- 1Key Laboratory of Receptors-Mediated Gene Regulation and Drug Discovery, People's Hospital of Hebi, School of Medicine, Henan University, Henan, China
- 2State Key Laboratory of Chemical Resource Engineering, College of Life Science and Technology, Beijing University of Chemical Technology, Beijing, China
- 3Key Laboratory of Molecular Pathology, School of Basic Medical Science, Inner Mongolia Medical University, Hohhot, China
Colorectal cancer (CRC) is the fourth leading cause of cancer death worldwide, and constitutive activation of the Wnt signaling pathway is universal in most CRC cases. Wnt ligands (Wnts) are secreted glycoproteins and fundamentally essential for the transduction of Wnt signaling pathway. However, the 19 members of Wnts in humans imply a daunting complexity of Wnt signaling and biological effects, and our understanding of their roles in CRC tumorigenesis is still quite rudimentary. This review will give an overview of the structural characteristics and maturation process of Wnts. The expression pattern of all human Wnts in CRC tissues, including Wnt1, Wnt2, Wnt2b, Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a, Wnt9b, Wnt10a, Wnt10b, Wnt11, and Wnt16, and their relationship with the tumorigenesis and the progression of CRC will be specifically summarized separately. Despite certain challenges, Wnt-based therapeutics for CRC emerge continuously and some are now in clinical trials. In conclusion, a deep understanding of Wnts is very helpful for a better management of this disease.
Introduction
Colorectal cancer (CRC) is the third most common malignancy and the fourth leading cause of cancer-related mortality worldwide, with more than 1.4 million new cases and 800,000 cancer-related deaths annually. The occurrence of CRC can be attributed to multiple lifestyle risk factors, such as diets high in fat and cholesterol, lack of exercise, excessive alcohol consumption and smoking, and other uncontrollable risk factors, including aging, type 2 diabetes, personal history of colonic polyps or inflammatory bowel disease, and some CRC-related hereditary syndromes. The routine use of fecal occult blood test, colonoscopy, and image evaluation has significantly improved the detection of CRC, and improved treatment options such as targeted therapy and immunotherapy have raised the 5-year survival rate to 65% for patients with CRC (1). Unfortunately, the tumor often reaches to the advanced stage or metastasizes without noticeable symptoms, and about 25% of CRC patients have metastatic diseases at initial diagnosis and their prognosis is still very poor (2). Therefore, an improved understanding of the underlying molecular mechanisms will contribute to the diagnostic and the therapeutic management of CRC.
Roles of Wnt Signaling in Tumorigenesis and the Progression of CRC
CRC is a highly heterogeneous disease, which is attributed to the complex interactions between genetic predisposition and environmental factors, and abnormalities in several crucial signal transduction pathways, such as Notch, TGFβ-Smads, Hedgehog, JAK-STAT, Ras-MAPK, PI3K-Akt, Wnt, p53, and DNA mismatch repair signaling pathways, play important roles in the initiation and the progression of CRC (3). Among them, Wnt signaling pathway attracts more attention due to its crucial role in a variety of biological processes, such as embryogenesis and tissue homeostasis. Abundant studies have proved that excessive activation of Wnt signaling was a major culprit in the carcinogenesis of most human malignancies, including CRC (4, 5). A genome-scale analysis has identified that more than 90% of CRC patients carried mutations of one or more downstream components of the Wnt signaling pathway, especially the loss-of-function mutations of adenomatous polyposis coli (APC) and the activating mutations of β-catenin or the extreme overexpression of some members such as frizzled (Fzd) receptors (6). Moreover, mutations of Wnt-dependent components, such as activating mutations of R-spondin (RSPO) family members and secreted Wnt agonists, occur in 10% of CRC cases carrying the wild-type APC allele (7). Additionally, the loss-of-function mutations of E3 ubiquitin ligases ring-finger protein 43 (RNF43), which lead to the excessive activation of Wnt signaling by blocking the ubiquitin-mediated degradation of Fzd receptors and LRP5/6 coreceptors, are dependent on Wnt secretion and frequently detected in CRC cases (8).
The activation of the Wnt signaling pathway, depending on the alteration of the Wnt pathway components and their functions, is indispensable for the initiation, the progression, and the metastasis of CRC. The Wnt signaling pathway transduction may be interrupted or exceedingly activated when the expression levels of crucial components change, especially in tumorigenesis (9). Even though the majority of the Wnt signaling pathway components have been determined, their functions in a specific tumor type or microenvironment remain intriguingly complicated and need to be understood deeply. The Wnt signaling pathway is mainly divided into β-catenin-dependent canonical signaling pathway, independent non-canonical Wnt/planar cell polarity, and Wnt/Ca2+ signaling pathways (Figure 1). It is still unclear by which mechanism Wnts choose to activate one specific signaling. A reasonable explanation is that the cell type and the signaling components expressed in cells may dictate the specificity of the signaling cascade and the downstream effectors (10). The identified transduction processes of the canonical Wnt signaling mainly include the secretion of Wnts, identification of Wnt coreceptors, silencing of β-catenin destruction complex, translocation of β-catenin into nucleus, recruitment of co-factors, and activation of target genes. An aberrant regulation of any of the steps mentioned above in canonical Wnt signaling could contribute to the development of human malignancies, and several studies have well-documented the impact of Wnt signaling on the carcinogenesis of CRC (9, 11). Besides that, abnormal feedback regulation of the Wnt pathway is also involved in the carcinogenesis of CRC. For instance, AXIN2 and DKK1 are direct targets and feedback inhibitors of the Wnt pathway in normal cells, whereas their inhibitory effect on activated Wnt signaling in CRC cells is invalid, and instead they become the promoters of CRC metastasis by activating epithelial–mesenchymal transition (EMT) pathways (12). Recently, Kang and colleagues found that phopholipase D isozymes, the direct targets and positive feedback regulators of the canonical Wnt pathway, could promote Wnt-driven growth and invasion of CRC cells (13). Moreover, complex interactions of Wnt pathways and many other signaling pathways, such as NF-κB, RAS-extra-cellular signal regulated kinase, and hypoxia-inducible factor-1α pathways, are commonly observed in most tissue types, and the aberrations within these pathways also contribute to the development of CRC, increasing the difficulty of designing better interventions against it (14–16). Overall, dysregulation of Wnt signaling is an important pathogenetic basis of CRC, and revealing detailed mechanisms of its action is critical for the treatment of this disease.
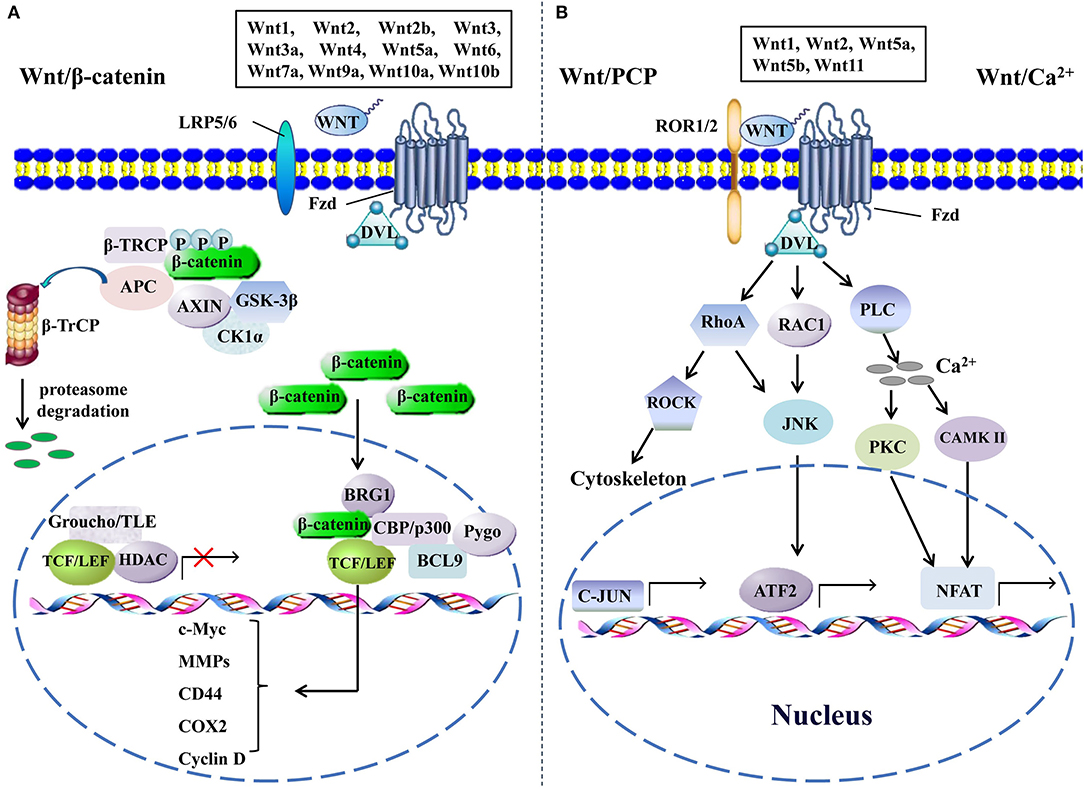
Figure 1. The canonical and the non-canonical Wnt signaling pathways. (A) In canonical Wnt signaling, β-catenin is phosphorylated by GSK-3β and CK1α in the absence of Wnts, followed by ubiquitination by β-TrCP and targeting for proteasomal degradation, without nuclear β-catenin; a repressive complex containing TCF/LEF and Groucho/TLE subsequently recruits HDACs to inhibit the transcriptional activation of β-catenin target genes. Conversely, the activation of canonical Wnt signaling is initiated from the binding of Wnts to Fzd and co-receptor LDL receptor-related protein (LRP5 or LRP6); then, the DVL is phosphorylated by GSK-3β and CK1α and begins to form a polymer that can inactivate the destruction complex through recruiting AXIN and GSK3β. Thereby, the accumulated β-catenin in the cytoplasm localizes to the nucleus and forms complexes with co-regulators of transcription factors such as TCF/LEF by removing Groucho/TLE complexes and recruiting transcriptional co-activators, including CBP/p300, BRG1, BCL9, and Pygopus. Next, downstream genes including cyclin D, MMPs, c-Myc, COX2, CD44, etc., are activated and give rise to the changes of the series of cellular activities, such as excessive cell proliferation, motility, and polarity. (B) The β-catenin-independent non-canonical Wnt signaling is initiated by binding certain Wnts and could regulate cellular polarity and migration-related signaling pathways. In the Wnt/PCP pathway, Wnts bind to the ROR1/2-Fzd complex to activate DVL, DVL binds to small Rho GTPases such as RAC1 and RhoA, RhoA and RAC1 together trigger JNK, and RhoA activates ROCK alone. This leads to the asymmetric cytoskeletal organization and/or coordination of cellular polarization via activating the transcription factors, such as c-JUN and ATF2. The Wnt/Ca2+ signaling triggers PLC activity and subsequently induces calcium influx; then, elevated Ca2+ activates several calcium-dependent signaling pathways, such as PKC and Ca2+/CAMKII, which finally leads to the accumulation of transcription factor NFAT in the nucleus. ATF2, activating transcription factor 2; BCL9, B-cell CLL/lymphoma 9; BRG1, brahma-related gene 1; β-TRCP, β-transducin repeat-containing protein; CAMKII, calmodulin-dependent protein kinase II; CK1α, casein kinase 1; COX2, cytochrome c oxidase subunit 2; DVL, disheveled; GSK-3β, glycogen synthase kinase; HDACs, histone deacetylases; JNK, JUN N-terminal kinase; LEF, lymphoid enhancer-binding factor; MMPs, matrix metalloproteinases; NFAT, nuclear factor of activated T cells; PCP, planar cell polarity; PKC, protein kinase C; PLC, phospholipase C; ROCK, rho kinase; ROR1/2, receptor tyrosine kinase-like orphan receptor 1/2; TCF, T-cell factor; TLE, transducin-like enhancer protein.
