Differences and similarities in the phytoplankton communities of two coupled transitional and marine ecosystems (the Lagoon of Venice and the Gulf of Venice - Northern Adriatic Sea)
- National Research Council of Italy, Institute of Marine Sciences (Cnr-Ismar), Venezia, Italy
The main aim of this paper is to paint an ecological picture of the phytoplankton communities of two adjacent and connected ecosystems, one transitional and one coastal marine, in the Northern Adriatic Sea: the Lagoon of Venice (LoV) and the Gulf of Venice (GoV). Based on 10 years (2011-2020) of monthly samplings, we compare the taxonomic composition, abundance and seasonal cycles of the two ecosystems. We focus on the inner zones of the LoV and on the coastal sea up to 8 nmi offshore, an area suitable for assessing the reciprocal influence of the lagoon and sea in terms of phytoplankton. Our main interest is to verify (i) whether the sea still affects the lagoon phytoplankton and (ii) whether the lagoon can provide organisms to the adjacent sea. Using a matrix composed of 466 samples, we performed various types of analysis to: (i) identify the prevalent features and seasonal patterns of abiotic factors and chlorophyll a, (ii) assess and compare taxonomic composition at each station and (iii) identify the generalist and specialist taxa. Our findings provide evidence that the prevalent structure of the communities in the selected areas of the two environments clearly differ concerning (i) seasonal succession, unimodal in the LoV (only one peak in summer) and multi-peak in the GoV (a succession of small peaks from spring to autumn), (ii) abundance and chlorophyll a, both much higher in the LoV (average: 6,009,593 cells l-1 and 4.1 µgl-1 respectively) than in the GoV (average 2,901,266 cells l-1 and 1,5 µgl-1 respectively), (iii) community composition, dominated by diatoms shared with benthic habitats (e.g. Thalassiosira, Nitzschia, Navicula) in the lagoon and by euplanktonic diatoms (e.g. Skeletonema, Chaetoceros, Pseudonitzschia) in the sea. The phytoplankton in the LoV appears to be affected by the marine phytoplankton of the adjacent sea and vice versa: the two environments share taxa that are both generalist (e.g. Skeletonema, Chaetoceros, Cyclotella, Pseudonitzschia) and specialist. Although the dominant factors in structuring the phytoplankton communities are local, dispersal rates, while not intense enough to generate transport of species that could significantly affect assemblage composition, are also at play.
1 Introduction
Recognizing the mechanisms underlying species distribution and community structure across time and space is a central issue of community ecology. Regarding phytoplankton, investigating and understanding these mechanisms is particularly important at the land-sea interfaces, i.e. in coastal areas, which are among the most productive and ecologically and socio-economically important systems on the planet (Vitousek et al., 1997; Harley et al., 2006). In these areas, considering the different habitats as components of a broader landscape or seascape is essential for understanding the range of processes driving community composition. For phytoplankton, these components appear complex and interconnected: the dispersal of planktonic organisms potentially forms a so-called a metacommunity of local communities (Leibold et al., 2004), in which both regional (e.g. dispersal, climate) and local (e.g. habitat heterogeneity, biotic interactions) phenomena are at play. Species coexistence and differentiation could be the multifaceted outcome of local adaptation and passive dispersal, which depends mainly on the rate of dispersal, connectivity and water retention time (de Wit and Bouvier, 2006; Vanormelingen et al., 2008; Declerck et al., 2013; Spatharis et al., 2019).
Lagoons and their adjacent marine waters constitute complex coupled ecosystems, where inputs from the watershed, tide cycles and currents generate spatially and temporally complex hydrological pathways, shaping plankton community structure (Melaku Canu et al., 2012; Newton et al., 2014; Ghezzo et al., 2015; Perez-Ruzafa et al., 2019; Spatharis et al., 2019). Understanding how local and broad-scale processes act and their relative roles has important practical implications for conservation and management (Pandit et al., 2009; Davis et al., 2014; Spatharis et al., 2019), since phytoplankton are included as a water quality indicator in two separate statutes regulating coastal and marine management currently in force in the European Union: the European Water Framework Directive (WFD; European Commission, 2000) and the Marine Strategy Framework Directive (MSFD; European Commission, 2008). Indeed, water quality assessment should consider broader-scale processes when interpreting the effect of local natural and/or anthropic influences, in particular whenever there is an important connection between the studied compartments (Borja et al., 2010; O’Hagan, 2020; Manea et al., 2020; Manea et al., 2021). By evaluating the effects of local conditions and dispersal on community features, it can be determined whether management efforts should concentrate on local conditions (e.g. reducing a certain stressor) or on the connectivity among communities (facilitating or limiting the exchange of species).
The Lagoon of Venice (LoV) and the Gulf of Venice (GoV), located in the Northern Adriatic Sea, are examples of coupled transitional and marine ecosystems. Both belong to LTER-Italy (www.lteritalia.it; Bastianini et al., 2021; Camatti et al., 2021; Capotondi et al., 2021), the national Long-Term Ecological Research (LTER) network. We therefore have consistent data and knowledge, regarding phytoplankton assemblages and related abiotic factors in particular (Bernardi Aubry et al., 2012; Bernardi Aubry et al., 2013; Acri et al., 2020; Bernardi Aubry et al., 2020; Bernardi Aubry et al., 2021), which may provide useful background information for answering specific questions about the mechanisms that shape community structure. The LoV is separated from the GoV by two long barrier islands, water exchange with the sea being enabled by three wide inlets. The lagoon is a highly heterogeneous ecosystem, characterized by a range of habitats with different environmental conditions, due to complex morphology and hydrodynamics (Tagliapietra et al., 2009; Solidoro et al., 2010). Salinity, water renewal and sediment type are the main factors delineating a hierarchical system of homogeneous environments within the lagoon. Its connection with the sea and spatial heterogeneity make the LoV a suitable case study for assessing the relative importance of environmental filtering and dispersal via water flow in the structuring of phytoplankton assemblages, on both spatial and temporal scales. Indeed, in coupled aquatic ecosystems, species sorting and species dispersal due to water flow (i.e. so-called mass effects) often coexist, and their balance determines the spatial pattern of phytoplankton (Rojo et al., 2016; Yang et al., 2018; Spatharis et al., 2019; Pineda et al., 2021). Spatial heterogeneity might be reduced by strong mass effects, whereas species sorting can predominate when the dispersal rate is low, leaving enough time for local communities to become established. In this paper we focus on this second aspect, by considering only the inner areas of the LoV, which are affected by low/moderate dispersal rates, and the sea up to 8 nmi offshore. This whole area can be considered suitable for assessing the limit of the reciprocal influence of the lagoon and the sea in terms of phytoplankton. Our main interest is to verify (a) whether the sea 8 nmi offshore still affects the lagoon phytoplankton and (b) whether the lagoon can provide organisms to the adjacent sea and with what effectiveness and persistence. To this purpose, based on 10 years (2011-2020) of monthly samplings, we compare phytoplankton species composition, diversity, abundance and seasonal cycles in the two ecosystems.
2 Materials and methods
2.1 Study area
The Adriatic Sea is a continental basin within the Mediterranean Sea, located between the Italian and Balkan peninsulas. The Northern Adriatic Sea, defined as the area lying north of a line drawn from Ancona to Zadar, is the shallowest (mean depth about 35 m) basin of the Mediterranean Sea and among the most productive (Salgado-Hernanz et al., 2022). It is characterized by a weak bathymetric gradient along its main axis and by high riverine inputs, mainly due to discharge from the Italian side. Its oceanography presents wide seasonal and inter-annual variability in terms of temperature, salinity and inorganic nutrient concentrations (Totti et al., 2019; Grilli et al., 2020). The GoV (Figure 1), the part of the Northern Adriatic lying to the west of Istria, is characterized by a highly dynamic frontal system, which separates the low-salinity and nutrient-rich coastal riverine waters from the more saline and oligotrophic waters of the southern Adriatic, and its trophic conditions are therefore highly variable on both spatial and temporal scales. The waters entering the lagoon from the Adriatic are prevalently oligo-mesotrophic (Bernardi Aubry and Acri, 2004; Solidoro et al., 2009): the plume of the Po, the biggest Italian river, which causes eutrophic conditions in the Western Adriatic, generally flows southwards, and only slightly affects the GoV and the coastal area close to the LoV.
The LoV (Figure 1) is located in the North-western part of the Adriatic Sea, in a densely inhabited area with an economy characterized by both industry and tourism, which hosts ports, shipyards, marinas, fisheries and aquaculture. It is the largest (550 km2) lagoon in Italy and one of the largest in the Mediterranean Sea. It is microtidal, poly- and euhaline (Ghezzo et al., 2011), classified as a transitional water body (European Commission, 2000). The lagoon has an average depth of 1 m and it is morphologically heterogeneous, with navigable canals (5–10 m deep) and a variety of habitats both aquatic (e.g. salt marshes, shoals, seagrass beds and mud flats) and terrestrial (e.g. islands, coasts).
The lagoon receives freshwater discharges from 12 major tributaries as well as other minor streams and artificially regulated channels, used primarily for agricultural drainage. The yearly average freshwater discharge is about 35 m3 s-1, with seasonal peaks in spring and autumn, and is highest in the northern part of the lagoon (Collavini et al., 2005). The drainage basin, with an area of around 2000 km2 lying mainly in the Veneto Region, is one of the most intensively cultivated areas in Italy and nutrient loads are about 5,000-6,000 t N y-1 and 300t P y-1 (Azzellino et al., 2013). Two long barrier islands separate the LoV from the GoV and allow water exchange through three large inlets (Lido, Malamocco and Chioggia), each corresponding to a specific basin inside the lagoon (north, central and south). Water circulation is driven primarily by the tide, whose average amplitude ranges from 20 cm at neap tide to 100 cm at spring tide. The tidal wave is mainly propagated via the major channels and extends to the shallow areas (Umgiesser et al., 2014). The residence time of LoV waters varies widely, from a few days close to the inlets up to 40 days in the internal areas, depending on the complex interactions of tide, wind, topography and meteorological conditions (Melaku Canu et al., 2012; Umgiesser et al., 2014; Ghezzo et al., 2015).
We considered four stations in total, two located in the LoV (St. SG and St. PR) and two in the sea (St. LI and St. PT). St. SG (San Giuliano, depth 2 m), in the central basin of the lagoon, receives freshwater inputs from a small channel and is influenced mainly by agricultural runoff; St. PR (Palude della Rosa, depth 2.7 m) is in the northern basin of the LoV, in an inland marshy area, a typical lagoon environment. The area in which the two stations lie has a fairly high degree of confinement (Melaku Canu et al., 2012), being moderately connected with the sea and other parts of the lagoon (Ghezzo et al., 2015). St. LI (Lido, depth 7 m) is in the northernmost inlet (Lido) of the LoV; St. PT (Platform, depth 16 m) is 8 nmi offshore, close to the “Acqua Alta” oceanographic platform (http://www.ismar.cnr.it/infrastrutture/piattaforma-acqua-alta/; Ravaioli et al., 2016).
2.2 Sampling strategy and laboratory methods
Monthly samplings were carried out for ten years (2011-2020) near the surface (1 m depth). The stations which are close together (Stations SG, PR and LI) were sampled on the same day, at neap tide mainly in the morning, keeping the duration of the sampling as short as possible, i.e. to a maximum of 4 hours in total. St. PT, which is some distance offshore and can only be reached by means of a suitable vessel, was necessarily sampled on a different day from the other three, although we sought to keep the sampling dates as close as possible. At the shallow stations (SG, PR and LI), where the water column is generally well mixed, with negligible salinity and temperature differences between surface and bottom, the near-surface waters are fairly representative of the water column as a whole (Bernardi Aubry et al., 2013). For St. PT, where the depth is greater and mixing seasonally alternates with stratification, the near-surface waters are representative of the upper part of the water column, which is well-illuminated and always mixed (Pugnetti et al., 2006).
Air temperature and incident irradiance for the whole area were obtained from the Cnr-Ismar (National Research Council of Italy-Institute of Marine Sciences) meteorological archive. At each station the following hydrochemical parameters were measured: transparency, temperature, salinity, dissolved nutrients including ammonia (N-NH3), nitrites (N-NO2), nitrates (N-NO3), orthophosphates (P-PO4), orthosilicates (Si-SiO4) and chlorophyll a (chl a). The methods used to measure the hydrochemical parameters are briefly reported in Table 1 and in Acri et al. (2020). Dissolved inorganic nitrogen (DIN) was obtained as the sum of ammonia, nitrites and nitrates. Analytical quality was assessed with the assistance of the Quality Assurance of Information for Marine Environmental Monitoring in Europe international laboratory proficiency-testing programme (QUASIMEME; http://www.quasimeme.org).
