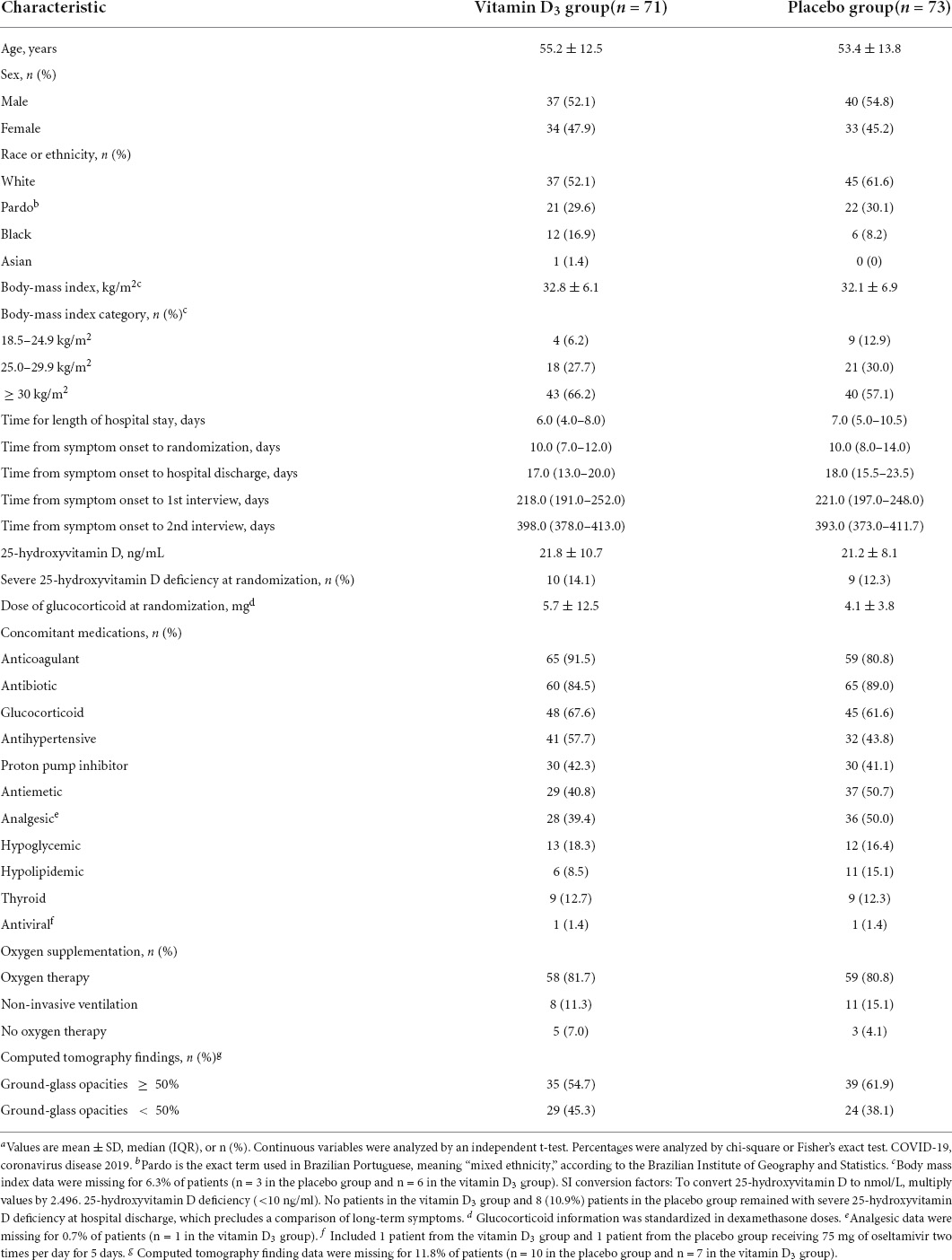
Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19
- Rheumatology Division, Faculdade de Medicina HCFMUSP, Hospital das Clinicas, Universidade de São Paulo, São Paulo, Brazil
Purpose: The aim of this study was to investigate the reported persistent or new symptoms 1 year after a single dose of 200,000 IU of vitamin D3 and hospitalization in patients with moderate to severe COVID-19.
Methods: This is a post-hoc, exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial from two hospitals in São Paulo, Brazil, registered in ClinicalTrials.gov, NCT04449718. Discharged patients were followed for up to 1 year and evaluated by telephone interviews at 6 and 12 months. The primary and secondary outcomes were previously published. These post-hoc exploratory secondary outcomes are the persistent or new symptoms and quality of life (QoL) at the post-viral stage of COVID-19. Generalized estimating equations (GEE) for repeated measures with Bonferroni’s adjustment were used for testing outcomes.
Results: Between 2 June and 27 August 2020, we randomized 240 patients of which 144 were included in this study [the vitamin D3 (n = 71) or placebo (n = 73) group]. The mean (SD) age was 54.3 (13.1) years, and body mass index (BMI) was 32.4 (6.5) kg/m2. Fever demonstrated a significant main effect of time (P < 0.001) with a reduction from baseline to 6 (52–0) and 12 months (52–0). No significant differences between groups were observed for fever, cough, fatigue, fever, myalgia, joint pain, runny nose, nasal congestion, sore throat, hypertension, diabetes, cardiovascular disease, rheumatic disease, asthma, chronic obstructive pulmonary, chronic kidney disease, QoL, and new or persistent symptoms up to 1-year of follow-up.
