Paraneoplastic Pemphigus Revealed by Anti-programmed Death-1 Pembrolizumab Therapy for Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa
- 1Department of Dermatology and Referral Center for Autoimmune Bullous Diseases MALIBUL, Avicenne Hospital, AP-HP, University Paris 13, Bobigny, France
- 2Laboratory of Immunology and Referral Center for Autoimmune Bullous Diseases MALIBUL, Bichat Hospital, AP-HP, Paris, France
- 3Department of Pathology, Avicenne Hospital, AP-HP, University Paris 13, Bobigny, France
A 64-year-old patient developed a widespread autoimmune mucocutaneous blistering disease 3 weeks after the initiation of the anti-programmed death-1 (anti-PD-1) pembrolizumab therapy administered for a locally advanced cutaneous squamous cell carcinoma (SCC) of the buttocks arising from hidradenitis suppurativa. A diagnosis of paraneoplastic pemphigus (PNP) was made based on the presence of a suprabasal acantholysis associated with intercellular deposits of immunoglobulin G and C3 on basement membrane zone. Analysis of the patient's sera was positive on monkey bladder and detected circulating antibodies against desmoglein 3 and desmoplakin I prior to the initiation of pembrolizumab. At that time, the patient had few localized blisters limited to the peri-tumoral skin of the buttocks with acantholysis but without in vivo immune deposits. Pembrolizumab therapy was discontinued and a complete remission of PNP was obtained using oral steroids. Reintroduction of pembrolizumab resulted in flare of PNP. Given the close temporal relation between pembrolizumab initiation and the subsequent clinical expression of a widespread PNP, the patient was diagnosed with pre-existing subclinical PNP exacerbated by PD-1 inhibitor. The extreme rarity of PNP in the setting of cutaneous SCC and the effects of challenge, dechallenge, and rechallenge of pembrolizumab argue in favor of a checkpoint inhibitor related adverse effect. Our case is the first PNP associated with anti-PD-1 therapy and serological follow-up suggest that one infusion of pembrolizumab is sufficient to allow clinical expression of underlying pemphigus auto-immunity.
Background
The immune system has developed sophisticated negative regulatory mechanisms to contain the development of autoimmunity. These regulatory mechanisms can be diverted by cancer cells to limit antitumor immunity. By counteracting these inhibitory signals, cancer immunotherapy aims to enhance immune responses to fight against tumors. Among several immunotherapeutic strategies tested these recent years, immune checkpoint inhibitors have come to the forefront in cancer treatment, showing remarkable benefit in the treatment of a wide range of cancer. By blocking negative regulators of lymphocyte activation, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand, programmed cell death ligand 1 (PD-L1), immune checkpoint inhibitors restore the function of effector T cells to target and destroy cancer cells. The drawback of inducing an effective immunity targeting cancers is the potential for autoimmune side effects, which are commonly termed immune-related adverse events (irAEs) (1). These manifest usually as organ-specific autoimmunity such as thyroiditis, colitis, hepatitis, or hypophysitis, among many others (2). A major challenge in the cancer immunotherapy field is therefore to increase the antitumor activity without promoting additional immune-related side effects.
