- 1School of Medicine, Western Sydney University, Sydney, NSW, Australia
- 2Neuroscience Research Australia, Sydney, NSW, Australia
- 3School of Health Sciences, RMIT University, Bundoora, VIC, Australia
We have previously reported that there are inter-individual differences in the cardiovascular responses to experimental muscle pain, which are consistent over time: intramuscular infusion of hypertonic saline, causing pain lasting ~60 min, increases muscle sympathetic nerve activity (MSNA)—as well as blood pressure and heart rate—in certain subjects, but decrease it in others. Here, we tested the hypothesis that baseline physiological parameters (resting MSNA, heart rate, blood pressure, heart rate variability) determine the cardiovascular responses to long-lasting muscle pain. MSNA was recorded from the common peroneal nerve, together with heart rate and blood pressure, during a 45-min intramuscular infusion of hypertonic saline solution into the tibialis anterior of 50 awake human subjects (25 females and 25 males). Twenty-four subjects showed a sustained increase in mean amplitude of MSNA (160.9 ± 7.3%), while 26 showed a sustained decrease (55.1 ± 3.5%). Between the increasing and decreasing groups there were no differences in baseline MSNA (19.0 ± 1.5 vs. 18.9 ± 1.2 bursts/min), mean BP (88.1 ± 5.2 vs. 88.0 ± 3.8 mmHg), HR (74.7 ± 2.0 vs. 72.8 ± 1.8 beats/min) or heart rate variability (LF/HF 1.8 ± 0.2 vs. 2.2 ± 0.3). Furthermore, neither sex nor body mass index had any effect on whether MSNA increased or decreased during tonic muscle pain. We conclude that the measured baseline physiological parameters cannot account for the divergent sympathetic responses during tonic muscle pain.
Introduction
Pain is important for survival by helping to avoid tissue damage, mobilizing all relevant homeostatic systems for a fight-and-flight response or, alternatively, promoting conservation of energy, and thus promoting healing (Craig, 2002). It is well known that pain originating in deep structures may evoke very different behavioral and cardiovascular responses than pain originating in superficial structures. Indeed, Lewis (1942) observed that pain originating in skin evokes “a rise of pulse rate” and a “sense of invigoration” whereas pain originating in deep structures evokes quiescence, a “slowing of the pulse” and “falling of the blood pressure” (Lewis, 1942). Subsequent studies confirmed Lewis' findings that muscle pain was associated with a fall in blood pressure and bradycardia in awake human subjects (Feinstein et al., 1954). However, since these early observations by Lewis and Feinstein, very few studies have examined the effects of pain on the cardiovascular system in awake human subjects.
We have been using subcutaneous or intramuscular injection of hypertonic saline—a specific stimulus for nociceptors (Graven-Nielsen and Mense, 2001)—to study the effects of acute pain on the cardiovascular system in awake human subjects. We showed that a bolus (0.5 ml) injection of hypertonic saline into the tibialis anterior muscle caused a sustained increase in muscle sympathetic nerve activity (MSNA), and a modest increase in blood pressure and heart rate (Burton et al., 2009a), while there was only a transient increase in skin sympathetic nerve activity (SSNA)—the latter being consistent with an arousal rather than reflex response (Burton et al., 2009b). More recently, we used intramuscular infusion to produce a sustained, steady-state, level of pain lasting for approximately 1 h (Fazalbhoy et al., 2012, 2014). We showed that about half of the subjects showed a sustained increase in MSNA, blood pressure, and heart rate during tonic muscle pain, while the other half showed sustained decreases (Fazalbhoy et al., 2012, 2014).
These data call into question the idea that noxious stimuli produce invariant responses and raise the prospect that these differential responses may be related to an individual's particular traits, which may be reproducible over time. That is, in some individuals muscle pain always evokes increases in MSNA, blood pressure and heart rate, whereas in others it consistently evokes decreases. Indeed, we recently showed that subjects who generate increases in MSNA, blood pressure and heart rate during one session also show increases in a second session; the same is true for those who show parallel decreases in MSNA, blood pressure and heart rate (Fazalbhoy et al., 2014). Moreover, we showed that there were no differences in resting MSNA, blood pressure or heart rate between the two recording sessions (Fazalbhoy et al., 2014), but we do not know whether differences in these baseline physiological parameters across individuals determines whether MSNA increases or decreases during tonic muscle pain. Indeed, in our first study we showed that resting levels of MSNA were higher in the group that showed an increase in MSNA than in the group that showed a decrease, but these differences failed to reach statistical significance—presumably because of the low subject numbers (n = 12) Against this background, the aim of the current study was to determine whether baseline physiological parameters—including resting MSNA, blood pressure and heart rate—could account for the divergent MSNA responses to tonic muscle pain. Our earlier studies (Fazalbhoy et al., 2012, 2014) were based on small subject numbers and were not sex-balanced. Here, we have studied a larger sample (n = 50), comprising 25 males and 25 females.