Conclusion: The findings do not support the use of 200,000 IU of vitamin D3 compared to placebo for the management of persistence or new symptoms, and QoL reported by moderate to severe patients after hospitalization for COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19) has been associated with manifestations in the post-viral stage that affect multiple organ systems (1). Emerging literature has reported residual effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection manifested by a wide spectrum of symptoms that persist or begin after the acute-viral stage called “long-term” of COVID-19 (2–4), “long COVID” (5, 6), or “post-COVID-19 syndrome” (7, 8). According to the guideline (9), “acute COVID-19” represents signs and symptoms for up to 4 weeks, while “post-COVID-19 syndrome, long-term or long COVID” comprises signs and symptoms that develop during or after an infection consistent with COVID-19 present for more than 12 weeks and is not attributable to alternative diagnoses (4, 9).
Persistent symptoms in survivors of COVID-19 who had been hospitalized tend to vary according to the time of assessment. Carfi et al. (10) showed a high proportion of individuals who still reported fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%), and chest pain (21.7%) 2 months after COVID-19 symptom onset. In a prospective cohort of patients interviewed by telephone after 4 months of hospital discharge by COVID-19, 51% reported the presence of at least one symptom that did not exist before the disease, being fatigue (31%), cognitive symptoms (21%), and new-onset dyspnea (16%) the most frequent manifestations (3).
Survivors of COVID-19 who had been hospitalized still present long-term health consequences at 6 months (2) or 1 year (11) after recovering from the acute phase of SARS-CoV-2 infection. Fatigue or muscle weakness (63%), sleep difficulties (26%), and anxiety or depression (23%) were the most common clinical manifestations after 6 months of symptom onset (2). A case report of a 50-year-old female nurse described a history of fatigue on minor exertion and persistent dysphonia without organic alterations after 1 year of mild COVID-19 (11).
Among numerous efforts to discover adjuvant therapeutic compounds against SARS-CoV-2 infection (12–14), it has been postulated through the effective increase in serum 25-hydroxyvitamin D (25[OH]D) levels after a single, safe high dose of vitamin D3 used in our study (15) the possible attenuate of the persistence or onset of symptoms. According to a previous systematic review (16), a single high dose of vitamin D3 significantly reduced levels of inflammatory cytokines (interleukin-6 and tumor necrosis factor-alpha, TNF-α) (17), improved endothelial function in patients with a history of stroke (18), type 2 diabetes (19), and cardiovascular function (20). Additionally, a single high dose of vitamin D3 promoted a good functional modified ranking scale with decreased mortality risk in ischemic stroke survivors with baseline serum 25(OH)D < 30 ng/ml at 6 months (21), while high concentrations of serum 25(OH)D were longitudinally associated with better quality of life (QoL) and less fatigue within 2 years after treatment (22).
Serum 25(OH)D levels are expected to remain sufficient for up to 4 months (23) following a single high dose of vitamin D3 of approximately 200,000 IU, even at baseline 25(OH)D levels 20 ≤ ng/ml (16). The main hypothesis was that a single high dose of vitamin D3 would sustain long-term serum 25(OH)D levels aiding in the modulation of inflammation, and innate and adaptative immune responses related to post-COVID-19 manifestation. In view of the biological plausibility and presumed benefit of vitamin D mitigating the persistent symptoms of COVID-19, this study investigates the reported persistent or new symptoms 1 year after a single high dose of vitamin D3 and hospitalization in patients with moderate to severe COVID-19.
Materials and methods
Study design
This is a post-hoc exploratory analysis of a multicenter, double-blind, placebo-controlled, randomized clinical trial (ClinicalTrials.gov, NCT04449718). The study and trial protocol were approved by the ethics committee and institutional review board of the Clinical Hospital of the School of Medicine of the University of São Paulo (Ethics Committee Approval Number 30959620.4.0000.0068) and were conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent before being enrolled in the study and agreed to participate in the telephone interview at the post-viral stage of COVID-19. Complete information about the trial concept and design, data acquisition, analysis, and interpretation were previously published (15). The manuscript had been critically revised for important intellectual content and was approved for submission for publication by all the authors.
Participants at the viral stage of COVID-19
Hospitalized patients were recruited from the Clinical Hospital of the School of Medicine of the University of São Paulo, and Ibirapuera Field Hospital from 2 June 2020 to 27 August 2020. The final follow-up occurred on 7 October 2020, and the screening criteria assumed were identical for both centers. All patients had a positive COVID-19 diagnosis confirmed by polymerase chain reaction (PCR) testing at the time of randomization or by serology assay (ELISA) to detect IgG against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to the time from symptom onset to study enrollment.
Patients were eligible for enrollment if they were aged 18 years or older and had a positive SARS-CoV-2 infection diagnosis by either nasopharyngeal swab PCR or chest computed tomography scan with compatible findings (bilateral multifocal ground-glass opacities with at least 50% lung involvement). Patients were considered moderate to severe COVID-19 if they met the following hospital admission criteria: diagnosis of flu syndrome indicative of hospitalization, presenting with a respiratory rate greater than 24 breaths/minute, oxygen saturation lower than 93% on room air, or risk factors for complications (e.g., heart disease, diabetes, systemic arterial hypertension, neoplasms, immunosuppression, pulmonary tuberculosis, and obesity), followed by COVID-19 confirmation. Patients were excluded if they were unable to read and sign the written informed consent; they were already admitted under invasive mechanical ventilation; they had a recent previous vitamin D3 supplementation (greater than 1,000 IU/day or weekly equivalent); they had renal failure requiring dialysis or creatinine above 2.0 mg/dl; they had hypercalcemia defined by total calcium greater than 10.5 mg/dl; they were pregnant or lactating women; or if they were expecting hospital discharge in less than 24 h. The criteria used for hospital discharge were the absence of fever in the previous 72 h, no need for supplemental oxygen in the previous 48 h, and oxygen saturation greater than 93% on room air without respiratory distress.
