Social Behaviour of Humpback Whales (Megaptera novaeangliae) in Hervey Bay, Eastern Australia, a Preferential Female Stopover During the Southern Migration
- 1The Oceania Project, Hervey Bay, QLD, Australia
- 2Marine Ecology Research Centre, School of Environment, Science and Engineering, Southern Cross University, Lismore, NSW, Australia
- 3StatPlan Consulting Pty., Ltd., Woodburn, NSW, Australia
- 4Department of Psychology, Department of Biology, University of Hawai‘i at Hilo, Hilo, HI, United States
- 5The Dolphin Institute, Hilo, HI, United States
- 6Seastar Scientific Inc., Vashon Island, WA, United States
Agonistic competitive social behaviour in humpback whales [Megaptera novaeangliae (Borowski, 1781)] has been extensively studied and reported in previous research. However, non-agonistic social behaviour in humpback whale pods has not been systematically studied. We investigated the social behaviour of 3,949 humpback whale pods over a period of 14 years during August, September, and October in Hervey Bay (Queensland, eastern Australia), a preferential female stopover early in the southern migration. Modelling and analyses of the data examined the factors influencing the occurrence and timing of non-agonistic social behaviour pods, agonistic competitive pods and newly associated pods. Non-agonistic social behaviour was observed more frequently during August when mature females, including early pregnant and resting females, co-occur and socially interact with immature males and females. Overall, relatively few mature males visit Hervey Bay. Agonistic competitive behaviour was observed with increasing frequency during September and October when mother-calf pods, with few escorts predominated. Mother-calf pods in Hervey Bay spent most of their time alone involved in maternal care. Agonistic competitive behaviour is related to the decreasing numbers of potentially oestrous females toward the end of the season. Non-agonistic social behaviour and agonistic competitive behaviour were more frequently observed in larger and newly associated pods. Overall, non-agonistic social behaviour pods were more prevalent than agonistic competitive social behaviour pods. The results of this study substantiate that non-agonistic social behaviour may be more prevalent than aggressive agonistic social behaviour in site-specific locations and habitats, depending upon the classes and timings of humpback whales using such habitats.
Introduction
Humpback whales (Megaptera novaeangliae) are a migratory species. Except for humpback whales in the Arabian Sea (Mikhalev, 1997), individual females and males, in all maturational classes, and different reproductive states, in all other populations, migrate from high-latitude summer/autumn feeding areas to low latitude winter/spring breeding grounds (Chittleborough, 1965; Dawbin, 1966; Clapham and Mead, 1999). Feeding is rare or absent in winter breeding grounds, when most behaviours are related to calving and mating. The latter includes singing of long, complex song by male humpbacks to either attract females and/or meditate intrasexual interactions with other males (Payne and McVay, 1971; Clapham, 1996; Darling et al., 2006; Herman, 2017).
Humpback whale social behaviour and demographics in the feeding areas and breeding grounds in the Northern Hemisphere, as well as along some migratory routes, have been well described (see summaries in Clapham, 1993, 2000; Herman, 2017). In contrast, there is relatively little understanding about the behaviours and demographics of humpback whales in so-called “stopover” habitats along migratory routes, to and from feeding areas and breeding grounds. The use of so-called “stopovers” for rest, refuelling and predator avoidance, is not uncommon in species that undergo relatively long migrations including insects (Kennedy, 1951; McCord and Davis, 2012), reptiles (Rice and Balazs, 2008; Baudouin et al., 2015; Dujon et al., 2017; Nivière et al., 2018), mammals (e.g., Sawyer and Kauffman, 2011), and numerous bird species (e.g., Alerstam and Hedenström, 1998; Weber et al., 1998; Schaub et al., 2001, 2008; Delmore et al., 2012; McCabe and Olsen, 2015; Zaynagutdinova et al., 2019).
Several recent studies have identified and investigated migratory stopovers of humpback whales involving shallow-water environments for resting mother-calf pods (Carvalho et al., 2011; Meynecke et al., 2013; Bruce et al., 2014; Franklin et al., 2018; Stack et al., 2020), coastal feeding areas (Gill et al., 1998; Stockin and Burgess, 2005; Stamation et al., 2007; Barendse et al., 2010, 2013; Owen et al., 2015), and sea mounts used for resting, early feeding and singing and as navigational aids (Garrigue et al., 2015; MacKay et al., 2016; Derville et al., 2020).
Social behaviour in humpback whales can be broadly characterised as either agonistic or non-agonistic. While physical agonistic behaviour has occasionally been observed from females apparently rejecting the advances of an escort (Clapham, 1996, 2000; Pack et al., 2002; Franklin, 2012), and between a singing male and a male joiner (Darling and Berube, 2001), most physical agonistic social behaviour in humpback whales occurs within “competitive groups” (Clapham et al., 1992). These groups consist of a single female with or without a calf and two or more male escorts competing through various displays and aggressive acts for position and presumably potential mating access to the female (Tyack and Whitehead, 1983; Baker and Herman, 1984b; Clapham et al., 1992). Most non-agonistic social behaviour (described in detail below) in humpback whales in the breeding grounds or along migratory routes occurs in lone mother-calf pairs, in mother-calf pairs accompanied by a single escort (e.g., Craig et al., 2002, 2014; Cartwright and Sullivan, 2009; Cartwright et al., 2012; Zoidis et al., 2014; Zoidis and Lomac-MacNair, 2017), in male-male dyads (Brown and Corkeron, 1995; Darling and Berube, 2001; Darling et al., 2006), in male-female dyads (Jones, 2010; Herman et al., 2011; Pack et al., 2012) and among singers and whales that join them (Darling et al., 2006; Herman, 2017).
Some types of associations of humpback whales are relatively long term. These include the relationship between a mother and calf, which typically lasts 11–12 months (Clapham, 1996), and the relationships of some individuals in cooperative feeding groups, which may continue for years (e.g., Weinrich and Kuhlberg, 1991; Sharpe, 2001; Sharpe et al., 2013). However, most associations are short lived and temporary (Mobley and Herman, 1985; Clapham, 1993, 2000). The modal size for pods involving a calf present in Hawaii was three, mother-calf and escort (Herman and Antinoja, 1977; Herman et al., 1980; Glockner and Venus, 1983). In contrast, in Hervey Bay in pods with calves present the modal size was two because of the significantly higher proportion of mothers alone with their calves (Franklin et al., 2011). In the Hawaiian breeding grounds, mother-calf pairs typically do not affiliate with each other, reflecting reports of a general trend of female avoidance of other females in Northern Hemisphere breeding grounds (Clapham, 2000; Darling, 2001; Pack et al., 2017).
Hervey Bay is a wide shallow coastal embayment (Ribbe, 2014), south of the presumed breeding grounds of eastern Australian humpback whales within the Great Barrier Reef (Simmons and Marsh, 1986; Paterson, 1991; Smith et al., 2012). Commercial industrial whaling during the 1950s and early 1960s, in Antarctica and along the east coast of Australia south of Hervey Bay (Clapham et al., 2009; Ivashchenko and Clapham, 2014), decimated to near extinction, the east Australian humpback whale population (Woinarski et al., 2014; Harrison and Woinarski, 2018). Historically there are no formal reports of whales in Hervey Bay prior to the late 1980s (see e.g., Chaloupka et al., 1999). Early research in Hervey Bay during the late-1980s and early 1990s established that humpback whales enter and leave the Bay from the north, aggregate in the shallow eastern part of the Bay along the western shore of Fraser Island and that mothers with calves are the last cohort to use the Bay (Corkeron, 1993; Corkeron et al., 1994; also see Figure 2 below). However, there were insufficient data to determine the importance of Hervey Bay for particular classes of humpback whales.
Subsequently, a long-term vessel-based photo-identification study of humpback whales was undertaken between 1992 and 2009 (see Franklin, 2012, 2014). Humpback whales use Hervey Bay as a stopover early in the southern migration during August, September, and October (Franklin et al., 2011, 2018). The estimated mean residency of humpback whales in Hervey Bay is constant by week within season and over years (Mean = 1.53 weeks, SE = 0.22 weeks, LCI 1.09 weeks: UCI 1.96 weeks; Franklin, 2014). Pod characteristics differ significantly in August compared to September and October, related to the different classes of humpback whales using the Bay (Franklin et al., 2011). Hervey Bay is a female preferential habitat (2.9:1 females to males, Franklin et al., 2018). Mature females, including resting and early pregnant females, occur during August, co-temporal with the immature male and female cohort (Franklin et al., 2018). During August immature males and females are actively involved in complex social interactions with each other and with mature females (e.g., see Franklin, 2012). Unescorted mother-calf pods predominate in Hervey Bay during September and October (Franklin et al., 2011). Overall, only a few mature males are present in Hervey Bay during August, September, and October (Franklin et al., 2018).
In the current study, we systematically investigated non-agonistic and agonistic social behaviour in pods of humpback whales in Hervey Bay to better understand the pod types, pod associations and social behaviours occurring in an area used as a stopover. Data collected over a 14-year period were used to (a) analyse and model the occurrence and timing of pod associations, non-agonistic social behaviour and agonistic competitive behaviour in pods, within season and between years; (b) determine the relative proportions of non-agonistic versus agonistic social behaviour; (c) reveal significant factors influencing agonistic and non-agonistic social behaviour; and (d) compare these observed behaviours at this stopover with those observed in the breeding grounds, feeding areas, and along migratory corridors.
Materials and Methods
Study Area and Timing of Vessel-Based Surveys
Hervey Bay, formed by Fraser Island and the mainland, is located at 25°S, 153°E on the east coast of Queensland (Figure 1). It is a wide shallow coastal embayment approximately 4,000 km2 in area with a mean depth of 20 m (Ribbe, 2014). Fraser Island is 126 km long; it lies along a northeasterly axis and its northern end bridges the continental shelf (Ribbe, 2014, also see Figure 2 below). The study area is in the eastern bay against the western shore of Fraser Island (Figure 1).