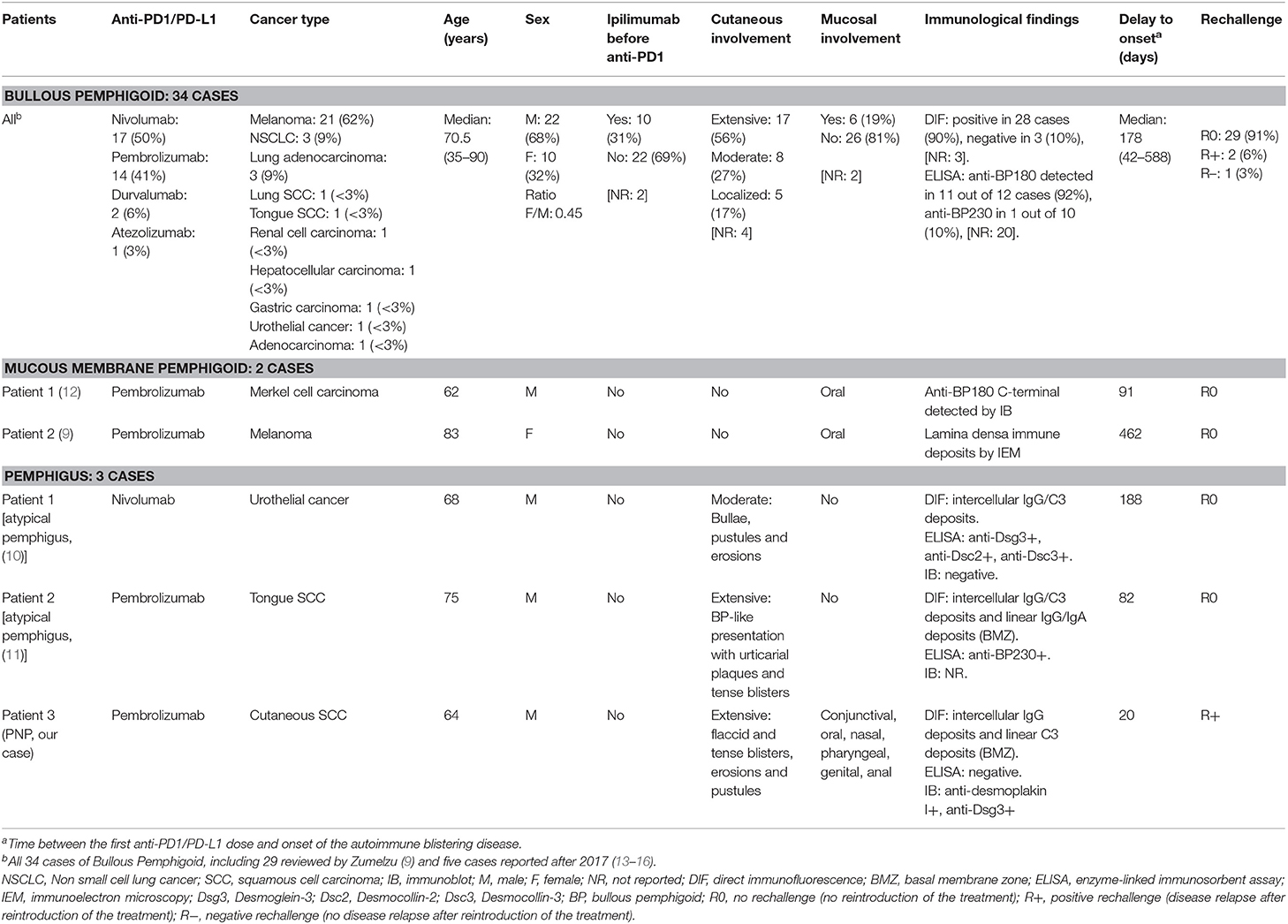
Cutaneous side effects are among the most frequent checkpoint inhibitor irAEs. Skin related toxicity of any grade may be seen in up to 25% of patients treated with antibodies targeting CTLA-4 (3) and around 20% of patients undergoing anti-PD-1 treatments (4). These cover a wide range of clinical presentation such as pruritus sine materia, maculopapular eruption, vesicular or pustular lesions, alopecia, and acneiform and neutrophilic dermatosis (5). Although skin toxicity of immune checkpoint inhibitors is usually mild in severity, there are several reports of life-threatening dermatologic reactions to checkpoints blockage including drug reaction with eosinophilia and systemic symptoms (6), Stevens-Johnson syndrome (7), and toxic epidermal necrolysis (8). Moreover, several cases of autoimmune blistering diseases have been observed in patients receiving checkpoint inhibitors. So far, 34 cases of bullous pemphigoid (BP) associated with anti-PD1/PD-L1 therapy have been reported, as well as 2 cases of mucous membrane pemphigoid (MMP) (9) and 2 cases of atypical pemphigus with no mucosal involvement (10, 11). Here, we report the first case of a widespread mucocutaneous paraneoplastic pemphigus (PNP) associated with pembrolizumab therapy given for a locally advanced cutaneous Squamous Cell Carcinoma (SCC) complicating Hidradenitis Suppurativa (HS).
