrenal mass and a history of malignancy constitute an important clinical problem. In patients with disseminated metastatic disease diagnosis of adrenal mass rarely alters treatment decision making. However, if an adrenal lesion is a unique sign of metastatic spread, it may have an essential impact on patient management.
Adrenalectomy is indicated for hormonally active tumors or tumors in which a benefit from potential malignancy treatment outweighs the risk of surgery, including treatment for isolated metastases from solid-organ cancers. Majority of adrenalectomies (81,5%), are performed for benign disease, mostly for pheochromocytoma followed by Cushing’s syndrome, Conn’s syndrome, and non-functioning adrenal adenoma. In malignancies, surgery is most often done for metastases, adrenocortical carcinoma and malignant pheochromocytoma (16). This seems more often to be the case in patients with a history of or synchronous malignancy (2). In an adrenal mass exceeding 4 cm, that grows in one-year follow up imaging, probability of metastatic character is up to 71% (17). In our series total risk of malignancy was 47.2%: 48.9% in patients without hormonal activity and 33.3% in hormonally active group.
Adrenocortical cancers occurred in women with a history of breast and endometrial cancers. Such coincidence may result from genetic mutations which may be found in Li-Fraumeni and Lynch syndromes (18, 19).
Most common primary cancers which metastasized to adrenals in our study were RCC (27.4%), CRC (18.9%) and NSCLC (18.9%) and this proportions are similar to previously reported. The most common primary sites reported are lung cancer (32%), RCC (22%) and melanoma (15%) (20). In another large series, most frequent primary tumors were lung (46,6%), colorectal (13,5%), and RCC (11,7%) (11). There are also reports with high prevalence of GI cancers (21).
Decision regarding surgery is usually based on diagnostic imaging. Risk of malignancy is determined by tumor size, radiographic features such as irregular margins, high density, slow contrast washout, presence of necrosis, area of hemorrhage and calcifications. CT is considered the most useful imaging technique in differential diagnosis of adrenal masses (22). Measurement of Hounsfield units (HU) in an unenhanced CT is very useful in discriminating benign from malignant mass. Recommended threshold for CT density of benign lipid-rich adenomas correctly is <=10 HU. At this threshold, pooled sensitivity and specificity were 71% and 98% respectively (23). Lipid-poor adenomas with attenuation value of less than 10 HU represent 10-40% of all adenomas (24). None of the metastatic tumors we found had native density lower than 10 HU. Characterization of adrenal mass with contrast-enhanced CT takes an advantage of specific perfusion pattern of different tumors. Malignant lesions including metastases enhance rapidly but demonstrate a slower washout of contrast medium (25). An absolute contrast washout of >60% and a relative contrast washout of >40% characterize an adenoma with sensitivity and specificity of 98 and 92% respectively (26, 27). It is worth to mention that more recent studies revealed that washout CT may be insufficient to reliably diagnose adrenal masses (28). Dedicated adrenal imaging including CT attenuation measurements with washout characteristic is highly recommended in patient with adrenal tumours previously treated for extra-adrenal malignancy (29). High precontrast HU (>36), and presence of metachronous mass should rise alert of metastasis in patients with extra-adrenal cancer who present with solitary adrenal mass (30). Our data revealed lower native density value cutoff (24 HU), above which risk of metastasis was statistically higher. It has been proven that adrenal tumour size is highly connected to malignancy. In unselected groups of patients with and without previous oncologic treatment, risk of malignancy increased with tumour size to 37.7% when lesion exceeded 6 cm (31). It had been suggested that an adrenal mass > 22 mm in patients with extra-adrenal cancer may be indicative of metastasis (sensitivity 73.1%; specificity 78.5%) (32). Our results confirm that adrenal mass bigger than 37 mm was related to higher risk of malignancy. This observation is in line with a recent study where tumor size > 3.2 cm, and features of malignancy on imaging were independent predictive factors for adrenal malignancy (33). Nevertheless in small and ambiguous tumors, CT may be insufficient to establish proper diagnosis and Positron Emission Tomography and Computed Tomography (PET-CT) may be necessary, although also has limitations. Many different benign adrenal lesions can show increased FDG uptake (34).
There were 11.3% of functional tumours in the study group. Regardless of concurrent or a history of malignancy, all patients with an adrenal mass should undergo standard hormonal screening to identify functional neoplasm, at least to carry out tests to exclude pheochromocytoma (formerly daily urinary metanephrines, nowadays mainly free plasma metanephrines), as it is then necessary to use alpha-blockade in preparation for surgery. However, limitations of the screening must be recognized. False-negative urinary test results in patients with final histopathological diagnosis of pheochromocytoma are common (35, 36). We identified 2 patients (2,1%) who had biochemically silent pheochromocytoma, which is significantly less than 6.57% as previously reported (37).