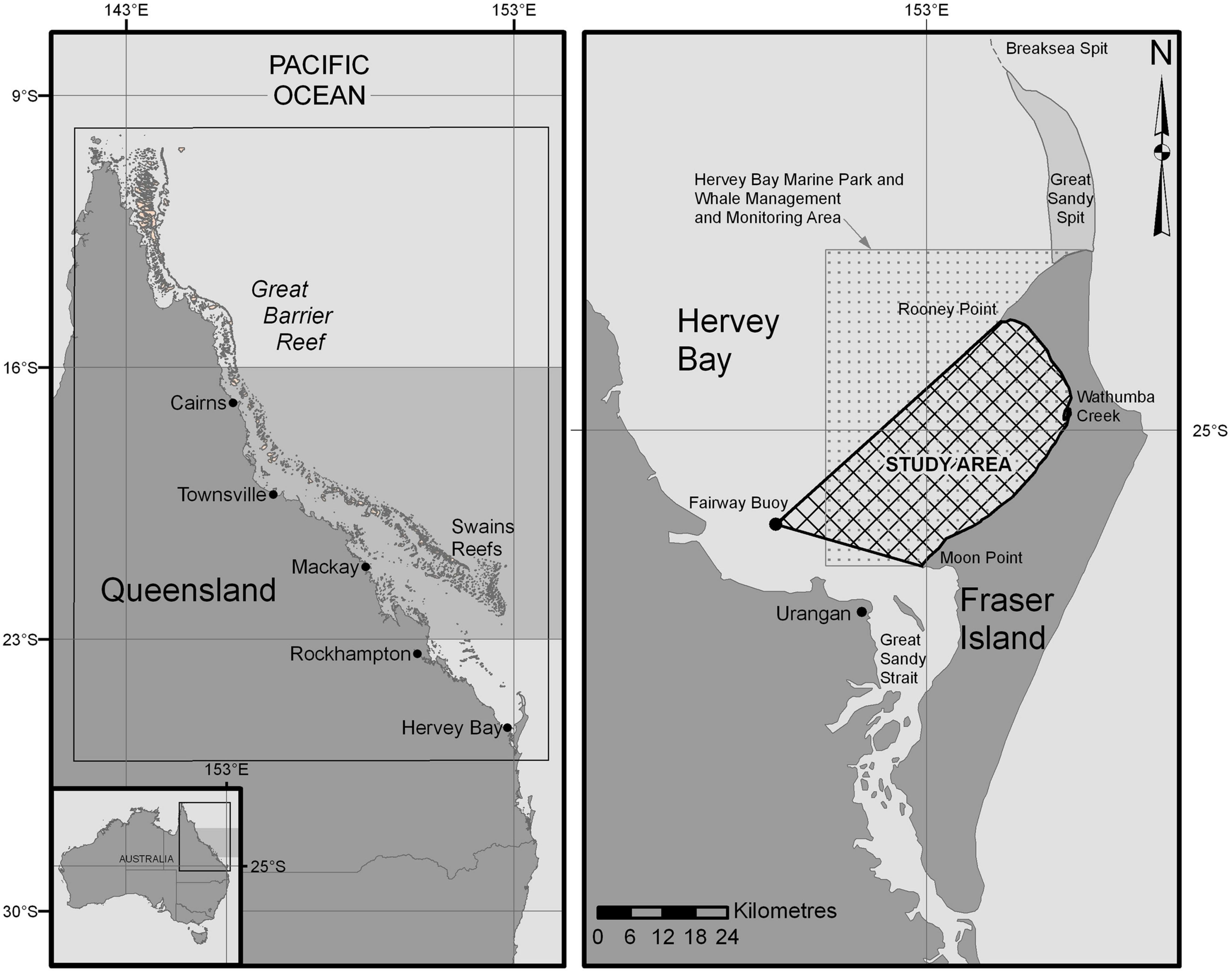
Figure 1. The location of Hervey Bay on the eastern coast of Australia and its geographic relationship to the Great Barrier Reef and presumed breeding grounds (16°S–23°S; shaded area) of humpback whales is shown on the left-side map. The study area and the Hervey Bay Marine Park boundaries are shown on the eastern side of Hervey Bay. A primary feeding area for eastern Australian humpback whales is around the Balleny Islands, approximately 5,000 km south of Hervey Bay (Franklin et al., 2012; Constantine et al., 2014); although, the feeding range of Southern Ocean humpback whales spreads widely across Antarctica from the Balleny islands, east (Dalla Rosa et al., 2012) and west (Franklin et al., 2017).
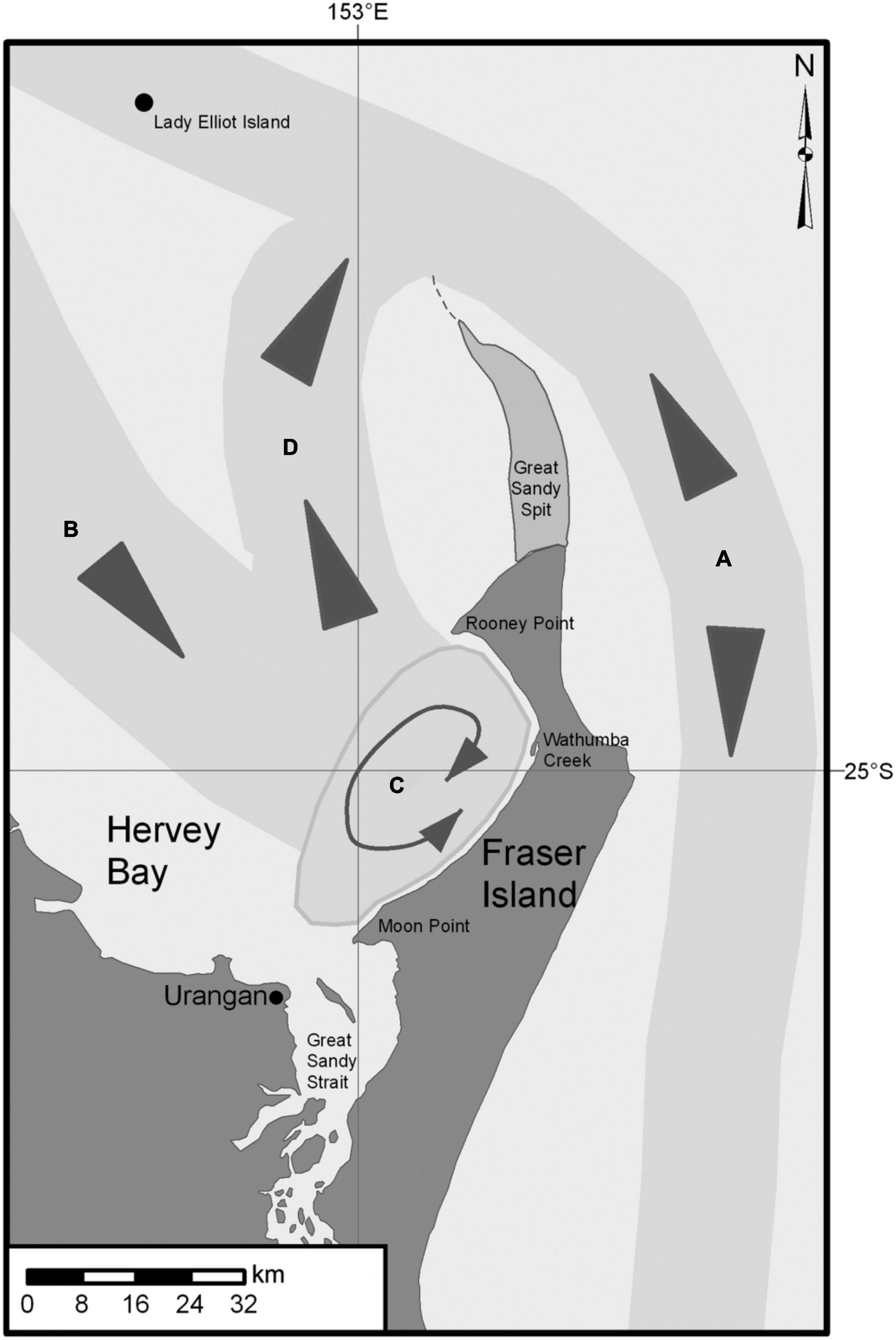
Figure 2. Humpback whale migratory pathways into and out of Hervey Bay. Hervey Bay’s northern entrance, east of the northern end of Great Sandy Spit, is 80 km wide with a mean depth of 20 m (Ribbe, 2014). Humpback whales enter (B) and leave Hervey Bay (D) to the north and aggregate in the eastern bay, off the western shore of Fraser Island (C) (Corkeron, 1993; Corkeron et al., 1994; Franklin et al., 2011, 2018). To enter Hervey Bay, humpback whales make a slight diversion from the primary north–south migratory pathway (A) (Paterson, 1991) and traverse shallow waters for approximately 40 km. They contend with 3 m tidal movements and do not return to deeper water until they pass north of Great Sandy Spit (Ribbe, 2014) and rejoin the primary southern migratory pathway (A).
Paterson (1991) reported that the southern migration from the Great Barrier Reef began in late July, with humpback whales moving into and out of Hervey Bay from early August to mid-October. Consequently, we conducted vessel-based observations of humpback whale pods in the Hervey Bay study area from early August until mid-October each year between 1992 and 2005 with consistent annual effort.
Four different motorised vessels were utilised as dedicated research platforms between 1992 and 2005: two were mono-hulls and two were catamarans, ranging in length from 11 to 27 m. The study area is approximately 27.8 km from Urangan Boat Harbour, Hervey Bay (Figure 1). Fieldwork was planned for 6 days each week, leaving Urangan harbour at 0800 each Sunday and returning at 1500 the following Friday. Planned daily operations were from 0930 to 1700 on Sunday, 0700 to 1700 Monday to Thursday, and from 0700 to 1330 on Friday.
Definitions of Terms in This Study
Given that a focus of this study is describing agonistic and non-agonistic social behaviour by pod types and pod associations, the following brief definitions of terms are provided here for clarity.
Singleton: a lone humpback whale.
Pod: is defined as either a singleton or two or more humpback whales within one to two body lengths of each other, generally moving in the same direction and at the same rate of travel (Whitehead, 1983; Clapham, 1993; Corkeron et al., 1994).
Initial pod: this is a pod as first encountered, prior to any change in pod size. If the initial pod joins, or is joined by, one or more pods during observation it is referred to as a newly associated pod.
Calf: an individual whale was considered to be a calf if it appeared to be less than half the length of a particular adult with which it maintained a constant and close relationship (Clapham et al., 1999; Pack et al., 2009). The adult in close proximity to the calf was assumed to be its mother.
Escort: was defined as a whale accompanying a mother with calf (Herman and Antinoja, 1977). In the breeding grounds, escorts have been identified as males, and their association with mother-calf pairs has been proposed as a tactic while prospecting for potential mating opportunities (Glockner and Venus, 1983; Tyack and Whitehead, 1983; Baker and Herman, 1984a; Mobley and Herman, 1985; Clapham, 1996; Craig et al., 2002).