As key factors to initiate the activation of Wnt signaling pathway, Wnts are expressed in all metazoan species, and humans carry 19 independent members sharing 40–90% amino acid sequence identity with each other. All Wnts are secreted glycosylated lipid-modified molecules. However, little is known regarding what determines the generation of various Wnts and the subsequent Wnt signaling pathways. Although the aberrant expression of Wnts is not the culprit for triggering majority of CRC, elucidating their specific roles in colorectal carcinogenesis is still beneficial for the diagnosis and the prevention of CRC. In the following parts, we will discuss the current insights into the characteristics of human Wnts and elaborate their roles in the pathogenesis of CRC. In addition, we will summarize the latest progress on the treatment of CRC by targeting Wnts.
The Structural Characteristics of Wnts
The structure of Wnts has been elaborately summarized previously (17, 18). In brief, Wnts consist of 350–400 amino acid residues and are ~40 kDa in size. The amino-terminal signal sequences are mainly hydrophobic amino acids in different lengths for secretion and may be cleaved for maturation (19). The amino terminus is predicted to determine which Wnt signaling will be activated (20), whereas other hypothesis suggests that Wnt signaling activity is perhaps conferred by specific cellular context based on the observations that some non-canonical ligands, such as Wnt5a and Wnt11, can activate the canonical Wnt/β-catenin in a certain context (19). Wnts also contain about 22 conserved cysteine residues which are postulated to form intramolecular S–S bonding and maintain the secondary structure. The high-resolution structural information of Wnts has puzzled scientists for decades, and this problem was solved by Garcia and colleagues. They crystallized the Xenopus Wnt8 (XWnt8) with the cysteine-rich domain (CRD) of Fzd8 and revealed that the amino-terminal domain (NTD) was composed of six α-helices from residues 1–250 and two β-strand hairpins, with five pairs of conserved cysteine residues to form disulfide bonds, and the carboxyl-terminal domain (CTD) from residues 261–338 rich in cysteine residues and constructed from four α-helices and two β-strand hairpins stabilized by six disulfide bonds. A serine residue (Ser187 of XWnt8) attached to a lipid group in NTD binds to a deep groove in Fzd8-CRD, and index finger forms conserved hydrophobic residues in CTD that contacts with a depressed region of Fzd8-CRD (21). Furthermore, a recent structural analysis on human Fzd5 and Fzd7 CRD also uncovered that the unsaturated fatty acyl group in Wnts was the common molecular mechanism for the recognition of multiple Fzd receptors and the subsequent dimerization (22). However, no structural information for human Wnts has been analyzed because the complicated lipid modification and combination with carrier proteins restrict their purification in natural form (23, 24).
The maturation of Wnts
Upon translation, Wnts are targeted to the endoplasmic reticulum where glycosylation and acylation take place. The number of glycosylation attachment endows the diversity of Wnts and controls over the subsequent acylation, folding, and secretion. Acylation is essential for the activity of Wnts, and nearly all Wnts are modified with unsaturated fatty acid such as palmitic acid at a conserved serine residue by acyltransferase called porcupine (PORCN) (67). Deletion or mutation in any porcupine isoforms will block the whole Wnt signaling transduction and lead to embryonic lethality in mice (68), and mutations in X-linked PORCN could cause a developmental disorder named focal dermal hypoplasia in human (69, 70). Additionally, other modifications such as O-sulfation at specific tyrosine residues are necessary for the hetero-oligomer of certain Wnts and canonical Wnt signaling activity (71). In Golgi apparatus, a conserved transmembrane protein Wntless (Wls) binds to Wnts and accompanies them to the cell surface for secretion (72, 73), and a conserved serine residue (in Wg S239) in Wnts is essential for their recognition by Wls (74). Coombs et al. proposed that vacuolar acidification was also required to release Wnts from Wls in secretory vesicles (75), and this anterograde secretory process also relies on p24 proteins which function as conserved cargo receptors (76). The release of Wnts from cells also depends on a lipocalin family member of extracellular transport proteins, which binds to Wnts with high affinity and maintains their solubility and activity (23). Once reaching the surface of receptor cells by autocrine or paracrine fashion, Wnts encounter multiple interacting molecules such as polyanionic compounds, glycans, and a myriad of protein-binding partners including Wnt inhibitory factor (WIF) and Fzd receptors to initiate the Wnt pathways, whereas massive questions concerning the switch mechanism of Wnt pathways needed to be answered.
Roles of Wnts in Tumorigenesis and the Progression of CRC
Although the function of Wnts varies in the initiation and the progression of CRC, their expression pattern can serve as an important diagnostic or prognostic indicator for patients with CRC. In the following paragraphs, the biological functions of each human Wnt in colorectal carcinogenesis will be discussed separately (Table 1).
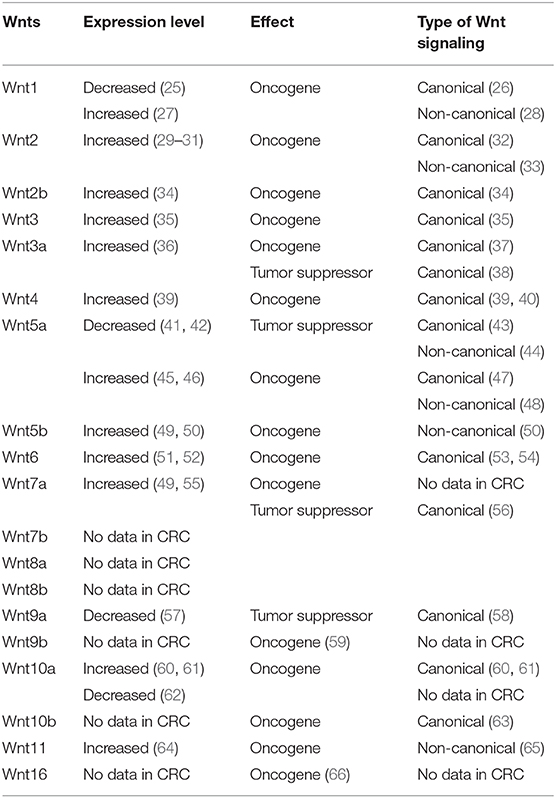
Table 1. Oncogenic and tumor suppressor Wnts regulating the canonical and the non-canonical Wnt signaling pathways in the pathogenesis of colorectal cancer (CRC).
Wnt1 is one of the ligands which mainly activate the canonical Wnt signaling cascade. The transcriptional activation of the Wnt1 (int-1) gene was firstly proved to be the initiating step in mammary gland hyperplasia and adenocarcinomas in mice (77). The transient or stable expression of Wnt1 could induce the formation of β-catenin–LEF1 complex and the persistent activation of canonical Wnt signaling in CRC cells (78). CRC cells expressing Wnt1 are resistant to cancer chemotherapy, and Wnt1 could inhibit the apoptosis by activating β-catenin/TCF transcription (26). Intriguingly, a latest study reported that exosomal Wnt1 could largely enhance the proliferation and the migration of CRC cells through activating the non-canonical Wnt signaling (28). Blockade of Wnt1 by WIF-1 or its antibody induced a significant apoptosis of human CRC cells containing mutations of APC, CTNNB1, and AXIN2 (79). Moreover, the ectopic expression of microRNA (miR)-200b-3p and miR-185 could significantly inhibit the proliferation and induce the apoptosis of CRC cells by targeting the canonical Wnt1/β-catenin signaling (80, 81). However, researches on the Wnt1 expression in human CRC tissues have yielded some conflicting results that the decrease or the increase of Wnt1 expression was detected in CRC tissues compared with normal colorectal mucosa (25, 27), and a study even found that the expression level of Wnt1 was decreased in human CRC tissues and CRC mice, whereas Wnt1 knockdown could still dramatically decrease the cell migration and the invasion of human CRC cells, and β-catenin expression was also enhanced in the tumors, indicating that Wnt1 expression could be regulated by more complicated mechanisms during CRC tumorigenesis (82).
