- 1Division of Thoracic Surgery, The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University, Changzhou, China
- 2School of Medicine, Dalian Medical University, Dalian, China
- 3Heart and Lung Disease Laboratory, The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University, Changzhou, China
Background: Approximately 80% of lung cancers are non-small cell lung cancers (NSCLC). Lung adenocarcinoma (LUAD) is the main subtype of NSCLC. The incidence and mortality of lung cancer are also increasing yearly. Myogenic differentiation family inhibitor (MDFI) as a transcription factor, its role in lung cancer has not yet been clarified.
Methods: LUAD data were downloaded from The Cancer Genome Atlas (TCGA) database and Gene Expression Omnibus (GEO), analyzed and plotted using the R language. Associations between Clinical information and MDFI expression were assessed using logistic regression analyses to explore the effects of MDFI on LUAD. Two sets of tissue microarrays (TMAs) further confirmed the overexpression of MDFI in LUAD and its impact on prognosis. In addition, we examined the correlation between MDFI and immune infiltration. To investigate the effect of MDFI on the biological behavior of LUAD tumor cells by GSEA and GO/KEGG analysis. The survival status and somatic mutational characteristics of patients according to MDFI levels were depicted and analyzed.
Results: Expression of high MDFI in LUAD tissues via analyzing TCGA dataset (P <0.001). Kaplan-Meier survival analysis indicated a poor prognosis for those patients with LUAD who had upregulated MDFI expression levels (P <0.001). This was also verified by two groups of TMAs (P=0.024). Using logistic statistics analysis, MDFI was identified as an independent predictive factor and was associated with poor prognosis in LUAD (P <0.001, P =0.021). Assessment of clinical characteristics, tumor mutation burden (TMB), and tumor microenvironment (TME) between high- and low-expression score groups showed lower TMB, richer immune cell infiltration, and better prognosis in the low-risk group.
Conclusion: This study showed that MDFI was overexpressed in LUAD and was significantly associated with poor prognosis, indicating that MDFI may be used as a potential novel biomarker for the diagnosis and prognosis of LUAD. MDFI is associated with immune infiltration of LUAD and it is reasonable to speculate that it plays an important role in tumor proliferation and spread. In view of the significant differences in MDFI expression between different biological activities, LUAD patients with MDFI overexpression may obtain more precise treatment strategies in the clinic.
Introduction
Lung cancer is one of the most common malignant tumors in the world, and is the malignant tumor with the highest mortality rate in the world. NSCLC accounts for approximately 80% of all lung cancers. Its cancer cells grow and divide slowly and spread and metastasize relatively late. About 75% of patients are in the middle and advanced stages, and the 5-year survival rate is very low (1). LUAD is the most common subtype of NSCLC (2), accounting for 50% of all lung cancer diagnoses, and its frequency is increasing (3, 4). Among all NSCLC subtypes, LUAD is the most heterogeneous and aggressive, and has a very high tumor mutational burden associated with EGFR, KRAS, BRAF, ERBB2, TP53, ALK, STK11, and TTE1 mutations (5, 6). In the past 50 years, China has reported a significant increase in the incidence and mortality of lung cancer. The incidence and mortality of male lung cancer rank first among all malignant tumors in China, and the incidence of female lung cancer ranks second and the mortality rate ranks second (7). However, 5 years survival for patients with stage I NSCLC is roughly 80%, and patients with stage II to stage III disease have a 5 years survival of 13–60% (8). Although the addition of adjuvant chemotherapy in patients with a specific stage can increase survival by 5-10%, there is a significant amount of toxicity associated with it (9). The opportunity to improve survival is evident in early-stage disease and is driving research to integrate targeted therapies and ICIs (1). There is space for improvement in the treatment of LUAD and scientists delve into the identification of molecular markers associated with tumors and combine them with pathological classifications that affect personalized treatment of patients. In order to highly accurately predict patient survival and/or response to individualized treatment, new biomarkers were identified in those with LUAD.
By excavating the TCGA database, we found that MDFI may be a novel lung cancer–related candidate target. MDFI is a Protein Coding gene. Diseases associated with MDFI include Erythema Infectiosum and Viral Exanthem (10). This protein is a transcription factor that negatively regulates other myogenic family proteins (11). MDFI is overexpressed in breast, colorectal, and liver cancers and may promote tumorigenesis in certain tissues (12–14). However, the role of MDFI in lung cancer has not yet been reported. We attempted to elaborate the prognostic value of MDFI in LUAD by exploring TCGA database in this study.
Methods and materials
Patient data acquisition
We searched the GEO database for high-throughput sequencing or microarray data on LUAD and selected 4 LUAD transcriptome datasets with different sample sizes: GSE43458, GSE62948, GSE116959, GSE139032 (15–18). All of these datasets including lung tumors and para-tumor tissues. We searched the TCGA database (19) and obtained patient data for the LUAD cohort based on legitimate research objectives (20). The TCGA-LUAD cohort contained a total of 599 participants, including 59 normal patients and 539 patients with lung adenocarcinoma tumors. This includes mRNA data, clinicopathological data, and somatic mutation data. Some patients with missing data were excluded, and 535 oncology patients were enrolled in the study (21).
Tissue microarray and analysis of immunohistochemical results
To further evaluate the expression of MDFI in NSCLC, we obtained a tissue microarray (TMA) from Superbiotek (Shanghai, China). It containing 60 pairs of NSCLC specimens and para-tumor tissues. In addition, we constructed a TMA containing 140 NSCLC tissues [included LUSC (n = 80), LUAD (n = 51), adenosquamous carcinoma (n = 5), Bronchioloalveolar carcinoma (n = 3), and sarcomatoid carcinoma (n = 1)] and 10 normal lung tissues. The reasonable tumor stage of these patients was determined based on the World Health Organization criteria and the International TNM classification (22). The patient did not receive radiotherapy or chemotherapy, and did not have other tumors within 5 years before surgery (Supplementary Table 1). Two groups of TMAs were deparaffinized with a conventional protocol and rehydrated according to a standard protocol for immunohistological (IHC) examination. Primary antibodies MDFI goat antibody (1:500, ProSci, PSI-42-165) were used (23, 24).
Then analyzing the staining results by Image Pro Plus 6 software (IPP6), the staining area (Area) and integrated optical density (IOD) can be obtained. The mean optical density (MOD) can be obtained by taking the ratio of the two, and the MOD value is positively correlated with the staining intensity of the tissue. By analyzing the MOD of MDFI in NSCLC, para tumor and normal tissues can be further studied. We mapped the immunostaining heat map of tumor tissue and normal lung tissue adjacent to the tumor, and labeled the corresponding expression scores. In addition, we also compared the expression levels of MDFI in the tumor and normal groups in 4 GEO validation sets.
Gene expression and survival time
High-volume data downloaded from the TCGA was managed using the R programming language (25). The results of unpaired and paired samples were analyzed by independent and paired sample t-test, respectively (26). We have selected several groups of characteristics with more obvious differences for comparative display. Boxplots plots, using Age, Pathologic stage, T stage, Smoker and so on as the variable, were generated to calculate differential expression of MDFI. Differences in global gene expression levels between the normal tissues and tumor tissues of patients with LUAD were analyzed with an R package and P < 0.05. Kaplan‐Meier analysis was used to evaluate the prognostic value of MDFI in LUAD patients (25).
Immune cell and immune function analysis
The gene set analysis of variance (GSVA) procedure of the ‘GSVA’ and ‘GSEA Base’ package of R software was used to calculate gene signature enrichment scores (GSVA scores) for immune cells and immune function in each sample, which is called relative immune cell abundance [10.1038/ng765.] (27, 28). Heat maps of immune cells and immune function within disease groups and normal groups have been established using the “pheat map” software package. We then performed a correlative analysis of immune cells and immune function separately using the corresponding package, which was calculated using the Pearson correlation method (29). The scores of immune cells and immune function were also compared in the MDFI high expression group and low expression group (30).
Functional enrichment analysis
First, we divided the TCGA-LUAD cohort patients into two groups with high and low MDFI expression levels according to the MDFI expression. Differential analysis between groups was performed using the ‘limma’ package of R software (31), and differential genes were screened according to the criteria of adj. P <0.05, |log FC| >0.5. To explore the functions of the differentially expressed genes in MDFI, the screened genes were enriched for Gene Ontology (32) and Kyoto Encyclopedia of Genes and Genomes (33) pathway terms, respectively (34). We used the “cluster Profiler” package in R software to carry out the analysis and plotted the enriched pathway results separately (35).
Somatic mutation analysis
We downloaded mutation data from TCGA for the LUAD patient cohort. To investigate the relationship between MDFI and mutations, we first divided the mutation profile of patients into high MDFI mutation group and low MDFI mutation group depending on the expression of MDFI. We assessed the mutations in the two groups separately using the R software “Maftools”, and plotted the waterfall of mutations (36). We analyzed differences in mutations in different MDFI expression groups, as well as the differences in the number and location of mutations in the same mutated gene due to different MDFI expression (37).
Predicted drugs
Finally, we used protein-drug interaction data from the DSigDB database (38) to identify potential drugs that could benefit lung adenocarcinoma. We adopted the following tests: t-test or wilcoxon test for differences between groups depending on the data, and pearson or spearman method for correlation analysis. Survival analysis was performed using Kaplan-Meier and Cox regression analysis, and the results were evaluated using LogRank test, respectively (25).
Statistical analysis
R software (version 4.1.1; The R Foundation for Statistical Computing) was used for all statistics of this article. All statistical tests were double-sided, and P values less than 0.05 were evaluated as significant (28).
Result
MDFI expression in TCGA dataset
Significantly increased levels of MDFI expression in LUAD and LUSC compared to normal lung tissue by pan-cancer analysis using TGCA data (P <0.001) (Figure 1A). Likewise, high expression of MDFI in LUAD and low expression in normal lung tissue was verified in four GEO validation sets (Figures 1B–E). However, in terms of Kaplan-Meier curve, LUAD with high MDFI expression has a significantly worse prognosis (P < 0.001), while the expression of MDFI was not significant with the prognosis of LUSC (P = 0.125). Therefore, the study focused on the role of MDFI in LUAD (Figures 1F–K).
