- 1Institute of Brain Science, Fudan University, Shanghai, China
- 2Centre for Rare Diseases, Institute of Medical Genetics and Applied Genomics, University Hospital and Faculty of Medicine, University of Tübingen, Tübingen, Germany
- 3Institute of Biology II - Functional Epigenetics in the Animal Model, RWTH Aachen University, Aachen, Germany
Editorial on the Research Topic
Neuroepigenetics of Neuropsychiatric Disease—Hope, Success and Obstacles for Translational Findings and Applications
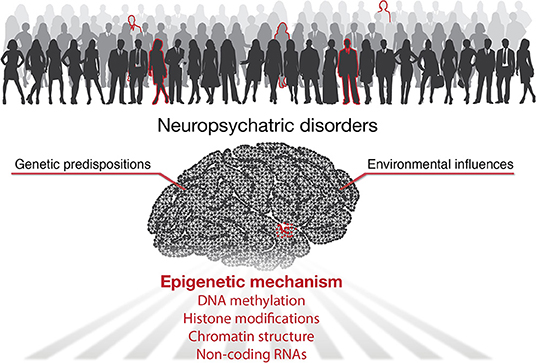
According to the World Health Organization, over one billion people worldwide suffer from neuropsychiatric diseases (World Health Organization, 2007). The lack of effective treatments is mainly due to our limited knowledge of the underlying pathogenesis. It has been well-accepted that besides genetic predispositions, environmental factors play essential roles in developing mental illness (Caspi and Moffitt, 2006). Indeed, clinical and animal studies have shown that many environmental factors participate in regulating mood behaviors, including chronic stress (Quello et al., 2005), major adverse life events (Corrarino, 2008), dietary factors (Owen and Corfe, 2017), drug or alcohol addiction (Lee et al., 2017; Lees et al., 2020), and endocrine disruptors (Tiffon, 2018; Rivollier et al., 2019). In addition to direct effects in adulthood, environmental insults can also influence embryonic and early brain development, thereby increasing the risk of mental disorders in adulthood (Serpeloni et al., 2019) (Figure 1).
Accumulating studies highlighted the essential role of epigenetics for gene-environment interactions with its implications for neuropsychiatric disorders (Tsuang et al., 2004; Lin and Tsai, 2020). Epigenetics refers to heritable changes in gene expression without changing the DNA sequence of genes. Epigenetic processes including DNA methylation, histone modifications, non-coding RNAs (ncRNAs), and higher-order chromatin organization. Typically, DNA methylation and histone modifications alter chromatin accessibility or serve as docking sites to recruit other functional proteins to turn genes “on” or “off.” ncRNAs work both on the transcriptional and post-transcriptional level to affect the production and stability of mRNAs. In addition, the organization of the 3D genome, in which spatial interactions of chromatin help bring linearly distant genes and regulatory elements into proximity (such as enhancer-promoter loops), has emerged as a new epigenetic mechanism in recent years (Rajarajan et al., 2016; Sun et al., 2021). In the current Research Topic, we present seven articles exploring different aspects of epigenetics for multiple neuropsychiatric conditions (Figure 1).
DNA methylation is one of the well-studied epigenetic mechanisms and collective evidence supports its role in various aspects of brain disorders (Bakusic et al., 2017). Dysregulation of excitation and inhibition balance (E/I balance) in the brain circuits is one of the significant neuropsychological changes for many mental illnesses (Sohal and Rubenstein, 2019; Molina et al., 2020). GABAergic inhibitory interneurons are essential to keep the E/I balance in the cortex (Tremblay et al., 2016), and DNA methylation is vital for the development and function of cortical interneurons. Linde and Zimmer-Bensch discuss the role of DNA methylation and its writers, DNA methyltransferases DNMT1 and DNMT3A, in regulating essential genes for GABAergic neuronal functions, as well as genes of the endocytosis process critical for synaptic neurotransmission.
