- 1Department of Medical Nanobiotechnology, Pirogov Russian State Medical University, Moscow, Russia
- 2The Mount Sinai Community Clinical Oncology Program, Mount Sinai Comprehensive Cancer Center, Mount Sinai Medical Center, Miami Beach, FL, USA
- 3Research Center for Children’s Health, Moscow, Russia
- 4Institute of General Pathology and Pathophysiology, Russian Academy of Sciences, Moscow, Russia
- 5Faculty of Medicine, School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
- 6School of Medicine, University of Western Sydney, Campbelltown, NSW, Australia
- 7Department of Oral and Diagnostic Sciences, Columbia University, New York, NY, USA
- 8Laboratory of Medical Genetics, Russian Cardiology Research and Production Complex, Moscow, Russia
The mucosal barriers are very sensitive to pathogenic infection, thereby assuming the capacity of the mucosal immune system to induce protective immunity to harmful antigens and tolerance against harmless substances. This review provides current information about mechanisms of induction of mucosal tolerance and about impact of gut microbiota to mucosal tolerance.
Introduction
The human intestine harbors a whole microbial ecosystem containing over 100 trillion microorganisms that collectively have a total genome (the microbiome) consisting of 100-fold more genes than the human genome (Tsai and Coyle, 2009). The gut microbiota lives in tight symbiosis and homeostasis with the host and plays an essential role in harvesting energy, minerals, and bioactive compounds from the food. The intestinal microbiota exists in reciprocal balance with the gut-associated lymphoid tissue (GALT), the largest immune system in the body. Gut microorganisms are the major source of natural antigens that continuously stimulate the GALT and induce mucosal immune tolerance (e.g., local or systemic immune unresponsiveness) to innocuous antigens such as food proteins and molecular components of commensal bacteria (Pabst and Mowat, 2012). In addition, the GALT is necessary for preventing acute proinflammatory immune responses against the microbiota resulting in inflammatory bowel diseases or against food protein causing food allergy and celiac disease. The mucosal barriers are very sensitive to pathogenic infection thereby assuming the capacity of the mucosal immune system to induce protective immunity to harmful antigens and tolerance against harmless materials (Weiner et al., 2011).
Oral tolerance is a phenomenon of suppressing immune responses in the gut and systemic immune system by orally administered antigens. Tolerance to intestinal bacteria and tolerance to food proteins differs by its effects on the immune system. Tolerance to food protein induced through the small intestine influences both local and systemic immune responses, while tolerance to gut bacteria in the colon does not attenuate systemic responses (Pabst and Mowat, 2012). Oral tolerance was extensively studied in rodents using many different antigens such as purified proteins, cellular antigens, and small haptens. The phenomenon of oral tolerance was also described in humans (Kraus et al., 2004; Kapp et al., 2010). The effects of oral tolerance on the immune system were usually evaluated as a suppression of cytokine production and T-cell proliferation and decrease in serum titers of circulating antibodies. The ability of oral tolerance to inhibit autoimmune and inflammatory diseases was demonstrated in rodent experimental models of type 1 diabetes, encephalomyelitis, arthritis, myasthenia, thyroiditis, and other pathology (Faria and Weiner, 2006). Thus, oral tolerance is able to diminish a broad spectrum of immune responses and hence could play a key role in maintaining peripheral immune homeostasis.
Mechanisms of Induction of Mucosal Tolerance
Intestinal Intake of Tolerogenic Antigen
The intestinal immune system is composed by several essential components such as GALT [Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs)] and gut-draining mesenteric lymph nodes (mLNs), which primarily contribute to the induction of mucosal tolerance through recognition of colon-derived bacterial and viral antigens (Brandtzaeg et al., 2008). In the epithelium of PPs and ILFs, specialized epithelial cells called as microfold cells (M cells) are involved in the permanent transfer of colon material from the gut lumen into the GALT (Miller et al., 2007). The intestinal antigen is then passed from M cells on to dendritic cells (DCs) that reside either below the epithelium or in a “pocket” created at the basolateral surface of the M cell. Antigen uptake by DCs in the lamina propria (LP) underlying regular villus epithelium was shown to be critical for inducing tolerance to soluble antigens in the small intestine (Chirdo et al., 2005).
There are several possible mechanisms of delivery of intestinal antigens from the lumen to DCs. Low-molecular antigens such as haptens and peptides could diffuse through the intestinal epithelium via pores in inter-epithelial tight junctions (Hossain and Hirata, 2008). High-molecular complexes can be taken across enterocytes by transcytosis or through exosome-mediated pathway associated with major histocompatibility complex (MHC) class II-dependent recognition and antigen processing (Menard et al., 2010). DCs were shown to efficiently uptake exosomes. The exosomes containing MHC class II associated with gut antigenic peptides were able to induce tolerance in recipient mice after isolation from serum of antigen-fed animals (Karlsson et al., 2001).
In fact, the antigen nature drives its path of uptake. Particulate material and microbiota are mainly delivered into the GALT by transcytosis throught M cells while soluble antigens induce oral tolerance after DC-mediated intake mostly in the LP and then in the GALT.
Dissemination of Gut Antigen within the Body
Orally administered antigens are likely to disseminate across the body through the circulation. For example, food protein can be found in the blood of humans soon after meal intake (Husby et al., 1985). The antigen entry to the bloodstream occurs not simply but is accompanied with detectable changes in the mucosal immune system including activation of C-type lectin (marker CD69) expression and T cells in mLNs and peripheral LNs (Smith et al., 2002). Furthermore, since serum-derived exosomes from antigen-fed animals could induce tolerance in naïve recipient animals, this phenomenon indicates the presence of tolerogenic material (Karlsson et al., 2001, 2010). Indeed, it is important to know where in the body the gut antigen induces oral tolerance.
The administration of an antigen into the portal vein induces tolerance that is specific to the antigen (Thomson and Knolle, 2010) whereas disruption of the intrahepatic blood flow by the portocaval shunt prevents oral tolerance induction (Yang et al., 1994). These findings support the liver as a likely tolerogenic site for gut antigen. Furthermore, the liver is anatomically located as the endpoint of the portal vein delivering blood directly from the intestine.
The liver is enriched with specialized antigen-presenting cells (APCs) that could be primarily involved in the tolerance induction. Kupffer cells and conventional hepatic DCs belong to professional APCs challenging immune responses against gut antigens in favor to inducing and maintaining tolerance (Thomson and Knolle, 2010). In addition, hepatic sinusoidal endothelial cells are able to collect circulating antigens and act as APCs in inducing tolerance (Limmer et al., 2005; Holz et al., 2010). In the liver, plasmacytoid DCs especially contribute to the induction of systemic tolerance to orally administered antigens by down-regulating and initiating anergy in antigen-specific CD4+ and CD8+ T cells (Goubier et al., 2008; Dubois et al., 2009).
In the spleen and peripheral LNs that are located beyond the liver, resident DCs could trigger local and systemic tolerance to the gut-derived antigen even the absence of costimulation through initiating anergy in effector T cells or inducing regulatory T cells (Tregs; Yamazaki et al., 2008) but with less efficiency than GALT-associated DCs do (Hashiguchi et al., 2011). However, it is likely that intestinal DCs play a key role in inducing systemic tolerance.