Wnt2 is also an oncogene with a potential to activate the canonical Wnt signaling during CRC tumorigenesis (83). Cancer-associated fibroblasts were identified as the main source of Wnt2, and Wnt2 could enhance the tumor growth and the invasion of CRC in a paracrine fashion (32). Meanwhile, the invasive activity of CRC cells was also induced by Wnt2 through a non-canonical Wnt pathway coupled to GSK-3β and c-Jun/AP-1 signaling (33). Wnt2 was expressed at high levels in all CRC tissue samples at different stages, including premalignant colorectal polyps and liver metastasis, and high Wnt2 expression levels indicated poor prognosis in human CRC, although this upregulation was not due to the mutation in its coding region (29–32). Another analysis demonstrated that Wnt2 was upregulated in the progression from colorectal adenoma to carcinoma, and in situ hybridization showed that Wnt2 was expressed predominantly in macrophages in the lamina propria/stroma regions (84). Depletion of endogenous Wnt2 or neutralizing secreted Wnt2 could suppress the proliferation of CRC cells by targeting the canonical Wnt signaling. Galectin-3 (Gal-3) is a multifunctional carbohydrate-binding protein and proven to interact with β-catenin (83). The combined inhibition of Wnt2 and Gal-3 has synergistic effects on destabilizing β-catenin and induce the apoptosis of human CRC cells (85). Wnt2 and Fzd7 are key players in CRC progression. In a recent study, Kalhor and colleagues assessed the three-dimensional structure of human Wnt2-Fzd7 CRD complex via bioinformatics approaches, and the data demonstrated a unique dynamic behavior of Wnt2 upon binding to Fzd7, which is highly useful in targeted therapy for Wnt2-related cancers (86). Wnt2b is a paralogue of Wnt2, with amino acid identity of 70%, and Wnt2b shows a different expression pattern in human malignancies (87). However, the role of Wnt2b in CRC development has been rarely reported; only a recent study demonstrated that Wnt2b was significantly increased in colon cancer cells compared with normal colon epithelial cells, and inhibiting the activity of a CRC-promoting nuclear factor, estrogen receptor, could significantly decrease the Wnt2b/β-catenin signaling in colon cells (34).
Wnt3 and Wnt3a are highly homologous proteins with 85% amino acid sequence identity. However, 15% of difference exerts a great influence on protein structure and dynamics under the same condition, which eventually leads to different biological functions (88). Wnt3 was highly expressed in colon cancer tissues, and autocrine Wnt3 secretion via Evi/Wls was required to maintain the Wnt activity in colon cancer cells. Interfering with the secretion of Wnt3 could impair the growth of colon cancer cells in vitro and in vivo (35). We previously reported that gastric tumors also expressed elevated levels of Wnt3, and silencing Wnt3 in gastric cancer cells could block cell proliferation and induce apoptosis through targeting the canonical Wnt pathway (89). Recently, we found that the upregulation of Wnt3 in human CRC cell lines was essential for CRC progression. The knockdown of Wnt3 in CRC cells suppressed the proliferation but enhanced the sensitivity to chemotherapeutics by inhibiting the canonical Wnt pathway and glycolytic pathway (90). A83-01 is a selective inhibitor of TGF-β receptor; it was shown to inhibit EMT in HER2-overexpressing breast cancer cells by interfering the TGF-β-induced upregulation of Wnt3 (91), whereas its application in treating CRC has not been reported.
The expression of Wnt3a was also elevated in CRC tissues and associated with EMT, for advanced stages as well as poor prognosis (36). Moreover, the expression of Wnt3a was higher in the primary sites than that in the metastatic sites of CRC tissues, suggesting that the expression of Wnt3a was induced in the initial period of CRC rather than emerging as the cancer progressed. The expression level of Wnt3a in primary tumors was positively correlated with lymph node involvement and the expression of certain metastatic related genes (92). Consistently, Schinzari et al. demonstrated that the concentration of secreted Wnt3a was much higher in conditioned medium from normal or tumor tissues obtained from CRC patients than that from healthy donors (93). Therefore, approaches to inhibit Wnt3a expression have been proposed to suppress CRC, and metformin was proved to attenuate the cell stemness and EMT in CRC cells by inhibiting the Wnt3a/β-catenin pathway (37). However, some exceptions revealed that the role of Wnt3a in CRC was not coupled. In a more recent study, Wnt3a was found to inhibit the proliferation and the migration capacities of human colon myofibroblasts, the latter of which has been recognized to promote CRC progression (38). Thus, the variable role of Wnt3a is probably due to the specific molecular and cellular characteristics of different CRC subgroups and its context-dependent nature.
Wnt4 regulates many crucial embryonic and developmental pathways through activating the canonical Wnt signaling and non-canonical mechanisms (94, 95). Dysregulation or variants of Wnt4 gene may disturb these host networks, leading to the malignant transformation of cells and the occurrence of many cancers. For example, Al-Tassan et al. have identified novel risk variants for CRC near Wnt4 gene (96). Interestingly, exosomes derived from hypoxic CRC cells could transfer Wnt4 to normoxic CRC cells to enhance pro-metastatic behaviors and promote angiogenesis in endothelial cells by activating the canonical Wnt signaling (39, 40), which demonstrates a novel mechanism for the development of CRC. Excitingly, the structural dynamic behavior of Wnt4 protein was analyzed by a comparative computational study, and a foundation for designing new Wnt4 inhibitors to combat its irregularities was established (97). Recently, tetramethylthiuram disulfide, an important pesticide extensively used in agriculture, was proved to reduce the growth performance of chickens by inhibiting the expression of Wnt4, whereas its application and efficacy in treating human malignancies have not been reported (98).
Wnt5a and Wnt5b are highly homologous proteins with 82% amino acid sequence identity. The orthologs of Wnt5a are evolutionarily conserved, whereas those of Wnt5b are significantly divergent (99). Traditionally, Wnt5a is believed to be the non-canonical Wnt ligand and activates Ca2+-dependent effectors and other non-canonical pathways through small Rho-GTPases and c-Jun-NH2-kinase (100). However, its role in the progression of CRC is complicated and seems to be contradictory. Several studies proved that Wnt5a was silenced in most CRC cell lines and specimens due to frequent methylation in its promoter region (41, 42), and Wnt5a acts as a tumor suppressor in human CRC by interfering with the canonical β-catenin signaling but activating the non-canonical signaling pathways (43, 44). The expression of Wnt5a was negatively correlated with the degree of tumor differentiation and the aggressive behavior (41, 101). Meanwhile, promoter methylation of Wnt5a was strongly associated with the microsatellite instability status of patients with CRC, and multiple histone modifications of Wnt5a were involved in Wnt5a silencing and might promote colon cancer metastasis, providing evidence that epigenetic events may promote Wnt5a-mediated signaling in CRC (102, 103). On the contrary, other studies demonstrated that Wnt5a was upregulated consistently in intestinal polyps and tumor samples, and increased Wnt5a expression predicted the early recurrence or metastasis in colon cancer patients (45, 46). Wnt5a could also promote the migration of CRC cells by activating Fzd7-driven non-canonical Wnt signaling and enhance the cell stemness of CRC through activating the canonical Wnt signaling (47, 48). Furthermore, Jiang et al. demonstrated that a higher Wnt5a methylation status could predict a better drug response and longer progression-free survival in 5-fluorouracil-treated CRC patients (104). Until recently, Bauer et al. found two isoforms of Wnt5a protein with opposite functions in cancers (105), and a subsequent study proved that the simultaneous reactivation of the downregulated Wnt5a-long mRNA isoform and knockdown of the upregulated Wnt5a-short mRNA isoform could induce the apoptosis of CRC cells by silencing the expression of β-catenin, providing a reasonable explanation for the obscure role of Wnt5a in CRC previously (106). Wnt5b plays a pivotal role during embryonic gut development (51), and its expression level is increased significantly in ulcerative colitis and CRC samples (49, 50). Moreover, the Wnt5b rs2010851 polymorphism predicts a high risk of tumor recurrence in patients with advance-stage colon cancer (107). Overexpression of Wnt5b increased the proliferation, migration, and invasion of CRC cells through activating the non-canonical Wnt/JNK signaling (50). Meanwhile, Wnt5b exosome released from CRC cells could stimulate the migration and the proliferation of other cancer cells in a paracrine manner (108). In contrast, downregulating Wnt5b signaling pathway by the knockdown of fatty acid synthase could contribute to the decrease in invasion and metastasis of CRC cells, indicating that targeting Wnt5b is a promising approach to treat CRC.
Wnt6 is most homologous to Wnt1, with 43% amino acid sequence identity. It is apparently upregulated during intestinal development and regeneration as well as in CRC cells (51, 52). Overexpression or activation of Wnt6 could promote CRC development via activating the canonical Wnt signaling (53, 54). Moreover, a significant upregulation of methylation in Wnt6 gene was also detected in CRC samples (109), and the Wnt6 rs6747776 polymorphism may participate in the increased risk of CRC associated with excessive saturated fat intake (110). These findings indicate that Wnt6 mainly functions as a carcinogenic factor in CRC progression and could be utilized as a potential therapeutic target.