Phytoplankton samples were fixed in formalin neutralized with hexamethylenetetramine (4% final concentration), identified and counted with an invertoscope after settling in 2–25-ml chambers for 12–24 h. Cell counting was carried out at 400x magnification along transects, the number of which varied depending on cell abundance. A minimum of 200 cells was aimed for (but often more than 500 were counted) in each sample (Utermöhl, 1958; Zingone et al., 2010). Over the study period, two different operators analysed the phytoplankton using the same technique, applying accurate intercalibration. Taxa composition was mainly established in accordance with Tomas and Hasle (1997) and Berard-Therriault et al. (1999). All taxa were reviewed and checked against synonyms with reference to the World Register of Marine Species (WoRMS, https://www.marinespecies.org, accessed on 21 June 2022) and the World’s Algae database (Algaebase, http://www.algaebase.org/, accessed on 21 June 2022). The analysis was confined to those forms that were detectable by light microscopy, i.e. up to 3 µm, and thus did not include the picophytoplankton fraction. All the undetermined organisms below 10 µm, which were mostly around 3–4 µm and mainly consisted of cryptophyceans, chrysophyceans, prymnesiophyceans (except coccolithophorids), chlorodendrophyceans and other undetermined forms, were assigned to the nanoflagellate group.
2.3 Data processing and statistical analysis
Statistical analyses were carried out on a matrix composed of 466 samples, after log-transformation of not normally distributed data (Sokal and Rohlf, 1981). We performed various types of analysis in order to: (i) identify the prevalent features and seasonal patterns of abiotic factors and chl a, (ii) assess and compare taxonomic composition and diversity at each station and (iii) identify the generalist and specialist taxa. We adopted a conventional division of the seasons (Bernardi Aubry et al., 2013; Bernardi Aubry et al., 2021) as follows: January–March = winter; April–June = spring; July–September = summer; October–December = autumn.
2.3.1 Seasonal patterns of abiotic factors and chlorophyll a
In order to describe the prevalent annual patterns, we used the 10-year monthly averages of abiotic factors and chl a at each station. For the principal component analysis (PCA, R-mode), we averaged the data, considering the 4 stations and the 4 seasons, obtaining 16 values for each parameter. Finally, the two-way ANOVA test was used to assess the statistical significance of the differences between the groups identified by PCA.
2.3.2 Comparison of taxonomic composition and diversity between stations and seasons
For these analyses we did not take account of the heterogeneous and abundant group of undetermined nanoflagellates, focusing only on those species for which taxonomic determination was available to at least the genus level. First of all, we analysed the whole data set for total taxonomic richness, richness at each station and taxa shared by all stations. We then ranked the taxa in decreasing order of abundance, selecting the most abundant taxa that made up 90% of the total abundance in each station and season. From this list we obtained information about evenness and dominance: the higher the number of taxa, the higher the evenness; the lower the number of taxa, the higher the dominance. Lastly, Principal Coordinate Analysis (PCoA), based on the Bray-Curtis distance, was performed on the averaged abundances for the 4 stations and the 4 seasons using PRIMER software. PCoA shows the relative similarities and differences in the species composition of the phytoplankton communities in time and space as the relative proximity and distance of samples.
2.3.3 Generalists and specialists
The generalists and the lagoon or sea specialists were identified by calculating the Indicator Value (IndVal; Dufrêne and Legendre, 1997), considering only those taxa that could be determined at the species level. The IndVal combines the relative abundance of a taxon with its relative frequency of occurrence at a given station: these two terms are multiplied and then scaled to 100 to express the Indicator Value of a species with respect to the cluster as a percentage. The higher the mean abundance and relative frequency of occurrence of a species at a station, the higher the IndVal. Thus, the taxa that best characterize each station are those with the highest IndVals. We classified taxa that were present in every station with high IndVals but no statistically significant differences between them as “generalist”; those with significantly higher IndVals in either the lagoon or in the sea as “specialist”. Among the specialists, we then highlighted two categories: (i) those that were also present, albeit with low abundances and IndVals, in the other environment and (ii) those found only in one of the two. The significance of the indicator values for each taxon was tested using a Monte Carlo permutation test (999 random permutations).
3 Results
3.1 Abiotic parameters and chlorophyll a
According to the Koppen–Geiger–Pohl Climate Classification (Geiger and Pohl, 1953), the Northern Adriatic Sea area corresponds to the “Cfa type” (i.e. humid subtropical, with hot summers and precipitation distributed throughout the year). Within the Mediterranean context, it is the only example of a Cfa climate experiencing non-negligible tides (Tagliapietra and Volpi Ghirardini, 2006). Incident irradiance and air temperature were obtained from the Cnr-Ismar meteorological archive. Monthly averages for the period 2011-2020, calculated from data recorded every 5’, are reported in Figure 2.
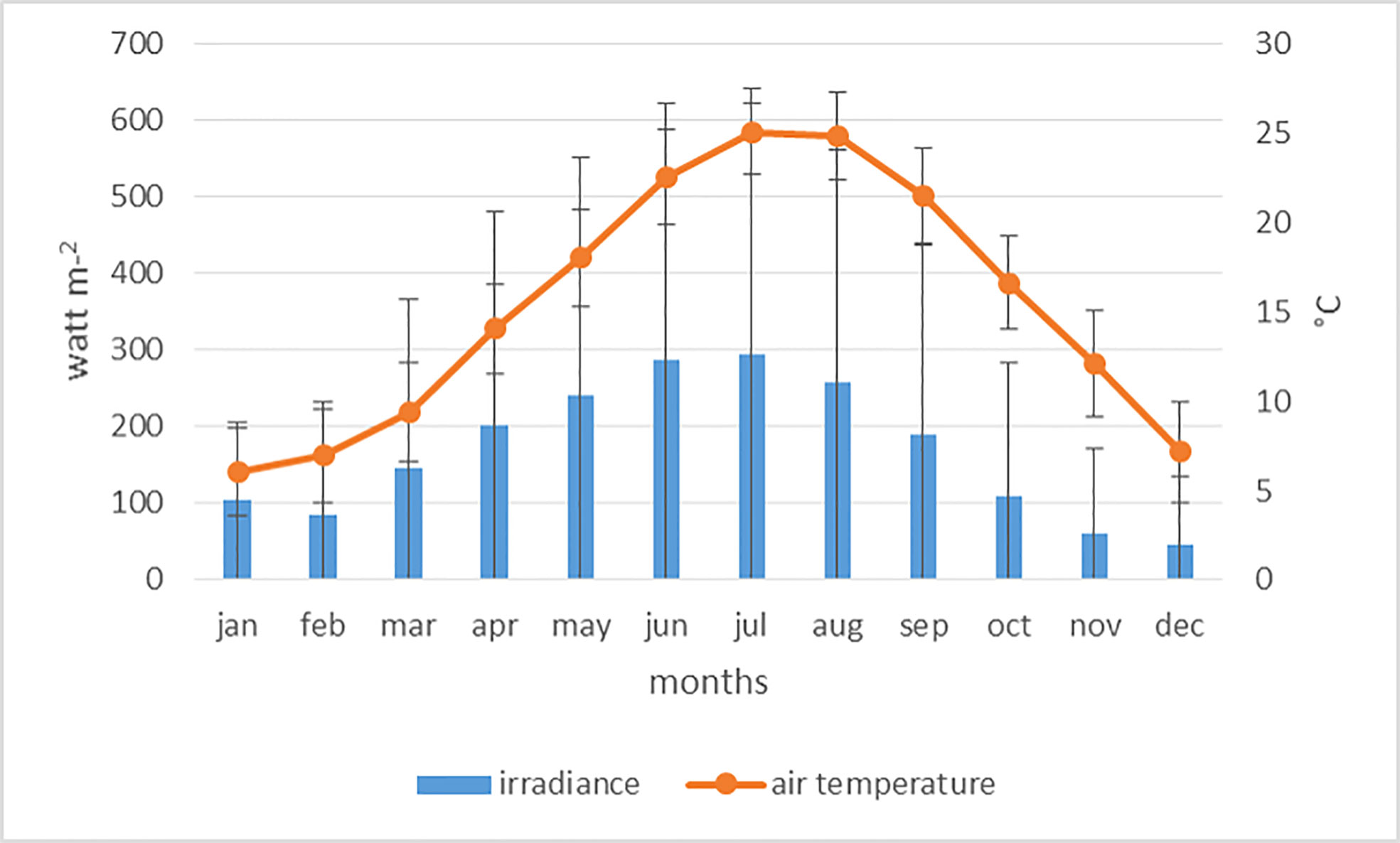
Figure 2 Monthly averages and standard deviations of the 10-year study period (2011-2020) of irradiance (bars) and air temperature (line).
PCA (Figure 3), performed on the samples’ abiotic parameters and chl a, highlighted a clear separation between the LoV and GoV stations (first component) and between the spring/summer and autumn/winter samples (second component). The bi-plot of the first two PCA components accounts for 84.5% of the total variance. The first axis explains most of the variance (60.8%) and it is related on one hand to nutrients and chl a and on the other to salinity and transparency. The second axis, which explains much less of the total variance (23.7%), is mainly related to temperature and is thus seasonal, separating the spring/summer samples from the autumn/winter ones, whatever the station. The average values of the four PCA clusters were compared by means of a two-way ANOVA (Table 2). The differences between the lagoon stations and the marine stations were statistically significant for salinity, nutrients, chl a and transparency; the differences between the seasons were significant for temperature, DIN, Si-SiO4 and chl a. The highest average values for nutrients and chl a and the lowest salinity and transparency were recorded at the stations in the LoV, specifically at St. SG; the lowest values for nutrients and chl a and the highest salinity and transparency were observed in the GoV, specifically at the offshore St. PT. On average, the seasonal water temperature cycle (Figure 4) was quite similar throughout the area, with the highest values seen in July and August. Salinity (Figure 4) did not show a clear seasonal pattern at any station, while nutrients (Figure 4) – taking DIN as representative – saw the lowest values in late spring and summer, from June to August in the LoV and from May to September in the GoV. At both stations in the LoV, the seasonal cycle of chl a (Figure 4) was characterized by a unimodal pattern, with values increasing in spring and maxima seen in summer. In the GoV the pattern was less regular, with peaks observed mainly in spring at St. LI and in late spring and early autumn at St. PT.