Methods
Experiments were performed on 25 female and 25 male healthy subjects, aged 18–39 years. Data from 35 new participants were pooled with those from 15 participants reported previously (Fazalbhoy et al., 2014). All subjects provided informed written consent to the experimental procedures, which were conducted under the approval of the Human Research Ethics Committee of the University of Western Sydney and satisfied the requirements of the Helsinki Declaration. No subject had a history of cardiovascular disease or former chronic musculoskeletal pain. Prior to commencement height, weight, body mass index (BMI), and total muscle mass were measured for each subject using a body-composition analyser (SA165A, Tanita, Japan).
Experimental Procedures
The subjects were seated in a comfortable reclined position with the legs supported in an extended position. The room was kept quiet and at a constant temperature of 22°C. The course of the common peroneal nerve was identified via external stimulation (2–10 mA) using a 1 mm surface probe which delivered 0.2 ms pulses at 1 Hz from an isolated stimulator (Stimulus Isolator; ADInstruments, Sydney, Australia). Spontaneous bursts of muscle sympathetic nerve activity (MSNA) were recorded from muscle fascicles of the common peroneal nerve supplying the ankle or toe extensor or foot everter muscles via tungsten microelectrodes (FHC, Bowdoin, ME, USA) inserted percutaneously at the level of the fibular head. Multi-unit neural activity was amplified (gain 20 000, bandpass 0.3–5.0 kHz) using an isolated amplifier (NeuroAmp EX, ADInstruments, Sydney, Australia) and stored on computer (10-kHz sampling) using a computer-based data acquisition and analysis system (PowerLab 16SP hardware and LabChart 7 software; ADInstruments, Sydney, Australia). ECG (0.3–1.0 kHz) was recorded with Ag–AgCl surface electrodes on the chest and sampled at 2 kHz. Blood pressure was recorded continuously using finger pulse plethysmography (Finometer Pro, Finapres Medical Systems, The Netherlands) and sampled at 400 Hz. Respiration (DC-100 Hz) was recorded using a strain-gauge transducer (Pneumotrace, UFI, Morro Bay CA, USA) wrapped around the chest.
Noxious Stimulation
A 7% hypertonic saline solution was prepared by diluting sterile, 20% hypertonic saline with sterile water. Two syringes of 10 ml each were filled with the 7% hypertonic saline, placed in an infusion pump (Harvard Instruments, USA), and connected to a three-way tap via a 75 cm extension tubing primed with hypertonic saline. A 23 gauge butterfly needle was then attached to the three-way tap via a cannula, primed, and inserted 1.5 cm deep into the belly of the ipsilateral tibialis anterior muscle, about 5 cm lateral and 10 cm inferior to the tibial tuberosity. The cannula was inserted as soon as a stable recording of spontaneous MSNA was achieved. Prior to infusion of the saline solution, a 5 min baseline recording of MSNA, blood pressure, respiration, and heart rate was obtained. Infusion of the 7% hypertonic saline solution was started at a time unknown to the subject, and was maintained for 45 min; as described previously (Fazalbhoy et al., 2012, 2014), the pain lasted for ~60 min. The initial rate of infusion was set at 0.25 ml/min and was constantly adjusted to maintain a pain level of 5–6/10 on a Numerical Rating Scale (NRS). Subjects were asked to rate their pain continuously by sliding a linear potentiometer (Response Meter, ADInstruments, Sydney, Australia) that was calibrated to the NRS, with a rating of “0” meaning “no pain/discomfort” at all, and a rating of “10” being equivalent to the “worst pain the subject ever had experienced.” When the pain level dropped below 4/10 or rose above 6/10, the infusion rate was changed by 0.02 ml/min accordingly. After the infusion was completed, the recording was continued until the pain stopped. At the conclusion of the experiment, each subject completed a McGill Pain Questionnaire, in which subjects described the quality of the pain using a standard set of descriptors.