Randomization and masking at the viral stage of COVID-19
Eligible patients were assigned in a 1:1 ratio to either the vitamin D3 group or the placebo group. The randomization list was created using a computer-generated code, in bloc sizes of 20 participants, which was managed by a staff member who had no role in the study.
The vitamin D3 group received on the same day of randomization a single oral dose of 200,000 IU of vitamin D3 diluted in a 10-ml peanut oil solution. The selected dose is within the recommended range that appears to be most effective in promoting vitamin D sufficiency (16). Patients enrolled in the placebo group received only 10 ml of peanut oil solution. The vitamin D3 and placebo solutions were identical in color, taste, smell, consistency, and container. They were prepared by the pharmacy unit of the Clinical Hospital. Both were labeled by a staff member who did not participate in the study, and allocation blindness was maintained until the final statistical analysis.
Procedures at the viral stage of COVID-19
Self-reported anthropometric characteristics (weight and height) and coexisting chronic diseases, acute COVID-19 symptoms, patients’ concomitant medications during hospitalization, oxygen supplementation requirement, and imaging features were assessed upon hospital admission. Self-reported coexisting chronic diseases and previous medications were checked according to the medical records for each patient. To provide a comprehensive demographic characterization, self-reported race/ethnicity data were also collected based on the following fixed categories: White, Black, Asian, and Pardo, the latter refers to people of mixed race/ethnicities, according to the Brazilian Institute of Geography and Statistics—IBGE (24).
Serum levels of 25-hydroxyvitamin D were assessed by a chemiluminescent immunoassay (ARCHITECT 25-OH Vitamin D 5P02; Abbott Diagnostics), at the same time by a blinded technician, following the manufacturer’s recommendations.
Outcomes
The primary outcome, length of hospital stay, was previously published and did not differ between the vitamin D3 and placebo groups (15). The prespecified secondary outcomes were the number and severity of symptoms at the viral stage of COVID-19. To provide a comprehensive understanding of the effect of vitamin D3 in the post-viral stage of COVID-19, a secondary and exploratory post-hoc analysis was performed following reports from patients interviewed by telephone about persistent or new symptoms at 6 months and 1 year after hospital discharge and QoL at 6 months from hospital discharge. The QoL was assessed using the 36-Item Short Form Health Survey (SF-36), a generic questionnaire that was chosen due to concerns about the measurement properties of multiple domains and accuracy (25). The scale ranges from 0 to 100 for each of the eight domains and summary scores, and higher scores reflect better QoL. Investigators were blinded to patient-reported persistent symptoms and SF-36 responses. Score analysis was performed after completed assessments.
Statistical analysis
The sample size was chosen based on feasibility and resources, as described in detail in a previous study (15). Generalized estimating equations (GEE) for repeated measures were used for testing possible differences in the persistence of symptoms and coexisting disease outcomes assuming group and time as fixed factors, with a binomial distribution, and a first-order autoregressive correlation matrix to test the main and interaction effects. Bonferroni’s adjustment was performed in GEE analyses to maintain a family-wise two-sided significance threshold of 0.05, considering 15 pairwise comparisons for all outcomes. Fever was handled by the McNemar test in comparisons within and between groups. Continuous variables were analyzed by an independent t-test. Percentages were analyzed by chi-square (χ2) or Fisher’s exact test. To consider potential confounders, GEE was handled by 3 models: unadjusted, adjusted by the length of hospital stay, and adjusted by the center (hospitals from which patients were recruited), using a per protocol approach. From 6 to 12 months, data were missing for 11.8% of patients (n = 9 in the placebo group and n = 8 in the vitamin D3 group) due to lack of contact during follow-up and were handled by GEE models, with no imputation for missing data.
Kaplan-Meier estimate curves for time (months) manifesting symptoms related to COVID-19 after hospital discharge were compared between vitamin D3 and placebo groups using the log-rank, Breslow, and Tarone-Ware, with cessation of the symptoms being right-censored in the analysis. To avoid bias regarding the different weights of the event in the survival curve analysis of each test, log-rank, Breslow, and Tarone-Ware were presented accordingly (26). Statistical analyses were performed using the IBM-SPSS software, version 20.0. The significance level was set at a two-sided p-value < 0.05.
Results
Of the 1,240 patients assessed for eligibility, 240 underwent randomization during the acute phase of SARS-CoV-2 infection, with 120 assigned to each group. Of the 110 patients discharged in the vitamin D3 group, 21 were excluded due to the absence of a telephone number, 8 were excluded due to lack of contact, 8 withdrew consent, and 2 did not receive vitamin D3. Of the 112 patients discharged in the placebo group, 25 were excluded due to the absence of telephone numbers, 10 were excluded due to lack of contact, 3 died after hospital discharge, and 1 withdrew consent (Supplementary Figure 1). Overall, mean (SD) age was 54.3 (13.1) years, BMI was 32.4 (6.5) kg/m2, 77 (53.5%) were men, 82 (56.9%) patients were White, 43 (29.9%) were Pardo, 18 (12.5%) were Black, and 1 (0.7%) was Asian (Table 1).
Fever demonstrated a significant main effect of time with a reduction from baseline to 6 months (52 to 0, P < 0.001) and from baseline to 12 months (52 to 0, P < 0.001) in the vitamin D3 group compared to the placebo group from baseline to 6 months (53 to 0, P < 0.001) and from baseline to 12 months (53 to 0, P < 0.001) (Table 2). No significant difference between the vitamin D3 and placebo groups for fever was observed at baseline (52 vs. 53, P = 1.00) (Table 2).
