- 1Department of Laboratory Medicine, University of California San Francisco, San Francisco, CA, United States
- 2Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA, United States
- 3Worldwide Innovative Network (WIN) for Personalized Cancer Therapy Consortium, Villejuif, France
- 4Division of Hematology Oncology, University of California San Francisco, San Francisco, CA, United States
Liquid biopsy biomarkers, such as circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), are noninvasive diagnostics that could complement predictive and prognostic tools currently used in the clinic. Recent trials of immunotherapy have shown promise in improving outcomes in a subset of breast cancer patients. Biomarkers could improve the efficacy of immune checkpoint inhibitors by identifying patients whose cancers are more likely to respond to immunotherapy. In this review, we discuss the current applications of liquid biopsy and emerging technologies for evaluation of immunotherapy response and outcomes in breast cancer. We also provide an overview of the status of immunotherapy in breast cancer.
1 Introduction
Predictive and prognostic biomarkers in oncology have played an important role in guiding treatment to improve patient outcomes (1, 2). The recent emergence of liquid biopsy-based biomarkers from blood—e.g., circulating tumor cells (CTCs) (3–5) and circulating tumor DNA (ctDNA) (6–10)—has offered minimally invasive approaches to assess tumor response and survival in early-stage and metastatic breast cancer (11, 12). Blood-based biomarkers have addressed the limitations poised by tissue-based biomarkers because they are more readily accessible than tissue (13). For example, blood markers offer several advantages over tissue assessment because of the ease of serial analysis via blood draws and the feasibility of monitoring of recurrence after surgical resection, when no clinically measurable disease is present (i.e., minimal residual disease) (14).
Breast cancer is the most common cancer in women and represents the leading cause of cancer-related deaths in women worldwide (15). A significant unmet need is effective treatment for triple negative breast cancer (TNBC), a particularly aggressive subtype of this disease. TNBC, defined by a lack of estrogen, progesterone, and human epidermal growth factor receptor 2 (HER2) receptors, accounts for 15% to 20% of all breast cancers and typically has a poor prognosis (16). Immunotherapy has revolutionized the management of multiple solid tumors. For TNBC, immune checkpoint inhibitor (ICI) agents targeting programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) and combined with chemotherapy have demonstrated significant clinical activity in early-stage and metastatic TNBC, leading to regulatory approval in the U.S. (17–20). However, in the metastatic setting, only a subgroup of patients responds to these agents, and in the early-stage setting it is important to identify those who do not need ICI for optimal outcome. Therefore, it is important to discover predictive biomarkers to identify breast cancer patients who will benefit from immunotherapy.
Currently, the only predictive test for first-line immunotherapy in patients with metastatic TNBC is immunohistochemical (IHC) testing for PD-L1 expression (17, 21, 22). PD-L1 testing of tumor tissue currently lacks standardization to encompass the heterogeneity in the assays, the diversity of antibodies for testing and the assessment platforms (instrumentation), and the thresholds for scoring PD-L1 status. Additionally, there is diversity in the tumor microenvironment compartments that are analyzed (tumor cells, immune cells, or both). In addition to prediction, it is important to detect resistance to immunotherapy and identify biomarkers to monitor breast cancer patients during immunotherapy. Evaluating patient immunotherapy response by imaging presents another challenge, as standard radiologic criteria for assessing response to ICI therapy could miss progression. One of the obstacles is pseudoprogression, described as radiologic enlargement of the tumor mass due to infiltration of leukocytes (23). There is an unmet need to identify sensitive and specific predictive biomarkers to select patients who will benefit from ICI therapy and to avoid unnecessary toxicities and cost. Liquid biopsies could be a potential approach to identify more robust biomarkers associated with ICI. Recent studies have shown that CTCs frequently express PD-L1 and are associated with worse prognosis, and thus, could serve as a useful non-invasive biomarker for real-time assessment of PD-L1 status and estimation of risk of disease relapse and progression (24–30).
In this review, we discuss liquid biopsy applications to guide immunotherapy to treat breast cancer. We highlight the promises and challenges of liquid biopsy biomarkers for breast cancer immunotherapy. Here, we focus our discussion on two liquid biopsy biomarkers, CTCs and ctDNA, and the clinical studies that examined their utility (Figure 1).
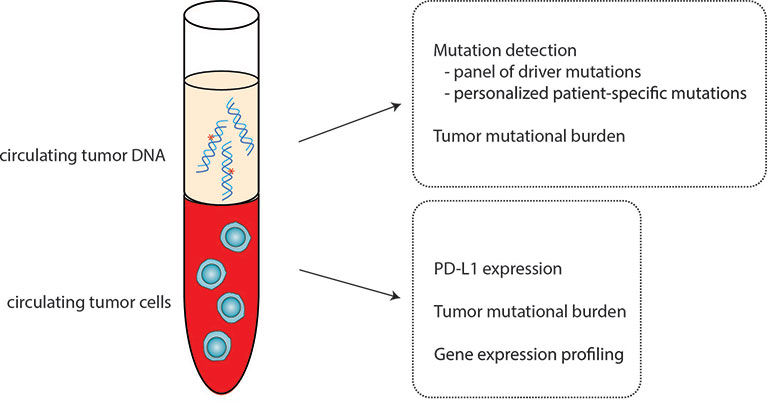
Figure 1 Circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) as biomarkers for immunotherapy. CTCs and ctDNA can serve as a noninvasive alternative for solid tissue assessment of candidate biomarkers to predict immunotherapy response and outcomes.
2 Liquid Biopsy Biomarkers: Characteristics and Technology Platforms for Analysis
The most established biomarkers for liquid biopsy assessment include CTCs (31) and ctDNA (6). Over the past decade or more, questions regarding the prognostic and predictive significance of these biomarkers have been actively studied (32–34). Below, we describe CTCs and ctDNA and discuss the detection platforms for each biomarker.
2.1 Circulating Tumor Cells
CTCs, defined as rare cells shed by primary tumors into the blood, are hypothesized to be precursors of distant metastases (35). Numerous studies have unequivocally demonstrated the prognostic value of these cells both in early-stage (3, 4) and metastatic breast cancer (5, 36). However, the clinical utility of these cells for guiding treatment to improve patient outcomes has yet to be fully established (37).
The many technologies for the detection and enumeration of CTCs have been reviewed in detail in recent articles (14, 38). To date, the only CTC detection platform to have received clearance from the US Food and Drug Administration (FDA) for enumeration of CTCs in breast cancer is the CellSearch™ system (31). CellSearch™ is a two-step method that involves: (1) immunomagnetic enrichment of cells expressing the epithelial cell adhesion marker (EPCAM), and (2) fluorescence microscopy detection of nucleated cells that are positive for cytokeratin (epithelial marker) and negative for CD45 (leukocyte marker) expression. The detection of 5 or more CTCs per 7.5 mL of blood has been demonstrated to be strongly prognostic for progression-free survival (PFS) and overall survival (OS) in patients with metastatic breast cancer (5, 31, 36). The prognostic value of CTCs in early-stage breast cancer, particularly in the neoadjuvant setting has been recently examined (4). Patients with one or more CTCs identified before neoadjuvant therapy have increased risk of local and distant recurrence as compared to those with no detectable CTCs (4).
Modifications to the standard CellSearch™ protocol for CTC enumeration has allowed for the reliable assessment of PD-L1 expression in CTCs (24, 25, 29, 30, 39). Researchers have added a fluorophore-conjugated antibody to PD-L1 (e.g., B7-H1) to the antibody cocktail (anti-cytokeratin and anti-CD45) for semi-quantitative analysis of PD-L1 expression in CTCs, using cancer cell lines with known PD-L1 expression levels as references. Strati and colleagues used RT-PCR to measure PD-L1 expression in CTC-enriched fractions after immunomagnetic enrichment using CellSearch (25). Others have used filter-based methods to enrich for CTCs prior to immunofluorescence staining to examine PD-L1 expression (26–28).
2.2 Circulating Tumor DNA and Cell-Free DNA
ctDNA are short fragments of DNA derived from a primary tumor, metastatic foci and/or circulating tumor cells. ctDNA can be detected in plasma and are present in an admixture of DNA derived mainly from normal blood cells. Collectively, this admixture is known as cell-free DNA (cfDNA). Examination of the size distribution of cfDNA reveals a predominant length of 166 bp with a series of peaks every 10 bp (40). The size and periodicity indicate an association with nucleosomes and suggest that cfDNA is released into circulation via apoptosis or necrosis of cells (41). It is unknown whether the mechanisms involved in the release of cfDNA are the same as those of ctDNA (41, 42).
Detection of ctDNA can be performed using several methods, including deep next generation sequencing (Figure 2) (14). The primary goal of deep sequencing is to detect rare mutated DNA copies shed by tumors (ctDNA) and differentiate them from wildtype copies that are simultaneously released from normal hematopoietic cells undergoing apoptosis. Comparative sequencing studies have shown that specific mutations in ctDNA vs. matched primary tumor tissue are generally concordant (43, 44), however, temporal spacing (e.g., timing of sample collection) and tumor heterogeneity could also lead to discrepancies (45). Overall, these data suggest that ctDNA can complement tissue sequencing to find actionable biomarkers. Initial approaches to detection of ctDNA involved digital droplet polymerase chain reaction (ddPCR) (46). However, ddPCR has become less favored (over sequencing) because of its limitations, particularly the restricted number of mutations that can be assessed in one experiment. Sequencing, on the other hand, can interrogate whole genomes, or a panel of genes that include driver mutations frequently observed in cancer, or a personalized list of mutations identified from a patient’s solid tumor (7, 10).
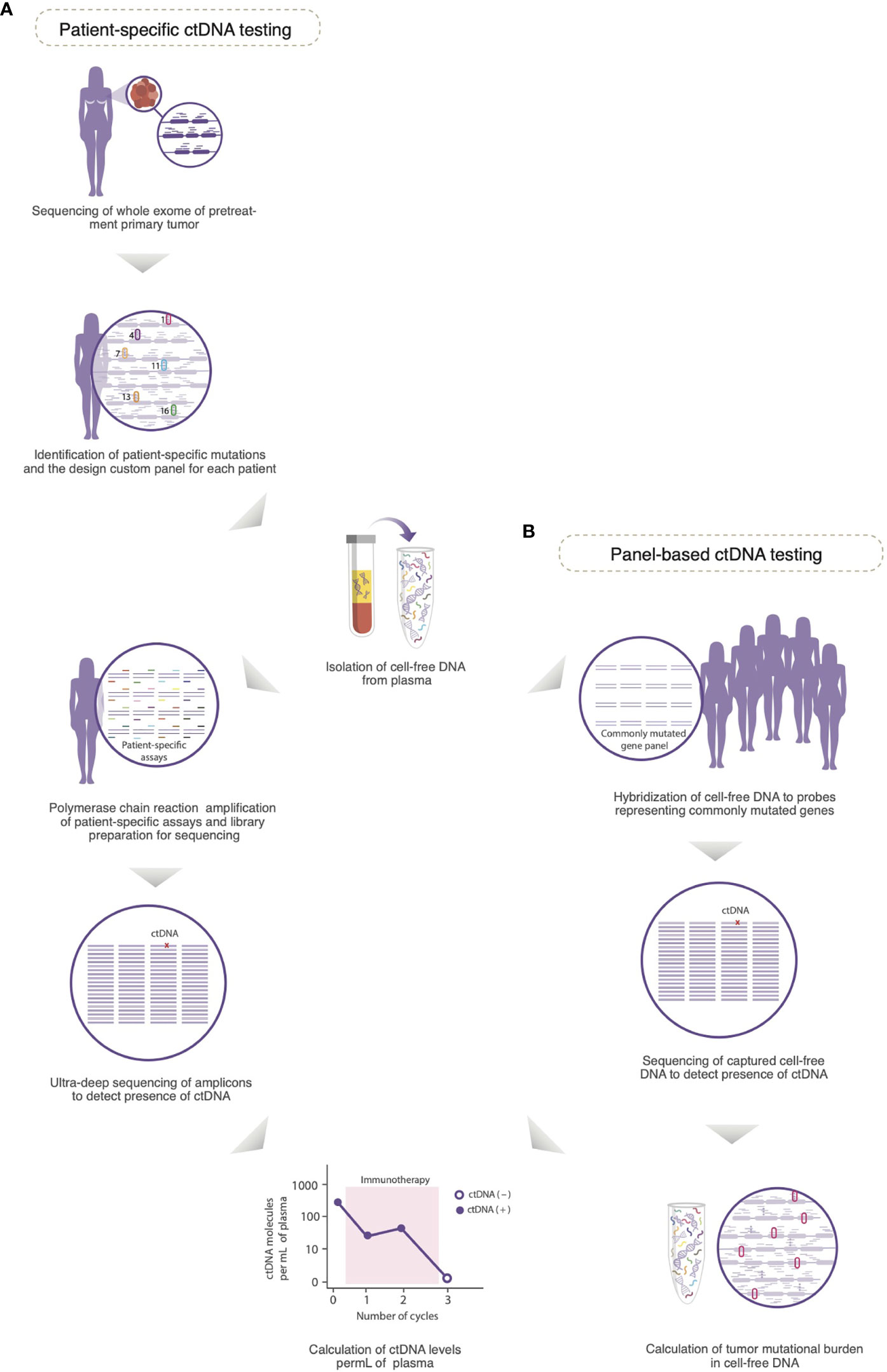
Figure 2 Detection of circulating tumor DNA (ctDNA) in plasma. (A) A customized panel containing multiplexed assays is designed to detect patient-specific mutations in cell-free DNA. The personalized panel is created from a list of mutations detected from whole exome sequencing of the untreated primary tumor. Matched germline DNA is also sequenced to exclude non-somatic mutations due to clonal hematopoiesis of indeterminate potential. Amplicons produced by polymerase chain reaction amplification of genomic regions that contain the selected mutations are subjected to ultra-deep sequencing to detect the presence of ctDNA. (B) In a panel-based approach, cell-free DNA is hybridized to probes that represent a panel of frequently mutated genes (e.g., PIK3CA and TP53), and therefore, the mutational profile of the corresponding solid tumor is not required for testing. The captured cell-free DNA molecules are then subjected to next generation sequencing to detect the presence of ctDNA. Because the panel of genes used for testing is consistent across all samples, and includes highly mutated genes, the tumor mutational burden in cell-free DNA can be calculated. In both approaches for testing of ctDNA, serial plasma can be prospectively collected to monitor the levels of ctDNA as a potential biomarker of response to immunotherapy [Modified with permissions from (10)].