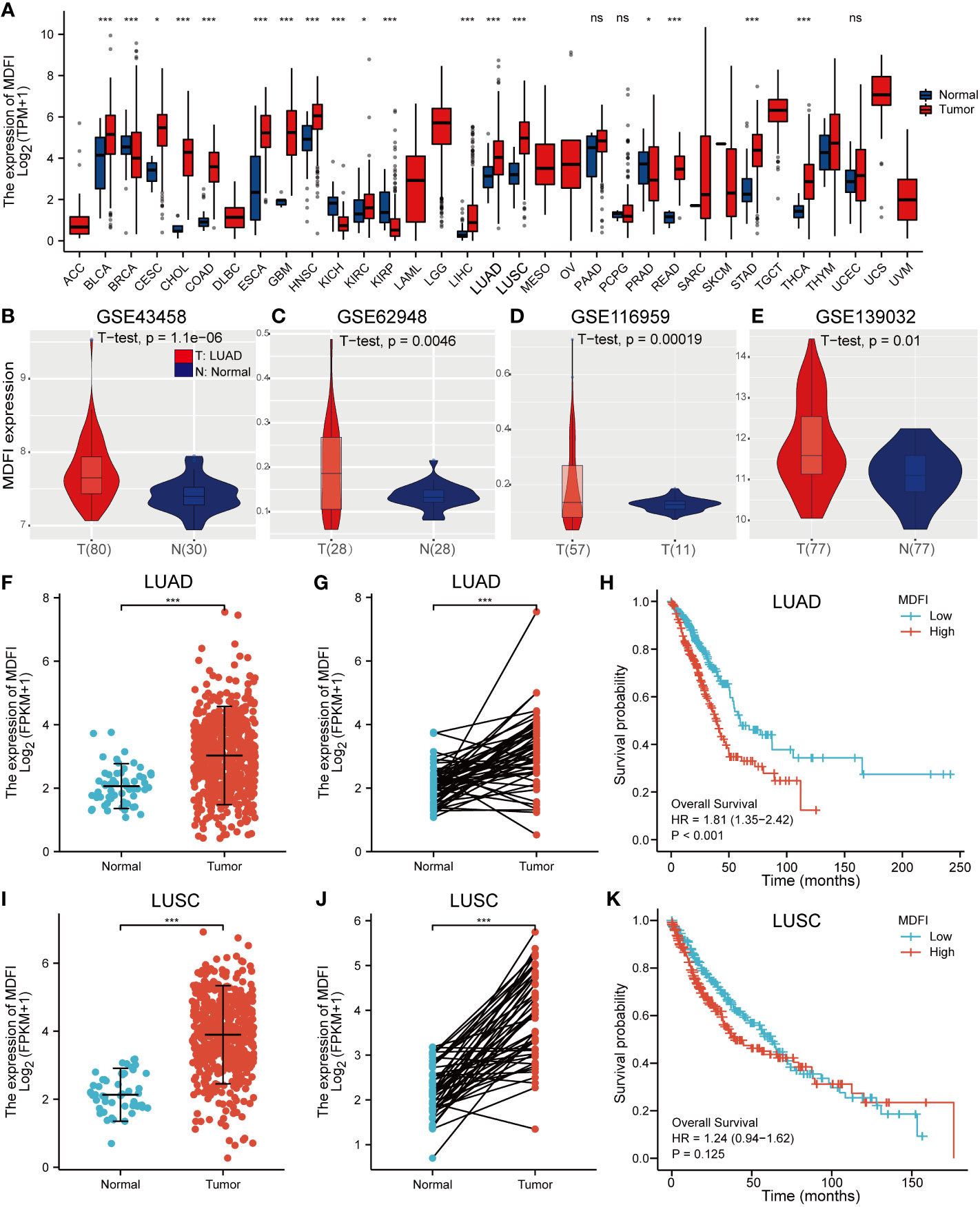
Figure 1 Analysis of MDFI expression in tumors, (A) Pan-cancer analysis of MDFI, (B–E) Expression analysis of MDFI in 4 sets of GEO datasets, (F) Expression analysis of MDFI in unpaired samples of LUAD, (G) MDFI in the LUAD expression analysis in paired sample, (H) Prognostic analysis of the survival of MDFI in LUAD, (I–K) Expression analysis of MDFI in LUSC. (A, F–K) Data from TCGA database. *p < 0.05, ***p < 0.001. ns, no significance.
Characteristics of patients
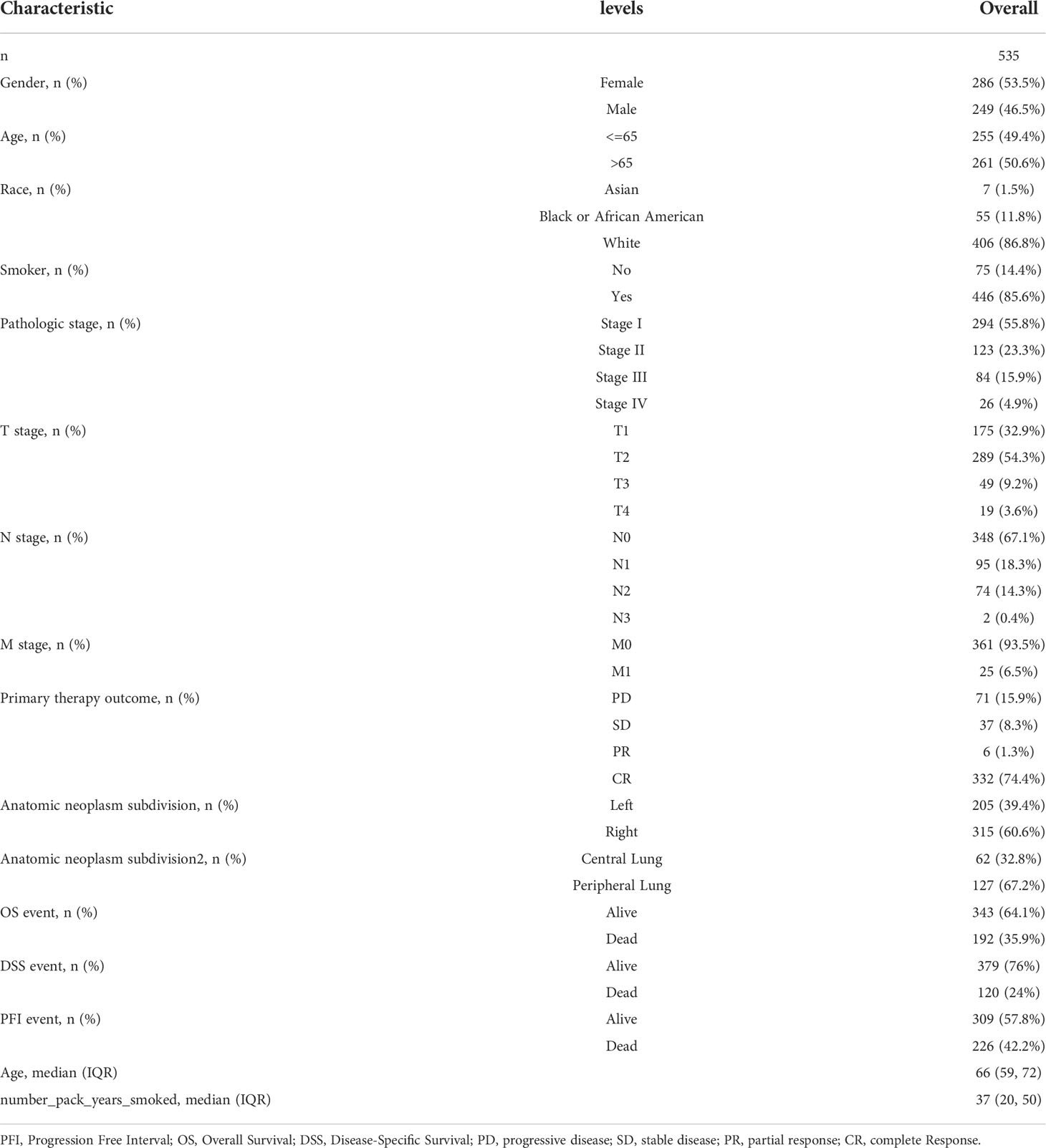
A total of 535 LUAD patients with the required clinical features were acquired from TCGA data portal in July 2022 (Table 1). Among them, 249 were male (46.5%) and 286 were female (53.5%). The median age of all patients was 66 years. In terms of LUAD pathologic stage, 294 patients were stage I (55.8%), 123 patients were stage II (23.3%), 84 patients were stage III (15.9%), and 26 patients were stage IV (4.9%). Subjects included 406 White (86.8%) and 62 non-White (13.2%). In terms of primary treatment outcome, PD + SD were 108 (24.2%), PR+CR were 338 (75.8%).
Immunohistochemical analysis
MDFI-specific antibody staining was performed on the two groups of TMAs constructed in advance, we could see various staining situations and plot 60 pairs of tumor and para-tumor immunostaining heatmap (Figures 2A–G). Meanwhile, we selected one column each of invasive breast cancer and clear cell renal cell carcinoma as the reference sample for the same staining procedure (Figures 2F–G). The staining results were processed by IPP6 software to obtain MOD values. By MOD analysis of 60 pairs of tumor and para-tumor, the results suggested that the expression of MDFI in tumors was indeed higher than that in normal tissues (P < 0.001) (Figure 2H). Further, the K-M curve suggested that high-expressing MDFI had a worse prognosis in LUAD (n = 51, P = 0.024), whereas the difference was not significant in LUSC (n = 80, P = 0.072) (Figures 2I–J). Our experimental results similarly corroborate previous results predicted by the TCGA database. High MDFI expression can be used as an independent prognostic marker in LUAD.