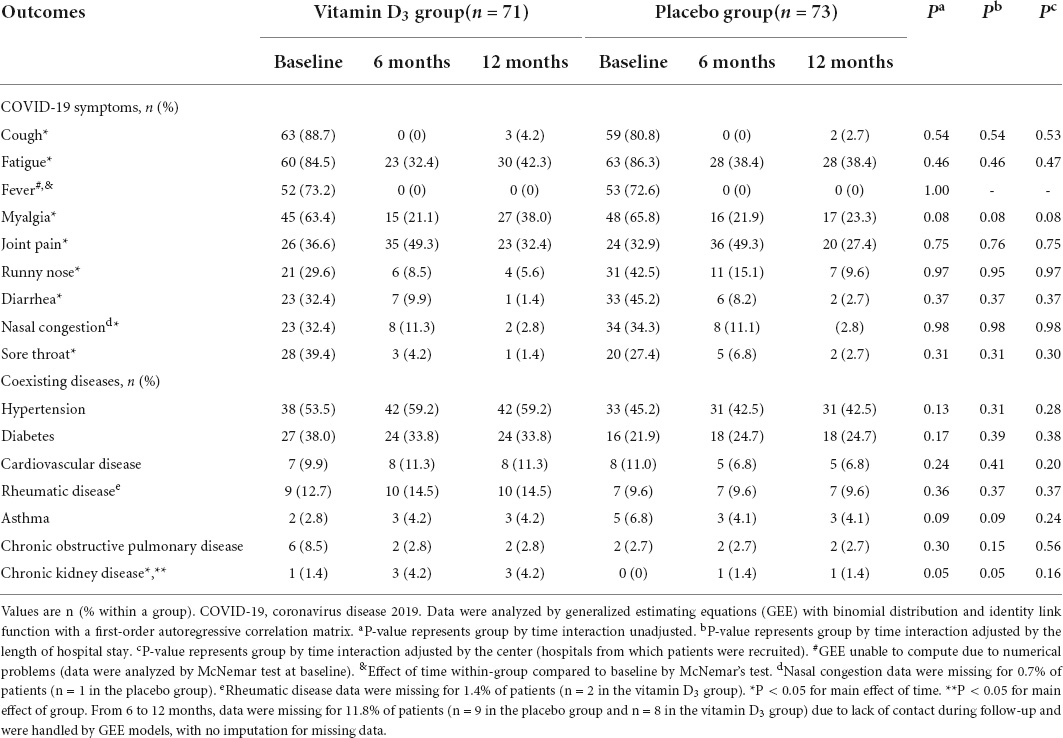
Table 2. Persistence of symptoms and coexisting diseases from baseline to 1 year after hospital discharge for COVID-19.
No significant differences between the vitamin D3 and placebo groups for cough, fatigue, fever, myalgia, joint pain, runny nose, nasal congestion, sore throat, hypertension, diabetes, cardiovascular disease, rheumatic disease, asthma, chronic obstructive pulmonary, and chronic kidney disease were observed (Table 2). In addition, no significant differences between groups were found for QoL and frequency of new symptoms (Figures 1A,B), nor Kaplan-Meier curves for time manifesting symptoms (Figure 2A) and frequency of participants manifesting at least one symptom (Figure 2B) related to COVID-19 up to 1 year of follow-up.
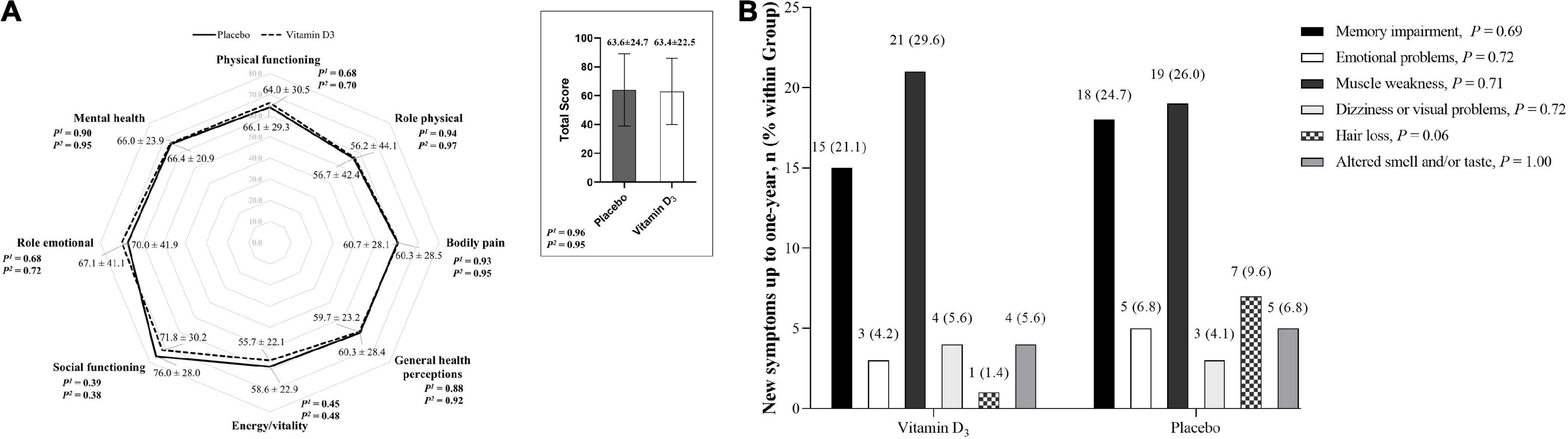
Figure 1. Quality of life and new symptoms related to COVID-19. (A) Quality of life was assessed using the 36-Item Short Form Health Survey (SF-36) at 6 months. Values are mean ± SD. Data were analyzed using an independent T-test and generalized estimating equations (GEE) with normal distribution and identity link function with the first-order autoregressive correlation matrix. 1P-value represents a 2-tailed independent t-test comparison. 2P-value represents the main effect of the group adjusted by the length of hospital stay. (B) Frequency of new symptoms from hospital discharge to 1 year of follow-up. Values are n (% within the group). Proportions were compared between groups using chi-square tests (χ2). New symptoms were missing for 11.8% of patients (n = 9 in the placebo group and n = 8 in the vitamin D3 group) due to a lack of contact during follow-up.