The presence of ctDNA in the blood of patients with early-stage breast cancer is associated with aggressive disease and portends poor clinical outcomes (10, 47). Failure to clear ctDNA during neoadjuvant or adjuvant therapy reflects treatment resistance and increased risk of metastatic recurrence (10, 47).
In the metastatic breast cancer setting, ctDNA testing is becoming a part of routine clinical practice because of the high prevalence of actionable mutations and its potential utility as a surrogate for tumor burden (48). A recently defined clinical use of ctDNA in metastatic breast cancer involves the detection of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations, which is already used to guide treatment and is now cleared by the Food and Drug Administration (FDA) (49). Studies are also evaluating the use of ctDNA to detect new mutations during treatment that might represent an early indication of resistance (50), e.g., the emergence of ESR1 mutations in metastatic breast cancer patients treated with CDK4/6 inhibitor in the PADA-1 Trial (51).
3 Immunotherapy in Breast Cancer
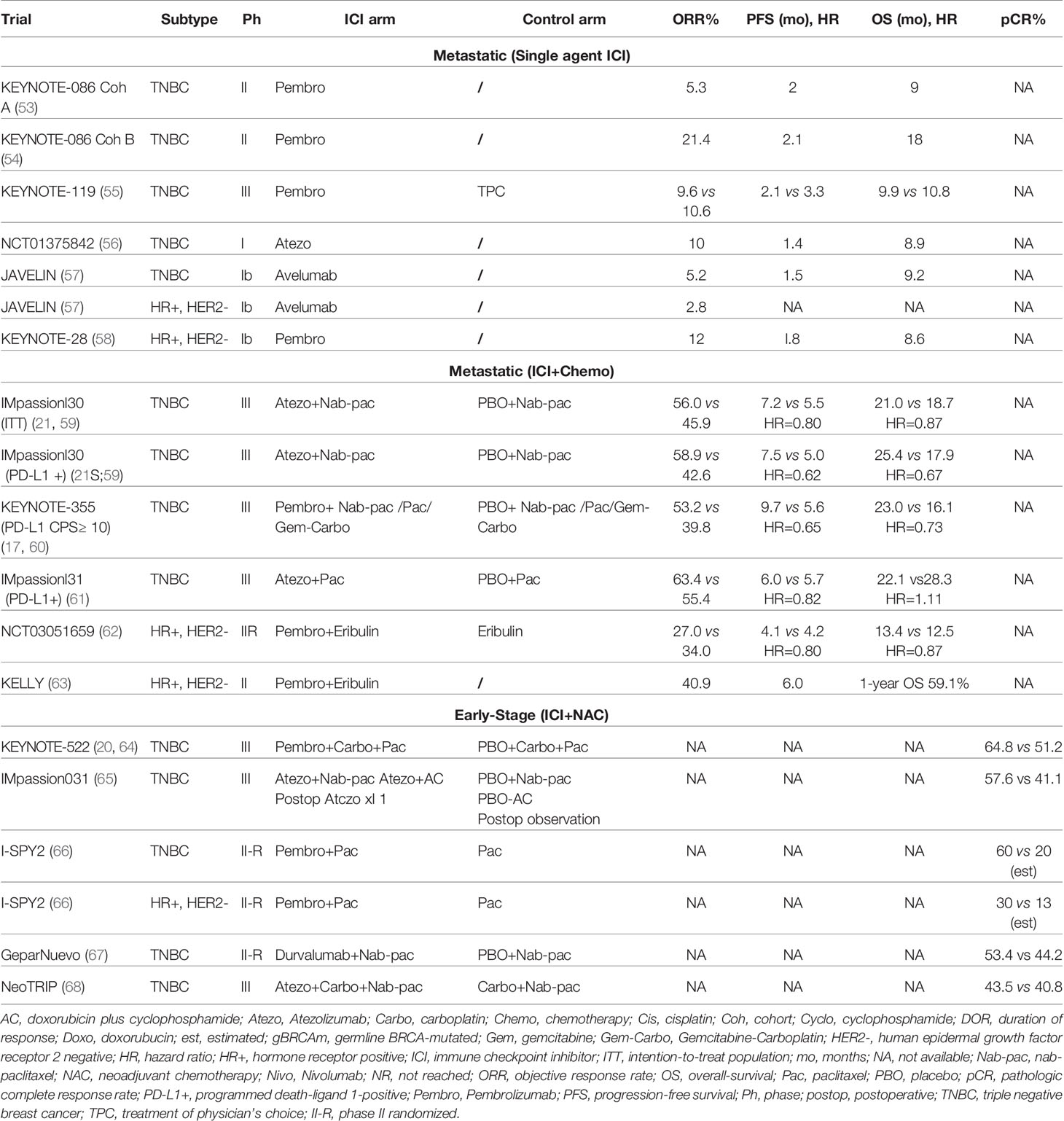
Immune checkpoint blockade, which helps the immune system recognize and attack tumor cells, is used to treat various cancers with durable responses compared to most chemotherapy and targeted agents. Inhibiting the PD-L1/PD-1 axis with monoclonal antibodies is a breast cancer treatment strategy that provides cell-mediated antitumor activity. The binding of PD-L1 to its receptor on T cells, PD-1, inhibits adaptive immune responses in the tumor microenvironment, enabling malignant cells to escape immunosurveillance. Immunotherapy drugs approved for the treatment of multiple tumor types include anti-PD-1 (pembrolizumab, nivolumab and cemiplimab), anti-PD-L1 (atezolizumab, durvalumab and avelumab), and the cytotoxic T-lymphocyte antigen 4 (anti-CTLA-4) (ipilimumab and tremelimumab) (52). In the U.S., only pembrolizumab is approved for the treatment of early-stage TNBC in the neoadjuvant setting combined with chemotherapy, followed by adjuvant single agent treatment, and in combination with chemotherapy for PD-L1+ metastatic breast cancer. Atezolizumab in combination with chemotherapy is approved in other countries in PD-L1+ metastatic disease. Multiple ongoing studies are evaluating ICI in all subtypes of breast cancer. Key trials that examined the efficacy of ICI are summarized in Table 1.
3.1 Unresectable Locally Advanced and Metastatic Breast Cancer
Some breast cancers are immunogenic with their tumor microenvironment (TME) enriched with tumor infiltrating lymphocytes (TILs). Increasing evidence suggests that triple negative and HER-2 positive subtypes are often associated with substantial infiltration of immune cells with a prognostic and predictive value (69).
3.1.1 Metastatic TNBC
The primary treatment for metastatic TNBC has been chemotherapy, with a median OS of 12 to 18 months (70). However, growing evidence suggests that immunotherapy is an effective treatment strategy for PD-L1-positive TNBC. Several key factors make TNBC more likely to respond to ICI than other subtypes of breast cancer, including higher levels of TILs, a greater number of nonsynonymous mutations, and higher levels of PD-L1 expression on both tumor and immune cells. High TIL levels are associated with PD-L1 expression on tumor and tumor immune cells (IC), and PD-L1+ tumors with high TILs have better outcomes (54, 71). The emergence of immunotherapy in breast cancer requires robust, sensitive, and specific predictive and prognostic biomarkers for clinical practice. Liquid biopsy could be a valuable tool to provide baseline information on the tumor and to monitor response to ICI therapy.
Although response is higher in TNBC than in hormone receptor positive (HR+) and HER2+ breast cancers, the efficacy of ICI monotherapy, while correlated with tumor and/or immune cell PD-L1 positivity, remains low. The response rates to atezolizumab and pembrolizumab monotherapy were about 5% in patients with pre-treated disease, and ~21% in untreated patients with metastatic TNBC (53, 54). Low response rates with ICI monotherapy led to the investigation of the efficacy of combination therapy with immunotherapy and chemotherapy.
The IMpassion130 trial was the first phase III trial to report positive data with ICI and chemotherapy for breast cancer, investigated the safety and efficacy of nab-paclitaxel +/- atezolizumab as first-line treatment. In this trial, in patients with PD-L1-positive disease, both PFS and OS were significantly improved with the addition of atezolizumab to nab-paclitaxel, by 2.5 (7.5 vs 5.0 months, Hazard Ratio (HR) 0.62; p<0.001) and 7.5 (25.4 vs 17.9 months, HR 0.67) months, respectively (21, 59). On March 18, 2019, the FDA granted accelerated approval for atezolizumab plus nab-paclitaxel to treat patients with unresectable, locally advanced or metastatic TNBC, whose tumor immune cells express PD-L1 at 1% or higher using the Ventana SP142 assay (72).
In a subsequent trial (IMpassion131), the addition of atezolizumab to paclitaxel in a similar setting failed to improve outcome in patients with PD-L1+ metastatic TNBC (61). Due to the inability to provide confirmatory data for IMpassion130, U.S. approval was withdrawn by the manufacturer (Roche) in August of 2021. The reason for the inconsistency in the results of IMpassion130 and IMpassion131 is not yet understood. A possible explanation for such inconsistency is that patients in the PD-L1-positive control arm in IMpassion131 have a non-stratified pathologic factor that predicts chemotherapy sensitivity (73). Additionally, more patients in IMpassion131 had received prior taxanes than those in IMpassion130, whereas more patients in IMpassion130 had de novo metastatic disease than those in IMpassion131 (18, 61). Although exposure to steroids had been considered a potential confounding factor, the use of steroids in the KEYNOTE-355 trial described below makes this unlikely (17, 19).
KEYNOTE-355 randomized patients with metastatic TNBC in the first-line setting to receive pembrolizumab or placebo in combination with physician’s choice of chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin). The success of this phase III trial resulted in the full regulatory approval of pembrolizumab in combination with chemotherapy in patients with PD-L1-positive TNBC, defined as a combined positive score (CPS) ≥10, and representing about 38% of patients with metastatic TNBC (74). Treatment with pembrolizumab compared to placebo resulted in a statistically significant improvement in PFS (9.7 vs 5.6 months, HR 0.65, p=0.0012 and OS (23 vs 16.1 months, HR 0.73, p=0.0093), as well as improving objective response rate (ORR, 53.2% vs 39.8%) and duration of response (19.3 vs 7.3 months) in patients whose tumors expressed PD-L1 (CPS ≥10) (17, 19).
Other chemotherapy agents have been combined with ICI in metastatic TNBC. The ENHANCE phase Ib/II trial evaluated eribulin mesylate (a microtubule-depolymerizing drug) in combination with pembrolizumab in 167 patients with metastatic TNBC, reporting an ORR of 23.4%, with median PFS of 4.1 months and median OS of 16.1 months (62, 75). A small study evaluated the combination of capecitabine with pembrolizumab. Thirty patients were enrolled (16 TNBC, 14 HR+, HER2 negative), reporting a median PFS of 4 months, similar to historic controls with capecitabine alone. Interestingly, the one-year PFS rate was 20.7%, suggesting durable responses in a subset of patients (76).
Preclinical data showed potential synergy with the combination of poly adenosine diphosphate-ribose polymerase (PARP) inhibition and ICI therapy. The phase Ib/II MEDIOLA trial evaluated the safety and efficacy of olaparib with durvalumab in patients with solid tumors, including 34 patients with germline BRCA1/2-mutated HER2 negative metastatic breast cancer. The median PFS was longer in patients who were treatment-naïve than in those with 2 prior lines of chemotherapy (11.7 vs 6.5 months; not clearly different than what has been seen with PARP inhibition alone in similar patient populations), and treatment was well tolerated (77, 78). Several other combinations of ICI with PARP inhibitors, AKT inhibitors, MEK inhibitors, antibody drug conjugates, and immunomodulatory drugs, among other drug classes, are under investigation to enhance the host immune response and broaden the subset of patients who could benefit from ICI in the metastatic setting (NCT03167619, NCT04191135). In addition, ICI are being actively studied in various combinations in patients with high-risk HR+ and HER2+ disease.
