- Laboratory of Animal Reproduction, Graduate School of Bioagricultural Sciences, Nagoya University, Nagoya, Japan
Endogenous opioid peptides have attracted attention as critical neuropeptides in the central mechanism regulating female reproduction ever since the discovery that arcuate dynorphin neurons that coexpress kisspeptin and neurokinin B (NKB), which are also known as kisspeptin/neurokinin B/dynorphin (KNDy) neurons, play a role as a master regulator of pulsatile gonadotropin-releasing hormone (GnRH) release in mammals. In this study, we first focus on the role of dynorphin released by KNDy neurons in the GnRH pulse generation. Second, we provide a historical overview of studies on endogenous opioid peptides. Third, we discuss how endogenous opioid peptides modulate tonic GnRH/gonadotropin release in female mammals as a mediator of inhibitory internal and external cues, such as ovarian steroids, nutritional status, or stress, on reproduction. Then, we discuss the role of endogenous opioid peptides in GnRH surge generation in female mammals.
Introduction
One of the most important findings on the role of endogenous opioid peptides in female reproduction over the last two decades is that dynorphin neurons in the hypothalamic arcuate nucleus (ARC) are involved in gonadotropin-releasing hormone (GnRH) pulse generation. More specifically, a majority of ARC dynorphin neurons coexpress kisspeptin and neurokinin B (NKB); thus, the neurons are also referred to as kisspeptin/neurokinin B/dynorphin (KNDy) neurons and act as master regulators of pulsatile GnRH release in mammals (Lehman et al., 2010a; Maeda et al., 2010; Okamura et al., 2013; Uenoyama et al., 2014, 2021b; Goodman et al., 2018; Moore et al., 2018; Ikegami et al., 2021; Nagae et al., 2021; Tsukamura, 2022). GnRH is intermittently secreted in the pituitary portal vessel (Clarke and Cummins, 1982; Moenter et al., 1992) and controls the tonic (pulsatile) release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior lobe of the pituitary gland. The tonic release of LH and FSH governs follicular development and corpus luteum function in the ovaries of female mammals. GnRH pulses are fundamental for reproduction in female mammals as a pioneer study demonstrated that circulating LH and FSH levels were maintained only when GnRH was applied in a pulsatile manner at physiological intervals in female rhesus monkeys after the blockade of endogenous GnRH release by a hypothalamic lesion (Belchetz et al., 1978). The neuronal circuit driving GnRH pulse generation has generally been termed the GnRH pulse generator (Lincoln et al., 1985; Maeda et al., 1995), and the intrinsic source of the generator has been a major enigma until very recently.
The present review mainly focuses on how endogenous opioid peptides regulate and/or modulate tonic GnRH/gonadotropin release, which is regulated by KNDy neurons, in female mammals. We also provide a historical overview of studies on endogenous opioid peptides and a summary of our recent understanding of the role of hypothalamic opioidergic neurons in the mechanism regulating female reproduction under normal and stressful conditions.
Kisspeptin/neurokinin B/dynorphin neurons as an intrinsic regulator of gonadotropin-releasing hormone pulses
Since the discovery of KNDy neurons, endogenous opioid peptides have attracted attention as critical neuropeptides in the central mechanism regulating female reproduction. Indeed, the discovery of KNDy neurons is one of the most exciting topics in reproductive neuroendocrinology over the last two decades. Using immunohistochemistry, Goodman and colleagues first demonstrated that dynorphin and NKB are largely coexpressed in a single population of ARC neurons in ewes (Foradori et al., 2006) and then revealed that kisspeptin is also expressed in the majority of the same neuronal population (Goodman et al., 2007). Importantly, Goodman and colleagues reported that none of the dynorphin neuronal populations located in the other hypothalamic regions, such as the paraventricular nucleus (PVN), supraoptic nucleus (SON), and preoptic area (POA), colocalized with NKB (Foradori et al., 2006) and kisspeptin (Goodman et al., 2007). Immediately thereafter, the coexpression of dynorphin, NKB, and kisspeptin in a population of ARC neurons was validated in several mammals, including goats (Wakabayashi et al., 2010), heifers (Hassaneen et al., 2016), rats (True et al., 2011; Murakawa et al., 2016), mice (Navarro et al., 2009; Ikegami et al., 2017), pigs (Harlow et al., 2021), and rhesus monkeys (Ramaswamy et al., 2010; True et al., 2017), as summarized in our recent article (Uenoyama et al., 2021b). These findings implied the physiological importance of KNDy neurons for mammalian reproduction beyond the species, although colocalization of dynorphin in ARC kisspeptin/NKB neurons was not evident yet in humans (Hrabovszky et al., 2012, 2019).
Importantly, dynorphin receptors (i.e., κ-opioid receptors; KORs) were found in a majority of rat and ovine KNDy neurons (Weems et al., 2016; Tsuchida et al., 2020) and a portion of KNDy neurons in female mice (Navarro et al., 2009; Ikegami et al., 2017). In addition, the NKB receptors (also known as NK3R) were found in a majority of rodent and ovine KNDy neurons (Navarro et al., 2009; Amstalden et al., 2010; Ikegami et al., 2017). On the other hand, kisspeptin receptors (also known as GPR54) were found in the majority of GnRH neurons and were scarcely found in KNDy neurons of mice and rats (Herbison et al., 2010; Higo et al., 2016). These findings suggest that KNDy neurons communicate with each other by dynorphin-KOR and NKB-NK3R signaling in an autocrine/paracrine manner. As shown in Figure 1, the most plausible interpretation of the cellular mechanism regulating synchronized KNDy neuronal activity to drive GnRH pulses is as follows: we envisage that dynorphin released from KNDy neurons arrests KNDy neuronal activity via the inhibitory Gi/o-coupled KOR, NKB initiates synchronized KNDy neuronal activity via stimulatory Gq-coupled NK3R to release kisspeptin, and kisspeptin, in turn, stimulates GnRH release via stimulatory Gq-coupled GPR54 expressed in GnRH neurons (Navarro et al., 2009; Lehman et al., 2010a,b; Okamura et al., 2013; Uenoyama et al., 2014, 2021b; Goodman et al., 2018; Moore et al., 2018; Ikegami et al., 2021). Indeed, in female goats, the frequency of multiple unit activity (MUA) volleys, which were recorded in the vicinity of ARC KNDy neurons and accompanied by LH pulses, was decreased by the central administration of dynorphin and increased by the administration of a KOR antagonist (nor-binaltorphimine; nor-BNI) or NKB (Ohkura et al., 2009; Wakabayashi et al., 2010). These findings suggest that dynorphin-KOR signaling and NKB-NK3R signaling play a role in determining the frequency of GnRH pulse generator activities. Furthermore, central or peripheral administration of dynorphin or NK3R antagonists (SB223412 and SB222200) suppressed LH pulses, whereas KOR antagonists (nor-BNI and PF-4455242); NKB, an NK3R agonist (senktide); and kisspeptin stimulated LH pulses in several mammalian species, such as rodents (Gottsch et al., 2004; Irwig et al., 2004; Kinoshita et al., 2005; Messager et al., 2005; Pheng et al., 2009; Navarro et al., 2011; Mostari et al., 2013; Ruiz-Pino et al., 2015) and ruminants (Messager et al., 2005; Ohkura et al., 2009; Sakamoto et al., 2012; Tanaka et al., 2012; Goodman et al., 2013; Naniwa et al., 2013; Yamamura et al., 2015; Nakamura et al., 2017; Sasaki et al., 2019, 2020).
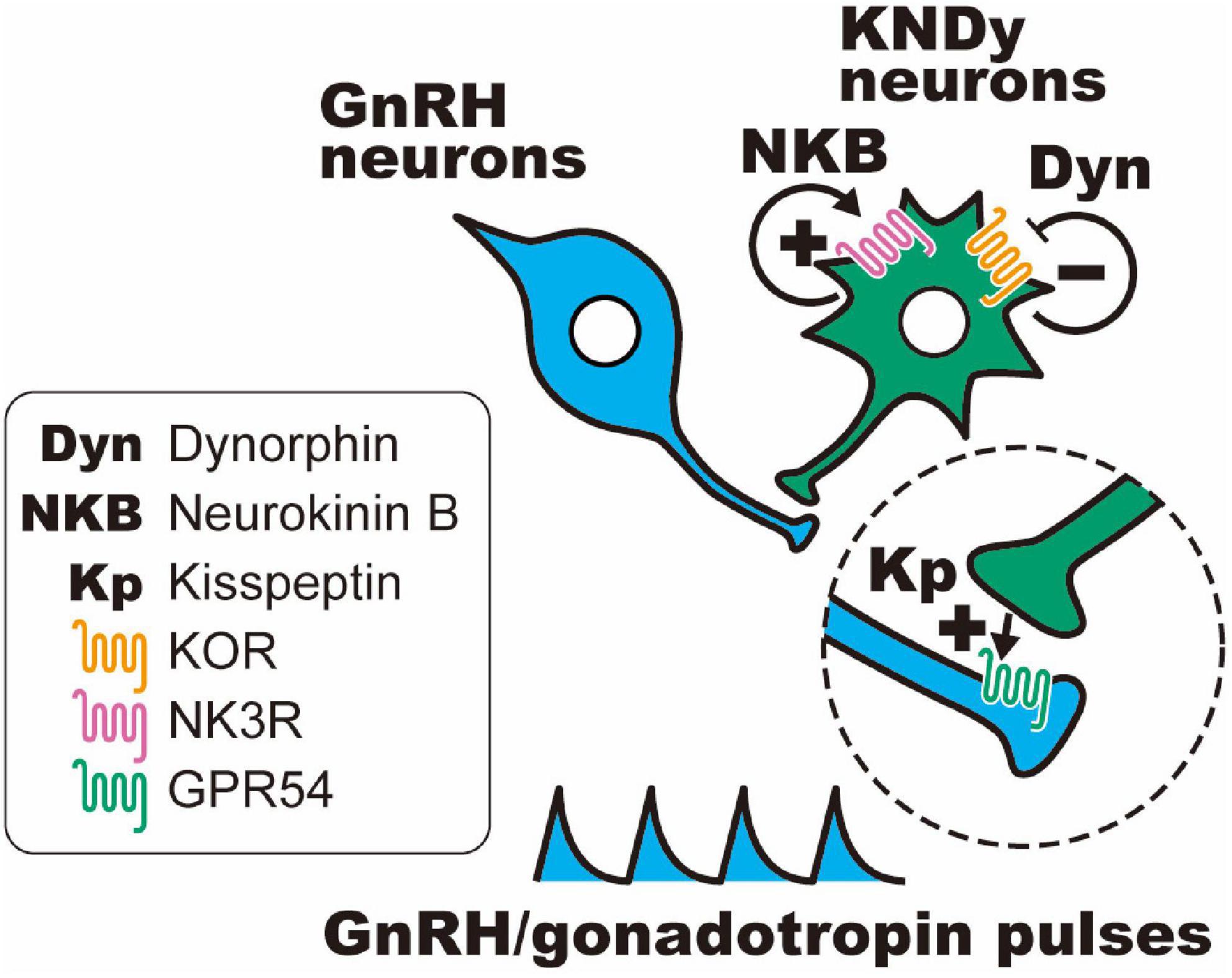
Figure 1. Schematic illustration of the hypothetical mechanism of gonadotropin-releasing hormone (GnRH) pulse generation in female mammals. Dynorphin (Dyn) released from KNDy neurons arrests KNDy neuronal activity via inhibitory Gi/o-coupled κ-opioid receptors (KORs), and neurokinin B (NKB) initiates synchronized KNDy neuronal activity via stimulatory Gq-coupled NKB receptors (also known as NK3R) to release kisspeptin, and kisspeptin, in turn, stimulates GnRH release via stimulatory Gq-coupled kisspeptin receptors (also known as GPR54) expressed in GnRH neurons.
Recently, we rescued Kiss1 (which is the gene that encodes kisspeptin) expression in ARC dynorphin/NKB neurons in global Kiss1-knockout rats utilizing adeno-associated virus (AAV) vectors carrying Kiss1 cDNA (Nagae et al., 2021). Rescuing Kiss1 expression in 20–50% of ARC NKB neurons could recover pulsatile LH release and folliculogenesis up to the preovulatory follicles in global Kiss1-knockout female rats. These findings provide direct evidence that ARC KNDy neurons serve as an intrinsic source of the GnRH pulse generator in female mammals.
