Spatial link between Adélie penguin foraging effort and krill swarm abundance and distribution
- 1Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tasmania, Australia
- 2Australian Antarctic Division, Kingston, Tasmania, Australia
Understanding how predator foraging behaviour is influenced by the distribution and abundance of prey is a fundamental challenge in marine foraging ecology. This is particularly relevant in Southern Ocean ecosystems where the relationships between select predator species and Antarctic krill (Euphausia superba) can inform ecosystem conservation and precautionary fisheries management. In this study, we examine the spatial associations between krill swarm characteristics and Adélie penguin (Pygoscelis adeliae) foraging effort at Béchervaise Island, a long-term monitoring site in East Antarctica. Spatially integrating two years of regional-scale krill acoustic data with contemporaneous horizontal and vertical movement information from chick-rearing adult Adélie penguins, we assessed how penguin foraging effort changed in relation to krill swarm abundance and distribution across the survey area. Our findings show that penguin diving effort was focused in areas with a high number of krill swarms, yet they did not focus their effort in areas with high krill biomass. These results suggest the spatial organisation of Adélie penguin foraging effort can provide an indication of krill presence (and/or availability) but may not reflect krill abundance. We discuss our results in the context of penguin foraging strategies, capturing single krill within the water column rather than the engulfment feeding strategy of larger marine mammals such as whales. Our work substantially improves understanding of penguin-krill dynamics in East Antarctica and provides a greater level of nuance regarding the utility of Adélie penguins as indicator species under CCAMLR’s Ecosystem Monitoring Programme (CEMP). Understanding these predator-prey linkages will become increasingly important for managing any expanding krill fisheries in the region or changes in the prey field under future climate change scenarios. Thus, our results can be interpreted alongside other ecological indicators to support management of the East Antarctic sector of the Southern Ocean ecosystem.
1 Introduction
Understanding how predator behaviour relates to the distribution and abundance of prey is a key objective in ecology (Hunsicker et al., 2011). This can be particularly challenging in marine environments, where predator-prey interactions occur in a dynamic three-dimensional environment (Bestley et al., 2015; Kuhn et al., 2015). Synchronous information on predator foraging behaviour and prey fields at spatiotemporal scales relevant to marine predators can be difficult to obtain. These survey efforts are both financially and logistically intensive, and are therefore, rarely undertaken (Grémillet et al., 2004; Bedford et al., 2015).
Studies investigating the fine-scale spatial overlap between marine predator foraging and prey fields have commonly coupled acoustic surveys with direct shipboard observations of predator density and feeding activity (Table 1). This can provide a snapshot of predator-prey spatial overlap in real time. However, these observational techniques have a very limited spatial coverage and only examine a small part of a predator’s foraging range. This limits inferences about predator-prey interactions occurring over larger spatial scales (Kuhn et al., 2015; Yamamoto et al., 2015). Furthermore, visual observations are generally restricted to surface foraging behaviour (in fair weather), omitting critical information on underwater foraging activity (Enstipp et al., 2007; Hazen et al., 2009). Bio-logging and telemetry devices are increasingly being used to provide a more detailed understanding of predator-prey relationships at a broader spatial resolution (horizontally and vertically) (Hunsicker et al., 2011). Marine surveys incorporating telemetry have developed understanding of predator-prey relationships in various temperate and polar ecosystems for a range of predator taxa (See Table 1 for examples).
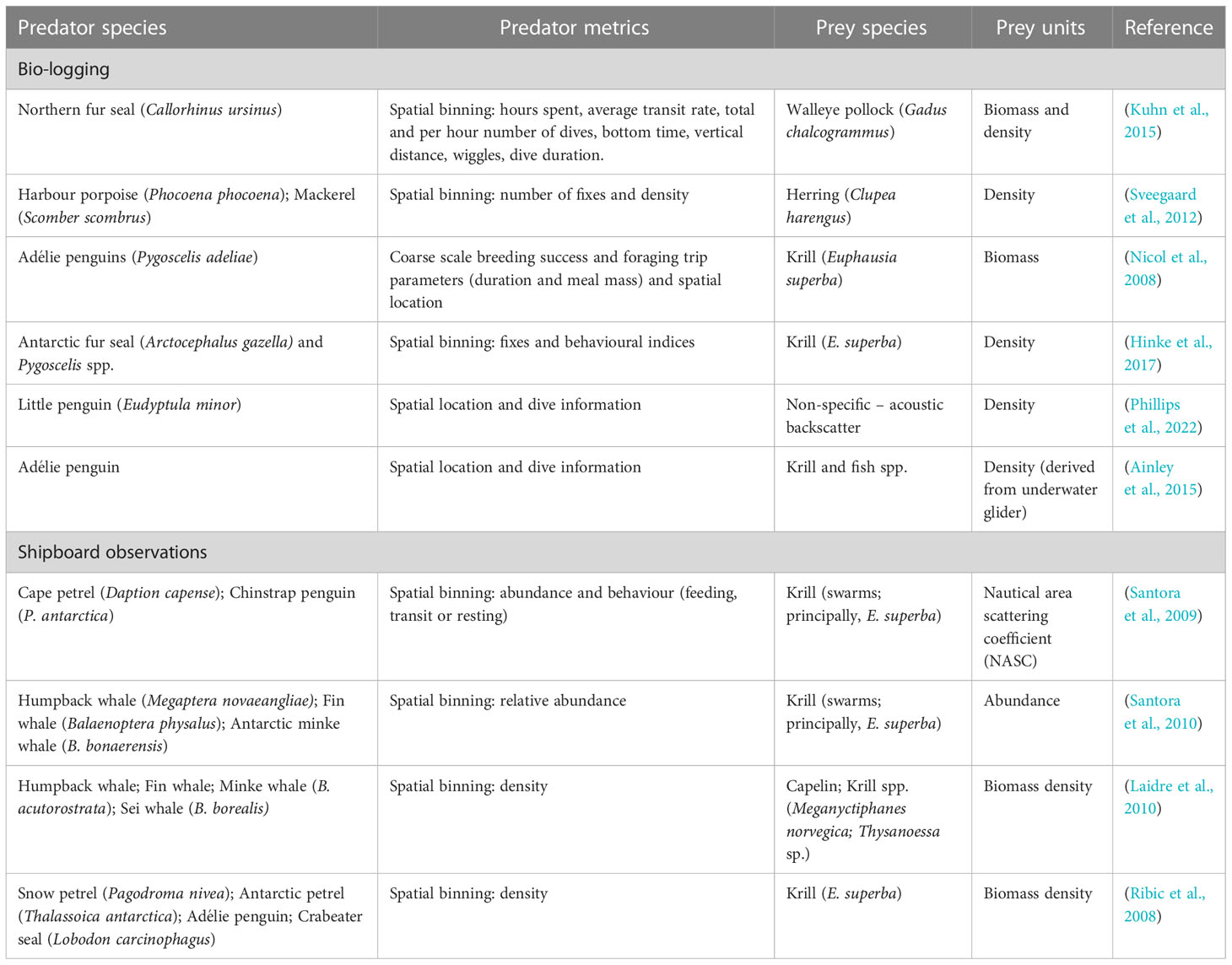
Table 1 Examples of studies on predator-prey relationship which couple prey field acoustic surveys with predator locations through biologging or shipboard observations.
Within the Southern Ocean, the area of interest in this study, regionally high abundances of Antarctic krill (Euphausia superba) support a diverse range of higher-order predators (Atkinson et al., 2009; Trathan and Hill, 2016). While the importance of krill-dominated energy pathways can vary over time and space, Antarctic krill (hereafter, krill) are an important forage resource for fish, squid, seabirds and marine mammals in Southern Ocean ecosystems (Croxall et al., 1999; Trathan et al., 2012). The substantial biomass of krill also supports a commercial fishery largely, concentrated around the Antarctic Peninsula (Watters et al., 2020).
A fundamental aspect of krill biology and ecology is their swarming behaviour resulting in their distribution being heterogenous and patchy (Tarling et al., 2009; Nicol and Brierley, 2010). In some regions, ~ 98% of krill biomass is contained in swarms (Fielding et al., 2014). Swarming plays an important role in krill reproduction (Watkins et al., 1992), foraging (Hamner and Hamner, 2000) and predator avoidance (Cox et al., 2009). Swarms can take a variety of shapes and can form large aggregations. Horizontal extent can span up to thousands of metres in length and can contain billions of krill in densities of several thousand individuals per cubic metre (Cox et al., 2010; Tarling and Fielding, 2016). The swarming behaviour of krill has a strong influence on the distribution and foraging strategies of Southern Ocean predators. Higher-order predators, such as seabirds and marine mammals, are reported to target and concentrate predation efforts in dense krill swarms (Veit et al., 1993; Santora et al., 2017).
In the Southern Ocean, krill harvesting is managed under the Commission for the Conservation of Marine Living Resources (CCAMLR). Through its ecosystem monitoring programme (CEMP), predator response parameters, such as breeding and foraging success, can be used to inform precautionary krill catch limits (Agnew, 1997; Constable et al., 2000). Adélie penguins (Pygoscelis adeliae) are a key indicator species under CEMP because they are important consumers of krill (Croxall et al., 2002). However, since the establishment of CEMP, there is increasing recognition Adélie penguin diets are more diverse than traditionally believed, with substantial spatial and temporal variability in the proportion of krill and fish consumed (Clarke et al., 2002; Ainley et al., 2003; Lynnes et al., 2004; Tierney et al., 2009; Gorman et al., 2021). The diet of Adélie penguin populations in the Scotia Sea, East Antarctic and regions of the Antarctic Peninsula are generally dominated by krill, while populations in the Ross Sea have a more varied diet and consume a mixture of fish and euphausiid species (Ratcliffe and Trathan, 2012). Despite establishing a greater understanding of the specific prey consumed, there remains a limited understanding of how the distribution and foraging behaviours of this indicator species are related to the spatiotemporal variability of krill patches (Ainley et al., 2015; Ford et al., 2015). Understanding foraging behaviour responses of Southern Ocean predators to krill distribution and abundance may provide insight into ecological factors influencing predator population dynamics.
Given the variability in krill abundance and density around coastal Antarctic waters (Atkinson et al., 2004; Jarvis et al., 2010) and variation in key physical and environmental features (i.e. sea ice and proximity of land-based colonies to shelf break), it is likely krill-predators adopt different foraging strategies at the regional-scale (Ford et al., 2015; Cimino et al., 2016). In East Antarctica, Nicol et al. (2008) conducted fine-scale acoustic surveys during 2001 and 2003 to assess krill distribution and abundance using a standard two-dimensional grid-based approach, where the acoustic data are integrated to depths of 250 m and averaged over one nautical mile intervals. The simultaneous collection of Adélie penguin spatial [horizontal] movements via telemetry also enabled key predator foraging parameters to be interpreted in the context of krill data. Using horizontal information, Nicol et al. (2008) relied upon area-restricted search (ARS) assumptions to make broad spatial associations between penguin movements and krill distribution and abundance. Results showed that penguin breeding success, foraging trip duration and meal mass were broadly related to overall krill biomass estimates in those two years. In this same area of East Antarctica, Emmerson et al. (2015) considered the spatiotemporal variability of penguin response parameters in relation to the two years using krill biomass estimates and proposed that krill availability was a function of its abundance in the water column and its accessibility, primarily related to the presence of extensive fast-ice. However, neither of these studies were able to consider their results in the context of krill in the vertical dimension; how predator foraging effort and prey abundance are distributed in the underwater environment; and the extent of their three-dimensional spatial overlap.