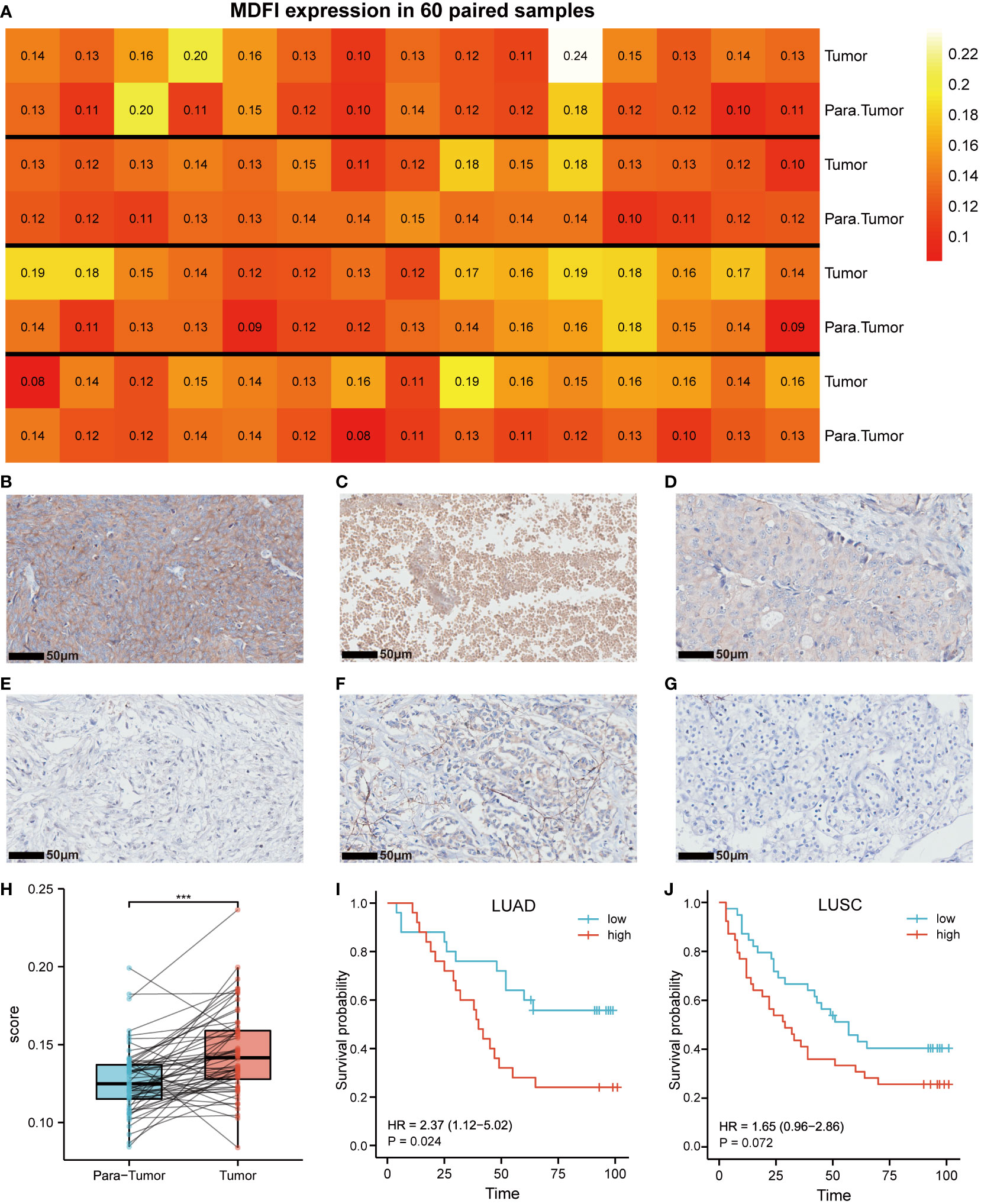
Figure 2 Expression of MDFI in tumor tissues. (A) Heat map of MDFI expression in 60 pairs of samples, (B) High expression of MDFI in lung cancer tissues, (C) MDFI is moderately expressed in lung cancer tissues, (D) Low expression of MDFI in lung cancer tissues, (E) Unstained normal tissue, (F) MDFI staining in invasive breast cancer tissue, (G) MDFI staining in clear cell renal cell carcinoma tissue, (H) Box plot of MDFI expression in 60 pairs of paired samples, (I) Prognastic analysis of MDFI in LUAD specimens, (J) prognostic analysis of MDFI in LUSC specimens, ***p < 0.001.
Correlation between MDFI expression and clinical features
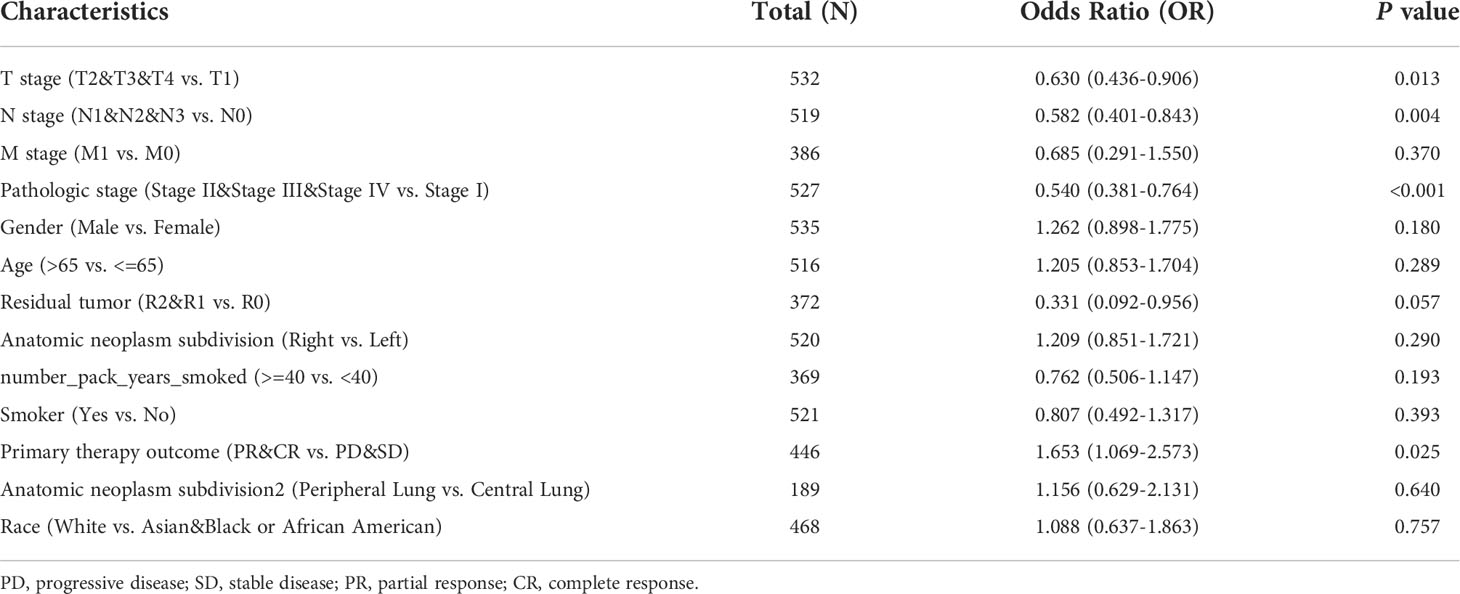
In LUAD patients, the relationship between MDFI and clinical characteristics is summarized in Table 2. Logistic regression analysis showed that MDFI gene expression is a categorical dependent variable associated with poor prognostic clinical features. High expression of MDFI was significantly associated with T stage (P =0.013), N stage (P =0.004), Pathologic Stage (P <0.001) and Primary therapy outcome (P =0.025) (Table 2). High expression of MDFI was significantly correlated with clinical stage (P <0.001), T stage (P <0.001), PFI event (P <0.05), OS event (P <0.001) and DSS event (P <0.01) (Figures 3A–H).
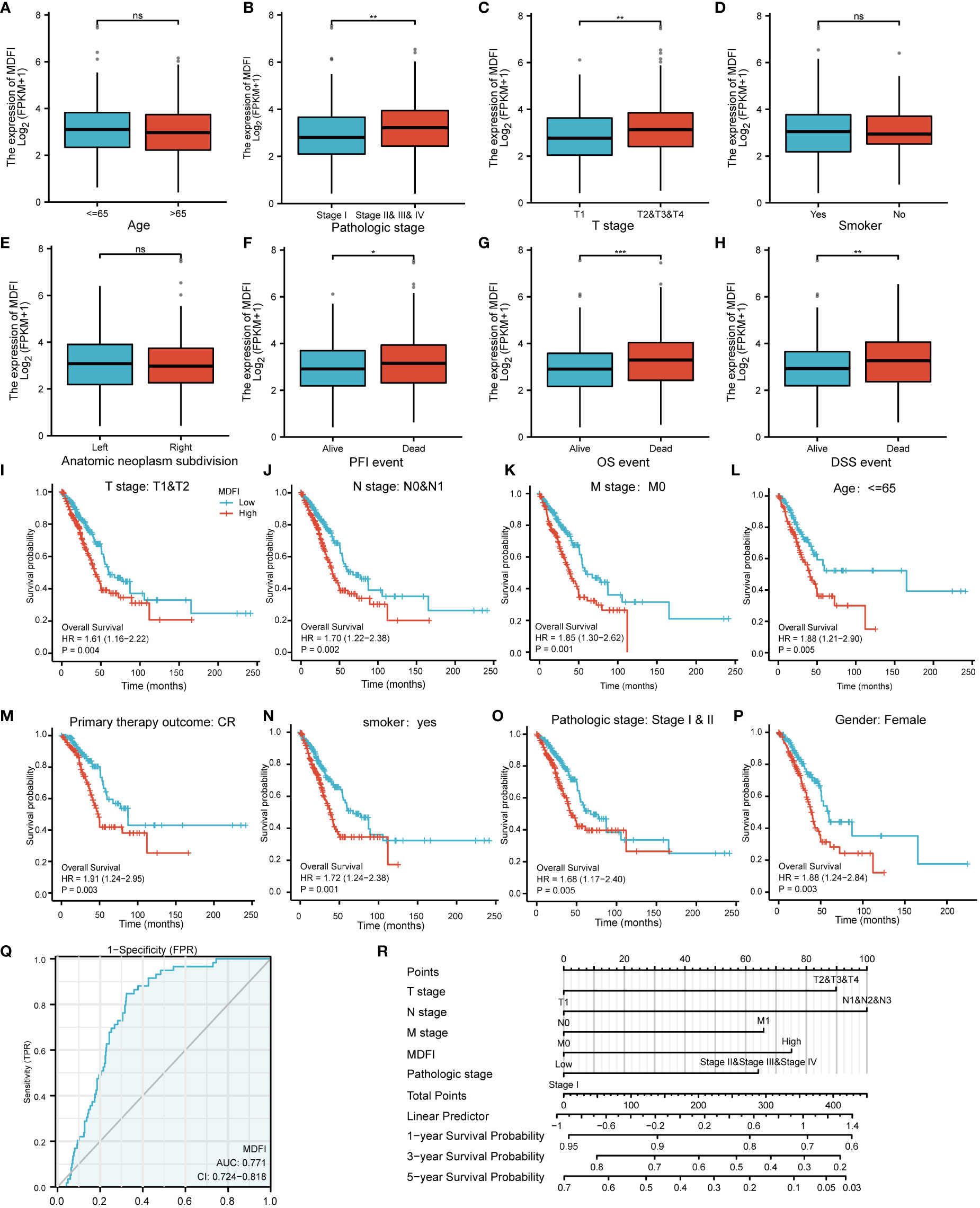
Figure 3 Association of MDFI expression with different LUAD characteristics and prognosis, date from TCGA, (A–H) Expression of MDFI in relation to different characteristics of LUAD, (I–P) Association between high MDFI expression in prognosis of LUAD with different characteristics, (Q) MDFI’s ROC curve analysis at LUAD, (R) Construction of a nomogram to assess the prognostic value of MDFI in LUAD. *p < 0.05, **p < 0.01, ***p < 0.001, ns, p > 0.05. PFI, Progression Free Interval; OS, Overall Survival; DSS, Diseases-Specific Survival; CR, complete response.