Besides perturbations in neurons, glia pathology also participates in psychiatric disorders (Cotter et al., 2002). For example, white matter lesions are observed in brains of patients with schizophrenia (SCZ) (Höistad et al., 2009). Oligodendrocytes are the myelinating cells in the central nervous system (CNS), and their disruption causes white matter (composed of myelinated nerve cells) damage. DNA methylation regulates oligodendrocyte differentiation during normal development (Moyon and Casaccia, 2017), and dysregulation of DNA methylation in myelinating glia is involved in aging and neurologic diseases (Arthur-Farraj and Moyon, 2020). Chen et al. provide new evidence for oligodendroglial DNA hypermethylation and SCZ-like behavioral deficits in adolescent mice with supply of l-methionine (met), a methyl-donor for DNA methylation. Besides oligodendrocytes, other glia cells, including astrocytes and microglia are implicated in psychiatric disorders. It will be interesting to check whether the supply of met affects other glia or maybe neurons as well, and thus contribute to the observed behavioral abnormalities.
Since DNA methylation is critically implicated in many neuropsychiatric diseases, it presents a potential target for treatment (Sales and Joca, 2016; Shirvani-Farsani et al., 2021). However, the development of specific therapeutic reagents is quite a challenge. Instead, researchers use DNA methylation to predict drug response in psychiatric disorders. Zhou et al. contribute a systematic review on published data of drug response-related DNA methylation in SCZ, bipolar disorder (BD), and major depressive disorder (MDD). Among all these studies, only the correlation between methylation at the BDNF gene locus and antidepressant effects in MDD was reproduced by multiple groups (Januar et al., 2015; Zhou et al.). Since the antidepressant effect of BDNF is well-established in animal studies (Lee and Kim, 2010), this provides evidence for using current animal models (stress-induced depressive-like behaviors in mice and rats) as valid tools for studying mental disorders. Meanwhile, cell-type specificity of epigenetic signatures may explain the limited agreement of current studies as they commonly collect complex peripheral tissue such as blood to profile DNA methylation.
Histone acetylation is another classic epigenetic mark for transcriptional activation (Hebbes et al., 1988). Histone deacetylases (HDACs) remove acetylation and thus exert transcriptionally repressive effects (Milazzo et al., 2020). Previous work from Cui et al. (2013) identified a missense mutation in HDACA786T that increases the risk for eating disorders (EDs). In the current topic, Davis et al. generated a transgenic mouse model carrying this mutation and revealed gender- and circadian-related behavioral deficits associated with EDs. This work provides evidence for Hdac4 in EDs and generates a valuable model for future studies on the neuropsychiatric basis of EDs.
In addition to changes at the level of genomic DNA and histone modifications, long non-coding RNAs (ncRNAs) serve as another layer of epigenetic regulatory mechanism (Cao, 2014). Circular RNAs (circRNAs) are a novel set of ncRNAs and highly expressed in the CNS, particularly important for regulating synaptic functions (Kocerha et al., 2015) and involved in psychiatric disorders (Yoshino and Dwivedi, 2020). Paudel et al. reported gender-specific changes of circRNAs after prenatal alcohol exposure (PAE) in the embryonic brain, and the expression of some circRNAs was correlated with neuronal and glial gene expression.
MDD is becoming one of the most severe health problems globally (Otte et al., 2016). Besides chronic stress that is well-accepted as top one risk factor for MDD (Breslau and Davis, 1986), other environmental factors, including dietary influences, contribute to the neuropathogenesis of depression (Firth et al., 2020). In this topic, Aly and Engmann reviewed current knowledge on some of these dietary factors, such as vitamins, fatty acids, and minerals, associated with MDD and may serve as potential antidepressant targets. Many nutritional factors are also reported to affect epigenetic events, especially for enzymes involved in DNA methylation and histone modifications (Maugeri and Barchitta, 2020). For example, vitamin B3 is one of the precursors for nicotinamide adenine dinucleotide (NAD), the essential cofactor for type III histone deacetylases (HDACs). In addition, S-Adenosyl Methionine (SAM), a methyl donor for DNA methylation and histone methylation, can be found in most dietary proteins. Vitamin B12 serves as a cofactor of methionine synthase, and vitamin B9 (also known as folic acid) participates in the vitamin B12-mediated SAM metabolic pathway once biologically activated in vivo (Crider et al., 2012). It would be interesting to provide direct evidence for connections between these essential nutritional components, epigenetic events, and depressive behaviors.