GALT-Associated DCs Play a Crucial Role in Inducing Oral Tolerance
Gut antigen-induced CD103+ DCs migrating from the LP to mLNs are responsible for major delivery and recognition of colon-derived antigens in the GALT (Pabst et al., 2007). The travel of DCs from LPs to mLNs is dependent on C-C chemokine receptor (CCR) 7, a chemokine receptor (Forster et al., 2008). The lack of all LNs and PP in lymphotoxin α-deficient mice leads to the loss of oral tolerance that could be restored by specifically induced mLN formation (Spahn et al., 2002). Similarly, surgical deletion of mLNs in mice abolishes the induction of oral tolerance (Worbs et al., 2006). These findings suggest that the intestine immune system and especially mLNs have a primary role in the induction of oral tolerance.
Gut-associated lymphoid tissue-associated DCs that express on their surface integrin chain-αE (CD103) never reach the circulation beyond mLNs (Milling et al., 2010). In LPs, intestinal CD103+ DCs recognize gut antigens and possess tolerogenic and immunoregulatory properties stimulating expression of homing molecules CCR7 and integrin-αIVβ7 on T cells resided in the mLNs and inducing Forkhead box protein 3 (FoxP3)-positive Tregs (Johansson-Lindbom et al., 2005; Sun et al., 2007; Jaensson et al., 2008; Worthington et al., 2011). Gut-derived vitamin A and other retinoids were shown to modulate homing-inducing and tolerogenic properties of CD103+cells by inducing synthesis of homing molecules CCR9 and CCR4 (Iwata et al., 2004; Jaensson-Gyllenbäck et al., 2011). Retinoic acid was reported to down-regulate experimentally induced intestinal inflammation (ileitis) by restoring the balance between proinflammatory Th17 cells and inducible CD4+FoxP3+ Tregs (iTregs) in the mouse (Collins et al., 2011). Furthermore, retinoic acid produced by CD103+DCs cooperates with transforming growth factor-β (TGF-β) in generation of iTregs from naive CD4+T cells (Sun et al., 2007). The integrin-αIVβ7 was found to be significantly up-regulated on the surface of CD103+DCs and activates latent TGF-β by releasing it from the complex with latent TGF-β-binding protein (LTBP) thereby mediating TGF-β-dependent induction of Tregs (Païdassi et al., 2011; Worthington et al., 2011). Intestinal inflammation impairs function of GALT-associated CD103+ cells and abolishes their tolerogenic activities (Laffont et al., 2010).
CD103+ DCs were observed to cooperate with other cell types presented in the intestinal mucosa to induce gut tropism in T cells and oral tolerance. In mLNs, non-hematopoietic stromal cells are essential for full display of ability of GALT-associated DCs to induce gut-homing T cells (Hammerschmidt et al., 2008). Notably, stromal cells taken from peripheral LNs failed to initiate gut tropism in T cells. Furthermore, mLN-derived stromal cells produce high levels of retinoic acid-metabolizing enzymes that are essential for retinoic acid-mediated induction of synthesis of homing molecules (Molenaar et al., 2009). Similarly, stromal cells from mLNs but not from skin-draining LNs support the generation of Foxp3+ Tregs (Cording et al., 2014). Thus, gut-specific environment and synergistic interactions of mLN-derived stromal and DCs play a crucial role in induction of intestinal T cell homing and gut-associated Tregs.
Recently, a new population of intestinal CD103– DCs was identified (Cerovic et al., 2013). Like CD103+ DCs, these cells are responsive to Flt3 (FMS-like tyrosine kinase 3), a regulatory factor crucial for the hematopoietic commitment and functional and phenotypic maintenance of DCs, and prime a gut-homing phenotype to naive T cells in the mLNs. CD103–CD11b+ CX3CR1int lymph DC subset induce the differentiation of proinflammatory interferon (IFN)-γ and interleukin (IL-17)-17-producing effector T cells (Cerovic et al., 2013). Administration of Flt3 ligand resulted in inducing CD103 expression in CD103– DCs and converting these cells from proinflammatory to tolerogenic CD103+ DCs that contributed to generation of CD4+FoxP3+ Tregs and attenuation of experimental Crohn’s-like ileitis (Collins et al., 2012). Therefore, local microenvironment and proinflammatory/anti-inflammatory stimuli could greatly influence the phenotype and function of GALT-associated DCs.
Mechanisms of Oral Tolerance Induction
Oral tolerance utilizes mechanisms similar with those of the peripheral immune tolerance including active impact of Tregs, clonal deletion, and clonal anergy of T cells. Antigen doses were shown to influence the choice of tolerogenic mechanisms and numbers of iTregs (Weiner et al., 2011). In exposure to a single high dose of antigen, clonal deletion or anergy are preferential tolerogenic mechanisms whereas numerous low antigen doses induce T cell anergy (Chen et al., 1996). In oral tolerance, the highest counts of Foxp3+ Tregs were induced after exposure to the high doses of an antigen (Siewert et al., 2008).
Inducible Tregs generated with contribution of intestinal CD103+ DCs and mLN-specific stromal cells appears to play a key role in induction and maintenance of oral tolerance. Several subsets of Tregs such as CD4+FoxP3+ iTregs, IL-10-producing regulatory type 1 cells (Tr1), and TGF-β-producing Th3 Tregs were shown to be involved in oral tolerance (Pabst and Mowat, 2012). Adoptive transfer of CD4+CD25+FoxP3+ Tregs from tolerogenic animals could induce oral tolerance in naïve mice whereas depletion of FoxP3+Tregs abolishes tolerance (Dubois et al., 2003). FoxP3+ and FoxP3– IL-10-producing Tregs were found in the gut mucosa (Maynard et al., 2007). Interestingly, all-trans retinoic acid was shown to have reciprocal effects on induction of Foxp3 and IL-10 in developing CD4+ Tregs. By enhancing TGF-β-dependent Foxp3 induction, all-trans retinoic acid inhibits TGF-β-dependent induction of IL-10-producing Tregs. Toll-like receptor (TLR)-9-mediated suppression of all-trans retinoic acid production by GALT-associated DCs alternately induces preferential expression of IL-10 in Tregs (Maynard et al., 2009).
Among subsets of Tregs, the role of FoxP3+ Tregs [e.g., natural CD4+CD25+FoxP3+ Tregs (nTregs) and iTregs] in oral tolerance is the best studied (Curotto de Lafaille et al., 2009). nTregs are selected in the thymus and are responsible for driving central tolerance whereas iTregs are induced in the periphery and are accordingly involved in the regulation of peripheral tolerance. In changing microenvironmental conditions, nTregs maintain a stable phenotype although their function could be impaired (Rubtsov et al., 2010). In contrast, iTregs have a plasticity to differentiate to other helper T cell types under inflammatory stimuli (Koenecke et al., 2009). However, in steady-state non-inflammatory conditions, FoxP3+ Tregs can induce and maintain oral tolerance for a long time. Interestingly, in a mouse model, nTregs were unable to induce tolerance to oral ovalbumin while iTregs did suggesting for the obligatory need in peripheral conversion of naïve CD4+ T cells to iTregs (Curotto de Lafaille et al., 2008).