High expression of MDFI is an independent risk factor foroverall survival
High MDFI expression was associated with poor prognosis, as shown in Kaplan-Meier survival analysis (Figures 3I–P). Analysis of different clinical characteristics showed that high MDFI expression was significantly associated with poor prognosis in LUAD patients with T1&T2 stage (p=0.004), N0&N1 stage (P =0.002), M0 stage (P =0.001), less than 65 years (P =0.005), Primary therapy outcome: CR (P =0.003), smoker (P =0.001), pathologic stage I&II (P =0.005) and female (P =0.003) (Figures 3B–I). Univariate Cox analysis demonstrated that high MDFI expression was significantly correlated with poor overall survival (P <0.001). Multivariate Cox analysis confirmed MDIF gene expression was an independent risk factor for overall survival in patients with LUAD (P =0.021) (Table 3).
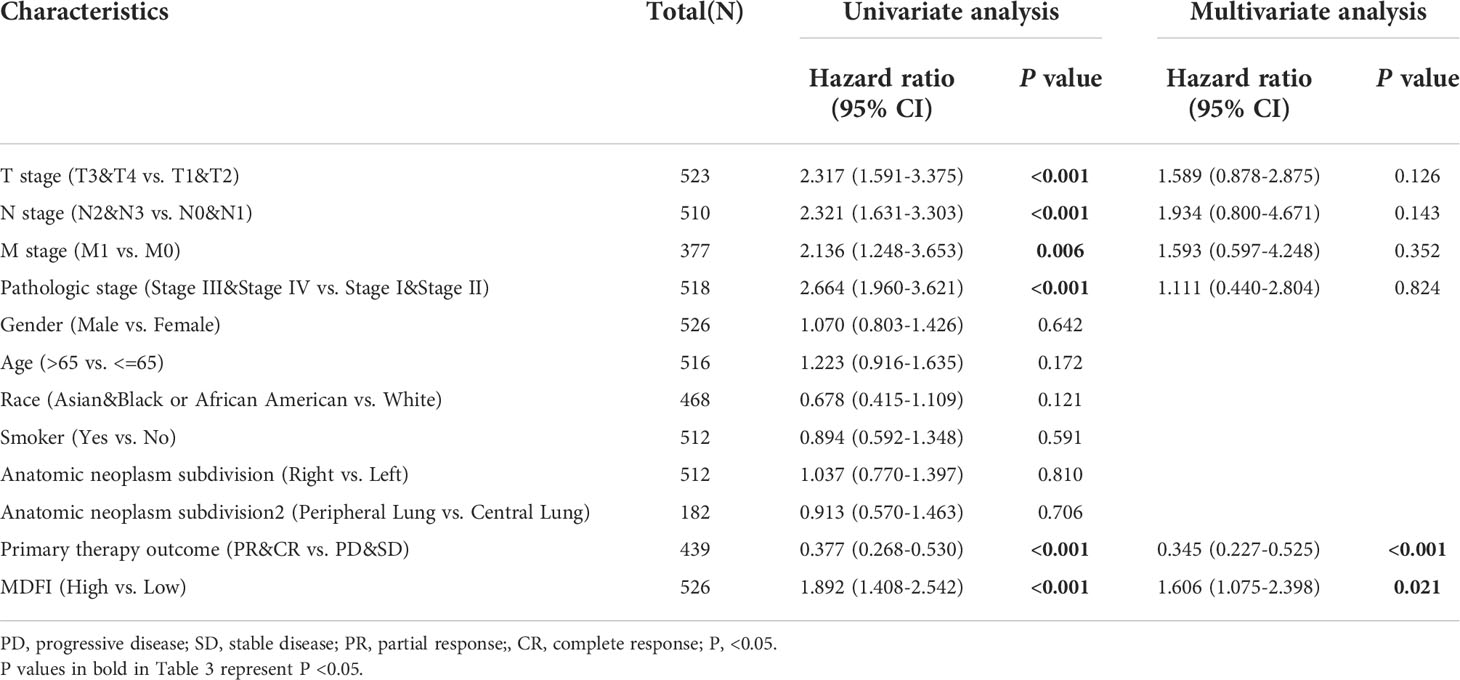
Table 3 Univariate and multivariate Cox regression analyses of clinical characteristics associated with overall survival.
Diagnostic value of MDFI expression in LUAD
We performed ROC curve analysis of MDFI gene expression data to evaluate the diagnostic value of this gene. The AUC area was 0.771 (CI =0.724-0.818). These results indicate that MDFI expression has certain value in the diagnosis of LUAD. A nomogram was constructed to predict the 1-, 3-, and 5-year survival probability of patients in combination with the expression level of MDFI, TNM stage, and pathological stage (Figures 3Q, R).
Immune infiltration by MDFI
To investigate the relationship between MDFI-associated immune cells and immune function, we screened the immune cell marker genes associated with the MDFI high expression group at P <0.05 (Figure 4A). We found that high MDFI expression was associated with most immune cells, with significant marker genes for Effector memory CD8 T cells and Monocyte, such as TRIB2 and MARCKSL1.
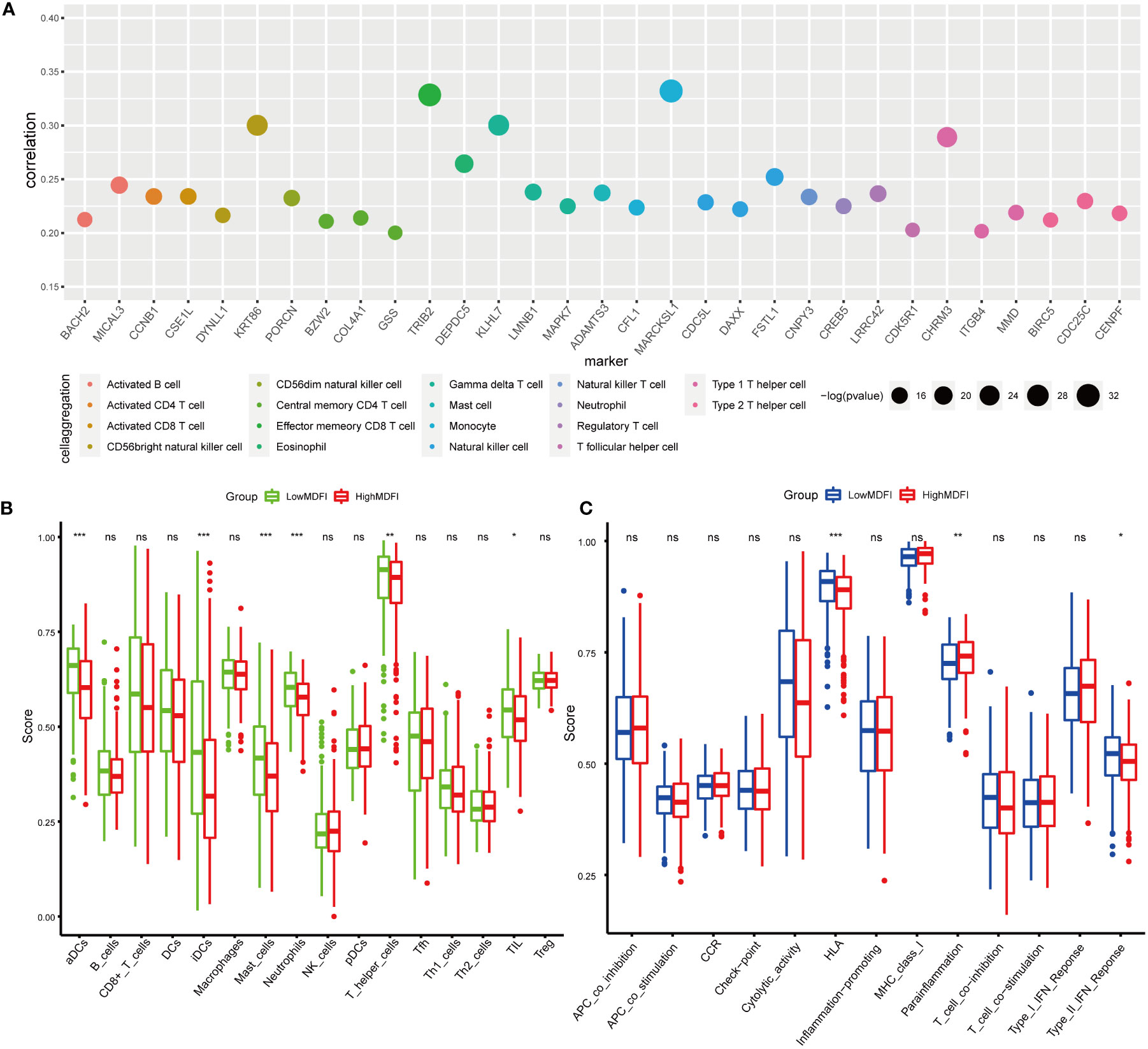
Figure 4 Immuno-infiltration analysis of MDFI in LUAD, data from TCGA, (A) Maker genes of immune cells associated with MDFI, (B) Relationship between the expression profile of MDFI and immune cells, (C) Relationship between the expression profile of MDFI and immune function. *p < 0.05, **p < 0.01, ***p < 0.001, ns, p > 0.05.