Results of surgical treatment for selected patients with isolated adrenal metastases of different types of cancers are satisfactory (38, 39). Resection of adrenal metastases in carefully chosen patients seems reasonable. There were no mortalities and only minor morbidity related to adrenalectomy in our series. Adrenalectomy is an effective treatment option in patients with good control of extra-adrenal disease, acceptable performance status and absence of significant comorbidities. We concur that all patients with a history of malignant disease and newly diagnosed adrenal lesion should be suspected for metastases and referred to surgical treatment with no delay (40, 41). Follow-up rules used for incidentalomas (42, 43) must not be applied to these cases since unnecessary delay may affect patient’s life expectancy. Factors such as sex, type of primary cancer, tumour size and CT characteristics may identify patients in whom treatment should be performed quickly.
Conclusions
In summary, analysis of this comprehensive series of patients with a history of extra-adrenal malignancy operated for adrenal tumours showed 47,2% risk of malignancy. In preoperative assessment type of primary cancer, adrenal tumour size and native density on CT should be taken into consideration as predictive factors of malignancy. Native density exceeding 24 HU was the strongest risk factor of adrenal malignancy (RR 3.23), followed by history of lung or renal cancer (RR 2.82) and maximum tumor diameter over 37 mm (RR 2.14).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Authors have obtained ethical approval from an ethics review board of Medical Centre of Postgraduate Education (30/2022), to perform and publish this study.
Author contributions
RS - Study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript. KN - Study conception and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript. LP - Study conception and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript. EK - Analysis and interpretation of data, critical revision of manuscript. KR-P - acquisition of data, drafting of manuscript, critical revision of manuscript. WS - acquisition of data, drafting of manuscript, critical revision of manuscript. TO - acquisition of data, analysis and interpretation of data, critical revision of manuscript. EC - acquisition of data, drafting of manuscript, critical revision of manuscript. AG - acquisition of data, analysis and interpretation of data, critical revision of manuscript. DL - acquisition of data, analysis and interpretation of data, critical revision of manuscript. AC - Study conception and design, acquisition of data, drafting of manuscript, critical revision of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research received no external funding. Autors hope to recive a refund for publication fee from Maria Sklodowska-Curie National Research Centre.
Acknowledgments
The authors are grateful to all of those with whom they have had the pleasure to work during this and other related projects. The authors would also like to extend their thanks to the technicians of the laboratory of the Department of Pathology, Maria Sklodowska-Curie National Research Institute of Oncology Warsaw Poland and Biochemical Laboratory of Bielański Hospital, Warsaw, Poland. for their help in offering the resources in running the program. The authors are also grateful for the insightful comments offered by the peer reviewers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RCC, renal cell cancer; CRC, colorectal cancer; NSCLC, non-small cell lung cancer; HA, hormonally active; HNA, hormonally non active; PM, primary malignancies; SM, malignancies secondary; CT, computed tomography; HU, Hounsfield units; MRI, magnetic resonance imaging; PET, Positron Emission Tomography; ROI, region of interest; ROC, receiver-operating characteristic.
References
1. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol (2008) 190(5):1163–8. doi: 10.2214/AJR.07.2799
2. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. (2006) 29(4):298–302. doi: 10.1007/BF03344099
3. Young WF Jr. Management approaches to adrenal incidentalomas. a view from Rochester, Minnesota. Endocrinol Metab Clin North Am (2000) 29(1):159–85, x. doi: 10.1016/s0889-8529(05)70122-5
4. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev (1995) 16(4):460–84. doi: 10.1210/edrv-16-4-460
5. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental indeterminate adrenal mass on CT (> 10 h) in patients without cancer: is further imaging necessary? follow-up of 321 consecutive indeterminate adrenal masses. AJR Am J Roentgenol (2007) 189(5):1119–23. doi: 10.2214/AJR.07.2167
6. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev (2004) 25(2):309–40. doi: 10.1210/er.2002-0031
7. Uberoi J, Munver R. Surgical management of metastases to the adrenal gland: Open, laparoscopic, and ablative approaches. Curr Urol Rep (2009) 10(1):67–72. doi: 10.1007/s11934-009-0012-0
8. Bullock WK, Hirst AE Jr. Metastatic carcinoma of the adrenal. Am J Med Sci (1953) 226(5):521–4. doi: 10.1097/00000441-195322650-00007
9. Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer (1950) 3(1):74–85. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7
10. Cingam SR, Mukkamalla SKR, Karanchi H. Adrenal metastasis. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2022).