3.1.2 Tumor Mutational Burden
Tumor mutational burden (TMB) is a promising tool to identify patients with TNBC who could benefit from ICI therapies. In 2020, the FDA granted accelerated approval to pembrolizumab monotherapy in previously treated, unresectable/metastatic solid tumors with high TMB, defined as ≥ 10 mutations per megabase, based upon the results of KEYNOTE-158, which showed an ORR of 29% among 102 patients with 27 tumor types (79). The phase III KEYNOTE-119 study randomized patients with 1-2 lines of prior therapy for metastatic TNBC to receive pembrolizumab vs chemotherapy of physician choice, with a primary endpoint of OS. Pembrolizumab did not improve OS, but an intriguing subset analysis demonstrated improved OS in the PD-L1 enriched population (CPS ≥20) (80). A further exploratory analysis suggested a potential positive association between TMB and clinical benefit with pembrolizumab but not with chemotherapy, particularly in patients whose TMB ≥10 mutations per megabase. High TMB is uncommon in breast cancer, representing up to 8% of patients with invasive lobular cancer (81).
3.1.3 Metastatic HR+, HER2 Negative Breast Cancer
HR+, HER2 negative breast cancers have lower TILs and PD-L1 expression levels, so these are traditionally considered immunologically cold tumors (82, 83). However, a minority of patients with cold tumors could have meaningful responses to immunotherapy. The phase Ib KEYNOTE-028 trial evaluated pembrolizumab monotherapy in heavily pretreated patients with HR+, HER2 negative metastatic breast cancer. PD-L1 positivity was defined with a tumor CPS ≥ 1, and among 261 patients, 48 (19.5%) had PD-L1-positive tumors. Of these, 25 patients were enrolled and treated with pembrolizumab. The ORR was 12%, but the median duration of response was 12 months (58). In the phase I JAVELIN trial, 168 patients with pretreated metastatic breast cancer of all subtypes received avelumab monotherapy, including 72 patients (42.9%) with HR+, HER2 metastatic breast cancer, regardless of PD-L1 status. The ORR for the entire cohort was only 3.0% (five patients), including three with TNBC and two with HR+, HER2 negative disease (57). Tolaney et al. conducted a phase II trial evaluating the addition of pembrolizumab to eribulin in HR+, HER2 negative metastatic breast cancer. The addition of pembrolizumab did not improve PFS, ORR, or OS compared to eribulin alone in both the ITT and PD-L1-positive (positivity was defined as modified proportion score ≥1) (84). A multicohort phase Ib study evaluated the efficacy and safety of the combination of pembrolizumab and abemaciclib in patients with HR+, HER2 negative metastatic breast cancer. Early data from 28 patients in the pembrolizumab and abemaciclib arm, all with tumors which had progressed on endocrine therapy, demonstrated an ORR of 29%, with partial response (PR) in 8 patients. Median PFS and OS were 8.9 months and 26.3 months, respectively (84). One arm of this study evaluating the safety and preliminary anti-tumor activity of abemaciclib plus pembrolizumab and anastrozole demonstrated a numerically higher rate of transaminase elevations and pneumonitis which were considered immunotherapy related toxicity (84). Ongoing trials will illuminate the role of immunotherapy in HR+, HER2 negative disease in the coming years (NCT03147287, NCT04895358).
3.1.4 Metastatic HER2 Positive Breast Cancer
Higher levels of TIL infiltration and PD-L1 expression have generated interest in the possible value of ICI in the treatment of HER2+ breast cancer (85). In the phase Ib/II PANACEA trial, pembrolizumab plus trastuzumab had modest efficacy; 6 of 40 (15%) patients with PD-L1-positive disease progressing on prior anti-HER2 targeted therapy achieved an objective response whereas no patients responded in the PD-L1-negative cohort (86). In the KATE2 phase II randomized trial, 202 patients with previously treated HER2+ metastatic breast cancer were randomized to receive atezolizumab or placebo with trastuzumab emtansine. The trial met its futility endpoint due to toxicity in the combination arm, and PFS was not improved with the addition of atezolizumab (87). There are ongoing trials evaluating ICI agents in patients with metastatic HER2+ breast cancer (NCT03199885, NCT02849496).
3.2 Early-Stage Breast Cancer
In the early-stage setting, neoadjuvant chemotherapy has resulted in significant improvements in the management of stage II and III TNBC and HER2+ breast cancer (88). Improvements in pathologic complete response (pCR) are associated with excellent outcome, and post-surgical treatment for patients without pCR has reduced the likelihood of recurrence in this high-risk patient population (89, 90). Use of ICI in early TNBC was driven by encouraging results in the phase II I-SPY2 trial (66), and the association of PD-L1 positivity and TILs with pCR (91).
Two phase III trials have evaluated the addition of ICI to neoadjuvant chemotherapy, then continued post-surgery. The largest trial is KEYNOTE-522, leading to the first regulatory approval of a checkpoint inhibitor in early-stage breast cancer. This phase III trial randomized 1174 patients with stage II or III breast cancer in a 2 to 1 ratio to receive neoadjuvant pembrolizumab or placebo in combination with paclitaxel/carboplatin followed by anthracycline/cyclophosphamide. Following surgery, patients continued with blinded pembrolizumab or placebo to complete one year of therapy. In the first 602 patients, the addition of pembrolizumab significantly improved pCR (from 51.2 to 64.8%, P=0.00055), independent of PD-L1 positivity. The trial was designed with dual primary endpoints, including both pCR and event free survival (EFS). At the 4th interim analysis, the addition of pembrolizumab improved EFS at three years (from 76.8% to 84.5%) (64, 80). Interestingly, EFS was improved with pembrolizumab, in the patients who did not achieve a pCR, whereas patients with a pCR had excellent outcome regardless of the post-neoadjuvant treatment arm. Immune-related adverse events (irAE) increased, with 3 deaths attributed to study therapy. Based on this data, the FDA approved pembrolizumab for high-risk, early-stage TNBC in combination with chemotherapy as neoadjuvant treatment, continued as a single agent as adjuvant treatment after surgery on July 26, 2021 (92, 93).
The second phase III trial, IMpassion031, randomized 333 patients with stage II or III TNBC to receive atezolizumab or placebo with neoadjuvant nab-paclitaxel followed by doxorubicin/cyclophosphamide. Following surgery, atezolizumab was continued in a non-blinded manner to complete one year of therapy. The addition of atezolizumab was associated with a significant increase in pCR (from 41.1% to 57.6%, p=0.0044) regardless of PD-L1 expression (65).
The phase II GeparNuevo trial evaluated the efficacy of durvalumab in combination with neoadjuvant chemotherapy in 174 patients. Although pCR was not significantly improved with the addition of durvalumab, invasive disease-free survival (iDFS), distant disease-free survival (DDFS) and OS were improved with long-term follow-up (67, 94). These results, although not definitive, have brought into question the optimal duration of ICI in the treatment of early-stage disease. Lastly, the NeoTRIP phase III trial evaluated the addition of atezolizumab to a non-anthracycline, nab-paclitaxel and carboplatin backbone in 280 patients did not show improvement in pCR although the primary endpoint is EFS, which is still pending (68).
Ongoing trials are evaluating the effectiveness of ICI in the adjuvant and post-neoadjuvant setting. NSABP B-59/GeparDouze is an ongoing phase III trial evaluating neoadjuvant administration of atezolizumab with neoadjuvant chemotherapy followed by adjuvant atezolizumab in patients with high-risk TNBC (NCT03281954). IMpassion030 is a phase III trial investigating the efficacy of and safety of atezolizumab in combination with standard anthracycline/taxane adjuvant chemotherapy in patients with early-stage TNBC (NCT03498716). The primary endpoint is iDFS. SWOG S1418/BR006 (NCT02954874) is a phase III trial that randomizes patients with TNBC and ± 1cm residual invasive breast cancer and/or positive lymph nodes after neoadjuvant chemotherapy to receive standard of care or pembrolizumab 1 year after surgery. The I-SPY2 trial, an adaptive, randomized phase II trial in the neoadjuvant setting also has immunotherapy arms including cemiplimab, cemiplimab plus REGN3767, triaciclibdostarlimab, dostarlimab plus oral paclitaxel/encequidar, and dostarlimab plus oral paclitaxel/encequidar±carboplatin (NCT01042379). Complementary approaches to enhance immunogenicity, including the addition of targeted therapies, novel agents, and induction therapies, have become the recent focus of various clinical trials in breast cancer.
Immune checkpoint blockade can lead to activation of autoreactive T cells, resulting in various irAEs. Although any organ system can be affected, irAEs most commonly involve the gastrointestinal tract, endocrine glands, skin and liver (95). Neurotoxicity, cardiotoxicity and pulmonary toxicity are relatively rare but can be fatal. Whether these adverse events are associated with the efficacy of immune checkpoint blockade remains controversial. The occurrence of irAEs is not required to obtain a benefit from ICI (96). However, specific adverse events may be related with treatment efficacy. For example, several studies including patients with melanoma have demonstrated an association between vitiligo and beneficial clinical outcomes (97, 98). Liquid biopsy biomarkers could also be developed to identify patients who are likely to experience irAEs.
4 Predictive and Prognostic Value of CTCs in Immunotherapy in Breast Cancer
Although the presence of PD-L1 has been shown to have good predictive value for ICI efficacy in metastatic TNBC, many challenges persist. First, PD-L1 immunohistochemistry assessment is not always possible due to the lack of available tissue or a low percentage of tumor cells in the tissue sample. Secondly, some patients with PD-L1-positive tumors may not respond to ICI, demonstrating the complexity and our incomplete understanding of the immunopathology of cancer (92). Some challenges are the heterogeneity and dynamic changes of PD-L1 expression in the tumor microenvironment, PD-L1 expression may vary between primary tumors and metastases, and in breast cancer immunotherapy trials, there were multiple assays for each antibody, multiple scoring systems, and different cut-offs to define PD-L1 positivity (99). To address these complexities, PD-L1 expression on the CTCs of metastatic breast cancer patients is actively under investigation as a predictive biomarker for PD-1/PD-L1 inhibition, potentially complementing or replacing PD-L1 detection on tumor cells and/or TILs in tumor tissue.
Liquid biopsy can identify potentially predictive biomarkers for various solid tumors. This approach is appealing since it is minimally invasive, cost-effective, and rapidly provides information to the clinician to guide therapeutic decision-making strategies (100). Liquid biopsy can be repeated longitudinally over the course of the disease, providing follow-up data for the patient during ICI therapy and beyond, and could help detect resistance mechanisms. CTCs can be isolated and analyzed using approaches designed for solid tissue biopsy, and therefore, could be a dynamic and promising strategy. Immune checkpoint proteins can be influenced by multiple factors, including micro-environmental, inflammatory, and therapeutic factors (27). CTCs may be derived from more than one tumor site and give a better systemic representation of PD-L1 expression than the evaluation of localized cells in tissue samples. There are some questions about the evaluation of PD-L1 expression on CTCs. The first is whether PD-L1 is expressed on all CTCs or only in a subpopulation of CTCs. The second is whether there is any discordance in PD-L1 expression between CTCs and the matched tissue biopsies. Lastly, does the prognosis and the predictive response to immunotherapy correlate with PD-L1 expression on CTCs at baseline or during the follow-up of treated patients (101)?
The evaluation of PD-L1 expression on CTCs has been reported in different solid tumor types including breast, lung, head and neck, colon, bladder and prostatic carcinoma (101). Previous studies evaluating the predictive and prognostic value of PD-L1-positive CTCs in patients treated with ICI have revealed provocative results. Nicolazzo et al. monitored CTCs in non-small cell lung cancer (NSCLC) during nivolumab treatment to investigate the association of PD-L1-positive CTCs with response to ICI therapy. At baseline, 20/24 (83%) patients were positive for CTCs with a very high prevalence of PD-L1 expression (100%). At 6 months of treatment, patients with PD-L1-negative CTCs all showed clinical benefit, while patients with PD-L1-positive CTCs experienced disease progression (39). Strati et al. including patients with head and neck cancer (HNC) reported that patients with CTCs overexpressing PD-L1 at the end of treatment had shorter PFS and OS (25). Similar findings were found by Guibert et al. in NSCLC. In this study, 96 patients with metastatic NSCLC receiving chemotherapy followed by ICI were included. PD-L1 was more highly expressed on CTCs (83%) than in matched tissue samples (41%). They found that patients with PD-L1-positive CTCs had lower response rates to nivolumab than those with PD-L1-negative CTCs. All patients who experienced disease progression had detectable PD-L1-positive CTCs (26). In another study including 71 patients with metastatic NSCLC, PD-L1 expression on CTCs and matched tissue biopsies were well correlated (27). Kulasinghe et al. isolated CTCs in 23 patients with HNC and in 33 patients with NSCLC. Positive PD-L1 expression was detected in 6/11 (54.4%) HNC samples and 11/17 (64.7%) NSCLC samples, respectively. PD-L1-positive CTC patients with HNC had shorter PFS while no significant difference in PFS was observed in the NSCLC cohort when stratified by PD-L1 CTC status (28). Another prospective study in 54 patients with advanced NSCLC evaluated the correlation with clinicopathological variables and prognostic value of PD-L1-positive CTC. CTCs and PD-L1-positive CTCs were detected in 43.4% and 9.4% of patients with NSCLC. The concordance of PD-L1 expression between tumor tissue and CTCs was low (54%). This study suggested that the presence of PD-L1-positive CTCs was associated with poor prognosis in patients with advanced NSCLC (30). Taken together, these studies demonstrate the feasibility of PD-L1 testing in CTCs and provide evidence of the predictive and prognostic value of CTCs expressing PD-L1.