Analysis
LabChart 7 Pro software (ADInstruments, Sydney, Australia) was used to record the following parameters: muscle sympathetic nerve activity (burst amplitude and frequency), heart rate, blood pressure, respiration, pulse pressure, heart rate variability (HRV), and pain ratings. Individual bursts of MSNA were displayed as a mean-voltage neurogram, computed as the root-mean-square (RMS) processed signal with a moving time average window of 200 ms. This signal was then analyzed using the “Peak Analysis” module of the LabChart 7 Pro software to calculate the amplitude of each burst. The absolute values were averaged into 5-min blocks and reported as percentages from the “baseline” values. An average of all blocks was taken to determine the direction of the response. Subjects with overall average MSNA amplitude 10% lower than baseline were arbitrarily assigned to the decreasing group; averages 10% higher than baseline were considered as increasing. Baseline MSNA amplitude was compared to the 5-min block with the mean value calculated over the entire infusion period, and to the highest average for the increasing group and to the lowest average value for the decreasing group. Changes in mean heart rate and mean blood pressure were also measured in 5 min epochs, normalized to the baseline value prior to the infusion of hypertonic saline. HRV was assessed over a 5-min steady state period before the infusion, and then again over 5 min when the subject experienced a steady-state level of pain during the infusion. The parameters of HRV that were analyzed included the low frequency (LF) and high frequency (HF) power, as well as the Root Mean Square Successive Difference of cardiac intervals (RMSSD). Statistical analysis—non-paired two-tailed t-tests for normally distributed data and Mann-Whitney tests for non-normally distributed data—was performed using Prism version 6 for Mac OS X (GraphPad software, San Diego, California, USA). All values are expressed as means and standard error. Probability levels of p < 0.05 were deemed significant.
Results
Subjective Experience of Tonic Muscle Pain
In all subjects intramuscular infusion of hypertonic saline induced a steady state level of muscle pain in the tibialis anterior muscle. The level of pain was kept constant, typically around 5 out of 10—throughout the period of infusion by adjusting the rate of infusion according to the subject's tracking of the pain level. The mean pain rating was 4.9 ± 0.1. Using the McGill Pain Questionnaire, 36 of the 50 subjects (72%) described the pain as “aching,” 48% described it as “heavy” and 48% as “dull.” After these, “throbbing,” “cramping,” “hurting,” “discomforting,” and “continuous” were the most frequent descriptions used.
Muscle Sympathetic Nerve Activity During Tonic Muscle Pain
Experimental records from two subjects are shown in Figures 1, 2. Muscle sympathetic nerve activity (MSNA) increased during tonic pain in the subject depicted in Figure 1; it is apparent that blood pressure also increased. Conversely, the subject illustrated in Figure 2 exhibited a sustained decrease in MSNA and blood pressure during the infusion.
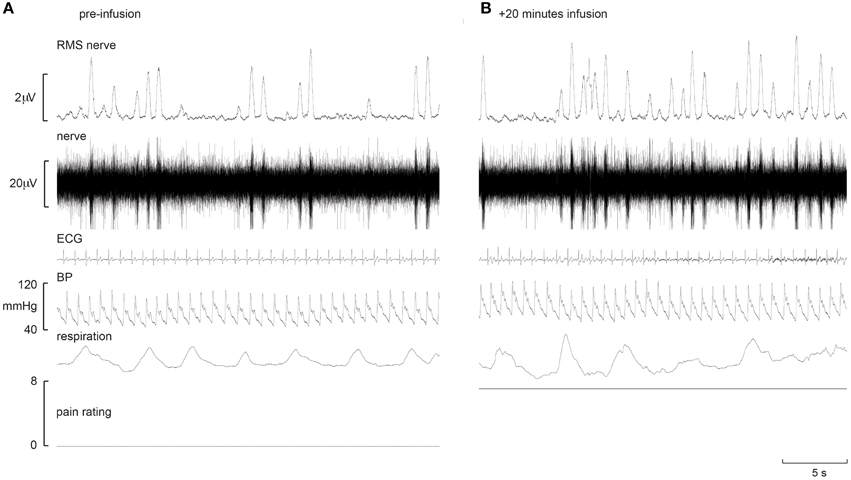
Figure 1. Experimental records from one subject in whom MSNA increased during intramuscular infusion of hypertonic saline. Baseline is shown in the left panel (A), while the right panel (B) shows a sample at which MSNA was at its maximum.