Immuno-infiltration analysis was then performed in the MDFI high and low expression groups, in which aDCs (P < 0.001), iDCs (P < 0.001), Mast cells (P < 0.001), Neutrophils (P < 0.001), T helper cells (P < 0.01) and TIL (P < 0.05) showed differential expression (Figure 4B). In terms of immune function, HLA (P < 0.001), Para-inflammation (P < 0.01) and Type II IFN Reponse (P < 0.05) were significant (Figure 4C).
Enrichment analysis of MDFI-related genes
To investigate the potential role of MDFI in LUAD, GO analysis and KEGG enrichment were used to analyze the function of MDFI differential genes. Among them, GO: BP/CC/MF showed 827 MDFI high expression group-related genes mainly acting in cornification, keratinization, cornified extracellular matrix and envelope structural constituent (Figures 5A, B). GSEA enrichment results were consistent with the direction of the above analysis, with up-regulated genes showing significant performance in regulation of actin cytoskeleton (P <0.001), focal adhesion (P <0.001) and extracellular matrix (ECM) receptor interaction (P =0.001) (Figures 5C–E).
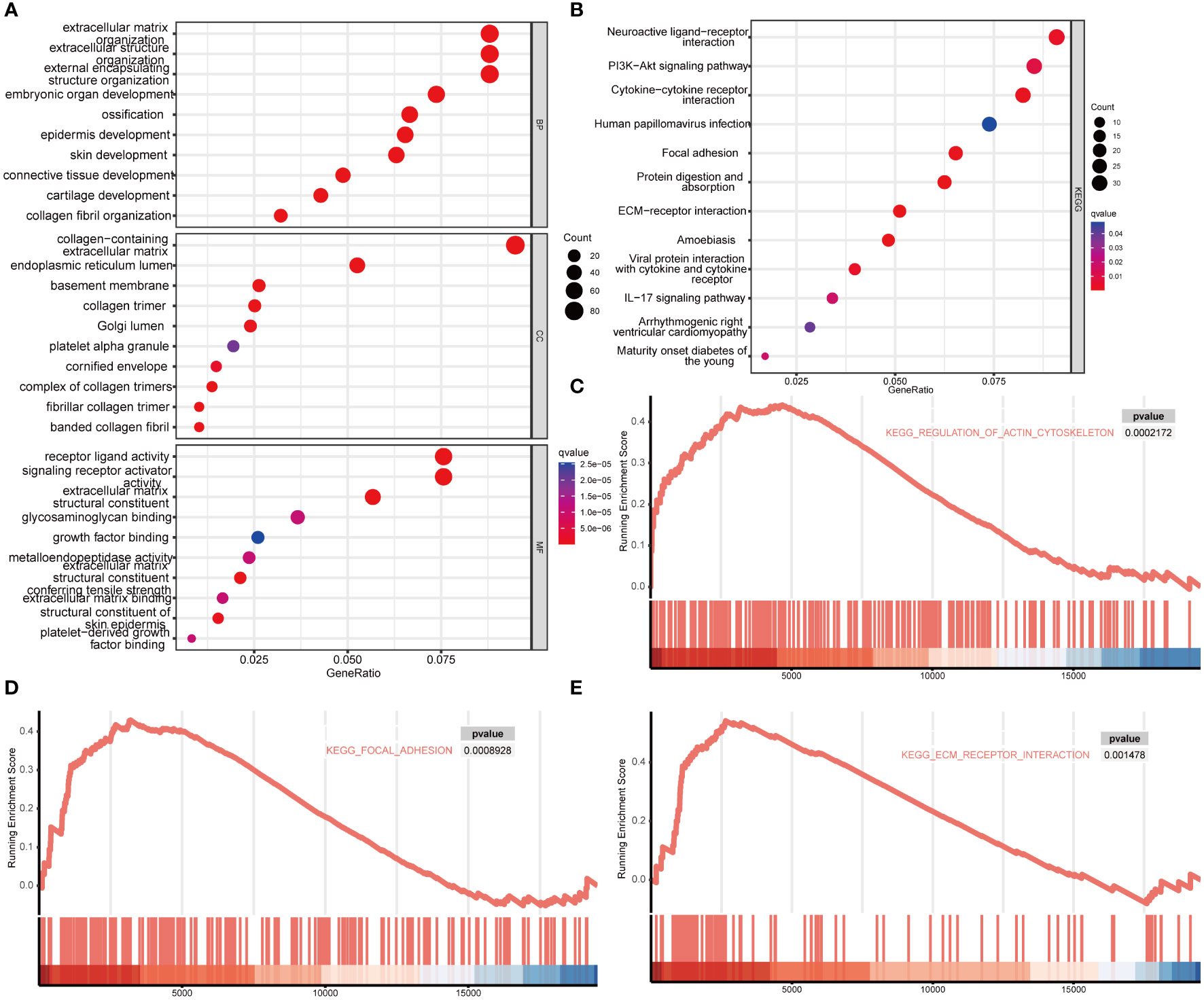
Figure 5 Enrichment analysis of MDFI in LUAD, data from TCGA. (A) GO. BP/CC/MF enrichment analysis, (B) KEGG enrichment analysis, (C–E) GSEA enrichment analysis.
MDFI-related gene co-expression analysis
We performed co-expression analysis of genes closely related to MDFI expression to characterize genes associated with MDFI expression in the LUAD cohort (Figure 6A). Further detailed analysis of the association of cellular Matrix Links and intermediate filament bioactivity-related genes with their MDFI revealed several prominent genes, such as TNS4, ITGB4, PL AUR, HMGA1, and FSCN1. Most of these genes are positively correlated with MDFI expression, and these genes have an impact on both LUAD and prognosis (Figures 6B–H). Next, the interaction networks of these co-expressed genes were visually analyzed to embody the association of these genes and biological functions. The network of these gene interactions suggests that the significantly associated biological functions are Cell differentiation, cytoskeleton, regulation of cell differentiation and positive regulation of cellular process (Figure 6I).
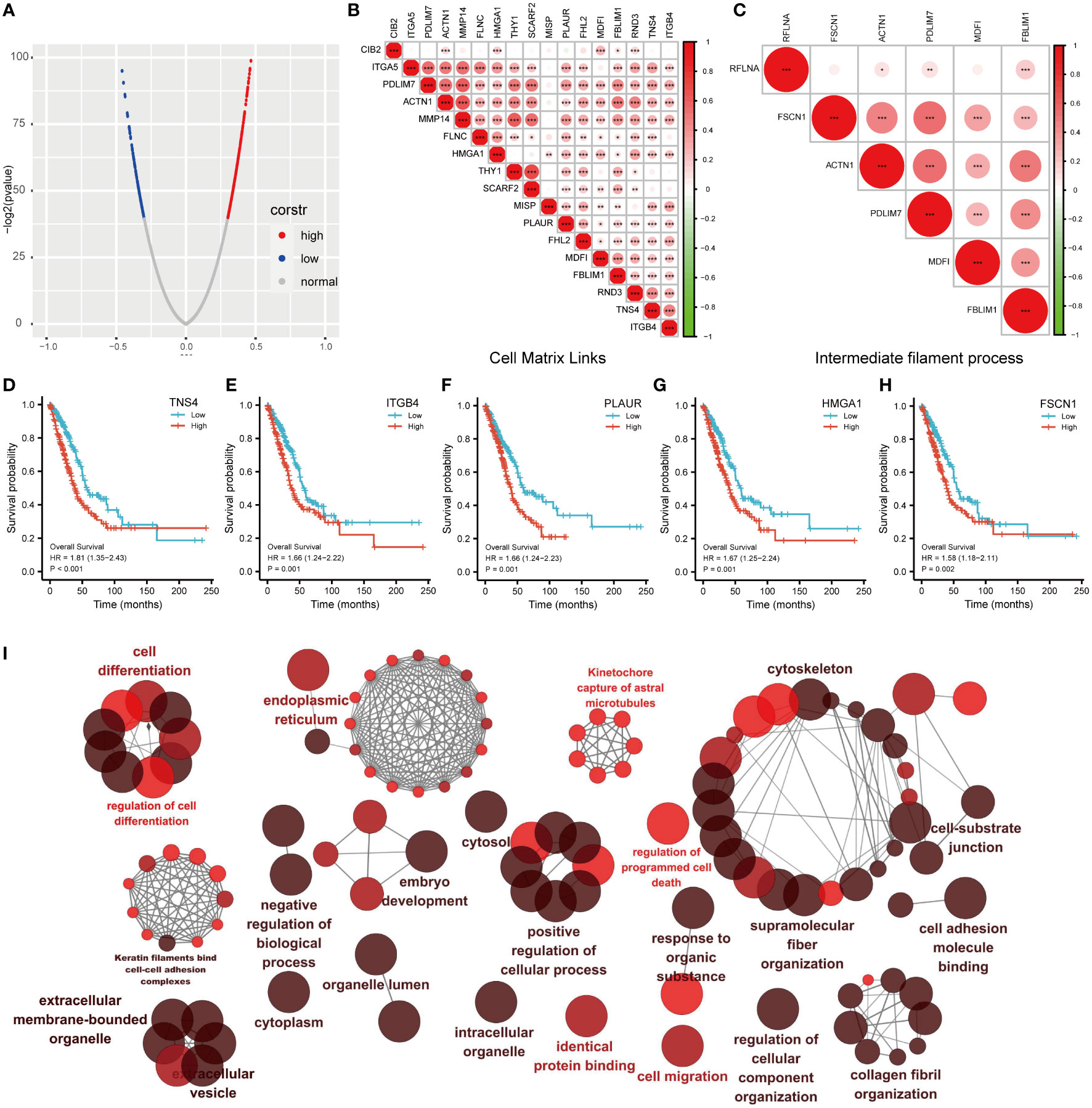
Figure 6 Correlation analysis of MDFI in LUAD (A) MDFI-related genes in LUAD, (B) Heat map of MDFI-related genes in Cell Matrix Links, (C) Heat map of MDFI-related genes in intermediate filament process, (D–H) Prognostic analysis of some MDFI-related genes in LUAD, (I) Visualization of the interaction network of genes strongly associated with MDFI by Cytoscape (ClueGo module). Node size indicates the mapped gene number; the node color schedule represents the p value, the darker the color, the smaller the p-value. (A–H) Data from TCGA. *p < 0.05, **p <0.01, ***p < 0.001.