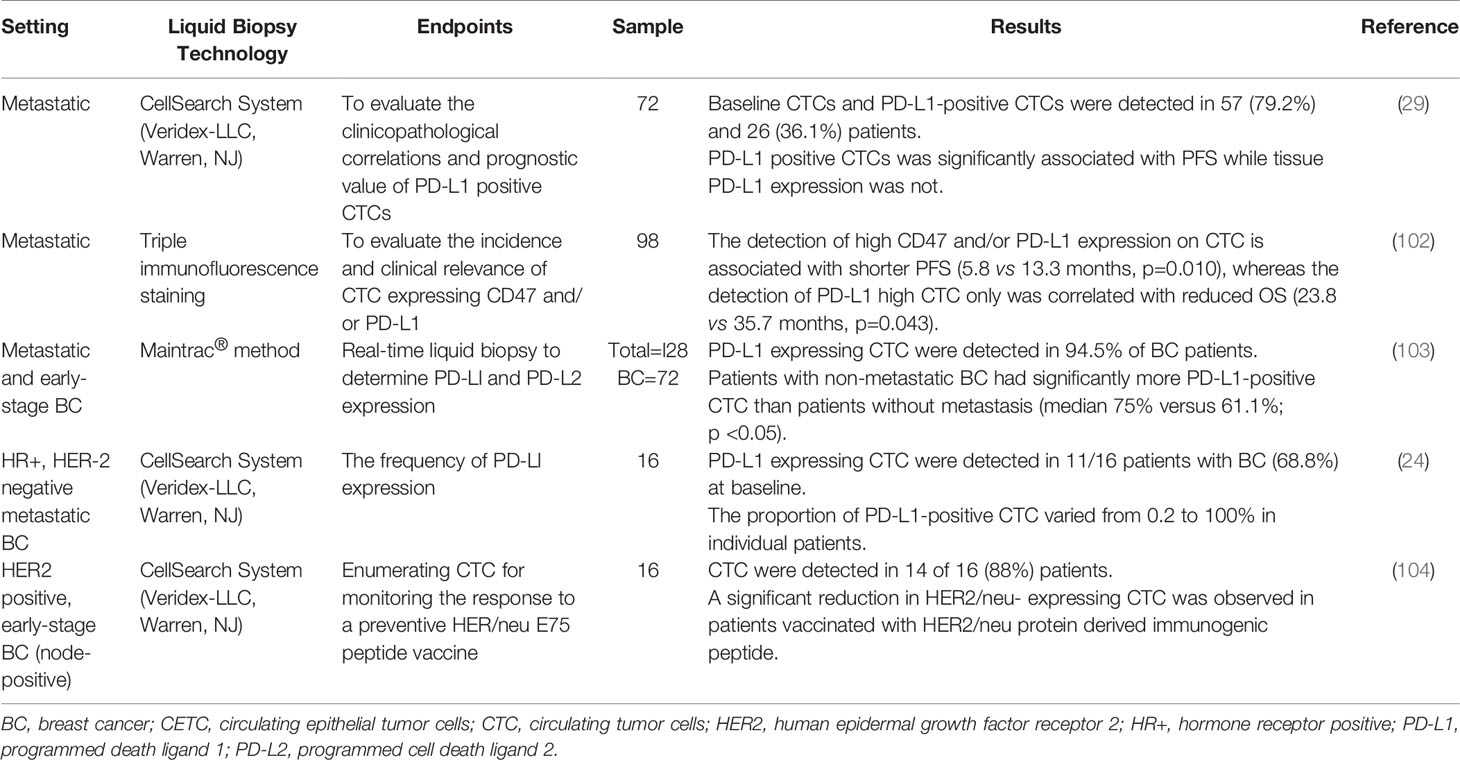
Studies on CTCs in breast cancer patients receiving immunotherapy are summarized in Table 2. Mazel et al. evaluated the frequency of PD-L1 expression in patients with HR+, HER2 negative breast cancer (24). PD-L1 expression on CTCs was evaluated in breast carcinoma patients using the EPCAM dependent CellSearch method as well as the B7-H1 PD-L1 monoclonal antibody (Figure 3). This study included 16 metastatic breast carcinomas with PD-L1-positive CTCs detected in 11 of 16 patients (68.8%), although the fraction of PD-L1-positive CTCs varied from 0.2 to 100% in individual patients. This study was the first report demonstrating the expression of PD-L1 on CTCs (24). The detection of CTCs expressing PD-L1 could be predictive of response to anti-PD-L1 therapy, and patients with a high percentage of PD-L1-positive CTCs could be potential candidates for anti-PD-L1 therapy. In a follow-up prospective study in 72 patients with metastatic breast cancer, CTCs and PD-L1-positive CTCs were detected in 57 (79.2%) and 26 (36.1%) patients before initiation of treatment (29). There was no statistically significant correlation between PD-L1 expression in tumors vs. that of CTCs. PD-L1-positive CTCs were significantly associated with PFS while tissue PD-L1 expression was not. Patients with metastatic breast cancer harboring PD-L1-positive CTCs had shorter PFS; however, this finding was not confirmed in multivariable analysis. Further studies are needed to investigate the predictive role of PD-L1 expression in tumor tissue and CTCs during ICI therapy (29).
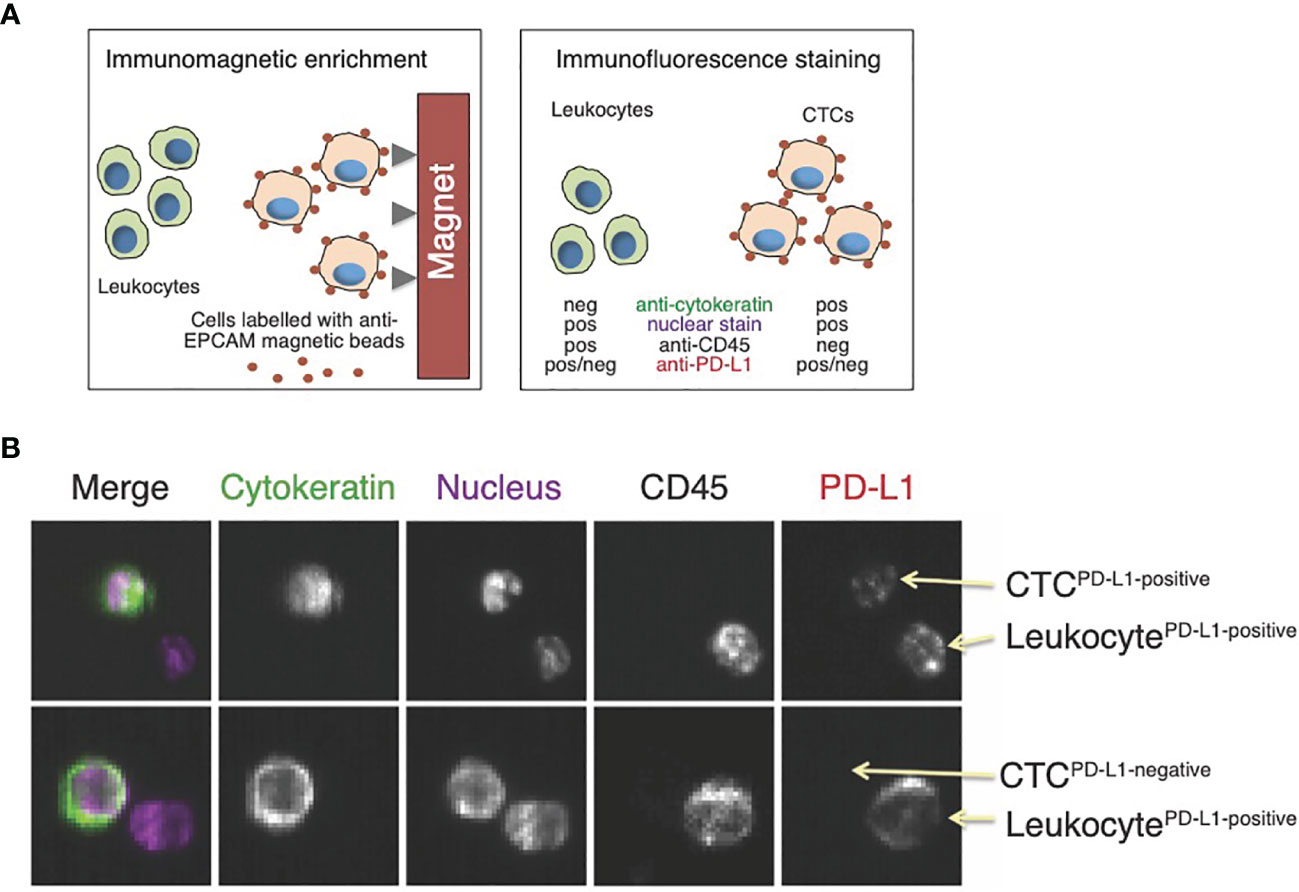
Figure 3 Assessment of PD-L1 expression in circulating tumor cells (CTCs). (A) CellSearch is a semi-automated two-step system used for CTC detection. First, monoclonal antibodies against the epithelial cell adhesion marker (EPCAM)-conjugated to iron beads are added to the blood sample. Magnetic capture allows for the enrichment of tumor cells expressing EPCAM. This is followed by immunofluorescence staining to distinguish CTCs from leukocytes and to detect PD-L1 expression; (B) Examples of images from the CellSearch gallery to identify CTCs expressing PD-L1. Modified with permission from (24).
Schott et al. examined PD-L1 and PD-L2 expression in CTCs of 72 patients with breast cancer (103). CTCs expressing PD-L1 were found in 94.5% of patients using the Maintrac® method. In patients expressing PD-L1 and PD-L2, the proportion of PD-L1-positive CTCs was significantly higher than that of PD-L2-positive CTCs (54.6% versus 28.7%; p <0.001). Furthermore, PD-L1-positive CTCs were detected in patients without metastatic disease, a finding that could extend the use of PD-L1 testing of CTCs in the early-stage setting. Additionally, patients with metastatic breast cancer had significantly more PD-L1-positive CTCs as compared to patients without metastasis (median 75% vs 61%; p<0.05). Dynamic monitoring of PD-L1 expression on CTCs during ICI therapy revealed that the number of CTCs and the percentage of the PD-L1-positive CTCs were reduced in patients that responded to ICI therapy. After discontinuing the ICI agent, the percentage of PD-L1-positive CTCs continuously increased. These findings demonstrated that the number of PD-L1-positive CTCs could be prognostic and correlates with tumor aggressiveness, as well as the potential response to immunotherapy (103).
CD47 is a key immune checkpoint which is highly expressed on a variety of cancer cells, making tumor cell resistant to host immune surveillance. Cell surface CD47 is a ligand for signal regulatory protein-α (SIRPα), a protein expressed on macrophages and dendritic cells, allowing cancer cells to send inhibitory signals to macrophages and impede phagocytosis and immune response (105, 106). Agelaki et al. evaluated the incidence and clinical relevance of CTCs expressing CD47 and/or PD-L1 in patients with metastatic breast cancer. Cytokeratin positive CTCs were detected in 22 of 98 patients (22.4%) with metastatic breast cancer. High CD47 and PD-L1 expression was identified in 41.9% and 11.6% of CTCs, respectively, with 9.1% of CTCs expressing high levels of both markers. High CD47 and/or high PD-L1 CTCs were associated with disease progression (27.8% vs 5.6%; p=0.005) and shorter PFS (5.8 vs 13.3 months; p=0.010), whereas the detection of high PD-L1 CTCs only was correlated with reduced OS (23.8 vs 35.7 months, p=0.043). The study showed that high CD47 and/or high PD-L1 CTCs were associated with increased risk of relapse and high PD-L1 CTCs were associated with high risk of death (HR 4.8; p=0.011). Patients with these CTC biomarker-positive populations could benefit from anti-CD47 and anti-PD-L1 immunotherapy strategies (102).
Quantification of CTCs to monitor response to the HER2/neu E75 peptide vaccine was evaluated in 16 patients with HER2+ breast cancer. Patients with node positive breast cancer were vaccinated monthly for six months after completion of standard therapy including surgery, chemotherapy, and radiotherapy. CTCs were detected in 14 of 16 (88%) patients at baseline. A significant reduction in HER2/neu- expressing CTCs was observed over the course of vaccination (104). This small pilot study suggested a potential role of CTCs enumeration in assessing response to vaccine-based therapy; however, these results were not validated in larger studies.
The persistence of PD-L1-positive CTCs in patients treated with ICI therapy in various cancer types has been associated with worse prognosis (39). However, there is no prospective data, and there are technical issues associated with the detection of CTCs: CTCs are rare and various methods might enrich CTCs populations differently, which could affect the PD-L1 assessment. However, liquid biopsy is a promising technique and a feasible strategy for dynamic assessment and sequential monitoring of PD-L1 expression in patients with breast cancer receiving ICI therapy. Given the small number of studies in patients with breast cancer, further studies are needed to understand the role of PD-L1 expression on CTCs during immunotherapy and to determine the relationship between the expression of PD-L1, CTCs, and tumor tissue.
5 Predictive and Prognostic Value of ctDNA in Immunotherapy In Breast Cancer
ctDNA detectable in blood has been demonstrated to reflect the mutational signatures of a primary tumor. ctDNA is emerging as a potential noninvasive biomarker to detect preclinical metastases and predict relapse following treatment for early-stage disease. ctDNA provides noninvasive access to cancer-specific somatic mutations and could be a technique used to identify specific mutations that are linked with therapeutic response (107, 108). However, ctDNA has not been used clinically for breast cancer patients treated with ICI.