Indeed, mLNs provide an essential gut-specific microenvironment for selective differentiation of iTregs (Hadis et al., 2011).
Maintenance of Oral Tolerance
Assuming that the mucosal immune system every day should respond to new intestinal antigens and oral tolerance to a specific antigen could be maintained for several months, iTregs are likely to be continuously generated in the GALT (Strobel and Ferguson, 1987). Induction and maintenance of oral tolerance is a multi-stage process involving lymphoid and mucosal tissues (Hadis et al., 2011). After induction in mLNs, iTregs should keep gut-specific homing for long-term supporting oral tolerance. Expression of homing molecules such as integrin-β7 and its ligand MadCAM-1 (mucosal vascular addressin cell adhesion molecule 1) is essential for supporting GALT-associated homing of newly generated iTregs (Wagner et al., 1996; Gorfu et al., 2009). Depletion of these two molecules results in greatly diminished and impaired oral tolerance that could be restored by adoptive transfer of integrin-β7-positive T cells (Hadis et al., 2011).
CCR9 was shown to be crucial for the maintenance of GALT-associated homing of T cells since CCR9-null mice have marked defects in oral tolerance (Cassani et al., 2011). CCR9 and integrin-αIVβ7 are both required for small intestine-specific homing of immune cells (Svensson et al., 2002; Stenstad et al., 2007). Retinoic acid is required for induction of CCR9 expression in T cells. Activation of the retinoic acid receptor (RAR) and retinoid X receptor (RXR) results in expression of high levels of CCR9, integrin-αIVβ7, and FoxP3 essential for differentiation of naïve T cells to iTregs and their homing in the small-intestine-associated GALT (Takeuchi et al., 2010).
In the small intestine LP, iTregs induced in mLNs are subjected to secondary expansion that is mediated by the chemokine receptor CX3CR. This receptor is also crucial for induction of oral tolerance because CX3CR-null mice lack oral tolerance. In the LP of CX3CR-null mice, myeloid cells secrete less IL-10, and its production could be rescued by adoptive transfer of wild-type macrophages that express IL-10 (Hadis et al., 2011). Due to decreased production of IL-10, generation of iTregs is suppressed in CX3CR-null mice, an event, which in turn abrogates the induction of oral tolerance (Murai et al., 2009). IL-10-producing intestinal mucosa-resident macrophages could therefore contribute to maintenance of iTregs and iTregs-dependent oral tolerance (Gonnella et al., 1998).
Indeed, oral tolerance associated with the small intestine is induced by the cooperative action of mLN-derived CD103+ DCs and stromal cells that produce retinoic acid required for imprinting of gut-homing molecules on specific T cells. Certain T cells differentiate to iTregs that leave the mLN and home to the small intestine where they undergo secondary expansion activated by IL-10-secreting CX3CRhigh myeloid cells and resident macrophages. Some secondarily expanded Tregs can possibly leave the GALT and enter the circulation via lymphatic vessels or directly to the bloodstream thereby contributing to expanding tolerance to orally administered antigen from local (e.g., gut-associated) to systemic tolerance. The mechanism of establishment of systemic oral tolerance could be similar with the trafficking of CD4+Foxp3+ Tregs from skin draining LNs to the skin where they display inhibitory effects and then come back to the LNs (Tomura et al., 2010).
Regular stimulation by gut antigen also contributes to maintaining Tregs in the gut. The T-cell receptor (TCR) repertoire of Tregs from the small intestinal LP is highly overlapping with the TCR repertoire of Tregs from gut-draining mLNs (Föhse et al., 2011). A substantial number of GALT-associated iTregs is likely to arise after induction by colonic microbiota-derived antigen (Lathrop et al., 2011). Thymic nTregs were shown to have the TCR repertoire that is skewed toward self-antigens while the TCR repertoire of iTregs is biased toward non-self-antigens (Hsieh et al., 2006). However, the TCR repertoire of thymus-derived Tregs in colon-associated lymphoid and non-lymphoid tissues is heavily influenced by the composition of the microbiota suggesting that nTregs are involved in mucosal tolerance to commensal microorganisms (Cebula et al., 2013).
Mechanisms of induction of mucosal tolerance are summarized in Figure 1.
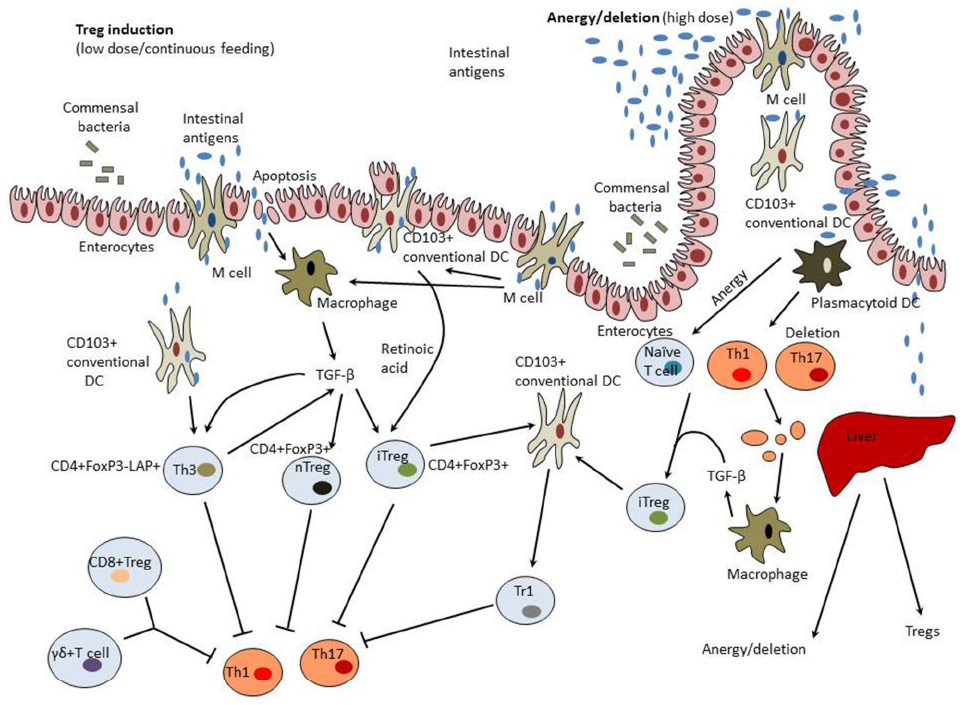
Figure 1. Mechanisms of induction of mucosal tolerance. Intestinal antigens could pass from the gut to the GALT through M cells, be collected by DCs, and taken up by enterocytes. GALT-associated DCs have a unique capacity to direct differentiation of regulatory T cells (Tregs) from forkhead box protein 3 (FoxP3)– T cells. These properties of tolerogenic dendritic cells (DCs) are modulated by commensal colon microbiota, transforming growth factor-β (TGF-β), and interleukin (IL-10) produced by enterocytes. Tolerogenic DCs secrete retinoic acid that is synthesized from dietary vitamin A and is essential for formation of inducible Tregs (iTregs). CD11b monocytes also contribute to the induction of Tregs. Treg induction occurs in mesenteric lymph nodes and involves C-C motif receptors (CCR)-7 and -9. Low doses of intestinal antigen lead to the induction of Tregs while high antigen doses result in tolerance induction preferentially through the mechanisms of anergy and deletion. Macrophages activated by clearance of apoptotic T cells and enterocytes become to produce TGF-β and therefore could possess tolerogenic properties. In the liver, high antigen doses could be taken up by tolerogenic plasmacytoid DCs that induce anergy/deletion and Tregs. A variety of Tregs could be induced including CD4+CD25+Foxp3+ natural Tregs (nTregs), CD4+CD25+Foxp3+ iTregs, latency-associated peptide (LAP)+ Tregs (Th3 cells), CD8+ Tregs, and γδT cells.