Relationship between somatic mutations and MDFI expression in LUAD
To investigate the critical role of MDFI in tumor progression and tumor cell dissemination and metastasis, we explored whether the distribution of mutations in the LUAD cohort was influenced by MDFI gene expression. We collected mutation profiles from the LUAD cohort in the TCGA database and plotted waterfalls according to MDIF expression. Interestingly, the top five mutated genes in the two groups of differential genes were the same TP53 (57%; 42%), TTN (46%; 40%), MUC16 (42%; 39%), CSMD3 (42%; 38%) and RYR2 (39%; 29%) (Supplementary Figures A, B).
Mutated genes were more frequent in the high-expression group, including MTCL1, HYDIN, DCHS1, FRMD3, TP53, SEMA3D, BTAF1, ENPP2, BNIP5, NPTX2, TANC1 and DPYS, whereas the low-expression group included ZNF268, SIGLEC10 and OLFM4 (Figure 7B).
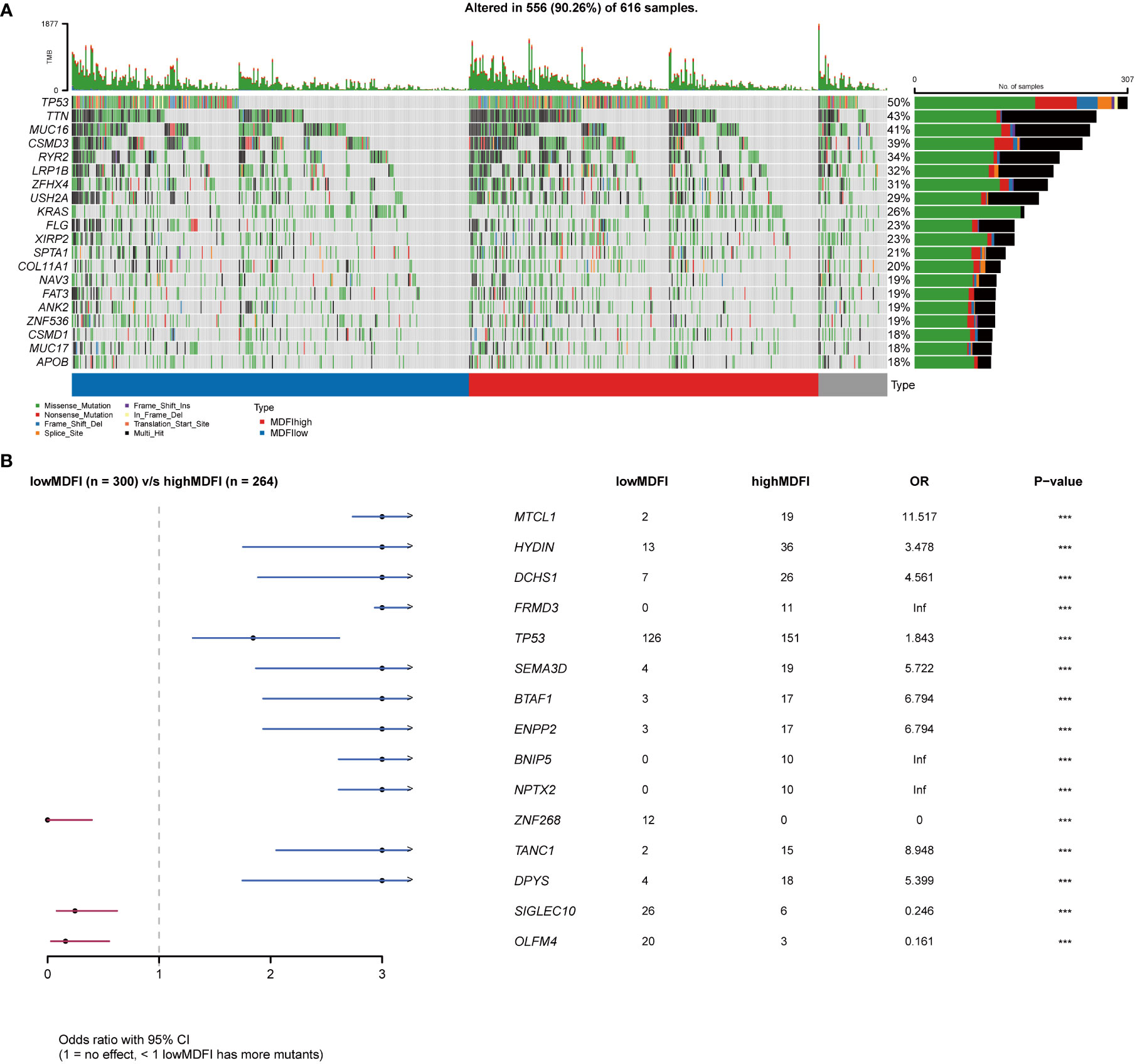
Figure 7 Relationship between MDFI and LUAD somatic mutation, data from TCGA. (A) Somatic mutation in MDFI-low and MDFI-high expression groups, (B) Comparison of mutation in MDFI high expression group and low expression group. ***p < 0.001.
Then we performed a visual analysis of mutations in the pathways involved in both sets of genes, including the number of genes mutated in the pathway and the number of samples mutated (Figures 8A, B). It can be seen that in terms of Pathway, the mutation in the MDFI low group was still lower than that in the MDFI high, and then the RTK-RAS pathway and WNT pathway of the first two pathways in the MDFI high group were separately plotted as waterfalls (Figures 8C, D).
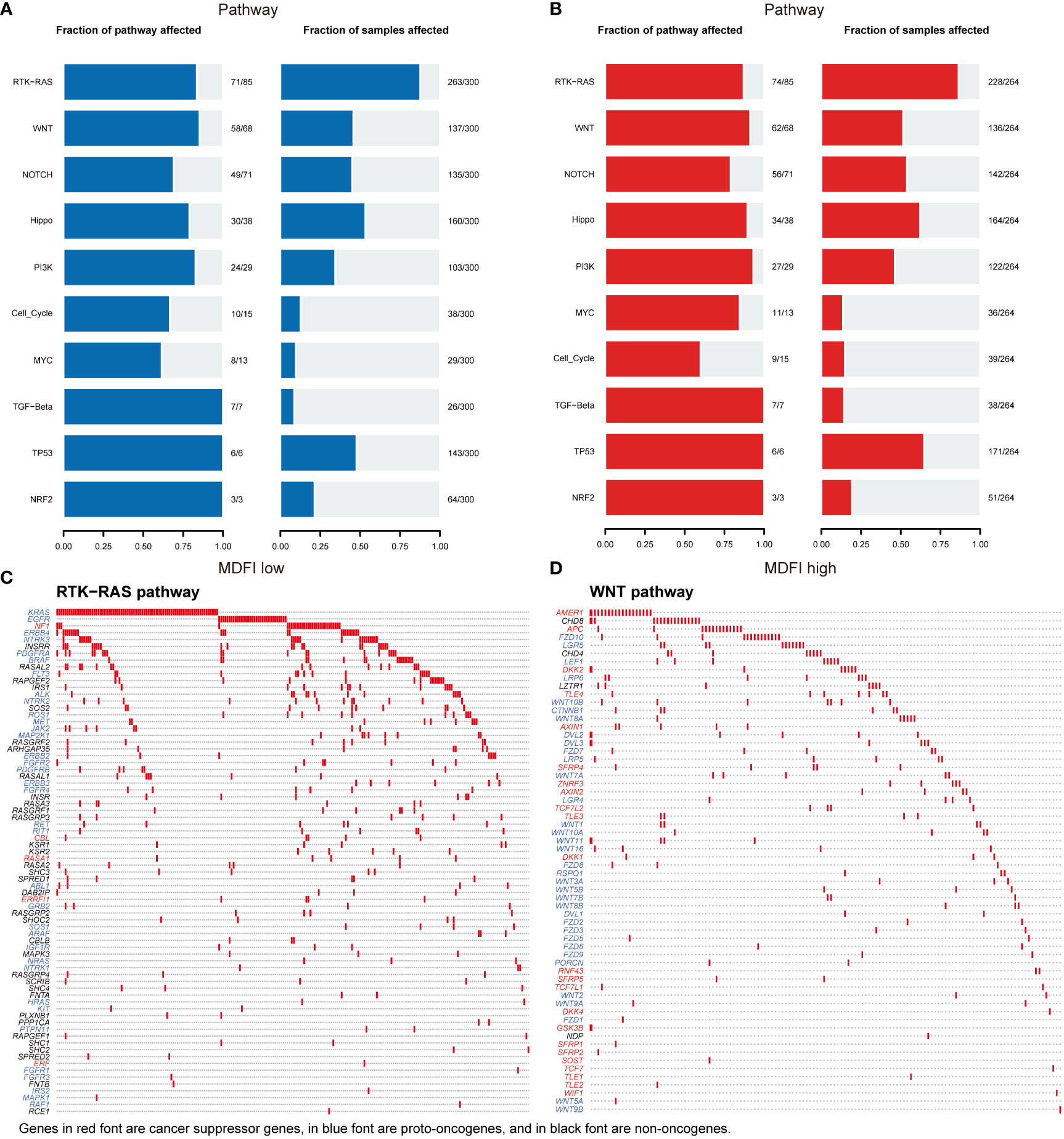
Figure 8 MDFI in LUAD involves mutations in the pathway data from TCGA (A) Pathway mutations of MDFI-low in LUAD, (B) Pathway mutations of MDFI-high in LUAD, (C) Mutations involved in RTK-RAS pathway, (D) Mutations involved in the WNT pathway.