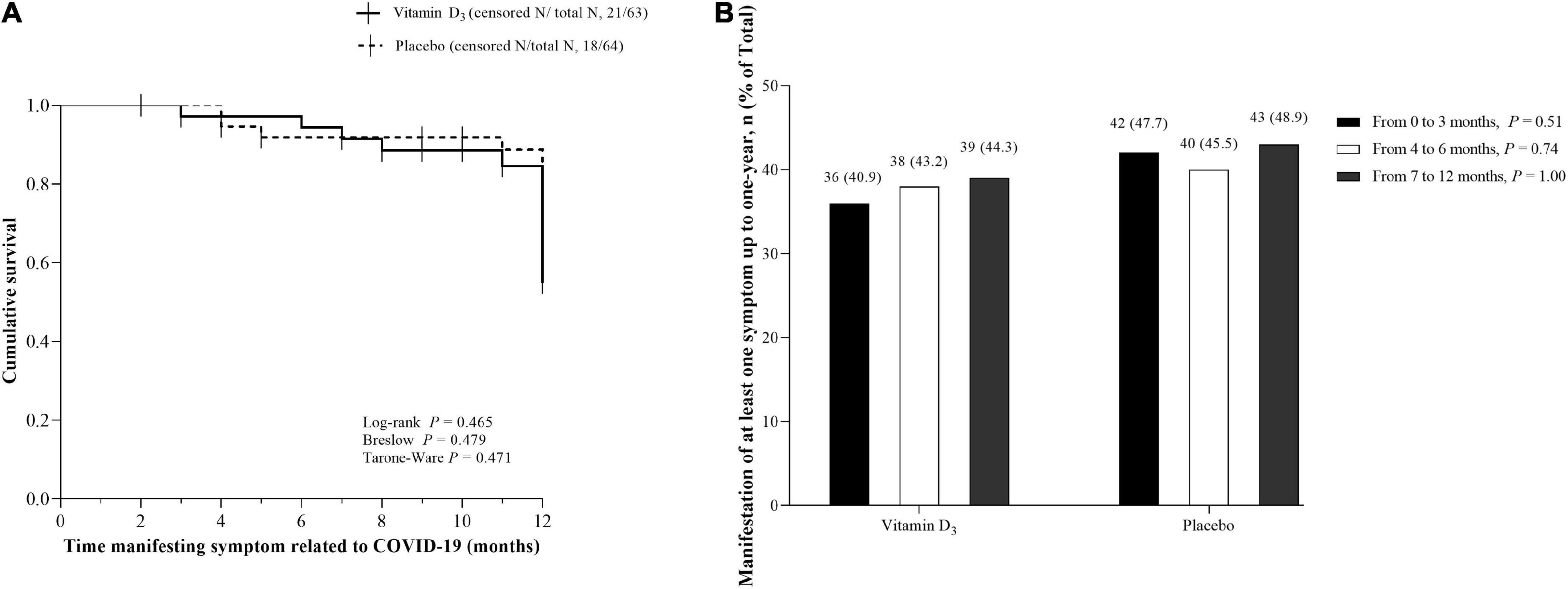
Figure 2. Kaplan-Meier curves for time manifesting symptoms and frequency of participants manifesting at least one symptom up to 1 year. (A) Cumulative survival for time (months) manifesting symptoms related to COVID-19 after hospital discharge in the vitamin D3 (42/63) and placebo groups (46/64). Vertical bars present single censored events (stop manifesting the symptom) in the vitamin D3 (n = 21) and placebo (n = 18) groups. (B) Frequency of participants manifesting at least one symptom from hospital discharge to 1-year follow-up. Values are n (% within the group). Proportions were compared between groups using chi-square tests (χ2). In panels A and B, data were missing for 11.8% of patients (n = 9 in the placebo group and n = 8 in the vitamin D3 group) due to lack of contact during follow-up, representing 127 patients in total.
Discussion
In this post-hoc exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial, a single high dose of vitamin D3 did not significantly differ from a placebo for COVID-19 symptoms, coexisting diseases, or QoL. To the best of our knowledge, this is the first randomized clinical trial to investigate the reported persistent or new symptoms in patients with moderate to severe COVID-19 1 year after a single high dose of vitamin D3 and hospitalization.
Cough, fatigue, fever, myalgia, joint pain, runny nose, diarrhea, nasal congestion, and sore throat presented a significant main effect of time with reduced frequency from baseline to 6 and 12 months in both groups, except for the increase in chronic kidney disease, not differing between vitamin D3 and placebo.
The findings demonstrated manifestation of at least one symptom related to COVID-19 persisting for up to 1 year after the acute phase of SARS-CoV-2 infection in 44.3% of the participants in the vitamin D3 group and 48.9% in the placebo group. Furthermore, our results showed that from the first trimester after hospital discharge, at least 40% of participants did manifest any symptoms related to COVID-19 for both groups, in line with previous evidence (2, 3, 10), although we did not observe a significant difference in time to manifesting symptoms between the vitamin D3 and placebo groups.