Impact of Gut Microbiota to Mucosal Tolerance
The Stable Gut Microbiota is Beneficial for Human Health
The regular intake of beneficial microorganisms (probiotics) is believed to confer health advances on the host. The probiotics such as lactobacilli and bifidobacteria exhibit a range of beneficial effects on the host health including competition with pathogenic microbes for nutrients, supply with vitamin K, inactivation of xenobiotics, stimulating colon peristalsis, digestion of indigestible food fibers, and modulation of the host’s immune system (Hardy et al., 2013). In addition, non-pathogenic commensal bacteria such as Escherichia coli were shown to inhibit growth and expansion of pathogens through secretion of antimicrobial peptides (bacteriocin, colicin M, microsin S; Kamenšek and Žgur-Bertok, 2013). Furthermore, several probiotics could cooperate (e.g., form synbiotics) in order to selectively promote the growth of one or more beneficial probiotic species (van Zanten et al., 2012).
The human body plays host to communities of beneficial commensal microorganisms (gut microbiota) that mediate key physiological processes in exchange for nutrients and a sheltered habitat in which they are able to reproduce. In the human intestinal commensal microbiota, the Firmicutes and the Bacteroidetes are the two prevalent phylogenetic types (Eckburg et al., 2005). The stomach contains low numbers of commensal bacteria with predominating species of Lactobacillus, Streptococcus, and Helicobacter pylori. In the small intestine, Streptococcus and Lactobacillus species are predominant. In the large intestine and distal gut, the Bacteroides, Clostridium, Fusobacterium, and Bifidobacterium are prevalent (Eckburg et al., 2005). Indeed, since strain and density range of commensal bacteria greatly vary along the gastrointestinal tract, antimicrobial peptide production and effects of microbiota to the host’s immune system also markedly vary from one location to another in the gut. In pathogenic conditions such as inflammatory bowel disease, these beneficial stable microbiomes are subjected to dramatic changes associated with decreased microbial diversity and unfavorable shift toward increased numbers of gram-negative bacteria (Qin et al., 2010).
The colonization of the newborn intestine is the important stage in the development of future stable gut microbiota. Lower numbers of initial colonizing bacteria, in particular bifidobacteria, may impede establishment of a stable gut microbiota during a critical period of immune education and development. Human milk have was found to provide early immune education and passive immunity through the cooperative action of various bioactive molecules and cells including immunoglobulins, lysozyme, lactoferrin (Grzelak et al., 2014) as well as being a source of commensals such as L. acidophilus, L. gasseri, Bifidobacterium bifidum, and Bifidobacterium breve (Martín et al., 2003).
The generation of germ-free mice provided a good option for evaluating the role of the microbiota composition on the immune system. The lack of microbial stimulation leads to maturation defects in lymphoid organs such as PPs and spleen, abnormalities in numbers of immune cells, and altered cytokine expression (Pollard and Sharon, 1970; Bartizal et al., 1984; Williams et al., 2006). Some gut bacteria were shown to play a significant role in the development of GALT. Bacteroides fragilis (B. fragilis) and Bacillus subtilis could stimulate transcytosis in M cells through induction of stress responses to secretion of bacterial protein YqxM essential for sporulation and biofilm formation (Rhee et al., 2004; Chu et al., 2006).
The effects of imbalanced microbiota are not restricted to gastrointestinal abnormalities but could have systemic impact on immunity especially in allergic disorders (Russell et al., 2013) and autoimmune diseases such as multiple sclerosis (Ochoa-Repáraz et al., 2010) and type 1 diabetes (King and Sarvetnick, 2011). Such findings further support the “hygiene hypothesis” suggesting that the absence of immune challenges, result in the insufficient maturation of the immune system and predispose to certain allergic and autoimmune disorders such as celiac disease, asthma, inflammatory bowel disease, type 1 diabetes, etc. (Strachan, 1989).
Intestinal Microbiota and Immune Homeostasis
The intestinal microbiota is a crucial component of the immunologic milieu that creates the substrate for oral tolerance (Strober, 2009). The recognition of intestinal bacteria triggers the choice of the subsequent GALT-mediated immune response that should be positive (e.g., stimulatory) in case of pathogens or negative (e.g., tolerogenic) in case of commensal microbes. The host innate immunity of host organism is primarily responsible for recognition of pathogens.
Toll-like receptors and other pathogen-sensing molecules that are highly expressed by enterocytes and mucosal APCs (DCs and macrophages) respond to pathogen-associated molecular patterns (PAMPs; Chu and Mazmanian, 2013). The recognition of pathogens results in induction of antiviral or proinflammatory response against infection.
In the gut epithelium, expression of TLRs is down-regulated on the apical membrane compared to the basolateral side. Low expression of TLR2 and TLR4 is observed on the apical membrane. These receptors prime tolerance to cell wall constituents of commensal bacteria such as lipopolysaccharides (LPS) and peptidoglycans (Fukase et al., 2003; Abreu, 2010). Normally, E. coli flagellin does not induce any TLR5-mediated inflammatory response. However, basolateral activation of TLR5 by flagellin derived from pathogenic bacteria such as Salmonella results in induction of the acute intestinal inflammation associated with transfer of pathogenic flagellin through gut epithelial cells (Gewirtz et al., 2001). Therefore, when stable and normal microbiota is present in the gut, intestinal epithelial cells are irresponsive to flagellin while flagellin-dependent stimulation of the basolateral epithelial surface is recognized as pathogenic and stimulates TLR5-mediated proinflammatory response (Maaser et al., 2004).
Gram-negative commensal bacteria are major LPS producers in the gut. The epithelial alkaline phosphatase dephosphorylates bacterial LPS that become tolerogenic due to inability to stimulate TLR9 (Lee et al., 2007). In the intestinal epithelium, alkaline phosphatase is concentrated closely to the apical membrane thereby contributing to induction of either inflammatory or immunosuppressive tolerogenic response (Lee et al., 2006). Indeed, bacterial PAMPs are involved not only in the induction of activatory immune responses but also in induction of intestinal tolerance.