Predicted drugs
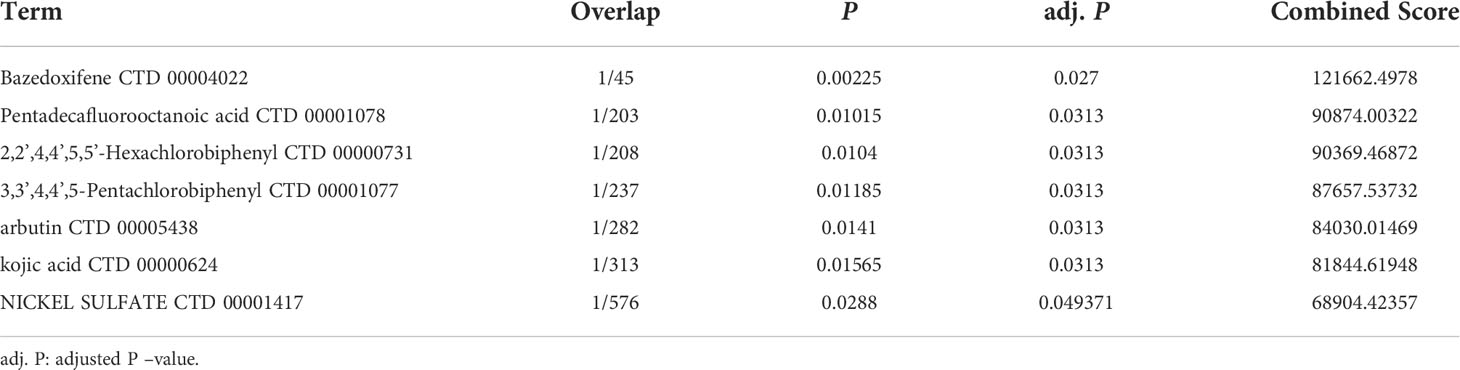
Bazedoxifene (adj. P =0.027), Pentadecafluorooctanoic acid (adj. P =0.0313), Hexachlorobiphenyl (adj. P =0.0313), Pentachlorphenyl (adj. P =0.0313), Arbutin (adj. P =0.0313), Kojic acid (adj. P =0.0313) and Nickel sulfate (adj. P =0.049371) were screened for potential drugs that may be beneficial in lung adenocarcinoma using protein-drug interaction data from the DSigDB database, and may be effective in LUAD patients with MDFI overexpression (Table 4).
Discussion
MDFI (Myogenic differentiation Family Inhibitor) is a Protein Coding gene (39). This protein is a transcription factor that negatively regulates other myogenic family proteins (11). It is highly expressed in a variety of cancer tissues, including Liver hepatocellular carcinoma, Breast invasive carcinoma, Colon adenocarcinoma/Rectum adenocarcinoma, etc (12–14). There is currently limited literature on the potential prognostic impact of MDFI in NSCLC. Therefore, we conducted a study on the potential role of MDFI in NSCLC, analyzing MDFI expression in NSCLC cohorts for the first time. In the context of TCGA data, we retrospectively analyzed 535 LUAD patients. The experimental results highlight that in LUAD patients, MDFI expression was significantly higher in tumors than in para-tumor. Similarly, it was validated in 4 GEO datasets and external immunohistochemistry experiments. Further, the expression levels of MDFI also differed in clinical stage, T stage, PFI event and OS event. We found that both RNA-seq data and TMA results showed that the expression level of MDFI was related to clinical prognosis. LUAD patients with high MDFI expression have a worse prognosis. Moreover, the K-M curves of female patients aged < 65 years with earlier tumor stage also supported the above results. Similarly, multivariate analysis showed that MDFI can be used as an independent prognostic factor in LUAD and is associated with poor prognosis in LUAD. Moreover, the AUC in our plotted ROC curve indicates that MDFI can be used as an indicator to predict LUAD.
The analysis of CIBERSORT showed that the expression level of MDFI was positively correlated with the infiltration level of most immune cells in LUAD, including CD4 T cells, Activated CD8 T cells, Effector memory CD8 T cells and Monocyte. The marker genes of these immune cells play an important role in tumor immune response, immune escape, proliferation, migration and invasion, promoting the progression of this LUAD. TRIB2 and MARCKSL1 are the most prominent marker genes in these immune cells. Critical role of TRIB2 in cancer and drug resistance to therapy, TRIB2 interacts with MAPKK, AKT and NFkB proteins and participates in cell survival, proliferation and immune responses (40, 41). Ectopic or intrinsic high expression of TRIB2 induces drug resistance by promoting phosphorylation of AKT through its COP1 structural domain. significantly increased expression of TRIB2 in tumor tissue correlates with increased phosphorylation of AKT, FOXO3a, MDM2 and impaired treatment response. This ultimately led to extremely poor clinical outcomes (42). When MARCKSL1 phosphorylation is inhibited, actin mobility is increased, filamentous sodium formation is impaired, and laminar lipid formation is enhanced, as is cell migration, and we speculate that the same process may be going on in LUAD, thereby promoting tumor cell proliferation, migration, and invasion (43). MARCKSL1 promoted the progression of lung adenocarcinoma by regulating epithelial–mesenchymal transition (EMT) (44). These results suggest that MDFI may play an important role in immune escape in the LUAD microenvironment (45). Moreover, it can be seen that the HLA presentation pathway is more pronounced in the low MDFI expression group, while tumor cells are more likely to evade immune detection in the absence of the HLA presentation pathway, thereby promoting tumor progression (46).
Keratin, the major intermediate filament protein of epithelial cells, and the cytoskeleton play multiple key roles in the cell, from cell migration to organelle dynamics (47, 48). They not only have a positive biological role in tumor progression and tumor cell dissemination and metastasis, but also often maintain their specific expression pattern during malignant transformation of cancer, so they are also widely used as tumor markers in cancer diagnosis. Both GO/KEGG enrichment and GSEA analysis indicate that MDFI has a prominent performance in the above biological processes. Keratins act as epithelial cell markers, which makes their role in cancer progression, diagnosis and treatment an important focus of research. Among them, keratin 1(K1) can act as a cell surface receptor in cancer, and KEGG enrichment analysis also shows that cytokine receptor action is more significant (49, 50); while keratin 17 (K17) plays a role in DNA damage response and tumor initiation. Moreover, keratin is an essential element of the cytoskeleton in normal and malignant epithelial cells (49, 51). Cancers often maintain their specific keratin expression pattern during malignant transformation, and therefore keratin is widely used as a tumor marker in cancer diagnosis. Keratin plays an active biological role in tumor cell dissemination and metastasis (52).
We speculate that detection and treatment for MDFI may allow earlier diagnosis of tumors and limit further tumor growth. Several genes that were highly correlated and positively correlated in the subsequent co-expression analysis of MDFI showed the same differential performance for the prognosis of LUAD, suggesting that low expression is better for the prognosis. It further strengthens the important role and predictive value of MDFI in the prognosis of LUAD. The network of these gene interactions suggests Cell differentiation, cytoskeleton, regulation of cell differentiation and positive regulation of cellular process that are considered to be important process processes of tumor progression (53). In the analysis of somatic mutations in the high and low groups, the proportion of the top five mutated genes in the low expression group was lower than that in the high expression group, and it was speculated that the tumor cells with high expression of MDFI produced a large number of DNA replication errors in the proliferation and spread, indicating a worse prognosis (54). We mapped the RTK-RAS pathway and the WNT pathway of the first two pathways in the high MDFI group as waterfalls, respectively, LUAD-derived Wnts increase the proliferation/stemness potential of cancer cells, and LUAD cells use paracrine Wnt1 signaling to induce immune resistance, which could provide a new pathway therapeutic option for LUAD with high MDFI expression (55–57). Finally, we predicted that Bazedoxifene performed most prominently in MDFI-related LUAD. Bazedoxifene, or combined with oxaliplatin, significantly induced apoptosis, inhibited cell viability, colony formation, and cell migration in colon cancer cells, and we speculated that it may have the same effect in LUAD (58).
The above data and experimental results all suggest that MDFI is a closely related gene in LUAD, but our study still has some limitations. Our exploration of the role of MDFI in LUAD is based on data already available in GEO and TCGA databases, coupled with external data collected for validation and the specimens are old, and there are individual unstained conditions. And, no in vivo and in vitro experiments were performed to further verify the role of MDFI in immune escape and proliferation and spread of tumors, which also points the direction for future work.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Changzhou Second People’s Hospital Affiliated to Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KY designed the research. XS and ZG performed the research and collected the data. ZQ analyzed the data. PC drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Changzhou Sci & Tech Program (Grant number: CZ20220025), Changzhou High-Level Medical Talents Training Project (Grant number: 2022CZBJ069), “333 Project” of Jiangsu Province (Grant number: BRA2020157), 333 High-Level Talent Training Project (Grant number: 2022-2), “Six One Project,” Research Projects of High-level Medical Personnel of Jiangsu Province (Grant number: LGY2019025), High-level Talent Selection and Training Project of the 16th Batch of “Six Talent Peak” in Jiangsu Province (Grant number: WSN-245), Medical Scientific Research Foundation of Jiangsu Commission of Health (Grant number: H2018083).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1005962/full#supplementary-material
References
1. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Nagy-Mignotte H, Guillem P, Vesin A, Toffart AC, Colonna M, Bonneterre V, et al. Primary lung adenocarcinoma: Characteristics by smoking habit and sex. Eur Respir J (2011) 38(6):1412–9. doi: 10.1183/09031936.00191710
4. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health (2019) 85(1):1–16. doi: 10.5334/aogh.2419
5. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol (2011) 6(2):244–85. doi: 10.1097/JTO.0b013e318206a221
6. Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol (2015) 16(7):e342–51. doi: 10.1016/S1470-2045(15)00077-7
7. Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in people's Republic of China. J Thorac Oncol (2020) 15(10):1567–76. doi: 10.1016/j.jtho.2020.04.028
8. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009
9. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol (2008) 26(21):3552–9. doi: 10.1200/JCO.2007.13.9030
10. European Virology 2000. 17-21 September 2000, Glasgow, scotland. Abstracts. J Clin Virol (2000) 18(1-3):1–330. doi: 10.1016/S1386-6532(00)00098-6.