Here, we further explore the concept of krill availability to penguins, specifically in relation to krill swarm distribution and abundance by reanalysing krill biomass estimates reported by Nicol et al. (2008) and integrating these estimates with the recent processing of extensive multi-year Adélie penguin dive data (Riaz et al., 2020) and quantitative integration of spatial location estimates from the same years (Riaz et al., 2021). Methods for swarm-based acoustic analyses (Tarling et al., 2009; Cox et al., 2010) offer an opportunity to re-examine acoustic prey-field data to characterise krill swarm structure (e.g., internal density, height, length) and location (depth) in the water column. Integrating this with penguin dive and location data provides an opportunity to examine where penguin underwater [vertical] foraging efforts are concentrated along horizontal movement trajectories in relation to krill swarm distribution and abundance. The benefits of examining krill abundance and distribution through a swarm-based analysis are twofold: (1) processing of acoustic information can be concentrated around the natural aggregation structures of krill swarms, reducing pre-processing, subjective noise removal and associated loss of acoustic information; (2) broad-scale summary information of the krill prey-field (e.g. swarm biomass and depth) are provided at a scale in which predator foraging decisions and prey encounters occur (Cox, 2017; Bestley et al., 2018). Combining horizontal-vertical predator movement with three-dimensional krill prey-field information can improve understanding of predator-prey spatial overlap and interactions (Ainley et al., 2015).
In this study, we investigated the spatial associations of krill swarm characteristics and Adélie penguin foraging effort by integrating predator-prey data streams collected simultaneously (in the same area and during the same seasons). We provide a novel reassessment of the krill acoustic data collected during surveys conducted in two years (2001 and 2003), using a swarm-based approach to estimate krill swarm distribution and physical structure. To understand how penguin foraging relates to krill swarm abundance and distribution we integrate contemporaneous horizontal and vertical movement information from chick-rearing Adélie penguins at Béchervaise Island, East Antarctica, which were dual-tagged with platform terminal transmitters (PTTs) and time-depth recorders (TDRs). We test whether foraging dive effort (summarised dive activity) changed in relation to krill swarm biomass and number across the gridded offshore survey domain. We expected all indices of underwater foraging effort would increase in areas with a greater krill abundance and number of swarms. Through this work, we provide an improved understanding of predator-prey interactions for this CEMP indicator species, and the prey-field characteristics critical to penguin foraging success at this colony.
2 Materials and methods
2.1 Prey-field data: Krill swarms
Shipboard acoustic surveys were performed off the coast of Béchervaise Island (67°35 S, 67°49 E) in East Antarctica in 2001 (KACTAS – Krill Availability, Community Trophodynamics and AMISOR Survey) and 2003 (KAOS – Krill Acoustics and Oceanography Survey). These surveys were conducted from 12 to 23 January, and from 16 January to 1 February, respectively. For both KACTAS and KAOS, the survey areas ranged from 66° to 67°S and 61.8° to 64.6°E, covering an estimated 11,921 km2. Due to operational curtailment, only 75% of transects within the survey area were completed during KACTAS. Full details of the survey design and acoustic data collection can be found in Nicol et al. (2008).
The acoustic data processing was carried out using Echoview (v5.4 Myriax, Hobart, Australia). The swarm-based acoustic analysis used 38 and 120 kHz frequencies of calibrated Simrad (Horten, Norway) EK500 echosounder for the KACTAS survey and EK60 for the KAOS survey. Echosounder transducers were hull-mounted split-beam transducers with a 7° beam width. Krill swarms were identified up to a 250 m depth limit at a 2 Hz pulse repetition rate. The mean vessel speed was 7.8 knots with a mean inter-pin space of 2 m. Surface noise, seabed and false seabed returns were isolated and removed from acoustic observations. Calibrations parameters applied to acoustic data are provided in Table S1. Time varied gain noise correction was performed using the procedure described in De Robertis and Higginbottom (2007).
Individual krill swarms were isolated in Echoview using the schools detection algorithm of Barange (1994). School detection was carried out on a 7 x 7 identity matrix convolution of the 120 kHz pre-processed data using the detection parameters established in Tarling et al. (2009) and a mean volume backscattering strength (Sv) threshold of -70 dB re 1 m-1, equivalent to a krill wet mass density of 0.9 g m-3. A description of acoustic terminology, symbols and units are provided by MacLennan et al. (2002). These aggregations are assigned a krill or non-krill status by applying a validated ‘dB-difference’ technique to the 7 x 7 convolution of 120 - 38 kHz pre-processed data falling within the detected aggregation boundaries (Madureira et al., 1993). As implemented here, the dB-difference technique is a binary classification, with aggregations falling within a dB difference range deemed to be krill. Outside of this range an aggregation is classified as coming from other species and excluded from further analysis. The dB-difference ranges were calculated using the distorted-wave Born approximation (DWBA) krill acoustic target strength model developed by McGehee et al. (1998), and extended by Conti and Demer (2006) to account for stochastic variation in the received signal phase. Consistent with CCAMLR recommendations, the Calise and Skaret (2011) krill target strength model was applied, using a tilt angle distribution, a wrapped normal distribution with mean -28° (head down) and standard deviation 20°. Stochastic DWBA krill target strength model parameters were set at their default values as given in Calise and Skaret (2011).
Once an aggregation was identified as krill, volume integrations were carried out on 120 kHz data falling within the boundary of individual krill swarms at a -80 dB re 1 m-1 threshold, equivalent to average (across length frequency clusters) krill wet mass density of 0.09 g m-3, and swarm internal density (ρ) was calculated using ρ=10(Sv−TSkg)/10 , where TSkg is the krill length cluster specific target strength of 1 kg (wet mass) of krill. Once krill swarms were identified, a range of physical properties were calculated within the Echoview software. For each individual swarm, this included the mean depth (m), cross-sectional area (m2), length (m), height (m), internal density (g wet mass per m3) and nautical area scattering coefficient (NASC) (m2 n mile-2). To calculate swarm biomass, we assumed swarms had a cylindrical shape an applied the following [Equation 1]:
We note that in some instances, the cylindrical shape assumption may underestimate swarm biomass, since swarms can take complex 3D shapes and structures which have volumes larger than cylindrical or ellipsoid shapes (Brierley and Cox, 2010).
2.2 Predator data: Movement and diving
Béchervaise Island is an Adélie penguin nesting site located in East Antarctica home to over 2000 breeding pairs. It is a CEMP site which has been the focus of a long-term Adélie penguin monitoring program since 1990 (Kerry et al., 2000). During 2001 and 2003, spatial location and dive data were collected from adult chick-rearing Adélie penguins at the Béchervaise Island colony through dual-tagging of individuals with PTTs and TDRs. These data were comprised of 18 dual-tagged individuals over 19 foraging trips during the guard and crèche chick-rearing periods (one individual from the 2003 season recorded two foraging trips). Individuals tagged in the two years were different individuals. Penguin movement data ranged from 12 to 25 January in 2001, and 9 to 26 January in 2003. Full details of PTT and TDR deployments and data processing are provided by Riaz et al. (2020); Riaz et al. (2021).
Archived dive data were downloaded using Wildlife Computers software packages. A zero-offset correction and surface noise exclusion (< 3 m) were applied to dive profiles (Riaz et al., 2020). All subsequent processing and analyses of data was performed using R statistical software version 3.5.1 (R Core Team, 2018). A series of dive indices were calculated for each dive. This included the maximum dive depth (m); bottom duration of dives (s) (defined as the maximum time spent within 50% of maximum dive depth and where the rate of change in depth during descent or ascent did not exceed 50%); and wiggles (the number of undulations in a dive profile > 2 m in depth).
Raw Argos location estimates were subjected to several quality control measures. Location estimates were removed when they occurred within 120 s of each other (n = 200), and we ensured our dataset did not contain foraging trips with fewer than 10 location fixes or lasting less than 1 day in duration. After quality control processing, PTT location estimates were fit with a continuous-time correlated random walk state-space model (SSM) using the ‘foieGras’ package (version 0.7.6; Jonsen and Patterson, 2021). This accounted for observation error in tracking data and provided location estimates at regular time intervals (Jonsen et al., 2013; Ropert-Coudert et al., 2020). Process models and equations are established in Jonsen et al. (2020). Spatial locations were regularised to 1-hour time steps, consistent with Riaz et al. (2021).
We binned diving activity into 1-hour periods corresponding to SSM location estimates. At each hourly location estimate along regularised tracks, we quantified underwater foraging effort by summing dive parameters (Pütz et al., 2006; Zimmer et al., 2008). The sum of the maximum dive depth (m), bottom duration (s), number of dives, and number of wiggles for each hour of an individual’s foraging trip were calculated. Summed dive parameters over regular time-steps are commonly used to quantify marine predator foraging effort underwater (Pütz et al., 2006; Zimmer et al., 2010; Kuhn et al., 2015; Riaz et al., 2021).
2.3 Predator-prey statistical integration
To examine the spatial associations of krill swarm characteristics and penguin foraging effort, we integrated the horizontal-vertical movements of Adélie penguins with krill survey estimates. We summarised our prey and predator indices across the entire survey area at a 0.05° latitude x 0.38° longitude resolution (approximately 5.5 x 17 km). The size of our spatial grid was chosen for three reasons. First, krill swarm characteristics are expected to have a greater variability over latitudinal gradients (i.e. offshore-inshore), associated with oceanographic conditions and food availability (Klevjer et al., 2010); second, it generally captures survey estimates from two adjacent transect lines, which were longitudinally spaced at approximately 5 – 10 nautical mile intervals (Nicol et al., 2008); and third, it is sufficiently broad to cater for prey horizontal transport through time, accommodating the temporal mismatch between survey estimates and penguin location estimates, which is a common challenge of survey efforts linking predators and prey (Santora et al., 2010; Kuhn et al., 2015; Hinke et al., 2017). The gridded approach adopted here is analogous to other studies examining prey-fields in the context of predator-prey relationships (Santora and Reiss, 2011; Sveegaard et al., 2012).
Using the raw acoustic transects, we calculated the geometric mean [hereafter referred to as ‘mean’] swarm biomass within defined 0.05° x 0.38° grid cells. The geometric mean is demonstrated to be an effective parameter in describing skewed prey-field data, as is it less sensitive to large outlier values (Cade et al., 2021). We also calculated the total number of swarms per grid cell and standardised this metric by the total length of the survey transect in each spatial bin to account for survey effort.
Gridded krill indices were overlaid with the horizontal-vertical movements of chick-rearing Adélie penguins at Béchervaise Island. To quantify foraging effort in corresponding 0.05° x 0.38° bins, for each penguin we calculated (1) total dive depth, (2) total number of wiggles, (3) total bottom duration, and (4) total number of dives per grid cell. By gridding the predator-prey data, spatial autocorrelation present within the high-resolution along-transect and along-track datasets is reduced (Warwick-Evans et al., 2022).
With the integrated final predator-prey dataset, we make inferences about how penguin foraging effort is associated with krill metrics using generalised linear mixed effects models to (‘glmmTMB’ package; version 1.1.4; Brooks et al., 2017). For independent models of each of the four penguin diving response variables [depth, bottom duration, number of wiggles, and number of dives], we fitted as predictor variables the two krill swarm metrics [mean krill biomass and number of swarms]. Prior to analysis, predictor variables were assessed for any collinearity issues [Pearson correlation coefficient = -0.13] (‘corrplot’ package; version 0.92; Wei and Simko, 2021). All four models were configured with individual penguin ID nested within year (i.e. Year/Bird ID) to allow relationships to vary among individuals. The small sample size of penguin individuals from 2003 (due to logistical, financial, and field constraints) precluded us from examining the influence of year as a fixed effect. To account for overdispersion and right skew in data, number of wiggles and dives (count data) models were fitted with a negative binomial distribution, while depth and bottom duration were configured with a gamma distribution and log link function. Both krill predictor metrics were scaled and centred to aid model convergence. Model covariates were considered significant at p-values< 0.05.
3 Results
3.1 KACTAS characteristics
The KACTAS voyage performed eight north-south acoustic transects spanning a 9374 km2 area (Figure 1). At a 0.05° latitude x 0.38° longitude resolution, there were 106 grid cells across the survey area (Figure 2). A total of 5792 krill swarms were recorded with a total estimated biomass of 8123 t. The mean krill swarm biomass was 3.83 kg. On average, swarms were 124.7 m2 in area (where area is the intersection between the acoustic beam and the swam) with an internal density of 5.87 g m-3. The mean NASC was 53.4 m2n mile-2, with 1% of swarms exceeding 5000 m2n mile-2 (see Table 2 for means and 95% CI).
