- 1Department of Pharmacology, University of Washington, Seattle, WA, United States
- 2Department of Biomedical Sciences of Cells & Systems, University Medical Centre Groningen, University of Groningen, Groningen, Netherlands
Depression is a severe mental disorder that places a significant economic burden on public health. The reciprocal link between the trillions of bacteria in the gut, the microbiota, and depression is a controversial topic in neuroscience research and has drawn the attention of public interest and press coverage in recent years. Mounting pieces of evidence shed light on the role of the gut microbiota in depression, which is suggested to involve immune, endocrine, and neural pathways that are the main components of the microbiota-gut-brain axis. The gut microbiota play major roles in brain development and physiology and ultimately behavior. The bidirectional communication between the gut microbiota and brain function has been extensively explored in animal models of depression and clinical research in humans. Certain gut microbiota strains have been associated with the pathophysiology of depression. Therefore, oral intake of probiotics, the beneficial living bacteria and yeast, may represent a therapeutic approach for depression treatment. In this review, we summarize the findings describing the possible links between the gut microbiota and depression, focusing mainly on the inflammatory markers and sex hormones. By discussing preclinical and clinical studies on probiotics as a supplementary therapy for depression, we suggest that probiotics may be beneficial in alleviating depressive symptoms, possibly through immune modulation. Still, further comprehensive studies are required to draw a more solid conclusion regarding the efficacy of probiotics and their mechanisms of action.
Gut Microbiota in Health
The trillions of bacteria inhabiting our gastrointestinal (GI) tract are referred to as “gut microbiota” and are well known to exert a marked influence on the host during homeostasis (Thursby and Juge, 2017). Approximately 160 species of bacteria are living in the human colon, with Bacteroidetes and Firmicutes being the dominant bacterial phyla in healthy individuals (Qin et al., 2010; Huttenhower et al., 2012; Sommer and Backhed, 2013; Rajilic-Stojanovic and de Vos, 2014). The gut microbiota are separated from immune cells by two mucus layers and a single layer of epithelial cells (Johansson et al., 2013; Mowat and Agace, 2014), allowing the regulation of the immune system and vice versa (Round and Mazmanian, 2009; Gensollen et al., 2016). They benefit the host by strengthening the gut integrity (Natividad and Verdu, 2013), providing nutrients such as vitamins (LeBlanc et al., 2013; Rowland et al., 2018), promoting resistance to colonization by pathogenic species (Cameron and Sperandio, 2015; Pacheco and Sperandio, 2015; Sassone-Corsi and Raffatellu, 2015; Baumler and Sperandio, 2016) and harvesting energy (den Besten et al., 2013). Therefore, a pathological alteration of the gut microbiota composition, known as dysbiosis, can have serious consequences on the health of the host, ranging from chronic GI diseases to neuropsychiatric disorders (Guinane and Cotter, 2013; Petersen and Round, 2014; Schroeder and Backhed, 2016).
The high-throughput and low-cost sequencing methods that have become available over the last decade enabled the investigation of the gut microbiota (Thursby and Juge, 2017). The bacterial species are nowadays easily distinguished via targeting the bacterial 16S ribosomal RNA gene that is present in all bacteria with enough sequence conservation and nine highly variable regions for phylogenetic analyses (Mizrahi-Man et al., 2013; Poretsky et al., 2014). To characterize the nature of the human microbiota and pave the way for a better understanding of human health and disease, the Human Microbiome Project (HMP) was carried out over 10 years and two phases (Turnbaugh et al., 2007; Proctor et al., 2019). Additionally, it can identify new diagnostic biomarkers of health, which will enhance our knowledge of the nutritional requirements of humans (Turnbaugh et al., 2007).
Different factors are known to shape the gut microbiota compositions including age (Odamaki et al., 2016; Hill et al., 2017; Aleman and Valenzano, 2019), host genetics (Kovacs et al., 2011; Org et al., 2016; Elderman et al., 2018; Kim et al., 2020), drugs (Wilson and Nicholson, 2009; Maier et al., 2018; Vich Vila et al., 2020), body mass index (BMI) (Ley et al., 2005; Yun et al., 2017; Gao et al., 2018; Stanislawski et al., 2018; Bai et al., 2019), diet, mode of delivery (Salminen et al., 2004; Rutayisire et al., 2016; Akagawa et al., 2019; Reyman et al., 2019), and environmental factors (Li et al., 2014; Osadchiy et al., 2019) (Figure 1). In particular, dietary modification exert a large effect on the gut microbiota (Walker et al., 2011; Zoetendal et al., 2012; David et al., 2014; Carmody et al., 2015) and can produce a shift in several bacterial species within 24 h (Wu et al., 2011). Also, antibiotics affect microbiota composition by depleting resident microbiota and subsequent enrichment of certain antibiotic-resistant strains, leading to pathogenic effects (Morgun et al., 2015). Other non-antibiotic drugs, such as proton-pump inhibitors, but also several psychiatric drugs, are known to have an extensive impact on the human gut microbiota (Maier et al., 2018).
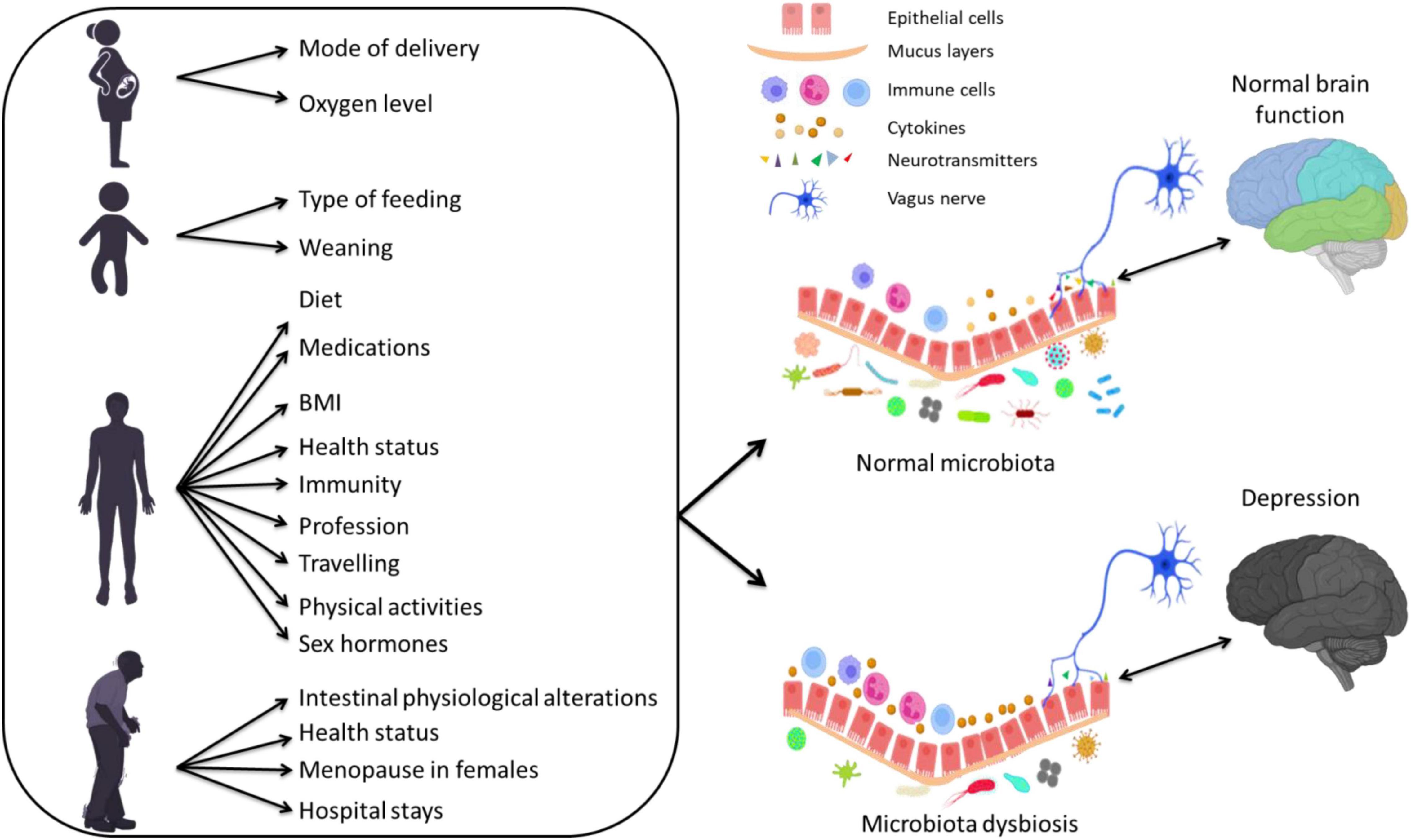
Figure 1. The effect of several factors during different stages of life on the gut microbiota composition and their association with depression via altering inflammatory responses.
Microbiota Dysbiosis in Depression
Depression is a debilitating neuropsychiatric disorder that involves persistent low mood and loss of interest that can be long-lasting or recurrent, substantially decreasing the quality of life (Wittchen et al., 2011; Lim et al., 2012; Murray et al., 2013; Whiteford et al., 2013). Symptoms usually are a reduced interest or pleasure in previously pleasurable activities, loss of sexual desire, changes in appetite, weight loss or gain, sleep disorders and motor retardation, along with recurrent thoughts of guilt and death (McCarter, 2008). It affects approximately 4.4% of the world’s population with an incidence rate above the rate of global population growth (Flux and Lowry, 2020). Despite the high prevalence rate of depression and the ongoing efforts to enhance the skills of healthcare providers, this mental disorder remains underdiagnosed and undertreated (Williams et al., 2017). The late diagnosis and treatment of depression are associated with a reduced treatment outcome, underscoring the importance of early intervention (Riedel et al., 2011).
The development of depression involves a complex interplay of biological, genetic, and environmental factors (Lopizzo et al., 2015). In addition to the strong association between gut microbiota and autism, schizophrenia and attention deficit hyperactivity disorder (for a review, see Eltokhi et al., 2020), accumulating shreds of evidence over the past 10 years support the hypothesis that gut microbiota may determine the initial risk and persistence of depression and contribute to treatment and resilience (for reviews, see Mangiola et al., 2016; Rogers et al., 2016; Flux and Lowry, 2020). The gut microbiota can signal to the CNS by way of neurohormones, vagal tonus, immune activation and metabolites that can alter eating behavior and mood (Martin et al., 2018). Moreover, healthy diets such as the Mediterranean one are suggested to reduce the risk of depression (Sanchez-Villegas et al., 2007; Skarupski et al., 2013; Pagliai et al., 2018; Konstantinos and Evangelia, 2019; Lassale et al., 2019) via modulation of the gut microbiota composition (De Filippis et al., 2016; Garcia-Mantrana et al., 2018; Krznaric et al., 2019; Nagpal et al., 2019). Confirming the role of gut microbiota in depression, a large epidemiological study revealed that the destabilization of the gut microbiota composition by antibiotics resulted in a 20–50% increased risk of depression (Lurie et al., 2015). Moreover, the underrepresentation of Firmicutes in the gut has been associated with depression (Huang et al., 2018), and 25.8% of individuals with inflammatory bowel disease (IBD) suffered from depression in the previous year (Lai and Cai, 2018).