Case Presentation
A 64-year-old man of Algerian origin presented with a 20-year history of sinuses and abscesses of the buttocks that had been treated with several minor surgical procedures over the past 10 years. A family history was noted because the patient's son had similar early-stage lesions. Clinical examination revealed a diffuse involvement of the buttocks, perineal, and perianal regions, with multiple interconnecting tracts and abscesses across entire areas (Figure 1A). He was diagnosed with severe Hurley stage III HS. Multiple ulcerated lesions on his buttocks and large inguinal lymphadenopathies were noted. The computed tomodensitometry revealed deep tumor infiltration associated with large inguinal and external iliac lymphadenopathies, multiple abscesses of the gluteal fold, internal obturator muscles and gluteus maximus, and a sacrococcygeal osteomyelitis. Histological examination of surgical biopsies performed on skin lesions revealed moderate or well-differentiated SCC. Given the extensive local spread, the tumor was deemed inoperable.
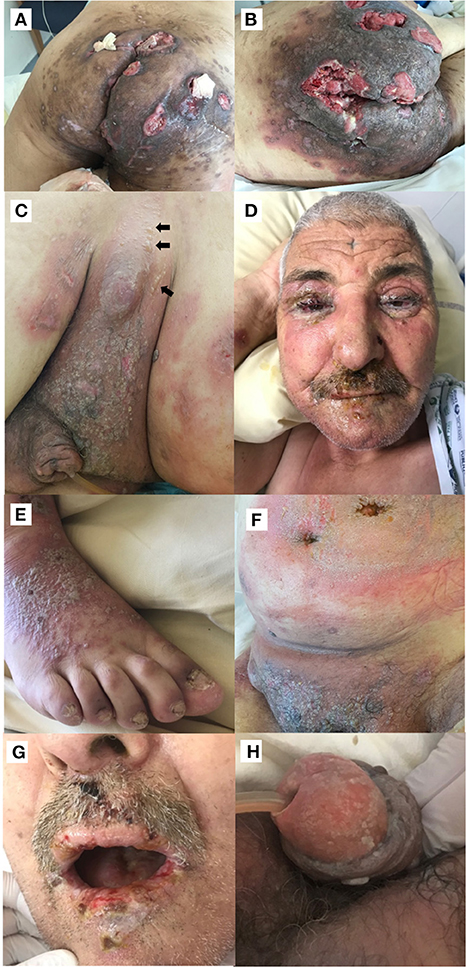
Figure 1. (A) Initial clinical presentation of the patient's buttocks showing severe hidradenitis suppurativa lesions complicated with multifocal squamous cell carcinoma. (B) Inflammatory swelling of the patient's buttocks and surrounding squamous cell carcinoma localization, 1 week after pembrolizumab administration. (C–H) Widespread mucocutaneous blistering disease 3 weeks after pembrolizumab with tense blisters (indicated by arrows) (C), erythema and edema of the face, pseudomembranous conjunctivitis and eyelid erosions (D), pustular lesions (E), large erythematous plaques (F), erosive stomatitis (G), and erosions of the glans penis (H).
Physical examination also revealed few small intact bullae on the buttocks and painful erosions of the buccal mucosa. There were no vesicular or bullous lesions on the upper trunk, arms, legs or extremities, and no history of a prior bullous eruption. Mouth swabs were positive for herpes simplex virus 1 (HSV-1) DNA. Histopathological examination of a buttock blister revealed intraepidermal blistering and suprabasal acantholysis. Direct immunofluorescence (DIF) was negative for deposits of immunoglobulin (Ig) G, IgA, IgM, and C3. A diagnosis of pemphigus could therefore not be confirmed. The patient was treated with oral valaciclovir, which resulted in the almost complete healing of the oral and cutaneous lesions.
Due to the poor performance status (stage 4 in ECOG scale) and high-risk of sepsis-related complications, cytotoxic chemotherapy was contraindicated for SCC treatment, and a treatment by anti-programmed cell death protein 1 (anti PD-1) was decided. One week after the first pembrolizumab administration (2 mg per kg), an intense inflammatory swelling affecting the HS areas was noted, followed by a relapse of the bullous cutaneous eruption (Figures 1B,C). Three weeks after the infusion, the patient developed erythema and edema of the face, bilateral pseudomembranous conjunctivitis with mucus discharge, and eyelid erosions (Figure 1D). Concomitantly, widespread polymorphic cutaneous lesions including flaccid and tense blisters, erosions, erythematous plaques, and pustular lesions occurred (Figures 1E,F). Mucosal examination revealed a severe stomatitis, crusting and bleeding erosions on the lips that extended beyond the vermilion border (Figure 1G), as well as erosions of the glans penis (Figure 1H). Ear, nose and throat endoscopic examination demonstrated erosions affecting the nasal mucosa, the arytenoids (75% of the surface), and the aryepiglottic folds (75%). The clinical differential diagnosis included PNP, pemphigus vulgaris (PV) and toxic epidermal necrolysis.