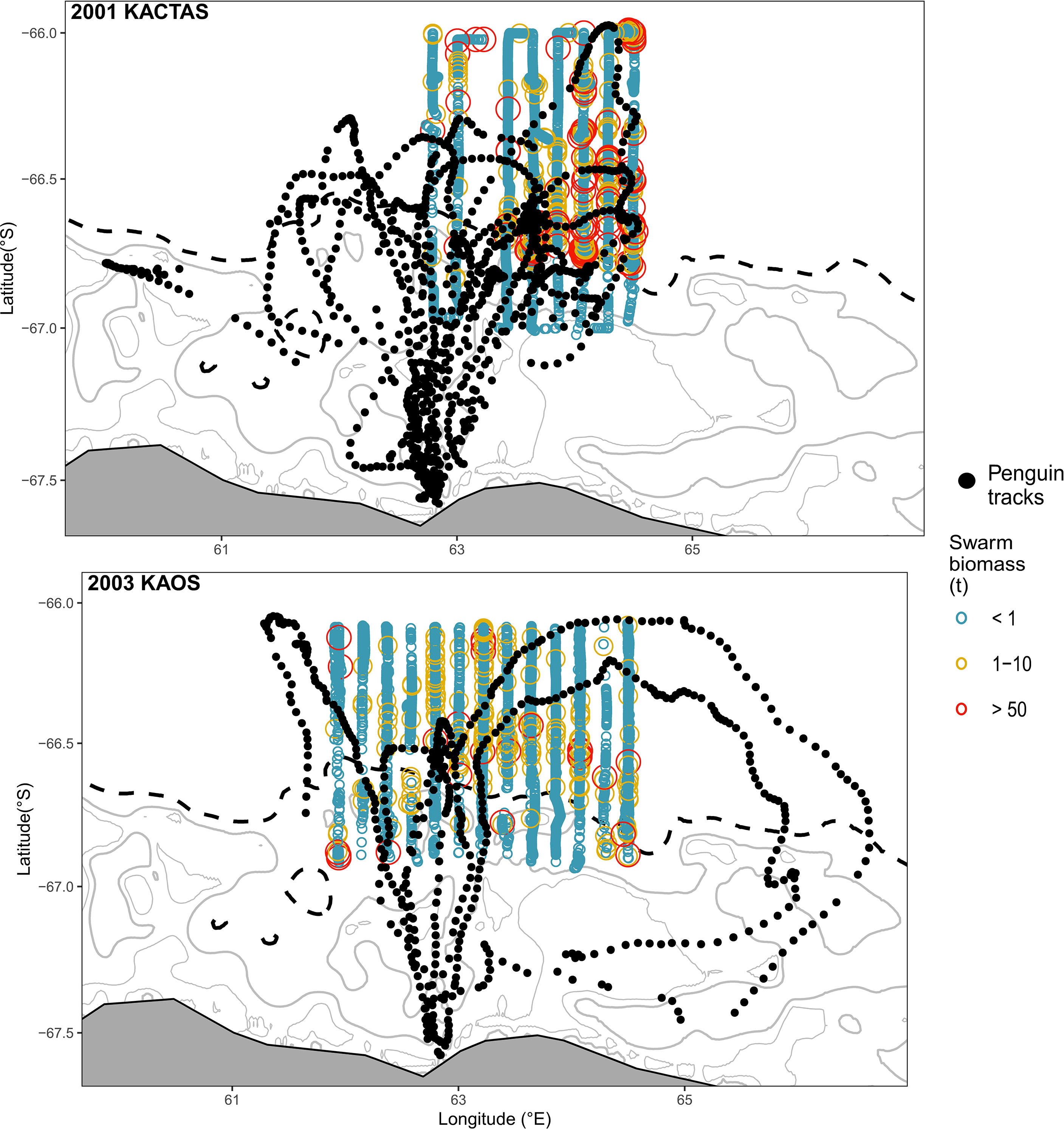
Figure 1 Distribution of krill swarm biomass in the waters off Béchervaise Island collected during KACTAS and KAOS. To aid visual presentation, krill swarms are grouped in discrete biomass categories. Swarms within transects are overlaid with SSM-filtered location estimates for chick-rearing Adélie penguins (n = 18 individuals on n = 19 foraging trips). Major land features are in grey and major bathymetric features (shelf break and other bathymetric features > 1000m) are illustrated by black dashed lines. Bathymetric contours are displayed at 100m intervals. Inset panel in (A) shows the study region (red circle) in East Antarctica.
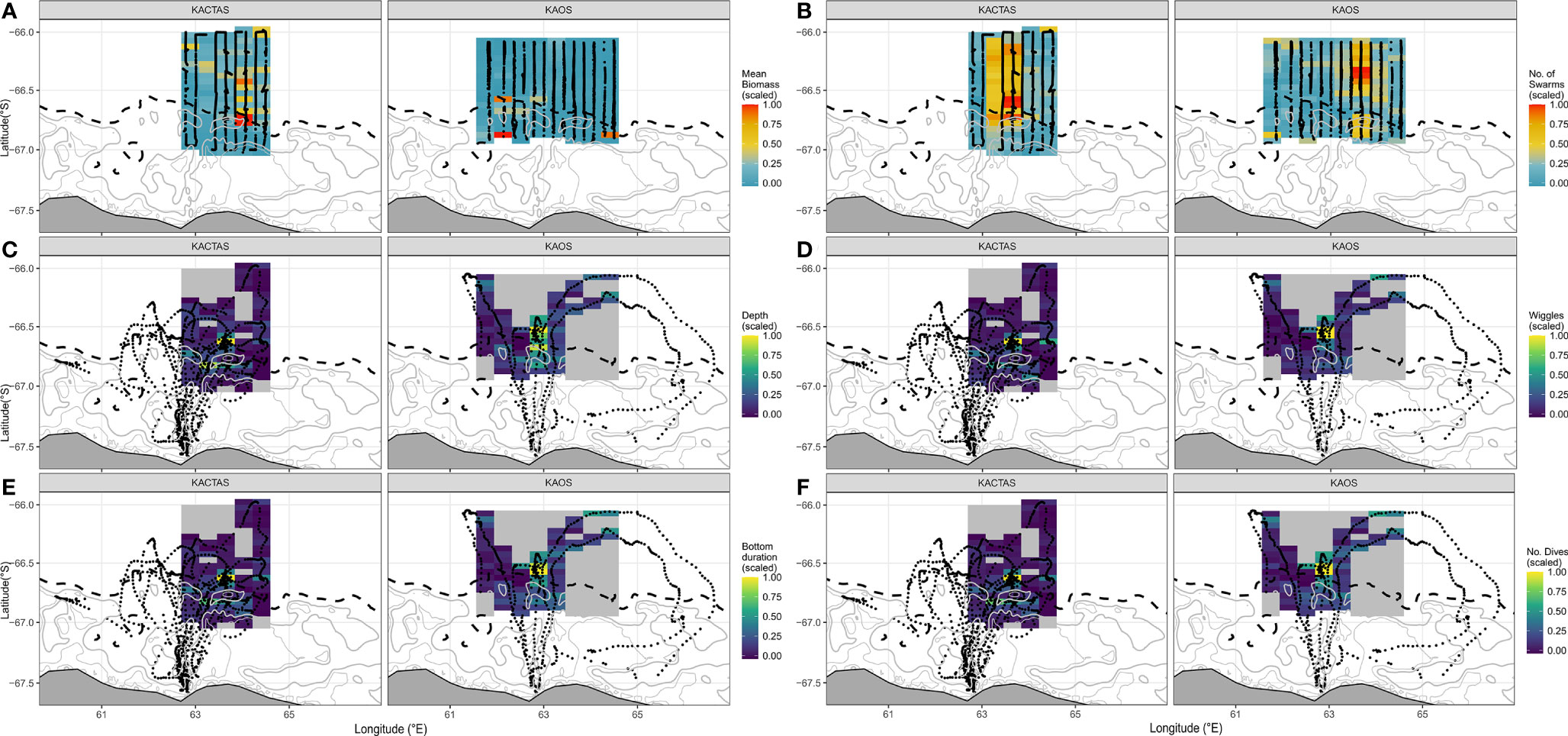
Figure 2 Maps displaying the distribution of krill metrics (mean biomass and number of swarms) and four penguin diving metrics (depth, wiggles, bottom duration and # of dives) over the KACTAS and KAOS survey area. Grid cell metrics are binned at a 0.05˚ latitude x 0.38˚ longitude spatial resolution. The black points represent krill transect (A, B) and penguin tracking observations for krill and penguin plots (C–F), respectively. Maps displayed illustrate spatially grided predator-prey data, as inputed into models. Land and bathymetric features are displayed as per Figure 1.
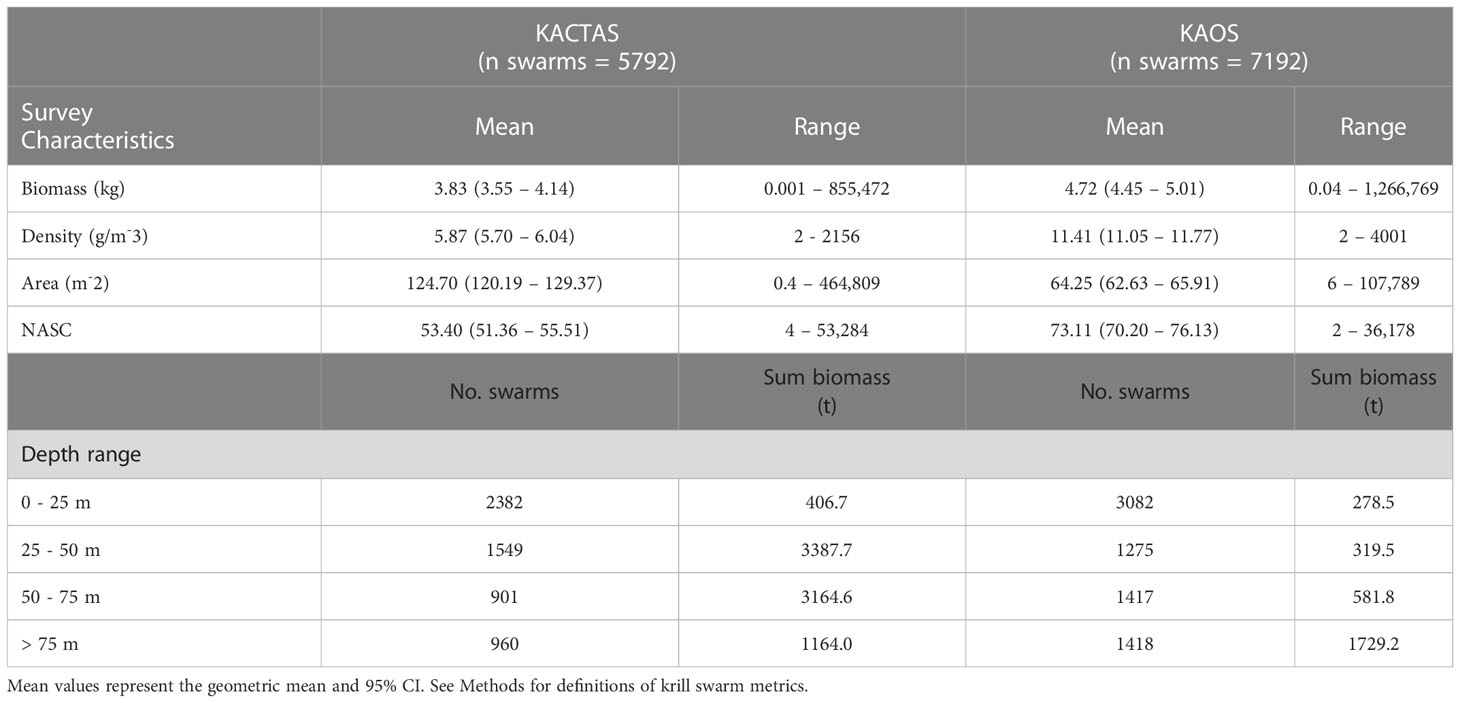
Table 2 Summaries of physical properties of krill swarms and the depth-stratified total number of swarms and biomass recorded during KACTAS and KAOS voyages that occurred over the entire survey area.