Wnt7a and Wnt7b share 78% amino acid sequence identity. Wnt7a is considered to be a crucial ligand for the canonical Wnt signaling, whereas its role in tumorigenesis is also controversial. Wnt7a promotes the progression of bladder, ovarian, tongue, and pancreatic cancers. However, it inhibits the growth of lung, breast, endometrial, renal, and gastric cancers, and there are few studies about its effect on CRC development. A significant increase of Wnt7a expression was detected in ulcerative colitis specimens and CRC cells, implying a potential carcinogenic effect (49, 55), whereas Becer et al. found that Colchicum pusillum exerted anticancer activities through activating the Wnt7a/β-catenin pathway, and another study also proved that the loss of Wnt7a expression contributed to tumor progression and predicted a poor prognosis of CRC, which indicates a protective role of Wnt7a during CRC carcinogenesis (56, 111). Thus, the exact role of Wnt7a in CRC progression still needs to be studied further. Wnt7b is weakly expressed in adult lung, brain, and prostate. Several studies have documented that the upregulation of Wnt7b was necessary for the growth, invasion, and metastasis of breast cancer and pancreatic adenocarcinoma through activating the canonical Wnt signaling (112, 113). Additionally, Wnt7b could promote the growth of prostate cancer through activating the non-canonical pathways (114). However, there has not been any report about the role of Wnt7b in the tumorigenesis of CRC, except a recent study which claimed that Wnt7b was highly expressed in CRC tissues by using bioinformatics analysis.
Wnt8a and Wnt8b are also secreted proteins with 63% amino acid sequence identity (115). At present, there is no report about the role of Wnt8a in cancer; only one study indirectly demonstrated that clofibrate could abrogate the binding of nuclear factor-κB to the Wnt8a promoter and downregulate the expression of Wnt8a and Wnt/β-catenin signaling activity, which ultimately sensitized pancreatic cancer cells to radiation (116). Compared with Wnt8a, Wnt8b attracts a little more attention due to its more conserved orthologs. However, more researches are focused on its indispensable role in the formation of certain organs (117, 118), and only one study showed that Wnt8b was significantly upregulated in gastric cancer cell lines and most primary gastric cancer tissues (119). Therefore, whether the upregulation of Wnt8a and Wnt8b also promote the progression of CRC remains an intriguing question.
Wnt9a and Wnt9b share 63% amino acid sequence identity. Wnt9a, known as Wnt14 formerly, is required for chondrogenesis and aortic amplification and identified as the ligand for both canonical and non-canonical Wnt signaling pathways (120, 121). Wnt9a is considered to be a tumor suppressor gene during CRC development. Ali et al. found that the LiCl-mediated induction of Wnt9a could suppress CRC proliferation and promote apoptosis through inhibiting the expression and the active form of β-catenin (58). Furthermore, hypermethylation and the resultant low expression of Wnt9a occur frequently in primary colon cancer and corresponding cell lines (57), suggesting that activating the Wnt9a-mediated pathway may have a therapeutic effect on colorectal cancer. Wnt9b was known as Wnt14b previously, and the Wnt9b-mediated activation of canonical and non-canonical Wnt pathways is required for the organogenesis of the mammalian urogenital system and nasal and maxillary processes (122–124). However, little is known about the role of Wnt9b in tumorigenesis, and only one study indirectly reported that the expression of Wnt9b was downregulated by a cancer-preventing glycoconjugate in CRC cells (59), indicating a potential carcinogenic property of Wnt9b.
Wnt10a and Wnt10b are closely related Wnts with 62% amino acid sequence identity. It is believed that they are notably expressed in various tissues for their formation through a β-catenin-dependent pathway (125, 126). Several studies demonstrated that Wnt10a was highly expressed in CRC tissues and several corresponding cell lines, and a higher Wnt10a expression level was associated with an advanced tumor stage. Hence, it is not surprising that the knockdown of Wnt10a could suppress the proliferation and the invasiveness of CRC cells through inactivating the canonical Wnt signaling (60, 61). However, a recent result was counter to previous findings which demonstrated a reduced expression of Wnt10a and a negative correlation between its expression and methylation in CRC tissues. Moreover, a higher Wnt10a methylation level was detected in CRC patients with advanced age, with distant metastasis, and diagnosed with mucinous adenocarcinoma (62). An explanation for this contradictory observation could be the different types of tissues collected in different groups. Additionally, polymorphisms of Wnt10a gene were strongly associated with the upper tertile of saturated fat intake and the resulting increase in CRC adenoma risk (110). Wnt10b also takes part in the progression of several digestive system malignancies, such as gastric, liver, and colon cancers (127–129). In human CRC cells, overexpression of the antineoplastic miR-148a could suppress cellular invasion and migration as well as tumor growth in vivo via blocking Wnt10b expression and β-catenin signaling activities (63). However, the precise role of Wnt10b in oncogenesis is not completely consistent. In the study of Yoshikawa et al., upregulated Wnt10b was found to activate the β-catenin/TCF pathway. Unexpectedly, it also suppressed the growth rate of HCC cells and tumorigenicity in nude mice through a β-catenin-independent mechanism, and the authors finally found that the fibroblast growth factor family proteins were the crucial factors to switch Wnt10b from its growth-suppressive effects to growth-stimulatory ones (127). These observations suggest that the role of Wnt10b remains obscure and still needs to be elucidated by further studies.
Wnt11 is most homologous to Wnt4, with 41% amino acid sequence identity. Initially, it was identified as a non-canonical Wnt ligand, and its characteristics and function have been summarized elaborately by Onganer et al. (130). The role of Wnt11 in CRC was firstly documented due to its high expression levels in some colorectal adenocarcinomas (64). Then, Wnt11 was found to stimulate the proliferation and the transformation abilities of intestinal epithelial cells by activating the non-canonical Wnt signaling pathway (65). Consistently, the expression of Wnt11 was obviously upregulated in patients with recurrence than those without, and Wnt11-transfected CRC cells showed increased phenotypes of tumors (131). Furthermore, Wnt11 was identified as a target of estrogen-related receptor α/β-catenin complex and increased the migratory capacity of CRC cells in an autocrine manner (132). On the contrary, Wnt11 was also involved in the maintenance of intestinal homeostasis by protecting intestinal epithelial cells from the invasion of pathogenic bacteria and suppressing the inflammation and the consequent apoptosis (133). In general, these findings indicate that Wnt11 may act as a tumor promoter in CRC progression and can be used as a cancer drug target.
Wnt16 shows no homology to any other Wnts but generates two mRNA isoforms, Wnt16a and Wnt16b. They are only different in the sequences of 5′-untranslational region and the first exons. Wnt16a is only expressed highly in pancreas, whereas Wnt16b is widely distributed in many organs such as kidney, brain, and heart (134). Therefore, most reports about Wnt16 mainly refer to Wnt16b. It is now accepted that Wnt16 contributes to skeletal development and postnatal bone homeostasis via activating the canonical and the non-canonical Wnt signaling cascades (135). Wnt16 is overexpressed in gastric adenocarcinoma, leiomyoma, and head and neck squamous cell carcinoma tissues (136, 137). Upregulation of Wnt16 induced by estrogen and progesterone treatment in uterine leiomyoma stem cells could promote the growth of uterine leiomyomas through activating the canonical Wnt pathway in a paracrine manner (138), and the enhanced β-catenin activities initiated by Wnt16 in prostate cancer cells could promote the malignant phenotypes and chemoresistance through preventing cell death (139). On the contrary, silencing Wnt16 by miR-374b could suppress the cellular proliferation and promote the chemotherapeutic agent-induced apoptosis in T-cell lymphoblastic lymphoma (140). However, there is still no direct evidence about the role of Wnt16 in colorectal carcinogenesis, except that a study indirectly reported that an ellagic acid derivative performed an antitumor action in CRC cells by downregulating the expression of Wnt16 in a dose-dependent manner (66), indicating a potential carcinogenic role of Wnt16 during tumorigenesis. Therefore, in-depth studies are still required to elucidate its role in CRC progression.
Opportunities and Challenges in Developing Wnt-based Therapeutics for CRC
According to the above information, most Wnts serve as primary determinants of colorectal carcinogenesis, and targeting the Wnt signaling pathway could be a promising therapeutic approach for CRC. For detailed information of targeting the Wnt signaling pathway in CRC, please refer to reviews by Sawa et al. and Bahrami et al. (141, 142). Herein we only give an overview of the current strategies to develop drugs directed at oncogenic Wnts for CRC treatment.
As mentioned earlier, porcupine is essential for the palmitoylation, secretion, and biological activity of Wnts. Theoretically, inhibiting its enzymatic activity could block all Wnt-driven cancers, and several small molecular inhibitors targeting porcupine have been developed for cancer treatment, including LGK974 (WNT974), Wnt-C59, ETC-159, IWP-O1, and GNF-6231 (143). Wnt-C59 and GNF-6231 are highly potent and orally available porcupine inhibitors capable of preventing the progression of mammary tumors in mice by downregulating Wnt1-mediated canonical signaling (144, 145). The pharmacological inhibition of the canonical Wnt signaling by Wnt-C59 and LGK974 could augment the cytotoxic effects of DNA-alkylating drug in CRC cells (146). It has been shown that Wnts secreted by fibroblast-exosomes protected differentiated CRC cells against chemotherapy, and their expression levels were correlated with the poor prognosis of patients with CRC. Therefore, blocking Wnt secretion by LGK974 treatment could diminish the clonogenic capacity and drug resistance of CRC cells in vitro and in vivo through decreasing the proportion of exosome Wnts (147). A phrase I trial of LGK974 is strikingly ongoing in patients with malignancies dependent on Wnts (NCT01351103), including BRAF mutant CRC. In addition, an orally available ETC-159 was proved to have a robust activity in CRC with RSPO mutations (148).
Furthermore, competitive receptors for Wnts were also explored to bind and sequester the free Wnts. Ipafricept (OMP-54F28) is a fusion protein that competes with native Fzd8 receptor for Wnts binding and blocks tumor growth in Wnt1-induced mice through antagonizing Wnt signaling. Moreover, ipafricept also impeded the growth of several solid tumors and selectively reduced the frequency of cancer stem cells (149). Intriguingly, in a phase I trial (NCT01608867) in patients with advanced solid tumors, ipafricept was well-tolerated with manageable toxicities, and a prolonged survival time was observed in a germ cell cancer and two desmoid tumor patients (150). Moreover, three phrase Ib clinical trials of ipafricept have just completed assessing its curative effect combined with different chemotherapeutic drugs in pancreatic cancer, ovarian cancer, and hepatocellular cancer. Additionally, some antibody-based inhibitors against specific Wnts have been produced to sequester the free Wnts. In the study of He et al., a monoclonal anti-Wnt1 antibody induced the apoptosis of human CRC cells expressing Wnt1 and showed great efficacy in treating primary tissue samples from patients with advanced CRC (79). Furthermore, the Wnt1 antibody also demonstrated an inhibitory effect on mesothelioma cells and the tumor growth of nude mice implanted with non-small cell lung cancer (NSCLC) cells. The inhibition of Wnt2 signaling by Wnt2 monoclonal antibody also similarly induced cell apoptosis and inhibited the tumor growth of several malignancies including melanoma, pleural mesothelioma, and NSCLC (151).