The most frequent symptoms at 6 and 12 months were joint pain (49.3 and 32.4%, respectively), fatigue (32.4 and 42.3%), and myalgia (21.1 and 38.0%) in the vitamin D3 group compared to joint pain (49.3 and 27.4%), fatigue (38.4 and 38.4%), and myalgia (21.9 and 23.3%) in the placebo group. These findings are very similar to 53.1% fatigue and 27.3% joint pain at 2 months demonstrated by Carfi et al. (10), 31% fatigue at 4 months by the Comebac study (3), and 63% fatigue or muscle weakness at 6 months by Huang et al. (2).
Regarding new symptoms after hospital discharge for COVID-19, muscle weakness and memory impairment were among the most frequent in the vitamin D3 group, whereas in the placebo group, in addition to the two aforementioned symptoms, a trend (P = 0.06) of greater hair loss was observed in comparison to the vitamin D3 group, being the third most frequent manifestation.
The technical term for this hair loss is reported telogen effluvium. Telogen effluvium is characterized by diffuse hair loss within months of a significant systemic stressor, such as the premature follicular transition from the anagen (active growth phase) to the telogen (resting phase), the latter phase lasting approximately 3 months, after which excessive hair loss ensues (27). In fact, some evidence has pointed to SARS-CoV-2 infection as a precursor to acute telogen effluvium (28–32).
A research letter presented 10 patients with telogen effluvium following SARS-CoV-2 infection (28). All patients were women with a median age of 55, had laboratory-confirmed COVID-19, and had no history of hair loss. Mild symptoms were reported in six of them and severe disease in four, requiring hospitalization for an average of 7 days, and they all experienced excessive hair loss within months after infection, which included hair coming out in large clumps and thinning along the frontal hairline (28). These data corroborate other reports showing, on average, the onset of hair loss 50 days after the first symptom of COVID-19 infection (29, 31), associated or not with trichodynia (30), and may present duration ranging from 12 to 100 days (32).
One of the attributions to vitamin D functions would be related to hair loss. Vitamin D plays an important dermatological and dermatotherapeutic role in affecting the hair cycle due to its anti-inflammatory and immunomodulatory properties, and regulation of keratinocyte differentiation and proliferation (33). Recently, the benefit of oral vitamin D3 (200,000 IU) therapy in patients suffering from diffuse hair loss (telogen effluvium) has been demonstrated (34). However, conclusive studies regarding the presumed benefit of vitamin D in hair loss are lacking. There are no clinical trials that have investigated the efficacy of vitamin D in managing hair loss.
With respect to the QoL, Jacobs et al. (35) reported persistent symptoms at 35 days after hospital discharge for COVID-19 infection associated with lower odds of rating QoL and its categories (general health, physical health, mental health, and social functioning). Similarly, Chen et al. (25) demonstrated a poor health-related QoL among COVID-19 patients, and those women were negatively associated with physical function, bodily pain, and emotional domain at the 1-month follow-up. Even 4 months after hospitalization for COVID-19, the median SF-36 was 46.9 (IQR, 31.2–68.8) for vitality and 57.5 (IQR, 40.0–75.0) for general health (3). Our results show a slight, but not significant, improvement at 6 months, although we have failed to observe differences between the vitamin D3 and placebo groups.
During follow-up interviews, participants were also asked whether they had been using medications and/or supplements. Only 3 participants in the vitamin D3 group and 2 in the placebo group reported using a commercially available multivitamin or vitamin D (∼200 IU). Statistical sensitivity analysis excluding the aforementioned participants showed no significant difference in persistence or new symptoms, and QoL reported up to 1 year of follow-up between the vitamin D3 (n = 68) and placebo (n = 71) groups.
The postulated role of vitamin D in SARS-CoV-2 infection suggests action on the innate and adaptive immune system while mitigating excessive signaling for local and systemic inflammation (36). Our group failed to observe an effect of a single high dose of 200,000 IU of vitamin D3 eliciting significant changes in systemic inflammatory cytokines, chemokines, and growth factors compared with placebo (37). Furthermore, this supplementation strategy was not able to significantly reduce the length of hospital stay compared to those who were supplemented with a placebo (15). Nevertheless, clinicians and researchers continue the search for supplements with therapeutic properties that effectively mitigate post-COVID-19 syndrome (38).
Aside from experimental design, the strength of this study includes being a prospective trial investigating the effects of vitamin D3 on persisted or new symptoms after hospitalization for COVID-19, and enrollment of moderate to severe patients followed up for 1 year.
This study has several limitations. First, this investigation did not assess 25(OH)D concentrations at 6 and 12 months. Second, it was not feasible to evaluate the patients in person, and it was necessary to rely on the patients’ memory to obtain the data retrospectively. Third, regarding the new symptom onset related to COVID-19, it is not possible to determine when it started or ended. Fourth, at the time, most patients were admitted to the hospital’s emergency room, some of whom did not remember their telephone numbers, and the hospital’s social care staff did not locate the patients’ or family’s telephone numbers at hospital/study admission or during hospitalization; therefore, the authors are not able to state whether approximately 20% of participants excluded due to absence of telephone numbers would be alive after hospital discharge.
This study does not rule out the possibility that intermediate doses of vitamin D3 administered during follow-up could imply different results or that a single high dose of vitamin D3 followed by treatment with a recommended standard intake of at least 400 IU/day (39) may result in positive effects in favor of vitamin D and therefore could be evaluated in the future. Therefore, further studies are needed to prospectively assess the efficacy of fractionated high doses of vitamin D3 on persistent or new symptoms after hospitalization for COVID-19.