Preclinical studies on rodents have investigated the link between gut microbiota and depression at a deeper level than clinical studies (Flux and Lowry, 2020). Studies mostly from the microbiota-devoid germ-free mice or mice treated with broad-spectrum antibiotics have shown that specific microbiota can impact brain physiology and behavior including deficiencies in learning, memory, recognition, and emotional behaviors (Gareau et al., 2011; Smith, 2015; Foster et al., 2017), accompanied by structural changes in the brain (Zhang et al., 2015; Principi and Esposito, 2016; Dinan and Cryan, 2017; Fung et al., 2017; Martin and Mayer, 2017). Different rodent models that showed high face validity to human depression have been used in microbiota research (Krishnan and Nestler, 2011). These models include olfactory bulbectomy (Harkin et al., 2003), social stress models (Toyoda, 2017), maternal separation (MS) models of early life adversity (Matthews and Robbins, 2003; Neumann et al., 2005; O’Mahony et al., 2009), repeated restraint stress models (Glavin et al., 1994; Bailey et al., 2010), chronic unpredictable mild stress (Duan et al., 2021; Zhang et al., 2021), and diet-induced obesity (Bruce-Keller et al., 2015; Bridgewater et al., 2017; Agusti et al., 2018; Soto et al., 2018) (for a review, see Flux and Lowry, 2020). The depression-like behaviors in rodents are measured by several behavioral experiments including forced swim, tail-suspension, sucrose preference, splash and learned helplessness tests (Eltokhi et al., 2018). Confirming the link between gut microbiota and depression, stressed germ-free mice showed high circulating levels of depression-sustaining hormones such as ACTH and corticosterone (Sudo et al., 2004). Moreover, the transplantation of fecal matter from depressed patients into microbiota-depleted rats exhibited depressive-like behavior in the rats (Kelly et al., 2016).
Clinical evidence for the role of the microbiota in depression is provided by an alteration in the number of microbiota and their diversity in individuals with depression when compared to healthy controls (for a summary, see Table 1). A study of fecal samples from patients with major depressive disorder (MDD) documented increased counts of Bacteroidales and decreased counts of Lachnospiraceae (Naseribafrouei et al., 2014). The analysis of fecal matters from MDD patients revealed a negative, albeit weak, correlation between the abundance of Faecalibacterium and the severity of the depressive symptoms (Jiang et al., 2015), and a positive correlation between Enterobacteriaceae and Alistipes counts and depression (Jiang et al., 2015). In another clinical study, lower Bifidobacterium and/or Lactobacillus counts were more common in patients with MDD compared to healthy controls (Aizawa et al., 2016). Two other studies revealed a different abundance of bacterial families and taxa between patients with MDD and healthy controls (Chen Z. et al., 2018; Chung et al., 2019). Moreover, in a Chinese MDD cohort, alterations of microbiota composition were found in MDD patients with an overrepresentation of Actinobacteria and underrepresentation of Bacteroidetes (Zheng et al., 2016). Investigating a group suffering from treatment-resistant MDD revealed a link between microbiota neuroactive capacity and the quality of life and depression (Valles-Colomer et al., 2019). Similar gut microbiota dysbiosis between Irritable bowel syndrome (IBS), depression, and IBS/depression co-morbid patients confirms the close link between the gut microbiota composition and depression (Liu et al., 2016). Although several previous studies demonstrated that depression was linked to marked alterations in gut microbiota composition, contradicting results regarding the enrichment of certain microbiota phyla in individuals with depression were obtained (Table 1). This inconsistency may have resulted from differences in study design, sample sizes, demographic and clinical severity of patients, unmeasured confounding factors and/or the used statistical methods. Additionally, most of these studies had several limitations including small sample sizes and a lack of data on the dietary habits of the tested subjects, which hinders obtaining a solid conclusion about the suitability of the microbiota as a biomarker of depression. It is still unsure whether changes in the microbiota are a cause or a result of depression. As depression is associated with changes in dietary habits, sleep and stress, the gut microbiota dysbiosis may well be a consequence rather than a cause. On the other hand, the depressive phenotype was shown to be transmissible by fecal microbiota transplantation (FMT) from MDD patients to germ-free rodents, revealing increased anxiety and depressive-like behavior in rodents (Kelly et al., 2016; Zheng et al., 2016). This strongly suggests that the alterations of the gut microbiota associated with depression may be a cause, rather than a consequence of the disorder.
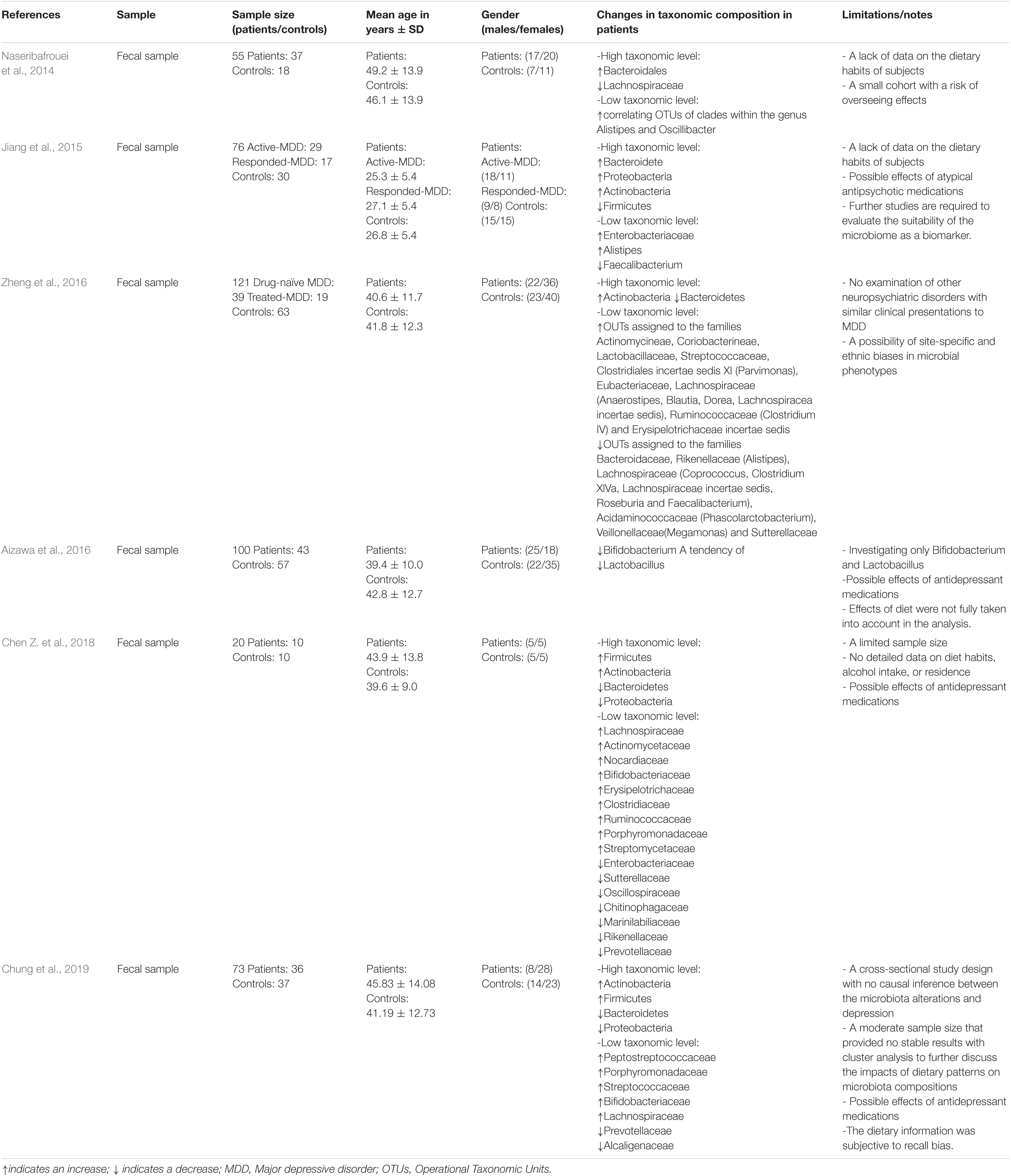
Table 1. Clinical studies investigating a correlation between the human fecal microbiota and depression.
Notably, several studies have shown that depression itself can affect the gut microbiota diversity via signals from the CNS to the gut environment, for example by changing secretion and motility in the stomach and gut. In an olfactory bulbectomy mouse model of depression, alteration of microbiota composition in the colon was suggested to be caused by increased activation of the stress response and alterations in colonic motility (Park et al., 2013). Moreover, diet disturbance in depression by the consumption of highly palatable foods is a major pathway from depression to gut microbiota dysbiosis (Rogers et al., 2016). Additionally, depression can reshape the gut microbiota composition by altering metabolic responses to food through hormones, inflammation, and autonomic alterations (Madison and Kiecolt-Glaser, 2019). For example, women suffering from depression had higher cortisol and fat oxidation levels (Kiecolt-Glaser et al., 2015), which can have a downstream effect on the gut microbiota (den Besten et al., 2013; Farzi et al., 2018). Other studies revealed that multiple classes of antidepressants also have anti-microbial properties against pathogenic bacteria (Macedo et al., 2017). For example, the antidepressant fluoxetine enriched the bacterial species associated with the regulation of BMI in mice (Lyte et al., 2019). The next-generation antidepressant ketamine plays a role in regulating the gut microbiota diversity (Yang et al., 2017) and showed increased Lactobacillus, Turicibacter, and Sarcina counts in the rat fecal microbiota and reduced opportunistic pathogens Mucispirillum and Ruminococcus (Getachew et al., 2018). To this end, the aforementioned studies suggest that the link between gut microbiota composition, antidepressants and depression is reciprocal.
The Effect of Gut Microbiota on Local and Circulating Inflammatory Markers and Its Relation to Depression
Depression has been associated with inflammation and its components such as inflammatory cytokines for more than 20 years (Ader and Cohen, 1985; Maes, 1995; Maes et al., 2012; Patel, 2013; Skaper et al., 2014; Miller and Raison, 2016; Leonard, 2018). Several gene variants are involved in both immune activation and depression (Barnes et al., 2017). Moreover, depression is known to be associated with polymorphisms in inflammation-related genes (Mikova et al., 2001; Kokai et al., 2002; Wong et al., 2008). Elevated levels of pro-inflammatory cytokines including Interleukin (IL)-1, IL-6, IL-8, and IL-12 were reported in individuals suffering from depression (Dowlati et al., 2010; Duivis et al., 2013; Lamers et al., 2013; Kim et al., 2016). Furthermore, IL-6, tumor necrosis factor-alpha (TNF-α), and IL-1β are implicated in the pathophysiology of depression (Brebner et al., 2000), and individuals with depression often have elevated circulating IL-1β, IL-6, and TNF with reduced levels of interferon-gamma (IFN-γ), IL-10, and IL-4 (Goldsmith et al., 2016). Longitudinal studies linked the high levels of pro-inflammatory cytokines with future risk for depression (van den Biggelaar et al., 2007; Gimeno et al., 2009). The pro-inflammatory cytokines have been connected to a pattern of sickness behaviors that include depressed mood, lethargy, heightened pain sensitivity, and sleep and appetite disturbances: all hallmarks of depression (Flux and Lowry, 2020). Moreover, non-specific inflammatory markers such as haptoglobin, fibrinogen, and C-reactive protein (CRP) are increased in depressed patients (Sluzewska et al., 1996).
Supporting the relationship between inflammation and depression, significant inflammatory activity in rodent models of depression has been identified (Song and Wang, 2011). Moreover, several antidepressants have been shown to reduce the endogenous production of pro-inflammatory cytokines along with a modification of immune reactivity in the CNS (Capuron et al., 2002; Nazimek et al., 2017; Gałecki et al., 2018). Tricyclic antidepressants inhibit the release of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α (Xia et al., 1996). Additionally, the next-generation antidepressant ketamine has been shown to decrease depressive symptoms via a decrease in circulating IL-1β levels (Tan et al., 2017), a protein known to cause neuroinflammation and depressive behaviors (Dunn et al., 2005; Dunn, 2006; Swiergiel and Dunn, 2007; Ransohoff and Brown, 2012). Conversely, anti-inflammatory drugs have been shown to enhance recovery in patients with depression (Mendlewicz et al., 2006; Muller et al., 2006), and depressive symptoms are observed clinically with the administration of IFNs (Baranyi et al., 2013; Mahajan et al., 2014). IL-10 knockout mice displayed a decreased latency to immobility in a forced swim test as a measure of depression, which was rescued by the administration of IL-10 (Mesquita et al., 2008) that is known to inhibit pro-inflammatory cytokine production (Ledeboer et al., 2000). Recent evidence indicated that microbiota dysbiosis is associated with the development of several chronic inflammatory disorders such as IBD (Ni et al., 2017). Notably, several gut microbial taxa with differential abundance patterns common to immune-mediated inflammatory diseases were identified (Forbes et al., 2018). Several genera including Lactobacillus, Bifidobacterium, and Faecalibacterium have been shown to stimulate the anti-inflammatory cytokine including IL-10 (Sokol et al., 2008) and downregulate inflammatory cytokines (Llopis et al., 2009).