Laboratory Investigations
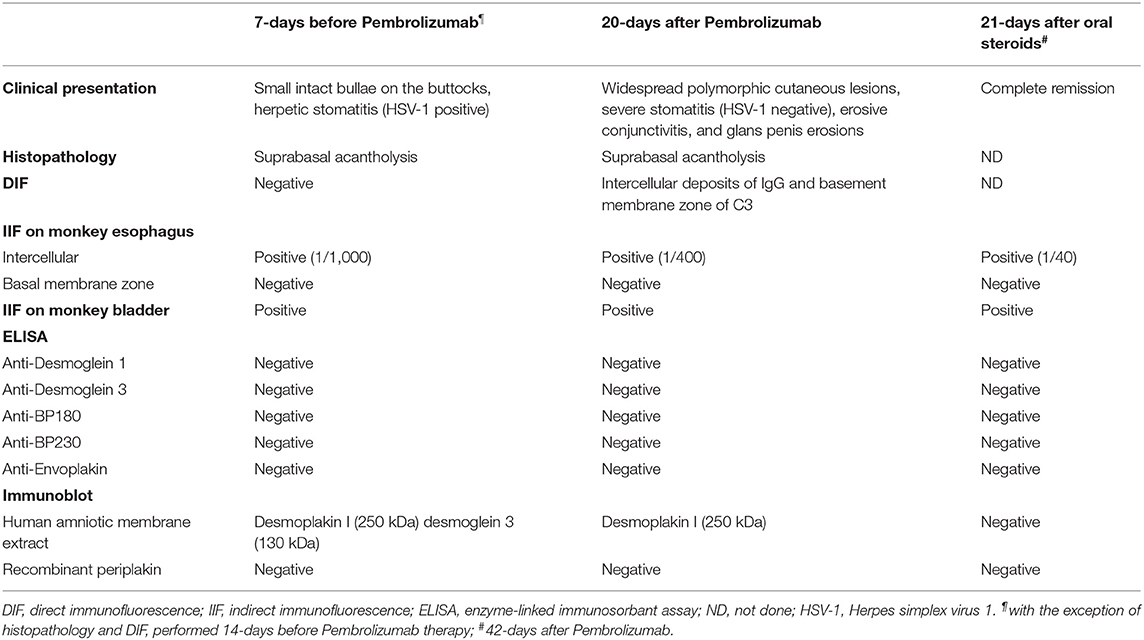
Several series of skin punch biopsies were performed for histopathologic examination and DIF studies (Table 1). Histological examination by hematoxylin and eosin staining showed a suprabasal acantholysis and intraepidermal blister formations, without keratinocyte necrosis (Figure 2A), supporting the hypothesis of a pemphigus and ruling out drug-induced toxic epidermal necrolysis. A second biopsy demonstrated a spongiotic dermatitis with exocytosis of eosinophils, intraepidermal pustule formation (Figure 2B), and superficial perivascular dermal infiltrate of eosinophils and lymphocytes. Scattered necrotic keratinocytes were observed in the second biopsy. DIF of the peribullous skin revealed intercellular deposits of IgG between keratinocytes, associated with linear deposits of C3 along the basement membrane, without IgA deposits. This combined pattern (intercellular and basement membrane staining) was suggestive of PNP. Indirect immunofluorescence (IIF) testing of the patient serum on monkey bladder epithelium revealed a staining of urothelial cell surface supporting the diagnosis of PNP. Characterization of circulating autoantibodies was performed using enzyme-linked immunosorbant assay (ELISA) and immunoblotting. ELISA did not detect IgG autoantibodies to desmoglein 1, desmoglein 3, BP180 (NC16A), BP230, or envoplakin. Immunoblotting using human amniotic membrane extracts revealed a 250 kDa band corresponding to desmoplakin I but did not detect autoantibodies to periplakin (Table 1). According to the clinical presentation, the immunofluorescence pattern and the immuno-serological profile, a diagnosis of probable PNP was made.