Baseline ctDNA concentration and genomic instability number have been shown to predict response to ICI, and ctDNA monitoring could become a valuable tool for therapy guidance in the future. Genetic analysis of ctDNA is feasible and thus permits the assessment of TMB, which could be a novel biomarker for cancer immunotherapy. Araujo et al. demonstrated that high TMB could predict ICI efficacy in patients with metastatic breast cancer. Among the 16 patients with detectable mutations in both formalin-fixed paraffin-embedded (FFPE) tumor tissue and ctDNA, a statistically significant correlation between blood-based TMB and tissue-based TMB was found (p=0.002) (109). Tumors with high microsatellite instability (MSI) can also be detected using ctDNA based assays (110). Previous studies demonstrated that high MSI from ctDNA is associated with a good response to ICI across various cancers (111). Additionally, the detection of somatic mutations in cfDNA modulating tumor-specific immune response might be helpful to identify non-responding patients. However, genomic analysis to detect mutations and TMB in blood could contain some mutations associated with clonal hematopoiesis, so these non-tumor mutations should be filtered out to prevent misleading results (112).
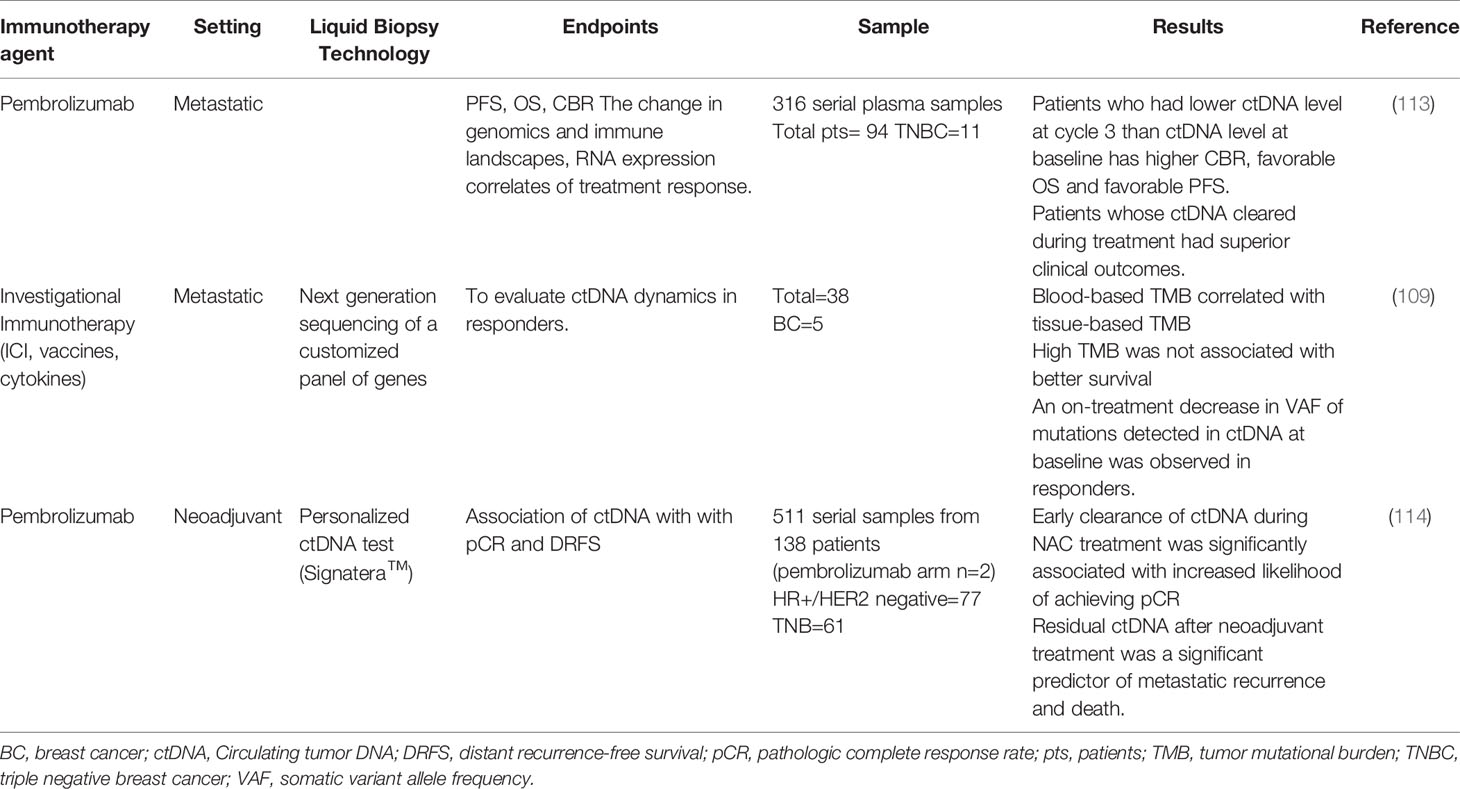
Studies on ctDNA in breast cancer patients receiving immunotherapy are summarized in Table 3. INSPIRE, a multicohort phase 2 trial, was conducted to evaluate the performance of an amplicon-based bespoke (personalized) ctDNA detection to predict response in patients treated with pembrolizumab (113). This study aimed to investigate if baseline ctDNA levels would be prognostic and whether early changes in ctDNA levels would precede imaging response to an ICI. Five cohorts of patients with advanced solid tumors were included. A total of 106 patients were enrolled; of them, 18 patients were TNBC. Researchers analyzed ctDNA levels at baseline and the beginning of cycle 3 of pembrolizumab treatment. Patients who had a lower ctDNA level at cycle 3 than at baseline, had a higher clinical benefit rate (CBR) and a more favorable OS and PFS. They monitored dynamic levels of ctDNA during pembrolizumab treatment to evaluate the predictive value of ctDNA. Among patients with at least two ctDNA measurements, any rise in ctDNA levels above baseline (n=45) during surveillance was associated with rapid disease progression in most patients and with poor survival (median OS=13.7 months). Patients whose ctDNA cleared during treatment (undetectable for at least one on-treatment time point) had superior clinical outcomes. This study showed that serial ctDNA analysis using the bespoke assay could be a monitoring strategy for patients treated with ICI. Changes in ctDNA levels and Response Evaluation Criteria in Solid Tumors (RECIST) from baseline to cycle 3 were discordant in 23% of cases, but the combination of these two metrics was superior to RECIST alone for predicting OS. This study suggests broad clinical utility for ctDNA based surveillance in patients treated with ICI (113). This is a noninvasive strategy to predict clinical benefit and long-term survival. Future large interventional studies are needed to confirm these results using ctDNA levels to guide ICI therapy.
In early-stage breast cancer, the addition of pembrolizumab to standard neoadjuvant chemotherapy improved pCR rates in patients with HR+, HER2 negative breast cancer and TNBC in the I-SPY2 trial (66). ctDNA levels were analyzed on 511 serial plasma samples during neoadjuvant treatment. The detection of ctDNA decreased over time in both the pembrolizumab arm and the control arm. All patients who achieved pCR (n=34) cleared their ctDNA prior to surgery. Among patients who failed to achieve pCR, the distant recurrence free survival (DRFS) rate was significantly better in patients who had ctDNA clearance prior to surgery compared to patients who were ctDNA positive (114).
6 Promises, Pitfalls, and Challenges of CTCs and ctDNA as Biomarkers for Breast Cancer Immunotherapy
CTCs are extremely rare, with an estimated frequency of 1 CTC per one billion blood cells and are difficult to detect in circulation. Counting (enumeration) them requires special reagents (e.g., immunomagnetic beads) and equipment (e.g., automated fluorescent microscope). Current CTC detection technologies, such as that of the CellSearch™ system, have limited sensitivity. Given that CTCs are relatively more abundant in blood of metastatic breast cancer patients, the analysis of CTCs may be more robust in the metastatic setting than in early-stage breast cancer. Even so, only about 50% of metastatic breast cancer patients are positive for CTC (5, 36).
The detection of ctDNA, on the other hand, is less technically challenging than that of CTCs. The isolation of cfDNA, which serves as the input material for sequencing, can be easily performed using commercially available purification kits. The downstream analysis to detect ctDNA in cfDNA generally requires only a next generation sequencer, instrumentation that is available in academic research settings and fee-for-service commercial sequencing companies or clinical reference labs.
Because CTCs can be isolated as live cells, other substrates for biomarker detection and discovery (e.g., DNA, RNA, proteins, and other macromolecules) are available for interrogation. This is a significant advantage of CTCs over ctDNA, which is limited to DNA-based profiling due to the nature of the biomarker (Table 4).
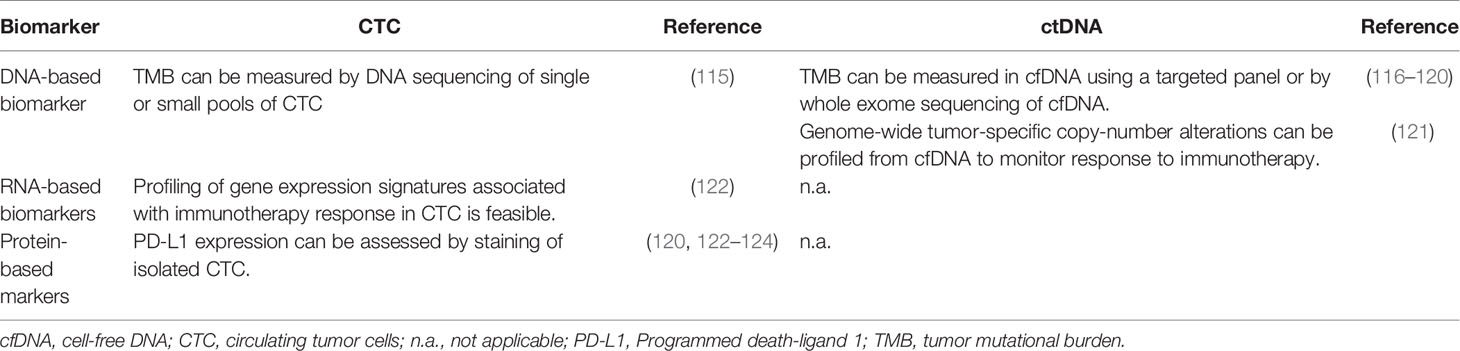
Table 4 Feasibility of assessment of candidate immunotherapy biomarkers in circulating tumor cells (CTC) and circulating tumor DNA (ctDNA).
7 Emerging Liquid Biopsy Technologies
In addition to CTCs and ctDNA, other blood-based biomarkers have been recently developed (125, 126). In this review, we will focus on emerging cfDNA-based biomarkers beyond mutation profiling.
Other cfDNA-based biomarkers, in addition to the detection of tumor mutant DNA molecules (i.e., ctDNA) are being developed. Cristiano and colleagues described an approach to profile genome-wide fragmentation patterns of cfDNA, also referred to as “fragmentomics” (127). The authors showed that fragmentation profiling, combined with mutation-based analysis, can accurately discriminate between cancer patients and healthy individuals. Another approach involves methylation sequencing of cfDNA (128). For example, Liu and colleagues showed that evaluation of the methylation patterns in more than 900 CpG sites in cfDNA detected the presence of cancer and identified the cancer type in patients with advanced cancers. Chromatin state or nucleosome footprint analysis of the cfDNA is another approach that is currently under development (129, 130). The positions of nucleosomes on DNA determine chromatin structure which in turn affect gene expression (131). This approach involves generating genome-wide maps that show nucleosome occupancy and the evaluation of transcription factor binding in small fragments of cfDNA (129). Using this approach, Ulz and colleagues found patient- and tumor-specific nucleosome occupancy patterns and were able to accurately predict subtypes in prostate cancer (130).
Mutation detection in cfDNA is challenging because rare tumor-derived mutated DNA molecules are present in an overwhelming background of normal DNA from hematopoietic cells. Detection is particularly challenging in cancers with low or moderate tumor mutational burden, like breast cancer (132). These new emerging platforms offer the opportunity to interrogate genome-wide or significantly more genomic loci than what is available for mutational profiling. For example, Jensen and colleagues describe the use of a genome-wide measure of genomic instability by low-coverage next generation sequencing of cfDNA, an assay that is validated for noninvasive prenatal testing, to detect tumor-specific copy number aberrations (13, 121, 133). Using this approach, the investigators developed a novel metric, genome instability number (GIN), that can be used to monitor response to immunotherapy drugs, including the differentiation of progression from pseudoprogression (121). The GIN assay and other novel technologies that interrogate the whole genome show promise in providing clinically relevant information above what ctDNA alone can provide. However, further testing to demonstrate their applications to guide immunotherapy, particularly in breast cancer, is warranted.
8 Future Directions and Summary
Immunotherapy has a defined role in the treatment of both early- and late-stage TNBC and is under active exploration in HER2+ as well as high-risk HR+ disease. Only a minority of patients in the metastatic setting are likely to benefit from adding ICI to standard chemotherapy, and outcome is particularly poor for patients with PD-L1-negative disease. In the early-stage setting, therapy is given with curative intent, so the balance of toxicity and efficacy is critical. In addition, ICI therapy is costly, and the duration of therapy has implications for both toxicity and patient quality of life. It is therefore of the utmost importance to identify better markers to predict efficacy. The analysis of PD-L1 expression on CTCs and the detection of ctDNA are actively under investigation. Confirming the predictive value of TMB in prospective trials and standardizing the assessment of TMB are critical next steps.