Competitive Pods: a group of three or more whales exhibiting agonistic “Competitive Group” surface behaviours, which typically consists of a single focal female, with or without a calf, and two or more male escorts, some of which compete with each other, presumably for access to the female (Tyack and Whitehead, 1983; Baker and Herman, 1984b; Clapham et al., 1992).
Non-Agonistic Pods: two or more whales, excluding unescorted mother-calf pairs, involved in spatially undirected surface activity and calm interactions with no high-energy actions, aggression or competitive behaviours occurring (Darling et al., 2006). Surface social behaviours involve slow coordinated movements (Herman and Antinoja, 1977; Tyack, 1981; Tyack and Whitehead, 1983). Surface social behaviours include head rising, spy-hops, rolling over ventral side up, pectoral fin extensions, tail fluke-extensions, breaching, pectoral fin slapping, lobtailing and milling.
Other Behaviour Pods: occurs with singletons and in pods that are neither a competitive pod nor a non-agonistic pod, as defined above. Surface behaviours in Hervey Bay may include surface travelling, resting (logging), or occasional surface activity, for example breaching, pectoral slapping, and/or lobtailing.
Field Procedures
Observations
A minimum of six research assistants, were rostered on morning and afternoon shifts. The roster duties were to scan and search for pods; take field notes, GPS positions, weather and environmental readings.
Pods were chosen for observation on a “first pod available” basis with no a priori selection of any particular pod class. During the weekly study period, a variable overnight anchorage within the study area was selected based on the location of the final pod observed, weather conditions, and tidal movements. Each morning, subject to weather and sea-state condition, travel was commenced in a direction that was different from the prior day, until a pod was sighted.
Each pod under observation was assigned an identification code that was recorded together with the date, time, and GPS location was taken at start and thereafter every 15 min until completion when a final GPS position was taken. The number of individuals and their sex (where possible, see below), pod composition, and surface behaviours within pods were also recorded throughout the duration of observation of each pod (continuous sampling; Altmann, 1974). All pod observations and behaviour data were recorded daily in field notes and entered into a FileMaker Pro database each evening. Behaviour that passes unobserved underwater or at night may be significantly different from that documented during the present study; we acknowledge this, but all observations reported here were necessarily made at the surface and during daylight.
Sex-Identification
To the extent possible, an individual whale’s sex was determined using one of two methods: from direct observation of the genital area – female humpback whales have mammary slits and a hemispheric lobe just posterior to the genital slit (True, 1904; Glockner, 1983) or sex was inferred from the whale’s previous sighting histories and/or its behavioural roles. For example, an adult-sized individual accompanying a calf consistently and providing it with nurturing behaviours has been verified to be female (Tyack and Whitehead, 1983; Pack et al., 2009), and escorts and singing whales have been verified to be males (Glockner and Venus, 1983; Tyack and Whitehead, 1983; Baker and Herman, 1984a; Clapham, 2000). Chu and Nieukirk (1988) verified that humpback whales with distinct vertical and horizontal dorsal fin and lateral body scars, resulting from competitive activity were male. Females never exhibit such marks (Franklin et al., 2020). With few exceptions (e.g., see Clapham et al., 1992), the whale designated from positioning and behaviour as the “focal whale” in competitive groups has been verified as female (Darling et al., 1983; Tyack and Whitehead, 1983; Baker and Herman, 1984b; Clapham et al., 1992, 1993; Clapham, 2000).
Pod Behaviour and Inter-Pod Association and Non-association Data
Each pod was surveyed until its composition, the number of whales present, individual sex (where possible), and the surface social activity and behaviours within pods throughout the period of observation were determined.
Upon commencement of observation of the initial pod (see Definitions above) a member of the observation team continually scanned for and reported on other pods within a radius of approximately a kilometre. If one or more of those pods were tracking toward the initial pod, or if the initial pod was tracking toward them, then the duration of observation was extended to observe and record associations as they occurred. The details of each associating pod, including time, GPS location, size, composition and surface social activity and behaviours were recorded. Each newly associated pod was designated as either a consecutive association (e.g., a mother-calf pod attracts one escort at time 1, time 2, time 3, etc.) or as a simultaneous association (e.g., three singletons approaching each other and associating at the same time).
Pods were firstly categorised into those that did not associate while under observation and those that did associate while under observation forming newly associated pods. All logged information on surface activity, behaviours and composition for each pod was reviewed and pods were categorised either as a non-agonistic pod, agonistic competitive pod or other behaviour pod, in accordance with the definitions above.
Statistical Analyses
The pod or singleton was used as the basic observational unit in analyses. The size of the newly associated pods by number of pods associating and size of the initial pod were reported. The duration of observations of competitive pods, non-agonistic pods and other behaviour pods were also reported.
The frequencies of competitive pods, non-agonistic pods and other behaviour pods, sorted by pods with no calves present and pods with calves present, by newly associated pods and pods that did not associate while under observation, and by number of whales (excluding calves) in pods (1, 2, and 3+) were reported. The data on non-agonistic pods, competitive pods, and newly associated pods, by week within season (1–10), together with the sub-set of pods used in statistical analysis and modelling were reported.
Variation in the proportion of newly associated pods by year, by week within year (1–10), by calf present or not present, and by pod size were assessed using chi-square analyses. The variation in the proportion of non-agonistic pods and competitive pods (independent analyses) by year, by week within year, by pods that did not associate while under observation and newly associated pods, and by pods with no calves present and pods with calves present were examined.
Chi-square analyses were used to document the univariate associations between the occurrence of non-agonistic pods and newly associated pods, year, week within year, number of whales (excluding calves) and presence of calf in the pod prior to fitting a binary logistic regression model to assess the joint effects of the above factors (explanatory variables) on the probability of occurrence of non-agonistic social behaviour in pods (response variable). Similarly, chi-square analyses were used to document the univariate associations between the occurrence of newly associated pods and agonistic competitive pods, year, week within year, number of whales (excluding calves) and presence of a calf in the pod prior to fitting a binary logistic regression model to assess the joint effects of the above factors (explanatory variable) on the probability of occurrence of agonistic competitive behaviour in pods (response variable).
The binary logistic regression models were fitted in SPSS (version 26; IBM Corporation, 2019) using the procedure Generalized Linear Models (GENLIN). Descriptions of the modelling process are included in the section “Results.” Goodness of fit was assessed in terms of model deviance, its degrees of freedom and Chi squared tests, and AIC values. Multicollinearity was considered from the perspective of how the model effects should be interpreted in terms of the significance of the terms in the model relative to the raw, univariate tests and changes between raw, univariate proportions in the categories of explanatory factors and the real-scale estimates from the models.
Results
Effort and Observations
A total of 139 6-day survey periods (Sunday to Friday) were conducted in the Hervey Bay study area (Figure 1 above) between 1992 and 2005. Data on pods were obtained on 770 of the planned 834 survey days. Total survey time was 6,160 h and observations of humpback whale pods were conducted for a total of 2,760 h.
Data Set
Data were collected on 4,506 pods (see Supplementary Appendix Data Sheet 1), 1,022 (22.7%) of which were pods that associated while under observation, involving associations of from 2 to 5 pods, becoming 465 newly associated pods (Supplementary Appendix Table 1), and 3,484 (77.3%), which were pods that did not associate while under observation, making a total of 3,949 pods used in the analyses (see Table 1 below and Supplementary Appendix Table 3).
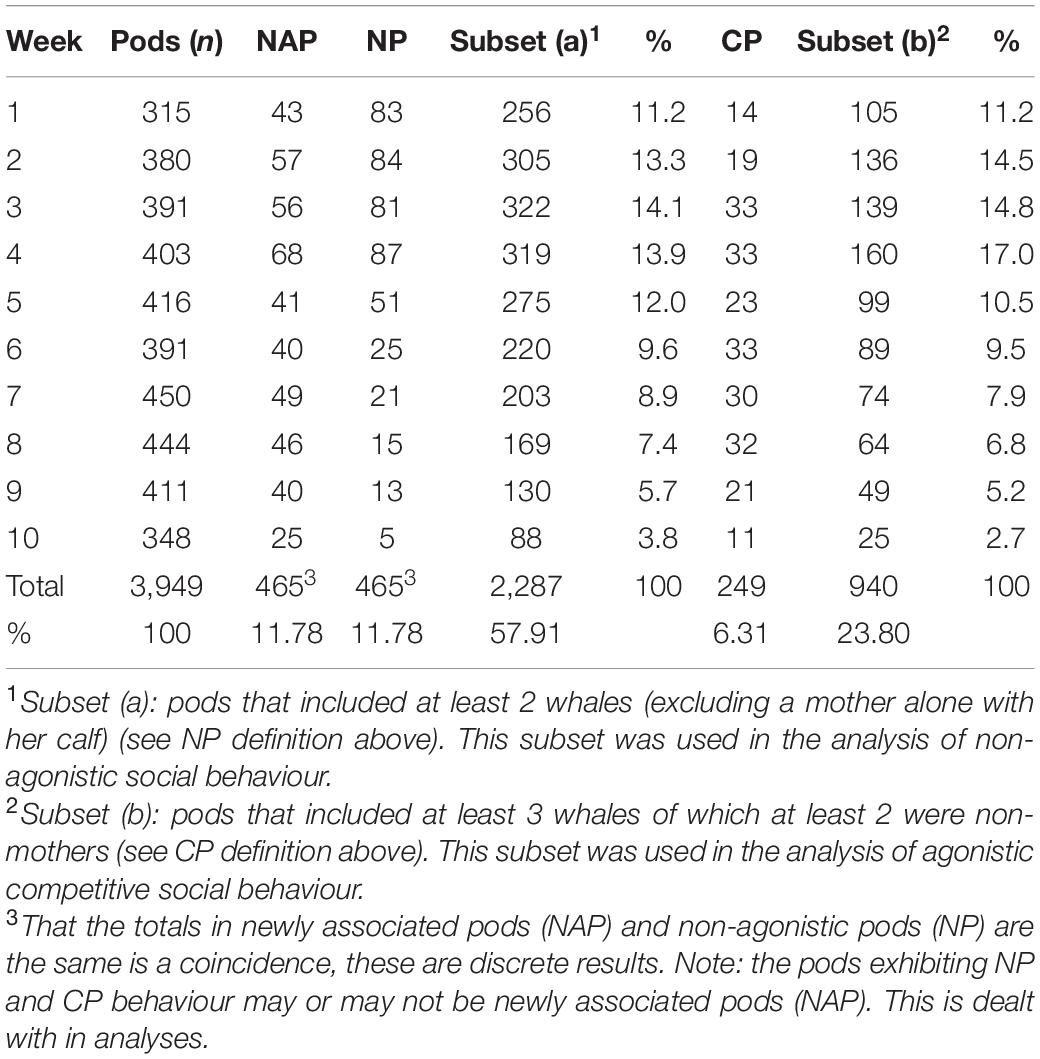
Table 1. Number of pods, week within year by pods (n), newly associated pods (NAP), non-agonistic pods (NP), the subset of pods used in analysis of non-agonistic social behaviour (Subset a), competitive pods (CP), the subset of pods used in analysis of agonistic competitive social behaviour (Subset b).