In recent years, the 3D configuration of chromatin has been recognized as one of the important strategies of epigenetic regulation. The linear genome is highly compacted and well-organized via chromatin loop interactions inside the nucleus (Zhao et al., 2019). Disturbance of the 3D genome is now considered an intriguing mechanism for psychiatric disorders (Rajarajan et al., 2018). Clustered protocadherin genes (cPcdh) encode a subfamily of cell adhesion molecules, predominantly expressed in the brain. The combination of different cPcdh expressions serves as an identity code for individual neurons (Wu and Maniatis, 1999). The cPcdh locus is one of the best-studied examples for regulating stochastic and combinatorial expression patterns via 3D chromatin organization (Wu and Jia, 2021). Previous seminal studies of Dr. Qiang Wu's group reported complex regulatory mechanisms for cPcdhs. More importantly, it described the role of chromatin organizer CTCF for the 3D chromatin organization at this locus (Wu et al., 2020). In the current topic, Jia and Wu provided a detailed review summarizing the biological function and regulatory mechanism for the cPcdh locus and its implications for various neuropsychological disorders.
The work presented in this Research Topic spans a variety of neuropsychiatric conditions, covering most of the important epigenetic mechanisms, including DNA methylation, histone modification, circRNAs, and higher-order chromatin organizations. Together, they would advance our understanding of neuroepigenetics in mental disorders and inspire the hope and efforts for successful translation to clinical care in the future.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was funded by grants of National Natural Science Foundation of China (No. 81971272) and Science and Technology Commission of Shanghai Municipality (No. 19ZR1405400 to YJ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arthur-Farraj, P., and Moyon, S. (2020). DNA methylation in Schwann cells and in oligodendrocytes. Glia 68, 1568–1583. doi: 10.1002/glia.23784
Bakusic, J., Schaufeli, W., Claes, S., and Godderis, L. (2017). Stress, burnout and depression: a systematic review on DNA methylation mechanisms. J. Psychosom. Res. 92, 34–44. doi: 10.1016/j.jpsychores.2016.11.005
Breslau, N., and Davis, G. C. (1986). Chronic stress and major depression. Arch. Gen. Psychiatry 43, 309–314. doi: 10.1001/archpsyc.1986.01800040015003
Cao, J. (2014). The functional role of long non-coding RNAs and epigenetics. Biol. Proced Online 16:11. doi: 10.1186/1480-9222-16-11
Caspi, A., and Moffitt, T. E. (2006). Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 7, 583–590. doi: 10.1038/nrn1925
Corrarino, J. E. (2008). Disaster-related mental health needs of women and children. MCN Am. J. Matern. Child Nurs. 33, 242–248. doi: 10.1097/01.NMC.0000326079.26870.e3
Cotter, D. R., Pariante, C. M., and Rajkowska, G. (2002). “Glial pathology in major psychiatric disorders,” in The Postmortem Brain in Psychiatric Research, eds. G. Agam, I. P. Everall, and R. H. Belmaker (Boston, MA: Springer US), 49–73.