In another approach, some natural compounds targeting Wnt signaling have shown potential application values in cancer treatment, and several reviews have made elegant descriptions (149). Flavonoids deserve more attention due to the efficiently protective role against CRC through modulating the Wnt signaling pathways (152). For example, taxifolin was shown to induce cell cycle arrest and tumor regression in CRC cells by targeting the canonical Wnt signaling (153). By the same token, as another extensively studied flavonoid, (-)-epigallocatechin-3-gallate (EGCG) is abundantly distributed in green tea and exerts a preventive and therapeutic effect on CRC by promoting the degradation of intracellular β-catenin and the subsequent silence of Wnt/β-catenin-dependent genes (154). Interestingly, the effect of EGCG on CRC could be attributed to the inhibition on CRC stem cells by suppressing the canonical Wnt signaling (155). Furthermore, other natural compounds such as calycosin, isobavachalcone, resveratrol, etc., were also proved to suppress the malignant phenotypes of CRC via inhibiting the Wnt signaling pathways (156–158), providing more feasible therapeutic options for CRC treatment. However, few natural compounds have been proven to suppress the development of CRC by directly targeting Wnts, and none of them is currently undergoing clinical testing.
Despite the great advantages of the abovementioned approaches targeting Wnts, several potential pitfalls are still blocking their clinical application in cancer treatment. For instance, the ubiquitous Wnt signaling networks and numerous effects complicate the blockade of Wnt signaling. Different Wnts may exert opposite functions during CRC development, and inhibition of a specific Wnt alone is insufficient to curb CRC progression. Moreover, data on the pharmacokinetic parameters and efficacy of most reported natural compounds are far from sufficient. Additionally, a potential off-target effect of the manipulation of Wnt signaling is common for some therapeutic drugs. For example, inhibitors or antibodies targeting Wnt-dependent components in canonical Wnt signaling, such as Fzd receptors, may have no ability to block non-canonical Wnt signaling. Although porcupine inhibitors offer an approach to overcome this limitation, they have potential toxicity to the gastrointestinal tract and could lead to alteration in bone remodeling due to the essential role of Wnts for the maintenance of normal tissue homeostasis (150). More importantly, most of CRC attributed to the mutations of downstream components in Wnt signaling, such as APC and β-catenin, might be insensitive to porcupine inhibitors (159). Therefore, only therapies with the combination of drugs targeting Wnts, mutated downstream components in Wnt signaling, and conventional chemotherapeutics could result in a cooperative inhibition of CRC progression. Fortunately, all these obstacles are being overcome with great efforts of scientists and the in-depth understanding of the Wnt signaling pathways, and Wnt-based therapeutics are still promising and will definitely provide therapeutic benefits for patients with CRC and other cancers.
Conclusion
The Wnt signaling pathway is essential for the regulation of embryogenesis and tissue homeostasis. However, dysregulation of the Wnt signaling pathway has been identified as the pathological basis of many human malignancies including CRC. Therefore, modulating this pathway is always a hotspot in the tumor field, and numerous approaches have been explored in preclinical and early clinical studies. In this review, we specifically described the recent findings on the structure and the maturation process of Wnts, discussed their functions during the tumorigenesis of human CRC separately, and summarized the current therapeutics targeting Wnts in CRC treatment and the existing challenges. We hope that the discussion of this topic will increase the knowledge of Wnts in CRC development and arouse the interest of researchers to design novel Wnt-based therapeutic strategies for CRC.
Author Contributions
XN wrote the manuscript. XN and HL revised the manuscript. HL and LL edited the manuscript and drew the figures. W-DC and Y-DW edited, modified, and revised the manuscript. XN, W-DC, and Y-DW secured funding for this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81970726 and 81472232) to W-DC, the Key Program for Science and Technology of Henan Province (Grant No. 202102310043) and the National Natural Science Foundation of China (Grant No. 81700731) to XN, the National Natural Science Foundation of China (Grant Nos. 81970551 and 81672433) to Y-DW, Program for Science and Technology Innovation Talents in Universities of Henan Province (HASTIT, Grant No. 13HASTIT024) and Plan for Scientific Innovation Talent of Henan Province to W-DC, and the Fundamental Research Funds for the Central Universities (Grant No. PT2001) to Y-DW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to colleagues whose work could not be cited due to space limitations.
References
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
2. Lee RM, Cardona K, Russell MC. Historical perspective: two decades of progress in treating metastatic colorectal cancer. J Surg Oncol. (2019) 119:549–63. doi: 10.1002/jso.25431
3. Pandurangan AK, Divya T, Kumar K, Dineshbabu V, Velavan B, Sudhandiran G. Colorectal carcinogenesis: insights into the cell death and signal transduction pathways: a review. World J Gastrointest Oncol. (2018) 10:244–59. doi: 10.4251/wjgo.v10.i9.244
4. van Neerven SM, Vermeulen L. The interplay between intrinsic and extrinsic Wnt signaling in controlling intestinal transformation. Differentiation. (2019) 108:17–23. doi: 10.1016/j.diff.2019.02.002
5. Nie X, Liu Y, Chen WD, Wang YD. Interplay of miRNAs and canonical Wnt signaling pathway in hepatocellular carcinoma. Front Pharmacol. (2018) 9:657. doi: 10.3389/fphar.2018.00657
6. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. (2012) 487:330–7. doi: 10.1038/nature11252
7. Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, et al. Recurrent R-spondin fusions in colon cancer. Nature. (2012) 488:660–4. doi: 10.1038/nature11282
8. Eto T, Miyake K, Nosho K, Ohmuraya M, Imamura Y, Arima K, et al. Impact of loss-of-function mutations at the RNF43 locus on colorectal cancer development and progression. J Pathol. (2018) 245:445–55. doi: 10.1002/path.5098
9. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. (2017) 36:1461–73. doi: 10.1038/onc.2016.304
10. Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. (2008) 4:68–75. doi: 10.4161/org.4.2.5851
11. Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. (2012) 4:1–13. doi: 10.1101/cshperspect.a008052
12. Tulchinsky E. Colorectal cancer cells use the negative feedback regulators of WNT signalling to activate epithelial-mesenchymal transition pathways. Gut. (2017) 66:563–4. doi: 10.1136/gutjnl-2016-313185
13. Kang DW, Min do S. Positive feedback regulation between phospholipase D and Wnt signaling promotes Wnt-driven anchorage-independent growth of colorectal cancer cells. PLoS ONE. (2010) 5:e12109. doi: 10.1371/journal.pone.0012109
14. Ma B, Hottiger MO. Crosstalk between Wnt/beta-Catenin and NF-kappaB signaling pathway during inflammation. Front Immunol. (2016) 7:378. doi: 10.3389/fimmu.2016.00378
15. Lee SK, Hwang JH, Choi KY. Interaction of the Wnt/beta-catenin and RAS-ERK pathways involving co-stabilization of both beta-catenin and RAS plays important roles in the colorectal tumorigenesis. Adv Biol Regul. (2018) 68:46–54. doi: 10.1016/j.jbior.2018.01.001
16. Giles RH, Lolkema MP, Snijckers CM, Belderbos M, van der Groep P, Mans DA, et al. Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene. (2006) 25:3065–70. doi: 10.1038/sj.onc.1209330
17. Takada S, Fujimori S, Shinozuka T, Takada R, Mii Y. Differences in the secretion and transport of Wnt proteins. J Biochem. (2017) 161:1–7. doi: 10.1093/jb/mvw071
18. Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. (2012) 4:a007864. doi: 10.1101/cshperspect.a007864
19. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. (2006) 4:e115. doi: 10.1371/journal.pbio.0040115
20. Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KL, et al. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell. (2012) 149:1565–77. doi: 10.1016/j.cell.2012.04.039
21. Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by frizzled. Science. (2012) 337:59–64. doi: 10.1126/science.1222879
22. Nile AH, Mukund S, Stanger K, Wang W, Hannoush RN. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc Natl Acad Sci USA. (2017) 114:4147–52. doi: 10.1073/pnas.1618293114
23. Mulligan KA, Fuerer C, Ching W, Fish M, Willert K, Nusse R. Secreted wingless-interacting molecule (Swim) promotes long-range signaling by maintaining wingless solubility. Proc Natl Acad Sci USA. (2012) 109:370–7. doi: 10.1073/pnas.1119197109
24. Willert KH. Isolation and application of bioactive Wnt proteins. Methods Mol Biol. (2008) 468:17–29. doi: 10.1007/978-1-59745-249-6_2
25. Stanczak A, Stec R, Bodnar L, Olszewski W, Cichowicz M, Kozlowski W, et al. Prognostic significance of Wnt-1, beta-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res. (2011) 17:955–63. doi: 10.1007/s12253-011-9409-4
26. Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. (2001) 152:87–96. doi: 10.1083/jcb.152.1.87
27. Yoshida N, Kinugasa T, Ohshima K, Yuge K, Ohchi T, Fujino S, et al. Analysis of Wnt and beta-catenin expression in advanced colorectal cancer. Anticancer Res. (2015) 35:4403–10.