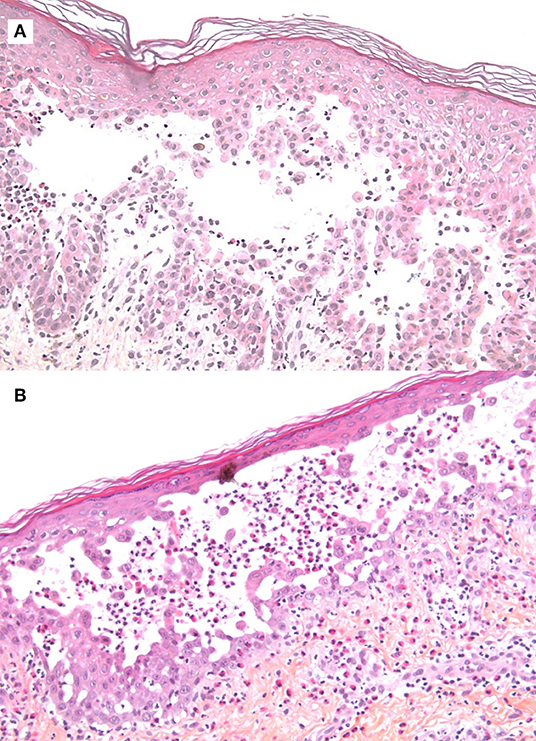
Figure 2. Histological examination by hematoxylin and eosin staining of skin biopsies. (A) Suprabasal acantholysis and intraepidermal blister formation (original magnification x100). (B) Intraepidermal pustule containing neutrophils and eosinophils, as well as epidermal spongiosis with exocytosis of eosinophils (original magnification x100).
Given the presence of a localized acantholytic disease prior to immunotherapy and the short delay (<3 weeks) between pembrolizumab initiation and the widespread mucocutaneous eruption, we suspected that PD-1 blockage was associated with the aggravation of a preexisting condition, rather than the development of a de novo autoimmune disease. Therefore, we performed immunoblot analysis on the patient's serum collected 7 days before initiation of pembrolizumab therapy. Immunoblotting indicated the presence of circulating antibodies against the desmosomal components desmoplakin I and desmoglein 3. Consistently, IIF performed on monkey bladder epithelium using the same serum revealed a positive staining. Together, these results supported the presence of a subclinical PNP before the initiation of pembrolizumab (Table 1).
The pembrolizumab therapy was withheld and oral prednisone at 1 mg per kg daily was started. After 3 weeks of steroid treatment, complete clinical remission of the PNP was obtained and anti-desmoplakin I antibodies were no more detectable by immunoblotting (Table 1). Given the rapid progression of the SCC and the lack of alternative options, pembrolizumab therapy was restarted 6 weeks after the initial infusion while the patient remained under high dose of oral steroids (1 mg per kg daily). Pembrolizumab (2 mg per kg) resuming was associated with a PNP relapse affecting the oral cavity and nasopharyngeal mucous membrane. Immunotherapy was definitely discontinued after the third pembrolizumab dose due to grade II/III (CTCAE classification v4.0) relapsing symptoms resulting from PNP. The patient died of sepsis 3 months after initiation of corticosteroids.
Discussion
A wide range of inflammatory skin disorders has been observed in patients treated with checkpoints inhibitors, including autoimmune blistering diseases. Current anti-PD1/PD-L1 therapy-associated autoimmune blistering diseases reported in the literature (including our patient) are presented in Table 2. To date, 34 cases of BP have been described in association with PD1 inhibitors, including 29 cases reviewed by Zumelzu (9) and five additional cases (13–16). In addition, a pharmacovigilance analysis performed on the Adverse Event Reporting System database of Food and Drug Administration recently demonstrated an increased risk to develop BP in patients treated by pembrolizumab or nivolumab (17); this signal was based on 35 case reports. Two cases of mild MMP limited to the oral cavity have also been described in patients under anti-PD1 therapy (9, 12). Apart from immune-mediated subepithelial blistering diseases, atypical suprabasal acantholytic dermatosis represents another emerging group of checkpoint inhibitor related skin toxicities. These are mainly Grover-like reactions (8 cases) and lichenoid dermatitis with suprabasal acantholysis (4 cases), without immune deposits or circulating antibodies targeting desmosomal components [reviewed in (11)]. Suprabasal acantholysis associated with immune deposits at the surface of keratinocytes has been reported in only two patients under anti-PD1 therapy. The first case reported by Ito et al. was a 68-year-old man with urothelial carcinoma treated with nivolumab who developed a polymorphic cutaneous eruption with bullae, pustules, and erosions. He had circulating autoantibodies targeting desmocollin-2 and -3, which are usually found in atypical types of pemphigus but not classical pemphigus (10). The second case was a 75-year-old man with SCC of the tongue who developed, under pembrolizumab therapy, a bullous eruption with a histopathology image and DIF pattern suggestive of PNP (11). Both cases did not show any mucosal involvement nor any other typical manifestations of PV or PNP. In contrast, our patient developed after pembrolizumab therapy a diffuse mucocutaneous eruption highly suggestive of PNP. The histopathology and serum analysis confirmed the diagnosis of PNP although immunological results were atypical by the absence of anti-envoplakine and periplakine antibodies, which are however only found in 60 and 90% of PNP cases (18). Our patient had anti-desmoplakin I antibodies which are associated with PNP in up to 47% of patients (19).