Brief history of studies on endogenous opioid peptides
Endogenous opioid peptides were found to be endogenous substances that produce the same analgesic effect as morphine, an opiate alkaloid derived from opium poppies (Brownstein, 1993; Snyder and Pasternak, 2003; Waldhoer et al., 2004; Gruber et al., 2007; Przewlocki, 2013; Devereaux et al., 2018). Opiate alkaloids have a long history of medicinal use since the time of ancient Greeks and Romans (Brownstein, 1993; Waldhoer et al., 2004; Gruber et al., 2007; Devereaux et al., 2018), and the active ingredient morphine was isolated in the middle of the 1800s (Devereaux et al., 2018). Morphine was introduced for pain treatment in the 1820s (Przewlocki, 2013; Devereaux et al., 2018), and then morphine, like original opiate alkaloids, was found to be an addictive drug (Brownstein, 1993; Przewlocki, 2013). In search of a safe analgesic, many opiate agonists and antagonists were developed (Brownstein, 1993; Gruber et al., 2007), and by the middle of the 1960s, it was becoming clear that the analgesic effect of morphine and opiate agonists could be explained by the presence of specific receptors for the opiates in the brain (Snyder and Pasternak, 2003; Devereaux et al., 2018). In 1973, a radioreceptor assay with tritium-labeled and non-labeled opiate agonists or antagonists (Table 1) revealed the stereospecific binding of opiates, namely, opiate or morphine receptors, in rat brain homogenates (Pert and Snyder, 1973; Simon et al., 1973; Terenius, 1973). These findings implied the presence of endogenous opioidergic ligand(s) as neurotransmitters in the central nervous systems of mammals. In 1975, two pentapeptides, Tyr-Gly-Gly-Phe-Met (termed Met-enkephalin) and Tyr-Gly-Gly-Phe-Leu (termed Leu-enkephalin), were found in the pig brain as endogenous ligands for opiate or morphine receptors (Hughes et al., 1975a,b). It soon became obvious that the Met-enkephalin sequence was present on the N terminus of another endogenous opioid peptide, that is, β-endorphin, in 1976 (Birdsall and Hulme, 1976; Li and Chung, 1976). Subsequently, the Leu-enkephalin sequence was found at the N terminus of another endogenous opioid peptide, dynorphin, in 1979 (Goldstein et al., 1979, 1981). To date, these endogenous opioid peptides have been classified into three families and were reported to be derived from three distinct precursors encoded by Pomc, Penk, and Pdyn genes (Nakanishi et al., 1979; Kakidani et al., 1982; Noda et al., 1982; Akil et al., 1984; Froehlich, 1997; Benarroch, 2012). Figure 2 shows three precursors—preproopiomelanocortin, preproenkephalin, and preprodynorphin—of endogenous opioid peptides, such as β-endorphin, Met- and Leu-enkephalins, and the dynorphin family [dynorphin A, and α- and β-neoendorphins, leumorphin, and rimorphin (also known as dynorphin B)], respectively, in humans and rats.

Figure 2. Schematic illustration of β-endorphin, Met- and Leu-enkephalins, and the dynorphin family (dynorphin A, α- and β-neoendorphins, leumorphin, and rimorphin) in their precursors in humans and rats based on UniProtKB (https://www.uniprot.org/uniprot/). The precursors comprise a signal peptide at the N-terminal. (A) β-Endorphin consists of 31 amino acids cleaved from the precursor preproopiomelanocortin in humans and rats. Note that the five N-terminal amino acids (YGGMF, yellow squares) of β-endorphin, identical to Met-enkephalin, are commonly found in the mammals examined. (B) Met- (YGGMF, yellow squares) and Leu-enkephalins (YGGML, orange squares) consist of five amino acids cleaved from the precursor preproenkephalin. Note that human and rat preproenkephalin possess six Met-enkephalin and one Leu-enkephalin motifs, and two of six Met-enkephalin motifs are processed to eight or seven amino acid peptides (Met-enkephalin-Arg-Gly-Leu and Met-enkephalin-Arg-Phe). (C) Dynorphin A, α- and β-neoendorphins, leumorphin, and rimorphin (also known as dynorphin B) consist of 8–28 amino acids cleaved from the single precursor preprodynorphin. Note that the five N-terminal amino acids (YGGML, orange squares) of all dynorphin family peptides are identical to Leu-enkephalin. The amino acid sequence of dynorphin A is identical among the mammals examined.
It is well known that β-endorphin, Met- and Leu-enkephalins, and the dynorphin family share three types of opioid receptors—μ-, δ-, and κ-opioid receptors (MOR, DOR, and KOR)—encoded by Oprm1, Oprd1, and Oprk1 genes, respectively (Snyder and Pasternak, 2003; Waldhoer et al., 2004; Stein, 2016). As shown in Table 2, β-endorphin has been reported to predominantly bind to both MOR and DOR with a similar affinity and with a lower affinity for KOR; Met- and Leu-enkephalins predominantly bind to the DOR with much higher affinity than the MOR and KOR; all dynorphin family peptides predominantly bind to the KOR, rather than the MOR and DOR (Yasuda et al., 1993; Raynor et al., 1994; Mansour et al., 1995b). We should note that morphine was reported to predominantly bind to the MOR, followed by the KOR, with a low affinity for the DOR (Mansour et al., 1995b). These opioid receptors were cloned in rats and mice during the early 1990s (Evans et al., 1992; Kieffer et al., 1992; Chen et al., 1993a,b; Fukuda et al., 1993; Li et al., 1993; Minami et al., 1993; Nishi et al., 1993; Thompson et al., 1993) and were found to belong to the large superfamily of seven-transmembrane G protein-coupled receptors. After the binding of an agonist, conformational changes of all three opioid receptors predominantly allow intracellular coupling of a heterotrimeric Gi/o protein (Connor and Christie, 1999; Waldhoer et al., 2004; Stein, 2016). Therefore, opioid receptor activation leads to inhibited adenylyl cyclase activity and reduced cAMP levels in target neurons (Connor and Christie, 1999; Waldhoer et al., 2004; Stein, 2016). In addition, opioid receptor activation leads to the opening of G protein-coupled inwardly rectifying K+ channels, thereby preventing neuronal excitation and/or propagation of action potentials of target neurons (Connor and Christie, 1999; Waldhoer et al., 2004; Stein, 2016). From these findings, it is well accepted that endogenous opioid peptides serve as inhibitory signals in the central nervous system via inhibitory Gi/o-coupled opioid receptors in mammals.
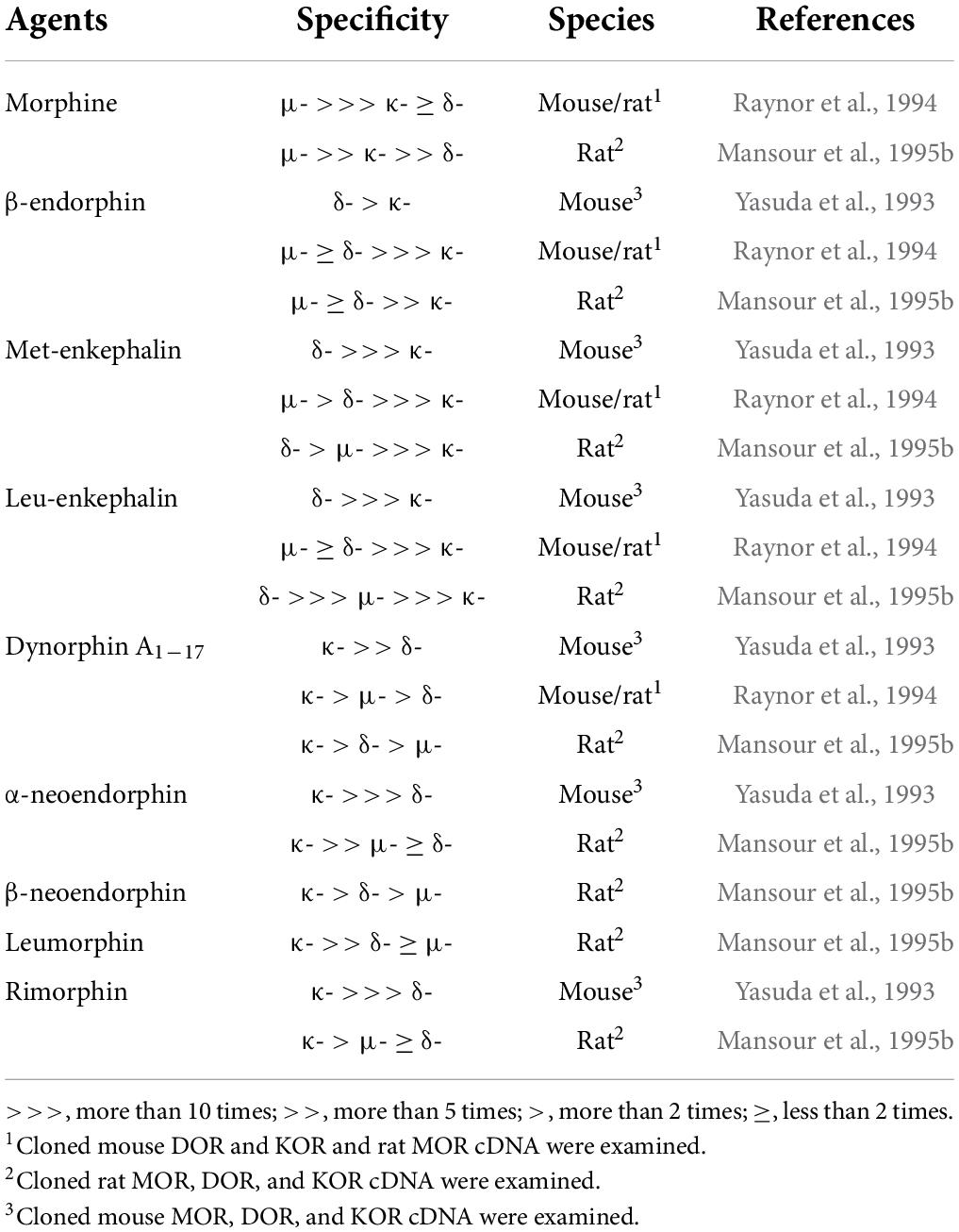
Table 2. Binding affinity and specificity of morphine and endogenous opioid peptides to opioid receptors.
Inhibitory roles of endogenous opioid peptides on tonic gonadotropin-releasing hormone/gonadotropin-releasing systems
Immediately after the isolation and characterization of the endogenous opioid peptides, the inhibitory effect of endogenous opioid peptides on pulsatile GnRH/gonadotropin release was intensively studied using the opioid receptor antagonist naloxone as a probe. As mentioned later in detail, peripheral and central administration of naloxone or other opioid antagonists facilitated tonic (pulsatile) LH release in female mammals at several stages of the reproductive cycle (Table 3) and under stressful conditions such as malnutrition and infection (Table 4). Thus, we envision that opioidergic neurons serve as mediators of inhibitory internal and external cues, such as ovarian steroids, nutritional status, or stress, on tonic GnRH/gonadotropin release in female mammals.
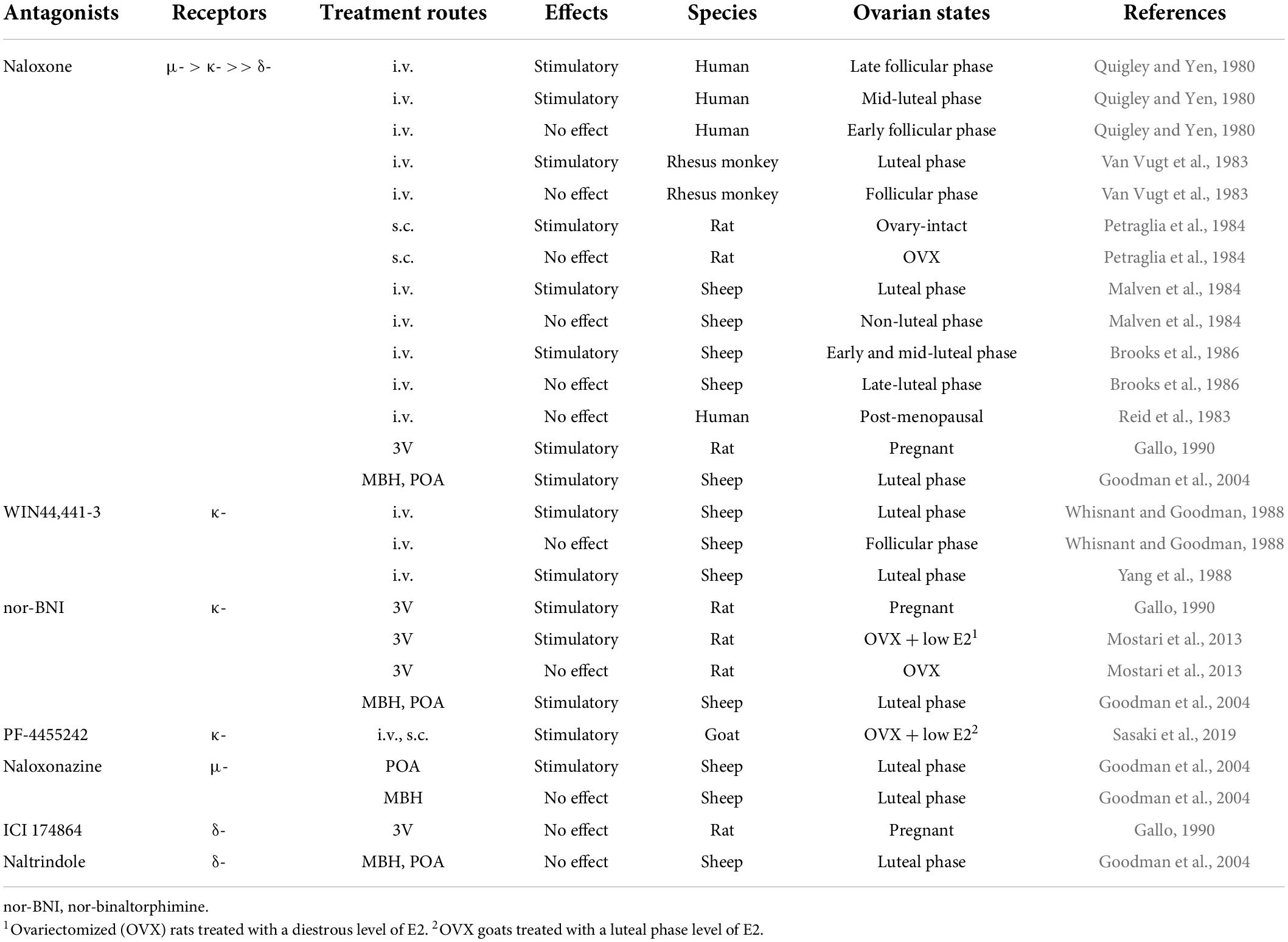
Table 3. Effects of opioid receptor antagonists on tonic luteinizing hormone (LH) secretion in female mammals.