28. Wang FW, Cao CH, Han K, Zhao YX, Cai MY, Xiang ZC, et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J Clin Invest. (2019) 129:727–43. doi: 10.1172/JCI122478
29. Vider BZ, Zimber A, Chastre E, Prevot S, Gespach C, Estlein D, et al. Evidence for the involvement of the Wnt 2 gene in human colorectal cancer. Oncogene. (1996) 12:153–8.
30. Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int J Oncol. (2001) 19:1003–7. doi: 10.3892/ijo.19.5.1003
31. Ma XR, Edmund Sim UH, Pauline B, Patricia L, Rahman J. Overexpression of Wnt2 and TSG101 genes in colorectal carcinoma. Trop Biomed. (2008) 25:46–57.
32. Kramer N, Schmollerl J, Unger C, Nivarthi H, Rudisch A, Unterleuthner D, et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. (2017) 36:5460–72. doi: 10.1038/onc.2017.144
33. Le Floch N, Rivat C, De Wever O, Bruyneel E, Mareel M, Dale T, et al. The proinvasive activity of Wnt-2 is mediated through a noncanonical Wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J. (2005) 19:144–6. doi: 10.1096/fj.04-2373fje
34. Liu S, Fan W, Gao X, Huang K, Ding C, Ma G, et al. Estrogen receptor alpha regulates the Wnt/beta-catenin signaling pathway in colon cancer by targeting the NOD-like receptors. Cell Signal. (2019) 61:86–92. doi: 10.1016/j.cellsig.2019.05.009
35. Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun. (2013) 4:2610. doi: 10.1038/ncomms3610
36. Qi L, Sun B, Liu Z, Cheng R, Li Y, Zhao X. Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J Exp Clin Cancer Res. (2014) 33:107. doi: 10.1186/s13046-014-0107-4
37. Zhang C, Wang Y. Metformin attenuates cells stemness and epithelialmesenchymal transition in colorectal cancer cells by inhibiting the Wnt3a/betacatenin pathway. Mol Med Rep. (2019) 19:1203–9. doi: 10.3892/mmr.2018.9765
38. Ferrer-Mayorga G, Niell N, Cantero R, Gonzalez-Sancho JM, Del Peso L, Munoz A, et al. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci Rep. (2019) 9:8085. doi: 10.1038/s41598-019-44574-9
39. Huang Z, Yang M, Li Y, Yang F, Feng Y. Exosomes derived from hypoxic colorectal cancer cells transfer Wnt4 to normoxic cells to elicit a prometastatic phenotype. Int J Biol Sci. (2018) 14:2094–102. doi: 10.7150/ijbs.28288
40. Huang Z, Feng Y. Exosomes derived from hypoxic colorectal cancer cells promote angiogenesis through Wnt4-induced beta-catenin signaling in endothelial cells. Oncol Res. (2017) 25:651–61. doi: 10.3727/096504016X14752792816791
41. Abdelmaksoud-Dammak R, Miladi-Abdennadher I, Saadallah-Kallel A, Khabir A, Sellami-Boudawara T, Frikha M, et al. Downregulation of WIF-1 and Wnt5a in patients with colorectal carcinoma: clinical significance. Tumour Biol. (2014) 35:7975–82. doi: 10.1007/s13277-014-2015-9
42. Hibi K, Mizukami H, Goto T, Kitamura Y, Sakata M, Saito M, et al. WNT5A gene is aberrantly methylated from the early stages of colorectal cancers. Hepatogastroenterology. (2009) 56:1007–9.
43. Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, et al. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. (2008) 14:55–61. doi: 10.1158/1078-0432.CCR-07-1644
44. Mehdawi LM, Prasad CP, Ehrnstrom R, Andersson T, Sjolander A. Non-canonical Wnt5A signaling up-regulates the expression of the tumor suppressor 15-PGDH and induces differentiation of colon cancer cells. Mol Oncol. (2016) 10:1415–29. doi: 10.1016/j.molonc.2016.07.011
45. Lai C, Robinson J, Clark S, Stamp G, Poulsom R, Silver A. Elevation of WNT5A expression in polyp formation in Lkb1+/- mice and peutz-Jeghers syndrome. J Pathol. (2011) 223:584–92. doi: 10.1002/path.2835
46. Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, et al. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis. (2013) 34:2629–38. doi: 10.1093/carcin/bgt215
47. Dong X, Liao W, Zhang L, Tu X, Hu J, Chen T, et al. RSPO2 suppresses colorectal cancer metastasis by counteracting the Wnt5a/Fzd7-driven noncanonical Wnt pathway. Cancer Lett. (2017) 402:153–65. doi: 10.1016/j.canlet.2017.05.024
48. Chen Z, Tang C, Zhu Y, Xie M, He D, Pan Q, et al. TrpC5 regulates differentiation through the Ca2+/Wnt5a signalling pathway in colorectal cancer. Clin Sci. (2017) 131:227–37. doi: 10.1042/CS20160759
49. You J, Nguyen AV, Albers CG, Lin F, Holcombe RF. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. (2008) 53:1013–9. doi: 10.1007/s10620-007-9973-3
50. Zhang Y, Lin L, Jin Y, Lin Y, Cao Y, Zheng C. Overexpression of WNT5B promotes COLO 205 cell migration and invasion through the JNK signaling pathway. Oncol Rep. (2016) 36:23–30. doi: 10.3892/or.2016.4772
51. Lickert H, Kispert A, Kutsch S, Kemler R. Expression patterns of Wnt genes in mouse gut development. Mech Dev. (2001) 105:181–4. doi: 10.1016/S0925-4773(01)00390-2
52. Sun LN, Yang HS, Chen MY, Xu DX. Cloning and expression analysis of Wnt6 and Hox6 during intestinal regeneration in the sea cucumber Apostichopus japonicus. Genet Mol Res. (2013) 12:5321–34. doi: 10.4238/2013.November.7.7
53. Zheng XL, Yu HG. Wnt6 contributes tumorigenesis and development of colon cancer via its effects on cell proliferation, apoptosis, cell-cycle and migration. Oncol Lett. (2018) 16:1163–72. doi: 10.3892/ol.2018.8729
54. Li N, Li D, Du Y, Su C, Yang C, Lin C, et al. Overexpressed PLAGL2 transcriptionally activates Wnt6 and promotes cancer development in colorectal cancer. Oncol Rep. (2019) 41:875–84. doi: 10.3892/or.2018.6914
55. Kirikoshi H, Katoh M. Expression of WNT7A in human normal tissues and cancer, and regulation of WNT7A and WNT7B in human cancer. Int J Oncol. (2002) 21:895–900. doi: 10.3892/ijo.21.4.895
56. Becer E, Hanoglu DY, Kabadayi H, Hanoglu A, Vatansever S, Yavuz DO, et al. The effect of Colchicum pusillum in human colon cancer cells via Wnt/beta-catenin pathway. Gene. (2019) 686:213–9. doi: 10.1016/j.gene.2018.11.047
57. Shu J, Jelinek J, Chang H, Shen L, Qin T, Chung W, et al. Silencing of bidirectional promoters by DNA methylation in tumorigenesis. Cancer Res. (2006) 66:5077–84. doi: 10.1158/0008-5472.CAN-05-2629
58. Ali I, Medegan B, Braun DP. Wnt9A induction linked to suppression of human colorectal cancer cell proliferation. Int J Mol Sci. (2016) 17:495. doi: 10.3390/ijms17040495
59. Krivohlava R, Grobarova V, Neuhoferova E, Fiserova A, Benson V. Interaction of colon cancer cells with glycoconjugates triggers complex changes in gene expression, glucose transporters and cell invasion. Mol Med Rep. (2018) 17:5508–17. doi: 10.3892/mmr.2018.8490
60. Kirikoshi H, Sekihara H, Katoh M. WNT10A and WNT6, clustered in human chromosome 2q35 region with head-to-tail manner, are strongly coexpressed in SW480 cells. Biochem Biophys Res Commun. (2001) 283:798–805. doi: 10.1006/bbrc.2001.4855
61. Li J, Zhang Z, Wang L, Zhang Y. The oncogenic role of Wnt10a in colorectal cancer through activation of canonical Wnt/beta-catenin signaling. Oncol Lett. (2019) 17:3657–64. doi: 10.3892/ol.2019.10035
62. Jiang H, Sun L, Hu C, Wang Y. Hypermethylated WNT10A and its clinical significance in colorectal cancer. Am J Transl Res. (2018) 10:4290–301.