PNP may have developed independently of pembrolizumab therapy. However, several data are suggestive of a checkpoint inhibitor related adverse effect in our patient: (1) PNP has never been reported previously in the setting of a cutaneous SCC, making the combination of PNP and cutaneous SCC an extremely rare phenomenon; (2) the close temporal relation between pembrolizumab initiation and the subsequent development of PNP clinical lesions (challenge); (3) the favorable outcome after pembrolizumab interruption and corticosteroids treatment (dechallenge); (4) the relapse after pembrolizumab reintroduction (rechallenge). Using the Begaud scoring system (20), we assessed the intrinsic accountability score of anti-PD1 therapy in PNP expression for our patient. With a chronological scoring (combining status of challenge, dechallenge, and rechallenge) of C3 (likely) and a symptomatological scoring of S2 (plausible), the intrinsic accountability scoring is high (I5) in our case. The two previously reported cases of pemphigus associated with anti-PD1 blockage also have high intrinsic accountability scores (I3 and I5), while <37% of induced BP reported cases were given high accountability scores (9). Although autoimmune diseases, such as inflammatory bowel diseases and spondyloarthritis, seems to occur more frequently in patients with HS, there is no association (to our knowledge) between HS and pemphigus. Interestingly, a recent article reports the development of a localized acantholytic disease associated with anti-desmoglein 1 and 3 circulating antibodies in a patient with HS of the buttocks (21). This case highlights the possibility that autoimmunity against desmosomal components might be triggered by a chronic skin inflammatory process like HS. Thus, we cannot rule out that PNP in our patient was induced by HS-related inflammation rather by the cutaneous SCC.
Several pathophysiologic models for PNP have been proposed. First, neoplasms arising from immune cell expansion have been shown to dysregulate B-cell homeostasis resulting in the subsequent production of autoantibodies targeting desmosome components. This is best illustrated by the identification of B-cell tumor clones producing antibodies targeting desmosomal plakin proteins in resected Castleman tumors (22), thymoma, and follicular dendritic cell sarcoma (23). However, this tumor antibody production hypothesis may not apply to other solid cancers associated with PNP. Alternatively, autoimmunity against keratinocytes may be the consequence of cross-reactivity between tumor antigens and normal epithelial proteins. Autoantigens routinely identified in PNP are expressed by epithelial malignancies. In our case, the patient had a skin SCC, a cancer arising from keratinocytes. Thus, antitumor immune responses to his carcinoma could cross-react with normal epidermal antigens. Therefore, distinct pathophysiologic mechanisms might explain PNP arising in the context of neoplasms involving B-cells vs. PNP associated to solid tissue epithelial tumors. Importantly, the fact that PNP is rarely associated with epithelial malignancies, which comprise the majority of human cancers, indicates that autoimmunity arising from tumor antigen cross-reactivity remains a marginal, very contained situation. This could be explained by at least two mechanisms. First, cancer cells adopt a variety of mechanisms to avoid immune recognition. Second, the immune system has evolved negative feedback mechanisms, known as checkpoints, to prevent autoimmunity. In both mechanisms, inhibitory receptors such as PD-1 and CTLA-4 have been found to play important functions (24). In our case, PD-1 blockage was rapidly associated with the expression of a severe PNP, supporting a central role of PD-1 signaling in the suppression of immune responses against antigens shared between normal and transformed keratinocytes. Because checkpoint inhibitors are now used in a growing number of human cancers, one could expect an increase in the future of cross-reactivity mediated paraneoplastic syndromes such as PNP associated to epithelial malignancies or paraneoplastic neurological syndromes triggered by the ectopic expression of neuronal antigens by tumors (25).