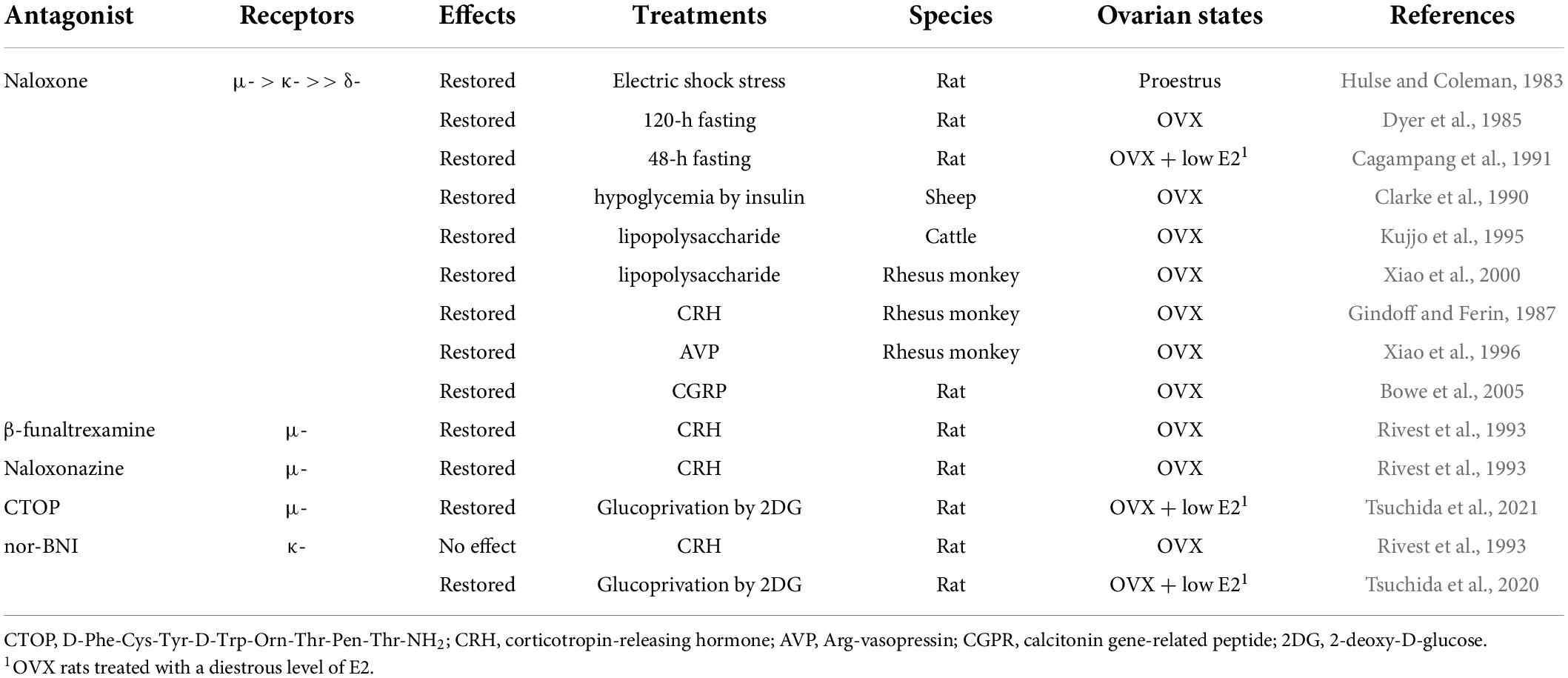
Table 4. Effects of opioid receptor antagonists on tonic luteinizing hormone (LH) secretion in female mammals under stressful conditions.
Involvement of endogenous opioid peptides in mediating the negative feedback action of ovarian steroids on tonic gonadotropin-releasing hormone/gonadotropin release
It is well established that the frequency of GnRH/gonadotropin pulses is fine-tuned by the negative feedback action of ovarian steroids such as estradiol-17β (E2) and progesterone (P4) to keep circulating LH and FSH at proper levels to promote follicular development in the follicular phase of the estrous/menstrual cycle and maintain corpus luteum function in the luteal phase and pregnancy period (Nishihara et al., 1999; Herbison, 2020; Uenoyama et al., 2021a). Endogenous opioid peptides are suggested to be mediators of the negative feedback action of gonadal steroids on tonic GnRH/gonadotropin release in female mammals (summarized in Table 3). An intravenous (IV) injection of naloxone increased plasma LH levels during the late follicular (E2 dominant) and mid-luteal (P4 dominant) phases of the menstrual cycle, but not during the early follicular phase, in humans (Quigley and Yen, 1980). In addition, an IV injection of naloxone increased serum LH levels during the luteal phase, but not the follicular phase, in rhesus monkeys (Van Vugt et al., 1983). Likewise, subcutaneous (SC) injection of naloxone increased plasma LH levels in ovary-intact rats (Petraglia et al., 1984). An IV injection of naloxone stimulated LH secretion during the luteal phase, but not during the non-luteal phase, in ewes (Malven et al., 1984; Brooks et al., 1986). Furthermore, it has been noted that IV or SC administration of naloxone was unable to increase plasma LH levels in post-menopausal women (Reid et al., 1983) and ovariectomized (OVX) rats (Petraglia et al., 1984). In addition, naloxone administration into the third cerebroventricle (3V) facilitated LH pulses in rats during pregnancy (Gallo, 1990). These findings suggest that endogenous opioid peptides mediate the negative feedback action of E2 and P4 on pulsatile GnRH/LH release in female mammals. Furthermore, the local implant of crystalline naloxone into the mediobasal hypothalamus (MBH) or POA facilitated pulsatile LH release during the luteal phase in ewes (Goodman et al., 2004), suggesting that the MBH and POA, in which KNDy and GnRH neurons were found, respectively, in ewes (Lehman et al., 1986, 2010b; Goodman et al., 2007), could be possible action sites of endogenous opioid peptides to exert the negative feedback action of ovarian steroids on tonic GnRH/gonadotropin release. The expression of opioid receptors in KNDy and GnRH neurons will be discussed later.
Both KOR and MOR signaling are considered to mediate the negative feedback action of ovarian steroids on GnRH/gonadotropin release in female mammals. An IV injection of WIN44,441-3 (a specific KOR antagonist) facilitated LH pulses during the luteal phase of the estrous cycle (Whisnant and Goodman, 1988; Yang et al., 1988) but failed to facilitate LH pulses during the follicular phase in ewes (Whisnant and Goodman, 1988). Likewise, a 3V injection of nor-BNI (another KOR antagonist), but not ICI 174864 (a specific DOR antagonist), facilitated LH pulses in pregnant rats (Gallo, 1990). Our previous study showed that a 3V injection of nor-BNI stimulated the baseline levels of LH pulses in OVX rats treated with a diestrous level of E2, but not in OVX rats (Mostari et al., 2013). In addition, IV and SC injections of PF-4455242 (another KOR antagonist) facilitated LH pulses in OVX goats treated with a luteal phase level of E2 (Sasaki et al., 2019). Furthermore, the local implant of crystalline nor-BNI into the MBH or POA and the local implant of crystalline naloxonazine (a specific MOR antagonist) in the POA facilitated pulsatile LH release during the luteal phase in ewes (Goodman et al., 2004). By contrast, the local implant of crystalline naltrindole (a specific DOR antagonist) failed to facilitate pulsatile LH release during the luteal phase in ewes (Goodman et al., 2004). These results are consistent with the finding that naloxone was reported to predominantly bind to the MOR, followed by the KOR, with a low affinity for the DOR (Mansour et al., 1995b). Taken together, these findings suggest that endogenous opioid peptides may mediate the negative feedback action of ovarian steroids via KOR signaling in the MBH and KOR and MOR signaling in the POA in female mammals.
Involvement of endogenous opioid peptides in mediating stress-induced suppression of tonic gonadotropin-releasing hormone/gonadotropin release
The frequency of GnRH/LH pulses is often suppressed under stressful conditions, such as malnutrition and infection (Chatterton, 1990; Tilbrook et al., 2000, 2002). Endogenous opioid peptides have attracted attention as mediators of the stress-induced suppression of GnRH/gonadotropin release in female mammals (summarized in Table 4). Previous studies have demonstrated that peripheral administration of naloxone blocks stress-induced LH suppression in several female mammals (Hulse and Coleman, 1983; Dyer et al., 1985; Clarke et al., 1990; Cagampang et al., 1991; Kujjo et al., 1995; Xiao et al., 2000). Concretely, an IV injection of naloxone blocked electric shock stress-induced LH suppression in proestrous female rats (Hulse and Coleman, 1983). Subcutaneous injections of naloxone blocked 48-h fasting-induced LH suppression in ovary-intact (Dyer et al., 1985) and OVX rats treated with a diestrous level of E2 (Cagampang et al., 1991). Our recent studies showed that the 3V administration of D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP, another MOR antagonist) or nor-BNI restored suppression of LH pulses induced by peripheral or central injection of 2-deoxy-D-glucose (2DG, an inhibitor of glucose utilization) in OVX rats treated with a diestrus level of E2 (Tsuchida et al., 2020, 2021). Furthermore, IV administration of naloxone restored LH pulses that were suppressed by insulin-induced hypoglycemia in OVX ewes (Clarke et al., 1990), and IV injections of naloxone restored LH pulses that were suppressed by the administration of an endotoxin lipopolysaccharide in OVX heifers (Kujjo et al., 1995) and OVX rhesus monkeys (Xiao et al., 2000). Taken together, these findings suggest that endogenous opioid peptides mediate acute stress-induced suppression of GnRH/LH pulses under stressful conditions, such as malnutrition and infection, in female mammals.
It is well known that the stress response is mainly driven by the hypothalamic–pituitary–adrenal axis in mammals (Brooks and Challis, 1989; Senn et al., 1995; Bale and Vale, 2004; Papadimitriou and Priftis, 2009). Accumulating evidence has demonstrated that both corticotropin-releasing hormone (CRH) and Arg-vasopressin (AVP) neurons located in the PVN govern pituitary corticotrophin release and adrenal functions in response to various stressors (Brooks and Challis, 1989; Senn et al., 1995; Bale and Vale, 2004; Papadimitriou and Priftis, 2009). Thus, administration of CRH and AVP has been used to mimic stressful conditions to determine the role of opioids as mediators. As shown in Table 4, IV and lateral ventricle (LV) administration of naloxone restored CRH- and AVP-induced suppression of the frequency of LH pulses in OVX rhesus monkeys, respectively (Gindoff and Ferin, 1987; Xiao et al., 1996). These findings suggest that opioidergic signaling may mediate CRH/AVP-induced suppression of tonic GnRH/gonadotropin release in female mammals. Specifically, administration of β-funaltrexamine and naloxonazine (specific MOR antagonists), but not nor-BNI (a KOR antagonist), into the POA partially restored CRH-induced LH suppression in OVX rats (Rivest et al., 1993), suggesting that MOR signaling mainly mediates the suppression. In addition, O’Byrne and colleagues (Bowe et al., 2005) demonstrated that the LV injection of naloxone restored LH pulses that were suppressed by LV administration of calcitonin gene-related peptide, another mediator of stress-induced LH suppression (Li et al., 2004), in OVX rats.