As the gut microbiota play a fundamental effect on the gut inflammatory and immune responses, it is highly likely that the role of gut microbiota dysbiosis in the pathophysiology of depression is induced by immune and inflammation responses (Slyepchenko et al., 2017). Evidence of increased bacterial translocation due to the disruption of tight junctions and barrier integrity of the GI tract has now surfaced in the pathogenesis of depression (Slyepchenko et al., 2017). The non-invasive bacterial translocation to the mesenteric lymph nodes, the lamina propria, and the peripheral blood results in immune activation and increased production of pro-inflammatory cytokines. Heightened IgA and IgM-mediated immune responses to lipopolysaccharide, a component of the cell walls of gram-negative bacteria, in MDD support the notion of increased gut microbiota translocation due to a leaky gut (Maes et al., 2008).
Evidence from rodent experiments confirms the link between microbiota and depression via inflammatory markers. Socially stressed mice showing increased fecal Oscillospira and a decreased fecal Firmicutes/Bacteroidetes ratio developed depression-like symptoms (Zhang et al., 2017). These symptoms were rescued via an intravenous treatment with an anti-mouse IL-6 receptor antibody (MR16-1) that significantly decreased Oscillospira counts and attenuated the decrease in the fecal Firmicutes/Bacteroidetes ratio (Zhang et al., 2017). Genetic deletion or pharmacological inhibition of Caspase-1 that cleaves IL-1β and IL-18 into their mature isoforms exhibited reduced depression- and anxiety-like behaviors in a chronic restraint stress model, combined with an increase in Akkermansia species that is associated with decreased inflammation and a rebalancing of the gut microbiota (Alcocer-Gomez et al., 2014; Anhe et al., 2015; Yang et al., 2015). Recent work has targeted the effects of the inflammasome in studying the relationship between the immune system, gut microbiota and depression (Inserra et al., 2018). It was hypothesized that the increased NLRP3 signaling due to stress/depression can lead to an overrepresentation of pro-inflammatory bacterial clades within the gut microbiota (Inserra et al., 2018). This pathological change in the gut microbiota composition may even strengthen the stress/depression phenotype and increase the risk of other NLRP3-related co-morbid disorders. Notably, several pieces of evidence have shown that the link between microbiota, inflammation and depression is indeed reciprocal (Figure 2). Microbiota dysbiosis in inflammatory diseases such as IBS can lead to depression (Tribbick et al., 2015; Liu et al., 2016). Conversely, repeated stress and/or depression increases pro-inflammatory signaling (Rohleder, 2014), leading to microbiota dysbiosis and increased representation of pro-inflammatory bacterial clades (Rogers et al., 2016; Wong et al., 2016; Zheng et al., 2016).
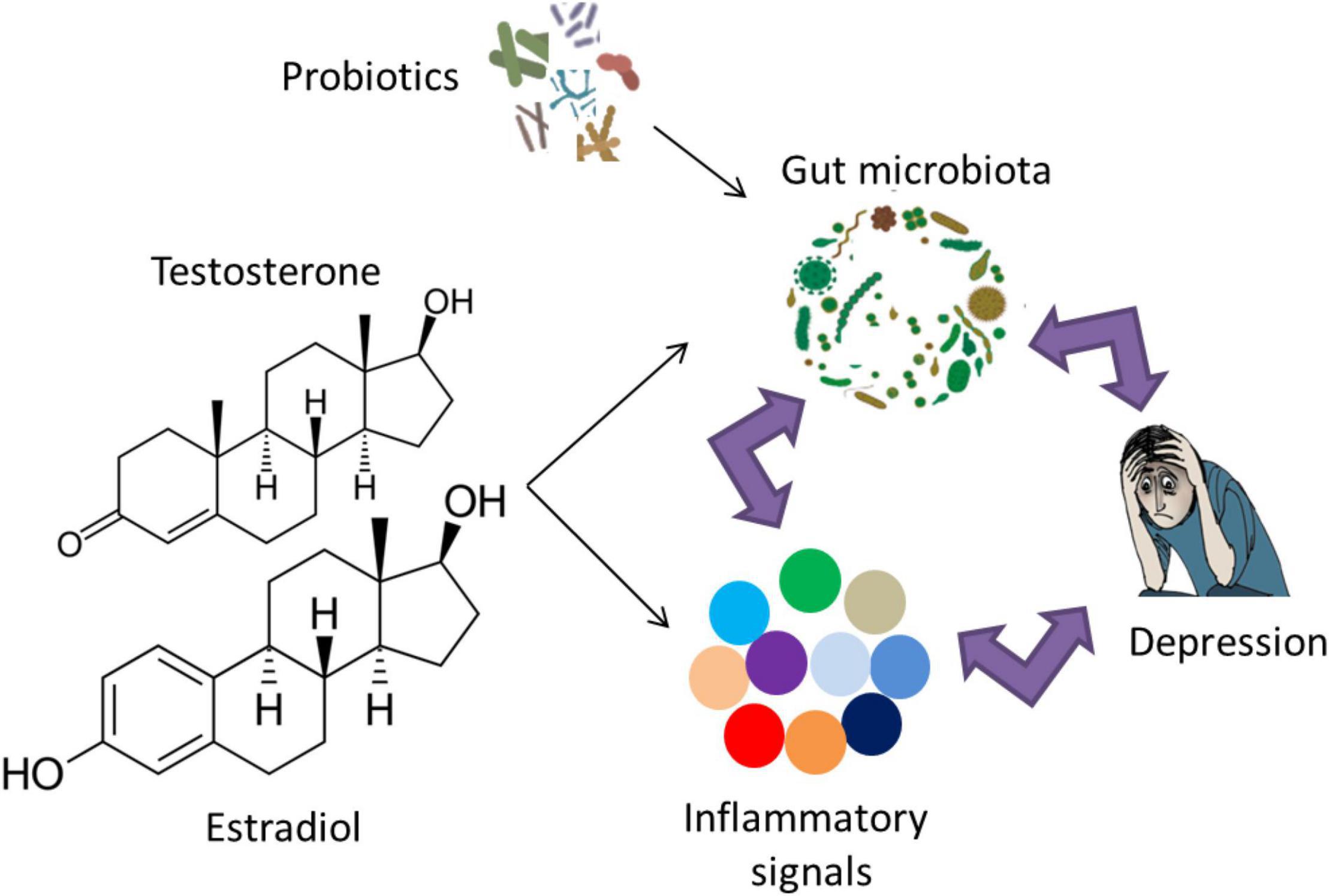
Figure 2. The reciprocal link between gut microbiota, inflammation and depression. The role of probiotic supplementation in turning the gut microbiota composition into a healthier form may lead to normal brain function and alleviation of depressive symptoms via modulation of inflammatory responses. Examples of probiotic genera include Lactobacillus, Bifidobacterium, Lactococcus, Propionibacterium, Bulgaricus, and Streptococcus. Notably, sex hormones affect gut microbiota and/or inflammatory signals, which may play a role in the sex bias of depression.
Influence of Sex Hormones on the Gut Microbiota Composition and Gender Bias in Depression
Depression is more frequent in women than men with a ratio of 2:1, which was reported globally and independent of race or ethnicity (Weissman and Klerman, 1977; Cyranowski et al., 2000; Andrade et al., 2003; Ford and Erlinger, 2004; Patten et al., 2006; Bromet et al., 2011; Salk et al., 2017). The finding of similar female: male prevalence ratios worldwide suggests that the differential risk is highly dependent on biological sex differences rather than race, culture or other potentially confounding social and economic factors (Albert, 2015). As the onset of depressive disorders in women peaks in their reproductive years, the increased prevalence of depression may be explained, in part, by sex hormones. Indeed, the female hormonal fluctuation during puberty, menstruation, pregnancy, and menopause is a trigger for depression (Albert, 2015). Estrogen and progesterone affect neurotransmitter, neuroendocrine, and circadian systems that have been implicated in mood disorders (Wharton et al., 2012). Androgens seem to have anxiolytic properties, whereas estrogen receptor activation has opposite consequences, with ERα having largely anxiogenic-like properties and ERβ serving to generate anxiolytic-like effects (Borrow and Handa, 2017). Furthermore, rates of depression in females correlate with the low levels of estrogen that occur throughout the life cycle (Albert et al., 2015). The risk of depression appears to increase during the perimenopausal transition (Cohen et al., 2006), with hormone replacement therapy being effective in the prevention of postmenopausal depression in women (Gordon and Girdler, 2014). In this light, a study has indicated that women who reported using an oral contraceptive showed reduced rates of MDD compared with non-users (Cheslack-Postava et al., 2015), suggesting a protective role of estrogen against depression. In contrast, other studies have shown depression and worsened mood as potential adverse effects of hormonal contraceptive use (Wiebe et al., 2011; Gingnell et al., 2013; Skovlund et al., 2016; de Wit et al., 2020). The heterogeneity in these findings may be explained by differences in study populations (de Wit et al., 2020).
Current evidence confirming the role of sex hormones on depression incidence relies mainly on rodent work. A recent study has explored the effect of treatment with a 5-α-reductase inhibitor that converts steroid hormones testosterone and progesterone into dihydrotestosterone and dihydroprogesterone, respectively on depression-like behavior in rats, as well as 1 month of treatment withdrawal (Diviccaro et al., 2019). The withdrawal from the 5-α-reductase inhibitor was associated with elevated depression-like symptoms, as measured by the forced swim test (Diviccaro et al., 2019). Interestingly, a change in sex hormone concentration is associated with an altered immune profile and a shift in inflammation responses (Figure 2; Kupelian et al., 2009; Casimir et al., 2010; Karim et al., 2010; Maggio et al., 2011; Bobjer et al., 2013; Park and Lee, 2020; Grandys et al., 2021) (for reviews, see Monteiro et al., 2014; Au et al., 2016; Mohamad et al., 2018; Slavich and Sacher, 2019). The alteration of inflammatory responses due to sex hormones suggests their combined role in the pathophysiology of depression.
Although gut microbiota differed in males and females, castrated male mice showed similar microbiota composition to females, suggesting an influence of androgens on the gut microbiota composition (Markle et al., 2013; Yurkovetskiy et al., 2013). Moreover, testosterone treatment after gonadectomy rescued the alteration in the gut microbiota composition that was observed in the untreated males (Org et al., 2016). Several studies have shown a direct effect of estrogen on the gut microbiota composition (Flores et al., 2012; Cox-York et al., 2015; Chen and Madak-Erdogan, 2016; Org et al., 2016). Treatment with a 5-α-reductase inhibitor was associated with increased Bacteroidetes and Prevotellaceae counts, and the withdrawal was associated with a reduction in the family Ruminococcaceae and the genera Oscillospira and Lachnospira (Diviccaro et al., 2019). Interestingly, there seems to be a reciprocal interaction between gut microbiota and sex hormones leading to a change in the level of sex hormones because of differences in gut microbiota (Markle et al., 2013) and that gut microbiota regulate the production and/or utilization of testosterone and cause a difference in metabolism (Colldén et al., 2019).