The GALT and intestinal epithelium can explore several options to down-regulate TLR-dependent immune stimulation including decrease of TLR expression, release of soluble immune receptors such as soluble TLR2, TLR4, and ST2 (Schmitz et al., 2005), and up-regulation of intracellular inhibitors of TLR signaling including MyD88s (a splice variant of myeloid differentiation factor 88), Toll-interacting protein (Tollip), TNF-related apoptosis-inducing ligand receptor (TRAIL-R), selective androgen receptor modulator (SARM), and others (Shibolet and Podolsky, 2007). Decoy receptors such as ST2 ligand and single Ig IL-1-related receptor (SIGIRR) also could contribute to colonic epithelial homeostasis by inhibiting TLR-induced gut inflammation (Xiao et al., 2007).
A growing number of evidence shows that intestinal commensal microbiota modulate Treg-mediated responses essential for establishing effective protection against pathogens and prevention of autoimmunity, food allergy, gut hypersensitivity to gut-derived antigens, and other unpleasant immunopathologic conditions. Germ-free mice colonized with commensal Clostridium species taken from the normal human microbiota developed IL-10-producing CD4+FoxP3+ Tregs (Atarashi et al., 2011, 2013). The community of 17 strains of Clostridia provided bacterial antigens and a TGF-β-rich environment to support induction, proliferation, and expansion of Tregs. Colonization of germ-free animals with human commensal B. fragilis induced development of FoxP3+ Tregs associated with production of anti-inflammatory cytokines (Round and Mazmanian, 2010). B. fragilis was found to secrete polysaccharide A. It has also been shown that B. fragilis mediates the conversion of CD4+ T cells into Foxp3+ Treg cells that produce IL-10 (Round and Mazmanian, 2010). Furthermore, bacterial polysaccharide A was not only able to prevent but also heal experimental colitis in animals suggesting for the involvement of gut commensal microbe B. fragilis to mucosal tolerance (Round and Mazmanian, 2010). In addition to live microbiota, LPS derived from the intestinal commensal bacteria could also modulate the mucosal immunity. In germ-free mice, LPS-rich sterile diet led to GALT-associated proliferation and expansion of CD4+ T cells and notably to expansion of Foxp3+ Tregs in mLNs. Both intestinal microbiota and LPS-rich diet were able to increase production of proinflammatory IL-12 and decrease production of IL-4 essential for differentiation of naïve T cells to pro-inflammatory Th2 cells (Hrncir et al., 2008).
Long-term treatment with antibiotics were shown to deplete normal gut microbiota causing systemic decrease in proliferation of CD4+ T cells while Foxp3+ Treg proliferation was only locally impaired in the GALT (Cording et al., 2013). In line with this, MyD88-null mice as well as animals deficient for various TLRs showed normal or even increased proliferation of conventional CD4+ T cells and Foxp3+ Tregs (Atarashi et al., 2011). Indeed, these findings suggest that TLR-mediated recognition of colon-derived bacterial components is not a major mechanism of induction of T cell homeostasis driven by commensal microbiota (Cording et al., 2013). Except for TLRs, other molecular sensors of microbe-derived antigens exist. Those include RIG-I-like receptors (RLPs), NOD-like receptors (NLRs), and DNA-sensing cytosolic receptors (Kumar et al., 2011; Jin and Flavell, 2013). Thus, other, TLR-independent PAMP-sensing mechanisms or metabolic influences could also be involved in the colonic microbiota-dependent control of T cell proliferation.
Animal models of experimentally induced autoimmune disorders suggest the link between the microbial inhabitants of the gastrointestinal tract and autoimmunity. Non-obese diabetic (NOD) mice, a model for type 1 diabetes, which lacks MyD88, an essential signaling component of innate immunity linking microbe-sensing immune receptors with immune signaling cascades is resistant to type 1 diabetes. However, germ-free MyD88-null NOD mice develop severe diabetes while colonization of germ-free MyD88-null NOD mice with microbiota mimicking normal human gut microbiota reduces diabetes (Wen et al., 2008).
In the model of experimental autoimmune encephalomyelitis (EAE), oral administration of Lactobacillus was shown to attenuate disease (Maassen and Claassen, 2008) and cause changes in gut microbiota, decrease in proinflammatory cytokines, and increase in production of anti-inflammatory cytokines IL-10 and IL-13 (Ochoa-Repáraz et al., 2009). Similarly, oral administration of a zwitterionic capsular polysaccharide A from human commensal bacterium B. fragilis was found to protect against EAE through induction of oral tolerance mediated by tolerogenic CD103+ DCs and IL-10-producing FoxP3+ Tregs in the GALT (Ochoa-Repáraz et al., 2010). In addition, non-filamentous ATP-producing gut microbiota (Atarashi et al., 2008) and intestinal segmental filamentous bacteria (Ivanov et al., 2008) were reported to specifically prime the development of proinflammatory Th17-polarizing DCs but did not affect IFN-γ-producing Th1 cells and FoxP3+ Tregs subsets (Ivanov et al., 2009). Intestinal filamentous bacteria were found to contribute to the pathogenesis of autoimmune arthritis through stimulation of proinflammatory Th17 cells (Wu et al., 2010). Therefore, filamentous bacteria represent an intriguing example of commensal microbiota capable of shifting the mucosal effector T cell inflammatory/tolerogenic balance and thus affect the immune fitness of the individual (Ivanov and Littman, 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank the Russian Scientific Foundation (grant 14-15-00112), Russian Federation for support of our work.
References
Abreu, M. T. (2010). Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10, 131–144. doi: 10.1038/nri2707
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Atarashi, K., Nishimura, J., Shima, T., Umesaki, Y., Yamamoto, M., Onoue, M., et al. (2008). ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812. doi: 10.1038/nature07240
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Atarashi, K., Tanoue, T., Oshima, K., Suda, W., Nagano, Y., Nishikawa, H., et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236. doi: 10.1038/nature12331
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Atarashi, K., Tanoue, T., Shima, T., Imaoka, A., Kuwahara, T., Momose, Y., et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. doi: 10.1126/science.1198469
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bartizal, K. F., Wostmann, B. S., and Wagner, M. (1984). Distribution and effects of a defined six-member murine-derived microbiota in gnotobiotic gerbils. Appl. Environ. Microbiol. 47, 746–751.