Associations and Disassociations of Pods
Newly associated pods, formed by the consecutive or simultaneous association of up to five pods during the period of observation, with calves present versus those without calves present are reported in Supplementary Appendix Table 1.
Of the 465 newly associated pods, 295 (63.4%) contained no calf, and 170 (36.6%) contained one or more calves. Of the newly associated pods, 368 (79.1%) were formed from the association of two pods (of one or more whales each), 85 (18.3%) from the association of three pods, 10 (2.2%) from the association of four pods and two (0.4%) from the association of five pods. Of the newly associated pods, 341 (73.3%) were consecutive associations and 124 (26.7%) were simultaneous associations. Disassociations were recorded in 171 (36.8%) of the newly associated pods, but not used in analyses.
Non-agonistic Pods, Competitive Pods, and Other Behaviour Pods
Duration of Observation
The duration of observations of competitive pods, non-agonistic pods and other behaviour pods are reported in Supplementary Appendix Table 2. Competitive pods were observed for 0.07–2.50 h (median = 0.73 h, mean = 0.91 h, SD = 0.46 h, n = 216), non-agonistic pods for 0.05–5.07 h (median = 0.73 h, mean = 0.85 h, SD = 0.55 h, n = 432), and other behaviour pods for 0.02–3.72 h (median = 0.43 h, mean = 0.56 h, SD = 0.46 h, n = 3268). Observation durations of pods that did not associate while under study (PDNA, Supplementary Appendix Table 3) ranged from 0.02 to 3.72 h (median = 0.45 h, mean = 0.56 h, SD = 0.48 h, n = 3,484), and for newly associated pods (NAP, Supplementary Appendix Table 3) ranged from 0.02 to 5.07 h (median = 0.83 h, mean = 0.97 h, SD = 0.58 h, n = 465).
Frequencies, Proportions and Pod Sizes
The frequencies, proportions and pod sizes of non-agonistic pods, competitive pods, and other behaviour pods are reported for all pods, non-calf pods, pods containing one or more calves, and by newly associated pods and pods that did not associate (Supplementary Appendix Table 3). The pod data by week within season used in analyses and modelling is presented in Table 1 above.
Avoidance and Repulsion Behaviour
There were instances in the “other behaviour pods” category (Supplementary Appendix Table 2) of agonistic behaviour that did not meet the definitions of a competitive pod. In 28 pods (0.71% of 3,949 pods), a mother actively repulsed or avoided the advances of a single escort (for description of these behaviours see Pack et al., 2002). All except one of these pods were trios, consisting of mother-calf and escort; the other pod was a mother in the company of two small calves repulsing an agonistic aggressive approach by a single escort. Between 1992 and 2005 there were only two sightings of a mother with two calves in Hervey Bay (Franklin et al., 2011). Of the 28 pods, 22 (78.6%) involved a mother avoiding the agonistic advances of a single escort and 6 pods (21.4%) involved her actively repulsing the agonistic advances of a single escort.
Non-agonistic Pods and Competitive Pods Within Season
The number of all observed pods, newly associated pods, non-agonistic pods and competitive pods are reported by week within season in Table 1.
Statistical Analysis and Modelling
Newly Associated Pods
The proportion of newly associated pods in Hervey Bay varied from 5.0 to 14.4% over the years, and from 16.9% to 7.2% over the weeks within year (Figures 3A,B, respectively). Although there was no systematic pattern to the variation over years, the proportion of newly associated pods over weeks within year was significantly greater in August compared to September and October (15.0%, 9.8%; Fisher’s exact test, P < 0.001).
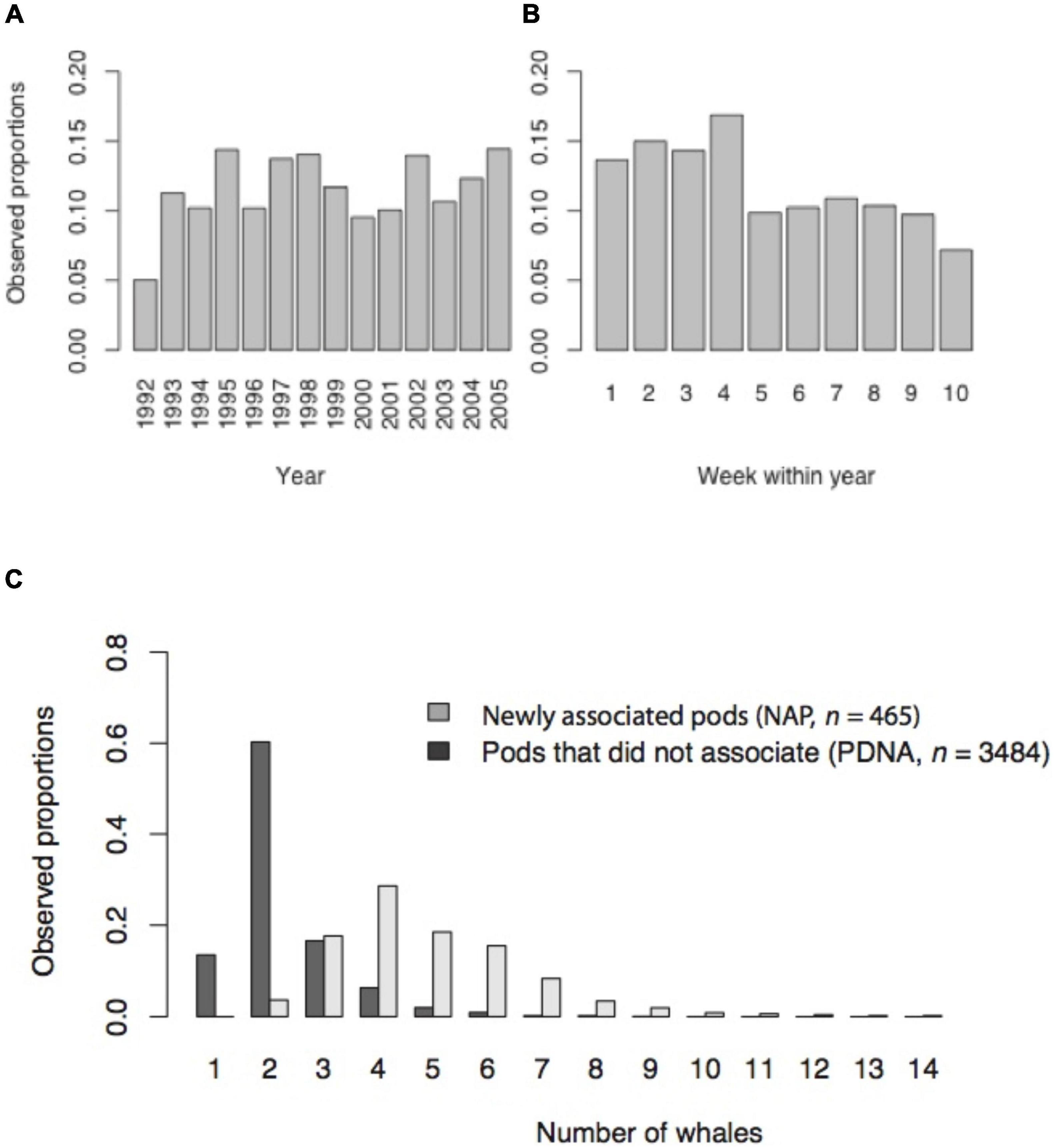
Figure 3. Observed proportions: (A) newly associated pods by year, (B) newly associated pods by week within year, (C) pods by number of whales in pods for newly associated pods (NAP) and pods that did not associate while under observation (PDNA).
Newly associated pods on average, as expected, were significantly larger than pods that did not associate with other whales while under observation (Mann–Whitney test, P < 0.001). Newly associated pods ranged in size from 2 to 14 whales (mode = 4, median = 5, mean = 4.9, SD = 1.85, n = 465) while pods that did not associate with other whales while under observation, ranged from 1 to 9 whales (mode = 2, median = 2, mean = 2.3, SD = 0.98, n = 3,484) (Figure 3C and Supplementary Appendix Table 3).
The proportion of pods with calves present increased rapidly from the last week in August, to the end of the season in mid-October (3.6–92.8%, Franklin et al., 2011). Pods that included a calf were less likely to associate than pods that did not include a calf (9.9%: 13.2%, χ2 = 10.57, df = 1, P < 0.001). However, when pods that included a calf did associate, they were more likely to associate with pods that also included a calf, than with pods that did not include a calf (70.4%: 29.6%; χ2 = 16.33, df = 1, P < 0.001).
Non-agonistic Pods
As non-agonistic pods and newly associated pods were closely related (see results below), the following analyses were conducted on the dataset in Table 1, which includes the data on newly associated pods and pods that did not associate while under observation. Of the 3,949 pods in the data set, 2,287 [57.9%, Subset (a), Table 1] included at least 2 whales (excluding a mother alone with her calf), and it was this subset that was analysed.
Non-agonistic social behaviour was observed in 465 (20.3%) of the 2,287 pods. Non-agonistic social behaviour in pods was:
1. Observed with greater frequency in newly associated pods (139/435 = 32.0%) than in pods that did not associate (326/1,852 = 17.6%), (χ2 = 44.79, df = 1, P < 0.001);
2. Significantly variable over years (χ2 = 44.79, df = 13, P < 0.001);
3. Observed significantly more often in pods with no calf present (421/1,759 = 23.9%) than in pods with a calf or calves present (44/528 = 8.3%) (χ2 = 61.02, df = 1, P < 0.001);
4. Observed to significantly increase in frequency with the number of whales in the pod (excluding calves) (176/1,319 = 13.3%, 125/446 = 28.0%, 76/276 = 27.5%, 88/246 = 35.8% in pods with 2, 3, 4, and 5+ whales, respectively, χ2 = 101.12, df = 3, P < 0.001);
5. Significantly variable by week within year (χ2 = 104.88, df = 9, P < 0.001).
However, these univariate effects were not independent. Consequently, a binary logistic regression model was fitted to assess the joint effects of newly associated pods (yes, no), presence of calf (present, not present), year, week within year and number of whales (excluding calves) (2, 3, 4, 5+) on the probability of observing non-agonistic social behaviour.