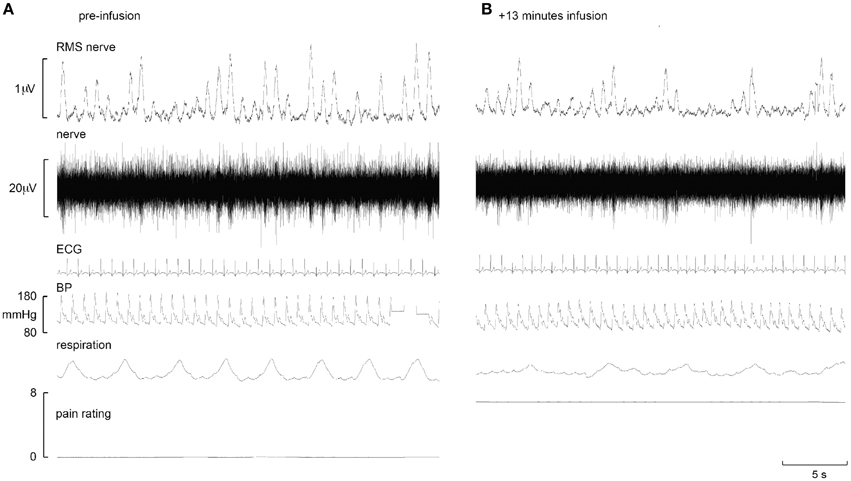
Figure 2. Experimental records from one subject in whom MSNA decreased during intramuscular infusion of hypertonic saline. Baseline is shown in the left panel (A), while the right panel (B) shows a sample at which MSNA was at its maximum.
As expected, when all subjects were analyzed according to their pattern of MSNA response to muscle pain two distinct groups of responses emerged: 24 subjects (48%) showed a significant increase in burst amplitude over the entire infusion period (132.6 ± 6.1% p < 0.0001, t-test), while 26 subjects (52%) showed a significant decrease (72.6 ± 3.0%, p < 0.0001, t-test), relative to baseline. The peak changes in the increasing and decreasing groups, measured over 5 min, were 160.9 ± 7.3% and 55.1 ± 3.5% respectively; these were significantly different from baseline (p < 0.0001, t-test). The time at which the peak increase in MSNA occurred (29 ± 3 min) in the increasing group, and the time at which the peak fall occurred (32 ± 3 min) in the decreasing group, were not significantly different (p = 0.5077, Mann-Whitney test). There was no significant difference in the mean pain rating in the increasing and decreasing groups (4.7 ± 0.2 vs. 5.1 ± 0.2, respectively; p = 0.18, t-test).
Blood Pressure and Heart Rate During Tonic Muscle Pain
Interestingly, those subjects who showed an increase in MSNA showed a significantly larger increase in blood pressure than those in whom MSNA decreased. Systolic pressure increased from 132.0 ± 5.5 (baseline) to 159.9 ± 5.8 mmHg (steady level of pain) in the increasing group but from only 133.0 ± 4.7 to 142.7 ± 5.3 in the decreasing group. Diastolic pressure increased from 70.2 ± 5.2 (baseline) to 86.6 ± 4.5 mmHg (steady level of pain) and from 75.1 ± 4.2 to 76.7 ± 4.3 in the increasing and decreasing groups, respectively. Relative changes in blood pressure, heart rate and MSNA in the two groups are presented in Figure 3. In the increasing group, data from two subjects were excluded from the calculated mean of all parameters as they showed much larger increases in amplitude of MSNA (396 and 520%), as defined by running an Outliers Test (Prism, GraphPad software), which would have skewed the results.
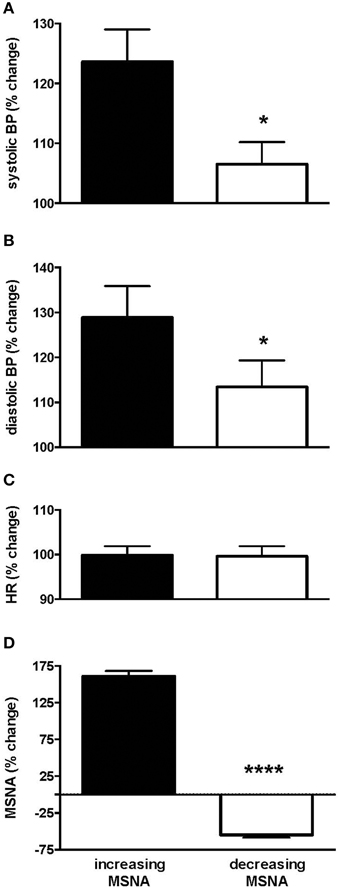
Figure 3. Changes in systolic (A) and diastolic (B) pressure, heart rate (C) and muscle sympathetic nerve activity (MSNA; D) in the group of subjects in whom MSNA amplitude increased during tonic muscle pain and the group in whom MSNA amplitude decreased, measured from the peak changes. Systolic and diastolic pressures were significantly higher in the increasing group as depicted by the asterisk. Results are compared to baseline levels (i.e., 100%).