Several studies have addressed the effects of sex on the gut microbiota in humans, which is suggested to be mediated, at least in part, by a difference in sex hormones levels (Figure 2; Mueller et al., 2006; Li et al., 2008; Ding and Schloss, 2014; Dominianni et al., 2015; Haro et al., 2016; Singh and Manning, 2016; Borgo et al., 2018; Gao et al., 2018; Sinha et al., 2019; Takagi et al., 2019) (for a recent review, see Kim et al., 2020). The sex differences in the gut microbiota composition mediated by sex hormones may exert adverse inflammatory and psychological effects related to depression and other neuropsychiatric disorders (Yurkovetskiy et al., 2013). For instance, adult women have reduced Bacteroidetes counts in their gut compared to age-matched men (Dominianni et al., 2015). Interestingly, the low levels of Bacteroidetes were previously observed in the fecal microbiota from patients diagnosed with clinical depression (Naseribafrouei et al., 2014). A study in a Chinese cohort revealed increased Actinobacteria levels in female MDD patients compared to female controls but reduced Bacteroidetes levels in male MDD patients compared to male controls (Chen J. J. et al., 2018).
In summary, the sex differences in the gut microbiota composition due to the effect of sex hormones may play a role in the sex bias of depression.
Probiotics as an Adjunctive Therapeutic Option for Depression
Given the strong association between the gut microbiota and the pathophysiology of depression, modulation of the gut microbiota composition can be a promising method for ameliorating the behavioral symptoms related to depression along with other neuropsychiatric disorders (Larroya-Garcia et al., 2019). One way for maintaining a healthy gut microbiota composition is mediated by the supplementation of probiotics; the consumable microbes intended to promote a healthy microbiota and can provide a benefit to the host when administered in adequate amounts (Butel, 2014) (Figure 2). The main bacterial genera used as probiotics in preclinical and clinical studies are the Lactobacillus and Bifidobacterium genera (Genedi et al., 2019). Studies involving animal models demonstrated that probiotics improved cognition, mood, anxiety, and stress (Sudo et al., 2004; Desbonnet et al., 2010; Bravo et al., 2011; Ait-Belgnaoui et al., 2014; Smith et al., 2014; Mohle et al., 2016; Bruce-Keller et al., 2018; Chunchai et al., 2018; Hadizadeh et al., 2019). Probiotics have also been studied in non-psychiatric individuals, and initial work showed improvements in cognitive function (Marotta et al., 2019) along with reducing constipation in different populations (Chmielewska and Szajewska, 2010; Miller and Ouwehand, 2013; Dimidi et al., 2014). Probiotic supplementation showed an enhancement in sleep, autonomic balance, and bowel habits and reduced stress and cortisol levels in Japanese medical students (Nishida et al., 2017). Moreover, healthy women who drank a probiotic-containing fermented milk product for 1 month showed lower brain activation when exposed to emotional stimuli (Tillisch et al., 2013). In a randomized controlled trial in healthy individuals, multispecies probiotics were able to reduce cognitive reactivity toward sad moods via a reduction in rumination and aggressive thoughts (Steenbergen et al., 2015). The Lactobacillus helveticus and Bifidobacterium longum probiotic mix given to healthy individuals for 1 month alleviated psychological distress compared to a control group, similar to the results seen in rats (Messaoudi et al., 2011). Moreover, the healthy individuals in the bottom third on the depressed/elated dimension reported improved mood after drinking probiotic-containing milk for 3 weeks (Benton et al., 2007).
The desire for a more effective treatment and a prevention of depression seems perpetually at the top of the list in terms of global health concerns to reduce the burden of this condition. The idea of treating depression with probiotics roots back to 1910 when Dr. George Porter Phillips reported that a gelatin-whey formula comprised of lactic-acid-producing bacteria decreased depressive symptoms in melancholic adults (Phillips, 1910). Preclinical studies and clinical trials have increasingly investigated the roles of probiotics in the treatment of depressive-like behaviors. In rodents, there is considerable evidence suggesting the effect of probiotics in decreasing the depression-like behaviors. Lactobacillus rhamnosus administration reduced stress-induced anxiety- and depressive-like behaviors in mice (Bravo et al., 2011). Bifidobacterium longum improved the depressive-like phenotype during the tail suspension test in a mouse model of heightened anxiety (Savignac et al., 2014). Likewise, daily Lactobacillus helveticus NS8 treatment reduced anxiety- and depressive-like behavioral dysfunctions in adult specific-pathogen-free rats facing chronic restraint stress in comparison to a selective serotonin reuptake inhibitor (SSRI) treatment (Liang et al., 2015). In a chronic mild stress model of depression, mice receiving a three-strain probiotic blend, Lactobacillus helveticus, Lactobacillus plantarum, and Bifidobacterium longum, displayed improved depression-like behavioral responses accompanied by a reduced TNF-α and interferon (IFN)-γ levels (Li et al., 2018). In another study, the administration of Faecalibacterium prausnitzii via oral gavage for 4 weeks in a chronic unpredictable mild stress model in rats resulted in decreased depression-like behaviors accompanied by increased IL-10 and decreased IL-6 and CRP levels (Hao et al., 2019). In another study, an administration of a probiotic formulation containing Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium breve, and Pediococcus pentosaceus alleviated depressive-like behaviors in mice and decreased corticosterone level by restoring the gut microbiota composition (Liu et al., 2020). In Fischer and Long Evans rats subjected to maternal deprivation, a probiotic mixture composed of Lactobacillus helveticus, Bifidobacterium longum, Lactococcus lactis, and Streptococcus thermophilus reduced anxiety- and depression-like behaviors accompanied by a change in the levels of certain metabolites (Daugé et al., 2020).
Clinical studies revealed contradicting results regarding the efficiency of probiotics in decreasing depressive symptoms (for a summary, see Table 2). In an 8-week, randomized, double-blind, placebo-controlled clinical trial, a triple-strain probiotic mix, Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum, resulted in improvements in depression scores in BDI in an MDD cohort (Akkasheh et al., 2016). Additionally, other beneficial metabolic effects were revealed including a significant reduction in inflammatory markers such as serum insulin, homeostasis model assessment of insulin resistance and serum hs-CRP (Akkasheh et al., 2016). Another 8-week, randomized, double-blind, placebo-controlled clinical trial examining the effect of a probiotic mix of Lactobacillus helveticus and Bifidobacterium longum on mild to moderate MDD reported a decrease in depression score in BDI (Kazemi et al., 2019). Interestingly, a randomized, placebo-controlled, double-blind study reported that daily Lactobacillus rhamnosus during pregnancy and into the post-partum period significantly decreased postnatal anxiety and depression scores (Slykerman et al., 2017). Another study testing the add-on effect of probiotic and prebiotic, the non-digestible plant-based carbohydrates that serve as nutrition for resident bacteria, on fluoxetine revealed a decrease in the score of hamilton rating scale for depression (HAM-D) compared to the placebo after 6 weeks of treatment (Ghorbani et al., 2018). One study provided petrochemical workers with either a probiotic capsule, probiotic yogurt, or conventional yogurt control, with both probiotic conditions resulting in a reduction of depressive symptoms on the general health questionnaire (GHQ) and depression anxiety and stress scale (DASS) scores as compared to control (Mohammadi et al., 2016). In another study, the efficacy of Bacillus coagulans administration on MDD in IBS patients was tested and revealed an improvement in HAM-D, Montgomery-Asberg Depression Rating Scale (MADRS), Center for Epidemiological Studies Depression Scale (CES-D) as well as reduced serum myeloperoxidase, an inflammatory biomarker (Majeed et al., 2018). In a recent open-label pilot study, a probiotic supplement containing Lactobacillus helveticus and Bifidobacterium longum administrated once per day for 8 weeks showed an improvement in affective clinical symptoms after 4 weeks, which was sustained till the end of the study (Wallace and Milev, 2021).
Other studies in the literature failed to show any improvement of depression scores in MADRS following 8 weeks of primary treatment with probiotics mix of Lactobacillus helveticus and Bifidobacterium longum (Romijn et al., 2017). Moreover, there was no change in psychological symptoms and the concentrations of CRP, IL-1β, IL-6, or TNF between baseline and the end of the study (Romijn et al., 2017). Worth notice is that individuals in this study were not taking any antidepressant medication at the time of the study, and their depressive history was entirely self-reported without a formal diagnostic interview (Romijn et al., 2017). In another study of individuals diagnosed with MDD, SSRI treatment supplemented with Lactobacillus plantarum did not exhibit any improvement in depressive symptoms and did not change the levels of circulating TNF, IL-6, or IL-1β, although there was an increase in cognitive functioning (Rudzki et al., 2019). In another study, the health properties of Lactobacillus reuteri DSM17938 were investigated in individuals older than 65 and revealed no improvement in digestive health, general wellbeing, stress, anxiety, or depression (Östlund-Lagerström et al., 2016). In a randomized clinical study in 2020, a combination of 9 bacterial species Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W22, Lactobacillus casei W56, Lactobacillus paracasei W20, Lactobacillus plantarum W62, Lactobacillus salivarius W24, and Lactobacillus lactis W19 in addition to biotin yielded no improvement in psychiatric symptoms in patients with MDD (Reininghaus et al., 2020).
Conclusion
Gut microbiota were incorporated into neurobiological models of depression via an effect on immune, endocrine, and nervous system responses, allowing a more comprehensive model of depression (Figure 2). The strong association between microbiota dysbiosis and depression could pave the way for enhancing diagnostic accuracy and patient phenotyping for treatment selection. Moreover, it can advance the treatment and prevention of depression by modifying the gut microbiota composition and offer an important future strategy in psychiatry via nutritional interventions, prebiotic or probiotic supplementations. Although preclinical mechanistic experimental data indicated that the manipulation of the gut microbiota with probiotics may have antidepressant and anxiolytic effects, there is limited clinical evidence for the efficacy of probiotics in depression at present. Notably, several probiotic studies that failed to show an improvement in depression revealed also no change in pro-inflammatory markers. Given the reciprocal link between microbiota, inflammation and depression discussed in this review, we believe that probiotics may partially exert their roles through a modification of the immune system. Generally, additional randomized clinical trials in patients with depression are necessary to fully evaluate their therapeutic potential on the clinical diagnosis and inflammatory biomarkers. Different factors should be kept into consideration to maximize the benefit of probiotic supplementation. The microbial diversity obtained by incorporating multiple strains of organisms is suggested to be more effective than using a single organism. Investigations at a finer taxonomic level coupled with multi-omic techniques such as transcriptomics, proteomics, and metabolomics are also needed to exclude confounding factors as much as possible. Since sex hormones can affect the inflammation biomarkers, with probiotics causing different inflammatory responses in female and male mice (Karunasena et al., 2014; Lee et al., 2017), sex should be taken into account in studies on gut microbiota, probiotics and depression.
Author Contributions
AE conceptualized and wrote the first draft. IES edited and reviewed the final draft. Both authors contributed to the article and approved the submitted version.
Funding
AE is partially supported by a postdoc stipend from the Fritz Thyssen Foundation. IES has received funding from the Stanley Medical Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
AE sincerely appreciates the continuous support from Shaimaa Madbouly and Rolf Sprengel.