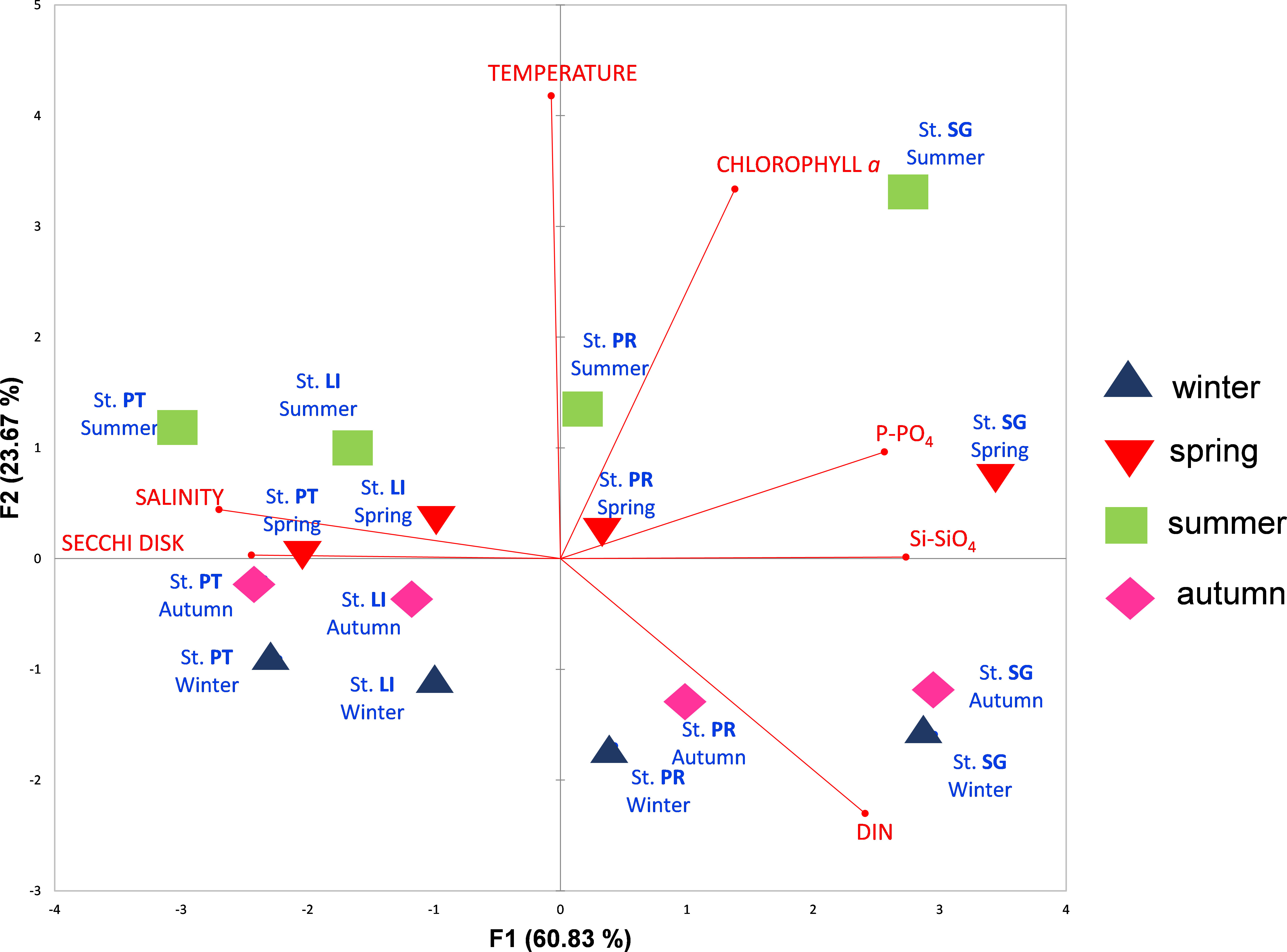
Figure 3 Biplot of samples (seasons and stations) based on PCA of the abiotic parameters and chl a. St. SG: San Giuliano station; St. PR, Palude della Rosa station; ST LI, Lido station; St. PT, Platform station. DIN, Dissolved Inorganic Nitrogen. Winter = January–March; Spring = April–June; Summer = July–September; Autumn = October–December.
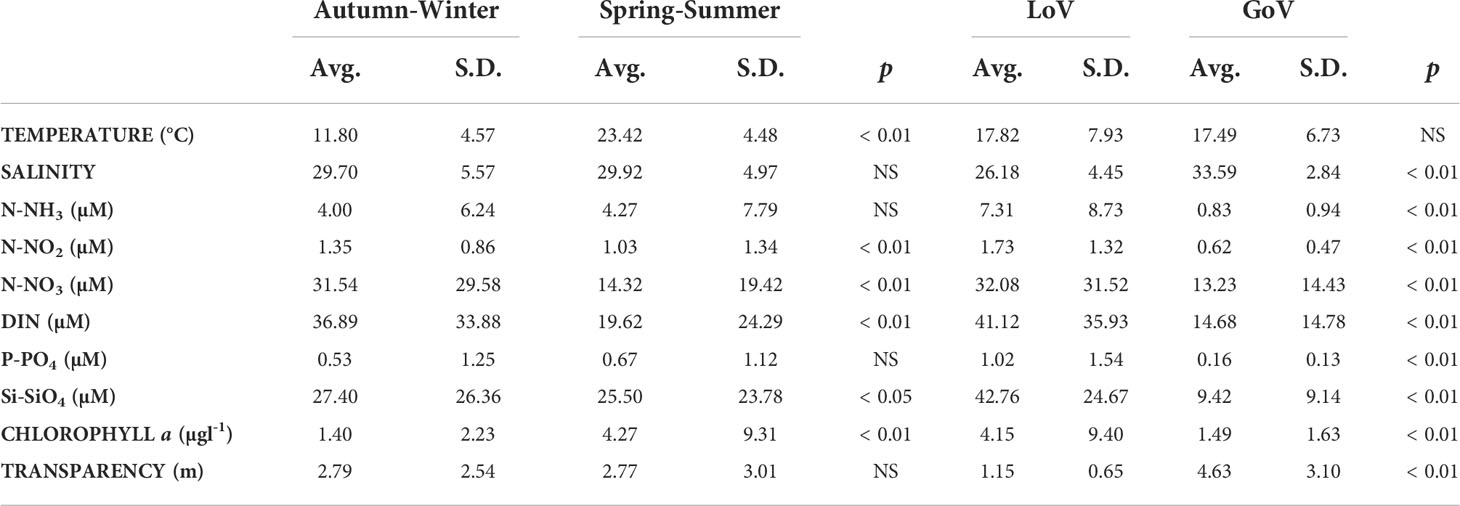
Table 2 Results of the ANOVA performed on the groups (seasons and stations) highlighted by the PCA. Avg, average values; S.D, standard deviations; p, probability; DIN, Dissolved Inorganic Nitrogen.
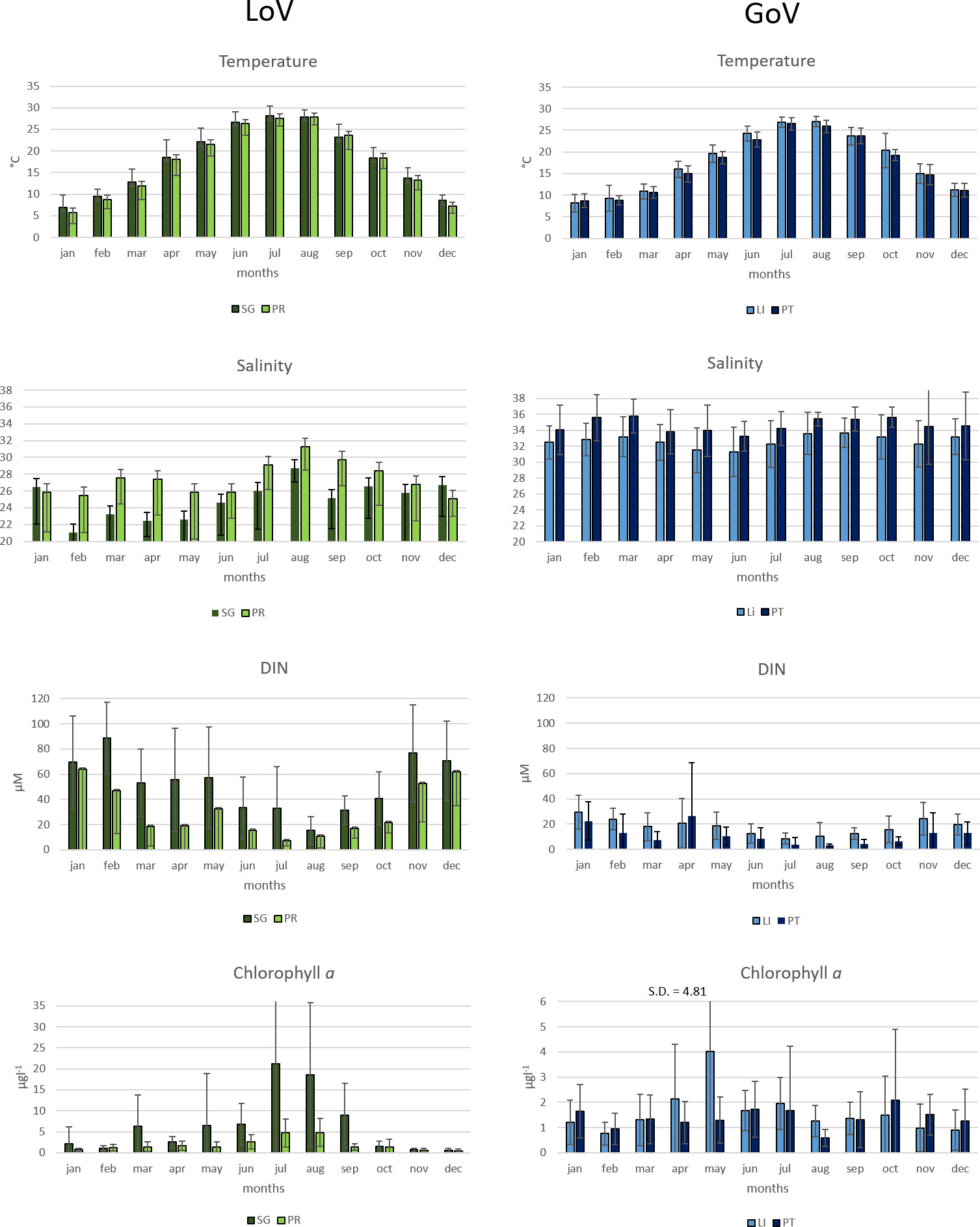
Figure 4 Monthly averages (boxes) and standard deviations (whiskers) of selected abiotic parameters: temperature, salinity, DIN (Dissolved Inorganic Nitrogen) and chlorophyll a at the four stations in the LoV (St. SG and St. PR; left) and GoV (St. LI and St. PT; right). SG, San Giuliano station; PR, Palude della Rosa station; LI, Lido station; PT, Platform station.
3.2 Phytoplankton community composition and diversity
For the whole period and the whole area, we identified 412 distinct taxa belonging to 19 divisions (Table S1): bacillariophyceae (diatoms; 233), dinophyceae (dinoflagellates; 78), chlorophyceae (24), prymnesiophyceae coccolithales (coccolithophorids; 23), trebouxiophyceae (10), pyramimonadophyceae (6), dictyochophyceae (6), cyanophyceae (6), chrysophyceae (5), euglenophyceae (5), raphidophyceae (3), prymnesiophyceae (3), cryptophyceae (2), thecofilosea (2), zignematophyceae (2), xantophyceae (1), chlorodendrophyceae (1), filosa (1), katablepharidophyceae (1). To these, the multitaxa category of undetermined flagellates may be added.
Considering the whole dataset, the highest taxa richness was found in the GoV, at St. PT (253 taxa) and St. LI (247); in the LoV, the number of taxa was higher at St. SG (222) than St. PR (187). At St. PT, the phytoplankton taxa were mainly diatoms (47%), followed by dinoflagellates (26%) coccolithophorids (8%), chlorophyceae (3%) and dictyochophyceae (2%). At St. LI, they were diatoms (58%), dinoflagellates (20%) and coccolithophorids (8%). In the LoV, at St. SG, diatoms prevailed (66%), followed by dinoflagellates (7%) and oligohaline taxa belonging to various classes: chlorophyceae (9%) trebouxiophyceae (4%), cyanophyceae (3%), euglenophyceae (2%) and zignematophyceae (1%). A similar situation was seen at St. PR, where the taxa were mainly diatoms (65%) together with dinoflagellates (13%) and oligohaline forms (chlorophyceae 6%, trebouxiophyceae 2%, cyanophyceae 2% and euglenophyceae 2%) (Figure 5).