Resting Levels of MSNA and BP
When comparing the increasing and decreasing groups, there were no differences in baseline MSNA (19.0 ± 1.5 vs. 18.9 ± 1.2 bursts/min; p = 0.99, t-test) that could account for these divergent responses. Moreover, as shown in Table 1, there were no differences in resting blood pressure parameters, heart rate or heart rate variability, and no effect of body mass index or total muscle mass.
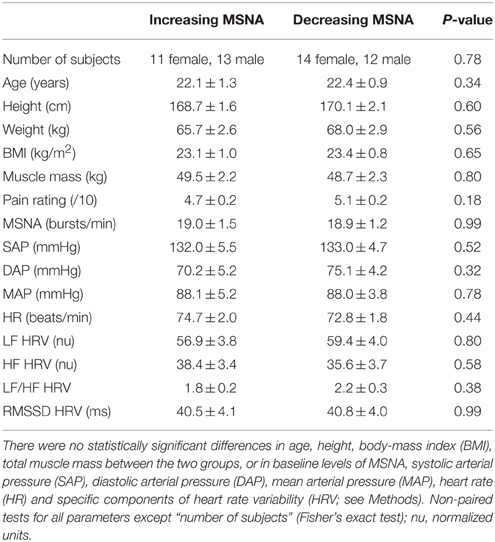
Table 1. Baseline data for the group showing an increase in MSNA (n = 24) during tonic muscle pain and the group showing a decrease (n = 26).
Sex Differences
Of the 24 subjects in whom MSNA increased, 11 were female and 13 were male, while there were 14 females and 12 males in whom MSNA decreased. These data indicate that there was no difference in the propensity of males or females to exhibit an increase or decrease in MSNA during long-lasting muscle pain (p = 0.78, Fisher's Exact test). Moreover, the data illustrated in Figure 4 show that there were no differences in the peak magnitude of change in MSNA between females and males in either the increasing group (158.0 ± 11.3% vs. 163.2 ± 10.0%; p = 0.40, Mann-Whitney) or the decreasing group (44.1 ± 4.7% vs. 46.4 ± 5.4%; p = 0.77, Mann-Whitney). There was no significant difference in the mean pain rating between females and males (4.9 ± 0.2 vs. 4.9 ± 0.2, respectively; p = 0.87, t-test).
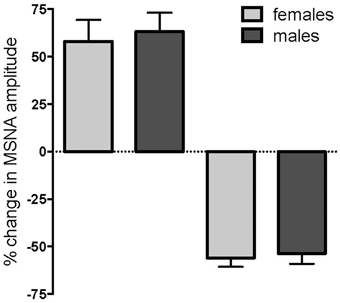
Figure 4. Changes in muscle sympathetic nerve activity (MSNA) in females and males during tonic muscle pain. There was no difference between females (light gray) and males (dark gray) in the propensity to show an increase or decrease in MSNA, and no differences in the magnitudes of the peak changes during muscle pain.
There were no statistically significant differences in resting MSNA between the female and male subjects (18.8 ± 1.5 vs. 20.2 ± 1.5 bursts/min; p = 0.19, unpaired Mann-Whitney test), and no significant differences in any of the other baseline cardiovascular parameters (Table 2). The only statistically significant differences between males and females were in BMI and muscle mass, both of which were significantly higher in the males (p = 0.05 and p < 0.0001, respectively), and age (p < 0.01)—on average, the females were one year older, though this is of no consequence because ages were not significantly different in the increasing and decreasing groups (cf Table 1).
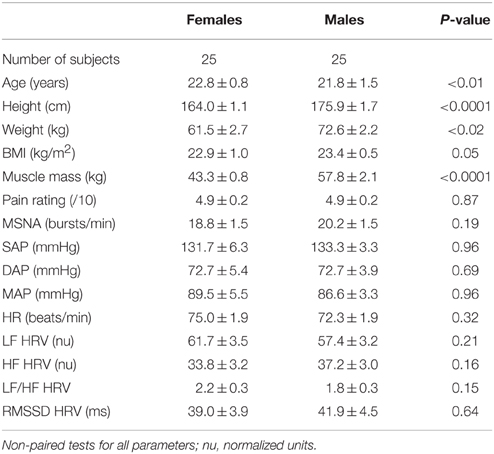
Table 2. Age, body-mass index (BMI), total muscle mass, MSNA frequency (normalized to baseline), systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), heart rate (HR) and specific components of heart rate variability (HRV; see Methods) at baseline, divided by sex.