Crider, K. S., Yang, T. P., Berry, R. J., and Bailey, L. B. (2012). Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv. Nutr. 3, 21–38. doi: 10.3945/an.111.000992
Cui, H., Moore, J., Ashimi, S. S., Mason, B. L., Drawbridge, J. N., Han, S., et al. (2013). Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J. Clin. Invest. 123, 4706–4713. doi: 10.1172/JCI71400
Firth, J., Gangwisch, J. E., Borisini, A., Wootton, R. E., and Mayer, E. A. (2020). Food and mood: how do diet and nutrition affect mental wellbeing? BMJ 369:m2382. doi: 10.1136/bmj.m2382
Hebbes, T. R., Thorne, A. W., and Crane-Robinson, C. (1988). A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7, 1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x
Höistad, M., Segal, D., Takahashi, N., Sakurai, T., Buxbaum, J. D., and Hof, P. R. (2009). Linking white and grey matter in schizophrenia: oligodendrocyte and neuron pathology in the prefrontal cortex. Front. Neuroanat. 3:9. doi: 10.3389/neuro.05.009.2009
Januar, V., Ancelin, M. L., Ritchie, K., Saffery, R., and Ryan, J. (2015). BDNF promoter methylation and genetic variation in late-life depression. Transl. Psychiatry 5:e619. doi: 10.1038/tp.2015.114
Kocerha, J., Dwivedi, Y., and Brennand, K. J. (2015). Noncoding RNAs and neurobehavioral mechanisms in psychiatric disease. Mol. Psychiatry 20, 677–684. doi: 10.1038/mp.2015.30
Lee, B. H., and Kim, Y. K. (2010). The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 7, 231–235. doi: 10.4306/pi.2010.7.4.231
Lee, K. H., Jun, J. S., Kim, Y. J., Roh, S., Moon, S. S., Bukonda, N., et al. (2017). Mental health, substance abuse, and suicide among homeless adults. J. Evid. Inf. Soc. Work 14, 229–242. doi: 10.1080/23761407.2017.1316221
Lees, B., Meredith, L. R., Kirkland, A. E., Bryant, B. E., and Squeglia, L. M. (2020). Effect of alcohol use on the adolescent brain and behavior. Pharmacol. Biochem. Behav. 192:172906. doi: 10.1016/j.pbb.2020.172906
Lin, E., and Tsai, S. J. (2020). Gene-environment interactions and role of epigenetics in anxiety disorders. Adv. Exp. Med. Biol. 1191, 93–102. doi: 10.1007/978-981-32-9705-0_6
Maugeri, A., and Barchitta, M. (2020). How dietary factors affect DNA methylation: lesson from epidemiological studies. Medicina (Kaunas) 56:374. doi: 10.3390/medicina56080374
Milazzo, G., Mercatelli, D., Di Muzio, G., Triboli, L., De Rosa, P., Perini, G., et al. (2020). Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, pharmacological actionability. Genes (Basel) 11:556. doi: 10.3390/genes11050556
Molina, J. L., Voytek, B., Thomas, M. L., Joshi, Y. B., Bhakta, S. G., Talledo, J. A., et al. (2020). Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 562–568. doi: 10.1016/j.bpsc.2020.02.004
Moyon, S., and Casaccia, P. (2017). DNA methylation in oligodendroglial cells during developmental myelination and in disease. Neurogenesis (Austin) 4:e1270381. doi: 10.1080/23262133.2016.1270381
Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., et al. (2016). Major depressive disorder. Nat. Rev. Dis. Primers 2:16065. doi: 10.1038/nrdp.2016.65
Owen, L., and Corfe, B. (2017). The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 76, 425–426. doi: 10.1017/S0029665117001057
Quello, S. B., Brady, K. T., and Sonne, S. C. (2005). Mood disorders and substance use disorder: a complex comorbidity. Sci. Pract. Perspect. 3, 13–21. doi: 10.1151/spp053113
Rajarajan, P., Gil, S. E., Brennand, K. J., and Akbarian, S. (2016). Spatial genome organization and cognition. Nat. Rev. Neurosci. 17, 681–691. doi: 10.1038/nrn.2016.124