The mapped distribution shows mean swarm biomass was higher in grid cells located over the eastern part of the shelf break, with high biomass also patchily distributed further north (Figure 2 upper panels). The number of krill swarms across the survey area showed a different and more consistent pattern, largely concentrated in the middle of the survey area in waters over the shelf break and north (Figure 2).
In vertical space, KACTAS krill swarms were observed at depths in the water column ranging between 4 – 246 m, with an average depth of 42 m. Most swarms (68%) were located in the upper 50 m (Figure 3) but the sum of the krill biomass was mostly concentrated between 25 – 50 m depths (81%) (Table 2).
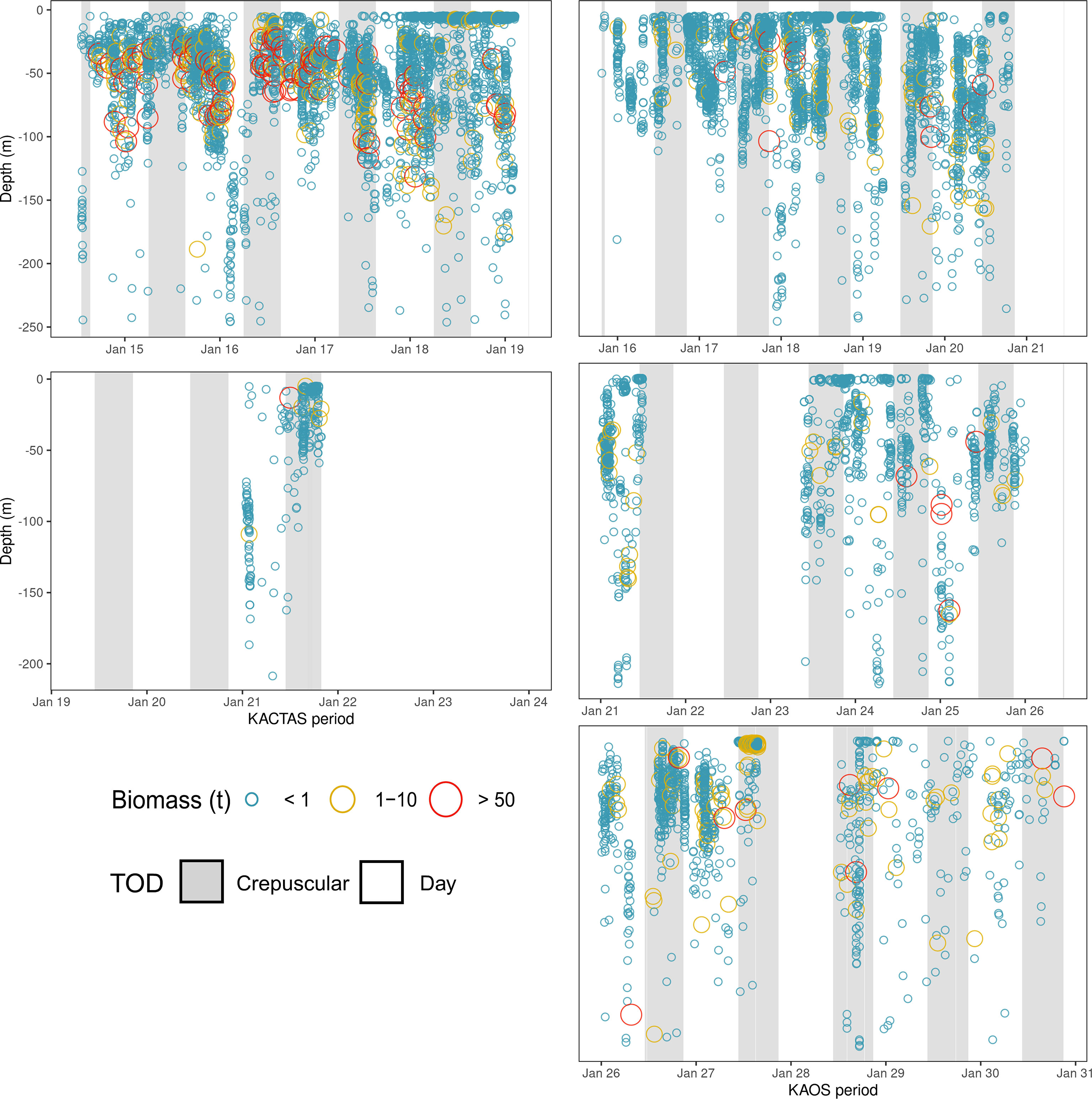
Figure 3 Time-series showing krill swarm distribution through time for KACTAS and KAOS (left and right panels, respectively). Each panel represents a vertical snapshot of the water column (0 – 250 m) over a specific period of time during survey efforts. Each circle represents a krill swarm observed and are sized and coloured in relation to their biomass values (see Methods for details). To aid visual presentation, biomass estimates are grouped into discrete categories. Shaded areas represent the diurnal period in which krill swarms were observed. Using the ‘maptools’ package, we calculate the solar position values during survey effort period (Bivand and Lewin-Koh, 2016). Solar positions values > 12° were assigned as day and those between -12° and 12° were assigned as crepuscular; at this time of year there are no night (< -12°) values.
3.2 KAOS characteristics
The KAOS voyage covered a larger spatial extent compared to KACTAS. Active acoustics were recorded over 13 transects, covering approximately 11,921 km2 (Figure 1). There were 144 grid cells when binned at a 0.05° latitude x 0.38° longitude spatial resolution (Figure 2). A greater number of swarms (n = 7192) were recorded compared to KACTAS, however total biomass (2909 t) was substantially lower. The mean biomass of krill swarms during KAOS was 4.80 kg. While the average internal density of krill swarms was also larger compared to KACTAS (11.41 g m-3), the mean swarm area was around half the size (64.63 m2). The mean NASC was 73.1 m2n mile-2 (see Table 2 for means and 95% CI). Similar to KACTAS, 1% of swarms exceeded 5000 m2n mile-2.
During KAOS, mean swarm biomass showed a relatively uniform distribution of low biomass across the survey area, with the majority of biomass concentrated across few grid cells. In comparison, the horizontal distribution of swarm frequency was patchily distributed along the eastern part of the shelf break and was most pronounced in grid cells located in more northern waters (Figure 2).
Over the KAOS survey area, swarms were observed within 6 – 248 m depths, but at 46 m on average. The distribution of swarms through the upper water column was relatively even, although 43% were recorded in waters shallower than 25 m. In contrast, most (66%) of the krill swarm biomass was in depths greater than 75 m (Figure 3).
3.3 Penguin activity in the survey area
Over the two breeding seasons, we recorded diving activity from 18 dual-tagged individuals on 19 foraging trips (Figure 1). Within the KACTAS and KAOS survey areas, we documented 12,278 dives spread across 787 at-sea locations. In 2001 (KACTAS), 14 individuals and 14 foraging trips were recorded. Within the KACTAS survey area, 8821 dives were logged over 500 spatial locations. This accounted for 48% of dives at 22% of locations over the entire foraging trip distribution. In 2003 (KAOS), 4 individuals and 5 foraging trips were recorded, and 3457 dives over 247 spatial locations occurred within the KAOS survey area (Figure 1). This comprised 40% and 31% of foraging trip dives and at-sea location, respectively.
At the trip level, mean dive effort (total depth travelled, bottom duration and number of wiggles and dives) across KACTAS and KAOS grid cells (n= 106 and n= 144 cells, respectively) were broadly similar but slightly higher during KACTAS (Table 3). Generally, higher values (i.e. greater dive effort, longer bottom duration, more wiggles and more dives) of all penguin dive metrics were centred in the vicinity of the shelf-break during both surveys (Figure 2).
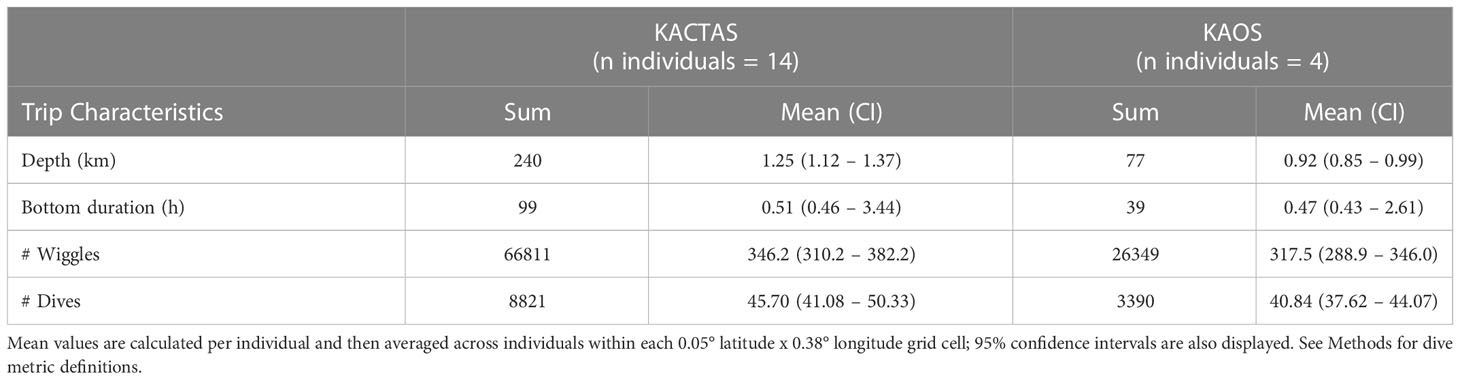
Table 3 Summaries of chick-rearing Adélie penguin activity at Béchervaise Island that occurred over the KACTAS and KAOS survey areas.
3.4 Predator-prey relationships
The total depth and bottom duration travelled, and number of wiggles and dives performed were consistently greater in areas where there were a higher number of krill swarms. In contrast, penguin foraging effort was not significantly related to mean swarm biomass in any model (Table 4; Figure 4).