Discussion
This study extends the recent work conducted in our laboratory on the effects of experimental muscle pain on the sympathetic nervous system (Burton et al., 2008, 2009a,b; Fazalbhoy et al., 2012, 2014; Hall et al., 2012). In our first study of 12 subjects we found that tonic muscle pain, produced by intramuscular infusion of hypertonic saline for 45 min created divergent changes in muscle sympathetic outflow: one group (n = 7) showing an increase in MSNA and another (n = 5) showing a decrease (Fazalbhoy et al., 2012). In a second study of 15 subjects, we had reported that 11 subjects showed consistent increases (n = 5) or decreases (n = 6) in MSNA when assessed on two occasions at least 2 weeks apart (Fazalbhoy et al., 2014). Here we have confirmed the findings of divergent sympathetic responses to long-lasting muscle pain, but with a much larger sample size (n = 50): one group of people (n = 24) showed an increase in MSNA and another group (n = 26) showed a decrease.
Baseline Physiological Parameters
The findings of the current study suggest that the cardiovascular responses to long-lasting muscle pain are not determined by our measured baseline physiological levels; both the direction of the response and the magnitude of change were independent of baseline MSNA, heart rate, blood pressure, heart rate variability, as well as age, sex, and BMI. This is consistent with studies showing comparable control and sensitivity of the sympathetic baroreflex in young men and young women (Tank et al., 2005; Studinger et al., 2009; Hart et al., 2011). Whether, these findings remain with increasing age is beyond the scope of this study. However, it would be interesting to know whether the pattern of response remains unchanged with age.
In this larger sample of subjects we found no correlation between MSNA and heart rate, unlike the parallel changes observed in the smaller data sets reported previously (Fazalbhoy et al., 2012, 2014). Because of the dual innervation of the heart, it may well be that the increase in sympathetic outflow to the vascular bed in muscle is matched by a parallel increase in cardiac sympathetic drive, which would increase heart rate, but that a competing parasympathetic influence via the vagus nerve counteracts this.
MSNA and Blood Pressure
Although, there was no difference in the changes in heart rate and the change in MSNA between the two groups, in the group of subjects in whom MSNA increased during tonic muscle pain blood pressure was significantly higher than in the group in whom MSNA decreased. This suggests that the increase in MSNA was driving the increase in blood pressure, as an increase in blood pressure should, via the baroreflex, lead to a fall in MSNA. Indeed, the latter mechanism may explain why in some subjects MSNA fell despite an increase in blood pressure: in these cases, it would appear that the increase in blood pressure was causing a baroreflex-mediated reduction in MSNA, while in other instances a reduction in both blood pressure and MSNA could be the result of a nociceptor-driven withdrawal of MSNA. However, for those subjects in whom both MSNA and blood pressure increased during tonic muscle pain, we would like to suggest that nociceptor-driven increases in blood pressure could potentially be a risk factor for the development of clinically significant high blood pressure in the future, given that some individuals with chronic pain go on to develop hypertension. Indeed, patients with post-surgical chronic pain have nearly twice the prevalence of clinical hypertension than medical patients without pain (Bruehl et al., 2005). Accordingly, we could postulate that a person who consistently exhibited increases in MSNA, blood pressure, and heart rate during experimental muscle pain may—if he or she developed chronic pain from an injury in the future—go on to develop hypertension.
Heart Rate Variability
Heart rate variability is widely reported to reflect the degree of sympathetic and parasympathetic control over the heart. The LF band is proposed to represent (primarily) sympathetic cardiac activation (Malliani et al., 1991), while the HF band is proposed to reflect vagal cardiac control (Bernston et al., 1997). Subsequently, the LF/HF ratio has been suggested as an index of the sympathovagal balance (Cohen et al., 2000; Martinez-Lavin, 2004; Staud, 2008; Reyes del Paso et al., 2011). The value of HRV in distinguishing between cardiac sympathetic and parasympathetic outflow is debatable (Goldstein et al., 2011). However, that there was no difference between any HRV parameters at either baseline or during tonic pain indicates that HRV is not related to whatever is responsible for the divergent sympathetic responses to muscle pain seen in this study.