Brandtzaeg, P., Kiyono, H., Pabst, R., and Russell, M. W. (2008). Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 1, 31–37. doi: 10.1038/mi.2007.9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cassani, B., Villablanca, E. J., Quintana, F. J., Love, P. E., Lacy-Hulbert, A., Blaner, W. S., et al. (2011). Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology 141, 2109–2118. doi: 10.1053/j.gastro.2011.09.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cebula, A., Seweryn, M., Rempala, G. A., Pabla, S. S., McIndoe, R. A., Denning, T. L., et al. (2013). Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497, 258–262. doi: 10.1038/nature12079
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cerovic, V., Houston, S. A., Scott, C. L., Aumeunier, A., Yrlid, U., Mowat, A. M., et al. (2013). Intestinal CD103– dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6, 104–113. doi: 10.1038/mi.2012.53
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, Y., Inobe, J., Kuchroo, V. K., Baron, J. L., Janeway, C. A. Jr., and Weiner, H. L. (1996). Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc. Natl. Acad. Sci. U.S.A. 93, 388–391. doi: 10.1073/pnas.93.1.388
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chirdo, F. G., Millington, O. R., Beacock-Sharp, H., and Mowat, A. M. (2005). Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 35, 1831–1840. doi: 10.1002/eji.200425882
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chu, F., Kearns, D. B., Branda, S. S., Kolter, R., and Losick, R. (2006). Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59, 1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chu, H., and Mazmanian, S. K. (2013). Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 14, 668–675. doi: 10.1038/ni.2635
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Collins, C. B., Aherne, C. M., Kominsky, D., McNamee, E. N., Lebsack, M. D., Eltzschig, H., et al. (2011). Retinoic acid attenuates ileitis by restoring the balance between T-helper 17 and T regulatory cells. Gastroenterology 141, 1821–1831. doi: 10.1053/j.gastro.2011.05.049
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Collins, C. B., Aherne, C. M., McNamee, E. N., Lebsack, M. D., Eltzschig, H., Jedlicka, P., et al. (2012). Flt3 ligand expands CD103+ dendritic cells and FoxP3+ T regulatory cells, and attenuates Crohn’s-like murine ileitis. Gut 61, 1154–1162. doi: 10.1136/gutjnl-2011-300820
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cording, S., Fleissner, D., Heimesaat, M. M., Bereswill, S., Loddenkemper, C., Uematsu, S., et al. (2013). Commensal microbiota drive proliferation of conventional and Foxp3+ regulatory CD4+ T cells in mesenteric lymph nodes and Peyer’s patches. Eur. J. Microbiol. Immunol. (Bp) 3, 1–10. doi: 10.1556/EuJMI.3.2013.1.1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cording, S., Wahl, B., Kulkarni, D., Chopra, H., Pezoldt, J., Buettner, M., et al. (2014). The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 7, 359–368. doi: 10.1038/mi.2013.54
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Curotto de Lafaille, M. A., Kutchukhidze, N., Shen, S., Ding, Y., Yee, H., and Lafaille, J. J. (2008). Adaptive Foxp3+ regulatory T cell-dependent and - independent control of allergic inflammation. Immunity 29, 114–126. doi: 10.1016/j.immuni.2008.05.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Curotto de Lafaille, M. A., and Lafaille, J. J. (2009). Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635. doi: 10.1016/j.immuni.2009.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dubois, B., Chapat, L., Goubier, A., Papiernik, M., Nicolas, J. F., and Kaiserlian, D. (2003). Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 102, 3295–3301. doi: 10.1182/blood-2003-03-0727
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dubois, B., Joubert, G., Gomez de Agüero, M., Gouanvic, M., Goubier, A., and Kaiserlian, D. (2009). Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology 137, 1019–1028. doi: 10.1053/j.gastro.2009.03.055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Faria, A. M., and Weiner, H. L. (2006). Oral tolerance: therapeutic implications for autoimmune diseases. Clin. Dev. Immunol. 13, 143–157. doi: 10.1080/17402520600876804
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Föhse, L., Suffner, J., Suhre, K., Wahl, B., Lindner, C., Lee, C. W., et al. (2011). High TCR diversity ensures optimal function and homeostasis of Foxp3+ regulatory T cells. Eur. J. Immunol. 41, 3101–3113. doi: 10.1002/eji.201141986
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Forster, R., Davalos-Misslitz, A. C., and Rot, A. (2008). CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371. doi: 10.1038/nri2297
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fukase, K., Kusumoto, S., Valvano, M. A., Foster, S. J., Mak, T. W., Nuñez, G., et al. (2003). An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4, 702–707. doi: 10.1038/ni945
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J., and Madara, J. L. (2001). Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885. doi: 10.4049/jimmunol.167.4.1882
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gonnella, P. A., Chen, Y., Inobe, J., Komagata, Y., Quartulli, M., and Weiner, H. L. (1998). In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J. Immunol. 160, 4708–4718.
Gorfu, G., Rivera-Nieves, J., and Ley, K. (2009). Role of β7 integrins in intestinal lymphocyte homing and retention. Curr. Mol. Med. 9, 836–850. doi: 10.2174/156652409789105525
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Goubier, A., Dubois, B., Gheit, H., Joubert, G., Villard-Truc, F., Asselin-Paturel, C., et al. (2008). Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475. doi: 10.1016/j.immuni.2008.06.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grzelak, T., Woźniak, U., and Czyżewska, K. (2014). The influence of natural feeding on human health: short- and long-term perspectives. Prz. Gastroenterol. 9, 4–10. doi: 10.5114/pg.2014.40843
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hadis, U., Wahl, B., Schulz, O., Hardtke-Wolenski, M., Schippers, A., Wagner, N., et al. (2011). Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246. doi: 10.1016/j.immuni.2011.01.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hammerschmidt, S. I., Ahrendt, M., Bode, U., Wahl, B., Kremmer, E., Förster, R., et al. (2008). Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490. doi: 10.1084/jem.20080039
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hardy, H., Harris, J., Lyon, E., Beal, J., and Foey, A. D. (2013). Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5, 1869–1912. doi: 10.3390/nu5061869
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hashiguchi, M., Hachimura, S., Ametani, A., Sato, T., Kojima, H., Kumagai, Y., et al. (2011). Naïve CD4+ T cells of Peyer’s patches produce more IL-6 than those of spleen in response to antigenic stimulation. Immunol. Lett. 141, 109–115. doi: 10.1016/j.imlet.2011.09.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Holz, L. E., Warren, A., Le Couteur, D. G., Bowen, D. G., and Bertolino, P. (2010). CD8+ T cell tolerance following antigen recognition on hepatocytes. J. Autoimmun. 34, 15–22. doi: 10.1016/j.jaut.2009.08.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hossain, Z., and Hirata, T. (2008). Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol. Biosyst. 4, 1181–1185. doi: 10.1039/b800402a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hrncir, T., Stepankova, R., Kozakova, H., Hudcovic, T., and Tlaskalova-Hogenova, H. (2008). Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol. 9:65. doi: 10.1186/1471-2172-9-65
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hsieh, C. S., Zheng, Y., Liang, Y., Fontenot, J. D., and Rudensky, A. Y. (2006). An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7, 401–410. doi: 10.1038/ni1318