References
Ader, R., and Cohen, N. (1985). High time for psychoimmunology. Nature 315, 103–104. doi: 10.1038/315103b0
Agusti, A., Moya-Pérez, A., Campillo, I., Montserrat-De La Paz, S., Cerrudo, V., et al. (2018). Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol. Neurobiol. 55, 5337–5352. doi: 10.1007/s12035-017-0768-z
Ait-Belgnaoui, A., Colom, A., Braniste, V., Ramalho, L., Marrot, A., Cartier, C., et al. (2014). Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 26, 510–520. doi: 10.1111/nmo.12295
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257. doi: 10.1016/j.jad.2016.05.038
Akagawa, S., Tsuji, S., Onuma, C., Akagawa, Y., Yamaguchi, T., Yamagishi, M., et al. (2019). Effect of delivery mode and nutrition on gut microbiota in neonates. Ann. Nutr. Metab. 74, 132–139. doi: 10.1159/000496427
Akkasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., Taghizadeh, M., et al. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. doi: 10.1016/j.nut.2015.09.003
Albert, K., Pruessner, J., and Newhouse, P. (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59, 14–24. doi: 10.1016/j.psyneuen.2015.04.022
Albert, P. R. (2015). Why is depression more prevalent in women? J. Psychiatry Neurosci. 40, 219–221. doi: 10.1503/jpn.150205
Alcocer-Gomez, E., De Miguel, M., Casas-Barquero, N., Nunez-Vasco, J., Sanchez-Alcazar, J. A., Fernandez-Rodriguez, A., et al. (2014). NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 36, 111–117. doi: 10.1016/j.bbi.2013.10.017
Aleman, F. D. D., and Valenzano, D. R. (2019). Microbiome evolution during host aging. PLoS Pathog. 15:e1007727. doi: 10.1371/journal.ppat.1007727
Andrade, L., Caraveo-Anduaga, J. J., Berglund, P., Bijl, R. V., De Graaf, R., Vollebergh, W., et al. (2003). The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int. J. Methods Psychiatr. Res. 12, 3–21. doi: 10.1002/mpr.138
Anhe, F. F., Roy, D., Pilon, G., Dudonne, S., Matamoros, S., Varin, T. V., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. doi: 10.1136/gutjnl-2014-307142
Au, A., Feher, A., Mcphee, L., Jessa, A., Oh, S., and Einstein, G. (2016). Estrogens, inflammation and cognition. Front. Neuroendocrinol. 40, 87–100. doi: 10.1016/j.yfrne.2016.01.002
Bai, J., Hu, Y., and Bruner, D. W. (2019). Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7-18 years old children from the American Gut Project. Pediatr. Obes. 14:e12480. doi: 10.1111/ijpo.12480
Bailey, M. T., Dowd, S. E., Parry, N. M. A., Galley, J. D., Schauer, D. B., and Lyte, M. (2010). Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect. Immun. 78, 1509–1519. doi: 10.1128/IAI.00862-09
Baranyi, A., Meinitzer, A., Stepan, A., Putz-Bankuti, C., Breitenecker, R. J., Stauber, R., et al. (2013). A biopsychosocial model of interferon-alpha-induced depression in patients with chronic hepatitis C infection. Psychother. Psychosom. 82, 332–340. doi: 10.1159/000348587
Barnes, J., Mondelli, V., and Pariante, C. M. (2017). Genetic contributions of inflammation to depression. Neuropsychopharmacology 42, 81–98. doi: 10.1038/npp.2016.169
Baumler, A. J., and Sperandio, V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. doi: 10.1038/nature18849
Benton, D., Williams, C., and Brown, A. (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 61, 355–361. doi: 10.1038/sj.ejcn.1602546
Bobjer, J., Katrinaki, M., Tsatsanis, C., Lundberg Giwercman, Y., and Giwercman, A. (2013). Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One 8:e61466. doi: 10.1371/journal.pone.0061466
Borgo, F., Garbossa, S., Riva, A., Severgnini, M., Luigiano, C., Benetti, A., et al. (2018). Body mass index and sex affect diverse microbial niches within the gut. Front. Microbiol. 9:213. doi: 10.3389/fmicb.2018.00213
Borrow, A. P., and Handa, R. J. (2017). Estrogen receptors modulation of anxiety-like behavior. Vitam. Horm. 103, 27–52. doi: 10.1016/bs.vh.2016.08.004
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 108, 16050. doi: 10.1073/pnas.1102999108
Brebner, K., Hayley, S., Zacharko, R., Merali, Z., and Anisman, H. (2000). Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology 22, 566–580. doi: 10.1016/S0893-133X(99)00166-9
Bridgewater, L. C., Zhang, C., Wu, Y., Hu, W., Zhang, Q., Wang, J., et al. (2017). Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 7:10776. doi: 10.1038/s41598-017-11069-4
Bromet, E., Andrade, L. H., Hwang, I., Sampson, N. A., Alonso, J., De Girolamo, G., et al. (2011). Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 9:90. doi: 10.1186/1741-7015-9-90
Bruce-Keller, A. J., Salbaum, J. M., and Berthoud, H. R. (2018). Harnessing gut microbes for mental health: getting from here to there. Biol. Psychiatry 83, 214–223. doi: 10.1016/j.biopsych.2017.08.014
Bruce-Keller, A. J., Salbaum, J. M., Luo, M., Blanchard, E. I. V., Taylor, C. M., Welsh, D. A., et al. (2015). Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 77, 607–615. doi: 10.1016/j.biopsych.2014.07.012
Butel, M. J. (2014). Probiotics, gut microbiota and health. Med. Mal. Infect. 44, 1–8. doi: 10.1016/j.medmal.2013.10.002
Cameron, E. A., and Sperandio, V. (2015). Frenemies: signaling and nutritional integration in pathogen-microbiota-host interactions. Cell Host Microbe 18, 275–284. doi: 10.1016/j.chom.2015.08.007
Capuron, L., Hauser, P., Hinze-Selch, D., Miller, A. H., and Neveu, P. J. (2002). Treatment of cytokine-induced depression. Brain Behav. Immun. 16, 575–580. doi: 10.1016/s0889-1591(02)00007-7
Carmody, R. N., Gerber, G. K., Luevano, J. M., Gatti, D. M., Somes, L., Svenson, K. L., et al. (2015). Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 17, 72–84. doi: 10.1016/j.chom.2014.11.010
Casimir, G. J., Mulier, S., Hanssens, L., Zylberberg, K., and Duchateau, J. (2010). Gender differences in inflammatory markers in children. Shock 33, 258–262. doi: 10.1097/SHK.0b013e3181b2b36b
Chen, Z., Li, J., Gui, S., Zhou, C., Chen, J., Yang, C., et al. (2018). Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 29, 417–425. doi: 10.1097/WNR.0000000000000985
Chen, J. J., Zheng, P., Liu, Y. Y., Zhong, X. G., Wang, H. Y., Guo, Y. J., et al. (2018). Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 14, 647–655. doi: 10.2147/NDT.S159322
Chen, K. L., and Madak-Erdogan, Z. (2016). Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol. Metab. 27, 752–755. doi: 10.1016/j.tem.2016.08.001
Cheslack-Postava, K., Keyes, K. M., Lowe, S. R., and Koenen, K. C. (2015). Oral contraceptive use and psychiatric disorders in a nationally representative sample of women. Arch. Womens Ment. Health 18, 103–111. doi: 10.1007/s00737-014-0453-4
Chmielewska, A., and Szajewska, H. (2010). Systematic review of randomised controlled trials: probiotics for functional constipation. World J. Gastroenterol. 16, 69–75. doi: 10.3748/wjg.v16.i1.69
Chunchai, T., Thunapong, W., Yasom, S., Wanchai, K., Eaimworawuthikul, S., Metzler, G., et al. (2018). Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflammation 15:11. doi: 10.1186/s12974-018-1055-2
Chung, Y. E., Chen, H. C., Chou, H. L., Chen, I. M., Lee, M. S., Chuang, L. C., et al. (2019). Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 111, 74–82. doi: 10.1016/j.jpsychires.2019.01.016
Cohen, L. S., Soares, C. N., Vitonis, A. F., Otto, M. W., and Harlow, B. L. (2006). Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch. Gen. Psychiatry 63, 385–390. doi: 10.1001/archpsyc.63.4.385
Colldén, H., Landin, A., Wallenius, V., Elebring, E., Fändriks, L., Nilsson, M. E., et al. (2019). The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 317, E1182–E1192. doi: 10.1152/ajpendo.00338.2019
Cox-York, K. A., Sheflin, A. M., Foster, M. T., Gentile, C. L., Kahl, A., Koch, L. G., et al. (2015). Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep. 3:e12488. doi: 10.14814/phy2.12488
Cyranowski, J. M., Frank, E., Young, E., and Shear, M. K. (2000). Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch. Gen. Psychiatry 57, 21–27. doi: 10.1001/archpsyc.57.1.21
Daugé, V., Philippe, C., Mariadassou, M., Rué, O., Martin, J.-C., Rossignol, M.-N., et al. (2020). A probiotic mixture induces anxiolytic- and antidepressive-like effects in fischer and maternally deprived long evans rats. Front. Behav. Neurosci. 14:581296. doi: 10.3389/fnbeh.2020.581296
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
De Filippis, F., Pellegrini, N., Vannini, L., Jeffery, I. B., La Storia, A., Laghi, L., et al. (2016). High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821. doi: 10.1136/gutjnl-2015-309957
de Wit, A. E., Booij, S. H., Giltay, E. J., Joffe, H., Schoevers, R. A., and Oldehinkel, A. J. (2020). Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry 77, 52–59. doi: 10.1001/jamapsychiatry.2019.2838
den Besten, G., Van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Desbonnet, L., Garrett, L., Clarke, G., Kiely, B., Cryan, J. F., and Dinan, T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188. doi: 10.1016/j.neuroscience.2010.08.005
Dimidi, E., Christodoulides, S., Fragkos, K. C., Scott, S. M., and Whelan, K. (2014). The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 100, 1075–1084. doi: 10.3945/ajcn.114.089151
Dinan, T. G., and Cryan, J. F. (2017). Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 595, 489–503. doi: 10.1113/JP273106
Ding, T., and Schloss, P. D. (2014). Dynamics and associations of microbial community types across the human body. Nature 509, 357–360. doi: 10.1038/nature13178
Diviccaro, S., Giatti, S., Borgo, F., Barcella, M., Borghi, E., Trejo, J. L., et al. (2019). Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 99, 206–215. doi: 10.1016/j.psyneuen.2018.09.021
Dominianni, C., Sinha, R., Goedert, J. J., Pei, Z., Yang, L., Hayes, R. B., et al. (2015). Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10:e0124599. doi: 10.1371/journal.pone.0124599
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. doi: 10.1016/j.biopsych.2009.09.033
Duan, J., Huang, Y., Tan, X., Chai, T., Wu, J., Zhang, H., et al. (2021). Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 11:303. doi: 10.1038/s41398-021-01428-1
Duivis, H. E., Vogelzangs, N., Kupper, N., De Jonge, P., and Penninx, B. W. (2013). Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology 38, 1573–1585. doi: 10.1016/j.psyneuen.2013.01.002
Dunn, A. J. (2006). Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 6, 52–68. doi: 10.1016/j.cnr.2006.04.002
Dunn, A. J., Swiergiel, A. H., and Beaurepaire, R. D. (2005). Cytokines as mediators of depression: What can we learn from animal studies? Neurosci. Biobehav. Rev. 29, 891–909. doi: 10.1016/j.neubiorev.2005.03.023
Elderman, M., Hugenholtz, F., Belzer, C., Boekschoten, M., Van Beek, A., De Haan, B., et al. (2018). Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 9:26. doi: 10.1186/s13293-018-0186-6
Eltokhi, A., Janmaat, I. E., Genedi, M., Haarman, B. C. M., and Sommer, I. E. C. (2020). Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J. Neurosci. Res. 98, 1335–1369. doi: 10.1002/jnr.24616
Eltokhi, A., Rappold, G., and Sprengel, R. (2018). Distinct phenotypes of Shank2 mouse models reflect neuropsychiatric spectrum disorders of human patients with SHANK2 Variants. Front. Mol. Neurosci. 11:240. doi: 10.3389/fnmol.2018.00240
Farzi, A., Fröhlich, E. E., and Holzer, P. (2018). Gut Microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22. doi: 10.1007/s13311-017-0600-5
Flores, R., Shi, J., Fuhrman, B., Xu, X., Veenstra, T. D., Gail, M. H., et al. (2012). Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med. 10:253. doi: 10.1186/1479-5876-10-253
Flux, M. C., and Lowry, C. A. (2020). Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiol. Dis. 135, 104578. doi: 10.1016/j.nbd.2019.104578
Forbes, J. D., Chen, C.-Y., Knox, N. C., Marrie, R.-A., El-Gabalawy, H., De Kievit, T., et al. (2018). A comparative study of the gut microbiota in immune-mediated inflammatory diseases—does a common dysbiosis exist? Microbiome 6:221. doi: 10.1186/s40168-018-0603-4
Ford, D. E., and Erlinger, T. P. (2004). Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 164, 1010–1014. doi: 10.1001/archinte.164.9.1010
Foster, J. A., Rinaman, L., and Cryan, J. F. (2017). Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124–136.