Involvement of endogenous opioid peptides in mediating pre-pubertal restraints of tonic gonadotropin-releasing hormone/gonadotropin release
It has been established that pre-pubertal restraints of GnRH/gonadotropin pulses are tightly associated with the negative feedback action of estrogen in rats and sheep (Foster and Ryan, 1979; Uenoyama et al., 2019). Endogenous opioid peptides are likely to mediate the estrogen-dependent pre-pubertal restraint of tonic GnRH/gonadotropin release in female mammals (summarized in Table 5). Ieiri et al. (1980) showed that SC administration of naloxone increased serum LH levels in pre-pubertal female rats. Ebling et al. (1989) suggested that endogenous opioidergic signaling mediates the estrogen-negative feedback action on pre-pubertal restraints of GnRH/gonadotropin pulses in lambs because IV administration of naloxone stimulated LH pulses in ovary-intact and E2-treated pre-pubertal OVX lambs. Ebling et al. (1989) also reported that naloxone was able to further increase the frequency of LH pulses shown in OVX pre-pubertal lambs in this study. Similarly, Wood et al. (1992) showed that naloxone was able to stimulate LH pulses in OVX pre-pubertal lambs in an estrogen-dependent manner. Furthermore, Nakahara et al. (2013) showed that chronic intraperitoneal infusion of nor-BNI increased LH pulses and hence advanced puberty onset in ovary-intact female rats. Similarly, Lopez et al. (2016) showed that LV infusion of non-BNI stimulated LH pulses in pre-pubertal E2-treated OVX lambs. Taken together, these findings suggest that central opioidergic signaling, at least KOR signaling, mediates the estrogen-dependent restraint of GnRH/gonadotropin pulses during the pre-pubertal period and may serve as a key determinant of puberty onset, at least in rats and sheep. It should be noted that Lopez et al. (2016) also showed that E2 replacement failed to increase dynorphin immunoreactivity in the ARC of pre-pubertal lambs, although P4 replacement increased dynorphin immunoreactivity in the ARC of post-pubertal female sheep. Thus, non-ARC dynorphin neurons may play a key role in the pre-pubertal restraint of GnRH/gonadotropin pulses in female sheep.
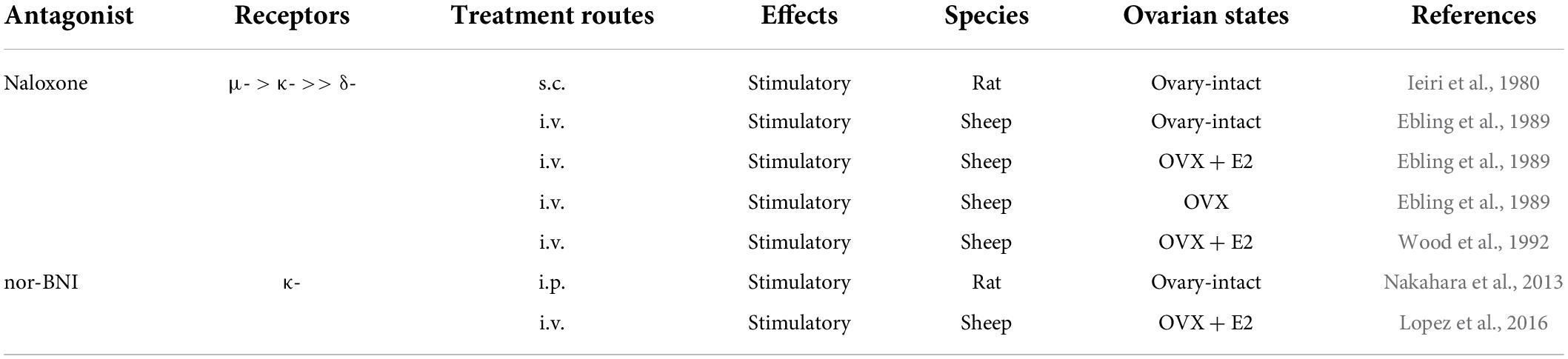
Table 5. Effects of opioid receptor antagonists on tonic luteinizing hormone (LH) secretion in pre-pubertal female mammals.
It has also been established that puberty onset is associated with body growth in mammals. Indeed, growth retardation resulted in delayed puberty onset in rats and sheep (Foster and Olster, 1985; Bronson, 1986; Majarune et al., 2019). Our previous study showed that chronic food restriction (negative energy balance) during the pre-pubertal phase caused suppression of ARC Pdyn and Kiss1 expression and subsequent pubertal failure in growth-retarded female rats and that ad libitum feeding (positive energy cues) caused an acute increase in the number of Pdyn- and Kiss1-expressing cells in the ARC, triggering puberty onset in growth-retarded female rats (Majarune et al., 2019). Similarly, Aerts et al. (2021) showed pubertal increases in Pdyn and Kiss1, but not Tac3, expression in the ARC of lambs. These findings suggest that dynorphin-KOR signaling and Kiss1 (as components of KNDy neurons) serve as critical regulators of GnRH pulse generation at the onset of puberty in female mammals. The completion of KNDy mRNA and peptide expression at puberty onset is likely a prerequisite. On the other hand, there might be species differences in pubertal changes in KNDy mRNA and peptide expression: Harlow et al. (2022) demonstrated that OVX lambs with all three KNDy mRNA and peptide expression showed apparent LH pulses, whereas OVX lambs under food restriction showed suppression of Kiss1/kisspeptin and NKB, but not Tac3 and Pdyn/dynorphin, expression and the suppression of LH pulses. It should also be noted that our and other previous studies showed that ARC Kiss1 expression was found even in neonates and did not alter peripubertal female pigs (Ieda et al., 2014; Harlow et al., 2021). Interestingly, Ebling et al. (1990) showed that IV administration of naloxone failed to affect pre-pubertal restraints of LH secretion in growth-retarded OVX lambs. Given that endogenous opioid peptides may mediate estrogen-dependent pre-pubertal suppression of GnRH/LH pulses in lambs, this finding suggests that inhibitory input(s), other than endogenous opioid peptides, may mainly mediate such steroid-independent inhibition of GnRH/LH secretion in pre-pubertal lambs under chronic malnutrition conditions.
Candidate populations of opioidergic neurons inhibiting tonic gonadotropin-releasing hormone/gonadotropin release
It is likely that dynorphin neurons in multiple hypothalamic nuclei—such as POA, anterior hypothalamus (AHA), and PVN—and β-endorphin neurons (also known as proopiomelanocortin neurons) located in the ARC serve as mediators of the inhibitory effect of ovarian steroids and/or stressors on GnRH/gonadotropin release in female mammals.
Foradori et al. (2005) showed that ovariectomy decreased the number of Pdyn-expressing neurons in the POA, AHA, and ARC compared to that in ewes at the luteal phase of the estrous cycle. The study also showed that P4 replacement restored the number of Pdyn-expressing cells in the POA and AHA, but not the ARC, to the level noticed in ewes at the luteal phase (Foradori et al., 2005). Our recent study showed that a systemic E2 implant that mimicked the diestrous stage significantly increased Pdyn-expressing cells in the PVN of OVX rats compared to OVX rats without E2 replacement (Tsuchida et al., 2020). Such a stimulatory effect of E2 on Pdyn mRNA expression was not found in the ARC and SON, in which dynorphin neurons were also abundantly found in female rats (Kanaya et al., 2017; Tsuchida et al., 2020). Taken together, these results suggest that POA, AHA, and/or PVN dynorphin neurons may mediate the negative feedback action of ovarian steroids on pulsatile GnRH/gonadotropin release in female mammals. It is likely that P4 directly activates Pdyn mRNA expression in the POA and AHA because previous studies using in situ hybridization or immunohistochemistry revealed that the majority of dynorphin neurons in the POA and AHA expressed nuclear progesterone receptors (PR) in ewes (Foradori et al., 2002). In addition, the majority of ARC dynorphin (KNDy) neurons expressed PR and estrogen receptor α (ERα) in ewes (Foradori et al., 2002; Franceschini et al., 2006; Smith et al., 2007) and ERα in rodents (Kinoshita et al., 2005; Smith et al., 2005; Adachi et al., 2007). It is still unclear whether PVN dynorphin neurons express ERα in rats.
Palkovits (2000) demonstrated that several stressors, such as immobilization and formalin injection, induced Pdyn expression in the PVN, and immobilization stress induced dynorphin-immunoreactivity in the SON of female rats. Our previous study showed that glucoprivation induced by central and peripheral injection of 2DG increased the number of activated (fos-positive) dynorphin neurons in the PVN in OVX rats treated with a diestrous level of E2 (Tsuchida et al., 2020). Thus, it might be possible that PVN and/or SON dynorphin neurons likely mediate the suppression of pulsatile GnRH/gonadotropin release induced by stress or malnutrition in female mammals.
Interestingly, both fasting and glucoprivation suppressed LH pulses in female rats in an estrogen-dependent manner (Cagampang et al., 1991; Nagatani et al., 1996). Our previous studies showed that 48-h fasting induced de novo ERα expression in the PVN (Estacio et al., 1996) and that the local E2 implant into the PVN is needed for the fasting-induced suppression of LH in OVX rats (Nagatani et al., 1994). Thus, it is tempting to speculate that PVN dynorphin neurons may integrate ovarian steroid-negative feedback and stressor-induced signals to suppress GnRH/gonadotropin pulses, although the detailed phenotype of PVN ERα-expressing cells is currently unknown.
Whisnant et al. (1992) and Broad et al. (1993) showed that both E2 and P4 increased Pomc mRNA levels in the ARC of OVX ewes, suggesting that ARC β-endorphin neurons may mediate the negative feedback action of ovarian steroids on pulsatile GnRH/gonadotropin release at least in sheep. On the other hand, Wilcox and Roberts (1985) showed that E2 decreased Pomc mRNA levels in the ARC of OVX rats, indicating that there is a potential species difference in the regulation of Pomc mRNA expression by ovarian steroids. Little is known about stress-induced Pomc mRNA upregulation in female rodents, while fasting increased β-endorphin release from the hypothalamic explant of male rats (Mitev et al., 1993). Thus, further studies are needed to clarify how ARC β-endorphin neurons mediate the inhibitory effect of ovarian steroids and/or stressors on tonic GnRH/gonadotropin release in female rodents.
Possible action sites of endogenous opioid peptides to inhibit tonic gonadotropin-releasing hormone/gonadotropin release
Receptors for endogenous opioid peptides are widely distributed in the brain of rodents (Mansour et al., 1988, 1993, 1994, 1995a; Desjardins et al., 1990; George et al., 1994). The receptor distribution was initially examined by autoradiography (at the brain nucleus level) and later examined by in situ hybridization (at the cell body level) and immunohistochemistry (at the cell body and fiber levels) (Mansour et al., 1995a). The difference between the localization of binding sites (detected by autoradiography) and mRNA expression (detected by in situ hybridization) could be explained by receptor transportation from the cell bodies to the axon terminals. It was reported that the MOR and KOR are widely distributed throughout the hypothalamus, whereas the DOR is scarcely distributed in the hypothalamus in rodents (Mansour et al., 1993, 1994, 1995a; George et al., 1994). Importantly, the distribution of opioid receptors are largely consistent between rodents and humans: the MOR and KOR mRNA are widely expressed, and DOR mRNA is rarely expressed in the human hypothalamus (Peckys and Landwehrmeyer, 1999).
Table 6 shows opioid receptor expression in GnRH neurons and KNDy neurons, which are considered a core component of the GnRH pulse generator, in female mammals (Lehman et al., 2010a; Okamura et al., 2013; Uenoyama et al., 2014, 2021b; Goodman et al., 2018; Moore et al., 2018; Ikegami et al., 2021). Immunohistochemical analysis revealed that the KOR is expressed in a large majority of ovine KNDy neurons and ovine and rat GnRH neurons (Lopez et al., 2016; Weems et al., 2016). In addition, in situ hybridization analyses revealed that KOR mRNA is expressed in the majority of ARC kisspeptin neurons in female rats (Tsuchida et al., 2020) and less than half of the ARC kisspeptin neurons in female mice (Navarro et al., 2009). Likewise, our quantitative RT-PCR analysis showed that KOR mRNA expression was detected in two of six pools of KNDy neurons (each pool consists of 10 green fluorescent protein-labeled kisspeptin cells) in female mice (Ikegami et al., 2017). On the other hand, previous in situ hybridization analyses revealed little MOR mRNA expression in both KNDy and GnRH neurons in female rats (Mitchell et al., 1997; Sannella and Petersen, 1997; Tsuchida et al., 2021), while MOR mRNA expression was observed in one-third of GnRH-immunoreactive cells in female guinea pigs (Zheng et al., 2005). MOR mRNA expression was found in a number of ARC non-KNDy and POA non-GnRH neurons in female rats (Mitchell et al., 1997; Sannella and Petersen, 1997; Tsuchida et al., 2021). Taken together, these findings suggest that dynorphin-KOR signaling in the majority of KNDy and GnRH neurons may mediate the negative feedback action of ovarian steroids and stress-induced suppression of tonic GnRH/gonadotropin release in female mammals (Figure 3). In addition, inhibitory β-endorphin-MOR signaling on interneurons may somehow transmit to KNDy and GnRH neurons to suppress tonic GnRH/gonadotropin release (Figure 3). To date, studies on DOR expression in KNDy or GnRH neurons are limited, while a previous study reported no DOR mRNA expression in GnRH neurons in female rats under various steroid milieus (Sannella and Petersen, 1997).