The five main effects were fitted as a block, which accounted for a significant proportion of variation (Likelihood ration χ2 = 321.41, df = 27, P < 0.001; AIC = 1217.846). None of the five predictors was redundant (All p ≤ 0.004). Adding the two-way interaction effects individually to the model showed that only the newly associated pods by number of whales in the pod interaction effect was significant (p < 0.05).
The selected model included the five main effects and the newly associated pods by number of whales (excluding calves) interaction effect (Likelihood ratio χ2 = 334.59, df = 30, P < 0.001; AIC = 1210.661).
Goodness of fit was assessed as the ratio of the deviance to its degrees of freedom, which indicated significant deviation of the data from the binomial model (Deviance/df = 1.110, Chi squared p = 0.021). The significance of this, however, is largely a consequence of a very large number of degrees of freedom and adjustment of residual variances would not affect which variables were significant (p < 0.05) in the model. Bootstrapped estimates (2000 iterations) were obtained to check on this and their standard errors were found to be substantially unaffected. Moreover, Wedderburn (1974) provides theoretical considerations to justify the usual MLEs (Maximum Likelihood Estimations) as (asymptotically) optimal point estimators of the model parameters, even when there is overdispersion in the data. Given these considerations, the MLE estimates are reported here.
The only redundant effect (p > 0.163) was for the number of whales in the pod. This variable participates in the newly associated pods by number of whales (excluding calves) interaction effect and must be included in the model although it would not be independently interpreted. It is not unexpected that main effects underlying an interaction effect may be non-significant. That all main effects other than the number of whales in the pod remained significant (all p < 0.001) indicates that such multicollinearity as may be present among the explanatory variables does not deprive any of them from making an important contribution to the model. Moreover, the estimated proportions in the categories of each explanatory variable from the model and the raw percentages reported above correspond reasonably well indicating that multicollinearity has not strongly or adversely affected interpretation of the effects (see Figure 4 below).
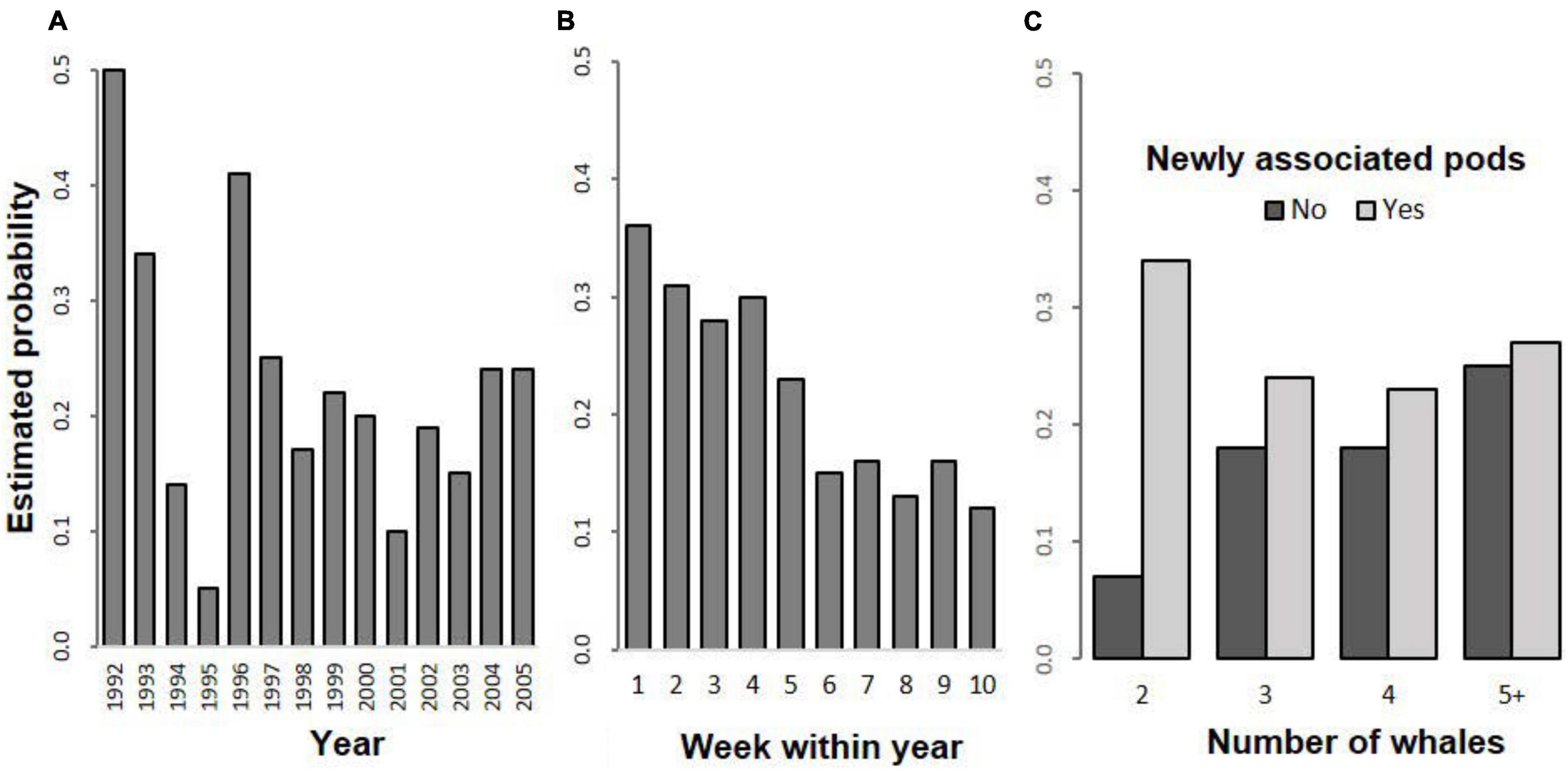
Figure 4. Estimated probabilities of observing non-agonistic social behaviour: (A) by year; (B) by week within year; (C) by number of whales (excluding calves), in newly associated pods (No, Yes).
The parameter estimates (logistic scale) and their standard errors are not reported. The parameter estimates were used to calculate the estimated probabilities of observing non-agonistic social behaviour by the factors in the model.
The estimated probability of observing non-agonistic social behaviour by year, week within year and by number of whales (excluding calves), in newly associated pods (No, Yes) are plotted in Figure 4.
The variation over years in the probability of observing non-agonistic social behaviour (Figure 4A) includes a rapid decline over the period 1992–1995 followed by a sudden increase in 1996. This was followed by a decline to 2001 and an increase after that to 2005.
The probability of observing non-agonistic social behaviour was highest during the first 4 weeks of the season (August) and declined rapidly from week 5 (Figure 4B). Although the effect of the presence of a calf in a pod is not shown in Figures 4A–C, there was a significant main effect in the model with the rate of occurrence of non-agonistic social behaviour being significantly lower in pods that included calves (8.3% with calves, 23.9% without calves, see Supplementary Appendix Table 3). That the presence of calf and week within year effects were significant in the model indicates that the calf effect is not simply due to the rapidly increasing proportion of pods that included calves later in the season (3.6% in late August to 92.8% by mid-October, Franklin et al., 2011). This indicates that the calf effect is over and above the decline in the rate of non-agonistic social behaviour shown in Figure 4B.
The probability of observing non-agonistic social behaviour increased with the number of whales (excluding calves) in the pod and was higher for pods of two whales (excluding calves) that were newly associated, than for pods of two whales that did not associate while under observation (Figure 4C). This difference largely accounts for the pod size effect. Thus, the effect of newly associated pods is largely confined to the difference between newly associated pods of two (two singletons associating) rather than newly associated pods of larger size.
Competitive Pods
As competitive pods and newly associated pods were closely related (see results below), the following analyses were conducted on the data set in Table 1, which included the data on newly associated pods and pods that did not associate while under observation. Of the 3,949 pods in the data set, 940 [23.8%, Subset (b), Table 1] were pods that included at least three whales, of which at least two were non-mothers; it is this subset that was analysed. Competitive behaviour was observed in 249 (26.5%) of these 940 pods.
The factors; newly associated pod, year, presence of calf, number of whales in pod (excluding calves), and week within year were each assessed for effects on the probability of observing competitive social behaviour. Competitive social behaviour in pods was:
1. Observed in a greater proportion of newly associated pods (140/376 = 37.2%) than in pods that did not associate while under observation (109/564 = 19.3%) (χ2 = 37.15, df = 1, P < 0.001);
2. Not significantly variable over years (χ2 = 13.55, df = 13, P = 0.406);
3. Significantly more frequent in pods with calves present (87/191 = 45.5%) than in pods with no calf or calves present (162/749 = 21.6%), (χ2 = 44.72, df = 1, P < 0.001);
4. Observed to significantly increase in frequency with the number of whales in the pod (excluding calves) (70/425 = 16.5%, 72/270 = 26.7%, and 107/245 = 43.7% for 3, 4, and 5+ whales, respectively, χ2 = 59.07, df = 2, P < 0.001);
5. Observed to significantly increase in frequency over weeks within year (from ∼12 to ∼45%), χ2 = 65.66, df = 9, P < 0.001).
However, these univariate effects were not independent. Consequently, a binary logistic regression model was fitted to assess the joint effects of newly associated pods (yes, no), presence of calf (present, not present), number of whales (excluding calves) (3, 4, 5+), and week within year (1, 2, …, 10) on the probability of observing competitive social behaviour.
Together the four main effects accounted for a significant proportion of variation in the rate of observation of competitive pods (Likelihood ratio χ2 = 137.23, df = 13, P < 0.001). However, the marginal Wald tests showed the calf effect to be non-significant in the context of the other effects (Wald Chi squared = 2.993, df = 1, P = 0.084). The non-significance of the calf effect was largely due to the strength of the association between the increasing proportion of calf pods and week within season. The presence of calf effect was removed from the model at this point.
An attempt to fit interaction effects required considerable collapsing of categories and failed to produce useful results. Consequently, the selected model included only the three main effects for newly associated pods, number of whales (excluding calves) and week within year (Likelihood ratio χ2 = 134.26, df = 12, P < 0.001; AIC = 230.72). Goodness of fit was assessed as the ratio of the deviance to its degrees of freedom, which indicated no significant deviation of the data from the binomial model (Deviance/df = 1.103, Chi squared p = 0.290).
The parameter estimates (logistic scale) and their standard errors are not reported. The parameter estimates were used to calculate the estimated probabilities of observing competitive social behaviour by the explanatory factor levels.