Limitations
The intramuscular infusion of hypertonic saline occurred at a time unknown to the subject, who was asked to continuously report the development of pain, as a rating out of 10, via the linear potentiometer provided. Infusion rates were titrated—by increasing or decreasing the rate of infusion in increments of 0.02 ml/min—to maintain a constant level of pain. Although we did not routinely record either the rate of infusion, or the total volume infused, in each subject, we never exceeded 20 ml (as noted in Methods we used two syringes of 10 ml each). Nevertheless, there were no differences in total muscle mass in the group in whom MSNA increased and the group in whom MSNA decreased and, given that the infusion caused a notable distension of the muscle belly in both groups, it is reasonable to assume that there any changes in plasma osmolality were limited to the muscle compartment and that comparable depolarization of small-diameter axons by the hypertonic saline occurred in the two groups. In other words, the noxious sensory input was the same in the two groups, as reflected in the fact that there were no significant differences in mean pain ratings between the two groups. The same was true when we separated the cohort into males and females: the only significant differences here being the higher BMI and lower total muscle mass in the females, both of which are expected. Of course, one could argue that the intramuscular infusion of hypertonic saline would have a greater effect in a smaller muscle (in the females), but in our experience we see no differences in mean pain ratings in small muscles (e.g., intrinsic muscles of the hand) and large muscles (e.g., flexor carpi radialis, deltoid, tibialis anterior), and pain ratings were the same in males and females.
Implications
We have shown, in a large sample of subjects (n = 50), that the baseline physiological parameters measured here do not predict whether an individual exhibits an increase or decrease in MSNA during long-lasting muscle pain. Furthermore, sex appears to play no role in determining the direction of response to muscle pain. Unlike the short-lasting pain we had previously induced by bolus injections (Burton et al., 2009a,b), we believe the physiological responses to tonic pain will more closely replicate episodes during which chronic pain patients are suffering and coping with their pain. Persistent deep pain in experimental animals has been shown to provoke a passive coping response—i.e., conservation/withdrawal (Keay and Bandler, 2002). Of course, while tonic muscle pain lasting only 20 min has been used as a model for chronic musculoskeletal pain (Capra and Ro, 2004), we should stress that this only reflects continuous nociceptive pain and not the neuropathic pain typically associated with chronic pain. Nevertheless, this method of inducing pain offers the advantage of allowing a controlled investigation into how pain may modulate MSNA, blood pressure, and heart rate. Conversely—assuming everything else is equal—one would need to know the level of MSNA in a person prior to the development of chronic pain in order to interpret any changes in muscle sympathetic outflow. Microelectrode recordings of sympathetic nerve traffic in human subjects have found no differences in sympathetic outflow to a painful limb compared to the contralateral non-painful limb in patients with complex regional pain syndrome, suspected to be sympathetically maintained because of the marked cutaneous vasoconstriction (Casale and Elam, 1992). In order to understand the neurophysiological basis of the divergent sympathetic responses to experimental muscle pain, further investigations are needed, as the current results fail to demonstrate that baseline physiological parameters, BMI or sex, play a role in the cardiovascular responses to long-lasting muscle pain in humans.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (GNT1029782).
References
Bernston, G. G., Bigger, J. T. Jr. Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., et al. (1997). Heart rate variability; origins, methods, and interpretive caveats. Psychophysiology 34, 623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x
Bruehl, S., Chung, O. Y., Jirjis, J. N., and Biridepalli, S. (2005). Prevalence of clinical hypertension in patients with chronic pain compared to nonpain general medical patients. Clin. J. Pain 21, 147–153. doi: 10.1097/00002508-200503000-00006
Burton, A., Brown, R., and Macefield, V. G. (2008). Selective activation of muscle and skin nociceptors does not trigger exaggerated sympathetic responses in spinal injured subjects. Spinal Cord 46, 660–665. doi: 10.1038/sc.2008.33
Burton, A. R., Birznieks, I., Bolton, P. S., Henderson, L. A., and Macefield, V. G. (2009a). Effects of deep and superficial experimentally induced acute pain on muscle sympathetic nerve activity in human subjects. J. Physiol. 587, 183–193. doi: 10.1113/jphysiol.2008.162230
Burton, A. R., Birznieks, I., Spaak, J., Henderson, L. A., and Macefield, V. G. (2009b). The effects of deep and superficial experimentally-induced acute pain on skin sympathetic nerve activity in human subjects. Exp. Brain Res. 195, 317–324. doi: 10.1007/s00221-009-1790-9
Capra, N. F., and Ro, J. Y. (2004). Human and animal experimental models of acute and chronic muscle pain: intramuscular algesic injection. Pain 10, 3–7. doi: 10.1016/j.pain.2004.04.033
Casale, R., and Elam, M. (1992). Normal sympathetic nerve activity in a reflex sympathetic dystrophy with marked skin vasoconstriction. J. Auton. Nerv. Syst. 41, 215–219. doi: 10.1016/0165-1838(92)90061-K
Cohen, H., Neumann, L., Shore, M., Amir, M., Cassuto, Y., and Buskila, D. (2000). Autonomic dysfuction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin. Arthritis Rheum. 29, 217–227. doi: 10.1016/S0049-0172(00)80010-4
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Fazalbhoy, A., Birznieks, I., and Macefield, V. G. (2012). Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp. Physiol. 97, 1084–1092. doi: 10.1113/expphysiol.2012.066191
Fazalbhoy, A., Birznieks, I., and Macefield, V. G. (2014). Consistent interindividual increases or decreases in muscle sympathetic nerve activity during experimental muscle pain. Exp. Brain Res. 232, 1309–13015. doi: 10.1007/s00221-014-3847-7
Feinstein, B., Langton, J. N., Jameson, R. M., and Schiller, F. (1954). Experiments on pain referred from deep somatic tissues. J. Bone Joint Surg. 36, 981–997.