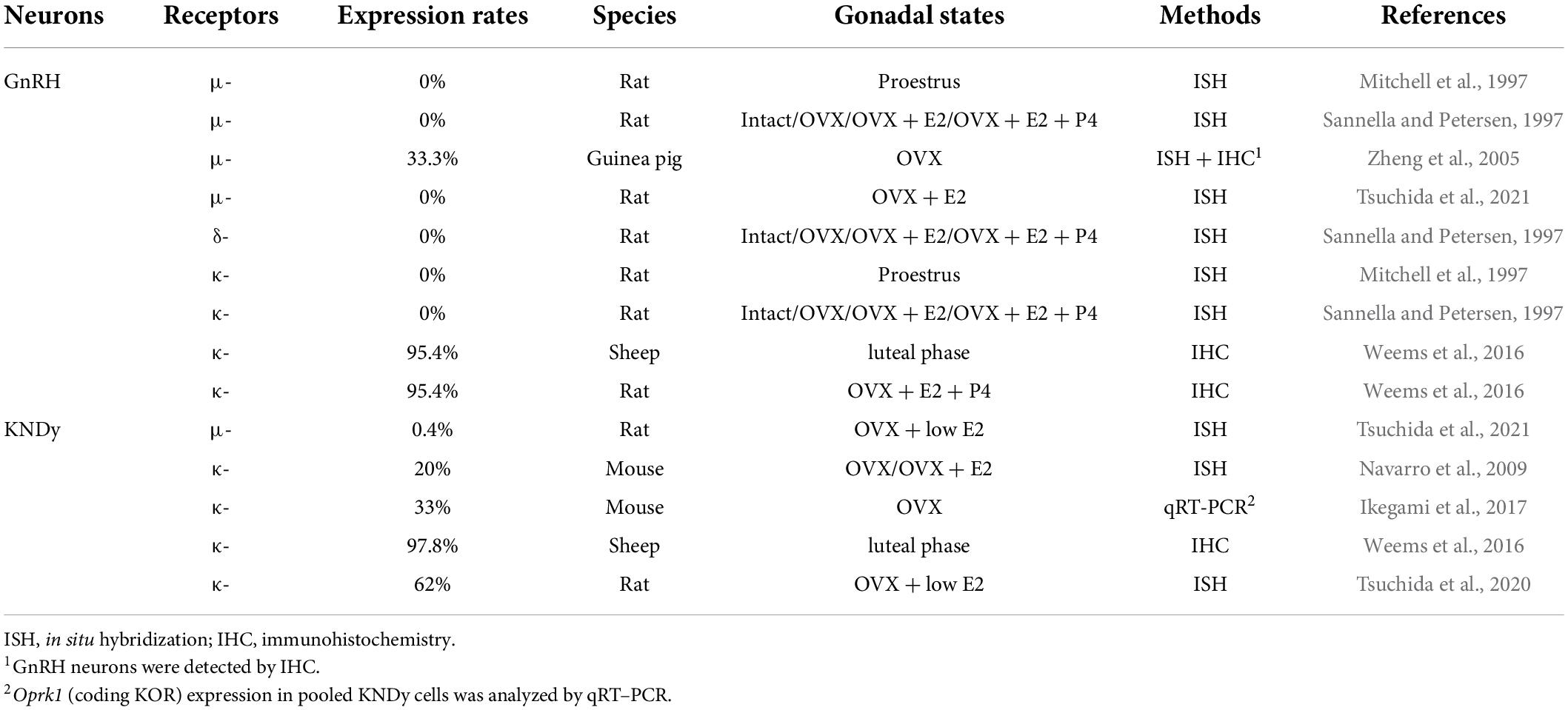
Table 6. Expression of opioid receptor mRNAs in gonadotropin-releasing hormone (GnRH) and KNDy neurons in female mammals.
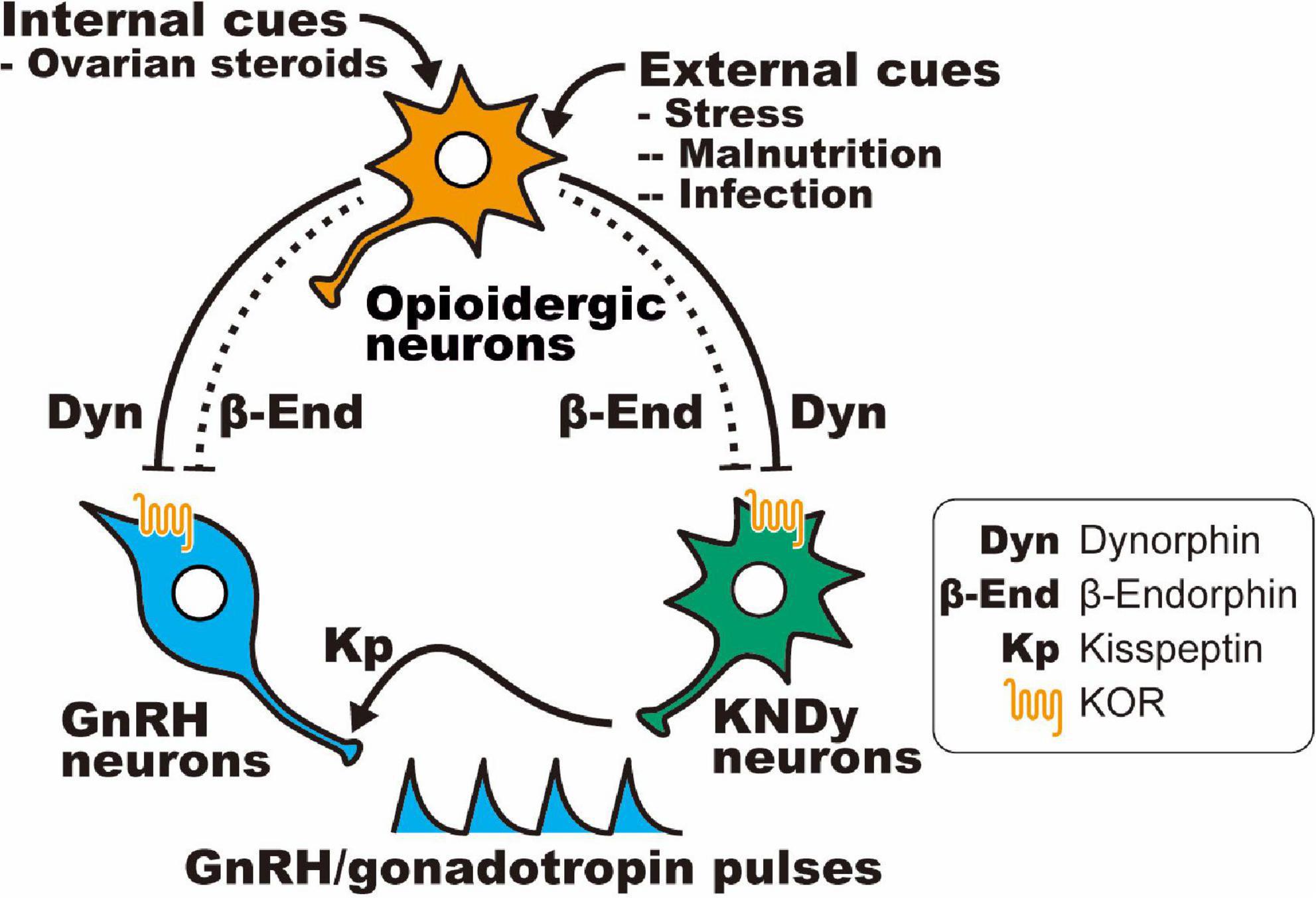
Figure 3. Schematic illustration showing a current interpretation of the opioidergic pathway in the regulation of GnRH/gonadotropin release in mammals: hypothalamic dynorphin and β-endorphin neurons serve as mediators of the inhibitory effect of ovarian steroids (internal cues) and/or stressors (e.g., malnutrition and infection; external cues) on GnRH/gonadotropin release in female mammals. It is likely that dynorphin directly acts on the majority of KNDy and GnRH neurons via the KOR, whereas β-endorphin indirectly (dotted line) acts on KNDy and GnRH neurons via μ-opioid receptor (MOR)-expressing interneurons.
Possible involvement of endogenous opioid peptides in gonadotropin-releasing hormone/luteinizing hormone surge generation
Previous studies suggest that a transient decrease in the endogenous opioid tone contributes to the initiation of the preovulatory LH surge in female mammals (Gabriel et al., 1983; Allen and Kalra, 1986; Rossmanith et al., 1988; Walsh and Clarke, 1996; Smith and Gallo, 1997). Concretely, IV or SC administration of naloxone advanced the onset of LH surge induction and increased the amplitude of LH surge in women with normal cycles (Rossmanith et al., 1988) and in proestrous or estradiol benzoate (EB)-treated OVX rats (Gabriel et al., 1983; Allen and Kalra, 1986). Furthermore, Smith and Gallo (1997) showed that nor-BNI infusion into the medial POA advanced the onset of LH surge in proestrous female rats. In addition, Walsh and Clarke (1996) showed that an MOR agonist, but not KOR and DOR agonists, delayed the onset of the EB-induced LH surge in OVX ewes. These findings suggest that endogenous opioid peptides may exert an inhibitory influence on GnRH/LH surge generation.
It is well accepted that another population of hypothalamic kisspeptin neurons, which are located in the anteroventral periventricular nucleus (AVPV) in rodents (Smith et al., 2005, 2006; Adachi et al., 2007; Clarkson et al., 2008) and the POA in several mammalian species, including macaque monkeys (Smith et al., 2010; Watanabe et al., 2014), sheep (Smith et al., 2009), goats (Matsuda et al., 2015), cattle (Hassaneen et al., 2016), and musk shrews (Inoue et al., 2011), as well as in the periventricular nucleus in pigs (Tomikawa et al., 2010), have been considered to serve as a target of estrogen-positive feedback action to induce GnRH surge in female mammals (see review article for details, Uenoyama et al., 2021a; Goodman et al., 2022; Tsukamura, 2022). Interestingly, previous studies showed coexpression of Penk/Met-enkephalin and Pdyn in the majority of AVPV kisspeptin neurons in female mice (Porteous et al., 2011; Stephens and Kauffman, 2021). To the best of our knowledge, little is known about the physiological roles of Met-enkephalin and dynorphin in AVPV kisspeptin neurons, although these findings tempt us to speculate that Met-enkephalin and/or dynorphin may have a role as a regulatory signal for LH surge generation in an autocrine/paracrine fashion in mice. Stephens and Kauffman (2021) showed that Pdyn expression was higher in OVX mice than E2-treated OVX mice, suggesting that dynorphin may suppress the onset of LH surge in an autocrine/paracrine fashion. Further studies are needed to uncover the precise mechanism by which endogenous opioid peptides regulate LH surge generation in female mammals.
Conclusion and perspectives
Based on the findings currently available, we can envisage that hypothalamic opioidergic neurons play several important roles in the brain mechanism, regulating reproduction in female mammals. In particular, ARC dynorphin neurons, which are now known as KNDy neurons because of the coexpression of NKB and kisspeptin, are recognized as the GnRH pulse generator that governs female reproduction by controlling tonic GnRH/gonadotropin release throughout the estrus/menstrual cycles. In addition, dynorphin neurons located in several hypothalamic nuclei, such as the POA, AHA, and/or PVN, are likely to serve as mediators of ovarian steroid-negative feedback action on tonic GnRH/gonadotropin release by suppressing KNDy and/or GnRH neuronal activity via the KOR expressed in KNDy and/or GnRH neurons in female mammals. It is also postulated that ARC β-endorphin neurons may also mediate ovarian steroid-negative feedback action and suppress KNDy and GnRH neuronal activity via MOR-positive interneurons. Furthermore, hypothalamic opioidergic neurons are also likely to serve as mediators of external adverse cues, such as malnutrition and infection, and suppress tonic GnRH/gonadotropin release under stressful conditions. To date, findings have mainly been accumulated for MOR and KOR signaling, and little is known about whether DOR signaling serves as a mediator of ovarian steroid-negative feedback action and/or stress-induced suppression of tonic GnRH/gonadotropin release in female mammals. To uncover the precise roles of hypothalamic opioidergic neurons in mammalian reproduction as a whole, further studies are needed to clarify precise opioidergic neural pathways that control KNDy and GnRH neuronal activity in female mammals.
Author contributions
YU and HTk collected the information and wrote the manuscript. HTc, MN, and NI critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by JSPS KAKENHI [Grant Numbers 21K19186 and 21H05031 (to HTk), 19H03103 (to NI), and 20H03127 (to YU)].
Acknowledgments
We acknowledge the great contributions of the late Kei-ichiro Maeda for his expert advice and supervision of our previous studies on kisspeptin and opioidergic regulation of female reproduction in mammals.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adachi, S., Yamada, S., Takatsu, Y., Matsui, H., Kinoshita, M., Takase, K., et al. (2007). Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 53, 367–378.
Aerts, E. G., Harlow, K., Griesgraber, M. J., Bowdridge, E. C., Hardy, S. L., Nestor, C. C., et al. (2021). Kisspeptin, neurokinin B, and dynorphin expression during pubertal development in female sheep. Biology 10:988. doi: 10.3390/biology10100988
Akil, H., Watson, S. J., Young, E., Lewis, M. E., Khachaturian, H., and Walker, J. M. (1984). Endogenous opioids: Biology and function. Annu. Rev. Neurosci. 7, 223–255. doi: 10.1146/annurev.ne.07.030184.001255
Allen, L. G., and Kalra, S. P. (1986). Evidence that a decrease in opioid tone may evoke preovulatory luteinizing hormone release in the rat. Endocrinology 118, 2375–2381. doi: 10.1210/endo-118-6-2375
Amstalden, M., Coolen, L. M., Hemmerle, A. M., Billings, H. J., Connors, J. M., Goodman, R. L., et al. (2010). Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 22, 1–12. doi: 10.1111/j.1365-2826.2009.01930.x
Bale, T. L., and Vale, W. W. (2004). CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 44, 525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410
Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J., and Knobil, E. (1978). Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202, 631–633.