Removal of the presence of calf from the model removed the most obvious cause of multicollinearity. All effects remaining in the model were significant (p ≤ 0.004) and the estimated proportions in the categories of each explanatory variable from the model and the raw percentages reported above correspond reasonably well indicating that multicollinearity has not strongly or adversely affected interpretation of the effects (see Figure 5 below).
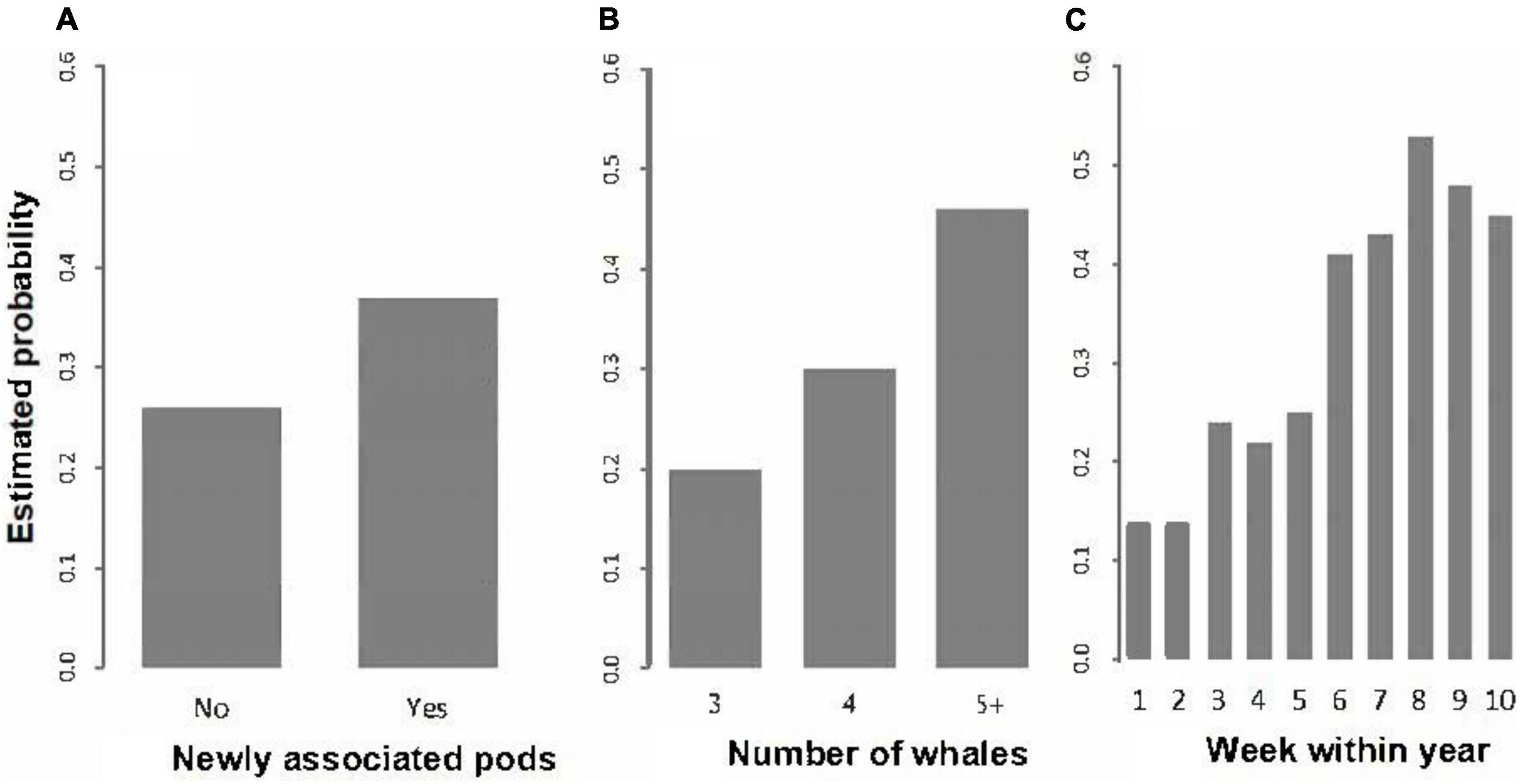
Figure 5. Estimated probabilities of observing competitive social behaviour: (A) by newly associated pods (No, Yes); (B) by number of whales (excluding calves); and (C) by week within year.
The mean probabilities of observing competitive social behaviour by newly associated pods (yes, no), number of whales (excluding calves) (3, 4, 5+) and for week within year are plotted in Figure 5.
That the effects of increasing pod size and newly associated pods are jointly significant indicates that the rate of competitive social behaviour is greater in newly associated pods than in pods that did not associate and is not simply a function of the increase in pod size following the formation of a newly associated pod. As shown in Figure 5B, larger pods were more likely to be competitive, with a larger increase in the frequency of competitive social behaviour between 4 and 5+ whales than between 3 and 4 whales. However, as shown in Figure 5A, if those pods have just associated, there is an approximately 11% increase in the frequency of competitive social behaviour compared to (i.e., over and above) pods of the same sizes that did not associate while under observation.
The calf effect was likely non-significant in the context of the other effects because the presence of calf and week within year were very strongly associated (χ2 = 349.76, df = 9, P < 0.001).
Discussion
Our results provide the first systematic seasonal study of non-agonistic social behaviour and agonistic competitive social behaviour in humpback whale pods in a site-specific female-biassed stopover along the southern migratory route from the eastern Australian breeding grounds. Behaviour in Hervey Bay is related to maternally directed philopatry, pod associations and the occurrence and timing of classes of humpback whales using the Bay. There are important differences and similarities with humpback whale behaviour in Hervey Bay compared with behaviour reported in Northern Hemisphere breeding grounds and feeding areas (see review in Clapham, 2000), and along some migratory corridors in the Southern Hemisphere.
A major contrast in humpback behaviour presented in this study, compared to reports of female avoidance from traditional breeding grounds and feeding areas (Clapham, 2000), is that mature females in Hervey Bay, non-lactating and lactating, are involved in multiple non-agonistic pod associations and complex social interactions with immature males and females and new season’s calves. The results presented in this study indicate that non-agonistic social behaviour may be more prevalent in humpback whale social organisation than previously reported (see Darling et al., 2006).
Differences and Similarities Between the Behaviour and the Social Interactions of Humpback Whales in Hervey Bay Compared to Northern Hemisphere Breeding and Feeding Grounds
Hervey Bay is neither a breeding nor a feeding ground, but a stopover early in the southern migration (Franklin et al., 2018), after humpback whales leave the putative breeding grounds north of Hervey Bay (Simmons and Marsh, 1986; Paterson, 1991; Chaloupka and Osmond, 1999; Smith et al., 2012). Burns et al. (2014) reported that eastern Australian humpback whales spend an average of 4 weeks in the breeding grounds, and the peak-breeding month in eastern Australia is August (Chittleborough, 1965). Calves are rarely seen in Hervey Bay during August (Franklin et al., 2011). Consequently, the calves entering Hervey Bay during September and October are likely to be larger, older and more robust than calves occurring in the breeding grounds and likely to be aged anywhere from 4 to 12 weeks.
It has been suggested that in feeding and breeding grounds a rarity of female-female associations may reflect avoidance and/or competition between females (Clapham, 2000). Furthermore, in the Hawaiian breeding ground mother-calf pods actively avoid encounters with other mother-calf pairs (Darling, 2001) and the modal size for pods having a calf present was three, mother-calf and escort (Herman and Antinoja, 1977; Herman et al., 1980, 2011; Glockner and Venus, 1983; Mobley and Herman, 1985). In Hervey Bay the modal size for pods was two, because of the significantly higher proportion of mothers alone with their calf (Franklin et al., 2011). In contrast to the above reports of female and mother-calf avoidance in feeding areas and breeding grounds, when pods that included a calf did associate in Hervey Bay, they were significantly more likely to associate with pods in which one or more mother-calf pairs were present than with pods not containing mother-calf pairs. Approximately 64% of “Other Behaviour” pods were mothers alone with their calves, while 29% involved associations of from two to seven mother-calf pods (see Supplementary Appendix Table 3).
The typical behaviour in these multiple mother-calf pod associations, usually involved highly surface-active calves, socially interacting with each other and with mothers carefully keeping the calves apart and possibly avoiding injury during these social interactions (Franklin, 2012). Moreover, last season’s calves and mature females were often involved in these multiple mother-calf pod associations (Franklin, 2012), with mothers constantly engaged in ensuring the safety of the calves by maintaining separation amongst calves during these extended social interactions. Calf surface activity involves early social opportunities and experience for older calves. The largest association of mother-calf pods observed in Hervey Bay consisted of seven mother-calf pairs, involving fourteen individual whales in which, all calves were involved in surface-active behaviours and ongoing social interactions. We suggest that the opportunity for multiple mother-calf pod associations and social interactions among those mother-calf pods, occurring in Hervey Bay during September and October, may contribute to preparing the calves for their return journey to join the cohort of new-seasons yearlings in Hervey Bay during August the following year.
It is well established that the association between a new calf and its mother endures for most, if not all, of the first year of the calf’s life (Clapham, 2000), and that calves learn from their mothers the migratory routes which will take them each season between breeding and feeding grounds (Baker and Herman, 1984a; Clapham, 2000; Franklin et al., 2018). Clapham (1993) reported that mothers spend 77% of their time alone with their calves in the feeding grounds, as the calves complete the weaning process to independent feeding. Similarly, during the stopover in Hervey Bay, prior to leaving for Antarctic feeding grounds, lactating females spend 69% of their time alone with their calves nursing, resting and engaging their offspring in surface behaviours (Franklin et al., 2011), the latter of which may assist in the development of muscular myoglobin (Cartwright et al., 2016).
Weinrich and Kuhlberg (1991) reported stable social associations among female humpback whales feeding in the southern Gulf of Maine and hypothesised that stable associations allow adult females to maximise their net energy gain through cooperative feeding. The structure of the annual migration with mature, resting and early pregnant, females leading the migration south from the breeding grounds, co-temporal with immature males and females and then lactating females with new calves being the last cohort to move south has been shown to be a constant feature of the social organisation of humpback whales (Dawbin, 1966, 1997; Franklin et al., 2018). As reported in this study, when mother-calf pods in Hervey Bay do associate, they are significantly more likely to associate with other mother-calf pods. We suggest that the behaviour of mature females in Hervey Bay involves cooperative social interactions with immature whales during August to maximise social development (Franklin, 2012) and as well, cooperative behaviour through separation among lactating females, during September and October (69% alone with calf, Franklin et al., 2011) which, may minimise the energetics of lactation (Lockyer, 1981, 1984).