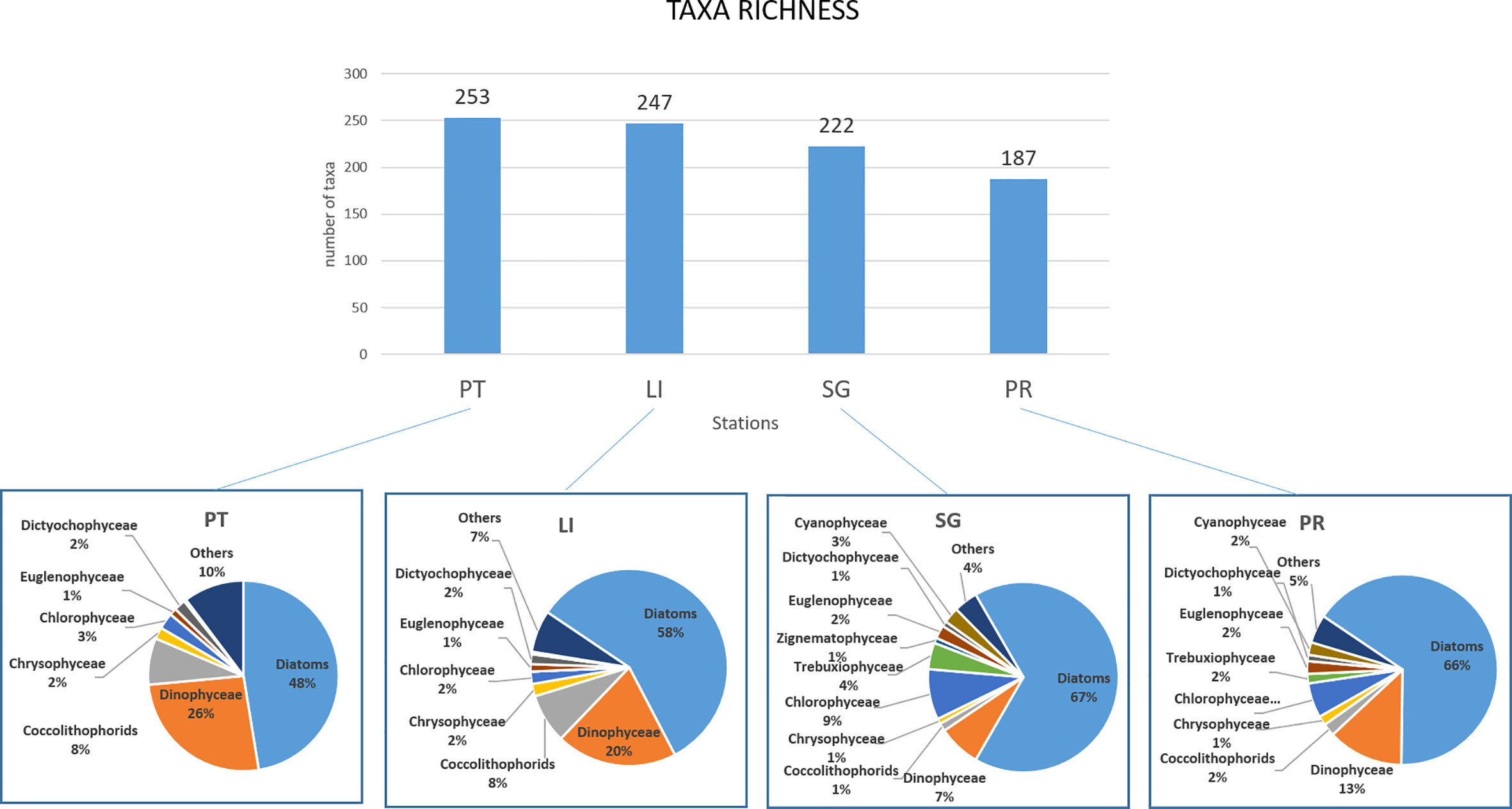
Figure 5 Number of species and mean contribution of the main phytoplankton taxa at each sampling station. St. PT, Platform station; ST LI, Lido station; St. SG, San Giuliano station; St. PR, Palude della Rosa station.
Average seasonal phytoplankton abundance (Figure 6) was lowest in autumn (1,657,201 cells l-1 in the GoV and 1,100,926 cells l-1 in the LoV) and winter (1,648,515 cells l-1 in the GoV and 1,907,868 cells l-1 in the LoV) at every station. It was highest in spring (4,188,436 cells l-1 in the GoV and 6,882,158 cells l-1 in the LoV) and summer (4,003,066 cells l-1 in the GoV and 14,079,056 in the LoV). Phytoplankton was most abundant at St. SG in every season (2,671,588, 10,453,316, 22,000,806 cells l-1 in winter, spring and summer respectively) except autumn, when they were most abundant at St. PT (2,323,826 cells l-1).
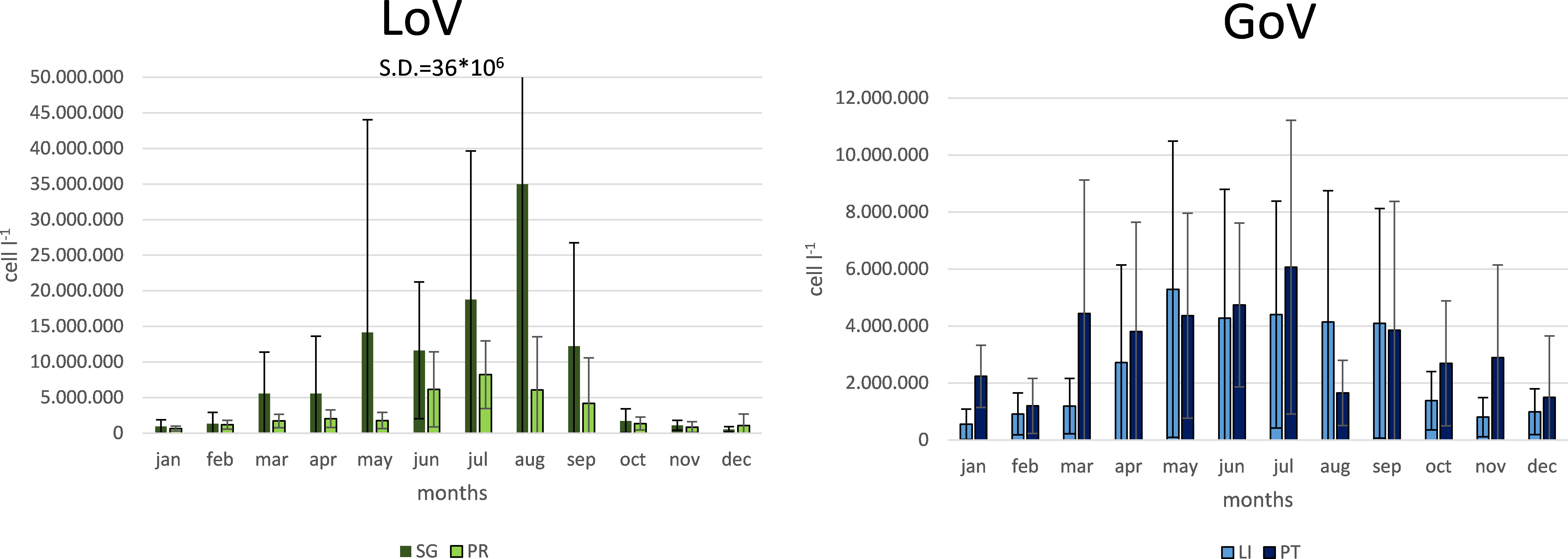
Figure 6 Monthly averages and standard deviations of phytoplankton abundance at the four stations in the LoV (St. SG and St. PR; left) and GoV (St. LI and St. PT; right). SG, San Giuliano station; PR, Palude della Rosa station; LI, Lido station; PT, Platform station.
The number of taxa that made up 90% of the total abundance, considering only those that individually accounted for at least 2% (Table 3), gives an indication of both the dominance (lowest number of taxa) and evenness (highest number of taxa) of the community. These numbers varied from season to season and between stations: the highest evenness was observed at all stations in autumn, the highest dominance at St. SG in all seasons. In the LoV, the lowest numbers of taxa (less than 10) were recorded in spring and summer, which also saw the highest abundances. In contrast, the lowest numbers of taxa in the GoV stations were observed in winter, when abundance was also lowest.

Table 3 Number of phytoplankton taxa that made up 90% of the total abundance and accounted for more than 2% (in italics) in each season and station; St. SG, San Giuliano station; St. PR, Palude della Rosa station; ST LI, Lido station; St. PT, Platform station.
Diatoms dominated the phytoplankton community in every season and station. At St. PT, Skeletonema marinoi, various Chaetoceros (mainly C. socialis and C. radicans) and Leptocylindrus danicus dominated in winter, with the first relative abundance peak of the year occurring at the end of the season, in March. After a slight decrease in April, phytoplankton increased again throughout the spring, with a mixed diatom community made up of Chaetoceros species (C. simplex and C. spp.), together with Cyclotella caspia and Bacteriastrum furcatum. After an early-summer decline, a peak was seen in July, mainly with Chaetoceros (C. socialis, C. radicans, C. simplex, C. curvisetus, C. calcitrans), and again in September, mainly with Pseudonitzschia (P. delicatissima, P. pseudodelicatissima complex), Chaetoceros socialis and Chaetoceros spp. In autumn the abundance declined, reaching the minimum in December when the diatom assemblage was mainly represented by Pseudonitzschia (P. pseudodelicatissima complex, P. delicatissima, P. galaxiae) and Chaetoceros (C. diversus, C. socialis, C. curvisetus, C. throndsenii, C. simplex). Diatoms were associated with the coccolithophorid Emiliania huxleyi and the dinoflagellate Gyrodinium flagellare in winter; with cryptophyceans, chrysophyceans (Ollicola vangoorii) and filosa (Paulinella ovalis) in spring and summer; and with coccolithophorids (Emiliania huxleyi), dinoflagellates (Heterocapsa rotundata), cryptophyceans and filosa (Paulinella ovalis) in autumn.
At St. LI, phytoplankton abundance progressively increased until May, when the yearly maximum was reached. It then remained fairly high throughout the summer, finally reaching the lowest values in autumn and winter. Diatom composition appears to have been similar to what was recorded at St. PT: the genus Chaetoceros was dominant in all seasons, together with Skeletonema marinoi in late winter and Pseudonitzschia in summer and autumn. The diatoms were associated with the coccolithophorid Emiliania huxleyi in autumn and winter; with undetermined cryptophyceans in winter, spring and autumn; with the chrysophyceans Ollicola vangoorii and the filosa Paulinella ovalis in autumn; and with the dinoflagellate Prorocentrum minimum in spring.
Phytoplankton abundance showed a unimodal cycle at St. SG: it increased from winter to summer, reaching the yearly maximum in August, and then decreased from late summer to winter. The winter community was mainly characterized by dictyochophyceans (Pseudopedinella pyriformis), euglenophyceans (Eutreptia globulifera), undetermined cryptophyceans, Skeletonema marinoi and a mixed community of pennate diatoms. Diatoms dominated in spring (Thalassiosira pseudonana, Chaetoceros socialis, Phaeodactylum tricornutum, Nitzschia frustulum, Leptocylindrus minimus, Navicula cryptocephala) and summer (Nitzschia frustulum, Thalassiosira pseudonana, Cylindrotheca closterium, Leptocylindrus minimus, Chaetoceros socialis, Chaetoceros spp.), together with the chlorophycean Coelastrum spp. The August peak was mainly due to Nitzschia frustulum and Thalassiosira pseudonana. Diatoms (Nitzschia frustulum, Skeletonema tropicum, Cocconeis scutellum, Navicula cryptocephala, Cylindrotheca closterium, Pseudonitzschia galaxiae, Thalassiosira pseudonana and undetermined pennates) also characterized the autumn community, together with the cyanobacteria Pseudoanabaena limnetica, the chrysophycean Ollicola vangoorii and undetermined cryptophyceans.
The abundance pattern was also unimodal at St. PR, with the highest values in summer. Autumn and winter were characterized by a mixed community of undetermined cryptophyceans, together with pennate diatoms (Navicula cryptocephala, Halamphora veneta, Cocconeis scutellum). Katablepharidophyceae (Leucocryptos marina), chlorodendrophyceae (Tetraselmis spp.), Chrysophyceans (Ollicola vangoorii), planktonic diatoms (Skeletonema marinoi, Cyclotella caspia, Thalassiosira pseudonana, Chaetoceros spp.) and a mixed community of pennates (Nitzschia frustulum, Navicula spp.) were found in late winter and spring. Diatoms prevailed throughout summer, composed mainly of Thalassiosira pseudonana, Nitzschia frustulum, Pseudonitzschia galaxiae, Chaetoceros socialis, C. calcitrans, Cyclotella caspia, together with the filosa Paulinella ovalis.
The PCoA, which computes the samples considering the taxa in each station and season (Figure 7), basically confirms what was shown by the PCA performed on the abiotic parameters and chl a (Figure 3): a clear separation between the LoV and GoV stations, highlighted by the second axis, which explains the highest proportion of the variance (40.2%), and between the spring/summer and autumn/winter samples along the first axis (15.7% of the variance).