Despite the trend toward greater hair loss (telogen effluvium) as the third most frequent new symptom in the placebo group, we did not rule out the possibility that vitamin D could prevent marked telogen effluvium if applied as a booster dose around 3 months after hospital discharge. Our findings advance in showing that a single high dose of vitamin D3 did not support the initial hypothesis of an eventual role in innate and adaptive immune responses modulating long-term COVID-19, even in view of the expected significant reduction in COVID-19 symptoms from baseline up to 6 months and 1 year. Therefore, a single high dose of 200,000 IU of vitamin D3 compared to placebo did not interfere with the persistence or new symptoms, and QoL reported by moderate to severe patients after hospitalization for COVID-19 up to 6 months and 1 year of follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Deidentified participant data of this study must be requested from the corresponding author RP, rosamariarp@yahoo.com. The codebook of this study will be made available upon request by qualified clinical researchers for specific purposes dependent on the nature of the request and the intention use of the data, with investigator support. The request must include a statistician. The lead author (RP) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Clinical Hospital of the School of Medicine of the University of São Paulo (approval numbers: 30959620.4.0000.0068). The patients/participants provided their written informed consent to participate in this study.
Author contributions
RP: full access to all the data in the study and responsibility for the integrity of the data and the accuracy of the data analysis and funding and supervision. AF, IM, and RP: concept and design. AF and RP: drafting of the manuscript and statistical analysis. LS, MS, and VC: administrative, technical, or material support. All authors: acquisition, analysis, and interpretation, and critical revision of the manuscript for important intellectual content.
Funding
AF reports receiving grant support from the Sao Paulo Research Foundation (FAPESP, grant 2020/11102-2; 2020/07098-0); IM reports receiving grant support from FAPESP (grant 2019/24782-4); and RP reports receiving grants support from FAPESP (grant 2020/05752-4) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 305556/2017-7). The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Acknowledgments
We are thankful to Monica Pinheiro and Roberta Costa (Ibirapuera field hospital) for assistance with the study; Cleuber Esteves Chaves (pharmacy unit of the clinical hospital) for the vitamin D3 and placebo solution preparation; Ana J. Pinto, Karla F. Goessler, Bruno Gualano (Applied Physiology and Nutrition Research Group, School of Medicine of University of São Paulo), Camila S. C. Duran, Carla B. R. Silva, André S. Franco, Marina B. Macedo, Henrique H. H Dalmolin, Janaina Baggio, Guilherme G. M Balbi, Caroline C. dos Santos (Rheumatology Division, School of Medicine of University of São Paulo), and Leila Antonangelo (Clinical Pathology Division, Clinical Hospital of the School of Medicine of University of São Paulo) for technical support; all of the staff members from both centers. None of these individuals received compensation for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.979667/full#supplementary-material
Supplementary Figure 1. Trial CONSORT diagram. All analyses were performed according to the patient’s randomization group using the intention-to-treat approach. There were missing data for 11.8% of patients (n = 9 in the placebo group and n = 8 in the vitamin D3 group) due to a lack of contact from the first interview at 6 months and the second interview at 1 year.
Abbreviations
BMI, body mass index; COVID-19, coronavirus disease 2019; GEE, generalized estimating equations; IBGE, Brazilian Institute of Geography and Statistics; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SF-36, 36-Item Short Form Health Survey; TNF-α, tumor necrosis factor alpha; 25(OH)D, 25-hydroxyvitamin D.
References
1. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. (2020) 26:1017–32. doi: 10.1038/s41591-020-0968-3
2. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
3. Writing Committee for the COMEBAC Study Group, Morin L, Savale L, Pham T, Colle R, Figueiredo S, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. (2021) 325:1525–34. doi: 10.1001/jama.2021.3331
4. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. (2021) 372:n136. doi: 10.1136/bmj.n136
5. Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. (2021) 9:129. doi: 10.1016/S2213-2600(21)00031-X
7. Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne). (2021) 8:653516. doi: 10.3389/fmed.2021.653516
8. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
9. National Institute for Health and Care Excellence [NICE].COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. London: National Institute for Health and Care Excellence (NICE) (2020).
10. Carfi A, Bernabei R, Landi F, GEMELLI Against Covid-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. (2020) 324:603–5. doi: 10.1001/jama.2020.12603
11. Buselli R, Corsi M, Necciari G, Pistolesi P, Baldanzi S, Chiumiento M, et al. Sudden and persistent dysphonia within the framework of COVID-19: the case report of a nurse. Brain Behav Immun Health. (2020) 9:100160. doi: 10.1016/j.bbih.2020.100160
12. Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. (2021) 4:e210369. doi: 10.1001/jamanetworkopen.2021.0369
13. Fernandez-Lazaro D, Fernandez-Lazaro CI, Mielgo-Ayuso J, Adams DP, Garcia Hernandez JL, Gonzalez-Bernal J, et al. Glycophosphopeptical AM3 food supplement: a potential adjuvant in the treatment and vaccination of SARS-CoV-2. Front Immunol. (2021) 12:698672. doi: 10.3389/fimmu.2021.698672
14. Doaei S, Gholami S, Rastgoo S, Gholamalizadeh M, Bourbour F, Bagheri SE, et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J Transl Med. (2021) 19:128. doi: 10.1186/s12967-021-02795-5
15. Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. (2021) 325:1053–60. doi: 10.1001/jama.2020.26848
16. Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. (2014) 20:341–51. doi: 10.4158/EP13265.RA
17. Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. (2012) 66:1072–4. doi: 10.1038/ejcn.2012.82
18. Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients – a randomised controlled trial. Nutr Metab Cardiovasc Dis. (2012) 22:864–70. doi: 10.1016/j.numecd.2010.11.001
19. Hussin AM, Ashor AW, Schoenmakers I, Hill T, Mathers JC, Siervo M. Effects of vitamin D supplementation on endothelial function: a systematic review and meta-analysis of randomised clinical trials. Eur J Nutr. (2017) 56:1095–104. doi: 10.1007/s00394-016-1159-3
20. Yarlagadda K, Ma N, Dore S. Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Front Neurol. (2020) 11:384. doi: 10.3389/fneur.2020.00384
21. Gupta A, Prabhakar S, Modi M, Bhadada SK, Kalaivani M, Lal V, et al. Effect of vitamin D and calcium supplementation on ischaemic stroke outcome: a randomised controlled open-label trial. Int J Clin Pract. (2016) 70:764–70. doi: 10.1111/ijcp.12866
22. Koole JL, Bours MJL, van Roekel EH, Breedveld-Peters JJL, van Duijnhoven FJB, van den Ouweland J, et al. Higher serum vitamin D concentrations are longitudinally associated with better global quality of life and less fatigue in colorectal cancer survivors up to 2 years after treatment. Cancer Epidemiol Biomarkers Prev. (2020) 29:1135–44. doi: 10.1158/1055-9965.EPI-19-1522
23. Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. (2010) 53:2112–9. doi: 10.1007/s00125-010-1838-1
24. IPetruccelli JL, Saboia AL. Características Étnico-raciais da População: Classificações e Identidades. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística (IBGE) (2013).