63. Shi L, Xi J, Xu X, Peng B, Zhang B. MiR-148a suppressed cell invasion and migration via targeting WNT10b and modulating beta-catenin signaling in cisplatin-resistant colorectal cancer cells. Biomed Pharmacother. (2019) 109:902–9. doi: 10.1016/j.biopha.2018.10.080
64. Kirikoshi H, Sekihara H, Katoh M. Molecular cloning and characterization of human WNT11. Int J Mol Med. (2001) 8:651–6. doi: 10.3892/ijmm.8.6.651
65. Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. (2004) 279:26707–15. doi: 10.1074/jbc.M402877200
66. Ramirez de Molina A, Vargas T, Molina S, Sanchez J, Martinez-Romero J, Gonzalez-Vallinas M, et al. The ellagic acid derivative 4, 4'-di-O-methylellagic acid efficiently inhibits colon cancer cell growth through a mechanism involving WNT16. J Pharmacol Exp Ther. (2015) 353:433–44. doi: 10.1124/jpet.114.221796
67. Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. (2006) 11:791–801. doi: 10.1016/j.devcel.2006.10.003
68. Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. (2011) 355:275–85. doi: 10.1016/j.ydbio.2011.04.029
69. Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. (2007) 39:836–8. doi: 10.1038/ng2057
70. Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. (2007) 39:833–5. doi: 10.1038/ng2052
71. Cha SW, Tadjuidje E, White J, Wells J, Mayhew C, Wylie C, et al. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. (2009) 19:1573–80. doi: 10.1016/j.cub.2009.07.062
72. Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires EVI, a conserved transmembrane protein. Cell. (2006) 125:523–33. doi: 10.1016/j.cell.2006.04.009
73. Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. (2006) 125:509–22. doi: 10.1016/j.cell.2006.02.049
74. Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by WLS. Dev Biol. (2012) 361:392–402. doi: 10.1016/j.ydbio.2011.11.003
75. Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, et al. WLS-dependent secretion of WNT3A requires ser209 acylation and vacuolar acidification. J Cell Sci. (2010) 123:3357–67. doi: 10.1242/jcs.072132
76. Buechling T, Chaudhary V, Spirohn K, Weiss M, Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO Rep. (2011) 12:1265–72. doi: 10.1038/embor.2011.212
77. Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. (1988) 55:619–25. doi: 10.1016/0092-8674(88)90220-6
78. Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. (1997) 15:2833–9. doi: 10.1038/sj.onc.1201462
79. He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. (2005) 24:3054–8. doi: 10.1038/sj.onc.1208511
80. Zhang W, Sun Z, Su L, Wang F, Jiang Y, Yu D, et al. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Eur J Pharmacol. (2018) 825:75–84. doi: 10.1016/j.ejphar.2018.02.019
81. Chen L, Wang X, Zhu Y, Zhu J, Lai Q. miR200b3p inhibits proliferation and induces apoptosis in colorectal cancer by targeting Wnt1. Mol Med Rep. (2018) 18:2571–80. doi: 10.3892/mmr.2018.9287
82. Wang J, Lu R, Fu X, Dan Z, Zhang YG, Chang X, et al. Novel regulatory roles of Wnt1 in infection-associated colorectal cancer. Neoplasia. (2018) 20:499–509. doi: 10.1016/j.neo.2018.03.001
83. Jung YS, Jun S, Lee SH, Sharma A, Park JI. Wnt2 complements Wnt/beta-catenin signaling in colorectal cancer. Oncotarget. (2015) 6:37257–68. doi: 10.18632/oncotarget.6133
84. Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. (1999) 81:496–502. doi: 10.1038/sj.bjc.6690721
85. Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, et al. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. (2007) 121:1175–81. doi: 10.1002/ijc.22848
86. Kalhor H, Poorebrahim M, Rahimi H, Shabani AA, Karimipoor M, Akbari Eidgahi MR, et al. Structural and dynamic characterization of human Wnt2-Fzd7 complex using computational approaches. J Mol Model. (2018) 24:274. doi: 10.1007/s00894-018-3788-3
87. Katoh M. Differential regulation of WNT2 and WNT2B expression in human cancer. Int J Mol Med. (2001) 8:657–60. doi: 10.3892/ijmm.8.6.657
88. Mirza SB, Ekhteiari Salmas R, Fatmi MQ, Durdagi S. Discovery of Klotho peptide antagonists against Wnt3 and Wnt3a target proteins using combination of protein engineering, protein-protein docking, peptide docking and molecular dynamics simulations. J Enzyme Inhib Med Chem. (2017) 32:84–98. doi: 10.1080/14756366.2016.1235569
89. Wang HS, Nie X, Wu RB, Yuan HW, Ma YH, Liu XL, et al. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Onco Targets Ther. (2016) 9:3849–60. doi: 10.2147/OTT.S101782
90. Nie X, Xia F, Liu Y, Zhou Y, Ye W, Hean P, et al. Downregulation of Wnt3 suppresses colorectal cancer development through inhibiting cell proliferation and migration. Front Pharmacol. (2019) 10:1110. doi: 10.3389/fphar.2019.01110
91. Wu Y, Tran T, Dwabe S, Sarkissyan M, Kim J, Nava M, et al. A83-01 inhibits TGF-beta-induced upregulation of Wnt3 and epithelial to mesenchymal transition in HER2-overexpressing breast cancer cells. Breast Cancer Res Treat. (2017) 163:449–460. doi: 10.1007/s10549-017-4211-y
92. Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK, Kim HN. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer. (2014) 14:125. doi: 10.1186/1471-2407-14-125
93. Schinzari V, Timperi E, Pecora G, Palmucci F, Gallerano D, Grimaldi A, et al. Wnt3a/beta-catenin signaling conditions differentiation of partially exhausted t-effector cells in human cancers. Cancer Immunol Res. (2018) 6:941–52. doi: 10.1158/2326-6066.CIR-17-0712
94. Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, et al. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res. (2004) 298:369–87. doi: 10.1016/j.yexcr.2004.04.036
95. Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. (2011) 352:58–69. doi: 10.1016/j.ydbio.2011.01.012
96. Al-Tassan NA, Whiffin N, Hosking FJ, Palles C, Farrington SM, Dobbins SE, et al. A new GWAS and meta-analysis with 1000Genomes imputation identifies novel risk variants for colorectal cancer. Sci Rep. (2015) 5:10442. doi: 10.1038/srep10442
97. Hammad MA, Azam SS. Structural dynamics and inhibitor searching for Wnt-4 protein using comparative computational studies. Drug Des Devel Ther. (2015) 9:2449–61. doi: 10.2147/DDDT.S79784
98. Zhang H, Mehmood K, Jiang X, Yao W, Iqbal M, Waqas M, et al. Effect of tetramethyl thiuram disulfide (thiram) in relation to tibial dyschondroplasia in chickens. Environ Sci Pollut Res Int. (2018) 25:28264–74. doi: 10.1007/s11356-018-2824-2
99. Katoh M, Katoh M. Comparative genomics on Wnt5a and Wnt5b genes. Int J Mol Med. (2005) 15:749–53. doi: 10.3892/ijmm.15.4.749
100. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. (2005) 434:843–50. doi: 10.1038/nature03319
101. Cao YC, Yang F, Liu XH, Xin X, Wang CC, Geng M. [Expression of Wnt5a, APC, beta-catenin and their clinical significance in human colorectal adenocarcinoma]. Zhonghua Zhong Liu Za Zhi. (2012) 34:674–8. doi: 10.3760/cma.j.issn.0253-3766.2012.09.007
102. Rawson JB, Mrkonjic M, Daftary D, Dicks E, Buchanan DD, Younghusband HB, et al. Promoter methylation of Wnt5a is associated with microsatellite instability and BRAF V600E mutation in two large populations of colorectal cancer patients. Br J Cancer. (2011) 104:1906–12. doi: 10.1038/bjc.2011.165
103. Li Q, Chen H. Silencing of Wnt5a during colon cancer metastasis involves histone modifications. Epigenetics. (2012) 7:551–8. doi: 10.4161/epi.20050
104. Jiang G, Lin J, Wang W, Sun M, Chen K, Wang F. WNT5A promoter methylation is associated with better responses and longer progression-free survival in colorectal cancer patients treated with 5-fluorouracil-based chemotherapy. Genet Test Mol Biomarkers. (2017) 21:74–9. doi: 10.1089/gtmb.2016.0162
105. Bauer M, Benard J, Gaasterland T, Willert K, Cappellen D. WNT5A encodes two isoforms with distinct functions in cancers. PLoS ONE. (2013) 8:e80526. doi: 10.1371/journal.pone.0080526
106. Huang TC, Lee PT, Wu MH, Huang CC, Ko CY, Lee YC, et al. Distinct roles and differential expression levels of Wnt5a mRNA isoforms in colorectal cancer cells. PLoS ONE. (2017) 12:e0181034. doi: 10.1371/journal.pone.0181034
107. Paez D, Gerger A, Zhang W, Yang D, Labonte MJ, Benhanim L, et al. Association of common gene variants in the WNT/beta-catenin pathway with colon cancer recurrence. Pharmacogenomics J. (2014) 14:142–50. doi: 10.1038/tpj.2013.20
108. Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. (2017) 108:42–52. doi: 10.1111/cas.13109
109. Farkas SA, Vymetalkova V, Vodickova L, Vodicka P, Nilsson TK. DNA methylation changes in genes frequently mutated in sporadic colorectal cancer and in the DNA repair and Wnt/beta-catenin signaling pathway genes. Epigenomics. (2014) 6:179–91. doi: 10.2217/epi.14.7
110. Galbraith RL, Poole EM, Duggan D, Muehling J, Hsu L, Makar K, et al. Polymorphisms in WNT6 and WNT10A and colorectal adenoma risk. Nutr Cancer. (2011) 63:558–64. doi: 10.1080/01635581.2011.542539
111. Jang SM, Wi YC, Kim Y, Bang SS, Sim J, Ahn BK, et al. Loss of Wnt7a expression correlates with tumor progression and poor prognosis in colorectal carcinoma. Int J Clin Exp Pathol. (2018) 11:4967–76.