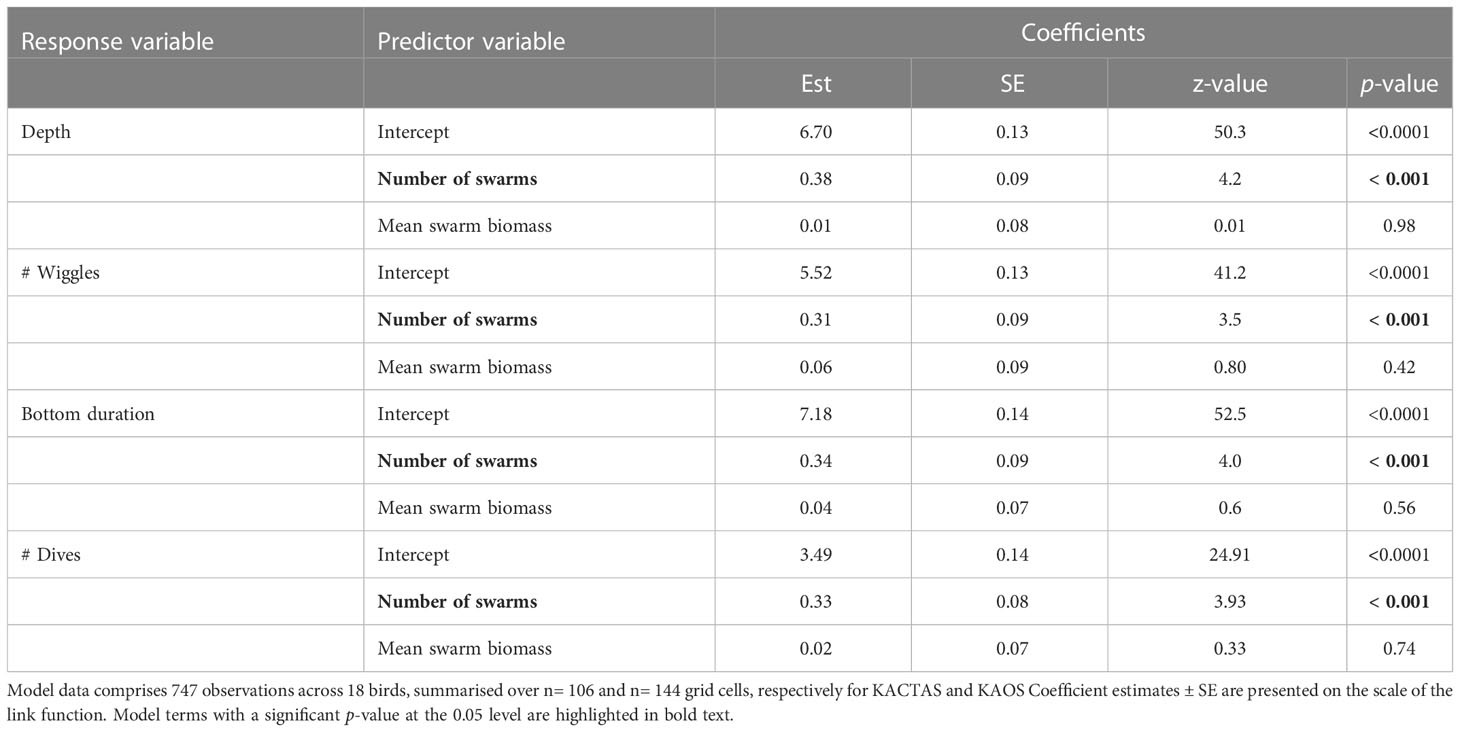
Table 4 Results of the four generalised linear mixed effects models for each dive metrics in relation to krill swarm metrics (number of swarms and mean swarm biomass).
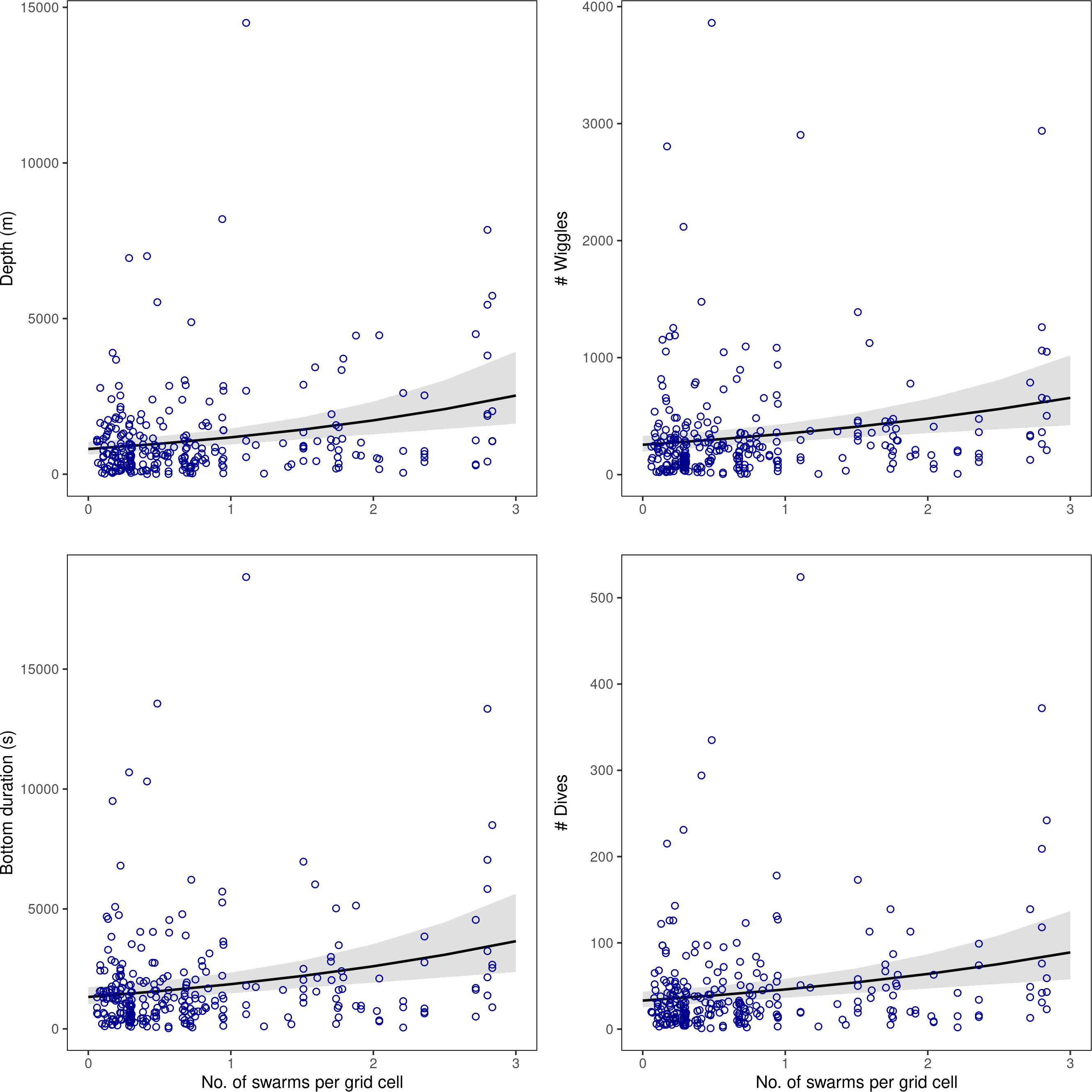
Figure 4 Results from the generalised linear mixed effects models showing the diving responses [summed depth, bottom duration, number of wiggles, and number of dives over each spatial grid; y-axes] modelled in relation to krill swarm number within spatial grid cells across the acoustic survey domain. Diving parameters showed no association with krill swarm biomass; full model results are given in Table 4.
Within a given grid cell (covering approximately 100 km), a change of 0 to 3 krill swarms would correspond with a predicted average change in penguin behaviour from 33 to 89 dives, and 255 to 656 wiggles. Similarly, predicted summed vertical dive distance would increase from 810m to 2527 m, and bottom time would increase from 1331 s (~ 22 mins) to 3656 s (~ 61 mins) (Figure 4).
4 Discussion
By spatially integrating krill swarm data with location and dive data for Adélie penguins, this study advances our understanding of Southern Ocean predator-prey relationships and contributes to a longstanding objective in ecosystem monitoring efforts. Our findings show areas of increased penguin diving effort corresponded with high krill swarm numbers. In contrast, areas of increased mean krill biomass did not influence the spatial distribution of penguin foraging activity. These results indicate that while the spatial distribution of penguin underwater foraging effort could be used as a proxy for krill presence (and/or availability), it may not necessarily indicate krill abundance. We discuss this in the context of Adélie penguin foraging ecology and how this information can be used to inform ecosystem assessment and management.
4.1 Acoustic prey field characteristics
Shipboard acoustic surveys provide a powerful means to record krill abundance and swarming characteristics over vast three-dimensional spatial scales (Cox et al., 2009; Cox et al., 2010). Our swarm-based analyses indicated substantial differences in krill biomass between the 2001 KACTAS and 2003 KAOS survey efforts. KACTAS recorded 8123 t of krill biomass within swarms, and KAOS recorded only 2909 t. While KAOS covered a spatial area that was 21% larger than KACTAS, it recorded only 36% of the biomass observed during KACTAS. Differences in biomass magnitude between survey years were consistent with previous grid-based analyses in Nicol et al. (2008), which characterised KACTAS and KAOS as krill rich and krill poor years, respectively.
For 2001 KACTAS, the biomass distribution was broadly similar to what was in reported in Nicol et al. (2008), occurring mostly over the eastern survey area. However, our analyses indicated the horizontal distribution of 2003 KAOS biomass to be aggregated in relatively small patches over the central and western area of the survey box, whereas previous analyses located biomass largely over the eastern survey area. These qualitative differences between swarm- and grid-based analyses were unexpected. Preliminary comparisons of the two approaches have yielded broadly similar density estimations (Cox, 2017), however comprehensive comparisons of krill distribution and abundance parameters are yet to be performed. Further work is needed to quantitatively assess the comparability in prey-field characteristics generated by swarm- and grid-based acoustic techniques; particularly if swarm-based approaches are likely to be the future of krill biomass mapping.
The swarm-based reanalysis showed that the greatest aggregations of swarm biomass generally occurred near the shelf break. Around the Antarctic coastline, this bathymetric feature is widely reported as an area where high krill biomass predictably occurs (Trathan et al., 2003; Jarvis et al., 2010; Silk et al., 2016; Bestley et al., 2018). These spatial patterns are driven by multiple factors, including krill food abundance and predictability, and oceanographic dynamics (Nicol et al., 2008; Silk et al., 2016). We found swarm biomass was highly patchy, with the majority of biomass concentrated in a small number of swarms (Lascara et al., 1999; Lawson et al., 2008; Klevjer et al., 2010). Hence the prey field in this region is largely comprised of an abundance of small, low-biomass krill aggregations, consistent with observations from other surveys in East Antarctica (Pauly et al., 2000; Jarvis et al., 2010) and elsewhere in the Southern Ocean (Lascara et al., 1999; Lawson et al., 2008).
Comparisons of krill swarm parameters across studies can be challenging due to differences in techniques and reported metrics (Nicol et al., 2008; Klevjer et al., 2010). During krill surveys in 2001 and 2003, swarms were mostly recorded at ~ 40 m depth, but were also observed as deep as the 250 m acoustic detection limit. The mean and range of water column depths occupied by krill swarms were similar to reports in the Scotia Sea and East Antarctic (Tarling et al., 2009; Bestley et al., 2018). Similarly, surface area values were broadly similar to observations from other Antarctic regions (Cox et al., 2009; Cox et al., 2010; Klevjer et al., 2010). However, the internal density of swarms recorded during both surveys in this part of East Antarctica was higher than elsewhere in Antarctica with several krill swarms having an internal density greater than 1000 g m-3, which is three times higher than previous swarm density observations in the region (Bestley et al., 2018). Further research is needed to understand the biophysical factors influencing krill swarm dynamics and aggregation structures in this region of East Antarctica.
4.2 Penguin foraging strategies
Examining the spatial links between prey distribution and abundance and predator foraging effort can provide critical insight into ecosystem-level trophodynamics and response to environmental change associated with harvesting and climate scenarios (Forcada and Trathan, 2009; Lynch et al., 2012). In this region, broad-scale predator foraging parameters (e.g. trip duration and meal mass) have been widely used to understand the role of krill in supporting penguin energetic needs during the breeding season (Clarke, 2001; Clarke et al., 2002; Nicol et al., 2008; Tierney et al., 2009; Emmerson et al., 2015; Southwell et al., 2015). Here, we extend these analyses by quantitatively integrating empirical data on penguin movement and krill abundance and distribution.
Our findings indicate the foraging effort of the Adélie penguin, one of the most abundant seabirds in East Antarctica, increases in areas where there are more krill swarms. All penguin dive metrics (total depth travelled, dive bottom time, number of wiggles and dives performed) were greater in areas corresponding with a higher number of swarms. This suggests chick-rearing penguins preferentially target and forage in areas where krill aggregations are more frequently encountered. This highlights the importance of krill availability (i.e., encounter frequency) to penguin foraging effort, and by association, foraging success. In contrast to our expectations, penguins did not increase their foraging activity in areas with a high mean swarm biomass. Through highlighting the spatial importance of krill swarm number, our results extend the concept of krill availability provided in Emmerson et al. (2015), which was centred on prey abundance and ice-driven access to foraging grounds.