11. Duh QY. Resecting isolated adrenal metastasis: Why and how? Ann Surg Oncol (2003) 10(10):1138–9. doi: 10.1245/aso.2003.10.916
12. Wachtel H, Roses RE, Kuo LE, Lindeman BM, Nehs MA, Tavakkoli A, et al. Adrenalectomy for secondary malignancy: Patients, outcomes, and indications. Ann Surg (2021) 274(6):1073–80. doi: 10.1097/SLA.0000000000003876
13. Eldeiry LS, Alfisher MM, Callahan CF, Hanna NN, Garber JR. The impact of an adrenal incidentaloma algorithm on the evaluation of adrenal nodules. J Clin Transl Endocrinol (2018) 13:39–45. doi: 10.1016/j.jcte.2018.07.001
14. Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer (1998) 82(2):389–94. doi: 10.1002/(SICI)1097-0142(19980115)82:2<395::AID-CNCR20>3.0.CO;2-T
15. Samsel R, Cichocki A, Roszkowska-Purska K, Papierska L, Koalasińska-Ćwikła A, Karpeta E, et al. Adrenal metastases - long-term results of surgical treatment, single-centre experience. Contemp Oncol (Pozn). (2020) 24(1):29–33. doi: 10.5114/wo.2020.93679
16. Patel N, Egan RJ, Carter BR, Scott-Coombes DM, Stechman MJ. British Association of endocrine and thyroid surgeons. outcomes of surgery for benign and malignant adrenal disease from the British association of endocrine and thyroid surgeons' national registry. Br J Surg (2019) 106(11):1495–503. doi: 10.1002/bjs.11297
17. Frilling A, Tecklenborg K, Weber F, Kühl H, Müller S, Stamatis G, et al. Importance of adrenal incidentaloma in patients with a history of malignancy. Surgery (2004) 136(6):1289–96. doi: 10.1016/j.surg.2004.06.060
19. Lim A, Rao P, Matin SF. Lynch syndrome and urologic malignancies: A contemporary review. Curr Opin Urol (2019) 29(4):357–63. doi: 10.1097/MOU.0000000000000639
20. Gunjur A, Duong C, Ball D, Siva S. Surgical and ablative therapies for the management of adrenal 'oligometastases' - a systematic review. Cancer Treat Rev (2014) 40(7):838–46. doi: 10.1016/j.ctrv.2014.04.001
21. Lam KY, Lo CY. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) (2002) 56(1):95–101. doi: 10.1046/j.0300-0664.2001.01435.x
22. Baltzer P, Clauser P, Klatte T, Walz J. Work-up of the incidental adrenal mass. Eur Urol Focus (2016) 1(3):217–22. doi: 10.1016/j.euf.2015.12.003
23. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol (2003) 149(4):273–85. doi: 10.1530/eje.0.1490273
24. Birsen O, Akyuz M, Dural C, Aksoy E, Aliyev S, Mitchell J, et al. A new risk stratification algorithm for the management of patients with adrenal incidentalomas. Surgery (2014) 156(4):959–65. doi: 10.1016/j.surg.2014.06.042
25. Szolar DH, Kammerhuber FH. Adrenal adenomas and nonadenomas: Assessment of washout at delayed contrast-enhanced CT. Radiology (1998) 207(2):369–75. doi: 10.1148/radiology.207.2.9577483
26. Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: Use of washout characteristics at contrast-enhanced CT. Radiology (2000) 217(3):798–802. doi: 10.1148/radiology.217.3.r00dc29798
27. Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer (2007) 14(3):587–99. doi: 10.1677/ERC-07-0045
28. Schloetelburg W, Ebert I, Petritsch B, Weng AM, Dischinger U, Kircher S, et al. Adrenal wash-out CT: Moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol (2021) 186(2):183–93. doi: 10.1530/EJE-21-0650
29. Hammarstedt L, Muth A, Sigurjónsdóttir HÁ, Almqvist E, Wängberg B, Hellström M, et al. Adrenal lesions in patients with extra-adrenal malignancy - benign or malignant? Acta Oncol (2012) 51(2):215–21. doi: 10.3109/0284186X.2011.608084
30. Byeon KH, Ha YS, Choi SH, Kim BS, Kim HT, Yoo ES, et al. Predictive factors for adrenal metastasis in extra-adrenal malignancy patients with solitary adrenal mass. J Surg Oncol (2018) 118(8):1271–6. doi: 10.1002/jso.25272