In summary, we reported a patient with extensive skin SCC who developed PNP after one infusion of pembrolizumab. The presence of autoantibodies directed against desmoplakin I and desmoglein 3 was demonstrated before the anti-PD-1 therapy. PNP in this patient was clearly induced since rechallenge was positive. So PNP should be added to the list of immune-related adverse events associated with checkpoint blockade such as BP.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AY, GB, and FC designed the study. SG-M conducted the immunological analyses. CP-S and AM performed the pathological studies. MA and CL collected clinical data. AY wrote the first draft of the manuscript. GB did modifications. FC and EM corrected the final version. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
FC is a consultant for Principia Biopharma. EM is consultant for MSD and has received support for congress from BMS and MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank physicians who referred this patient to our department and the physicians of our multidisciplinary team who did ENT and stomatological examinations (Dr. Isaac Soued and Dr. Francis Pascal).
Abbreviations
Anti-PD-1, anti-programmed death-1; BP, bullous pemphigoid; BP180, 180 kDa bullous pemphigoid antigen; BP230, 230 kDa bullous pemphigoid antigen; kDa, kilodalton; CTLA-4, cytotoxic T-lymphocyte antigen 4; DIF, direct immunofluorescence; DRESS, drug reaction with eosinophilia and systemic symptoms; ELISA, enzyme-linked immunosorbant assay; ENT, ear, nose and throat; HS, Hidradenitis Suppurativa; HSV-1, Herpes simplex virus 1; IgG, immunoglobulin G; IIF, indirect immunofluorescence; irAEs, immune-related adverse events; PD1, programmed cell death 1; PD-L1, programmed cell death ligand 1; PNP, paraneoplastic pemphigus; MMP, mucous membrane pemphigoid; SCC, squamous cell carcinoma.
References
1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
2. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389
3. Minkis K, Garden BC, Wu S, Pulitzer MP, Lacouture ME. The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. (2013) 69:e121–8. doi: 10.1016/j.jaad.2012.12.963
4. Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2015) 16:375–84. doi: 10.1016/S1470-2045(15)70076-8
5. Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. (2017) 44:158–76. doi: 10.1111/cup.12858
6. Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE. (2013) 8:e53745. doi: 10.1371/journal.pone.0053745
7. Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. (2017) 81:237–9. doi: 10.1016/j.ejca.2017.03.026
8. Vivar KL, Deschaine M, Messina J, Divine JM, Rabionet A, Patel N, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. (2017) 44:381–4. doi: 10.1111/cup.12876
9. Zumelzu C, Alexandre M, Le Roux C, Weber P, Guyot A, Levy A, et al. Mucous membrane pemphigoid, bullous pemphigoid, and anti-programmed death-1/ programmed death-ligand 1: a case report of an elderly woman with mucous membrane pemphigoid developing after pembrolizumab therapy for metastatic melanoma and review of the literature. Front Med. (2018) 5:268. doi: 10.3389/fmed.2018.00268
10. Ito M, Hoashi T, Endo Y, Kimura G, Kondo Y, Ishii N, et al. Atypical pemphigus developed in a patient with urothelial carcinoma treated with nivolumab. J Dermatol. (2019) 46:e90–2. doi: 10.1111/1346-8138.14601
11. Chen WS, Tetzlaff MT, Diwan H, Jahan-Tigh R, Diab A, Nelson K, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: a spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. (2018) 45:764–73. doi: 10.1111/cup.13312
12. Haug V, Behle V, Benoit S, Kneitz H, Schilling B, Goebeler M, et al. Pembrolizumab-associated mucous membrane pemphigoid in a patient with Merkel cell carcinoma. Br J Dermatol. (2018) 179:993–4. doi: 10.1111/bjd.16780
13. Fontecilla NM, Khanna T, Bayan CY, Antonov NA, Geskin LJ. Bullous pemphigoid associated with a new combination checkpoint inhibitor immunotherapy. J Drugs Dermatol. (2019) 18:103–4.