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Husby, S., Jensenius, J. C., and Svehag, S. E. (1985). Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand. J. Immunol. 22, 83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ivanov, I. I., Atarashi, K., Manel, N., Brodie, E. L., Shima, T., Karaoz, U., et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. doi: 10.1016/j.cell.2009.09.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ivanov, I. I., Frutos Rde, L., Manel, N., Yoshinaga, K., Rifkin, D. B., Sartor, R. B., et al. (2008). Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349. doi: 10.1016/j.chom.2008.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ivanov, I. I., and Littman, D. R. (2010). Segmented filamentous bacteria take the stage. Mucosal Immunol. 3, 209–212. doi: 10.1038/mi.2010.3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Iwata, M., Hirakiyama, A., Eshima, Y., Kagechika, H., Kato, C., and Song, S. Y. (2004). Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538. doi: 10.1016/j.immuni.2004.08.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jaensson, E., Uronen-Hansson, H., Pabst, O., Eksteen, B., Tian, J., Coombes, J. L., et al. (2008). Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149. doi: 10.1084/jem.20080414
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jaensson-Gyllenbäck, E., Kotarsky, K., Zapata, F., Persson, E. K., Gundersen, T. E., Blomhoff, R., et al. (2011). Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 4, 438–447. doi: 10.1038/mi.2010.91
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jin, C., and Flavell, R. A. (2013). Innate sensors of pathogen and stress: linking inflammation to obesity. J. Allergy Clin. Immunol. 132, 287–294. doi: 10.1016/j.jaci.2013.06.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Johansson-Lindbom, B., Svensson, M., Pabst, O., Palmqvist, C., Marquez, G., Förster, R., et al. (2005). Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073. doi: 10.1084/jem.20051100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kamenšek, S., and Žgur-Bertok, D. (2013). Global transcriptional responses to the bacteriocin colicin M in Escherichia coli. BMC Microbiol. 13:42. doi: 10.1186/1471-2180-13-42
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kapp, K., Maul, J., Hostmann, A., Mundt, P., Preiss, J. C., Wenzel, A., et al. (2010). Modulation of systemic antigen-specific immune responses by oral antigen in humans. Eur. J. Immunol. 40, 3128–3137. doi: 10.1002/eji.201040701
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Karlsson, C., Ahrné, S., Molin, G., Berggren, A., Palmquist, I., Fredrikson, G. N., et al. (2010). Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis 208, 228–233. doi: 10.1016/j.atherosclerosis.2009.06.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Karlsson, M., Lundin, S., Dahlgren, U., Kahu, H., Pettersson, I., and Telemo, E. (2001). “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 31, 2892–2900. doi: 10.1002/1521-4141(2001010)31:10<2892::AID-IMMU2892>3.0.CO;2-I
King, C., and Sarvetnick, N. (2011). The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS ONE 6:e17049. doi: 10.1371/journal.pone.0017049
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koenecke, C., Czeloth, N., Bubke, A., Schmitz, S., Kissenpfennig, A., Malissen, B., et al. (2009). Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur. J. Immunol. 39, 3091–3096. doi: 10.1002/eji.200939432
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kraus, T. A., Toy, L., Chan, L., Childs, J., and Mayer, L. (2004). Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology 126, 1771–1778. doi: 10.1053/j.gastro.2004.03.076
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kumar, H., Kawai, T., and Akira, S. (2011). Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34. doi: 10.3109/08830185.2010.529976
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Laffont, S., Siddiqui, K. R., and Powrie, F. (2010). Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 40, 1877–1883. doi: 10.1002/eji.200939957
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lathrop, S. K., Bloom, S. M., Rao, S. M., Nutsch, K., Lio, C. W., Santacruz, N., et al. (2011). Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254. doi: 10.1038/nature10434
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, J., Mo, J. H., Katakura, K., Alkalay, I., Rucker, A. N., Liu, Y. T., et al. (2006). Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 8, 1327–1336. doi: 10.1038/ncb1500
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, J., Mo, J. H., Shen, C., Rucker, A. N., and Raz, E. (2007). Toll-like receptor signaling in intestinal epithelial cells contributes to colonic homoeostasis. Curr. Opin. Gastroenterol. 23, 27–31. doi: 10.1097/MOG.0b013e3280118272
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Limmer, A., Ohl, J., Wingender, G., Berg, M., Jüngerkes, F., Schumak, B., et al. (2005). Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur. J. Immunol. 35, 2970–2981. doi: 10.1002/eji.200526034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maaser, C., Heidemann, J., von Eiff, C., Lugering, A., Spahn, T. W., Binion, D. G., et al. (2004). Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J. Immunol. 172, 5056–5062. doi: 10.4049/jimmunol.172.8.5056
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maassen, C. B., and Claassen, E. (2008). Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine 26, 2056–2057. doi: 10.1016/j.vaccine.2008.02.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martín, R., Langa, S., Reviriego, C., Jimínez, E., Marín, M. L., Xaus, J., et al. (2003). Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 143, 754–758. doi: 10.1016/j.jpeds.2003.09.028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maynard, C. L., Harrington, L. E., Janowski, K. M., Oliver, J. R., Zindl, C. L., Rudensky, A. Y., et al. (2007). Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3– precursor cells in the absence of interleukin 10. Nat. Immunol. 8, 931–941. doi: 10.1038/ni1504
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maynard, C. L., Hatton, R. D., Helms, W. S., Oliver, J. R., Stephensen, C. B., and Weaver, C. T. (2009). Contrasting roles for all-trans retinoic acid in TGF-β-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 206, 343–357. doi: 10.1084/jem.20080950
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Menard, S., Cerf-Bensussan, N., and Heyman, M. (2010). Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 3, 247–259. doi: 10.1038/mi.2010.5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miller, H., Zhang, J., Kuolee, R., Patel, G. B., and Chen, W. (2007). Intestinal M cells: the fallible sentinels? World J. Gastroenterol. 13, 1477–1486. doi: 10.3748/wjg.v13.i10.1477
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Milling, S., Yrlid, U., Cerovic, V., and MacPherson, G. (2010). Subsets of migrating intestinal dendritic cells. Immunol. Rev. 234, 259–267. doi: 10.1111/j.0105-2896.2009.00866.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Molenaar, R., Greuter, M., van der Marel, A. P., Roozendaal, R., Martin, S. F., Edele, F., et al. (2009). Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J. Immunol. 183, 6395–6402. doi: 10.4049/jimmunol.0900311
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murai, M., Turovskaya, O., Kim, G., Madan, R., Karp, C. L., Cheroutre, H., et al. (2009). Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 10, 1178–1184. doi: 10.1038/ni.1791
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ochoa-Repáraz, J., Mielcarz, D. W., Ditrio, L. E., Burroughs, A. R., Foureau, D. M., Haque-Begum, S., et al. (2009). Role of gut commensal microbiota in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050. doi: 10.4049/jimmunol.0900747
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ochoa-Repáraz, J., Mielcarz, D. W., Wang, Y., Begum-Haque, S., Dasgupta, S., Kasper, D. L., et al. (2010). A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495. doi: 10.1038/mi.2010.29
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pabst, O., Bernhardt, G., and Forster, R. (2007). The impact of cell-bound antigen transport on mucosal tolerance induction. J. Leukoc. Biol. 82, 795–800. doi: 10.1189/jlb.0307144
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pabst, O., and Mowat, A. M. (2012). Oral tolerance to food protein. Mucosal Immunol. 5, 232–239. doi: 10.1038/mi.2012.4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Païdassi, H., Acharya, M., Zhang, A., Mukhopadhyay, S., Kwon, M., Chow, C., et al. (2011). Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141, 1813–1820. doi: 10.1053/j.gastro.2011.06.076
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pollard, M., and Sharon, N. (1970). Responses of the Peyer’s patches in germ-free mice to antigenic stimulation. Infect. Immun. 2, 96–100.