Further clinical studies are warranted to demonstrate the role of liquid biopsy in guiding immunotherapy in breast cancer. Blood biomarkers can monitor disease trajectory during and after therapy and have the potential to reveal mutational shifts and resistance mechanisms. These biomarkers reflect, in part, the changes in tumor burden during treatment. However, the correlation between tumor burden/response and the levels of CTCs and ctDNA is not perfect; therefore, additional biomarkers are needed to refine their predictive and prognostic value. Ongoing clinical trials involving the assessment of liquid biopsy technologies in patients with breast cancer receiving immunotherapy are listed in Table 5.
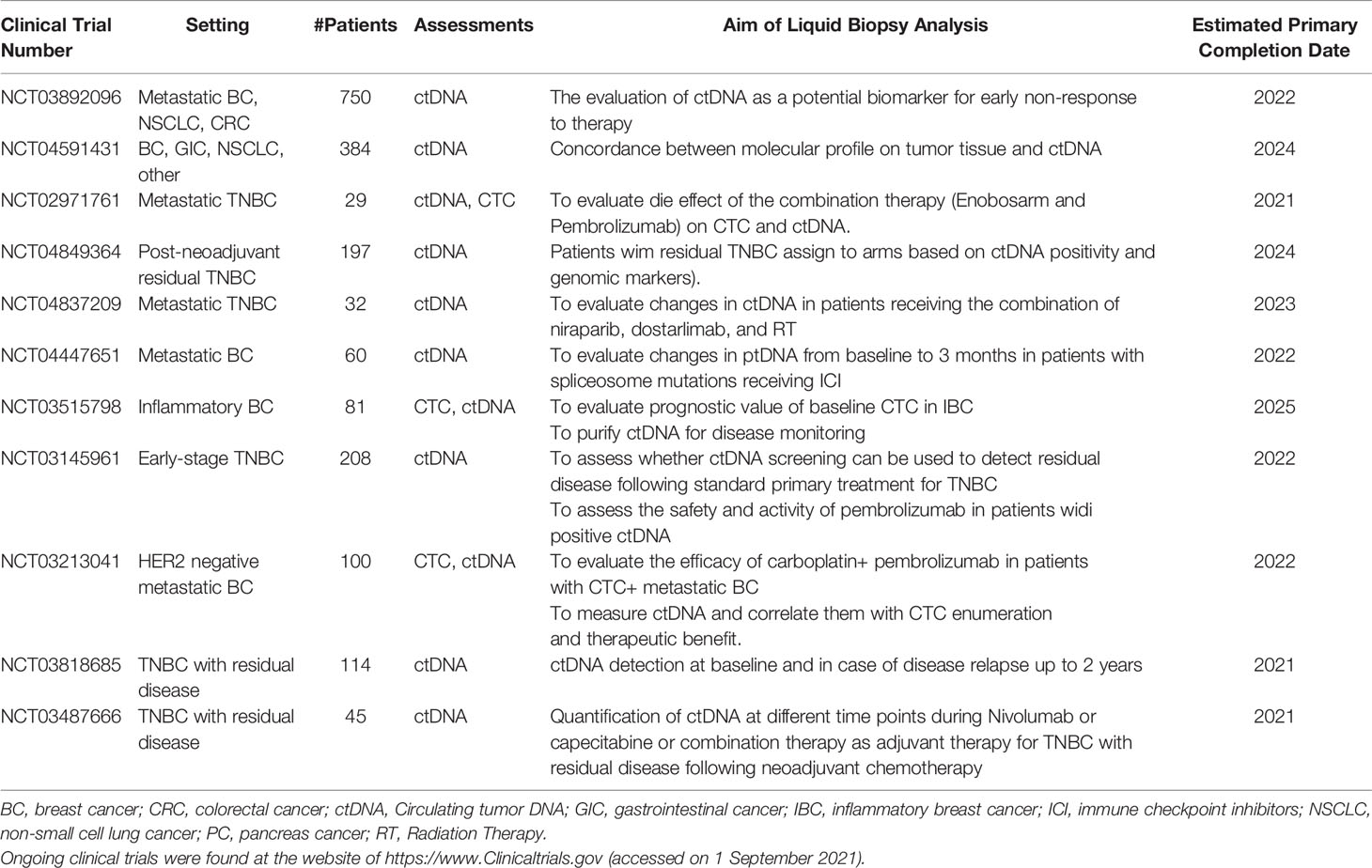
Table 5 Overview of ongoing clinical trials of liquid biopsy techniques in breast cancer undergoing immunotherapy.
In conclusion, liquid biopsy applications to guide immunotherapy in breast cancer have not yet been implemented in clinical practice, but promising data and rapidly advancing technologies indicate that this approach has the potential to select patients who would benefit from immunotherapy.
Author Contributions
The authors MM and OG contributed to the study conception, data collection and interpretation, and manuscript preparation. HR wrote sections of the manuscript. HR, RK, and LVV reviewed the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The study was funded in part by the grants from the NIH (R01 CA255442), the Breast Cancer Research Foundation (BCRF-18-142), and the California Breast Cancer Research Program IDEA award; and a fellowship awarded to MM by the Cancer Cell Mapping Initiative (U54 CA209891).
Conflict of Interest
HR received research support for clinical trials through the University of California: Pfizer, Merck, Novartis, Lilly, Roche, Odonate, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, Astra Zeneca, OBI, Gilead, and Ayala; honoraria: Puma, Mylan, Samsung, and Napo. RK has received research funding from Biological Dynamics, Daiichi Sankyo, Inc., EISAI, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc., CureMetrix, and IDbyDNA; serves on the Board of CureMatch and CureMetrix,and is a co-founder of CureMatch. LVV is a co-founder, stockholder and part-time employee of Agendia NV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Amy L. Delson of the UCSF Breast Science Advocacy Group for her critical review of the paper.
References
1. Pritzker KP. Predictive and Prognostic Cancer Biomarkers Revisited. Expert Rev Mol Diagn (2015) 15:971–4. doi: 10.1586/14737159.2015.1063421
2. Nicolini A, Ferrari P, Duffy MJ. Prognostic and Predictive Biomarkers in Breast Cancer: Past, Present and Future. Semin Cancer Biol (2018) 52:56–73. doi: 10.1016/j.semcancer.2017.08.010
3. Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating Tumor Cells Predict Survival in Early Average-to-High Risk Breast Cancer Patients. J Natl Cancer Inst (2014) 106(5):dju066. doi: 10.1093/jnci/dju066
4. Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-Analysis. J Natl Cancer Inst (2018) 110:560–7. doi: 10.1093/jnci/djy018
5. Magbanua MJM, Hendrix LH, Hyslop T, Barry WT, Winer EP, Hudis C, et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. J Natl Cancer Inst (2021) 113:443–52. doi: 10.1093/jnci/djaa113
6. Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N Engl J Med (2013) 368:1199–209. doi: 10.1056/NEJMoa1213261
7. Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin Cancer Res (2019) 25:4255–63. doi: 10.1158/1078-0432.CCR-18-3663
8. Cullinane C, Fleming C, O'leary DP, Hassan F, Kelly L, O'sullivan MJ, et al. Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open (2020) 3:e2026921. doi: 10.1001/jamanetworkopen.2020.26921
9. Magbanua MJM, Li W, Wolf DM, Yau C, Hirst GL, Swigart LB, et al. Circulating Tumor DNA and Magnetic Resonance Imaging to Predict Neoadjuvant Chemotherapy Response and Recurrence Risk. NPJ Breast Cancer (2021) 7:32. doi: 10.1038/s41523-021-00239-3
10. Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating Tumor DNA in Neoadjuvant-Treated Breast Cancer Reflects Response and Survival. Ann Oncol (2021) 32:229–39. doi: 10.1016/j.annonc.2020.11.007
11. Agashe R, Kurzrock R. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers (Basel) (2020) 12. doi: 10.3390/cancers12092361
12. Adashek JJ, Janku F, Kurzrock R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers (Basel) (2021) 13(14):3600. doi: 10.3390/cancers13143600
13. Jensen TJ, Goodman AM, Ellison CK, Holden KA, Kato S, Kim L, et al. Genome-Wide Sequencing of Cell-Free DNA Enables Detection of Copy-Number Alterations in Patients With Cancer Where Tissue Biopsy Is Not Feasible. Mol Cancer Ther (2021) 20(11):2274–9. doi: 10.1158/1535-7163.MCT-20-1066
14. Pantel K, Alix-Panabieres C. Liquid Biopsy and Minimal Residual Disease - Latest Advances and Implications for Cure. Nat Rev Clin Oncol (2019) 16:409–24. doi: 10.1038/s41571-019-0187-3
15. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
16. Cheang MC, Voduc D, Bajdik C, Leung S, Mckinney S, Chia SK, et al. Basal-Like Breast Cancer Defined by Five Biomarkers Has Superior Prognostic Value Than Triple-Negative Phenotype. Clin Cancer Res (2008) 14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658
17. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet (2020) 396:1817–28. doi: 10.1016/S0140-6736(20)32531-9
18. Schmid P, Rugo H, Adams S, Schneeweiss A, Barrios C, Iwata H, et al. Impassion 130 Investigators Atezolizumab Plus Nab-Paclitaxel as First-Line Treatment for Unresectable, Locally Advanced or Metastatic Triple-Negative Breast Cancer (IMpassion130): Updated Efficacy Results From a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2020) 21:44–59. doi: 10.1016/S1470-2045(19)30689-8
19. Cortés J, Cescon D, Rugo H, Im S, Yusof MM, Gallardo C, et al. LBA16 KEYNOTE-355: Final Results From a Randomized, Double-Blind Phase III Study of First-Line Pembrolizumab+ Chemotherapy vs Placebo+ Chemotherapy for Metastatic TNBC. Ann Oncol (2021) 32:S1289–90. doi: 10.1016/j.annonc.2021.08.2089
20. Schmid P, Cortes J, Dent R, Pusztai L, Mcarthur H, Kümmel S, et al. VP7-2021: KEYNOTE-522: Phase III Study of Neoadjuvant Pembrolizumab+ Chemotherapy vs. Placebo+ Chemotherapy, Followed by Adjuvant Pembrolizumab vs. Placebo for Early-Stage TNBC. Ann Oncol (2021) 32:1198–200. doi: 10.1016/j.annonc.2021.06.014
21. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
22. Emens LA, Adams S, Barrios CH, Dieras V, Iwata H, Loi S, et al. LBA16 IMpassion130: Final OS Analysis From the Pivotal Phase III Study of Atezolizumab+ Nab-Paclitaxel vs Placebo+ Nab-Paclitaxel in Previously Untreated Locally Advanced or Metastatic Triple-Negative Breast Cancer. Ann Oncol (2020) 31:S1148. doi: 10.1016/j.annonc.2020.08.2244
23. Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol (2015) 33:3541. doi: 10.1200/JCO.2015.61.6870
24. Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, et al. Frequent Expression of PD-L1 on Circulating Breast Cancer Cells. Mol Oncol (2015) 9:1773–82. doi: 10.1016/j.molonc.2015.05.009
25. Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, Economopoulou P, et al. Prognostic Significance of PD-L1 Expression on Circulating Tumor Cells in Patients With Head and Neck Squamous Cell Carcinoma. Ann Oncol (2017) 28:1923–33. doi: 10.1093/annonc/mdx206
26. Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 Expression in Circulating Tumor Cells of Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Lung Cancer (2018) 120:108–12. doi: 10.1016/j.lungcan.2018.04.001
27. Ilie M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvee S, Selva E, et al. Detection of PD-L1 in Circulating Tumor Cells and White Blood Cells From Patients With Advanced Non-Small-Cell Lung Cancer. Ann Oncol (2018) 29:193–9. doi: 10.1093/annonc/mdx636
28. Kulasinghe A, Kapeleris J, Kimberley R, Mattarollo SR, Thompson EW, Thiery JP, et al. The Prognostic Significance of Circulating Tumor Cells in Head and Neck and Non-Small-Cell Lung Cancer. Cancer Med (2018) 7:5910–9. doi: 10.1002/cam4.1832
29. Jacot W, Mazel M, Mollevi C, Pouderoux S, D'hondt V, Cayrefourcq L, et al. Clinical Correlations of Programmed Cell Death Ligand 1 Status in Liquid and Standard Biopsies in Breast Cancer. Clin Chem (2020) 66:1093–101. doi: 10.1093/clinchem/hvaa121
30. Sinoquet L, Jacot W, Gauthier L, Pouderoux S, Viala M, Cayrefourcq L, et al. Programmed Cell Death Ligand 1-Expressing Circulating Tumor Cells: A New Prognostic Biomarker in Non-Small Cell Lung Cancer. Clin Chem (2021) 67:1503–12. doi: 10.1093/clinchem/hvab131
31. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N Engl J Med (2004) 351:781–91. doi: 10.1056/NEJMoa040766
32. Ignatiadis M, Lee M, Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res (2015) 21:4786–800. doi: 10.1158/1078-0432.CCR-14-1190
33. Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discovery (2016) 6:479–91. doi: 10.1158/2159-8290.CD-15-1483
34. Yoo TK. Liquid Biopsy in Breast Cancer: Circulating Tumor Cells and Circulating Tumor DNA. Adv Exp Med Biol (2021) 1187:337–61. doi: 10.1007/978-981-32-9620-6_17
35. Keller L, Pantel K. Unravelling Tumour Heterogeneity by Single-Cell Profiling of Circulating Tumour Cells. Nat Rev Cancer (2019) 19:553–67. doi: 10.1038/s41568-019-0180-2
36. Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical Validity of Circulating Tumour Cells in Patients With Metastatic Breast Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol (2014) 15:406–14. doi: 10.1016/S1470-2045(14)70069-5
37. Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, et al. Circulating Tumor Cells: Clinical Validity and Utility. Int J Clin Oncol (2017) 22:421–30. doi: 10.1007/s10147-017-1105-2
38. Rushton AJ, Nteliopoulos G, Shaw JA, Coombes RC. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers (Basel) (2021) 13(5):970. doi: 10.3390/cancers13050970
39. Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 Positive Circulating Tumor Cells in Non-Small Cell Lung Cancer Patients Treated With the PD-1 Inhibitor Nivolumab. Sci Rep (2016) 6:1–8. doi: 10.1038/srep31726
40. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev (2016) 35:347–76. doi: 10.1007/s10555-016-9629-x
41. Papadopoulos N. Pathophysiology of ctDNA Release Into the Circulation and Its Characteristics: What Is Important for Clinical Applications. Recent Results Cancer Res (2020) 215:163–80. doi: 10.1007/978-3-030-26439-0_9
42. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, Necrosis, and Apoptosis Govern Circulating Cell-Free DNA Release Kinetics. Cell Rep (2020) 31:107830. doi: 10.1016/j.celrep.2020.107830
43. Moreno F, Gayarre J, López-Tarruella S, Monte-Millán MD, Picornell AC, Álvarez E, et al. Concordance of Genomic Variants in Matched Primary Breast Cancer, Metastatic Tumor, and Circulating Tumor DNA: The MIRROR Study. JCO Precis Oncol (2019) 1–16. doi: 10.1200/PO.18.00263
44. Shatsky R, Parker BA, Bui NQ, Helsten T, Schwab RB, Boles SG, et al. Next-Generation Sequencing of Tissue and Circulating Tumor DNA: The UC San Diego Moores Center for Personalized Cancer Therapy Experience With Breast Malignancies. Mol Cancer Ther (2019) 18:1001–11. doi: 10.1158/1535-7163.MCT-17-1038