Goldstein, D. S., Bentho, O., Park, M. Y., and Sharabi, Y. (2011). Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone by may be a measure of modulation of cardiac autonomic ouflows by baroreflexes. Exp. Physiol. 96, 1255–1261. doi: 10.1113/expphysiol.2010.056259
Graven-Nielsen, T., and Mense, S. (2001). The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin. J. Pain 17, 2–10. doi: 10.1097/00002508-200103000-00002
Hall, S., Fazalbhoy, A., Birznieks, I., and Macefield, V. G. (2012). Biphasic effects of tonic muscle pain on skin sympathetic nerve activity in human subjects. Exp. Brain Res. 221, 107–114. doi: 10.1007/s00221-012-3156-y
Hart, E. C., Wallin, B. G., Curry, T. B., Joyner, M. J., Karlsson, T., and Charkoudian, N. (2011). Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J. Physiol. 589, 3395–3404. doi: 10.1113/jphysiol.2011.208538
Keay, K. A., and Bandler, R. (2002). Distinct central representations of inescapable and escapable pain: observations and speculation. Exp. Physiol. 87, 275–279. doi: 10.1113/eph8702355
Malliani, A., Pagani, M., Lombardi, F., and Cerutti, S. (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation 84, 482–492. doi: 10.1161/01.CIR.84.2.482
Martinez-Lavin, M. (2004). Fibromyalgia as a sympathetically maintained pain syndrome. Curr. Pain Headache Rep. 8, 385–389. doi: 10.1007/s11916-996-0012-4
Reyes del Paso, G. A., Garrido, S., Pulgar, Á., and Duschek, S. (2011). Autonomic cardiovascular control and responses to experimental pain simulation in fibromyalgia syndrome. J. Psychosomat. Res. 70, 125–134. doi: 10.1016/j.jpsychores.2010.09.012
Staud, R. (2008). Heart rate variability as a biomarker of fibromyalgia syndrome. Fut. Rheumatol. 3, 475–483. doi: 10.2217/17460816.3.5.475
Studinger, P., Goldstein, R., and Taylor, J. A. (2009). Age- and fitness-related alterations in vascular sympathetic control. J. Physiol. 587, 2049–2057. doi: 10.1113/jphysiol.2009.170134
Keywords: blood pressure, HRV, MSNA, muscle pain, muscle sympathetic nerve activity
Citation: Kobuch S, Fazalbhoy A, Brown R and Macefield VG (2015) Inter-Individual Responses to Experimental Muscle Pain: Baseline Physiological Parameters Do Not Determine Whether Muscle Sympathetic Nerve Activity Increases or Decreases During Pain. Front. Neurosci. 9:471. doi: 10.3389/fnins.2015.00471
Received: 14 August 2015; Accepted: 24 November 2015;
Published: 17 December 2015.
Edited by:
James J. Galligan, Michigan State University, USAReviewed by:
Christopher J. Madden, Oregon Health and Science University, USAFlorian Beissner, Hannover Medical School, Germany
Copyright © 2015 Kobuch, Fazalbhoy, Brown and Macefield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vaughan G. Macefield, v.macefield@westernsydney.edu.au
 Sophie Kobuch
Sophie Kobuch Azharuddin Fazalbhoy
Azharuddin Fazalbhoy Rachael Brown
Rachael Brown Vaughan G. Macefield
Vaughan G. Macefield