31. Cyranska-Chyrek E, Szczepanek-Parulska E, Olejarz M, Ruchala M. Malignancy risk and hormonal activity of adrenal incidentalomas in a Large cohort of patients from a single tertiary reference center. Int J Environ Res Public Health (2019) 16(10):1872. doi: 10.3390/ijerph16101872
32. Lee JH, Kim EK, Hong AR, Roh E, Bae JH, Kim JH, et al. Radiographic characteristics of adrenal masses in oncologic patients. Endocrinol Metab (Seoul) (2016) 31(1):147–52. doi: 10.3803/EnM.2016.31.1.147
33. Tan L, Ye Y, Xiao K, Xu X, Liang H, Zheng F, et al. A clinicopathological analysis of adrenal tumors in patients with history of extra-adrenal cancers. BMC Cancer (2019) 19(1):838. doi: 10.1186/s12885-019-6061-y
34. Dong A, Cui Y, Wang Y, Zuo C, Bai Y. (18)F-FDG PET/CT of adrenal lesions. AJR Am J Roentgenol (2014) 203(2):245–52. doi: 10.2214/AJR.13.11793
35. Lenert JT, Barnett CC Jr, Kudelka AP, Sellin RV, Gagel RF, Prieto VG, et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery (2001) 130(6):1060–7. doi: 10.1067/msy.2001.118369
36. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Endocrine society. pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2014) 99(6):1915–42. doi: 10.1210/jc.2014-1498
37. Vural V, Kılınç EM, Sarıdemir D, Gök İB, Hüseynov A, Akbarov A, et al. Association between tumor size and malignancy risk in hormonally inactive adrenal incidentalomas. Cureus (2020) 12(1):e6574. doi: 10.7759/cureus.6574
38. Krumeich LN, Roses RE, Kuo LE, Lindeman BM, Nehs MA, Tavakkoli A, et al. Survival after adrenalectomy for metastatic lung cancer. Ann Surg Oncol (2022) 29(4):2571–9. doi: 10.1245/s10434-021-11192-7
39. Sarela AI, Murphy I, Coit DG, Conlon KC. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol (2003) 10(10):1191–6. doi: 10.1245/aso.2003.04.020
40. Marangos IP, Kazaryan AM, Rosseland AR, Røsok BI, Carlsen HS, Kromann-Andersen B, et al. Should we use laparoscopic adrenalectomy for metastases? Scandinavian multicenter study. J Surg Oncol (2009) 100(1):43–7. doi: 10.1002/jso.21293
41. Strong VE, D'Angelica M, Tang L, Prete F, Gönen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol (2007) 14(12):3392–400. doi: 10.1245/s10434-007-9520-7
42. Kasperlik-Zeluska AA, Rosłonowska E, Słowinska-Srzednicka J, Migdalska B, Jeske W, Makowska A, et al. Incidentally discovered adrenal mass (incidentaloma): Investigation and management of 208 patients. Clin Endocrinol (Oxf). (1997) 46(1):29–37. doi: 10.1046/j.1365-2265.1997.d01-1751.x
Keywords: adrenal tumors, adrenal malignancy, adrenal metastases, adrenalectomy, risk of adrenal malignancy
Citation: Samsel R, Nowak K, Papierska L, Karpeta E, Roszkowska-Purska K, Smiertka W, Ostrowski T, Chrapowicki E, Grabowski A, Leszczyńska D and Cichocki A (2023) Risk of malignancy in adrenal tumors in patients with a history of cancer. Front. Oncol. 13:1018475. doi: 10.3389/fonc.2023.1018475
Received: 13 August 2022; Accepted: 09 March 2023;
Published: 27 March 2023.
Edited by:
Longfei Liu, Xiangya Hospital, Central South University, Changsha, ChinaReviewed by:
Mariarita Tarallo, Sapienza University of Rome, ItalyShreyamsa M., King George’s Medical University, India
Copyright © 2023 Samsel, Nowak, Papierska, Karpeta, Roszkowska-Purska, Smiertka, Ostrowski, Chrapowicki, Grabowski, Leszczyńska and Cichocki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Radosław Samsel, radeksamsel@o2.pl
†ORCID: Radosław Samsel, orcid.org/0000-0001-5925-3115
 Radosław Samsel
Radosław Samsel Karolina Nowak
Karolina Nowak Lucyna Papierska
Lucyna Papierska Edyta Karpeta3,4
Edyta Karpeta3,4