Benarroch, E. E. (2012). Endogenous opioid systems: Current concepts and clinical correlations. Neurology 79, 807–814. doi: 10.1212/WNL.0b013e3182662098
Birdsall, N. J., and Hulme, E. C. (1976). C fragment of lipotropin has a high affinity for brain opiate receptors. Nature 260, 793–795. doi: 10.1038/260793a0
Bowe, J. E., Li, X. F., Kinsey-Jones, J. S., Paterson, S., Brain, S. D., Lightman, S. L., et al. (2005). Calcitonin gene-related peptide-induced suppression of luteinizing hormone pulses in the rat: The role of endogenous opioid peptides. J. Physiol. 566, 921–928. doi: 10.1113/jphysiol.2005.085662
Broad, K. D., Kendrick, K. M., Sirinathsinghji, D. J., and Keverne, E. B. (1993). Changes in pro-opiomelanocortin and pre-proenkephalin mRNA levels in the ovine brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. J. Neuroendocrinol. 5, 711–719. doi: 10.1111/j.1365-2826.1993.tb00544.x
Bronson, F. H. (1986). Food-restricted, prepubertal, female rats: Rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology 118, 2483–2487. doi: 10.1210/endo-118-6-2483
Brooks, A. N., and Challis, J. R. (1989). Effects of CRF, AVP and opioid peptides on pituitary-adrenal responses in sheep. Peptides 10, 1291–1293. doi: 10.1016/0196-9781(89)90024-7
Brooks, A. N., Lamming, G. E., Lees, P. D., and Haynes, N. B. (1986). Opioid modulation of LH secretion in the ewe. J. Reprod. Fertil. 76, 693–708. doi: 10.1530/jrf.0.0760693
Brownstein, M. J. (1993). A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. U. S. A. 90, 5391–5393. doi: 10.1073/pnas.90.12.5391
Cagampang, F. R., Maeda, K.-I., Tsukamura, H., Ohkura, S., and Ota, K. (1991). Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J. Endocrinol. 129, 321–328. doi: 10.1677/joe.0.1290321
Chatterton, R. T. (1990). The role of stress in female reproduction: Animal and human considerations. Int. J. Fertil. 35, 8–13.
Chen, Y., Mestek, A., Liu, J., Hurley, J. A., and Yu, L. (1993a). Molecular cloning and functional expression of a μ-opioid receptor from rat brain. Mol. Pharmacol. 44, 8–12.
Chen, Y., Mestek, A., Liu, J., and Yu, L. (1993b). Molecular cloning of a rat κ opioid receptor reveals sequence similarities to the μ and δ opioid receptors. Biochem. J. 295, 625–628. doi: 10.1042/bj2950625
Clarke, I. J., and Cummins, J. T. (1982). The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111, 1737–1739. doi: 10.1210/endo-111-5-1737
Clarke, I. J., Horton, R. J., and Doughton, B. W. (1990). Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology 127, 1470–1476. doi: 10.1210/endo-127-3-1470
Clarkson, J., d’Anglemont, de Tassigny, X., Moreno, A. S., Colledge, W. H., and Herbison, A. E. (2008). Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 28, 8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008
Connor, M., and Christie, M. D. (1999). Opioid receptor signalling mechanisms. Clin. Exp. Pharmacol. Physiol. 26, 493–499. doi: 10.1046/j.1440-1681.1999.03049.x
Desjardins, G. C., Brawer, J. R., and Beaudet, A. (1990). Distribution of mu, delta, and kappa opioid receptors in the hypothalamus of the rat. Brain Res. 536, 114–123. doi: 10.1016/0006-8993(90)90015-4
Devereaux, A. L., Mercer, S. L., and Cunningham, C. W. (2018). DARK Classics in Chemical Neuroscience: Morphine. ACS Chem. Neurosci. 9, 2395–2407. doi: 10.1021/acschemneuro.8b00150
Dyer, R. G., Mansfield, S., Corbet, H., and Dean, A. D. (1985). Fasting impairs LH secretion in female rats by activating an inhibitory opioid pathway. J. Endocrinol. 105, 91–97. doi: 10.1677/joe.0.1050091
Ebling, F. J., Schwartz, M. L., and Foster, D. L. (1989). Endogenous opioid regulation of pulsatile luteinizing hormone secretion during sexual maturation in the female sheep. Endocrinology 125, 369–383. doi: 10.1210/endo-125-1-369
Ebling, F. J., Wood, R. I., Karsch, F. J., Vannerson, L. A., Suttie, J. M., Bucholtz, D. C., et al. (1990). Metabolic interfaces between growth and reproduction. III. Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb. Endocrinology 126, 2719–2727. doi: 10.1210/endo-126-5-2719
Estacio, M. A., Yamada, S., Tsukamura, H., Hirunagi, K., and Maeda, K.-I. (1996). Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 717, 55–61.
Evans, C. J., Keith, D. E. Jr., Morrison, H., Magendzo, K., and Edwards, R. H. (1992). Cloning of a delta opioid receptor by functional expression. Science 258, 1952–1955. doi: 10.1126/science.1335167
Foradori, C. D., Amstalden, M., Goodman, R. L., and Lehman, M. N. (2006). Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J. Neuroendocrinol. 18, 534–541. doi: 10.1111/j.1365-2826.2006.01445.x
Foradori, C. D., Coolen, L. M., Fitzgerald, M. E., Skinner, D. C., Goodman, R. L., and Lehman, M. N. (2002). Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143, 4366–4374. doi: 10.1210/en.2002-220586
Foradori, C. D., Goodman, R. L., Adams, V. L., Valent, M., and Lehman, M. N. (2005). Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146, 1835–1842. doi: 10.1210/en.2004-1326
Foster, D. L., and Olster, D. H. (1985). Effect of restricted nutrition on puberty in the lamb: Patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology 116, 375–381. doi: 10.1210/endo-116-1-375
Foster, D. L., and Ryan, K. D. (1979). Mechanisms governing onset of ovarian cyclicity at puberty in the lamb. Ann. de Biologie Animale Biochimie Biophysique 19, 1369–1380.
Franceschini, I., Lomet, D., Cateau, M., Delsol, G., Tillet, Y., and Caraty, A. (2006). Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci. Lett. 401, 225–230. doi: 10.1016/j.neulet.2006.03.039
Fukuda, K., Kato, S., Mori, K., Nishi, M., and Takeshima, H. (1993). Primary structures and expression from cDNAs of rat opioid receptor δ- and μ-subtypes. FEBS Lett. 327, 311–314. doi: 10.1016/0014-5793(93)81011-n
Gabriel, S. M., Simpkins, J. W., and Kalra, S. P. (1983). Modulation of endogenous opioid influence on luteinizing hormone secretion by progesterone and estrogen. Endocrinology 113, 1806–1811. doi: 10.1210/endo-113-5-1806
Gallo, R. V. (1990). Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J. Neuroendocrinol. 2, 685–691. doi: 10.1111/j.1365-2826.1990.tb00465.x
George, S. R., Zastawny, R. L., Briones-Urbina, R., Cheng, R., Nguyen, T., Heiber, M., et al. (1994). Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem. Biophys. Res. Commun. 205, 1438–1444. doi: 10.1006/bbrc.1994.2826
Gindoff, P. R., and Ferin, M. (1987). Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology 121, 837–842. doi: 10.1210/endo-121-3-837
Goldstein, A., Fischli, W., Lowney, L. I., Hunkapiller, M., and Hood, L. (1981). Porcine pituitary dynorphin: Complete amino acid sequence of the biologically active heptadecapeptide. Proc. Natl. Acad. Sci. U. S. A. 78, 7219–7223. doi: 10.1073/pnas.78.11.7219
Goldstein, A., Tachibana, S., Lowney, L. I., Hunkapiller, M., and Hood, L. (1979). Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc. Natl. Acad. Sci. U. S. A. 76, 6666–6670. doi: 10.1073/pnas.76.12.6666
Goodman, R. L., Coolen, L. M., Anderson, G. M., Hardy, S. L., Valent, M., Connors, J. M., et al. (2004). Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145, 2959–2967. doi: 10.1210/en.2003-1305
Goodman, R. L., Herbison, A. E., Lehman, M. N., and Navarro, V. M. (2022). Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J. Neuroendocrinol. 34:e13094. doi: 10.1111/jne.13094
Goodman, R. L., Hileman, S. M., Nestor, C. C., Porter, K. L., Connors, J. M., Hardy, S. L., et al. (2013). Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 154, 4259–4269. doi: 10.1210/en.2013-1331
Goodman, R. L., Lehman, M. N., Smith, J. T., Coolen, L. M., de Oliveira, C. V., Jafarzadehshirazi, M. R., et al. (2007). Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760. doi: 10.1210/en.2007-0961
Goodman, R. L., Ohkura, S., Okamura, H., Coolen, L. M., and Lehman, M. N. (2018). “KNDy hypothesis for generation of GnRH pulses: Evidence from sheep and goats,” in The GnRH neuron and its control, eds A. E. Herbison and T. M. Plant (Hoboken, NJ: Wiley), 289–324.
Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., et al. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077.
Gruber, S. A., Silveri, M. M., and Yurgelun-Todd, D. A. (2007). Neuropsychological consequences of opiate use. Neuropsychol. Rev. 17, 299–315. doi: 10.1007/s11065-007-9041-y
Harlow, K., Griesgraber, M. J., Seman, A. D., Shuping, S. L., Sommer, J. R., Griffith, E. H., et al. (2022). The impact of undernutrition on KNDy (kisspeptin/neurokinin B/dynorphin) neurons in female lambs. J. Neuroendocrinol. 34:e13135. doi: 10.1111/jne.13135
Harlow, K., Renwick, A. N., Shuping, S. L., Sommer, J. R., Lents, C. A., Knauer, M. T., et al. (2021). Evidence that pubertal status impacts kisspeptin/neurokinin B/dynorphin neurons in the giltdagger. Biol. Reprod. 105, 1533–1544. doi: 10.1093/biolre/ioab189
Hassaneen, A. S. A., Naniwa, Y., Suetomi, Y., Matsuyama, S., Kimura, K., Ieda, N., et al. (2016). Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J. Reprod. Dev. 62, 471–477. doi: 10.1262/jrd.2016-075
Herbison, A. E. (2020). A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front. Neuroendocrinol. 57:100837. doi: 10.1016/j.yfrne.2020.100837
Herbison, A. E., de Tassigny, X., Doran, J., and Colledge, W. H. (2010). Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151, 312–321. doi: 10.1210/en.2009-0552
Higo, S., Honda, S., Iijima, N., and Ozawa, H. (2016). Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localisation with oxytocin in the paraventricular nucleus. J. Neuroendocrinol. 28, doi: 10.1111/jne.12356
Hrabovszky, E., Sipos, M. T., Molnar, C. S., Ciofi, P., Borsay, B. A., Gergely, P., et al. (2012). Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 153, 4978–4989. doi: 10.1210/en.2012-1545
Hrabovszky, E., Takacs, S., Gocz, B., and Skrapits, K. (2019). New perspectives for anatomical and molecular studies of kisspeptin neurons in the aging human brain. Neuroendocrinology 109, 230–241. doi: 10.1159/000496566
Hughes, J., Smith, T., Morgan, B., and Fothergill, L. (1975a). Purification and properties of enkephalin - the possible endogenous ligand for the morphine receptor. Life Sci. 16, 1753–1758. doi: 10.1016/0024-3205(75)90268-4
Hughes, J., Smith, T. W., Kosterlitz, H. W., Fothergill, L. A., Morgan, B. A., and Morris, H. R. (1975b). Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258, 577–580. doi: 10.1038/258577a0
Hulse, G. K., and Coleman, G. J. (1983). The role of endogenous opioids in the blockade of reproductive function in the rat following exposure to acute stress. Pharmacol. Biochem. Behav. 19, 795–799. doi: 10.1016/0091-3057(83)90083-7
Ieda, N., Uenoyama, Y., Tajima, Y., Nakata, T., Kano, M., Naniwa, Y., et al. (2014). KISS1 gene expression in the developing brain of female pigs in pre- and peripubertal periods. J. Reprod. Dev. 60, 312–316. doi: 10.1262/jrd.2013-129
Ieiri, T., Chen, H. T., and Meites, J. (1980). Naloxone stimulation of luteinizing hormone release in prepubertal female rats; role of serotonergic system. Life Sci. 26, 1269–1274. doi: 10.1016/0024-3205(80)90072-7
Ikegami, K., Minabe, S., Ieda, N., Goto, T., Sugimoto, A., Nakamura, S., et al. (2017). Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 29, doi: 10.1111/jne.12480
Ikegami, K., Watanabe, Y., Nakamura, S., Goto, T., Inoue, N., Uenoyama, Y., et al. (2021). Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals. Front. Neuroendocrinol. 64:100968. doi: 10.1016/j.yfrne.2021.100968
Inoue, N., Sasagawa, K., Ikai, K., Sasaki, Y., Tomikawa, J., Oishi, S., et al. (2011). Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc. Natl. Acad. Sci. U. S. A. 108, 17527–17532. doi: 10.1073/pnas.1113035108
Irwig, M. S., Fraley, G. S., Smith, J. T., Acohido, B. V., Popa, S. M., Cunningham, M. J., et al. (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80, 264–272.