45. Chae YK, Davis AA, Jain S, Santa-Maria C, Flaum L, Beaubier N, et al. Concordance of Genomic Alterations by Next-Generation Sequencing in Tumor Tissue Versus Circulating Tumor DNA in Breast Cancer. Mol Cancer Ther (2017) 16:1412–20. doi: 10.1158/1535-7163.MCT-17-0061
46. Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation Tracking in Circulating Tumor DNA Predicts Relapse in Early Breast Cancer. Sci Transl Med (2015) 7:302ra133. doi: 10.1126/scitranslmed.aab0021
47. Garcia-Murillas I, Chopra N, Comino-Mendez I, Beaney M, Tovey H, Cutts RJ, et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol (2019) 5:1473–8. doi: 10.1001/jamaoncol.2019.1838
48. Davis AA, Jacob S, Gerratana L, Shah AN, Wehbe F, Katam N, et al. Landscape of Circulating Tumour DNA in Metastatic Breast Cancer. EBioMedicine (2020) 58:102914. doi: 10.1016/j.ebiom.2020.102914
49. Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
50. Jacob S, Davis AA, Gerratana L, Velimirovic M, Shah AN, Wehbe F, et al. The Use of Serial Circulating Tumor DNA to Detect Resistance Alterations in Progressive Metastatic Breast Cancer. Clin Cancer Res (2021) 27:1361–70. doi: 10.1158/1078-0432.CCR-20-1566
51. Bidard FC, Callens C, Dalenc F, Pistilli B, Rouge TDLM, Clatot F, et al. Prognostic Impact of ESR1 Mutations in ER+ HER2- MBC Patients Prior Treated With First Line AI and Palbociclib: An Exploratory Analysis of the PADA-1 Trial. J Clin Oncol (2020) 38:1010. doi: 10.1200/JCO.2020.38.15_suppl.1010
52. Lee HT, Lee SH, Heo Y-S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules (2019) 24:1190. doi: 10.3390/molecules24061190
53. Adams S, Schmid P, Rugo H, Winer E, Loirat D, Awada A, et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort A of the Phase II KEYNOTE-086 Study. Ann Oncol (2019) 30:397–404. doi: 10.1093/annonc/mdy517
54. Adams S, Loi S, Toppmeyer D, Cescon D, De Laurentiis M, Nanda R, et al. Pembrolizumab Monotherapy for Previously Untreated, PD-L1-Positive, Metastatic Triple-Negative Breast Cancer: Cohort B of the Phase II KEYNOTE-086 Study. Ann Oncol (2019) 30:405–11. doi: 10.1093/annonc/mdy518
55. Cortés J, Lipatov O, Im S-A, Im S-A, Gonçalves A, Lee KS, Schmid P, et al. KEYNOTE-119: Phase III Study of Pembrolizumab (Pembro) Versus Single-Agent Chemotherapy (Chemo) for Metastatic Triple Negative Breast Cancer (mTNBC). Annals Oncology (2019) 30:v859–60. doi: 10.1093/annonc/mdz394.010
56. Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-Term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncology (2019) 5:74–82. doi: 10.1001/jamaoncol.2018.4224
57. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H-T, Forero-Torres A, et al. Avelumab, an Anti-PD-L1 Antibody, in Patients With Locally Advanced or Metastatic Breast Cancer: A Phase 1b JAVELIN Solid Tumor Study. Breast Cancer Res Treat (2018) 167:671–86. doi: 10.1007/s10549-017-4537-5
58. Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. Clin Cancer Res (2018) 24:2804–11. doi: 10.1158/1078-0432.CCR-17-3452
59. Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-Line Atezolizumab Plus Nab-Paclitaxel for Unresectable, Locally Advanced, or Metastatic Triple-Negative Breast Cancer: IMpassion130 Final Overall Survival Analysis. Ann Oncol (2021) 32:983–93. doi: 10.1016/j.annonc.2021.05.355
60. Rugo HS, Schmid P, Cescon DW, Nowecki Z, Im S-A, Yusof MM, et al. Abstract GS3-01: Additional Efficacy Endpoints From the Phase 3 KEYNOTE-355 Study of Pembrolizumab Plus Chemotherapy vs Placebo Plus Chemotherapy as First-Line Therapy for Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer. AACR (2021). doi: 10.1158/1538-7445.SABCS20-GS3-01
61. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary Results From IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel With or Without Atezolizumab for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer. Ann Oncol (2021) 32:994–1004. doi: 10.1016/j.annonc.2020.08.2243
62. Tolaney SM, Kalinsky K, Kaklamani VG, D'adamo DR, Aktan G, Tsai ML, et al. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin Cancer Res (2021) 27(11):3061–8. doi: 10.1158/1078-0432.CCR-20-4726
63. Pérez-García JM, Llombart-Cussac A, Gion M, Curigliano G, López-Miranda E, et al. Pembrolizumab Plus Eribulin in Hormone-Receptor-Positive, HER2-Negative, Locally Recurrent or Metastatic Breast Cancer (KELLY): An Open-Label, Multicentre, Single-Arm, Phase II Trial. Eur J Cancer (2021) 148:382–94. doi: 10.1016/j.ejca.2021.02.028
64. Schmid P, Cortes J, Pusztai L, Mcarthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. New Engl J Med (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
65. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant Atezolizumab in Combination With Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy Versus Placebo and Chemotherapy in Patients With Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet (2020) 396:1090–100. doi: 10.1016/S0140-6736(20)31953-X
66. Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol (2020) 6:676–84. doi: 10.1001/jamaoncol.2019.6650
67. Loibl S, Untch M, Burchardi N, Huober J, Sinn B, Blohmer J-U, et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann Oncol (2019) 30:1279–88. doi: 10.1093/annonc/mdz158
68. Gianni L, Huang C-S, Egle D, Bermejo B, Zamagni C, Thill M, et al. "Abstract GS3-04: Pathologic Complete Response (pCR) to Neoadjuvant Treatment With or Without Atezolizumab in Triple Negative, Early High-Risk and Locally Advanced Breast Cancer. NeoTRIPaPDL1 Michelangelo Randomized Study". AACR (2020) 80(4 Supplement):GS3-04-GS3-04. doi: 10.1158/1538-7445.SABCS19-GS3-04
69. Nathan MR, Schmid P. The Emerging World of Breast Cancer Immunotherapy. Breast (2018) 37:200–6. doi: 10.1016/j.breast.2017.05.013
70. Den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival With Metastatic Breast Cancer Based on Initial Presentation, De Novo Versus Relapsed. Breast Cancer Res Treat (2017) 161:549–56. doi: 10.1007/s10549-016-4080-9
71. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol Res (2014) 2:361–70. doi: 10.1158/2326-6066.CIR-13-0127
72. U.S. Food and Drug Administration. FDA Approves Atezolizumab for PD-L1 Positive Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer (2019). Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (Accessed 01/21/2022).
73. Gumusay O, Wabl CA, Rugo HS. Trials of Immunotherapy in Triple Negative Breast Cancer. Curr Breast Cancer Rep (2021) 13:171–85. doi: 10.1007/s12609-021-00418-y
74. Merck. KEYTRUDA® (Pembrolizumab) Prescribing Information (2021). Available at: https://merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (Accessed 01/21/2022).
75. Tolaney S, Kalinsky K, Kaklamani V, Savulsky C, Olivo M, Aktan G, et al. "Abstract PD6-13: Phase 1b/2 Study to Evaluate Eribulin Mesylate in Combination With Pembrolizumab in Patients With Metastatic Triple-Negative Breast Cancer". AACR (2018) 78(4 Supplement):PD6-13-PD6-13. doi: 10.1158/1538-7445.SABCS17-PD6-13
76. Shah AN, Flaum L, Helenowski I, Santa-Maria CA, Jain S, Rademaker A, et al. Phase II Study of Pembrolizumab and Capecitabine for Triple Negative and Hormone Receptor-Positive, HER2– Negative Endocrine-Refractory Metastatic Breast Cancer. J Immunother Cancer (2020) 8(1):e000173. doi: 10.1136/jitc-2019-000173
77. Domchek S, Postel-Vinay S, Im S-A, Park Y, Delord J-P, Italiano A, et al. Phase II Study of Olaparib (O) and Durvalumab (D)(MEDIOLA): Updated Results in Patients (Pts) With Germline BRCA-Mutated (gBRCAm) Metastatic Breast Cancer (MBC). Ann Oncol (2019) 30:v477. doi: 10.1093/annonc/mdz253.017
78. Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A, et al. Olaparib and Durvalumab in Patients With Germline BRCA-Mutated Metastatic Breast Cancer (MEDIOLA): An Open-Label, Multicentre, Phase 1/2, Basket Study. Lancet Oncol (2020) 21:1155–64. doi: 10.1016/S1470-2045(20)30324-7
79. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of Tumour Mutational Burden With Outcomes in Patients With Advanced Solid Tumours Treated With Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
80. Winer EP, Lipatov O, Im S-A, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab Versus Investigator-Choice Chemotherapy for Metastatic Triple-Negative Breast Cancer (KEYNOTE-119): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22:499–511. doi: 10.1016/S1470-2045(20)30754-3
81. Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, et al. Prevalence and Mutational Determinants of High Tumor Mutation Burden in Breast Cancer. Annals Oncology (2020) 31:387–394. doi: 10.1016/j.annonc.2019.11.010
82. Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol (2016) 2:1354–60. doi: 10.1001/jamaoncol.2016.1061
83. Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. "Expression of PD-L1 and Prognosis in Breast Cancer: A Meta-Analysis. Oncotarget (2017) 8:31347–54. doi: 10.18632/oncotarget.15532
84. Rugo HS, Kabos P, Beck JT, Chisamore MJ, Hossain A, Chen Y, et al. "A Phase Ib Study of Abemaciclib in Combination With Pembrolizumab for Patients With Hormone Receptor Positive (HR+), Human Epidermal Growth Factor Receptor 2 Negative (HER2-) Locally Advanced or Metastatic Breast Cancer (MBC)(NCT02779751): Interim Results". Am Soc Clin Oncol (2020) 38(15_suppl):1051. doi: 10.1158/1538-7445.AM2020-CT108
85. Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat Rev Clin Oncol (2016) 13:228–41. doi: 10.1038/nrclinonc.2015.215
86. Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab Plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b–2 Trial. Lancet Oncol (2019) 20:371–82. doi: 10.1016/S1470-2045(18)30812-X
87. Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim SB, et al. Trastuzumab Emtansine Plus Atezolizumab Versus Trastuzumab Emtansine Plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol (2020) 21:1283–95. doi: 10.1016/S1470-2045(20)30465-4
88. Boughey JC, Mccall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor Biology Correlates With Rates of Breast-Conserving Surgery and Pathologic Complete Response After Neoadjuvant Chemotherapy for Breast Cancer: Findings From the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg (2014) 260:608. doi: 10.1097/SLA.0000000000000924
89. Von Minckwitz G, Untch M, Blohmer J-U, Costa SD, Eidtmann H, Fasching PA, et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J Clin Oncol (2012) 30:1796–804. doi: 10.1200/JCO.2011.38.8595
90. Untch M, Konecny GE, Paepke S, Von Minckwitz G. Current and Future Role of Neoadjuvant Therapy for Breast Cancer. Breast (2014) 23:526–37. doi: 10.1016/j.breast.2014.06.004
91. Yamaguchi R, Tanaka M, Yano A, Gary MT, Yamaguchi M, Koura K, et al. Tumor-Infiltrating Lymphocytes Are Important Pathologic Predictors for Neoadjuvant Chemotherapy in Patients With Breast Cancer. Hum Pathol (2012) 43:1688–94. doi: 10.1016/j.humpath.2011.12.013
92. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther (2015) 14:847–56. doi: 10.1158/1535-7163.MCT-14-0983
93. Merck. FDA Approves KEYTRUDA (Pembrolizumab) for Treatment of Patients With High-Risk Early-Stage Triple-Negative Breast Cancer in Combination With Chemotherapy as Neoadjuvant Treatment, Then Continued as Single Agent as Adjuvant Treatment After Surgery [Online] (2021). Available at: https://bit.ly/2Vc0rgX (Accessed 01/21/2022).