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rhee, K. J., Sethupathi, P., Driks, A., Lanning, D. K., and Knight, K. L. (2004). Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 172, 1118–1124. doi: 10.4049/jimmunol.172.2.1118
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Round, J. L., and Mazmanian, S. K. (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209. doi: 10.1073/pnas.0909122107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rubtsov, Y. P., Niec, R. E., Josefowicz, S., Li, L., Darce, J., Mathis, D., et al. (2010). Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671. doi: 10.1126/science.1191996
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Russell, S. L., Gold, M. J., Willing, B. P., Thorson, L., McNagny, K. M., and Finlay, B. B. (2013). Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4, 158–164. doi: 10.4161/gmic.23567
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schmitz, J., Owyang, A., Oldham, E., Song, Y., Murphy, E., McClanahan, T. K., et al. (2005). IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490. doi: 10.1016/j.immuni.2005.09.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shibolet, O., and Podolsky, D. K. (2007). TLRs in the gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1469–G1473. doi: 10.1152/ajpgi.00531.2006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Siewert, C., Lauer, U., Cording, S., Bopp, T., Schmitt, E., Hamann, A., et al. (2008). Experience-driven development: effector/memory-like αE+Foxp3+ regulatory T cells originate from both naive T cells and naturally occurring naive-like regulatory T cells. J. Immunol. 180, 146–155. doi: 10.4049/jimmunol.180.1.146
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smith, K. M., Davidson, J. M., and Garside, P. (2002). T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology 106, 144–158. doi: 10.1046/j.1365-2567.2002.01427.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spahn, T. W., Weiner, H. L., Rennert, P. D., Lügering, N., Fontana, A., Domschke, W., et al. (2002). Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur. J. Immunol. 32, 1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K
Stenstad, H., Svensson, M., Cucak, H., Kotarsky, K., and Agace, W. W. (2007). Differential homing mechanisms regulate regionalized effector CD8αβ+ T cell accumulation within the small intestine. Proc. Natl. Acad. Sci. U.S.A. 104, 10122–10127. doi: 10.1073/pnas.0700269104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Strachan, D. P. (1989). Hay fever, hygiene, and household size. BMJ 299, 1259–1260. doi: 10.1136/bmj.299.6710.1259
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Strobel, S., and Ferguson, A. (1987). Persistence of oral tolerance in mice fed ovalbumin is different for humoral and cell-mediated immune responses. Immunology 60, 317–318.
Strober, W. (2009). The multifaceted influence of the mucosal microbiota on mucosal dendritic cell responses. Immunity 31, 377–388. doi: 10.1016/j.immuni.2009.09.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, C. M., Hall, J. A., Blank, R. B., Bouladoux, N., Oukka, M., Mora, J. R., et al. (2007). Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785. doi: 10.1084/jem.20070602
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Svensson, M., Marsal, J., Ericsson, A., Carramolino, L., Brodén, T., Márquez, G., et al. (2002). CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121. doi: 10.1172/JCI15988
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Takeuchi, H., Yokota, A., Ohoka, Y., Kagechika, H., Kato, C., Song, S. Y., et al. (2010). Efficient induction of CCR9 on T cells requires coactivation of retinoic acid receptors and retinoid X receptors (RXRs): exaggerated T Cell homing to the intestine by RXR activation with organotins. J. Immunol. 185, 5289–5299. doi: 10.4049/jimmunol.1000101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thomson, A. W., and Knolle, P. A. (2010). Antigen-presenting cell function in the tolerogenic liver environment Nat. Rev. Immunol. 10, 753–766. doi: 10.1038/nri2858
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tomura, M., Honda, T., Tanizaki, H., Otsuka, A., Egawa, G., Tokura, Y., et al. (2010). Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 120, 883–893. doi: 10.1172/JCI40926
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsai, F., and Coyle, W. J. (2009). The microbiome and obesity: is obesity linked to our gut flora? Curr. Gastroenterol. Rep. 11, 307–313. doi: 10.1007/s11894-009-0045-z
van Zanten, G. C., Knudsen, A., Röytiö, H., Forssten, S., Lawther, M., Blennow, A., et al. (2012). The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. PLoS ONE 7:e47212. doi: 10.1371/journal.pone.0047212
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wagner, N., Löhler, J., Kunkel, E. J., Ley, K., Leung, E., Krissansen, G., et al. (1996). Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370. doi: 10.1038/382366a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weiner, H. L., da Cunha, A. P., Quintana, F., and Wu, H. (2011). Oral tolerance. Immunol. Rev. 241, 241–259. doi: 10.1111/j.1600-065X.2011.01017.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wen, L., Ley, R. E., Volchkov, P. Y., Stranges, P. B., Avanesyan, L., Stonebraker, A. C., et al. (2008). Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113. doi: 10.1038/nature07336
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Williams, A. M., Probert, C. S., Stepankova, R., Tlaskalova-Hogenova, H., Phillips, A., and Bland, P. W. (2006). Effects of microbiota on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 119, 470–478. doi: 10.1111/j.1365-2567.2006.02458.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Worbs, T., Bode, U., Yan, S., Hoffmann, M. W., Hintzen, G., Bernhardt, G., et al. (2006). Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527. doi: 10.1084/jem.20052016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Worthington, J. J., Czajkowska, B. I., Melton, A. C., and Travis, M. A. (2011). Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology 141, 1802–1812. doi: 10.1053/j.gastro.2011.06.057
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wu, H. J., Ivanov, I. I., Darce, J., Hattori, K., Shima, T., Umesaki, Y., et al. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827. doi: 10.1016/j.immuni.2010.06.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xiao, H., Gulen, M. F., Qin, J., Yao, J., Bulek, K., Kish, D., et al. (2007). The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26, 461–475. doi: 10.1016/j.immuni.2007.02.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yamazaki, S., Dudziak, D., Heidkamp, G. F., Fiorese, C., Bonito, A. J., Inaba, K., et al. (2008). CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181, 6923–6933. doi: 10.4049/jimmunol.181.10.6923
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: intestinal microbiota, immune system, microflora, tolerance, harmful antigens
Citation: Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA and Orekhov AN (2015) Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front. Microbiol. 5:781. doi: 10.3389/fmicb.2014.00781
Received: 06 October 2014; Accepted: 19 December 2014;
Published online: 13 January 2015.
Edited by:
Hao Shen, University of Pennsylvania School of Medicine, USAReviewed by:
Qibin Leng, Institut Pasteur of Shanghai, Chinese Academy of Sciences, ChinaSukanya Narasimhan, Yale University School of Medicine, USA
Copyright © 2015 Chistiakov, Bobryshev, Kozarov, Sobenin and Orekhov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuri V. Bobryshev, Faculty of Medicine, School of Medical Sciences, University of New South Wales, High Street, Kensington, Sydney, NSW 2052, Australia e-mail: y.bobryshev@unsw.edu.au
 Dimitry A. Chistiakov
Dimitry A. Chistiakov