Rajarajan, P., Jiang, Y., Kassim, B. S., and Akbarian, S. (2018). Chromosomal conformations and epigenomic regulation in schizophrenia. Prog. Mol. Biol. Transl. Sci. 157, 21–40. doi: 10.1016/bs.pmbts.2017.11.022
Rivollier, F., Krebs, M. O., and Kebir, O. (2019). Perinatal exposure to environmental endocrine disruptors in the emergence of neurodevelopmental psychiatric diseases: a systematic review. Int. J. Environ. Res. Public Health 16:1318. doi: 10.3390/ijerph16081318
Sales, A. J., and Joca, S. R. (2016). Effects of DNA methylation inhibitors and conventional antidepressants on mice behaviour and brain DNA methylation levels. Acta Neuropsychiatr. 28, 11–22. doi: 10.1017/neu.2015.40
Serpeloni, F., Radtke, K. M., Hecker, T., Sill, J. V, Vukojevic de Assis, S. G., et al. (2019). Does prenatal stress shape postnatal resilience? - An epigenome-wide study on violence and mental health in humans. Front. Genet. 10:269. doi: 10.3389/fgene.2019.00269
Shirvani-Farsani, Z., Maloum, Z., Bagheri-Hosseinabadi, Z., Vilor-Tejedor, N., and Sadeghi, I. (2021). DNA methylation signature as a biomarker of major neuropsychiatric disorders. J. Psychiatr. Res. 141, 34–49. doi: 10.1016/j.jpsychires.2021.06.013
Sohal, V. S., and Rubenstein, J. L. R. (2019). Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 24, 1248–1257. doi: 10.1038/s41380-019-0426-0
Sun, D., Weng, J., Dong, Y., and Jiang, Y. (2021). Three-dimensional genome organization in the central nervous system, implications for neuropsychological disorders. J. Genet. Genomics 48, 1045–1056. doi: 10.1016/j.jgg.2021.06.017
Tiffon, C. (2018). The impact of nutrition and environmental epigenetics on human health and disease. Int. J. Mol. Sci. 19:3425. doi: 10.3390/ijms19113425
Tremblay, R., Lee, S., and Rudy, B. (2016). GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. doi: 10.1016/j.neuron.2016.06.033
Tsuang, M. T., Bar, J. L., Stone, W. S., and Faraone, S. V. (2004). Gene-environment interactions in mental disorders. World Psychiatry 3, 73–83.
World Health Organization. (2007). Neurological disorders affect millions globally. J. Environ. Health 69, 70–71.
Wu, Q., and Jia, Z. (2021). Wiring the brain by clustered protocadherin neural codes. Neurosci. Bull. 37, 117–131. doi: 10.1007/s12264-020-00578-4
Wu, Q., Liu, P., and Wang, L. (2020). Many facades of CTCF unified by its coding for three-dimensional genome architecture. J. Genet. Genomics 47, 407–424. doi: 10.1016/j.jgg.2020.06.008
Wu, Q., and Maniatis, T. (1999). A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97, 779–790. doi: 10.1016/S0092-8674(00)80789-8
Yoshino, Y., and Dwivedi, Y. (2020). Non-coding RNAs in psychiatric disorders and suicidal behavior. Front. Psychiatry 11:543893. doi: 10.3389/fpsyt.2020.543893
Keywords: circRNA, nutrition, DNA methylation, BDNF, HDAC4, protocadherins
Citation: Jiang Y, Schulze-Hentrich JM and Jakovcevski M (2022) Editorial: Neuroepigenetics of Neuropsychiatric Disease—Hope, Success and Obstacles for Translational Findings and Applications. Front. Neurosci. 16:886695. doi: 10.3389/fnins.2022.886695
Received: 28 February 2022; Accepted: 07 March 2022;
Published: 01 April 2022.
Edited and reviewed by: Sarah H. Elsea, Baylor College of Medicine, United States
Copyright © 2022 Jiang, Schulze-Hentrich and Jakovcevski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mira Jakovcevski, mira.jakovcevski@gmail.com
 Yan Jiang
Yan Jiang Julia M. Schulze-Hentrich
Julia M. Schulze-Hentrich Mira Jakovcevski
Mira Jakovcevski