Kakidani, H., Furutani, Y., Takahashi, H., Noda, M., Morimoto, Y., Hirose, T., et al. (1982). Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature 298, 245–249. doi: 10.1038/298245a0
Kanaya, M., Iwata, K., and Ozawa, H. (2017). Distinct dynorphin expression patterns with low- and high-dose estrogen treatment in the arcuate nucleus of female rats. Biol. Reprod. 97, 709–718. doi: 10.1093/biolre/iox131
Kieffer, B. L., Befort, K., Gaveriaux-Ruff, C., and Hirth, C. G. (1992). The δ-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc. Natl. Acad. Sci. U. S. A. 89, 12048–12052. doi: 10.1073/pnas.89.24.12048
Kinoshita, M., Tsukamura, H., Adachi, S., Matsui, H., Uenoyama, Y., Iwata, K., et al. (2005). Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146, 4431–4436. doi: 10.1210/en.2005-0195
Kujjo, L. L., Bosu, W. T., and Perez, G. I. (1995). Opioid peptides involvement in endotoxin-induced suppression of LH secretion in ovariectomized Holstein heifers. Reprod. Toxicol. 9, 169–174. doi: 10.1016/0890-6238(94)00068-9
Lehman, M. N., Coolen, L. M., and Goodman, R. L. (2010a). Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151, 3479–3489. doi: 10.1210/en.2010-0022
Lehman, M. N., Merkley, C. M., Coolen, L. M., and Goodman, R. L. (2010b). Anatomy of the kisspeptin neural network in mammals. Brain Res. 1364, 90–102. doi: 10.1016/j.brainres.2010.09.020
Lehman, M. N., Robinson, J. E., Karsch, F. J., and Silverman, A. J. (1986). Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J. Comp. Neurol. 244, 19–35. doi: 10.1002/cne.902440103
Li, C. H., and Chung, D. (1976). Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc. Natl. Acad. Sci. U. S. A. 73, 1145–1148. doi: 10.1073/pnas.73.4.1145
Li, S., Zhu, J., Chen, C., Chen, Y. W., Deriel, J. K., Ashby, B., et al. (1993). Molecular cloning and expression of a rat κ opioid receptor. Biochem. J. 295, 629–633. doi: 10.1042/bj2950629
Li, X. F., Bowe, J. E., Mitchell, J. C., Brain, S. D., Lightman, S. L., and O’Byrne, K. T. (2004). Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: A novel neural action for calcitonin gene-related peptide. Endocrinology 145, 1556–1563. doi: 10.1210/en.2003-1609
Lincoln, D. W., Fraser, H. M., Lincoln, G. A., Martin, G. B., and McNeilly, A. S. (1985). Hypothalamic pulse generators. Recent Prog. Horm. Res. 41, 369–419.
Lopez, J. A., Bedenbaugh, M. N., McCosh, R. B., Weems, P. W., Meadows, L. J., Wisman, B., et al. (2016). Does dynorphin play a role in the onset of puberty in female sheep? J. Neuroendocrinol. 28, doi: 10.1111/jne.12445
Maeda, K.-I., Ohkura, S., Uenoyama, Y., Wakabayashi, Y., Oka, Y., Tsukamura, H., et al. (2010). Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 1364, 103–115. doi: 10.1016/j.brainres.2010.10.026
Maeda, K.-I., Tsukamura, H., Ohkura, S., Kawakami, S., Nagabukuro, H., and Yokoyama, A. (1995). The LHRH pulse generator: A mediobasal hypothalamic location. Neurosci. Biobehav. Rev. 19, 427–437. doi: 10.1016/0149-7634(94)00069-d
Majarune, S., Nima, P., Sugimoto, A., Nagae, M., Inoue, N., Tsukamura, H., et al. (2019). Ad libitum feeding triggers puberty onset associated with increases in arcuate Kiss1 and Pdyn expression in growth-retarded rats. J. Reprod. Dev. 65, 397–406. doi: 10.1262/jrd.2019-048
Malven, P. V., Bossut, D. F., and Diekman, M. A. (1984). Effects of naloxone and electroacupuncture treatment on plasma concentrations of LH in sheep. J. Endocrinol. 101, 75–80. doi: 10.1677/joe.0.1010075
Mansour, A., Hoversten, M. T., Taylor, L. P., Watson, S. J., and Akil, H. (1995b). The cloned μ, δ and κ receptors and their endogenous ligands: Evidence for two opioid peptide recognition cores. Brain Res. 700, 89–98. doi: 10.1016/0006-8993(95)00928-j
Mansour, A., Fox, C. A., Akil, H., and Watson, S. J. (1995a). Opioid-receptor mRNA expression in the rat CNS: Anatomical and functional implications. Trends Neurosci. 18, 22–29. doi: 10.1016/0166-2236(95)93946-u
Mansour, A., Fox, C. A., Burke, S., Meng, F., Thompson, R. C., Akil, H., et al. (1994). Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J. Comp. Neurol. 350, 412–438. doi: 10.1002/cne.903500307
Mansour, A., Khachaturian, H., Lewis, M. E., Akil, H., and Watson, S. J. (1988). Anatomy of CNS opioid receptors. Trends Neurosci. 11, 308–314. doi: 10.1016/0166-2236(88)90093-8
Mansour, A., Thompson, R. C., Akil, H., and Watson, S. J. (1993). Delta opioid receptor mRNA distribution in the brain: Comparison to delta receptor binding and proenkephalin mRNA. J. Chem. Neuroanat. 6, 351–362. doi: 10.1016/0891-0618(93)90010-2
Matsuda, F., Nakatsukasa, K., Suetomi, Y., Naniwa, Y., Ito, D., Inoue, N., et al. (2015). The LH surge-generating system is functional in male goats as in females: Involvement of kisspeptin neurones in the medial preoptic area. J. Neuroendocrinol. 27, 57–65. doi: 10.1111/jne.12235
Messager, S., Chatzidaki, E. E., Ma, D., Hendrick, A. G., Zahn, D., Dixon, J., et al. (2005). Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. U. S. A. 102, 1761–1766. doi: 10.1073/pnas.0409330102
Minami, M., Toya, T., Katao, Y., Maekawa, K., Nakamura, S., Onogi, T., et al. (1993). Cloning and expression of a cDNA for the rat κ-opioid receptor. FEBS Lett. 329, 291–295. doi: 10.1016/0014-5793(93)80240-u
Mitchell, V., Prevot, V., Jennes, L., Aubert, J. P., Croix, D., and Beauvillain, J. C. (1997). Presence of μ and κ opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport 8, 3167–3172. doi: 10.1097/00001756-199709290-00032
Mitev, Y., Almeida, O. F., and Patchev, V. (1993). Pituitary-adrenal function and hypothalamic beta-endorphin release in vitro following food deprivation. Brain Res. Bull. 30, 7–10. doi: 10.1016/0361-9230(93)90033-8
Moenter, S. M., Brand, R. M., Midgley, A. R., and Karsch, F. J. (1992). Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130, 503–510.
Moore, A. M., Coolen, L. M., Porter, D. T., Goodman, R. L., and Lehman, M. N. (2018). KNDy Cells Revisited. Endocrinology 159, 3219–3234. doi: 10.1210/en.2018-00389
Mostari, M. P., Ieda, N., Deura, C., Minabe, S., Yamada, S., Uenoyama, Y., et al. (2013). Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J. Reprod. Dev. 59, 266–272. doi: 10.1262/jrd.2012-193
Murakawa, H., Iwata, K., Takeshita, T., and Ozawa, H. (2016). Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci. Lett. 612, 161–166. doi: 10.1016/j.neulet.2015.12.008
Nagae, M., Uenoyama, Y., Okamoto, S., Tsuchida, H., Ikegami, K., Goto, T., et al. (2021). Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. U. S. A. 118:e2009156118. doi: 10.1073/pnas.2009156118
Nagatani, S., Bucholtz, D. C., Murahashi, K., Estacio, M. A., Tsukamura, H., Foster, D. L., et al. (1996). Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology 137, 1166–1170. doi: 10.1210/endo.137.4.8625885
Nagatani, S., Tsukamura, H., and Maeda, K.-I. (1994). Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology 135, 870–875.
Nakahara, T., Uenoyama, Y., Iwase, A., Oishi, S., Nakamura, S., Minabe, S., et al. (2013). Chronic peripheral administration of kappa-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J. Reprod. Dev. 59, 479–484. doi: 10.1262/jrd.2013-046
Nakamura, S., Wakabayashi, Y., Yamamura, T., Ohkura, S., and Matsuyama, S. (2017). A neurokinin 3 receptor-selective agonist accelerates pulsatile luteinizing hormone secretion in lactating cattle. Biol. Reprod. 97, 81–90. doi: 10.1093/biolre/iox068
Nakanishi, S., Inoue, A., Kita, T., Nakamura, M., Chang, A. C., Cohen, S. N., et al. (1979). Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature 278, 423–427. doi: 10.1038/278423a0
Naniwa, Y., Nakatsukasa, K., Setsuda, S., Oishi, S., Fujii, N., Matsuda, F., et al. (2013). Effects of full-length kisspeptin administration on follicular development in Japanese Black beef cows. J. Reprod. Dev. 59, 588–594. doi: 10.1262/jrd.2013-064
Navarro, V. M., Castellano, J. M., McConkey, S. M., Pineda, R., Ruiz-Pino, F., Pinilla, L., et al. (2011). Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am. J. Physiol. Endocrinol. Metab. 300, E202–E210. doi: 10.1152/ajpendo.00517.2010
Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., and Steiner, R. A. (2009). Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009
Nishi, M., Takeshima, H., Fukuda, K., Kato, S., and Mori, K. (1993). cDNA cloning and pharmacological characterization of an opioid receptor with high affinities for κ-subtype-selective ligands. FEBS Lett. 330, 77–80. doi: 10.1016/0014-5793(93)80923-i
Nishihara, M., Takeuchi, Y., Tanaka, T., and Mori, Y. (1999). Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev. Reprod. 4, 110–116. doi: 10.1530/ror.0.0040110
Noda, M., Furutani, Y., Takahashi, H., Toyosato, M., Hirose, T., Inayama, S., et al. (1982). Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature 295, 202–206. doi: 10.1038/295202a0
Ohkura, S., Takase, K., Matsuyama, S., Mogi, K., Ichimaru, T., Wakabayashi, Y., et al. (2009). Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J. Neuroendocrinol. 21, 813–821. doi: 10.1111/j.1365-2826.2009.01909.x
Okamura, H., Tsukamura, H., Ohkura, S., Uenoyama, Y., Wakabayashi, Y., and Maeda, K.-I. (2013). “Kisspeptin and GnRH pulse generation,” in Kisspeptin signaling in reproductive biology. advances in experimental medicine and biology, eds A. Kauffman and J. Smith (New York: Springer), 297–323.
Palkovits, M. (2000). Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur. J. Pharmacol. 405, 161–166. doi: 10.1016/s0014-2999(00)00549-5
Papadimitriou, A., and Priftis, K. N. (2009). Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16, 265–271. doi: 10.1159/000216184
Peckys, D., and Landwehrmeyer, G. B. (1999). Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: A 33P in situ hybridization study. Neuroscience 88, 1093–1135. doi: 10.1016/s0306-4522(98)00251-6
Pert, C. B., and Snyder, S. H. (1973). Opiate receptor: Demonstration in nervous tissue. Science 179, 1011–1014. doi: 10.1126/science.179.4077.1011
Petraglia, F., Locatelli, V., Penalva, A., Cocchi, D., Genazzani, A. R., and Muller, E. E. (1984). Gonadal steroid modulation of naloxone-induced LH secretion in the rat. J. Endocrinol. 101, 33–39. doi: 10.1677/joe.0.1010033
Pheng, V., Uenoyama, Y., Homma, T., Inamoto, Y., Takase, K., Yoshizawa-Kumagaye, K., et al. (2009). Potencies of centrally- or peripherally-injected full-length kisspeptin or its C-terminal decapeptide on LH release in intact male rats. J. Reprod. Dev. 55, 378–382. doi: 10.1262/jrd.20240
Porteous, R., Petersen, S. L., Yeo, S. H., Bhattarai, J. P., Ciofi, P., de Tassigny, X. D., et al. (2011). Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J. Comp. Neurol. 519, 3456–3469. doi: 10.1002/cne.22716
Przewlocki, R. (2013). “Opioid peptides,” in Neuroscience in the 21st Century, ed. D. W. Pfaff (New York: Springer), 1525–1553.