25. Chen KY, Li T, Gong FH, Zhang JS, Li XK. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front Psychiatry. (2020) 11:668. doi: 10.3389/fpsyt.2020.00668
26. Hazra A, Gogtay N. Biostatistics series module 9: survival analysis. Indian J Dermatol. (2017) 62:251–7. doi: 10.4103/ijd.IJD_85_17
27. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen effluvium: a review of the literature. Cureus. (2020) 12:e8320. doi: 10.7759/cureus.8320
28. Mieczkowska K, Deutsch A, Borok J, Guzman AK, Fruchter R, Patel P, et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. (2021) 60:122–4. doi: 10.1111/ijd.15313
29. Olds H, Liu J, Luk K, Lim HW, Ozog D, Rambhatla PV. Telogen effluvium associated with COVID-19 infection. Dermatol Ther. (2021) 34:e14761. doi: 10.1111/dth.14761
30. Starace M, Iorizzo M, Sechi A, Alessandrini AM, Carpanese M, Bruni F, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. (2021) 5:11–8. doi: 10.1016/j.jdin.2021.07.006
31. Rizzetto G, Diotallevi F, Campanati A, Radi G, Bianchelli T, Molinelli E, et al. Telogen effluvium related to post severe Sars-Cov-2 infection: clinical aspects and our management experience. Dermatol Ther. (2021) 34:e14547. doi: 10.1111/dth.14547
32. Abrantes TF, Artounian KA, Falsey R, Simao JCL, Vano-Galvan S, Ferreira SB, et al. Time of onset and duration of post-COVID-19 acute telogen effluvium. J Am Acad Dermatol. (2021) 85:975–6. doi: 10.1016/j.jaad.2021.07.021
33. Saini K, Mysore V. Role of vitamin D in hair loss: a short review. J Cosmet Dermatol. (2021) 20:3407–14. doi: 10.1111/jocd.14421
34. Sattar F, Almas U, Ibrahim NA, Akhtar A, Shazad MK, Akram S, et al. Efficacy of oral vitamin D3 therapy in patients suffering from diffuse hair loss (Telogen Effluvium). J Nutr Sci Vitaminol (Tokyo). (2021) 67:68–71. doi: 10.3177/jnsv.67.68
35. Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. (2020) 15:e0243882. doi: 10.1371/journal.pone.0243882
36. Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. (2020) 183:R133–47. doi: 10.1530/EJE-20-0665
37. Fernandes AL, Murai IH, Reis BZ, Sales LP, Santos MD, Pinto AJ, et al. Effect of a single high dose of Vitamin D3 on cytokines, chemokines and growth factor in patients with moderate to severe COVID-19. Am J Clin Nutr. (2022) 115:790–8. doi: 10.1093/ajcn/nqab426
38. Di Stadio A, D’Ascanio L, Vaira LA, Cantone E, De Luca P, Cingolani C, et al. Ultramicronized palmitoylethanolamide. and luteolin supplement combined with olfactory training to treat post-COVID-19 olfactory impairment: a multi-center double-blinded randomized placebo-controlled clinical trial. Curr Neuropharmacol. (2022) 20:2001–12. doi: 10.2174/1570159X20666220420113513
39. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and middle east countries and strategies to prevent vitamin D deficiency: a position statement of the European calcified tissue society. Eur J Endocrinol. (2019) 180:23–54. doi: 10.1530/EJE-18-0736
Keywords: post-viral stage, SARS-CoV-2, persistent symptoms, post-COVID-19, vitamin D, quality of life
Citation: Fernandes AL, Sales LP, Santos MD, Caparbo VF, Murai IH and Pereira RMR (2022) Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19. Front. Nutr. 9:979667. doi: 10.3389/fnut.2022.979667
Received: 27 June 2022; Accepted: 15 August 2022;
Published: 13 September 2022.
Edited by:
Laurel M. Wentz, Appalachian State University, United StatesReviewed by:
Diego Fernández Lázaro, University of Valladolid, SpainEvelyn Frias-Toral, Catholic University of Santiago de Guayaquil, Ecuador
Copyright © 2022 Fernandes, Sales, Santos, Caparbo, Murai and Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosa M. R. Pereira, rosamariarp@yahoo.com
 Alan L. Fernandes
Alan L. Fernandes Lucas P. Sales
Lucas P. Sales Mayara D. Santos
Mayara D. Santos  Igor H. Murai
Igor H. Murai Rosa M. R. Pereira
Rosa M. R. Pereira