14. Thomsen K, Diernaes J, Ollegaard TH, Spaun E, Vestergaard C. Bullous pemphigoid as an adverse reaction to pembrolizumab: two case reports. Case Rep Dermatol. (2018) 10:154–7. doi: 10.1159/000489661
15. Lopez AT, Geskin L. A case of nivolumab-induced bullous pemphigoid: review of dermatologic toxicity associated with programmed cell death protein-1/programmed death ligand-1 inhibitors and recommendations for diagnosis and management. Oncologist. (2018) 23:1119–26. doi: 10.1634/theoncologist.2018-0128
16. Hirotsu K, Chiou AS, Chiang A, Kim J, Kwong BY, Pugliese S. Localized bullous pemphigoid in a melanoma patient with dual exposure to PD-1 checkpoint inhibition and radiation therapy. JAAD Case Rep. (2017) 3:404–6. doi: 10.1016/j.jdcr.2017.06.004
17. Aggarwal P. Disproportionality analysis of bullous pemphigoid adverse events with PD-1 inhibitors in the FDA adverse event reporting system. Expert Opin Drug Saf. (2019) 18:623–33. doi: 10.1080/14740338.2019.1619693
18. Poot AM, Diercks GF, Kramer D, Schepens I, Klunder G, Hashimoto T, et al. Laboratory diagnosis of paraneoplastic pemphigus. Br J Dermatol. (2013) 169:1016–24. doi: 10.1111/bjd.12479
19. Oursler JR, Labib RS, Ariss-Abdo L, Burke T, O'Keefe EJ, Anhalt GJ. Human autoantibodies against desmoplakins in paraneoplastic pemphigus. J Clin Invest. (1992) 89:1775–82. doi: 10.1172/JCI115781
20. Miremont-Salame G, Theophile H, Haramburu F, Begaud B. Causality assessment in pharmacovigilance: the French method and its successive updates. Therapie. (2016) 71:179–86. doi: 10.1016/j.therap.2016.02.010
21. Kurihara Y, Furue M. Gluteal hidradenitis suppurativa presenting pemphigus-like findings: case report. BMC Dermatol. (2019) 19:11. doi: 10.1186/s12895-019-0091-7
22. Wang L, Bu D, Yang Y, Chen X, Zhu X. Castleman's tumours and production of autoantibody in paraneoplastic pemphigus. Lancet. (2004) 363:525–31. doi: 10.1016/S0140-6736(04)15539-6
23. Wang J, Bu DF, Li T, Zheng R, Zhang BX, Chen XX, et al. Autoantibody production from a thymoma and a follicular dendritic cell sarcoma associated with paraneoplastic pemphigus. Br J Dermatol. (2005) 153:558–64. doi: 10.1111/j.1365-2133.2005.06599.x
24. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. (2018) 8:86. doi: 10.3389/fonc.2018.00086
Keywords: paraneoplastic pemphigus, immune checkpoints inhibitors, anti-programmed-death-1, pembrolizumab, cutaneous squamous cell carcinoma, hidradenitis suppurativa
Citation: Yatim A, Bohelay G, Grootenboer-Mignot S, Prost-Squarcioni C, Alexandre M, Le Roux-Villet C, Martin A, Maubec E and Caux F (2019) Paraneoplastic Pemphigus Revealed by Anti-programmed Death-1 Pembrolizumab Therapy for Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa. Front. Med. 6:249. doi: 10.3389/fmed.2019.00249
Received: 10 July 2019; Accepted: 16 October 2019;
Published: 05 November 2019.
Edited by:
Karin Loser, University of Münster, GermanyReviewed by:
Thilo Gambichler, University Hospitals of the Ruhr-University of Bochum, GermanyGiovanni Di Zenzo, Institute of Dermatology Immaculate (IRCCS), Italy
Copyright © 2019 Yatim, Bohelay, Grootenboer-Mignot, Prost-Squarcioni, Alexandre, Le Roux-Villet, Martin, Maubec and Caux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frédéric Caux, frederic.caux@aphp.fr
†These authors have contributed equally to this work as co-first authors
 Ahmad Yatim1†
Ahmad Yatim1†  Gérôme Bohelay
Gérôme Bohelay Catherine Prost-Squarcioni
Catherine Prost-Squarcioni Marina Alexandre
Marina Alexandre Frédéric Caux
Frédéric Caux