Intercepting many small krill swarms likely represent a reliable prey-field feature which Adélie penguins can exploit. From the perspective of a penguin, a profitable krill prey field in this region appears to be dependent upon a high encounter rate with swarms rather than their specific biomass. We suggest this is likely due to opportunistic diving and prey-capture attempts in areas where krill patches are frequent and closely spaced, which maximises food intake and reduces energy loss associated with commuting between forage opportunities (Santora et al., 2009; Bernard and Steinberg, 2013; Ford et al., 2015). These behavioural results are consistent with Adélie penguins feeding continuously and opportunistically during foraging trips (Ford et al., 2015; Warwick-Evans et al., 2019; Riaz et al., 2021). Larger predators, such as baleen whales, must consume relatively high densities of krill to satisfy their high energetic requirements. For example, humpback whales are conservatively estimated to each consume 390 – 874 kg of krill per day (i.e. a substantial biomass) (Reilly et al., 2004). Adopting an engulfment-feeding strategy and consuming bulk quantities of krill during high velocity lunges (Goldbogen et al., 2013), baleen whales maximise efficiency by selectively targeting large/dense krill aggregations (Santora et al., 2010; Goldbogen et al., 2012; Miller et al., 2019; Harrison et al., 2020). In contrast, penguins forage on individual krill, even when foraging within a swarm (Watanabe et al., 2014), and require a much smaller energy load (total prey kg consumed). Bioenergetics models for breeding Adélie penguins at Béchervaise Island indicate that foraging success is achieved if daily per capita ingested energy is equivalent to 579 - 635 g of krill (Southwell et al., 2015). Our results suggest that penguins at this colony do not necessarily need to selectively target large krill aggregations to satisfy their energy requirements. Frequent prey encounters are likely to be an efficient way to meet the required energy intake. While penguins consume individual prey items at a time, they have been observed foraging together in flocks of varying size (Ainley et al., 2015). Smaller and more frequent krill swarm encounters may be conducive to penguin group foraging strategies. It is also plausible the foraging effort of whales disperses large biomass krill swarms, creating a krill prey-field more favourable to penguins. Further work is needed to assess how predation pressure exerted by penguins and other marine predators alter the physical structure, size and distribution of krill swarms in the region, and how this affects penguin foraging behaviour (Ainley et al., 2015).
Our findings provide valuable insight regarding how Adélie penguins respond to krill swarm distribution, abundance and biomass. These results provide insight for CEMP which assumes that foraging efforts of land-based predators, such as Adélie penguins, respond to krill availability, and can therefore assist in monitoring ecosystem change associated with climate change or harvesting (Nicol et al., 2008). Although our study only examined data from two seasons and may not reflect the full range of Adélie penguin response parameters (Emmerson et al., 2015), our results suggest Adélie penguin foraging effort may not reliably indicate total krill biomass. Instead, we provide evidence that the spatial distribution of foraging effort reflects krill distribution in terms of the number of swarms present. While studies in this area indicate that penguin foraging trip durations, meal mass and breeding success vary in relation to overall krill biomass (Clarke et al., 2003; Nicol et al., 2008; Emmerson et al., 2015; Southwell et al., 2017), our study extends understanding of predator-prey dynamics within the water column as a first step towards a mechanistic understanding of penguin response reflected in different demographic parameters. This is particularly relevant amidst potential changes in prey availability associated with climate change (Bestley et al., 2020; McBride et al., 2021), and renewed interests to expand krill fishing operations in the Mawson region (Kelly et al., 2018; Watters et al., 2020).
4.3 Spatiotemporal predator-prey linkages
When investigating the spatial overlap of marine predators and their prey, selection of appropriate spatiotemporal scales is critical (Fauchald et al., 2000). This can be challenging when trying to integrate predator and prey information that are derived from separate data streams and recorded at different spatial and temporal resolutions. Inferences regarding predator-prey relationship can vary depending on the spatial scales selected to assess these interactions (Rose and Leggett, 1990; Reid et al., 2004; Bailey and Thompson, 2009; Kuhn et al., 2015). Addressing temporal disparities arising from predator-prey data via spatial design should be guided by an informed understanding of how prey dynamics shift over space and time (Hunsicker et al., 2011). In terms of krill distribution, horizontal displacements over time are poorly understood. A key driver of horizontal advection is obviously oceanographic currents (Bestley et al., 2018; McBride et al., 2021; Nocera et al., 2021). However, krill can also swim at high speeds for extended periods of time against local currents (Krafft et al., 2015). Clearly, understanding rates of advection and structural persistence of swarms in the Southern Ocean is a complex and challenging task (Tarling and Thorpe, 2014). Furthermore, it is unknown how krill swarm horizontal-vertical distribution, aggregation and structure may be altered by penguin foraging activity and predation pressure through time (Ainley et al., 2015).
Through our gridded approach to summarising penguin foraging effort and krill swarm information, we assume the large spatial area chosen to bin predator-prey information adequately captures the spatiotemporal krill displacements over the penguin foraging period (Sveegaard et al., 2012; Kuhn et al., 2015; Hinke et al., 2017). We also assume the entire prey field within grid cells are accessible and available to penguins during their foraging efforts, disregarding the swarm depths during surveys. While this enables us to make broad-scale conclusions regarding predator-prey spatial overlap, further research is needed to understand finer-scale spatiotemporal associations (real- or near-time representation). The complementary use of devices capable of recording contemporaneous three-dimensional predator-prey interactions, such as underwater gliders (Ainley et al., 2015; Reiss et al., 2021) or animal-borne echosounders (Goulet et al., 2019) may advance this field.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Australian Antarctic Division Animal Ethics Committee.
Author contributions
SB, MC, LE and JR conceived the study. JR analysed the data and drafted the manuscript. SB, MC, SW and LE provided supervision and assistance during the analyses and results interpretation. All authors contributed to the article and approved the submitted version
Funding
SB was supported under the Australian Research Council Discovery Early Career Research Award (DECRA) project DE180100828. This work is a contribution to Australian Antarctic Science project AAS # 4518 drawing on data from projects #2205 and 2722.
Acknowledgments
We thank the ship-based field teams and engineers during KACTAS and KAOS who assisted with data collection and led by Steve Nicol. Krill swarm analyses for the KACTAS and KAOS data were performed by St Andrews. We thank the penguin monitoring field staff, Knowles Kerry and Judy Clarke for instigating and overseeing the initial years of the penguin monitoring programme. We also thank Julien Freyer for his help synthesising and processing the penguin dive data, Natalie Kelly for her assistance unpacking the reprocessed krill acoustic data, and Colin Southwell and So Kawaguchi for providing their ecological wisdom. We thank the Australian Antarctic Division for logistics and financial support. All animal handling procedures and biologging devices attached to penguins were with approval from the Australian Antarctic Division Animal Ethics Committee to AAS projects # 2205 and 2722.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1060984/full#supplementary-material
References
Agnew D. J. (1997). The CCAMLR ecosystem monitoring programme. Antarctic Sci. 9 (3), 235–242. doi: 10.1017/S095410209700031X
Ainley D. G., Ballard G., Barton K. J., Karl B. J., Rau G. H., Ribic C. A., et al. (2003). Spatial and temporal variation of diet within a presumed metapopulation of Adélie penguins. Condor 105 (1), 95–106. doi: 10.1093/condor/105.1.95
Ainley D. G., Ballard G., Jones R. M., Jongsomjit D., Pierce S. D., Smith W. O. Jr., et al. (2015). Trophic cascades in the western Ross Sea, Antarctica: revisited. Mar. Ecol. Prog. Ser. 534, 1–16. doi: 10.3354/meps11394
Atkinson A., Siegel V., Pakhomov E., Jessopp M., Loeb V. (2009). A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. Part I: Oceanographic Res. Papers 56 (5), 727–740. doi: 10.1016/j.dsr.2008.12.007
Atkinson A., Siegel V., Pakhomov E., Rothery P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432 (7013), 100–103. doi: 10.1038/nature02996
Bailey H., Thompson P. M. (2009). Using marine mammal habitat modelling to identify priority conservation zones within a marine protected area. Mar. Ecol. Prog. Ser. 378, 279–287. doi: 10.3354/meps07887
Barange M. (1994). Acoustic identification, classification and structure of biological patchiness on the edge of the Agulhas Bank and its relation to frontal features. South African Journal of Marine Science. 14 (1), 333–47. doi: 10.2989/025776194784286969
Bedford M., Melbourne-Thomas J., Corney S., Jarvis T., Kelly N., Constable A. (2015). Prey-field use by a Southern Ocean top predator: enhanced understanding using integrated datasets. Mar. Ecol. Prog. Ser. 526, 169–181. doi: 10.3354/meps11203
Bernard K. S., Steinberg D. K. (2013). Krill biomass and aggregation structure in relation to tidal cycle in a penguin foraging region off the Western Antarctic peninsula. ICES J. Mar. Sci. 70 (4), 834–849. doi: 10.1093/icesjms/fst088
Bestley S., Jonsen I., Hindell M., Harcourt R., Gales N. (2015). Taking animal tracking to new depths: synthesizing horizontal–vertical movement relationships for four marine predators. Ecology 96 (2), 417–427. doi: 10.1890/14-0469.1
Bestley S., Raymond B., Gales N. J., Harcourt R. G., Hindell M., Jonsen I. D., et al. (2018). Predicting krill swarm characteristics important for marine predators foraging off East Antarctica. Ecography 41 (6), 996–1012. doi: 10.1111/ecog.03080
Bestley S., Ropert-Coudert Y., Bengtson Nash S., Brooks C. M., Cotté C., Dewar M., et al. (2020). Marine ecosystem assessment for the Southern Ocean: Birds and marine mammals in a changing climate. Front. Ecol. Evol. 8 (338). doi: 10.3389/fevo.2020.566936
Bivand R., Lewin-Koh N. (2016). Maptools: Tools for reading and handling spatial objects, R package version 0.8-39. Available at: https://cran.r-project.org/web/packages/maptools/index.html
Brierley A. S., Cox M. J. (2010). Shapes of krill swarms and fish schools emerge as aggregation members avoid predators and access oxygen. Curr. Biol. 20 (19), 1758–1762. doi: 10.1016/j.cub.2010.08.041
Brooks M. E., Kristensen K., Van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9 (2), 378–400. doi: 10.32614/RJ-2017-066
Cade D. E., Seakamela S. M., Findlay K. P., Fukunaga J., Kahane-Rapport S. R., Warren J. D., et al. (2021). Predator-scale spatial analysis of intra-patch prey distribution reveals the energetic drivers of rorqual whale super-group formation. Funct. Ecol. 35 (4), 894–908. doi: 10.1111/1365-2435.13763
Calise L., Skaret G. (2011). Sensitivity investigation of the SDWBA Antarctic krill target strength model to fatness, material contrasts and orientation. CCAMLR Sci 18, 97–122
Conti S.G., Demer D.A. (2006). Improved parameterization of the SDWBA for estimating krill target strength. ICES Journal of Marine Science 63 (5), 928–935. doi: 10.1016/j.icesjms.2006.02.007
Cimino M. A., Moline M. A., Fraser W. R., Patterson-Fraser D. L., Oliver M. J. (2016). Climate-driven sympatry may not lead to foraging competition between congeneric top-predators. Sci. Rep. 6, 18820. doi: 10.1038/srep18820
Clarke J. (2001). Partitioning of foraging effort in Adélie penguins provisioning chicks at Béchervaise island, Antarctica. Polar Biol. 24 (1), 16–20. doi: 10.1007/s003000000168
Clarke J., Emmerson L., Townsend A., Kerry K. (2003). Demographic characteristics of the Adélie penguin population on Béchervaise island after 12 years of study. CCAMLR Sci. 10, 53–74.