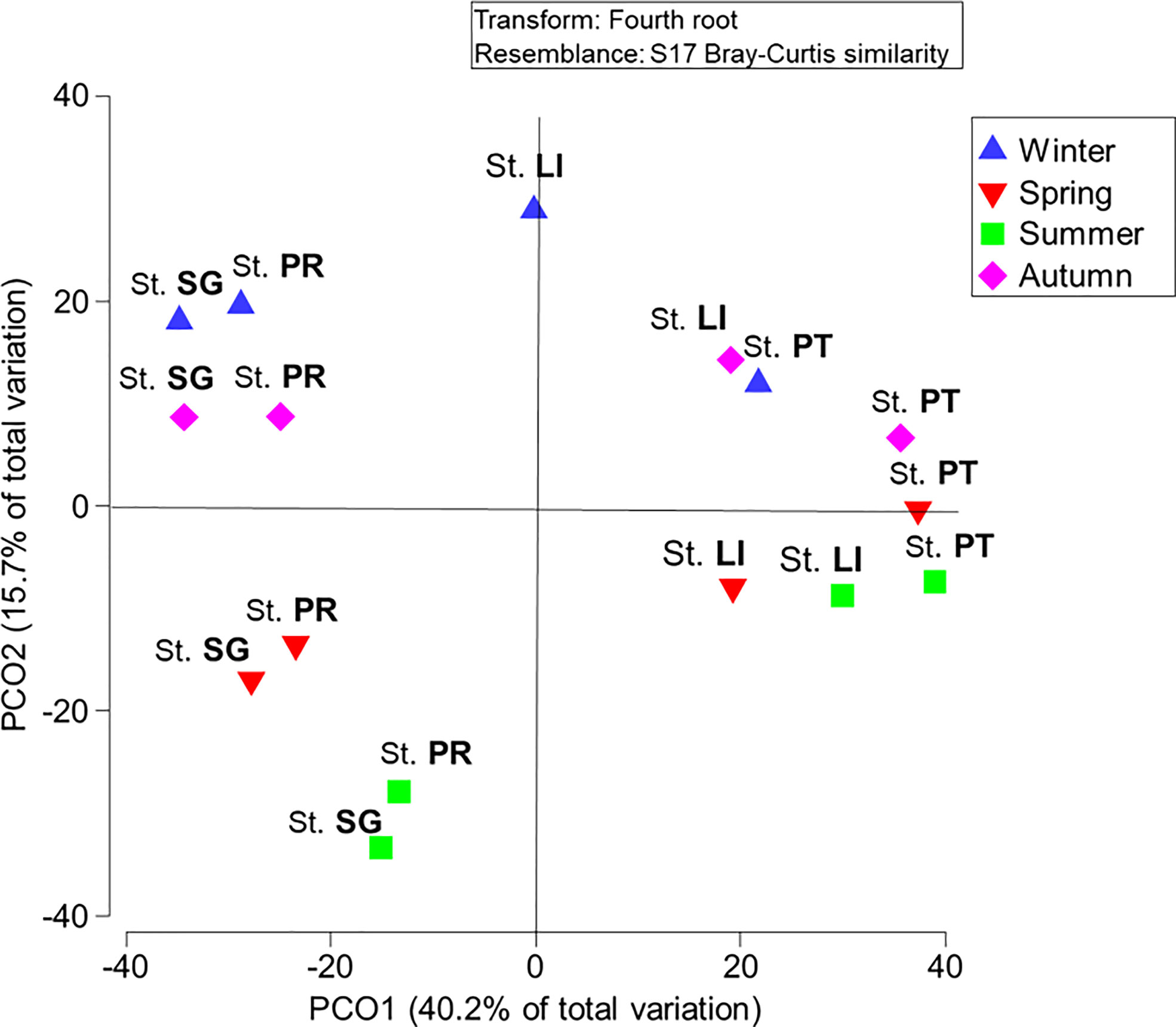
Figure 7 Biplot of samples (seasons and stations) based on PCoA of the phytoplankton taxa. St. SG, San Giuliano station; St. PR, Palude della Rosa station; ST LI, Lido station; St. PT, Platform station.
3.3 Generalists and specialists
About 10% of all taxa (i.e. 43) were shared by all stations; they include: diatoms (67%), dinoflagellates (9%), chrysophyceae, euglenophyceae, dictyochophyceae (5% each), coccolithophorids, katablepharidophyceae, chlorophyceae and filosa (2% each).
Calculation of the IndVal allowed us to assess which of the most abundant species (collectively accounting for > 90%), could be considered generalists or lagoon/sea specialists (Figure 8). Few taxa appear to be generalists, including in winter only Skeletonema marinoi, in spring Cyclotella caspia and two flagellates, the katablepharidophyceae Leucocryptos marina and the chrysophyceans Ollicola vangoorii, in summer two Chaetoceros species (C. socialis and C. calcitrans), and the filosa Paulinella ovalis and in summer and autumn Pseudonitzschia galaxiae and the chrysophycean Ollicola vangoorii.
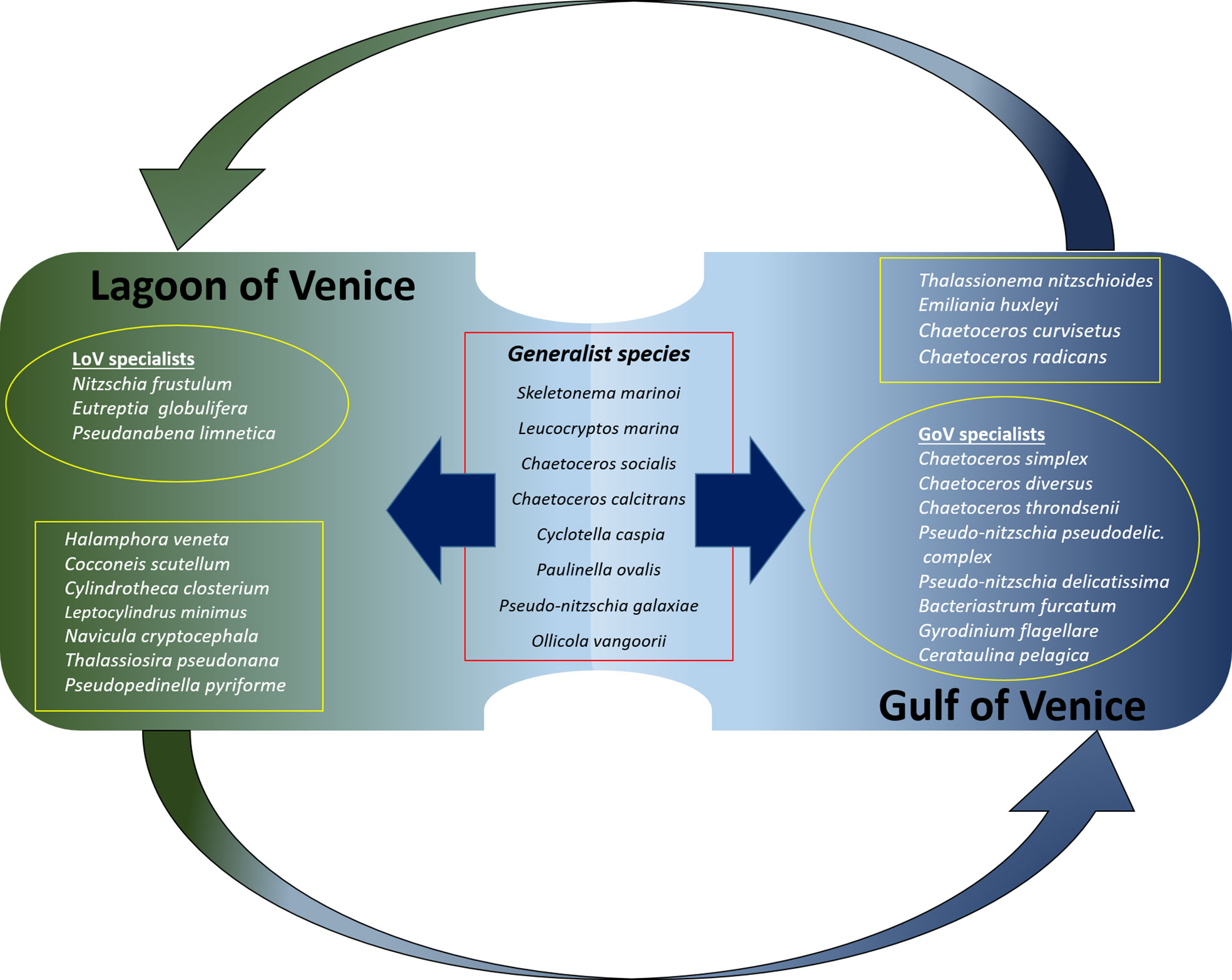
Figure 8 Diagram showing the main generalists (in the centre of the picture) and specialists in the LoV and in the GoV. The specialists framed in the squares are those that are also found, in low abundance and at low frequency, in the other environment; those in the circles are found only in one of the two environments.
There were far more specialists than generalists and they had a higher number of representatives in the sea than in the lagoon. The sea specialists (Figure 8) were mainly diatoms: Chaetoceros radicans in winter, Bacteriastrum furcatum, Cerataulina pelagica, Chaetoceros simplex and Thalassionema nitzschioides in spring, Chaetoceros curvisetus, C. radicans, Pseudonitzschia pseudodelicatissima complex and Cerataulina pelagica in summer and Pseudonitzschia pseudodelicatissima complex, P. delicatissima, Chaetoceros diversus, C. curvisetus and C. throndsenii in autumn. In addition to the diatoms, Emiliania huxleyi is a sea specialist in autumn and winter, as is Gyrodinium flagellare in autumn.
The lagoon specialists were mainly pennate diatoms: Cylindrotheca closterium and Halamphora veneta in winter, Cocconeis scutellum in autumn and winter, Navicula cryptocephala in autumn, winter and spring and Nitzschia frustulum in summer. The specialists also include two flagellates (Eutreptia globulifera and Pseudopedinella pyriforme) in winter, a centric diatom (Thalassiosira pseudonana) in spring and summer and Cyanophyceae (Pseudoanabaena limnetica) in autumn and winter.
Some of these “specialist” taxa were found only in the lagoon (e.g. Eutreptia globulifera, Pseudoanabaena limnetica, Nitzschia frustulum) or the sea (e.g. Chaetoceros diversus, C. simplex, C. throndsenii, Bacteriastrum furcatum, Cerataulina pelagica, Pseudonitzschia pseudodelicatissima complex, P. delicatissima and Gyrodinium flagellare). Others, while significantly more abundant and frequent in either the sea or the lagoon, could also be found, albeit in low quantities and at low frequencies, in the other environment. These include the sea specialists Emiliania huxleyi, Thalassionema nitzschioides, Chaetoceros curvisetus and C. radicans; and the lagoon specialists Navicula cryptocephala, Cylindrotheca closterium, Halamphora veneta, Cocconeis scutellum, Leptocylindrus minimus and Thalassiosira pseudonana.
4 Discussion
4.1 Abiotic parameters and seasonal pattern of chlorophyll a
The LoV and the GoV are adjacent coastal ecosystems, connected through three large inlets: the inputs from the watershed, the tide and the current regimes generate a spatially and temporally complex environment in terms of hydrological connectivity and confinement, which contributes to shape phytoplankton community structure. In this study we analysed phytoplankton taxonomic composition, abundance and seasonal cycles based on a ten-year (2011-2020) data series of monthly samplings. The phytoplankton community of transitional and coastal marine waters is characterized by an intrinsically high level of variability, with a low signal-to-noise ratio due to multiple processes and multi-scale regulation (Cloern and Jassby, 2010). The availability of multiannual ecological data series represents a precious tool with which to determine “baselines” against which to evaluate local or large-scale changes and multiannual trends (Edwards et al., 2010; Karl, 2010). Indeed, we used the ten-year series not to assess interannual variability or trends, but to identify and compare patterns in the lagoon and sea assemblages.