In contrast to reports of female avoidance from breeding and feeding grounds in the Northern Hemisphere, when mature non-lactating and lactating females were observed interacting with each other during the Hervey Bay stopover, they were involved in non-agonistic social interactions and multiple pod associations. Overall, mature females in Hervey Bay are involved in non-agonistic cooperative social interactions.
Differences and Similarities Between the Behaviour and Social Interactions of Humpback Whales in Hervey Bay Compared to Other Site-Specific Migratory Corridor Locations
Baker et al. (1990) reported a marked segregation of mitochondrial DNA haplotypes among subpopulations of humpback whales on different feeding and wintering grounds and interpreted this segregation to be the consequence of maternally directed fidelity to migratory breeding and feeding destinations. Photo-identification of individual humpback whales over long periods of time has documented maternally directed fidelity to feeding destinations (Martin et al., 1984; Clapham and Mayo, 1987; Katona and Beard, 1990; Clapham et al., 1993; Palsbøll et al., 1997).
In the Southern Hemisphere, humpback whales predominantly migrate along the extensive continental coastlines and nearshore islands of eastern and western Australia, Africa, and South America, en route to and from tropical breeding grounds (Chittleborough, 1965; Dawbin, 1966, 1997). Franklin et al. (2018) reported that the site-specific female-biassed sex ratio occurring in Hervey Bay (25°S) involved female philopatry and high levels of survival and site fidelity, of all classes of humpback whales using Hervey Bay. They suggested that Hervey Bay was a socially and ecologically important habitat for mature females, accompanying and socially interacting with immature males and females in August and lactating females with older calves involved in maternal and social activities during September and October. The behaviour in Hervey Bay reported in this study is consistent with previous observations of the occurrence and timing of classes of humpback whales using Hervey Bay as a stopover early in the southern migration.
Several studies have reported site-specific behaviour along Southern Hemisphere coastal migratory corridors, including resting, nursing and early feeding locations. In contrast to Hervey Bay, Brown et al. (1995) reported a male-biassed sex ratio in the migratory corridor off Stradbroke Island, southeast Queensland (27°S), Australia, during the northern and southern winter migration. Brown and Corkeron (1995) investigated pod size on the northern migration from late-May to mid-August and reported that most pods travel north in pods of one to two whales with pod sizes ranging up to nine whales. Whereas in Hervey Bay on the southern migration, pod size range between two and fourteen whales, with the larger average pod sizes reflecting the high rates of pod associations in the Bay. Overall, 12.7% of pods biopsied off Stradbroke were classified as competitive (Brown and Corkeron, 1995), almost double the 6.3% of competitive pods observed in Hervey Bay. Furthermore, Brown and Corkeron (1995) reported that during the southern migration the social relationship between most males was characterised by non-agonistic and occasionally cooperative interactions. Franklin et al. (2018) suggested that habitat preferences and differential migration of females and males provides a plausible explanation for site-specific sex-bias in breeding grounds, migratory stopovers, and along migratory corridors.
The only other female-biassed sex ratio reported in a migratory corridor, other than Hervey Bay, was in an early feeding area off the southwestern coast of Africa (33°S), (Barendse et al., 2010). Barendse et al. (2013) also reported a female-dominated presence in the same area including non-nursing (possibly pregnant) females and yearlings, which suggested female-derived site fidelity, likely involving culturally transferred fidelity to a feeding area.
Meynecke et al. (2013) studied humpback whales using the Gold Coast Bay, southeast Queensland (28°S) as a temporary stopover during the northern and southern migration and reported resting behaviour during the southern migration. They reported similar patterns in pod sizes and timing of classes of whales during the southern migration to those recorded in Hervey Bay in this study. Consistent with Hervey Bay data, sightings of mothers with calves were highest in October with fewer sightings in August and September. Bruce et al. (2014) investigated the spatial use of Jervis Bay (35°S) off the coast of southern NSW by humpback whales during the late-2000s. They suggested that, associated with increases in the population, calf and non-calf pods were using Jervis Bay as a resting area with mother-calf pods preferring shallower waters.
Site-specific early feeding locations early in the southern migration have been reported within the migratory corridor off eastern Australia at Cape Morton, southeastern Queensland (27°S, Stockin and Burgess, 2005), Eden, off the coast of southern NSW (37°S, Stamation et al., 2007; Owen et al., 2015), and off the eastern coast of Tasmania (43°S, Gill et al., 1998). Garrigue et al. (2015) investigated the migratory movement of humpback whales in the southern waters of New Caledonia using satellite-monitored tags deployed between 21°S and 23°S. In contrast to Hervey Bay, they suggested that seamounts probably serve multiple and important roles as breeding locations, resting areas, navigational landmarks or even supplemental feeding grounds [also see MacKay et al. (2016) and Derville et al. (2020) for reports on use of seamounts by humpback whales]. Together these data suggest that site-specific stopover habitats in the Southern Hemisphere migratory corridors along the extensive coastlines of eastern and western Australia, South Africa, and South America; may enhance reproductive success (e.g., see Franklin, 2014; Noad et al., 2019) and therefore recovery of humpback whale populations using these habitats. Moreover, observed behaviour at these site-specific locations will be related to the occurrence and timing of classes of humpback whales using these locations.
Pod Associations and Non-agonistic Behaviour in August
While there was no systematic pattern in the frequency of newly associated pods over years in Hervey Bay, the rate of formation of newly associated pods within season was significantly higher during August compared to September and October (see Figures 3A,B). This result, together with the significant differences in pod characteristics and composition within season reported in Franklin et al. (2011), confirm that there are differences in the maturational and reproductive classes of humpback whales present in Hervey Bay in August compared to September and October (also see Franklin et al., 2018).
In Hervey Bay, 52% of singleton pods occurred in August when calves were rarely seen and 69% of 3 and 4+ larger pods with no calves present also occurred in August (Franklin et al., 2011). Overall, in Hervey Bay singletons and pairs predominated in the formation of newly associated pods (Supplementary Appendix Table 1). The social interactions occurring among singletons, involved in non-agonistic social behaviour in August, is reflected in the markedly higher probability of observing two singletons forming pairs in newly associated pods (Figure 3C). Consequently, the presence of socially active immature males and females, involved in increasing social interactions with each other and with mature females (e.g., see Franklin, 2012; Franklin et al., 2018) are likely to contribute to the higher rate of newly associated pods and the occurrence of non-agonistic pods during August.
There was significant variability in the occurrence of non-agonistic social behaviour over years and within season (see Figure 4 above). Franklin et al. (2011) reported a significant increase in pods with 3+ whales over years in Hervey Bay. They suggested that as the population increased, larger groups became more common and were likely to have generated a skewed distribution in the population toward younger whales. Therefore, the variability of non-agonistic social behaviour over years may be related to the relative proportions of age, sex, reproductive and maturational classes of humpback whales entering Hervey Bay in any given year.
Franklin (2012) reported the social interactions of known-age individuals from calves to maturity and the complex social interactions between immature males and females, with non-lactating and lactating mature females. Pack et al. (2012) found that many male-female dyads were comprised of immature whales and suggested that these pairings were important for social learning and development. The social behaviour of mature females and immature males and females is reflected in the higher frequency of non-agonistic pods in newly associated pods and as pod size increases during August.
The probability of observing non-agonistic social behaviour increased with the number of whales in the pod (Figure 3C). Franklin et al. (2011) suggested that because whales enter and leave Hervey Bay from the north, the density and movements of whales increased the likelihood of interactions among pods, contributing to the formation of larger pods or to the probability of encountering recently aggregated pods. Consequently, the higher levels of non-agonistic social behaviour pods observed during August is related to pod associations and the complex social interactions occurring among immature males and females and mature females (Franklin, 2012).
Average residency of humpback whales in Hervey Bay is from 1.5 to 2 weeks (Franklin, 2014). Hervey Bay offers mature females an important habitat for social activity conducive to social development (Franklin, 2012; Franklin et al., 2018), and for physical development of immature male and female whales and calves (Cartwright et al., 2016). We suggest that Hervey Bay is an area of aggregation early in the southern migration, for mature females travelling with the new season’s yearlings and the immature cohort during August.
Competitive Behaviour and Males Maximising Mating Opportunities
Only a low proportion of pods in Hervey Bay were involved in agonistic competitive behaviour. The probability of observing competitive pods in Hervey Bay was at its lowest during August and increased significantly throughout the season. Franklin et al. (2011) reported that pod characteristics early in the season in Hervey Bay were consistent with the presence of immature males and females, while Franklin et al. (2018) confirmed that mature, resting or early pregnant, females use Hervey Bay during August, co-temporal with immature male and female humpback whales with few mature males present. The absence of mature males relative to mature and immature females in August may contribute to the observed lower levels of competitive pods during August.
Although the probability of observing competitive pods was highest from mid-September onward, the number of pods available to engage in competitive behaviour was relatively small, as most pods were composed of mothers alone with their calves (Franklin et al., 2011). Chittleborough (1958, 1965) reported that post-partum oestrous may occur in a minority of cases and this would likely occur 1 month after parturition, and that August is the peak-birthing month. However, mothers with calves are rarely present in Hervey Bay during August and begin moving into the bay with older calves in early September (Franklin et al., 2011, 2018). Consequently, the occurrence of competitive pods from September onward in Hervey Bay, is likely to be related to some males seeking to maximise mating opportunities due to the presence of potentially oestrous mature, and the possibility of post-partum oestrous lactating females (e.g., see Franklin et al., 2018; Pallin et al., 2018). Baker and Herman (1984a) and Craig et al. (2002) reported increased competitive activity of males toward females with a calf at the end of the season in the Hawaiian breeding grounds, related to the declining numbers of non-lactating oestrous females. Consequently, the potential decline in availability of non-lactating oestrous females in Hervey Bay as the season progresses, may be a major factor influencing male behaviour leading to an increased rate of occurrence of competitive pods involving mother-calf pairs toward the end of the season.
Craig et al. (2014) reported that females with calves in the Hawaiian breeding grounds favoured shallow waters to avoid energetically costly male harassment. Overall, few mature male humpback whales use Hervey Bay (Franklin et al., 2018), and the relatively shallow waters of Hervey Bay (Ribbe, 2014) may be beneficial in minimising harassment of mature females from mature males prospecting for mating opportunities among mature females.