Clarke J., Kerry K., Irvine L., Phillips B. (2002). Chick provisioning and breeding success of Adélie penguins at Béchervaise island over eight successive seasons. Polar Biol. 25 (1), 21–30. doi: 10.1007/s003000100307
Constable A. J., de la Mare W. K., Agnew D. J., Everson I., Miller D. (2000). Managing fisheries to conserve the Antarctic marine ecosystem: Practical implementation of the convention on the conservation of Antarctic marine living resources (CCAMLR). ICES J. Mar. Sci. 57 (3), 778–791. doi: 10.1006/jmsc.2000.0725
Cox M. J., Demer D. A., Warren J. D., Cutter G. R., Brierley A. S. (2009). Multibeam echosounder observations reveal interactions between Antarctic krill and air-breathing predators. Mar. Ecol. Prog. Ser. 378, 199–209. doi: 10.3354/meps07795
Cox M. J., Warren J. D., Demer D. A., Cutter G. R., Brierley A. S. (2010). Three-dimensional observations of swarms of Antarctic krill (Euphausia superba) made using a multi-beam echosounder. Deep Sea Res. Part II: Topical Stud. Oceanography 57 (7), 508–518. doi: 10.1016/j.dsr2.2009.10.003
Croxall J., Reid K., Prince P. (1999). Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 177, 115–131. doi: 10.3354/meps177115
Croxall J. P., Trathan P., Murphy E. (2002). Environmental change and Antarctic seabird populations. Science 297 (5586), 1510–1514. doi: 10.1126/science.1071987
De Robertis A., Higginbottom I. (2007). A post-processing technique to estimate the signal-to-noise ratio and remove echosounder background noise. ICES Journal of Marine Science 64 (6), 1282–1291. doi: 10.1093/icesjms/fsm112
Emmerson L., Southwell C., Clarke J., Tierney M., Kerry K. (2015). Adélie penguin response parameters signal reduced prey accessibility: implications for predator–prey response curves. Mar. Biol. 162 (6), 1187–1200. doi: 10.1007/s00227-015-2661-5
Enstipp M. R., Grémillet D., Jones D. R. (2007). Investigating the functional link between prey abundance and seabird predatory performance. Mar. Ecol. Prog. Ser. 331, 267–279. doi: 10.3354/meps331267
Fauchald P., Erikstad K. E., Skarsfjord H. (2000). Scale-dependent predator–prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology 81 (3), 773–783. doi: 10.1890/0012-9658(2000)081[0773:SDPPIT]2.0.CO;2
Fielding S., Watkins J. L., Trathan P. N., Enderlein P., Waluda C. M., Stowasser G., et al. (2014). Interannual variability in Antarctic krill (Euphausia superba) density at south Georgia, Southern Ocean: 1997–2013. ICES J. Mar. Sci. 71 (9), 2578–2588. doi: 10.1093/icesjms/fsu104
Forcada J., Trathan P. N. (2009). Penguin responses to climate change in the Southern Ocean. Global Change Biol. 15 (7), 1618–1630. doi: 10.1111/j.1365-2486.2009.01909.x
Ford R. G., Ainley D., Lescroël A., Lyver P. O. B., Toniolo V., Ballard G. (2015). Testing assumptions of central place foraging theory: a study of Adélie penguins Pygoscelis adeliae in the Ross Sea. J. Avian Biol. 46 (2), 193–205. doi: 10.1111/jav.00491
Goldbogen J. A., Calambokidis J., Croll D. A., McKenna M. F., Oleson E., Potvin J., et al. (2012). Scaling of lunge-feeding performance in rorqual whales: mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct. Ecol. 26 (1), 216–226. doi: 10.1111/j.1365-2435.2011.01905.x
Goldbogen J. A., Friedlaender A. S., Calambokidis J., McKenna M. F., Simon M., Nowacek D. P. (2013). Integrative approaches to the study of baleen whale diving behavior, feeding performance, and foraging ecology. BioScience 63 (2), 90–100. doi: 10.1525/bio.2013.63.2.5
Gorman K. B., Ruck K. E., Williams T. D., Fraser W. R. (2021). Advancing the Sea ice hypothesis: Trophic interactions among breeding pygoscelis penguins with divergent population trends throughout the Western Antarctic peninsula. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.526092
Goulet P., Guinet C., Swift R., Madsen P. T., Johnson M. (2019). A miniature biomimetic sonar and movement tag to study the biotic environment and predator-prey interactions in aquatic animals. Deep Sea Res. Part I: Oceanographic Res. Papers 148, 1–11. doi: 10.1016/j.dsr.2019.04.007
Grémillet D., Kuntz G., Delbart F., Mellet M., Kato A., Robin J. P., et al. (2004). Linking the foraging performance of a marine predator to local prey abundance. Funct. Ecol. 18 (6), 793–801. doi: 10.1111/j.0269-8463.2004.00914.x
Hamner W. M., Hamner P. P. (2000). Behavior of Antarctic krill (Euphausia superba): schooling, foraging, and antipredatory behavior. Can. J. Fisheries Aquat. Sci. 57, 192–202. doi: 10.1139/f00-195
Harrison L.-M. K., Goetz K., Cox M. J., Harcourt R. (2020). A Southern Ocean archipelago enhances feeding opportunities for a krill predator. Mar. Mammal Sci. 36 (1), 260–275. doi: 10.1111/mms.12645
Hazen E. L., Friedlaender A. S., Thompson M. A., Ware C. R., Weinrich M. T., Halpin P. N., et al. (2009). Fine-scale prey aggregations and foraging ecology of humpback whales Megaptera novaeangliae. Mar. Ecol. Prog. Ser. 395, 75–89. doi: 10.3354/meps08108
Hinke J. T., Cossio A. M., Goebel M. E., Reiss C. S., Trivelpiece W. Z., Watters G. M. (2017). Identifying risk: concurrent overlap of the Antarctic krill fishery with krill-dependent predators in the Scotia Sea. PloS One 12 (1), e0170132. doi: 10.1371/journal.pone.0170132
Hunsicker M. E., Ciannelli L., Bailey K. M., Buckel J. A., Wilson White J., Link J. S., et al. (2011). Functional responses and scaling in predator–prey interactions of marine fishes: contemporary issues and emerging concepts. Ecol. Lett. 14 (12), 1288–1299. doi: 10.1111/j.1461-0248.2011.01696.x
Jarvis T., Kelly N., Kawaguchi S., van Wijk E., Nicol S. (2010). Acoustic characterisation of the broad-scale distribution and abundance of Antarctic krill (Euphausia superba) off East Antarctica (30-80°E) in January-march 2006. Deep Sea Res. Part II: Topical Stud. Oceanography 57 (9), 916–933. doi: 10.1016/j.dsr2.2008.06.013
Jonsen I., Basson M., Bestley S., Bravington M., Patterson T., Pedersen M. W., et al. (2013). State-space models for bio-loggers: A methodological road map. Deep Sea Res. Part II: Topical Stud. Oceanography 88, 34–46. doi: 10.1016/j.dsr2.2012.07.008
Jonsen I. D., Patterson T. A. (2021) foieGras: Fit continuous-time state-space and latent variable models for filtering Argos satellite (and other) telemetry data and estimating movement behaviour. Available at: https://CRAN.R-project.org/package=foieGras.
Jonsen I. D., Patterson T. A., Costa D. P., Doherty P. D., Godley B. J., Grecian W. J., et al. (2020). A continuous-time state-space model for rapid quality control of argos locations from animal-borne tags. Movement Ecol. 8 (1), 1–13. doi: 10.1186/s40462-020-00217-7
Kelly N., Emmerson L., Kawaguchi S., Southwell C., Welsford D. (2018). An ecological risk assessment of current conservation measures for krill fishing in East Antarctica (CCAMLR divisions 58.4.1 and 58.4.2), WG-EMM-18/37). Available online at: https://www.ccamlr.org/en/wg-emm-18/37.
Kerry K., Meyer L., Papps W., Clarke J., Irvine L. (2000). Béchervaise island, MacRobertson land, Antarctica–CCAMLR ecosystem monitoring program (CEMP) monitoring site: description, maps and colony photographs. CCAMLR Science Abstracts No. WG-EMM-00/32, p 9.
Klevjer T., Tarling G., Fielding S. (2010). Swarm characteristics of Antarctic krill euphausia superba relative to the proximity of land during summer in the Scotia Sea. Mar. Ecol. Prog. Ser. 409, 157–170. doi: 10.3354/meps08602
Krafft B. A., Skaret G., Knutsen T. (2015). An Antarctic krill (Euphausia superba) hotspot: population characteristics, abundance and vertical structure explored from a krill fishing vessel. Polar Biol. 38 (10), 1687–1700. doi: 10.1007/s00300-015-1735-7
Kuhn C. E., Sterling J. T., Zeppelin T. K. (2015). Linking northern fur seal behavior with prey distributions: the impact of temporal mismatch between predator studies and prey surveys. Anim. Biotelemetry 3 (1), 1–12. doi: 10.1186/s40317-015-0064-5
Laidre K. L., Heide-Jørgensen M. P., Heagerty P., Cossio A., Bergström B., Simon M. (2010). Spatial associations between large baleen whales and their prey in West Greenland. Mar. Ecol. Prog. Ser. 402, 269–284. doi: 10.3354/meps08423
Lascara C. M., Hofmann E. E., Ross R. M., Quetin L. B. (1999). Seasonal variability in the distribution of Antarctic krill, Euphausia superba, west of the Antarctic peninsula. Deep Sea Res. Part I: Oceanographic Res. Papers 46 (6), 951–984. doi: 10.1016/S0967-0637(98)00099-5
Lawson G. L., Wiebe P. H., Ashjian C. J., Stanton T. K. (2008). Euphausiid distribution along the Western Antarctic peninsula–part b: distribution of euphausiid aggregations and biomass, and associations with environmental features. Deep Sea Res. Part II: Topical Stud. Oceanography 55 (3-4), 432–454. doi: 10.1016/j.dsr2.2007.11.014
Lynch H. J., Naveen R., Trathan P. N., Fagan W. F. (2012). Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic peninsula. Ecology 93 (6), 1367–1377. doi: 10.1890/11-1588.1
Lynnes A., Reid K., Croxall J. (2004). Diet and reproductive success of Adélie and chinstrap penguins: linking response of predators to prey population dynamics. Polar Biol. 27 (9), 544–554. doi: 10.1007/s00300-004-0617-1
MacLennan D.N., Fernandes P.G., Dalen J.. (2002). A consistent approach to definitions and symbols in fisheries acoustics. ICES Journal of Marine Science 59 (2), 365–369. doi: 10.1006/jmsc.2001.1158
Madureira L.S., Everson I., Murphy E.J.. (1993). Interpretation of acoustic data at two frequencies to discriminate between Antarctic krill (Euphausia superba Dana) and other scatterers. Journal of Plankton Research 15 (7), 787–802. doi: 10.1093/plankt/15.7.787
McBride M. M., Stokke O. S., Renner A. H., Krafft B. A., Bergstad O. A., Biuw M., et al. (2021). Antarctic Krill Euphausia superba: spatial distribution, abundance, and management of fisheries in a changing climate. Mar. Ecol. Prog. Ser. 668, 185–214. doi: 10.3354/meps13705
McGehee D., O’Driscoll R.L., Traykovski L.M. (1998). Effects of orientation on acoustic scattering from Antarctic krill at 120 kHz. Deep Sea Research Part II: Topical Studies in Oceanography 45 (7), 1273–1294. doi: 10.1016/S0967-0645(98)00036-8
Miller E. J., Potts J. M., Cox M. J., Miller B. S., Calderan S., Leaper R., et al. (2019). The characteristics of krill swarms in relation to aggregating Antarctic blue whales. Sci. Rep. 9 (1), 16487. doi: 10.1038/s41598-019-52792-4
Nicol S., Brierley A. S. (2010). Through a glass less darkly–new approaches for studying the distribution, abundance and biology of euphausiids. Deep Sea Res. Part II: Topical Stud. Oceanography 57 (7-8), 496–507. doi: 10.1016/j.dsr2.2009.10.002
Nicol S., Clarke J., Romaine S., Kawaguchi S., Williams G., Hosie G. (2008). Krill (Euphausia superba) abundance and Adélie penguin (Pygoscelis adeliae) breeding performance in the waters off the Béchervaise island colony, East Antarctica in 2 years with contrasting ecological conditions. Deep Sea Res. Part II: Topical Stud. Oceanography 55 (3-4), 540–557. doi: 10.1016/j.dsr2.2007.11.013
Nocera A. C., Giménez E. M., Diez M. J., Retana M. V., Winkler G. (2021). Krill diel vertical migration in southern Patagonia. J. Plankton Res. 43 (4), 610–623. doi: 10.1093/plankt/fbab047
Pauly T., Nicol S., Higginbottom I., Hosie G., Kitchener J. (2000). Distribution and abundance of Antarctic krill (Euphausia superba) off East Antarctica (80–150 e) during the austral summer of 1995/1996. Deep Sea Res. Part II: Topical Stud. Oceanography 47 (12-13), 2465–2488. doi: 10.1016/S0967-0645(00)00032-1
Phillips L. R., Carroll G., Jonsen I., Harcourt R., Brierley A. S., Wilkins A., et al. (2022). Variability in prey field structure drives inter-annual differences in prey encounter by a marine predator, the little penguin. R. Soc. Open Sci. 9 (9), 220028. doi: 10.1098/rsos.220028
Pütz K., Rey A. R., Huin N., Schiavini A., Pütz A., Lüthi B. H. (2006). Diving characteristics of southern rockhopper penguins (Eudyptes c. chrysocome) in the southwest Atlantic. Mar. Biol. 149 (2), 125–137. doi: 10.1007/s00227-005-0179-y
Ratcliffe N., Trathan P. (2012). A review of the diet and at-sea distribution of penguins breeding within the CAMLR convention area. CCAMLR Sci. 19, 75–114.