The two areas that we investigated appear to be clearly distinct in terms of abiotic factors, as highlighted by the PCA: nutrient levels are significantly higher in the LoV, while salinity and transparency are lower. The trophic state varies from meso- to eutrophic in the LoV and from oligo- to mesotrophic in the GoV, in accordance with other studies (Bernardi Aubry and Acri, 2004; Solidoro et al., 2009; Bonometto et al., 2022). The seasonal patterns of phytoplankton chl a and abundance are also quite different in the two areas, although the regional meteoclimatic conditions are obviously the same. In both areas, phytoplankton saw the highest chl a and abundance in spring and summer. However, the seasonal pattern of phytoplankton abundance in the LoV is prevalently unimodal, with a summer peak, which can be considered typical of this environment (Bernardi Aubry et al., 2013; Bernardi Aubry et al., 2021) and of temperate shallow and nutrient-enriched enclosed coastal ecosystems in general (Cebrian and Valiela, 1999; Cloern and Jassby, 2008; Winder and Cloern, 2010; Zingone et al., 2010b). Indeed, this feature could be related to the high nutrient concentrations in the LoV, which reduces the seasonal constraint on phytoplankton growth, thus making the seasonal climate-based cycles of temperature and light the main driver of phytoplankton abundance. In the GoV, at the offshore station in particular, the seasonal phytoplankton cycle appears more irregular: after a late-winter/spring growth phase, collapse and growth then alternate from late spring to early autumn, until reaching the late autumn and winter minima. This is fairly common in coastal sites (Cebrian and Valiela, 1999; Cloern and Jassby, 2008; Winder and Cloern, 2010; Zingone et al., 2010b), and in the GoV it appears to be mainly modulated by the alternation of nutrient depletion and sporadic nutrient inputs, typical of this area (Bernardi Aubry et al., 2012).
4.2 Phytoplankton community composition and seasonal succession of prevalent taxa
The LoV and GoV phytoplankton also shows marked differences in terms of composition and the seasonal succession of prevalent taxa. The LoV is mostly characterized by diatoms, especially pennates and cryptophyceans. Also important, albeit sporadic, is the presence of a few freshwater species, such as the chlorophycean Coelastrum, the chlorodendrohycean Tetraselmis and the cyanophycean Pseudoanabaena limnetica, which are signs of riverine influence. Most of the diatom taxa found in the LoV belong to the benthic habitat, having been resuspended in the water column from sediments. Close benthic-pelagic coupling is a well-known and typical feature of the LoV (Facca et al., 2002; Facca and Sfriso, 2007; Bernardi Aubry et al., 2013; Bernardi Aubry et al., 2017; Bernardi Aubry et al., 2021) and other shallow transitional ecosystems (Brito et al., 2012; Rubino and Belmonte, 2021), where the resuspension of microphytobenthos represents an essential element of phytoplankton dynamics. The constant interactions between water column and sediment blur the boundary between the two habitats, so that the microalgal populations recorded in the water column do not differ significantly from those found in the upper sediment layer (Facca et al., 2002). Many taxa that we found in the water column are also cited in the literature as common in the sediments, including Halamphora veneta, Cocconeis scutellum and various species belonging to the genera Navicula and Nitzschia. The two diatom species responsible for the summer peaks, Nitzschia frustulum and Thalassiosira pseudonana, are also observed in benthic habitats, and they can apparently thrive in both the sediment and the water column (Facca et al., 2002; Facca and Sfriso, 2007). Specifically, Thalassiosira is the only centric diatom genus that is also frequently found as a common (and sometimes dominant) benthic inhabitant in the LoV (Facca and Sfriso, 2007). Another typical component of the LoV phytoplankton is undetermined nanoflagellates, which represent the most abundant or second most abundant group in the LoV throughout the seasonal cycle. Nanoflagellates, which are impossible to identify with light microscopy in routine long-term phytoplankton monitoring, are reported as being important components of other transitional ecosystems too (Glibert et al., 2010; Bec et al., 2011; Durante et al., 2013; Pachés et al., 2014; Leruste et al., 2016). Recent studies of LoV protists based on metabarcoding (Armeli Minicante et al., 2019; Armeli Minicante et al., 2020) showed that nanoflagellates are represented by several distinct taxa, some of which (e.g. Picochlorum, Micromonas) were recorded for the first time in the LoV in that study.
Undetermined nanoflagellates were also observed to be a significant component of the GoV phytoplankton, and this is also reported in other parts of the Northern Adriatic Sea, such as the area of the GoV further offshore (Bernardi Aubry et al., 2012), the sea opposite Ancona (Neri et al., 2022) and the Gulf of Trieste (Cabrini et al., 2012; Mozetic et al., 2012), where they may represent the most abundant group throughout the year. In our study, planktonic diatoms constituted the main taxa across the seasons in the GoV. The classic diatom-dinoflagellate succession, considered typical of many temperate marine systems (Margalef, 1978), was not actually observed in this study, which in contrast recorded the prevalence of diatoms throughout the year. This feature may however be considered typical of the whole of the Northern Adriatic Sea, where dinoflagellates rarely exceed 2% of total annual abundance (Bernardi Aubry et al., 2012; Cabrini et al., 2012; Bosak et al., 2016; Neri et al., 2022), and of other coastal marine environments (Alves-De-Souza et al., 2008; Carstensen et al., 2015). In this study, it could also be an effect of the sampling depth, limited to near-surface waters and therefore representative mainly of the upper mixed layer, where the light and turbulence conditions are more favourable to diatoms (Dell’Aquila et al., 2017). During our 10-year study, the most representative GoV taxa were Chaetoceros from spring to summer and Pseudonitzschia from summer to autumn. Chaetoceros is one of the largest and most diverse diatom genera (Hasle and Syvertsen, 1997; Rines and Theriot, 2003). It is considered a typical inhabitant of the Northern Adriatic Sea, both in the coastal area (Bernardi Aubry et al., 2012; Bosak et al., 2016) and offshore (Neri et al., 2022). Pseudonitzschia was present mainly as P. delicatissima, a species that has been reported as typical of late winter (Bernardi Aubry and Acri, 2004; Totti et al., 2019; Giulietti et al., 2021a) and autumn (Neri et al., 2022) in the Northern Adriatic. It should be emphasized that the genus Pseudonitzschia actually includes several cryptic species, which might exhibit seasonal differences in behaviour even in geographically close areas (Turk Dermastia et al., 2020; Giulietti et al., 2021b). In our study, another characteristic component of the GoV was the diatom Skeletonema marinoi. This species has marked seasonal behaviour, extensively observed in the area, being a prevalent member of the assemblage, whose growing season starts in late winter. It can be found frequently from February to April, as a regular and blooming component of the northern Adriatic late winter-early spring assemblages (Degobbis et al., 2000; Bernardi Aubry et al., 2012; Pfannkuchen et al., 2018; Neri et al., 2022).
4.3 Generalists and specialists
S. marinoi is also among the generalist taxa that we have been able to identify in this study: in late winter and spring it was recorded in our samples at all four stations. The LoV and GoV share 10% of the total taxa recorded in the study period. However, if we consider only those accounting for more than 2% of the total, the number of shared species is quite low, limited to a few flagellates and diatoms (Figure 8). Besides S. marinoi, we should mention Chaetoceros socialis, which in late spring and summer can be found at all four stations, accounting for 9% to 35% of total abundances.
On the whole, the most abundant taxa are prevalently specialists of one of the two environments. However, it is important to point out that some of them can be found, in low quantities and at low frequencies, at all stations (Figure 8). This suggests that dispersal from the sea to the lagoon (and the other way round) does in fact occur in these areas, notwithstanding the observed marked differences in the phytoplankton assemblages. Few studies, whether experimental (Bianchi et al., 2000; Bernardi Aubry and Acri, 2004) or based on a modelling approach (Melaku Canu et al., 2012; Ghezzo et al., 2015), have addressed phytoplankton exchanges at the inlets and the degree of connectivity with the sea in the inner areas of the LoV. The exchange of taxa through the inlets, at flood and ebb tide, appears substantial and seasonally variable. Bernardi Aubry and Acri (2004) measured higher phytoplankton abundances at flood than at ebb tide, when they were mainly characterized by neritic species (e.g. Chaetoceros decipiens, Cerataulina pelagica, Hemiaulus hauckii, Asterionellopsis glacialis, Pseudonitzschia delicatissima, Emiliania huxleyi). It was only in late summer to early autumn that the reverse occurred, with higher abundances at ebb tide and dominance of species associated with the LoV (Cocconeis scutellum, Navicula cryptocephala, Navicula spp., Nitzschia frustulum). The study by Bianchi et al. (2000) of the effect of tide on the inner lagoon areas, including St. PR, highlighted inputs from the sea that were linked to the tidal cycle and were particularly marked in spring and summer, although variability in the area appeared mainly driven by the annual meteoclimatic cycle. The model-based assessment (Melaku Canu et al., 2012) of water flows through the inlets of the LoV and the consequent degree of confinement (i.e. the extent to which a sector of a lagoon is subject to the renewing influence of marine waters) simulated various scenarios. The estimates of confinement varied substantially from month to month in the individual LoV subareas, with complex changes in space and time, depending on several interconnected factors, mainly meteorological conditions, river inputs and exchanges at the lagoon inlets. The LoV subareas where the present study was carried out were classified as having a high degree of confinement, which could reach 30 days but was around 15 days on average. Assessment of the connectivity of the LoV with the adjacent sea, by means of a Lagrangian particle-tracking model coupled with a hydrodynamic model by Ghezzo et al. (2015), allowed us to classify the subareas where stations SG and PR are located as moderately connected to the sea, and to show that they mainly act as particle sinks. The assessment also showed that the sea appears to receive “particles” from all the lagoon subareas with almost identical probability, while it gives “particles” with the lowest intensity to the northern part of the LoV.
The overall picture emerging from our study shows environmental heterogeneity as the main driver of the phytoplankton’s marked spatial differentiation, due to the moderate dispersal rates, which enhance the confinement of the inner areas of the lagoon. Our results are in agreement with a previous study (Armeli Minicante et al., 2019), which considered the whole protistan community in accordance with a metabarcoding approach. Although based on only 4 sampling dates (one for each season), specific features of the lagoon and sea communities were highlighted, indicating that environmental heterogeneity was sufficient for ecological segregation.
5 Concluding remarks
In this paper we sought to analyse a specific aspect of the complex interconnections that affect the phytoplankton community in coupled marine aquatic ecosystems, such as the GoV and LoV, specifically the limit of the reciprocal influence of the lagoon and the sea in terms of phytoplankton composition and seasonal pattern. Our findings provide evidence that the prevalent pattern and structure of the communities in the selected areas of the two environments clearly differ concerning:
(i) the seasonal succession, unimodal in the LoV and characterized by a series of peaks in the GoV,
(ii) phytoplankton abundance and chl a, both much higher in the LoV than in the GoV,
(iii) the composition of the community, dominated by diatoms shared with the benthic habitat in the lagoon and by euplanktonic diatoms in the sea.
However, the phytoplankton in the inner areas of the LoV still appear to be affected by the marine phytoplankton of the adjacent sea and vice versa: the two environments share both generalist and specialist species. Although local factors dominate in structuring the two phytoplankton communities, dispersal rates, while not intense enough to generate transport of species that could significantly affect assemblage composition, are still at play.
Data availability statement
The datasets presented in this article are not readily available because the data are at the ISMAR-CNR repositories and could be made available by the Authors upon request. Requests to access the datasets should be directed to Fabrizio Bernardi Aubry, fabrizio.bernardi@ismar.cnr.it.
Author contributions
FBA, FA and MB designed the experiment. FBA, FA, MB and SF performed the samplings and all the laboratory activities. FBA and AP conceptualized and proposed the idea and drafted the manuscript. All authors contributed to the processing and interpretation of the results, to the discussion, to the revision and the finalization of the manuscript.
Funding
The research activities were carried out in the framework of the Italian Long-Term Ecological Research network LTER-Italy, with no specific dedicated funding.