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Gałecki, P., Mossakowska-Wójcik, J., and Talarowska, M. (2018). The anti-inflammatory mechanism of antidepressants – SSRIs, SNRIs. Prog. Neuropsychopharmacol. Biol. Psychiatry 80, 291–294. doi: 10.1016/j.pnpbp.2017.03.016
Gao, X., Zhang, M., Xue, J., Huang, J., Zhuang, R., Zhou, X., et al. (2018). Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 9:1250. doi: 10.3389/fmicb.2018.01250
Garcia-Mantrana, I., Selma-Royo, M., Alcantara, C., and Collado, M. C. (2018). Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 9:890. doi: 10.3389/fmicb.2018.00890
Gareau, M. G., Wine, E., Rodrigues, D. M., Cho, J. H., Whary, M. T., Philpott, D. J., et al. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. doi: 10.1136/gut.2009.202515
Genedi, M., Janmaat, I. E., Haarman, B., and Sommer, I. E. C. (2019). Dysregulation of the gut-brain axis in schizophrenia and bipolar disorder: probiotic supplementation as a supportive treatment in psychiatric disorders. Curr. Opin. Psychiatry 32, 185–195. doi: 10.1097/YCO.0000000000000499
Gensollen, T., Iyer, S. S., Kasper, D. L., and Blumberg, R. S. (2016). How colonization by microbiota in early life shapes the immune system. Science 352, 539–544. doi: 10.1126/science.aad9378
Getachew, B., Aubee, J. I., Schottenfeld, R. S., Csoka, A. B., Thompson, K. M., and Tizabi, Y. (2018). Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 18:222. doi: 10.1186/s12866-018-1373-7
Ghorbani, Z., Nazari, S., Etesam, F., Nourimajd, S., Ahmadpanah, M., and Razeghi Jahromi, S. (2018). The Effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch. Neurosci. 5:e60507.
Gimeno, D., Kivimaki, M., Brunner, E. J., Elovainio, M., De Vogli, R., Steptoe, A., et al. (2009). Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 39, 413–423. doi: 10.1017/S0033291708003723
Gingnell, M., Engman, J., Frick, A., Moby, L., Wikström, J., Fredrikson, M., et al. (2013). Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill–a double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology 38, 1133–1144. doi: 10.1016/j.psyneuen.2012.11.006
Glavin, G. B., Paré, W. P., Sandbak, T., Bakke, H.-K., and Murison, R. (1994). Restraint stress in biomedical research: An update. Neurosci. Biobehav. Rev. 18, 223–249. doi: 10.1016/0149-7634(94)90027-2
Goldsmith, D. R., Rapaport, M. H., and Miller, B. J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709. doi: 10.1038/mp.2016.3
Gordon, J. L., and Girdler, S. S. (2014). Hormone replacement therapy in the treatment of perimenopausal depression. Curr. Psychiatry Rep. 16:517. doi: 10.1007/s11920-014-0517-1
Grandys, M., Majerczak, J., Zapart-Bukowska, J., Duda, K., Kulpa, J. K., and Zoladz, J. A. (2021). Lowered serum testosterone concentration is associated with enhanced inflammation and worsened lipid profile in men. Front. Endocrinol. 12:735638. doi: 10.3389/fendo.2021.735638
Guinane, C. M., and Cotter, P. D. (2013). Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 6, 295–308. doi: 10.1177/1756283X13482996
Hadizadeh, M., Hamidi, G. A., and Salami, M. (2019). Probiotic supplementation improves the cognitive function and the anxiety-like behaviors in the stressed rats. Iran. J. Basic Med. Sci. 22, 506–514. doi: 10.22038/ijbms.2019.33956.8078
Hao, Z., Wang, W., Guo, R., and Liu, H. (2019). Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 104, 132–142. doi: 10.1016/j.psyneuen.2019.02.025
Harkin, A., Kelly, J., and Leonard, B. (2003). A revew of the relevance and validity of olfactory bulbectomy as a model of depression. Clin. Neurosci. Res. 3, 253–262. doi: 10.1016/s1566-2772(03)00087-2
Haro, C., Rangel-Zuniga, O. A., Alcala-Diaz, J. F., Gomez-Delgado, F., Perez-Martinez, P., Delgado-Lista, J., et al. (2016). Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS One 11:e0154090. doi: 10.1371/journal.pone.0154090
Hill, C. J., Lynch, D. B., Murphy, K., Ulaszewska, M., Jeffery, I. B., O’shea, C. A., et al. (2017). Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 5:4.
Huang, Y., Shi, X., Li, Z., Shen, Y., Shi, X., Wang, L., et al. (2018). Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 14, 3329–3337. doi: 10.2147/NDT.S188340
Huttenhower, C., Gevers, D., Knight, R., Abubucker, S., Badger, J. H., Chinwalla, A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Inserra, A., Rogers, G. B., Licinio, J., and Wong, M.-L. (2018). The Microbiota-inflammasome hypothesis of major depression. Bioessays 40:1800027. doi: 10.1002/bies.201800027
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Johansson, M. E., Sjövall, H., and Hansson, G. C. (2013). The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361. doi: 10.1038/nrgastro.2013.35
Karim, R., Stanczyk, F., Hodis, H., Cushman, M., Lobo, R., Hwang, J., et al. (2010). Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause 17, 785–790. doi: 10.1097/gme.0b013e3181cc50b2
Karunasena, E., Mcmahon, K. W., Chang, D., and Brashears, M. M. (2014). Host responses to the pathogen Mycobacterium avium subsp. paratuberculosis and beneficial microbes exhibit host sex specificity. Appl. Environ. Microbiol. 80, 4481–4490. doi: 10.1128/AEM.01229-14
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38, 522–528. doi: 10.1016/j.clnu.2018.04.010
Kelly, J. R., Borre, Y., O’ Brien, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Kiecolt-Glaser, J. K., Habash, D. L., Fagundes, C. P., Andridge, R., Peng, J., Malarkey, W. B., et al. (2015). Daily stressors, past depression, and metabolic responses to high-fat meals: a novel path to obesity. Biol. Psychiatry 77, 653–660. doi: 10.1016/j.biopsych.2014.05.018
Kim, Y. K., Na, K. S., Myint, A. M., and Leonard, B. E. (2016). The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 277–284. doi: 10.1016/j.pnpbp.2015.06.008
Kim, Y. S., Unno, T., Kim, B. Y., and Park, M. S. (2020). Sex differences in gut microbiota. World J. Mens Health 38, 48–60.
Kokai, M., Kashiwamura, S., Okamura, H., Ohara, K., and Morita, Y. (2002). Plasma interleukin-18 levels in patients with psychiatric disorders. J. Immunother. 25(Suppl. 1), S68–S71. doi: 10.1097/00002371-200203001-00011
Konstantinos, A., and Evangelia, M. (2019). Adherence to Mediterranean diet and risk of depression later in life. A cross sectional study in East Attica, Greece. Glob. Psychiatry 2, 201–209. doi: 10.2478/gp-2019-0012
Kovacs, A., Ben-Jacob, N., Tayem, H., Halperin, E., Iraqi, F. A., and Gophna, U. (2011). Genotype is a stronger determinant than sex of the mouse gut microbiota. Microb. Ecol. 61, 423–428. doi: 10.1007/s00248-010-9787-2
Krishnan, V., and Nestler, E. J. (2011). Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 7, 121–147. doi: 10.1007/7854_2010_108
Krznaric, Z., Vranesic Bender, D., and Mestrovic, T. (2019). The Mediterranean diet and its association with selected gut bacteria. Curr. Opin. Clin. Nutr. Metab. Care 22, 401–406. doi: 10.1097/MCO.0000000000000587
Kupelian, V., Chiu, G., Araujo, A., Williams, R., Clark, R., and Mckinlay, J. (2009). Association of sex hormones and C-reactive protein levels in men. Clin. Endocrinol. 72, 527–533. doi: 10.1111/j.1365-2265.2009.03713.x
Lai, W., and Cai, S. (2018). Comment on “Prevalence of Anxiety and Depression in Patients with Inflammatory Bowel Disease”. Can. J. Gastroenterol. Hepatol. 2018, 6747630–6747630. doi: 10.1155/2018/6747630
Lamers, F., Vogelzangs, N., Merikangas, K. R., De Jonge, P., Beekman, A. T., and Penninx, B. W. (2013). Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18, 692–699. doi: 10.1038/mp.2012.144
Larroya-Garcia, A., Navas-Carrillo, D., and Orenes-Pinero, E. (2019). Impact of gut microbiota on neurological diseases: diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 59, 3102–3116. doi: 10.1080/10408398.2018.1484340
Lassale, C., Batty, G. D., Baghdadli, A., Jacka, F., Sánchez-Villegas, A., Kivimäki, M., et al. (2019). Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol. Psychiatry 24, 965–986. doi: 10.1038/s41380-018-0237-8
LeBlanc, J. G., Milani, C., De Giori, G. S., Sesma, F., Van Sinderen, D., and Ventura, M. (2013). Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168. doi: 10.1016/j.copbio.2012.08.005
Ledeboer, A., Breve, J. J., Poole, S., Tilders, F. J., and Van Dam, A. M. (2000). Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia 30, 134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3
Lee, J. Y., Kim, N., Nam, R. H., Sohn, S. H., Lee, S. M., Choi, D., et al. (2017). Probiotics reduce repeated water avoidance stress-induced colonic microinflammation in Wistar rats in a sex-specific manner. PLoS One 12:e0188992. doi: 10.1371/journal.pone.0188992
Leonard, B. E. (2018). Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 30, 1–16. doi: 10.1017/neu.2016.69
Ley, R. E., Backhed, F., Turnbaugh, P., Lozupone, C. A., Knight, R. D., and Gordon, J. I. (2005). Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 102, 11070–11075. doi: 10.1073/pnas.0504978102
Li, J., Jia, H., Cai, X., Zhong, H., Feng, Q., Sunagawa, S., et al. (2014). An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32, 834–841. doi: 10.1038/nbt.2942
Li, M., Wang, B., Zhang, M., Rantalainen, M., Wang, S., Zhou, H., et al. (2008). Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. U.S.A. 105, 2117–2122. doi: 10.1073/pnas.0712038105
Li, N., Wang, Q., Wang, Y., Sun, A., Lin, Y., Jin, Y., et al. (2018). Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 12:266. doi: 10.3389/fnbeh.2018.00266
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi: 10.1016/j.neuroscience.2015.09.033
Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. doi: 10.1016/S0140-6736(12)61766-8
Liu, Q. F., Kim, H. M., Lim, S., Chung, M. J., Lim, C. Y., Koo, B. S., et al. (2020). Effect of probiotic administration on gut microbiota and depressive behaviors in mice. Daru 28, 181–189. doi: 10.1007/s40199-020-00329-w
Liu, Y., Zhang, L., Wang, X., Wang, Z., Zhang, J., Jiang, R., et al. (2016). Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 14, 1602–1611.e5. doi: 10.1016/j.cgh.2016.05.033
Llopis, M., Antolin, M., Carol, M., Borruel, N., Casellas, F., Martinez, C., et al. (2009). Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 15, 275–283. doi: 10.1002/ibd.20736
Lopizzo, N., Bocchio Chiavetto, L., Cattane, N., Plazzotta, G., Tarazi, F. I., Pariante, C. M., et al. (2015). Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry 6:68. doi: 10.3389/fpsyt.2015.00068
Lurie, I., Yang, Y. X., Haynes, K., Mamtani, R., and Boursi, B. (2015). Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J. Clin. Psychiatry 76, 1522–1528. doi: 10.4088/JCP.15m09961
Lyte, M., Daniels, K. M., and Schmitz-Esser, S. (2019). Fluoxetine-induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ 7:e6199. doi: 10.7717/peerj.6199
Macedo, D., Filho, A. J. M. C., Soares, De Sousa, C. N., Quevedo, J., Barichello, T., et al. (2017). Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 208, 22–32. doi: 10.1016/j.jad.2016.09.012
Madison, A., and Kiecolt-Glaser, J. K. (2019). Stress, depression, diet, and the gut microbiota: human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 28, 105–110. doi: 10.1016/j.cobeha.2019.01.011
Maes, M. (1995). Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 19, 11–38. doi: 10.1016/0278-5846(94)00101-m
Maes, M., Berk, M., Goehler, L., Song, C., Anderson, G., Galecki, P., et al. (2012). Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 10:66. doi: 10.1186/1741-7015-10-66
Maes, M., Kubera, M., and Leunis, J. C. (2008). The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 29, 117–124.