R Core Team (2018). “R: a language and environment for statistical computing,” in R foundation for statistical computing(Vienna).
Reid K., Sims M., White R. W., Gillon K. W. (2004). Spatial distribution of predator/prey interactions in the Scotia Sea: implications for measuring predator/fisheries overlap. Deep Sea Res. Part II: Topical Stud. Oceanography 51 (12-13), 1383–1396. doi: 10.1016/j.dsr2.2004.06.007
Reilly S., Hedley S., Borberg J., Hewitt R., Thiele D., Watkins J., et al. (2004). Biomass and energy transfer to baleen whales in the south Atlantic sector of the Southern Ocean. Deep Sea Res. Part II: Topical Stud. Oceanography 51 (12), 1397–1409. doi: 10.1016/j.dsr2.2004.06.008
Reiss C. S., Cossio A. M., Walsh J., Cutter G. R., Watters G. M. (2021). Glider-based estimates of meso-zooplankton biomass density: a fisheries case study on Antarctic krill (Euphausia superba) around the northern Antarctic peninsula. Front. Mar. Sci. 8, 256. doi: 10.3389/fmars.2021.604043
Riaz J., Bestley S., Wotherspoon S., Emmerson L. (2021). Horizontal-vertical movement relationships: Adélie penguins forage continuously throughout provisioning trips. Movement Ecol. 9 (1), 43. doi: 10.1186/s40462-021-00280-8
Riaz J., Bestley S., Wotherspoon S., Freyer J., Emmerson L. (2020). From trips to bouts to dives: temporal patterns in the diving behaviour of chick-rearing Adélie penguins, East Antarctica. Mar. Ecol. Prog. Ser. 654, 177–194. doi: 10.3354/meps13519
Ribic C. A., Chapman E., Fraser W. R., Lawson G. L., Wiebe P. H. (2008). Top predators in relation to bathymetry, ice and krill during austral winter in Marguerite bay, Antarctica. Deep Sea Res. Part II: Topical Stud. Oceanography 55 (3-4), 485–499. doi: 10.1016/j.dsr2.2007.11.006
Ropert-Coudert Y., Van de Putte A. P., Reisinger R. R., Bornemann H., Charrassin J.-B., Costa D. P., et al. (2020). The retrospective analysis of Antarctic tracking data project. Sci. Data 7 (1), 1–11. doi: 10.1038/s41597-020-0406-x
Rose G. A., Leggett W. C. (1990). The importance of scale to predator-prey spatial correlations: An example of Atlantic fishes. Ecology 71 (1), 33–43. doi: 10.2307/1940245
Santora J. A., Dorman J. G., Sydeman W. J. (2017). Modeling spatiotemporal dynamics of krill aggregations: size, intensity, persistence, and coherence with seabirds. Ecography 40 (11), 1300–1314. doi: 10.1111/ecog.02250
Santora J. A., Reiss C. S. (2011). Geospatial variability of krill and top predators within an Antarctic submarine canyon system. Mar. Biol. 158 (11), 2527–2540. doi: 10.1007/s00227-011-1753-0
Santora J. A., Reiss C. S., Cossio A. M., Veit R. R. (2009). Interannual spatial variability of krill (Euphausia superba) influences seabird foraging behavior near elephant island, Antarctica. Fisheries Oceanography 18 (1), 20–35. doi: 10.1111/j.1365-2419.2008.00490.x
Santora J. A., Reiss C. S., Loeb V. J., Veit R. R. (2010). Spatial association between hotspots of baleen whales and demographic patterns of Antarctic krill Euphausia superba suggests size-dependent predation. Mar. Ecol. Prog. Ser. 405, 255–269. doi: 10.3354/meps08513
Silk J. R., Thorpe S. E., Fielding S., Murphy E. J., Trathan P. N., Watkins J. L., et al. (2016). Environmental correlates of Antarctic krill distribution in the Scotia Sea and southern drake passage. ICES J. Mar. Sci. 73 (9), 2288–2301. doi: 10.1093/icesjms/fsw097
Southwell D., Emmerson L., Forcada J., Southwell C. (2015). A bioenergetics model for estimating prey consumption by an Adélie penguin population in East Antarctica. Mar. Ecol. Prog. Ser. 526, 183–197. doi: 10.3354/meps11182
Southwell C., Emmerson L., Takahashi A., Barbraud C., Delord K., Weimerskirch H. (2017). Large-Scale population assessment informs conservation management for seabirds in Antarctica and the Southern Ocean: A case study of Adélie penguins. Global Ecol. Conserv. 9, 104–115. doi: 10.1016/j.gecco.2016.12.004
Sveegaard S., Nabe-Nielsen J., Stæhr K.-J., Jensen T. F., Mouritsen K. N., Teilmann J. (2012). Spatial interactions between marine predators and their prey: herring abundance as a driver for the distributions of mackerel and harbour porpoise. Mar. Ecol. Prog. Ser. 468, 245–253. doi: 10.3354/meps09959
Tarling G. A., Fielding S. (2016). “Swarming and behaviour in Antarctic krill,” in Biology and ecology of Antarctic krill (Springer), 279–319. doi: 10.1007/978-3-319-29279-3_8
Tarling G. A., Klevjer T., Fielding S., Watkins J., Atkinson A., Murphy E., et al. (2009). Variability and predictability of Antarctic krill swarm structure. Deep Sea Res. Part I: Oceanographic Res. Papers 56 (11), 1994–2012. doi: 10.1016/j.dsr.2009.07.004
Tarling G. A., Thorpe S. E. (2014). Instantaneous movement of krill swarms in the Antarctic circumpolar current. Limnology Oceanography 59 (3), 872–886. doi: 10.4319/lo.2014.59.3.0872
Tierney M., Emmerson L., Hindell M. (2009). Temporal variation in Adélie penguin diet at Béchervaise island, east Antarctica and its relationship to reproductive performance. Mar. Biol. 156 (8), 1633–1645. doi: 10.1007/s00227-009-1199-9
Trathan P., Brierley A., Brandon M., Bone D., Goss C., Grant S., et al. (2003). Oceanographic variability and changes in Antarctic krill (Euphausia superba) abundance at south Georgia. Fisheries oceanography 12 (6), 569–583. doi: 10.1046/j.1365-2419.2003.00268.x
Trathan P. N., Hill S. L. (2016). “The importance of krill predation in the Southern Ocean,” in Biology and ecology of Antarctic krill (Springer), 321–350. doi: 10.1007/978-3-319-29279-3_9
Trathan P., Ratcliffe N., Masden E. (2012). Ecological drivers of change at south Georgia: the krill surplus, or climate variability. Ecography 35 (11), 983–993. doi: 10.1111/j.1600-0587.2012.07330.x
Veit R. R., Silverman E. D., Everson I. (1993). Aggregation patterns of pelagic predators and their principal prey, Antarctic krill, near south Georgia. J. Anim. Ecol. 62 (3), 551–564. doi: 10.2307/5204
Warwick-Evans V., Downie R., Santos M., Trathan P. N. (2019). Habitat preferences of Adélie Pygoscelis adeliae and chinstrap penguins Pygoscelis antarctica during pre-moult in the weddell Sea (Southern Ocean). Polar Biol. 42 (4), 703–714. doi: 10.1007/s00300-019-02465-9
Warwick-Evans V., Fielding S., Reiss C., Watters G., Trathan P. N. (2022). Estimating the average distribution of Antarctic krill Euphausia superba at the northern Antarctic peninsula during austral summer and winter. Polar Biol. 45 (5), 857–871. doi: 10.1007/s00300-022-03039-y
Watanabe Y. Y., Ito M., Takahashi A. (2014). Testing optimal foraging theory in a penguin–krill system. Proc. R. Soc. B: Biol. Sci. 281 (1779), 20132376. doi: 10.1098/rspb.2013.2376
Watkins J. L., Buccholz F., Priddle J., Morris D., Ricketts C. (1992). Variation in reproductive status of Antarctic krill swarms; evidence for a size-related sorting mechanism? Mar. Ecol. Prog. Ser. 82, 163–174. doi: 10.3354/meps082163
Watters G. M., Hinke J. T., Reiss C. S. (2020). Long-term observations from Antarctica demonstrate that mismatched scales of fisheries management and predator-prey interaction lead to erroneous conclusions about precaution. Sci. Rep. 10 (1), 1–9. doi: 10.1038/s41598-020-59223-9
Wei T., Simko V. (2021). R package 'corrplot': Visualization of a correlation matrix; version 0.92. Available at: https://cran.r-project.org/web/packages/corrplot/index.html.
Yamamoto T., Watanuki Y., Hazen E. L., Nishizawa B., Sasaki H., Takahashi A. (2015). Statistical integration of tracking and vessel survey data to incorporate life history differences in habitat models. Ecol. Appl. 25 (8), 2394–2406. doi: 10.1890/15-0142.1
Zimmer I., Wilson R. P., Beaulieu M., Ropert-Coudert Y., Kato A., Ancel A., et al. (2010). Dive efficiency versus depth in foraging emperor penguins. Aquat. Biol. 8 (3), 269–277. doi: 10.3354/ab00213
Keywords: foraging behaviour, Pygoscelis adeliae, Euphausia superba, krill swarm, dive behaviour, predator-prey
Citation: Riaz J, Bestley S, Wotherspoon S, Cox MJ and Emmerson L (2023) Spatial link between Adélie penguin foraging effort and krill swarm abundance and distribution. Front. Mar. Sci. 10:1060984. doi: 10.3389/fmars.2023.1060984
Received: 04 October 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Bernardo Antonio Perez Da Gama, Fluminense Federal University, BrazilReviewed by:
Kristen B. Gorman, University of Alaska Fairbanks, United StatesDavid Ainley, H.T. Harvey & Associates, United States
Copyright © 2023 Riaz, Bestley, Wotherspoon, Cox and Emmerson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javed Riaz, javed.riaz@utas.edu.au
 Javed Riaz
Javed Riaz Sophie Bestley
Sophie Bestley Simon Wotherspoon
Simon Wotherspoon Martin J. Cox2
Martin J. Cox2