94. Loibl S, Schneeweiss A, Huober JB, Braun M, Rey J, Blohmer JU, et al. "Durvalumab Improves Long-Term Outcome in TNBC: Results From the Phase II Randomized GeparNUEVO Study Investigating Neodjuvant Durvalumab in Addition to an Anthracycline/Taxane Based Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer (TNBC)". Wolters Kluwer Health (2021) 39(15_suppl):506. doi: 10.1200/JCO.2021.39.15_suppl.506
95. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 35:785–92. doi: 10.1200/JCO.2015.66.1389
96. Postow MA, Hellmann MD. Adverse Events Associated With Immune Checkpoint Blockade. New Engl J Med (2018) 378:1165–5. doi: 10.1056/NEJMc1801663
97. Teulings H-E, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-Like Depigmentation in Patients With Stage III-IV Melanoma Receiving Immunotherapy and Its Association With Survival: A Systematic Review and Meta-Analysis. J Clin Oncol (2015) 33:773–81. doi: 10.1200/JCO.2014.57.4756
98. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol (2016) 152:45–51. doi: 10.1001/jamadermatol.2015.2707
99. Gonzalez-Ericsson PI, Stovgaard ES, Sua LF, Reisenbichler E, Kos Z, Carter JM, et al. The Path to a Better Biomarker: Application of a Risk Management Framework for the Implementation of PD-L1 and TILs as Immuno-Oncology Biomarkers in Breast Cancer Clinical Trials and Daily Practice. J Pathol (2020) 250:667–84. doi: 10.1002/path.5406
100. Muinelo-Romay L, García-González J, León-Mateos L. Lung Cancer and Liquid Biopsy: Realities and Challenges in Routine Clinical Practice. Arch Bronconeumol (Engl Ed) (2019) 55(6):289-90. doi: 10.1016/j.arbres.2018.11.011.
101. Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid Biopsy in the Era of Immuno-Oncology: Is It Ready for Prime-Time Use for Cancer Patients? Ann Oncol (2019) 30:1448–59. doi: 10.1093/annonc/mdz196
102. Agelaki S, Papadaki MA, Tsoulfas PG, Aggouraki D, Monastirioti AA, Merodoulaki KA, et al. "Role of the Expression of PD-L1 and CD47 on Circulating Tumor Cells (CTCs) in the Prediction of Outcome in Metastatic Breast Cancer (mBC) Patients". Am Soc Clin Oncol (2019) 37(15_suppl):e14045–e14045. doi: 10.1200/JCO.2019.37.15_suppl.e14045
103. Schott DS, Pizon M, Pachmann U, Pachmann K. Sensitive Detection of PD-L1 Expression on Circulating Epithelial Tumor Cells (CETCs) Could Be a Potential Biomarker to Select Patients for Treatment With PD-1/PD-L1 Inhibitors in Early and Metastatic Solid Tumors. Oncotarget (2017) 8:72755. doi: 10.18632/oncotarget.20346
104. Stojadinovic A, Mittendorf EA, Holmes JP, Amin A, Hueman MT, Ponniah S, et al. Quantification and Phenotypic Characterization of Circulating Tumor Cells for Monitoring Response to a Preventive HER2/neu Vaccine-Based Immunotherapy for Breast Cancer: A Pilot Study. Ann Surg Oncol (2007) 14:3359–68. doi: 10.1245/s10434-007-9538-x
105. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell (2009) 138:271–85. doi: 10.1016/j.cell.2009.05.046
106. Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, et al. A CD47-Associated Super-Enhancer Links Pro-Inflammatory Signalling to CD47 Upregulation in Breast Cancer. Nat Commun (2017) 8:14802. doi: 10.1038/ncomms14802
107. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of Circulating Tumor DNA in Early-and Late-Stage Human Malignancies. Sci Trans Med (2014) 6:224ra224–224ra224. doi: 10.1158/1538-7445.AM2014-5606
108. Bardelli A, Pantel K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell (2017) 31:172–9. doi: 10.1016/j.ccell.2017.01.002
109. Araujo DV, Wang A, Torti D, Leon A, Marsh K, Mccarthy A, et al. Applications of Circulating Tumor DNA in a Cohort of Phase I Solid Tumor Patients Treated With Immunotherapy. JNCI Cancer Spectr (2021) 5:pkaa122. doi: 10.1093/jncics/pkaa122
110. Mayrhofer M, De Laere B, Whitington T, Van Oyen P, Ghysel C, Ampe J, et al. Cell-Free DNA Profiling of Metastatic Prostate Cancer Reveals Microsatellite Instability, Structural Rearrangements and Clonal Hematopoiesis. Genome Med (2018) 10:1–13. doi: 10.1186/s13073-018-0595-5
111. Weiss GJ, Beck J, Braun DP, Bornemann-Kolatzki K, Barilla H, Cubello R, et al. Tumor Cell–Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin Cancer Res (2017) 23:5074–81. doi: 10.1158/1078-0432.CCR-17-0231
112. Ptashkin RN, Mandelker DL, Coombs CC, Bolton K, Yelskaya Z, Hyman DM, et al. Prevalence of Clonal Hematopoiesis Mutations in Tumor-Only Clinical Genomic Profiling of Solid Tumors. JAMA Oncol (2018) 4:1589–93. doi: 10.1001/jamaoncol.2018.2297
113. Bratman SV, Yang SC, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. Personalized Circulating Tumor DNA Analysis as a Predictive Biomarker in Solid Tumor Patients Treated With Pembrolizumab. Nat Cancer (2020) 1:873–81. doi: 10.1038/s43018-020-0096-5
114. Magbanua MJM, Wolf D, Renner D, Shchegrova S, Swigart LB, Yau C, et al. Abstract PD9-02: Personalized ctDNA as a Predictive Biomarker in High-Risk Early Stage Breast Cancer (EBC) Treated With Neoadjuvant Chemotherapy (NAC) With or Without Pembrolizumab (P). Cancer Res (2021) 81:PD9-02-PD09-02. doi: 10.1158/1538-7445.SABCS20-PD9-02
115. Rodriguez A, Lee J, Sutton R, Jiles R, Wang Y, Landers M, et al. Abstract A061: Tumor Mutation Burden (TMB), Microsatellite Instability (MSI), and Chromosomal Instability (CIN) Analysis Using Low Pass Whole Genome Sequencing of Single Circulating Tumor Cell (CTC). Mol Cancer Ther (2018) 17:A061–1. doi: 10.1158/1535-7163.TARG-17-A061
116. Koeppel F, Blanchard S, Jovelet C, Genin B, Marcaillou C, Martin E, et al. Whole Exome Sequencing for Determination of Tumor Mutation Load in Liquid Biopsy From Advanced Cancer Patients. PloS One (2017) 12:e0188174. doi: 10.1371/journal.pone.0188174
117. Fenizia F, Pasquale R, Roma C, Bergantino F, Iannaccone A, Normanno N. Measuring Tumor Mutation Burden in Non-Small Cell Lung Cancer: Tissue Versus Liquid Biopsy. Transl Lung Cancer Res (2018) 7:668–77. doi: 10.21037/tlcr.2018.09.23
118. Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, et al. Clinical Implications of Circulating Tumor DNA Tumor Mutational Burden (ctDNA TMB) in Non-Small Cell Lung Cancer. Oncologist (2019) 24:820–8. doi: 10.1634/theoncologist.2018-0433
119. Qiu P, Poehlein CH, Marton MJ, Laterza OF, Levitan D. Measuring Tumor Mutational Burden (TMB) in Plasma from mCRPC Patients Using Two Commercial NGS Assays. Sci Rep (2019) 9:114. doi: 10.1038/s41598-018-37128-y
120. Friedlaender A, Nouspikel T, Christinat Y, Ho L, Mckee T, Addeo A. Tissue-Plasma TMB Comparison and Plasma TMB Monitoring in Patients With Metastatic Non-small Cell Lung Cancer Receiving Immune Checkpoint Inhibitors. Front Oncol (2020) 10:142. doi: 10.3389/fonc.2020.00142
121. Jensen TJ, Goodman AM, Kato S, Ellison CK, Daniels GA, Kim L, et al. Genome-Wide Sequencing of Cell-Free DNA Identifies Copy-Number Alterations That Can Be Used for Monitoring Response to Immunotherapy in Cancer Patients. Mol Cancer Ther (2019) 18:448–58. doi: 10.1158/1535-7163.MCT-18-0535
122. Ntzifa A, Strati A, Kallergi G, Kotsakis A, Georgoulias V, Lianidou E. Gene Expression in Circulating Tumor Cells Reveals a Dynamic Role of EMT and PD-L1 During Osimertinib Treatment in NSCLC Patients. Sci Rep (2021) 11:2313. doi: 10.1634/theoncologist.2019-0557
123. Bergmann S, Coym A, Ott L, Soave A, Rink M, Janning M, et al. Evaluation of PD-L1 Expression on Circulating Tumor Cells (CTCs) in Patients With Advanced Urothelial Carcinoma (UC). Oncoimmunology (2020) 9:1738798. doi: 10.1080/2162402X.2020.1738798
124. Khattak MA, Reid A, Freeman J, Pereira M, Mcevoy A, Lo J, et al. PD-L1 Expression on Circulating Tumor Cells May Be Predictive of Response to Pembrolizumab in Advanced Melanoma: Results from a Pilot Study. Oncologist (2020) 25:e520–7. doi: 10.1634/theoncologist.2019-0557
125. Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating Tumor DNA and Liquid Biopsy in Oncology. Nat Cancer (2020) 1:276–90. doi: 10.1038/s43018-020-0043-5
126. Keller L, Belloum Y, Wikman H, Pantel K. Clinical Relevance of Blood-Based ctDNA Analysis: Mutation Detection and Beyond. Br J Cancer (2021) 124:345–58. doi: 10.1038/s41416-020-01047-5
127. Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-Wide Cell-Free DNA Fragmentation in Patients With Cancer. Nature (2019) 570:385–9. doi: 10.1038/s41586-019-1272-6
128. Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Consortium C. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann Oncol (2020) 31:745–59. doi: 10.1016/j.annonc.2020.04.013
129. Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-Free DNA Comprises an In Vivo Nucleosome Footprint That Informs Its Tissues-Of-Origin. Cell (2016) 164:57–68. doi: 10.1016/j.cell.2015.11.050
130. Ulz P, Perakis S, Zhou Q, Moser T, Belic J, Lazzeri I, et al. Inference of Transcription Factor Binding From Cell-Free DNA Enables Tumor Subtype Prediction and Early Detection. Nat Commun (2019) 10:4666. doi: 10.1038/s41467-019-12714-4
131. Pranckeviciene E, Hosid S, Liang N, Ioshikhes I. Nucleosome Positioning Sequence Patterns as Packing or Regulatory. PloS Comput Biol (2020) 16:e1007365. doi: 10.1371/journal.pcbi.1007365
132. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of Mutational Processes in Human Cancer. Nature (2013) 500:415–21. doi: 10.1038/nature12477
Keywords: breast cancer, circulating tumor cells, circulating tumor DNA, liquid biopsy, immunotherapy, biomarkers
Citation: Magbanua MJM, Gumusay O, Kurzrock R, van ‘t Veer LJ and Rugo HS (2022) Immunotherapy in Breast Cancer and the Potential Role of Liquid Biopsy. Front. Oncol. 12:802579. doi: 10.3389/fonc.2022.802579
Received: 26 October 2021; Accepted: 03 February 2022;
Published: 15 March 2022.
Edited by:
Tomoharu Sugie, Kansai Medical University Hospital, JapanReviewed by:
Arutha Kulasinghe, The University of Queensland, AustraliaElham Sajjadi, University of Milan, Italy
Copyright © 2022 Magbanua, Gumusay, Kurzrock, van ‘t Veer and Rugo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Jesus M. Magbanua, mark.magbanua@ucsf.edu; Ozge Gumusay, ozgebostankolu@hotmail.com
†These authors have contributed equally to this work and share first authorship
 Mark Jesus M. Magbanua
Mark Jesus M. Magbanua Ozge Gumusay
Ozge Gumusay Razelle Kurzrock3
Razelle Kurzrock3