Maggio, M., Ceda, G. P., Lauretani, F., Bandinelli, S., Corsi, A. M., Giallauria, F., et al. (2011). SHBG, sex hormones, and inflammatory markers in older women. J. Clin. Endocrinol. Metab. 96, 1053–1059. doi: 10.1210/jc.2010-1902
Mahajan, S., Avasthi, A., Grover, S., and Chawla, Y. K. (2014). Incidence of depression in patients with chronic hepatitis C receiving combination therapy of pegylated interferon-alpha and ribavirin. Psychother. Psychosom. 83, 308–309. doi: 10.1159/000358527
Maier, L., Pruteanu, M., Kuhn, M., Zeller, G., Telzerow, A., Anderson, E. E., et al. (2018). Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628. doi: 10.1038/nature25979
Majeed, M., Nagabhushanam, K., Arumugam, S., Majeed, S., and Ali, F. (2018). Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 62:1218. doi: 10.29219/fnr.v62.1218
Mangiola, F., Ianiro, G., Franceschi, F., Fagiuoli, S., Gasbarrini, G., and Gasbarrini, A. (2016). Gut microbiota in autism and mood disorders. World J. Gastroenterol. 22, 361–368. doi: 10.3748/wjg.v22.i1.361
Markle, J. G., Frank, D. N., Mortin-Toth, S., Robertson, C. E., Feazel, L. M., Rolle-Kampczyk, U., et al. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. doi: 10.1126/science.1233521
Marotta, A., Sarno, E., Del Casale, A., Pane, M., Mogna, L., Amoruso, A., et al. (2019). Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front. Psychiatry 10:164. doi: 10.3389/fpsyt.2019.00164
Martin, C. R., and Mayer, E. A. (2017). Gut-brain axis and behavior. Nestle Nutr. Inst. Workshop Ser. 88, 45–53.
Martin, C. R., Osadchiy, V., Kalani, A., and Mayer, E. A. (2018). The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 6, 133–148.
Matthews, K., and Robbins, T. W. (2003). Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci. Biobehav. Rev. 27, 45–55. doi: 10.1016/s0149-7634(03)00008-3
Mendlewicz, J., Kriwin, P., Oswald, P., Souery, D., Alboni, S., and Brunello, N. (2006). Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 21, 227–231. doi: 10.1097/00004850-200607000-00005
Mesquita, A. R., Correia-Neves, M., Roque, S., Castro, A. G., Vieira, P., Pedrosa, J., et al. (2008). IL-10 modulates depressive-like behavior. J. Psychiatr. Res. 43, 89–97. doi: 10.1016/j.jpsychires.2008.02.004
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., et al. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764. doi: 10.1017/S0007114510004319
Mikova, O., Yakimova, R., Bosmans, E., Kenis, G., and Maes, M. (2001). Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur. Neuropsychopharmacol. 11, 203–208. doi: 10.1016/s0924-977x(01)00081-5
Miller, A. H., and Raison, C. L. (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34. doi: 10.1038/nri.2015.5
Miller, L. E., and Ouwehand, A. C. (2013). Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J. Gastroenterol. 19, 4718–4725. doi: 10.3748/wjg.v19.i29.4718
Mizrahi-Man, O., Davenport, E. R., and Gilad, Y. (2013). Taxonomic classification of bacterial 16S rRNA genes using short sequencing reads: evaluation of effective study designs. PLoS One 8:e53608. doi: 10.1371/journal.pone.0053608
Mohamad, N., Wong, S. K., Hasan, W., Jolly, J., Fozi, F., Ima-Nirwana, S., et al. (2018). The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22, 1–12. doi: 10.1080/13685538.2018.1482487
Mohammadi, A. A., Jazayeri, S., Khosravi-Darani, K., Solati, Z., Mohammadpour, N., Asemi, Z., et al. (2016). The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 19, 387–395. doi: 10.1179/1476830515y.0000000023
Mohle, L., Mattei, D., Heimesaat, M. M., Bereswill, S., Fischer, A., Alutis, M., et al. (2016). Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956. doi: 10.1016/j.celrep.2016.04.074
Monteiro, R., Teixeira, D., and Calhau, C. (2014). Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014:615917. doi: 10.1155/2014/615917
Morgun, A., Dzutsev, A., Dong, X., Greer, R. L., Sexton, D. J., Ravel, J., et al. (2015). Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 64, 1732–1743. doi: 10.1136/gutjnl-2014-308820
Mowat, A. M., and Agace, W. W. (2014). Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685. doi: 10.1038/nri3738
Mueller, S., Saunier, K., Hanisch, C., Norin, E., Alm, L., Midtvedt, T., et al. (2006). Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006
Muller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Muller, B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684. doi: 10.1038/sj.mp.4001805
Murray, C. J., Atkinson, C., Bhalla, K., Birbeck, G., Burstein, R., Chou, D., et al. (2013). The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608. doi: 10.1001/jama.2013.13805
Nagpal, R., Shively, C., Register, T., Craft, S., and Yadav, H. (2019). Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res. 8:699. doi: 10.12688/f1000research.18992.1
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linlokken, A., Wilson, R., et al. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 26, 1155–1162. doi: 10.1111/nmo.12378
Natividad, J. M., and Verdu, E. F. (2013). Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol. Res. 69, 42–51. doi: 10.1016/j.phrs.2012.10.007
Nazimek, K., Strobel, S., Bryniarski, P., Kozlowski, M., Filipczak-Bryniarska, I., and Bryniarski, K. (2017). The role of macrophages in anti-inflammatory activity of antidepressant drugs. Immunobiology 222, 823–830. doi: 10.1016/j.imbio.2016.07.001
Neumann, I. D., Wigger, A., Krömer, S., Frank, E., Landgraf, R., and Bosch, O. J. (2005). Differential effects of periodic maternal separation on adult stress coping in a rat model of extremes in trait anxiety. Neuroscience 132, 867–877. doi: 10.1016/j.neuroscience.2005.01.034
Ni, J., Wu, G. D., Albenberg, L., and Tomov, V. T. (2017). Gut microbiota and IBD: causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 14, 573–584. doi: 10.1038/nrgastro.2017.88
Nishida, K., Sawada, D., Kuwano, Y., Tanaka, H., Sugawara, T., Aoki, Y., et al. (2017). Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 36, 112–121. doi: 10.1016/j.jff.2017.06.031
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J.-Z., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16:90. doi: 10.1186/s12866-016-0708-5
O’Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A.-M., Quigley, E. M. M., et al. (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263–267. doi: 10.1016/j.biopsych.2008.06.026
Org, E., Mehrabian, M., Parks, B. W., Shipkova, P., Liu, X., Drake, T. A., et al. (2016). Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322. doi: 10.1080/19490976.2016.1203502
Osadchiy, V., Martin, C. R., and Mayer, E. A. (2019). The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 17, 322–332. doi: 10.1016/j.cgh.2018.10.002
Östlund-Lagerström, L., Kihlgren, A., Repsilber, D., Björkstén, B., Brummer, R. J., and Schoultz, I. (2016). Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr. J. 15:80. doi: 10.1186/s12937-016-0198-1
Pacheco, A. R., and Sperandio, V. (2015). Enteric pathogens exploit the microbiota-generated nutritional environment of the gut. Microbiol. Spectr. 3:10.1128/microbiolspec.MBP-0001-2014. doi: 10.1128/microbiolspec.MBP-0001-2014
Pagliai, G., Sofi, F., Vannetti, F., Caiani, S., Pasquini, G., Molino Lova, R., et al. (2018). Mediterranean diet, food consumption and risk of late-life depression: the mugello study. J. Nutr. Health Aging 22, 569–574. doi: 10.1007/s12603-018-1019-3
Park, A. J., Collins, J., Blennerhassett, P. A., Ghia, J. E., Verdu, E. F., Bercik, P., et al. (2013). Altered colonic function and microbiota profile in a mouse model of chronic depression. J. Neurogastroenterol. Motil. 25, 733–e575. doi: 10.1111/nmo.12153
Park, J. M., and Lee, Y. J. (2020). Serum oestradiol levels are inversely associated with C-reactive protein levels in premenopausal women, but not postmenopausal women. J. Int. Med. Res. 48:300060520961228. doi: 10.1177/0300060520961228
Patel, A. (2013). Review: the role of inflammation in depression. Psychiatr. Danub. 25(Suppl. 2), S216–S223.
Patten, S. B., Wang, J. L., Williams, J. V., Currie, S., Beck, C. A., Maxwell, C. J., et al. (2006). Descriptive epidemiology of major depression in Canada. Can. J. Psychiatry 51, 84–90. doi: 10.1177/070674370605100204
Petersen, C., and Round, J. L. (2014). Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 16, 1024–1033. doi: 10.1111/cmi.12308
Phillips, J. G. P. (1910). The treatment of melancholia by the lactic acid Bacillus. J. Ment. Sci. 56, 422–430. doi: 10.1192/bjp.56.234.422
Poretsky, R., Rodriguez, R. L., Luo, C., Tsementzi, D., and Konstantinidis, K. T. (2014). Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One 9:e93827. doi: 10.1371/journal.pone.0093827
Principi, N., and Esposito, S. (2016). Gut microbiota and central nervous system development. J. Infect. 73, 536–546. doi: 10.1016/j.jinf.2016.09.010
Proctor, L. M., Creasy, H. H., Fettweis, J. M., Lloyd-Price, J., Mahurkar, A., Zhou, W., et al. (2019). The integrative human microbiome project. Nature 569, 641–648. doi: 10.1038/s41586-019-1238-8
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Rajilic-Stojanovic, M., and de Vos, W. M. (2014). The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 38, 996–1047. doi: 10.1111/1574-6976.12075
Ransohoff, R. M., and Brown, M. A. (2012). Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171. doi: 10.1172/jci58644
Reininghaus, E. Z., Platzer, M., Kohlhammer-Dohr, A., Hamm, C., Mörkl, S., Bengesser, S. A., et al. (2020). PROVIT: supplementary probiotic treatment and vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 12:3422. doi: 10.3390/nu12113422
Reyman, M., Van Houten, M. A., Van Baarle, D., Bosch, A. A. T. M., Man, W. H., Chu, M. L. J. N., et al. (2019). Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 10:4997.