Quigley, M. E., and Yen, S. S. (1980). The role of endogenous opiates in LH secretion during the menstrual cycle. J. Clin. Endocrinol. Metab. 51, 179–181. doi: 10.1210/jcem-51-1-179
Ramaswamy, S., Seminara, S. B., Ali, B., Ciofi, P., Amin, N. A., and Plant, T. M. (2010). Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151, 4494–4503. doi: 10.1210/en.2010-0223
Raynor, K., Kong, H., Chen, Y., Yasuda, K., Yu, L., Bell, G. I., et al. (1994). Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol. Pharmacol. 45, 330–334.
Reid, R. L., Quigley, M. E., and Yen, S. S. (1983). The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. J. Clin. Endocrinol. Metab. 57, 1107–1110. doi: 10.1210/jcem-57-6-1107
Rivest, S., Plotsky, P. M., and Rivier, C. (1993). CRF alters the infundibular LHRH secretory system from the medial preoptic area of female rats: Possible involvement of opioid receptors. Neuroendocrinology 57, 236–246. doi: 10.1159/000126365
Rossmanith, W. G., Mortola, J. F., and Yen, S. S. (1988). Role of endogenous opioid peptides in the initiation of the midcycle luteinizing hormone surge in normal cycling women. J. Clin. Endocrinol. Metab. 67, 695–700. doi: 10.1210/jcem-67-4-695
Ruiz-Pino, F., Garcia-Galiano, D., Manfredi-Lozano, M., Leon, S., Sanchez-Garrido, M. A., Roa, J., et al. (2015). Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology 156, 576–588. doi: 10.1210/en.2014-1026
Sakamoto, K., Murata, K., Wakabayashi, Y., Yayou, K. I., Ohkura, S., Takeuchi, Y., et al. (2012). Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J. Reprod. Dev. 58, 700–706. doi: 10.1262/jrd.2011-038
Sannella, M. I., and Petersen, S. L. (1997). Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or δ opiate receptors. Endocrinology 138, 1667–1672.
Sasaki, T., Ito, D., Sonoda, T., Morita, Y., Wakabayashi, Y., Yamamura, T., et al. (2019). Peripheral administration of κ-opioid receptor antagonist stimulates gonadotropin-releasing hormone pulse generator activity in ovariectomized, estrogen-treated female goats. Domest. Anim. Endocrinol. 68, 83–91. doi: 10.1016/j.domaniend.2018.12.011
Sasaki, T., Sonoda, T., Tatebayashi, R., Kitagawa, Y., Oishi, S., Yamamoto, K., et al. (2020). Peripheral administration of SB223412, a selective neurokinin-3 receptor antagonist, suppresses pulsatile luteinizing hormone secretion by acting on the gonadotropin-releasing hormone pulse generator in estrogen-treated ovariectomized female goats. J. Reprod. Dev. 66, 351–357. doi: 10.1262/jrd.2019-145
Senn, M., Maier, P. M., and Langhans, W. (1995). ACTH, cortisol and glucose responses after administration of vasopressin in cattle and sheep. J. Comp. Physiol. B. 164, 570–578. doi: 10.1007/BF00261398
Simon, E. J., Hiller, J. M., and Edelman, I. (1973). Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc. Natl. Acad. Sci. U. S. A. 70, 1947–1949. doi: 10.1073/pnas.70.7.1947
Smith, J. T., Clay, C. M., Caraty, A., and Clarke, I. J. (2007). KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148, 1150–1157. doi: 10.1210/en.2006-1435
Smith, J. T., Cunningham, M. J., Rissman, E. F., Clifton, D. K., and Steiner, R. A. (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692. doi: 10.1210/en.2005-0488
Smith, J. T., Li, Q., Pereira, A., and Clarke, I. J. (2009). Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150, 5530–5538. doi: 10.1210/en.2009-0712
Smith, J. T., Popa, S. M., Clifton, D. K., Hoffman, G. E., and Steiner, R. A. (2006). Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 26, 6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006
Smith, J. T., Shahab, M., Pereira, A., Pau, K. Y., and Clarke, I. J. (2010). Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol. Reprod. 83, 568–577. doi: 10.1095/biolreprod.110.085407
Smith, M. J., and Gallo, R. V. (1997). The effect of blockade of kappa-opioid receptors in the medial preoptic area on the luteinizing hormone surge in the proestrous rat. Brain Res. 768, 111–119. doi: 10.1016/s0006-8993(97)00622-7
Snyder, S. H., and Pasternak, G. W. (2003). Historical review: Opioid receptors. Trends Pharmacol. Sci. 24, 198–205. doi: 10.1016/S0165-6147(03)00066-X
Stein, C. (2016). Opioid Receptors. Annu. Rev. Med. 67, 433–451. doi: 10.1146/annurev-med-062613-093100
Stephens, S. B. Z., and Kauffman, A. S. (2021). Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology 162:bqab080. doi: 10.1210/endocr/bqab080
Tanaka, T., Ohkura, S., Wakabayashi, K., and Okamura, H. (2012). Effect of peripherally administered kisspeptin-10 on GnRH neurosecretion into the hypophyseal portal circulation in ovariectomized goat does. Small Ruminant Res. 105, 273–276.
Terenius, L. (1973). Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol. Toxicol. 33, 377–384. doi: 10.1111/j.1600-0773.1973.tb01539.x
Thompson, R. C., Mansour, A., Akil, H., and Watson, S. J. (1993). Cloning and pharmacological characterization of a rat μ opioid receptor. Neuron 11, 903–913. doi: 10.1016/0896-6273(93)90120-g
Tilbrook, A. J., Turner, A. I., and Clarke, I. J. (2000). Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev. Reprod. 5, 105–113. doi: 10.1530/ror.0.0050105
Tilbrook, A. J., Turner, A. I., and Clarke, I. J. (2002). Stress and reproduction: Central mechanisms and sex differences in non-rodent species. Stress 5, 83–100. doi: 10.1080/10253890290027912
Tomikawa, J., Homma, T., Tajima, S., Shibata, T., Inamoto, Y., Takase, K., et al. (2010). Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol. Reprod. 82, 313–319. doi: 10.1095/biolreprod.109.079863
True, C., Kirigiti, M., Ciofi, P., Grove, K. L., and Smith, M. S. (2011). Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J. Neuroendocrinol. 23, 52–64. doi: 10.1111/j.1365-2826.2010.02076.x
True, C., Takahashi, D., Kirigiti, M., Lindsley, S. R., Moctezuma, C., Arik, A., et al. (2017). Arcuate nucleus neuropeptide coexpression and connections to gonadotrophin-releasing hormone neurones in the female rhesus macaque. J. Neuroendocrinol. 29, doi: 10.1111/jne.12491
Tsuchida, H., Kawai, N., Yamada, K., Takizawa, M., Inoue, N., Uenoyama, Y., et al. (2021). Central μ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology 162:bqab140. doi: 10.1210/endocr/bqab140
Tsuchida, H., Mostari, P., Yamada, K., Miyazaki, S., Enomoto, Y., Inoue, N., et al. (2020). Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology 161:bqaa161. doi: 10.1210/endocr/bqaa161
Tsukamura, H. (2022). Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen. Comp. Endocrinol. 315:113755. doi: 10.1016/j.ygcen.2021.113755
Uenoyama, Y., Inoue, N., Nakamura, S., and Tsukamura, H. (2019). Central mechanism controlling pubertal onset in mammals: A triggering role of kisspeptin. Front. Endocrinol. 10:312. doi: 10.3389/fendo.2019.00312
Uenoyama, Y., Nagae, M., Tsuchida, H., Inoue, N., and Tsukamura, H. (2021b). Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front. Endocrinol. 12:724632. doi: 10.3389/fendo.2021.724632
Uenoyama, Y., Inoue, N., Nakamura, S., and Tsukamura, H. (2021a). Kisspeptin neurons and estrogen-estrogen receptor α signaling: Unraveling the mystery of steroid feedback system regulating mammalian reproduction. Int. J. Mol. Sci. 22:9229. doi: 10.3390/ijms22179229
Uenoyama, Y., Tsukamura, H., and Maeda, K.-I. (2014). KNDy neuron as a gatekeeper of puberty onset. J. Obstet. Gynaecol. Res. 40, 1518–1526. doi: 10.1111/jog.12398
Van Vugt, D. A., Bakst, G., Dyrenfurth, I., and Ferin, M. (1983). Naloxone stimulation of luteinizing hormone secretion in the female monkey: Influence of endocrine and experimental conditions. Endocrinology 113, 1858–1864. doi: 10.1210/endo-113-5-1858
Wakabayashi, Y., Nakada, T., Murata, K., Ohkura, S., Mogi, K., Navarro, V. M., et al. (2010). Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 30, 3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010
Waldhoer, M., Bartlett, S. E., and Whistler, J. L. (2004). Opioid receptors. Annu. Rev. Biochem. 73, 953–990. doi: 10.1146/annurev.biochem.73.011303.073940
Walsh, J. P., and Clarke, I. J. (1996). Effects of central administration of highly selective opioid mu-, delta- and kappa-receptor agonists on plasma luteinizing hormone (LH), prolactin, and the estrogen-induced LH surge in ovariectomized ewes. Endocrinology 137, 3640–3648. doi: 10.1210/endo.137.9.8756528
Watanabe, Y., Uenoyama, Y., Suzuki, J., Takase, K., Suetomi, Y., Ohkura, S., et al. (2014). Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinizing hormone surge in male and female Japanese monkeys. J. Neuroendocrinol. 26, 909–917. doi: 10.1111/jne.12227
Weems, P. W., Witty, C. F., Amstalden, M., Coolen, L. M., Goodman, R. L., and Lehman, M. N. (2016). Kappa opioid receptor is co-localized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology 157, 2367–2379. doi: 10.1210/en.2015-1763
Whisnant, C. S., Curto, K., and Goodman, R. L. (1992). Immunocytochemical localization of beta endorphin and gonadal steroid regulation of proopiomelanocortin messenger ribonucleic acid in the ewe. Neuroendocrinology 56, 812–821. doi: 10.1159/000126311
Whisnant, C. S., and Goodman, R. L. (1988). Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol. Reprod. 39, 1032–1038. doi: 10.1095/biolreprod39.5.1032
Wilcox, J. N., and Roberts, J. L. (1985). Estrogen decreases rat hypothalamic proopiomelanocortin messenger ribonucleic acid levels. Endocrinology 117, 2392–2396. doi: 10.1210/endo-117-6-2392
Wood, R. I. I, Anson, H., Ebling, F. J., and Foster, D. L. (1992). Opioid inhibition of luteinizing hormone secretion compared in developing male and female sheep. Neuroendocrinology 56, 822–830. doi: 10.1159/000126312
Xiao, E., Xia-Zhang, L., and Ferin, M. (2000). Inhibitory effects of endotoxin on LH secretion in the ovariectomized monkey are prevented by naloxone but not by an interleukin-1 receptor antagonist. Neuroimmunomodulation 7, 6–15. doi: 10.1159/000026415
Xiao, E., Xia-Zhang, L., Thornell, D., Shalts, E., and Ferin, M. (1996). The luteinizing hormone but not the cortisol response to arginine vasopressin is prevented by naloxone and a corticotropin-releasing hormone antagonist in the ovariectomized rhesus monkey. Neuroendocrinology 64, 225–232. doi: 10.1159/000127121
Yamamura, T., Wakabayashi, Y., Ohkura, S., Navarro, V. M., and Okamura, H. (2015). Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J. Reprod. Dev. 61, 20–29. doi: 10.1262/jrd.2014-109
Yang, K., Haynes, N. B., Lamming, G. E., and Brooks, A. N. (1988). Ovarian steroid hormone involvement in endogenous opioid modulation of LH secretion in mature ewes during the breeding and non-breeding seasons. J. Reprod. Fertil. 83, 129–139. doi: 10.1530/jrf.0.0830129
Yasuda, K., Raynor, K., Kong, H., Breder, C. D., Takeda, J., Reisine, T., et al. (1993). Cloning and functional comparison of κ and δ opioid receptors from mouse brain. Proc. Natl. Acad. Sci. U. S. A. 90, 6736–6740. doi: 10.1073/pnas.90.14.6736
Keywords: endogenous opioid peptides, dynorphin, β-endorphin, enkephalin, GnRH pulse generator, GnRH surge generator, KNDy neurons, Kiss1
Citation: Uenoyama Y, Tsuchida H, Nagae M, Inoue N and Tsukamura H (2022) Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front. Neurosci. 16:958377. doi: 10.3389/fnins.2022.958377
Received: 31 May 2022; Accepted: 19 July 2022;
Published: 11 August 2022.
Edited by:
Víctor M. Navarro, Harvard Medical School, United StatesReviewed by:
Stan Hileman, West Virginia University, United StatesCasey Nestor, North Carolina State University, United States
Copyright © 2022 Uenoyama, Tsuchida, Nagae, Inoue and Tsukamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshihisa Uenoyama, uenoyama@nagoya-u.jp
 Yoshihisa Uenoyama
Yoshihisa Uenoyama Hitomi Tsuchida
Hitomi Tsuchida Mayuko Nagae
Mayuko Nagae Naoko Inoue
Naoko Inoue Hiroko Tsukamura
Hiroko Tsukamura