11. Chen CM, Kraut N, Groudine M. Weintraub h. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell (1996) 86(5):731–41. doi: 10.1016/s0092-8674(00)80148-8
12. Lin ZY, Chuang WL. Genes responsible for the characteristics of primary cultured invasive phenotype hepatocellular carcinoma cells. BioMed Pharmacother (2012) 66(6):454–8. doi: 10.1016/j.biopha.2012.04.001
13. Soran A, Ozbas S, Ozcinar B, Isik A, Dogan L, Senol K, et al. Intervention for hepatic and pulmonary metastases in breast cancer patients: Prospective, multi-institutional registry study-IMET, protocol MF 14-02. Ann Surg Oncol (2022) 29(10): 6337–38. doi: 10.1245/s10434-022-12320-7
14. Sui Y, Li X, Oh S, Zhang B, Freeman WM, Shin S, et al. Opposite roles of the JMJD1A interaction partners MDFI and MDFIC in colorectal cancer. Sci Rep (2020) 10(1):8710. doi: 10.1038/s41598-020-65536-6
15. Kabbout M, Garcia MM, Fujimoto J, Liu DD, Woods D, Chow CW, et al. ETS2 mediated tumor suppressive function and MET oncogene inhibition in human non-small cell lung cancer. Clin Cancer Res (2013) 19(13):3383–95. doi: 10.1158/1078-0432.CCR-13-0341
16. Mansfield AS, Wang L, Cunningham JM, Jen J, Kolbert CP, Sun Z, et al. DNA Methylation and RNA expression profiles in lung adenocarcinomas of never-smokers. Cancer Genet (2015) 208(5):253–60. doi: 10.1016/j.cancergen.2014.12.002
17. Enfield KSS, Marshall EA, Anderson C, Ng KW, Rahmati S, Xu Z, et al. Epithelial tumor suppressor ELF3 is a lineage-specific amplified oncogene in lung adenocarcinoma. Nat Commun (2019) 10(1):5438. doi: 10.1038/s41467-019-13295-y
18. Moreno Leon L, Gautier M, Allan R, Ilie M, Nottet N, Pons N, et al. The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress. Oncogene (2019) 38(46):7146–65. doi: 10.1038/s41388-019-0935-y
19. The cancer genome atlas (2022). Available at: https://cancergenome.nih.gov/.
20. Hutter C, Zenklusen JC. The cancer genome atlas: Creating lasting value beyond its data. Cell (2018) 173(2):283–5. doi: 10.1016/j.cell.2018.03.042
21. Zhao J, Guo C, Ma Z, Liu H, Yang C, Li S. Identification of a novel gene expression signature associated with overall survival in patients with lung adenocarcinoma: A comprehensive analysis based on TCGA and GEO databases. Lung Cancer (2020) 149:90–6. doi: 10.1016/j.lungcan.2020.09.014
22. Wittekind C, Brierley JD, Gospodarowicz MK. TNM classification of malignant Tumours 2017. (2016) New Jersey: Wiley-Blackwell.
23. Liu J, Tian Y, Yi L, Gao Z, Lou M, Yuan K. High KIF11 expression is associated with poor outcome of NSCLC. Tumori (2022) 108(1):40–6. doi: 10.1177/0300891620988342
24. Waldvogel HJ, Curtis MA, Baer K, Rees MI, Faull RL. Immunohistochemical staining of post-mortem adult human brain sections. Nat Protoc (2006) 1(6):2719–32. doi: 10.1038/nprot.2006.354
25. Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell (2018) 173(2):400–16 e11. doi: 10.1016/j.cell.2018.02.052
27. Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf (2013) 14:7. doi: 10.1186/1471-2105-14-7
28. Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, Gentleman R. ShortRead: A bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics. (2009) 25(19):2607–8. doi: 10.1093/bioinformatics/btp450
29. Adler J, Parmryd I. Recent review on colocalization seem to misunderstand the Pearson correlation coefficient. J Microsc (2007) 227(Pt 1):83. doi: 10.1111/j.1365-2818.2007.01788.x
30. Liu XS, Zhou LM, Yuan LL, Gao Y, Kui XY, Liu XY, et al. NPM1 is a prognostic biomarker involved in immune infiltration of lung adenocarcinoma and associated with m6A modification and glycolysis. Front Immunol (2021) 12:724741. doi: 10.3389/fimmu.2021.724741
31. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res (2015) 43(7):e47. doi: 10.1093/nar/gkv007
32. Gene Ontology (2022). Available at: http://www.geneontology.org. [Accessed July 2022]
33. Kyoto Encyclopedia Genes Genomes. (2022). Available at: http://www.genome.jp/kegg/pathway.html. [Accessed July 2022]
34. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA (2005) 102(43):15545–50. doi: 10.1073/pnas.0506580102
35. Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) (2021) 2(3):100141. doi: 10.1016/j.xinn.2021.100141
36. Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res (2018) 28(11):1747–56. doi: 10.1101/gr.239244.118
37. Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature (2008) 455(7216):1069–75. doi: 10.1038/nature07423
38. DSigDB Database (2022). Available at: http://dsigdb.tanlab.org/DSigDBv1.0/. [Accessed July 2022]
39. Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell (1987) 51(6):987–1000. doi: 10.1016/0092-8674(87)90585-X
40. Mayoral-Varo V, Jimenez L, Link W. The critical role of TRIB2 in cancer and therapy resistance. Cancers (Basel) (2021) 13(11):1–17. doi: 10.3390/cancers13112701
41. Yokoyama T, Nakamura T. Tribbles in disease: Signaling pathways important for cellular function and neoplastic transformation. Cancer Sci (2011) 102(6):1115–22. doi: 10.1111/j.1349-7006.2011.01914.x
42. Hill R, Madureira PA, Ferreira B, Baptista I, Machado S, Colaco L, et al. TRIB2 confers resistance to anti-cancer therapy by activating the serine/threonine protein kinase AKT. Nat Commun (2017) 8:14687. doi: 10.1038/ncomms14687
43. Bjorkblom B, Padzik A, Mohammad H, Westerlund N, Komulainen E, Hollos P, et al. C-jun n-terminal kinase phosphorylation of MARCKSL1 determines actin stability and migration in neurons and in cancer cells. Mol Cell Biol (2012) 32(17):3513–26. doi: 10.1128/MCB.00713-12
44. Liang W, Gao R, Yang M, Wang X, Cheng K, Shi X, et al. MARCKSL1 promotes the proliferation, migration and invasion of lung adenocarcinoma cells. Oncol Lett (2020) 19(3):2272–80. doi: 10.3892/ol.2020.11313
45. Zhang C, Zhang J, Xu FP, Wang YG, Xie Z, Su J, et al. Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol (2019) 14(11):1912–23. doi: 10.1016/j.jtho.2019.07.031
46. Hazini A, Fisher K, Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer (2021) 9(8):1–17. doi: 10.1136/jitc-2021-002899
47. Schiavon CR, Zhang T, Zhao B, Moore AS, Wales P, Andrade LR, et al. Actin chromobody imaging reveals sub-organellar actin dynamics. Nat Methods (2020) 17(9):917–21. doi: 10.1038/s41592-020-0926-5
48. Ehrlich F, Fischer H, Langbein L, Praetzel-Wunder S, Ebner B, Figlak K, et al. Differential evolution of the epidermal keratin cytoskeleton in terrestrial and aquatic mammals. Mol Biol Evol (2019) 36(2):328–40. doi: 10.1093/molbev/msy214
49. Nair RR, Hsu J, Jacob JT, Pineda CM, Hobbs RP, Coulombe PA. A role for keratin 17 during DNA damage response and tumor initiation. Proc Natl Acad Sci USA (2021) 118(13):1–10. doi: 10.1073/pnas.2020150118
50. Ogunnigbagbe O, Bunick CG, Kaur K. Keratin 1 as a cell-surface receptor in cancer. Biochim Biophys Acta Rev Cancer (2022) 1877(1):188664. doi: 10.1016/j.bbcan.2021.188664
51. Perone Y, Farrugia AJ, Rodriguez-Meira A, Gyorffy B, Ion C, Uggetti A, et al. SREBP1 drives keratin-80-dependent cytoskeletal changes and invasive behavior in endocrine-resistant ERalpha breast cancer. Nat Commun (2019) 10(1):2115. doi: 10.1038/s41467-019-09676-y
52. Werner S, Keller L, Pantel K. Epithelial keratins: Biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med (2020) 72:100817. doi: 10.1016/j.mam.2019.09.001
53. Otasek D, Morris JH, Boucas J, Pico AR, Demchak B. Cytoscape automation: Empowering workflow-based network analysis. Genome Biol (2019) 20(1):185. doi: 10.1186/s13059-019-1758-4
54. Lenormand C, Lipsker D. Somatic mutations in "Benign" disease. N Engl J Med (2021) 385(11):e34. doi: 10.1056/NEJMc2110545
55. Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann Oncol (2021) 32(9):1101–10. doi: 10.1016/j.annonc.2021.06.001
56. Skronska-Wasek W, Gosens R, Konigshoff M, Baarsma HA. WNT receptor signalling in lung physiology and pathology. Pharmacol Ther (2018) 187:150–66. doi: 10.1016/j.pharmthera.2018.02.009
57. Kerdidani D, Chouvardas P, Arjo AR, Giopanou I, Ntaliarda G, Guo YA, et al. Wnt1 silences chemokine genes in dendritic cells and induces adaptive immune resistance in lung adenocarcinoma. Nat Commun (2019) 10(1):1405. doi: 10.1038/s41467-019-09370-z
Keywords: MDFI, LUAD, TCGA, high expression, prognosis
Citation: Chen P, Quan Z, Song X, Gao Z and Yuan K (2022) MDFI is a novel biomarker for poor prognosis in LUAD. Front. Oncol. 12:1005962. doi: 10.3389/fonc.2022.1005962
Received: 28 July 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Qingqing Zhu, The First Affiliated Hospital of Soochow University, ChinaReviewed by:
Depeng Jiang, Second Affiliated Hospital of Chongqing Medical University, ChinaSong Xu, Tianjin Medical University General Hospital, China
Lai Baochang, Xi’an Jiaotong University Health Science Center, China
Copyright © 2022 Chen, Quan, Song, Gao and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Yuan, yuankai1978@163.com
†These authors have contributed equally to this work and share first authorship
 Pengyu Chen
Pengyu Chen Zhen Quan
Zhen Quan Xueyu Song
Xueyu Song Zhaojia Gao1,3
Zhaojia Gao1,3