Acknowledgments
The LoV and the GoV are included in the Italian (LTER-Italy), European (LTER-Europe) and International (LTER-International) Long-Term Ecological Research (LTER) networks: the time series analysed in this paper was gathered in the context of these networks. The authors wish to thank F. Bianchi, E. Camatti, M. Pansera and L. Dametto for their technical support during samplings and fieldwork, and the crew of the M/B “Litus” M. Penzo, D. Penzo and G. Zennaro. We thank Christian Ferrarin and Michol Ghezzo for their helpful comments during the drafting of the manuscript. Dedicated to Giorgio Socal, beloved colleague and friend, who recently passed away.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.974967/full#supplementary-material
References
Acri F., Braga F., Bernardi Aubry F. (2020). Long-term dynamics in nutrients, chlorophyll a and water quality parameters in the lagoon of Venice. Sci. Mar. 84, 215–225. doi: 10.3989/scimar.05022.30A
Alves-De-Souza C., Gonzalez M. T., Iriarte J. L. (2008). Functional groups in marine phytoplankton assemblages dominated by diatoms in fjords of southern Chile. J. Plankton Res. 30 (11), 1233–1243. doi: 10.1093/plankt/fbn079
Armeli Minicante S., Piredda R., Finotto S., Bernardi Aubry F., Acri F., Pugnetti A., et al. (2020). Spatial diversity of planktonic protists in the lagoon of Venice (LTER-Italy) based on 18S rDNA. adv. Oceanogr. Limnol. 11, 8961. doi: 10.4081/aiol.2020.8961
Armeli Minicante S., Piredda R., Quero G. M., Finotto S., Bernardi Aubry F., Bastianini M., et al. (2019). Habitat heterogeneity and connectivity: Effects on the planktonic protist community structure at two adjacent coastal sites (the lagoon and the gulf of Venice, northern Adriatic Sea, Italy) revealed by metabarcoding. Front. Microbiol. 10, 2736. doi: 10.3389/fmicb.2019.02736
Azzellino A., Carpani M., Çevirgen S., Giupponi C., Parati P., Ragusa F., et al. (2013). Managing the nutrient loads of the Venice lagoon watershed: are the loads external to the watershed relevant under the WFD river basin district framework? J. Coast. Res. 65, 25–30. doi: 10.2112/SI65-005.1
Bastianini M., Bernardi Aubry F., Acri F., Camatti E., Pansera M., Finotto S., et al. (2021). “Golfo di venezia,” in La rete italiana per la ricerca ecologica di lungo termine. lo studio della biodiversità e dei cambiamenti. Eds. a cura di Capotondi L., Ravaioli M., Acosta A., Chiarini F., Lami A., Stanisci A., Tarozzi L., Mazzocchi M. G. (CNR-Edizioni, Roma), 403–406.
Bec B., Collos Y., Souchu P., Vaquer A., Lautier J., Fiandrino A., et al. (2011). Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquat. Microb. Ecol. 63, 29–45. doi: 10.3354/ame01480
Berard-Therriault L., Poulin M., Bossé L. (1999). Guide D’Identification du phytoplancton Marin de L’Estuaire et du golfe du saint-Laurent (Ottawa, ON, Canada: NRC Research Press), 387.
Bernardi Aubry F., Acri F. (2004). Phytoplankton seasonality and exchange at the inlets of the lagoon of Venice (July 2001-June 2002). J. Mar. Syst. 51, 65–76. doi: 10.1016/j.jmarsys.2004.05.008
Bernardi Aubry F., Acri F., Bianchi F., Pugnetti A. (2013). Looking for patterns in the phytoplankton community of the Mediterranean microtidal Venice lagoon: Evidence from ten years of observations. Sci. Mar. 77, 47–60. doi: 10.3989/scimar.03638.21A
Bernardi Aubry F., Acri F., Finotto S., Pugnetti A. (2021). Phytoplankton dynamics and water quality in the Venice lagoon. Water 13, 2780. doi: 10.3390/w13192780
Bernardi Aubry F., Acri F., Scarpa G. M., Braga F. (2020). Phytoplankton–macrophyte interaction in the lagoon of Venice (Northern Adriatic Sea, Italy). Water 12, 2810. doi: 10.3390/w12102810
Bernardi Aubry F., Cossarini G., Acri F., Bastianini M., Bianchi F., Camatti E., et al. (2012). Plankton communities in the northern Adriatic Sea: Patterns and changes over the last 30 years. Estuar. Coast. Shelf. Sci. 115, 125–137. doi: 10.1016/j.ecss.2012.03.011
Bernardi Aubry F., Pugnetti A., Roselli L., Stanca E., Acri F., Finotto F., et al. (2017). Phytoplankton morphological traits in a nutrient-enriched, turbulent Mediterranean microtidal lagoon. J. Plankton Res. 39 (3), 564–576. doi: 10.1093/plankt/fbx008
Bianchi F., Acri F., Alberighi L., Bastianini M., Boldrin A., Cavalloni B., et al. (2000). “Biological variability in the Venice lagoon,” in The Venice lagoon ecosystem. inputs and interactions between land and Sea. Eds. Lasserre P., Marzollo A. (UNESCO and Parthenon Publishing Press: Paris), 97–126.
Bonometto A., Ponis E., Cacciatore F., Riccardi E., Pigozzi S., Parati P., et al. (2022). A new multi-index method for the eutrophication assessment in transitional waters: Large-scale implementation in Italian lagoons. Environments 9, 41. doi: 10.3390/environments9040041
Borja A., Elliott M., Carstensen J., Heiskanen A. S., van de Bund W. (2010). Marine management–towards an integrated implementation of the European marine strategy framework and the water framework directives. Mar. pollut. Bull. 60 (12), 2175–2186. doi: 10.1016/j.marpolbul.2010.09.026
Bosak S., Godrijan J., Silovic T. (2016). Dynamics of the marine planktonic diatom family chaetocerotaceae in a Mediterranean coastal zone. Est. Coast. Shelf Sci. 180, 69–81. doi: 10.1016/j.ecss.2016.06.026
Brito A. C., Fernandes T. F., Newton A., Facca C., Tett P. (2012). Does microphytobenthos resuspension influence phytoplankton in shallow systems? a comparison through a Fourier series analysis. Est. Coast. Shelf Sci. 110, 77–84. doi: 10.1016/j.ecss.2012.03.028
Cabrini M., Fornasaro D., Cossarini G., Lipizer M., Virgilio D. (2012). Phytoplankton temporal changes in a coastal northern Adriatic site during the last 25 years. Est. Coast. Shelf Sci. 115, 113–124. doi: 10.1016/j.ecss.2012.07.007
Camatti E., Acri F., Anelli Monti M., Bernardi Aubry F., Bon M., Buosi A., et al. (2021). IT-16M Laguna di venezia. in: La rete italiana per la ricerca ecologica di lungo termine. lo studio della biodiversità e dei cambiamenti. Eds. a cura di Capotondi L., Ravaioli M., Acosta A., Chiarini F., Lami A., Stanisci A., Tarozzi L., Mazzocchi M. G. (Roma: CNR-Edizioni), 531–553.
Capotondi L., Ravaioli M., Acosta A., Chiarini F., Lami A., Stanisci A., et al. (2021). La rete italiana per la ricerca ecologica di lungo termine (Roma: Lo studio della biodiversità e dei cambiamenti CNR-Edizioni).
Carstensen J., Klais R., Cloern J. E. (2015). Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Est. Coast. Shelf Sci. 162, 98–109. doi: 10.1016/j.ecss.2015.05.005
Cebrian C., Valiela I. (1999). Seasonal patterns in phytoplankton biomass in coastal ecosystems. J. Plankton Res. 21 (3), 429–444. doi: 10.1093/plankt/21.3.429
Cloern J. E., Jassby A. D. (2008). Complex seasonal patterns of primary producers at the land–sea interface. Ecol. Lett. 11, 1294–1303. doi: 10.1111/j.1461-0248.2008.01244.x
Cloern J. E., Jassby A. D. (2010). Patterns and scales of phytoplankton variability in estuarine-coastal ecosystems. Estuaries Coasts 33, 230–241. doi: 10.1007/s12237-009-9195-3
Collavini F., Bettiol C., Zaggia L., Zonta R. (2005). Pollutant loads from the drainage basin to the Venice lagoon (Italy). Environ. Inter. 31, 939–947. doi: 10.1016/j.envint.2005.05.003
Davis B., Baker R., Sheaves M. (2014). Seascape and metacommunity processes regulate fish assemblage structure in coastal wetlands. Mar. Ecol. Prog. Ser. 500, 187–202. doi: 10.3354/meps10680
Declerck S. A. J., Winter C., Shurin J. B., Curtis A Suttle C. A., Matthews B. (2013). Effects of patch connectivity and heterogeneity on metacommunity structure of planktonic bacteria and viruses. ISME J. 7, 533–542. doi: 10.1038/ismej.2012.138
Degobbis D., Precali R., Ivancic I., Smodlaka N., Fuks D., Kveder S. (2000). Long-term changes in the northern Adriatic ecosystem related to anthropogenic eutrophication. Intern. J. Environ. Poll. 13, 495–533. doi: 10.1504/IJEP.2000.002332
Dell’Aquila G., Ferrante M. I., Gherardi M., Cosentino Lagomarsino M., Ribera d’Alcalà M., Iudicone D., et al. (2017). Nutrient consumption and chain tuning in diatoms exposed to storm-like turbulence. Sci. Rep. 7, 1828. doi: 10.1038/s41598-017-02084-6
de Wit R., Bouvier T. (2006). ‘Everything is everywhere, but, the environment selects’; what did Baas becking and Beijerinck really say? Environ. Microbiol. 8, 755–758. doi: 10.1111j.1462-2920.2006.01017.x
Dufrêne M., Legendre P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. doi: 10.2307/2963459
Durante G., Stanca E., Roselli L., Basset A. (2013). Phytoplankton composition in six northern Scotland lagoons (Orkney islands). Trans. Wat. Bull. 7, 159–174. doi: 10.1285/i1825229Xv7n2p159
Edwards M., Beaugrand G., Hays G. C., Koslow J. A., Richards A. J. (2010). Multi-decadal oceanic ecological datasets and their application in marine policy and management. Trends Ecol. Evol. 25, 602–610. doi: 10.1016/j.tree.2010.07.007
European Commission (2000). Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy (Water framework directive). Off. J. Eur. Union. 43, 1–74
European Commission (2008). Directive 2008/56/EC of the European parliament of the council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine strategy framework directive). Off. J. Eur. Union. 51, 1–45
Facca C., Sfriso A. (2007). Epipelic diatom spatial and temporal distribution and relationship with the main environmental parameters in coastal waters. Est. Coast. Shelf Sci. 75, 35–49. doi: 10.1016/j.ecss.2007.03.033
Facca C., Sfriso A., Socal G. (2002). Changes in abundance and composition of phytoplankton and microphytobenthos due to increased sediment fluxes in the Venice lagoon, Italy. Est. Coast. Shelf Sci. 54, 773–792. doi: 10.1006/ecss.2001.0848
Geiger R., Pohl W. (1953). Revision of koppen-Geiger climate maps of the earth (Darmstadt, Germany: Justus Perthes).
Ghezzo M., De Pascalis F., Umgiesser G., Zemlys P., Sigovini M., Marcos C., et al. (2015). Connectivity in three European coastal lagoons. Estuar. Coasts 38, 1764–1781. doi: 10.1007/s12237-014-9908-0
Ghezzo M., Sarretta A., Sigovini M., Guerzoni S., Tagliapietra D., Umgiesser. G. (2011). Modeling the inter-annual variability of salinity in the lagoon of Venice in relation to the water framework directive typologies. Ocean Cost. Manage. 54 (9), 706–719. doi: 10.1016/j.ocecoaman.2011.06.007
Giulietti S., Romagnoli T., Campanelli A., Totti C., Accoroni S. (2021a). Ecology and seasonality of Pseudo-nitzschia species (Bacillariophyceae) in the northwestern Adriatic Sea over a 30-year period, (1988-2020). Mediterr. Mar. Sci. 22 (3), 505–520. doi: 10.12681/mms.26021
Giulietti S., Romagnoli T., Siracusa M., Bacchiocchi S., Totti C., Accoroni S. (2021b). Integrative taxonomy of the Pseudo-nitzschia (Bacillariophyceae) populations in the NW Adriatic Sea, with a focus on a novel cryptic species in the P. delicatissima species complex. Phycologia 60 (3), 247–264. doi: 10.1080/00318884.2021.1899733
Glibert P. M., Boyer J. N., Heil C., Madden C. J., Sturgis B., Wazniak C. S. (2010). “Blooms in lagoons: Different from those of river dominated estuaries,” in Coastal lagoons: Critical habitats of environmental change, vol. pp . Eds. Kennish M., Paerl H. (Boca Raton, FL, USA: CRC Press), 91–114.
Grasshoff K., Ehrhardt M., Kremling K. (1983). Methods of seawater analysis. 2nd ed Vol. p (Weinheim, Germany: Wiley, Verlag Chemie), 419.