Riedel, M., Moller, H. J., Obermeier, M., Adli, M., Bauer, M., Kronmuller, K., et al. (2011). Clinical predictors of response and remission in inpatients with depressive syndromes. J. Affect. Disord. 133, 137–149. doi: 10.1016/j.jad.2011.04.007
Rogers, G. B., Keating, D. J., Young, R. L., Wong, M. L., Licinio, J., and Wesselingh, S. (2016). From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 21, 738–748. doi: 10.1038/mp.2016.50
Rohleder, N. (2014). Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 76, 181–189. doi: 10.1097/PSY.0000000000000049
Romijn, A. R., Rucklidge, J. J., Kuijer, R. G., and Frampton, C. (2017). A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 51, 810–821. doi: 10.1177/0004867416686694
Round, J. L., and Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323. doi: 10.1038/nri2515
Rowland, I., Gibson, G., Heinken, A., Scott, K., Swann, J., Thiele, I., et al. (2018). Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24. doi: 10.1007/s00394-017-1445-8
Rudzki, L., Ostrowska, L., Pawlak, D., Malus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi: 10.1016/j.psyneuen.2018.10.010
Rutayisire, E., Huang, K., Liu, Y., and Tao, F. (2016). The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 16:86. doi: 10.1186/s12876-016-0498-0
Salk, R. H., Hyde, J. S., and Abramson, L. Y. (2017). Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822. doi: 10.1037/bul0000102
Salminen, S., Gibson, G. R., Mccartney, A. L., and Isolauri, E. (2004). Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 53, 1388–1389. doi: 10.1136/gut.2004.041640
Sanchez-Villegas, A., Henríquez, P., Bes-Rastrollo, M., and Doreste, J. (2007). Mediterranean diet and depression. Public Health Nutr. 9, 1104–1109.
Sassone-Corsi, M., and Raffatellu, M. (2015). No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 194, 4081–4087. doi: 10.4049/jimmunol.1403169
Savignac, H. M., Kiely, B., Dinan, T. G., and Cryan, J. F. (2014). Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 26, 1615–1627. doi: 10.1111/nmo.12427
Schroeder, B. O., and Backhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089. doi: 10.1038/nm.4185
Singh, P., and Manning, S. D. (2016). Impact of age and sex on the composition and abundance of the intestinal microbiota in individuals with and without enteric infections. Ann. Epidemiol. 26, 380–385. doi: 10.1016/j.annepidem.2016.03.007
Sinha, T., Vich Vila, A., Garmaeva, S., Jankipersadsing, S. A., Imhann, F., Collij, V., et al. (2019). Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 10, 358–366. doi: 10.1080/19490976.2018.1528822
Skaper, S. D., Facci, L., and Giusti, P. (2014). Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol. Disord. Drug Targets 13, 1654–1666. doi: 10.2174/1871527313666141130224206
Skarupski, K. A., Tangney, C. C., Li, H., Evans, D. A., and Morris, M. C. (2013). Mediterranean diet and depressive symptoms among older adults over time. J. Nutr. Health Aging 17, 441–445. doi: 10.1007/s12603-012-0437-x
Skovlund, C. W., Mørch, L. S., Kessing, L. V., and Lidegaard, Ø. (2016). Association of hormonal contraception with depression. JAMA Psychiatry 73, 1154–1162. doi: 10.1001/jamapsychiatry.2016.2387
Slavich, G., and Sacher, J. (2019). Stress, sex hormones, inflammation, and major depressive disorder: extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology 236, 1–17. doi: 10.1007/s00213-019-05326-9
Sluzewska, A., Rybakowski, J., Bosmans, E., Sobieska, M., Berghmans, R., Maes, M., et al. (1996). Indicators of immune activation in major depression. Psychiatry Res. 64, 161–167. doi: 10.1016/s0165-1781(96)02783-7
Slyepchenko, A., Maes, M., Jacka, F. N., Kohler, C. A., Barichello, T., Mcintyre, R. S., et al. (2017). Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom. 86, 31–46. doi: 10.1159/000448957
Slykerman, R. F., Hood, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: a Randomised Double-blind Placebo-controlled Trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Smith, C. J., Emge, J. R., Berzins, K., Lung, L., Khamishon, R., Shah, P., et al. (2014). Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G793–G802. doi: 10.1152/ajpgi.00238.2014
Smith, P. A. (2015). The tantalizing links between gut microbes and the brain. Nature 526, 312–314. doi: 10.1038/526312a
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermudez-Humaran, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Sommer, F., and Backhed, F. (2013). The gut microbiota–masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238. doi: 10.1038/nrmicro2974
Song, C., and Wang, H. (2011). Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 760–768. doi: 10.1016/j.pnpbp.2010.06.020
Soto, M., Herzog, C., Pacheco, J. A., Fujisaka, S., Bullock, K., Clish, C. B., et al. (2018). Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol. Psychiatry 23, 2287–2301. doi: 10.1038/s41380-018-0086-5
Stanislawski, M. A., Dabelea, D., Wagner, B. D., Iszatt, N., Dahl, C., Sontag, M. K., et al. (2018). Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. mBio 9:e01751-18. doi: 10.1128/mBio.01751-18
Steenbergen, L., Sellaro, R., Van Hemert, S., Bosch, J. A., and Colzato, L. S. (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 48, 258–264. doi: 10.1016/j.bbi.2015.04.003
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275. doi: 10.1113/jphysiol.2004.063388
Swiergiel, A. H., and Dunn, A. J. (2007). Effects of interleukin-1β and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol. Biochem. Behav. 86, 651–659. doi: 10.1016/j.pbb.2007.02.010
Takagi, T., Naito, Y., Inoue, R., Kashiwagi, S., Uchiyama, K., Mizushima, K., et al. (2019). Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 54, 53–63. doi: 10.1007/s00535-018-1488-5
Tan, S., Wang, Y., Chen, K., Long, Z., and Zou, J. (2017). Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol. Pharm. Bull. 40, 1260–1267. doi: 10.1248/bpb.b17-00131
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836. doi: 10.1042/bcj20160510
Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., et al. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394. doi: 10.1053/j.gastro.2013.02.043
Toyoda, A. (2017). Social defeat models in animal science: What we have learned from rodent models. Anim. Sci. J. 88, 944–952. doi: 10.1111/asj.12809
Tribbick, D., Salzberg, M., Ftanou, M., Connell, W. R., Macrae, F., Kamm, M. A., et al. (2015). Prevalence of mental health disorders in inflammatory bowel disease: an Australian outpatient cohort. Clin. Exp. Gastroenterol. 8, 197–204. doi: 10.2147/CEG.S77567
Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R., and Gordon, J. I. (2007). The human microbiome project. Nature 449, 804–810.
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
van den Biggelaar, A. H., Gussekloo, J., De Craen, A. J., Frolich, M., Stek, M. L., Van Der Mast, R. C., et al. (2007). Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 42, 693–701. doi: 10.1016/j.exger.2007.01.011
Vich Vila, A., Collij, V., Sanna, S., Sinha, T., Imhann, F., Bourgonje, A. R., et al. (2020). Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11:362. doi: 10.1038/s41467-019-14177-z
Walker, A. W., Ince, J., Duncan, S. H., Webster, L. M., Holtrop, G., Ze, X., et al. (2011). Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5, 220–230. doi: 10.1038/ismej.2010.118
Wallace, C. J. K., and Milev, R. V. (2021). The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front. Psychiatry 12:618279. doi: 10.3389/fpsyt.2021.618279
Weissman, M. M., and Klerman, G. L. (1977). Sex differences and the epidemiology of depression. Arch. Gen. Psychiatry 34, 98–111. doi: 10.1001/archpsyc.1977.01770130100011
Wharton, W., Gleason, C. E., Olson, S. R., Carlsson, C. M., and Asthana, S. (2012). Neurobiological underpinnings of the estrogen - mood relationship. Curr. Psychiatry Rev. 8, 247–256. doi: 10.2174/157340012800792957
Whiteford, H. A., Degenhardt, L., Rehm, J., Baxter, A. J., Ferrari, A. J., Erskine, H. E., et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. doi: 10.1016/s0140-6736(13)61611-6
Wiebe, E. R., Brotto, L. A., and Mackay, J. (2011). Characteristics of women who experience mood and sexual side effects with use of hormonal contraception. J. Obstet. Gynaecol. Can. 33, 1234–1240. doi: 10.1016/S1701-2163(16)35108-8
Williams, S., Chung, G., and Muennig, P. (2017). Undiagnosed depression: a community diagnosis. SSM Popul. Health 3, 633–638. doi: 10.1016/j.ssmph.2017.07.012
Wilson, I. D., and Nicholson, J. K. (2009). The role of gut microbiota in drug response. Curr. Pharm. Des. 15, 1519–1523. doi: 10.2174/138161209788168173
Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jonsson, B., et al. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 21, 655–679. doi: 10.1016/j.euroneuro.2011.07.018
Wong, M. L., Dong, C., Maestre-Mesa, J., and Licinio, J. (2008). Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry 13, 800–812. doi: 10.1038/mp.2008.59
Wong, M. L., Inserra, A., Lewis, M. D., Mastronardi, C. A., Leong, L., Choo, J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21, 797–805. doi: 10.1038/mp.2016.46
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y.-Y., Keilbaugh, S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. doi: 10.1126/science.1208344
Xia, Z., Depierre, J. W., and Nassberger, L. (1996). Tricyclic antidepressants inhibit IL-6, IL-1 beta and TNF-alpha release in human blood monocytes and IL-2 and interferon-gamma in T cells. Immunopharmacology 34, 27–37. doi: 10.1016/0162-3109(96)00111-7
Yang, C., Qu, Y., Fujita, Y., Ren, Q., Ma, M., Dong, C., et al. (2017). Possible role of the gut microbiota–brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 7:1294. doi: 10.1038/s41398-017-0031-4
Yang, T., Santisteban, M. M., Rodriguez, V., Li, E., Ahmari, N., Carvajal, J. M., et al. (2015). Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340. doi: 10.1161/hypertensionaha.115.05315
Yun, Y., Kim, H.-N., Kim, S. E., Heo, S. G., Chang, Y., Ryu, S., et al. (2017). Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 17:151. doi: 10.1186/s12866-017-1052-0
Yurkovetskiy, L., Burrows, M., Khan, A. A., Graham, L., Volchkov, P., Becker, L., et al. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. doi: 10.1016/j.immuni.2013.08.013
Zhang, J. C., Yao, W., Dong, C., Yang, C., Ren, Q., Ma, M., et al. (2017). Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut–microbiota–brain axis. Transl. Psychiatry 7:e1138. doi: 10.1038/tp.2017.112
Zhang, M., Li, A., Yang, Q., Li, J., Wang, L., Liu, X., et al. (2021). Beneficial Effect of Alkaloids From Sophora alopecuroides L. on CUMS-induced depression model mice via modulating gut microbiota. Front. Cell. Infect. Microbiol. 11:665159. doi: 10.3389/fcimb.2021.665159
Zhang, Y. J., Li, S., Gan, R. Y., Zhou, T., Xu, D. P., and Li, H. B. (2015). Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16, 7493–7519. doi: 10.3390/ijms16047493
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. doi: 10.1038/mp.2016.44
Keywords: gut microbiota, inflammation, depression, sex hormones, probiotics
Citation: Eltokhi A and Sommer IE (2022) A Reciprocal Link Between Gut Microbiota, Inflammation and Depression: A Place for Probiotics? Front. Neurosci. 16:852506. doi: 10.3389/fnins.2022.852506
Received: 11 January 2022; Accepted: 18 March 2022;
Published: 25 April 2022.
Edited by:
He Wang, Fudan University, ChinaReviewed by:
Amy Mackos, The Ohio State University, United StatesValentina Caputi, University College Cork, Ireland
Copyright © 2022 Eltokhi and Sommer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Eltokhi, Eltokhi@uw.edu
 Ahmed Eltokhi
Ahmed Eltokhi Iris E. Sommer
Iris E. Sommer