112. Arensman MD, Kovochich AN, Kulikauskas RM, Lay AR, Yang PT, Li X, et al. WNT7B mediates autocrine Wnt/beta-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma. Oncogene. (2014) 33:899–908. doi: 10.1038/onc.2013.23
113. Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA, et al. Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. (2014) 74:2962–73. doi: 10.1158/0008-5472.CAN-13-2421
114. Zheng D, Decker KF, Zhou T, Chen J, Qi Z, Jacobs K, et al. Role of WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol Cancer Res. (2013) 11:482–93. doi: 10.1158/1541-7786.MCR-12-0520
115. Katoh M, Katoh M. Comparative genomics on Wnt8a and Wnt8b genes. Int J Oncol. (2005) 26:1129–33. doi: 10.3892/ijo.26.4.1129
116. Xue J, Zhu W, Song J, Jiao Y, Luo J, Yu C, et al. Activation of PPARalpha by clofibrate sensitizes pancreatic cancer cells to radiation through the Wnt/beta-catenin pathway. Oncogene. (2018) 37:953–62. doi: 10.1038/onc.2017.401
117. Smith R, Huang YT, Tian T, Vojtasova D, Mesalles-Naranjo O, Pollard SM, et al. The transcription factor Foxg1 promotes optic fissure closure in the mouse by suppressing Wnt8b in the nasal optic stalk. J Neurosci. (2017) 37:7975–93. doi: 10.1523/JNEUROSCI.0286-17.2017
118. Hasenpusch-Theil K, Watson JA, Theil T. Direct interactions between Gli3, Wnt8b, and Fgfs underlie patterning of the dorsal telencephalon. Cereb Cortex. (2017) 27:1137–48. doi: 10.1093/cercor/bhv291
119. Saitoh T, Mine T, Katoh M. Up-regulation of WNT8B mRNA in human gastric cancer. Int J Oncol. (2002) 20:343–8. doi: 10.3892/ijo.20.2.343
120. Spater D, Hill TP, O'Sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. (2006) 133:3039–49. doi: 10.1242/dev.02471
121. Grainger S, Richter J, Palazon RE, Pouget C, Lonquich B, Wirth S, et al. Wnt9a is required for the aortic amplification of nascent hematopoietic stem cells. Cell Rep. (2016) 17:1595–606. doi: 10.1016/j.celrep.2016.10.027
122. Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. (2009) 41:793–9. doi: 10.1038/ng.400
123. Jin YR, Han XH, Taketo MM, Yoon JK. Wnt9b-dependent FGF signaling is crucial for outgrowth of the nasal and maxillary processes during upper jaw and lip development. Development. (2012) 139:1821–30. doi: 10.1242/dev.075796
124. Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. (2011) 138:1247–57. doi: 10.1242/dev.057646
125. Wang J, Shackleford GM. Murine Wnt10a and Wnt10b: cloning and expression in developing limbs, face and skin of embryos and in adults. Oncogene. (1996) 13:1537–44.
126. Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibanez G, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. (2012) 50:477–89. doi: 10.1016/j.bone.2011.08.010
127. Yoshikawa H, Matsubara K, Zhou X, Okamura S, Kubo T, Murase Y, et al. WNT10B functional dualism: beta-catenin/Tcf-dependent growth promotion or independent suppression with deregulated expression in cancer. Mol Biol Cell. (2007) 18:4292–303. doi: 10.1091/mbc.e06-10-0889
128. Wu G, Fan X, Sun L. Silencing of Wnt10B reduces viability of heptocellular carcinoma HepG2 cells. Am J Cancer Res. (2015) 5:1911–20.
129. Wu XD, Bie QL, Zhang B, Yan ZH, Han ZJ. Wnt10B is critical for the progression of gastric cancer. Oncol Lett. (2017) 13:4231–37. doi: 10.3892/ol.2017.5992
130. Uysal-Onganer P, Kypta RM. Wnt11 in 2011 the regulation and function of a non-canonical Wnt. Acta Physiol. (2012) 204:52–64. doi: 10.1111/j.1748-1716.2011.02297.x
131. Nishioka M, Ueno K, Hazama S, Okada T, Sakai K, Suehiro Y, et al. Possible involvement of Wnt11 in colorectal cancer progression. Mol Carcinog. (2013) 52:207–17. doi: 10.1002/mc.21845
132. Dwyer MA, Joseph JD, Wade HE, Eaton ML, Kunder RS, Kazmin D, et al. WNT11 expression is induced by estrogen-related receptor alpha and beta-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. (2010) 70:9298–308. doi: 10.1158/0008-5472.CAN-10-0226
133. Liu X, Wu S, Xia Y, Li XE, Xia Y, Zhou ZD, et al. Wingless homolog Wnt11 suppresses bacterial invasion and inflammation in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. (2011) 301:G992–1003. doi: 10.1152/ajpgi.00080.2011
134. Fear MW, Kelsell DP, Spurr NK, Barnes MR. Wnt-16a, a novel Wnt-16 isoform, which shows differential expression in adult human tissues. Biochem Biophys Res Commun. (2000) 278:814–20. doi: 10.1006/bbrc.2000.3852
135. Gori F, Lerner U, Ohlsson C, Baron R. A new WNT on the bone: WNT16, cortical bone thickness, porosity and fractures. Bonekey Rep. (2015) 4:669. doi: 10.1038/bonekey.2015.36
136. Norollahi SE, Alipour M, Rashidy-Pour A, Samadani AA, Larijani LV. Regulatory fluctuation of WNT16 gene expression is associated with human gastric adenocarcinoma. J Gastrointest Cancer. (2019) 50:42–7. doi: 10.1007/s12029-017-0022-y
137. Le PN, Keysar SB, Miller B, Eagles JR, Chimed TS, Reisinger J, et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol Carcinog. (2019) 58:398–410. doi: 10.1002/mc.22937
138. Ono M, Yin P, Navarro A, Moravek MB, Coon JSt, Druschitz SA, et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci USA. (2013) 110:17053–8. doi: 10.1073/pnas.1313650110
139. Sun Y, Zhu D, Chen F, Qian M, Wei H, Chen W, et al. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene. (2016) 35:4321–34. doi: 10.1038/onc.2015.494
140. Qian D, Chen K, Deng H, Rao H, Huang H, Liao Y, et al. MicroRNA-374b suppresses proliferation and promotes apoptosis in T-cell lymphoblastic lymphoma by repressing AKT1 and Wnt-16. Clin Cancer Res. (2015) 21:4881–91. doi: 10.1158/1078-0432.CCR-14-2947
141. Sawa M, Masuda M, Yamada T. Targeting the Wnt signaling pathway in colorectal cancer. Expert Opin Ther Targets. (2016) 20:419–29. doi: 10.1517/14728222.2016.1098619
142. Bahrami A, Amerizadeh F, ShahidSales S, Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, et al. Therapeutic potential of targeting Wnt/beta-catenin pathway in treatment of colorectal cancer: rational and progress. J Cell Biochem. (2017) 118:1979–83. doi: 10.1002/jcb.25903
143. Zhang LS, Lum L. Chemical modulation of WNT signaling in cancer. Prog Mol Biol Transl Sci. (2018) 153:245–69. doi: 10.1016/bs.pmbts.2017.11.008
144. Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. (2013) 73:502–7. doi: 10.1158/0008-5472.CAN-12-2258
145. Cheng D, Liu J, Han D, Zhang G, Gao W, Hsieh MH, et al. Discovery of pyridinyl acetamide derivatives as potent, selective, and orally bioavailable porcupine inhibitors. ACS Med Chem Lett. (2016) 7:676–80. doi: 10.1021/acsmedchemlett.6b00038
146. Wickstrom M, Dyberg C, Milosevic J, Einvik C, Calero R, Sveinbjornsson B, et al. Wnt/beta-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. (2015) 6:8904. doi: 10.1038/ncomms9904
147. Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, et al. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. (2019) 38:1951–1965. doi: 10.1038/s41388-018-0557-9
148. Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. (2016) 35:2197–207. doi: 10.1038/onc.2015.280
149. Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. (2015) 146:1–11. doi: 10.1016/j.pharmthera.2014.08.005
150. Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, et al. A first-in-human phase I study of the anticancer stem cell agent Ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. (2017) 23:7490–7. doi: 10.1158/1078-0432.CCR-17-2157
151. Mazieres J, You L, He B, Xu Z, Twogood S, Lee AY, et al. Wnt2 as a new therapeutic target in malignant pleural mesothelioma. Int J Cancer. (2005) 117:326–32. doi: 10.1002/ijc.21160
152. Amado NG, Predes D, Moreno MM, Carvalho IO, Mendes FA, Abreu JG. Flavonoids and Wnt/beta-catenin signaling: potential role in colorectal cancer therapies. Int J Mol Sci. (2014) 15:12094–106. doi: 10.3390/ijms150712094
153. Razak S, Afsar T, Ullah A, Almajwal A, Alkholief M, Alshamsan A, et al. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/ beta -catenin signaling pathway. BMC Cancer. (2018) 18:1043. doi: 10.1186/s12885-018-4959-4
154. Oh S, Gwak J, Park S, Yang CS. Green tea polyphenol EGCG suppresses Wnt/beta-catenin signaling by promoting GSK-3beta- and PP2A-independent beta-catenin phosphorylation/degradation. Biofactors. (2014) 40:586–95. doi: 10.1002/biof.1185
155. Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y, Xie CF, et al. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/beta-Catenin Pathway. Nutrients. (2017) 9:572. doi: 10.3390/nu9060572
156. Wang Q, Lu W, Yin T, Lu L. Calycosin suppresses TGF-beta-induced epithelial-to-mesenchymal transition and migration by upregulating BATF2 to target PAI-1 via the Wnt and PI3K/Akt signaling pathways in colorectal cancer cells. J Exp Clin Cancer Res. (2019) 38:240. doi: 10.1186/s13046-019-1243-7
157. Li Y, Qin X, Li P, Zhang H, Lin T, Miao Z, et al. Isobavachalcone isolated from psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3beta/beta-catenin pathway in colorectal cancer cells. Drug Des Devel Ther. (2019) 13:1449–60. doi: 10.2147/DDDT.S192681
158. Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS ONE. (2013) 8:e78700. doi: 10.1371/journal.pone.0078700
Keywords: colorectal cancer, Wnts, canonical Wnt signaling pathway, non-canonical Wnt signaling pathway, Wnt-based therapeutics
Citation: Nie X, Liu H, Liu L, Wang Y-D and Chen W-D (2020) Emerging Roles of Wnt Ligands in Human Colorectal Cancer. Front. Oncol. 10:1341. doi: 10.3389/fonc.2020.01341
Received: 23 February 2020; Accepted: 26 June 2020;
Published: 14 August 2020.
Edited by:
Zhe-Sheng Chen, St. John's University, United StatesReviewed by:
Ethan David Cohen, University of Rochester School of Medicine and Dentistry, United StatesRamiro Malgor, Ohio University, United States
Copyright © 2020 Nie, Liu, Liu, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Dong Wang, ydwangbuct2009@163.com; Wei-Dong Chen, wdchen666@163.com
†These authors have contributed equally to this work
 Xiaobo Nie1†
Xiaobo Nie1† Yan-Dong Wang
Yan-Dong Wang Wei-Dong Chen
Wei-Dong Chen