Grilli F., Accoroni S., Acri F., Bernardi Aubry F., Bergami C., Cabrini M., et al. (2020). Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings using the EMODnet chemistry data portal. Water 12, 2280. doi: 10.3390/w12082280
Harley C. D. G., Hughes A. R., Hultgren K. M., Miner B. J., Sorte C. J. B., Thornber C. S., et al. (2006). The impacts of climate change in coastal marine systems: Climate change in coastal marine systems. Ecol. Lett. 9, 228–241. doi: 10.1111/j.1461-0248.2005.00871.x
Hasle G. R., Syvertsen E. E. (1997). “Marine diatoms,” in Identifying marine phytoplankton. Ed. Tomas C. R. (San Diego: Academic Press), 5–385.
Holm-Hansen O., Lorenzen C. J., Holmes R. W., Strickland J. D. H. (1965). Fluorometric determination of chlorophyll. ICES J. Mar. Sci. 30, 3–15. doi: 10.1093/icesjms/30.1.3
Karl D. M. (2010). Oceanic ecosystem time-series programs: Ten lessons learned. Oceanography 23 (3), 104–125. doi: 10.5670/oceanog.2010.27
Leibold M. A., Holyoak M., Mouquet N., Amarasekare P., Chase J. M., Hoopes M. F., et al. (2004). The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. doi: 10.1111/j.1461-0248.2004.00608.x
Leruste A., Malet N., Munaros D., Deroles V., Hatey E., Collos Y., et al. (2016). First steps of ecological restoration in Mediterranean lagoons: Shifts in phytoplankton communities. Estuar. Coast. Shelf Sci. 180, 190–2013. doi: 10.1016/j.ecss.2016.06.029
Manea E., Bergami C., Bongiorni L., Capotondi L., De Maio E., Oggioni A., et al. (2021). A transnational marine ecological observatory in the Adriatic Sea to harmonize a fragmented approach to monitoring and conservation. Adv. Oceanogr. Limnol. 12 (1), 9811. doi: 10.4081/aiol.2021.9811
Manea E., Bongiorni L., Bergami C., Pugnetti A. (2020). “Challenges for marine ecological observatories to promote effective GMS of natura 2000 network. the case study of ECOAdS in the Adriatic Sea,” in Governing future challenges in protected areas, vol. p . Eds. Alfaré L., Ruoss E. (Roma: CNR Edizioni), 23–39.
Margalef R. (1978). Life-forms of phytoplankton as survival alterna¬tives in an unstable environment. Oceanol. Acta 1, 493–509.
Melaku Canu D., Solidoro C., Umgiesser G., Cucco A., Ferrarin C. (2012). Assessing confinement in coastal lagoons. Mar. Poll. Bull. 64, 2391–23298. doi: 10.1016/j.marpolbul.2012.08.007
Mozetic P., Francé J., Kogovsek T., Talaber I., Malej A. (2012). Plankton trends and community changes in a coastal sea (northern adriatic): bottom-up vs. top-down control in relation to environmental drivers. Estuar. Coast. Shelf Sci. 115, 138–148. doi: 10.1016/j.ecss.2012.02.009
Neri F., Romagnoli T., Accoronia S., Campanelli A., Marini M., Grillic F., et al. (2022). Phytoplankton and environmental drivers at a long-term offshore station in the northern Adriatic Sea, (1988–2018). Cont. Shelf Res. 242, 104746. doi: 10.1016/j.csr.2022.104746
Newton A., Icely J., Cristina S., Brito A., Cardoso A. C., Colijn F., et al. (2014). An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Est. Coast. Shelf Sci. 140, 95–122. doi: 10.1016/j.ecss.2013.05.023
O’Hagan A. M. (2020). Ecosystem-based management (EBM) and ecosystem services in EU law, policy and governance. in ecosystem-based management, ecosystem services and aquatic biodiversity (Cham: Springer), 353–372.
Pachés M., Romero I., Martínez-Guijarro R., Martí C. M., Ferrer J. (2014). Changes in phytoplankton composition in a Mediterranean coastal lagoon in the cullera estany (Comunitat valenciana, Spain). Water Environ. J. 28, 135–144. doi: 10.1111/wej.12020
Pandit S. N., Kolasa J., Cottenie K. (2009). Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90 (8), 2253–2262. doi: 10.1890/08-0851.1
Perez-Ruzafa A., De Pascalis F., Ghezzo M., Quispe-Becerra J. I., Hernandez-García R., Munoz I., et al. (2019). Connectivity between coastal lagoons and sea: Asymmetrical effects on assemblages' and populations' structure. Est. Coast. Shelf Sci. 216, 171–186. doi: 10.1016/j.ecss.2018.02.031
Pfannkuchen D. M., Godrijan J., Smodlaka Tanković M., Baričević A., Kužat Djakovac N.T., Pustijanac E., et al. (2018). The ecology of one cosmopolitan, one newly introduced and one occasionally advected species from the genus Skeletonema in a highly structured ecosystem, the northern Adriatic. Microb. Ecol. 75, 674–687. doi: 10.1007/s00248-017-1069-9
Pineda A., Bortolini J. C., Rodrigues L. C. (2021). Effects of space and environment on phytoplankton distribution in subtropical reservoirs depend on functional features of the species. Aquat. Sci. 84, 5. doi: 10.1007/s00027-021-00837-0
Pugnetti A., Camatti E., Mangoni O., Morabito G., Oggioni A., Saggiomo V. (2006). Phytoplankton production in Italian freshwater and marine ecosystems: State of the art and perspectives. Chem. Ecol. 22: (sup1), S49–S69. doi: 10.1080/02757540600557330
Ravaioli M., Bergami C., Riminucci F., Langone L., Cardin V., Di Sarra A., et al. (2016). The RITMARE Italian fixed-point observatory network (IFON) for marine environmental monitoring: a case study. J. Oper. Oceanogr. 9, 202–214. doi: 10.1080/1755876X.2015.1114806
Rines J. E. B., Theriot E. C. (2003). Systematics of chaetocerotaceae (Bacillariophyceae). i. a phylogenetic analysis of the family. Phycol. Res. 51, 83e98. doi: 10.1046/j.1440-1835.2003.00297.x
Rojo C., Mesquita-Joanes F., Monrós J. S., Armengol J., Sasa M., Bonilla F., et al. (2016). Hydrology affects environmental and spatial structuring of microalgal metacommunities in tropical pacific coast wetlands. PloS One 11 (2), e0149505. doi: 10.1371/journal.pone.0149505
Rubino F., Belmonte G. (2021). Habitat shift for plankton: The living side of benthic-pelagic coupling in the mar piccolo of taranto (Southern Italy, Ionian Sea). Water 13, 3619. doi: 10.3390/w13243619
Salgado-Hernanz P. M., Regaudie-de-Gioux A., Antoine D., Basterretxeal G. (2022). Pelagic primary production in the coastal Mediterranean Sea: variability, trends, and contribution to basin-scale budgets. Biogeosciences 19, 47–69. doi: 10.5194/bg-19-47-2022
Sokal R., Rohlf J. (1981). Biometry: The principles and practice of statistics in biological research. 2nd ed. Ed. Freeman W. H. (San Francisco, CA, USA), 859.
Solidoro C., Bandelj V., Bernardi Aubry F., Camatti E., Ciavatta S., Cossarini G., et al. (2010). “Response of Venice lagoon ecosystem to natural and anthropogenic pressures over the last 50 years,” in Coastal lagoons: critical habitats of environmental change. Eds. Kennish M. J., Paerl H. W. (Boca Raton, FL: CRC Press), 483–511.
Solidoro C., Bastianini M., Bandelj V., Codermatz R., Cossarini G., Melaku Canu D., et al. (2009). Current state, scales of variability and decadal trends of biogeochemical properties in the northern Adriatic Sea. J. Geophys. Res. 114, C06026. doi: 10.1029/2008JC004838
Spatharis S., Lamprinou V., Meziti A., Kormas K. A., Danielidis D. D., Smeti E., et al. (2019). Everything is not everywhere: can marine compartments shape phytoplankton assemblages? Proc. R. Soc B 286, 20191890. doi: 10.1098/rspb.2019.1890
Tagliapietra D., Volpi Ghirardini A. (2006). Notes on coastal lagoon typology in the light of the EU water framework directive: Italy as a case study. aquatic conservation. Mar. Freshw. Ecosyst. 16, 457–467. doi: 10.1002/aqc.768
Tagliapietra D., Zanon V., Frangipane G., Umgiesser G., Sigovini M. (2009). “Physiographic zoning of the Venetian lagoon,” in Scientific research and safeguarding of Venice, vol. VII - 2007-2010 . Ed. Campostrini P., (CORILA: Venezia) 161–164.
Totti C., Romagnoli T., Accoroni S., Coluccelli A., Pellegrini M., Campanelli A., et al. (2019). Phytoplankton communities in the northwestern Adriatic Sea: Interdecadal variability over a 30-years period, (1988–2016) and relationships with meteoclimatic drivers. J. Mar. Syst. 193, 137–153. doi: 10.1016/j.jmarsys.2019.01.007
Turk Dermastia T., Cerino F., Stankovic D., France J., Ramsak A., Znidaric Tusek M., et al. (2020). Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo- nitzschia H. Peragallo in the northern Adriatic Sea. Harmful Algae 93, 101773. doi: 10.1016/j.hal.2020.101773
Umgiesser G., Ferrarin C., Cucco A., De Pascalis F., Bellafiore D., Ghezzo M., et al. (2014). Comparative hydrodynamics of 10 Mediterranean lagoons by means of numerical modeling. J. Geophys. Res. Oceans 119, 2212–2226. doi: 10.1002/2013JC009512
Utermöhl H. (1958). Zur vervollkomnung der quantitativen phytoplankton- methodik. Mitt. Int. Ver. Limnol. 9, 1–38.
Vanormelingen P., Cottenie K., Michels E., Muylaert K., Vyverman W. I. M., De Meester L. U. C. (2008). The relative importance of dispersal and local processes in structuring phytoplankton communities in a set of highly interconnected ponds. Freshw. Biol. 53, 2170–2183. doi: 10.1111/j.1365-2427.2008.02040.x
Vitousek P. M., Mooney H. A., Lubchenco J., Melillo J. M. (1997). Human domination of earth’s ecosystems. Science 277, 494–499. doi: 10.1126/science.277.5325.494
Winder M., Cloern J. E. (2010). The annual cycles of phytoplankton biomass. Phil. Trans. R. Soc B. 365, 3215–3226. doi: 10.1098/rstb.2010.0125
Yang Y., Niu H., Xiao L., Lin Q., Han B. P., Naselli-Flores L. (2018). Spatial heterogeneity of spring phytoplankton in a large tropical reservoir: could mass effect homogenize the heterogeneity by species sorting? Hydrobiologia 819, 109–122. doi: 10.1007/s10750-018-3651-7
Zingone A., Phlips E. J., Harrison P. A. (2010b). Multiscale variability of twenty-two coastal phytoplankton time series: A global scale comparison. Estuaries Coasts 33 (2), 224–229. doi: 10.1007/s12237-009-9261-x
Zingone A., Totti C., Sarno D., Cabrini M., Caroppo C., Giacobbe M. G., et al. (2010a). “Fitoplancton: metodiche di analisi quali-quantitativa,” in Metodologie di studio del plancton marino. Eds. Socal G., Buttino I., Cabrini M., Mangoni O., Penna A., Totti C. (SIBM Roma: Manuali e Linee Guida), 213–237.
Keywords: Lagoon of Venice, Gulf of Venice, LTER-Italy, phytoplankton assemblages, connectivity, confinement, habitat heterogeneity
Citation: Bernardi Aubry F, Acri F, Bastianini M, Finotto S and Pugnetti A (2022) Differences and similarities in the phytoplankton communities of two coupled transitional and marine ecosystems (the Lagoon of Venice and the Gulf of Venice - Northern Adriatic Sea). Front. Mar. Sci. 9:974967. doi: 10.3389/fmars.2022.974967
Received: 21 June 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Yunyan Deng, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Mostafa Mohamed El-Sheekh, Tanta University, EgyptRomina Kraus, Rudjer Boskovic Institute, Croatia
Chaofeng Wang, Institute of Oceanology Chinese Academy of Sciences, China
Copyright © 2022 Bernardi Aubry, Acri, Bastianini, Finotto and Pugnetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabrizio Bernardi Aubry, fabrizio.bernardi@ismar.cnr.it
 Fabrizio Bernardi Aubry
Fabrizio Bernardi Aubry Francesco Acri
Francesco Acri  Mauro Bastianini
Mauro Bastianini Alessandra Pugnetti
Alessandra Pugnetti