It has been reported that the reproductive success of long-lived mammals occurs over many breeding seasons and individual male humpback whales may behave to maximise their reproductive success over a lifetime (Clapham, 1996; Boness et al., 2002). Although Hervey Bay is south of the presumed breeding ground of eastern Australian humpback whales (Simmons and Marsh, 1986; Paterson, 1991; Chaloupka and Osmond, 1999; Smith et al., 2012), it is a habitat where predictable aggregations of females occur (Franklin et al., 2011, 2018). The proportion of competitive pods in Hervey Bay compared to other pod types was low (i.e., 6.3% of pods). However, the presence of some escorting behaviour combined with the occurrence of singing day and night (Mark Francis Franklin, unpublished data) indicates that some mature males are prospecting for mating opportunities in Hervey Bay with late, or post-partum, ovulating females.
Relative Proportions of Non-agonistic and Agonistic Behaviour
Competitive group behaviour has been well documented in the Northern Hemisphere predominantly in breeding grounds (Darling et al., 1983; Tyack and Whitehead, 1983; Baker and Herman, 1984b; Clapham et al., 1992; Clapham, 2000) and within a migratory corridor in the Southern Hemisphere (Brown and Corkeron, 1995). Darling et al. (2006) noted that competitive behaviour is more conspicuous than cooperative relationships, which are more difficult to identify and confirm. Non-agonistic and cooperative behaviour has been reported in various earlier studies (Herman and Antinoja, 1977; Tyack and Whitehead, 1983; Clapham et al., 1992; Brown and Corkeron, 1995). Darling et al. (2006) suggested that non-agonistic behaviour may be more prevalent in humpback whale interactions than has previously been reported, and that while competitive and non-agonistic relations do occur, the relative proportion of each type of behaviour in a humpback population is not known.
This current study provides a measure of the relative proportion of agonistic competitive behaviour and non-agonistic behaviour of humpback whales within season, in a preferential female stopover in Hervey Bay. Overall, agonistic behaviour (7.0%) occurred in competitive groups (6.3%), with only a very small proportion of repulsion or avoidance behaviour by mothers toward escorts (0.7%) occurring outside of competitive groups. However, it is important to note that 82.8% of pods in Hervey Bay were “other behaviour” pods, of which a third (1107 of 3268, 33.9% of pods; see Supplementary Appendix Table 3) were mothers alone with their calf, involved in non-agonistic social behaviour. Consequently, the results of this study substantiate that non-agonistic social behaviour may be more prevalent than aggressive agonistic social behaviour in site-specific locations and habitats, depending upon the classes and timing of humpback whales using such locations and habitats.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Animal Research Authorities issued by the Southern Cross University Animal Care and Ethics Committee.
Author Contributions
TF and WF conceived the fieldwork, designed the study, collected the data, and wrote the manuscript. LB analysed the data and undertook modelling, assisted by TF and WF with data curation and interpretation of results. All authors contributed critically to the manuscript drafts and gave final approval for publication.
Funding
The long-term study of humpback whales in Hervey Bay, being conducted by TF and WF, were supported by The Oceania Project’s paying internship program aboard the annual Whale Research Expeditions, and in part by an Australian Research Council Linkage grant with the Southern Cross University, Marine Ecology Research Centre (MERC) and International Fund for Animal Welfare (IFAW). This research in Hervey Bay was conducted under Scientific and Marine Park Permits issued by the Queensland Parks and Wildlife Service (permit numbers WISP03749806 and MP2006/020), and annual Animal Research Authorities issued by the Southern Cross University Animal Care and Ethics Committee.
Conflict of Interest
LB was employed by StatPlan Consulting Pty. Ltd. PC was employed by Seastar Scientific Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Tim Stevens for assistance in the implementation of the long-term study in Hervey Bay and Emeritus Peter Baverstock and Phil Clapham for their support of the research over many years, and the Research Assistants and the participants in The Oceania Project’s Internship program for their assistance and financial contribution to the study. We particularly thank Mark Cornish for his long-term involvement as a Research Assistant and for his financial support of The Oceania Project. We also thank Greg Luker and Daniele Cagnazzi from Southern Cross University, Marine Ecology Research Centre for assistance with figures. Finally, we thank the reviewers for their constructive comments and suggestions which, enhanced and strengthened the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.652147/full#supplementary-material
References
Alerstam, T., and Hedenström, A. (1998). The development of bird migration theory. J. Avian Biol. 29, 343–369. doi: 10.2307/3677155
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–267. doi: 10.1163/156853974x00534
Baker, C. S., and Herman, L. M. (1984b). Aggressive behaviour between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can. J. Zool. 62, 1922–1937. doi: 10.1139/z84-282
Baker, C. S., and Herman, L. M. (1984a). Seasonal contrasts in the social behavior of humpback whales. Cetus 5, 14–16.
Baker, C. S., Palumbi, S. R., Lambertsen, R. H., Weinrich, M. T., Calambokidis, J., and O Brien, S. J. (1990). Influence of seasonal migration on geographic distribution of mitochondrial DNA haplotypes in humpback whales. Nature 344, 238–240. doi: 10.1038/344238a0
Barendse, J., Best, P. B., Carvalho, I., and Pomilla, C. (2013). Mother knows best: occurrence and associations of Resighted humpback whales suggest maternally derived fidelity to a Southern hemisphere coastal feeding ground. PLoS One 8:e81238. doi: 10.1371/journal.pone.0081238
Barendse, J., Best, P. B., Thornton, M., Pomilla, C., Carvalho, I., and Rosenbaum, H. C. (2010). Migration redefined? Seasonality, movements and group composition of humpback whales Megaptera novaeangliae off the west coast of South Africa. Afr. J. Mar. Sci. 32, 1–22. doi: 10.3354/meps118001
Baudouin, M., de Thoisy, B., Chambault, P., Berzins, R., Entraygues, M., Kelle, L., et al. (2015). Identification of key marine areas for conservation based on satellite tracking of post-nesting migrating green turtles (Chelonia mydas). Biol. Conserv. 184, 36–41. doi: 10.1016/j.biocon.2014.12.021
Boness, D. J., Clapham, P. J., and Mesnick, S. L. (2002). “Life history and reproductive strategies,” in Marine Mammal Biology: An Evolutionary Approach, ed. A. R. Hoelzel (Oxford: Blackwell Science Ltd), 278–324.
Brown, M. R., Corkeron, P. J., Hale, P. T., Schultz, K. W., and Bryden, M. M. (1995). Evidence for a sex-segregated migration in the humpback whale (Megaptera novaeangliae). Proc. R. Soc. Lond. Ser. B Biol. Sci. 259, 229–234. doi: 10.1098/rspb.1995.0034
Brown, M., and Corkeron, P. (1995). Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian coast. Behaviour 132, 163–179. doi: 10.1163/156853995x00676
Bruce, E., Albright, L., Sheehan, S., and Blewitt, M. (2014). Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: a spatial analysis using geographical citizen science data. Appl. Geogr. 54, 83–95.
Burns, D., Brooks, L., Harrison, P., Franklin, T., Franklin, W., Paton, D., et al. (2014). Migratory movements of individual humpback whales photographed off the eastern coast of Australia. Mar. Mamm. Sci. 30, 562–578. doi: 10.1111/mms.12057
Cartwright, R., and Sullivan, M. (2009). Associations with multiple male groups increase the energy expenditure of humpback whale (Megaptera novaeangliae) female and calf pairs on the breeding grounds. Behaviour 146, 1573–1600. doi: 10.1163/156853909x458377
Cartwright, R., Gillespie, B., Labonte, K., Mangold, T., Venema, A., Eden, K., et al. (2012). Between a rock and a hard place: habitat selection in female-calf humpback whale (Megaptera novaeangliae) Pairs on the Hawaiian Breeding Grounds. PLoS One 7:e38004. doi: 10.1371/journal.pone.0038004
Cartwright, R., Newton, C., West, K. M., Rice, J., Niemeyer, M., Burek, K., et al. (2016). Tracking the development of muscular myoglobin stores in mysticete calves. PLoS One 11:e0145893. doi: 10.1371/journal.pone.0145893
Carvalho, I., Brito, C., Dos Santos, M. E., and Rosenbaum, H. C. (2011). The waters of São Tomé: a calving ground for West African humpback whales? Afr. J. Mar. Sci. 33, 91–97.
Chaloupka, M., and Osmond, M. (1999). “Spatial and seasonal distribution of humpback whales in the Great Barrier Reef region,” in Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals. American Fisheries Society symposium No. 23, ed. J. A. Musick (Bethesda MD: American Fisheries Society), 89–106.
Chaloupka, M., Osmond, M., and Kaufman, G. (1999). Estimating seasonal abundance trends and survival probabilities of humpback whales in Hervey Bay (east coast Australia). Mar. Ecol. Prog. Ser. 184, 291–301. doi: 10.3354/meps184291
Chittleborough, R. G. (1958). The breeding cycle of the female humpback whale, Megaptera nodosa, (Bonnaterre). Aust. J. Mar. Freshw. Res. 9, 1–18. doi: 10.1071/MF9580001
Chittleborough, R. G. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Aust. J. Mar. Freshw. Res. 16, 33–128. doi: 10.1071/mf9650033
Chu, K., and Nieukirk, S. (1988). Dorsal fin scars as indicators of age, sex, and social status in humpback whales (Megaptera novaeangliae). Can. J. Zool. 66, 416–420. doi: 10.1139/z88-059
Clapham, P. J. (1993). Social organization of humpback whales on a North Atlantic feeding ground. Symp. Zool. Soc. Lond. 66, 131–145. doi: 10.1093/oxfordjournals.jhered.a111340
Clapham, P. J. (1996). The social and reproductive biology of humpback whales – an ecological perspective. Mamm. Rev. 26, 27–49.
Clapham, P. J. (2000). “The humpback whale – Seasonal feeding and breeding in a baleen whale,” in Cetacean Societies: Field Studies of Dolphins And Whales, eds J. Mann, R. C. Connor, P. L. Tyack, and H. Whitehead (Chicago, IL: The University of Chicago), 173–196.
Clapham, P. J., and Mayo, C. A. (1987). Reproduction and recruitment of individually identified humpback whales, Megaptera novaeangliae, observed in Massachusetts Bay 1979-1985. Can. J. Zool. 65, 2853–2863. doi: 10.1139/z87-434
Clapham, P. J., and Mead, J. G. (1999). Megaptera novaeangliae. Mamm. Species 604, 1–9. doi: 10.2307/3504352
Clapham, P. J., Baraff, L. S., Carlson, C. A., Christian, M. A., Mattila, D. K., Mayo, C. A., et al. (1993). Seasonal occurrence and annual return of humpback whales, Megaptera novaeangliae, in the Southern Gulf of Maine. Can. J. Zool. 71, 440–443. doi: 10.1139/z93-063
Clapham, P. J., Palsboll, P. J., Mattila, D. K., and Oswaldo, V. (1992). Composition and dynamics of humpback whale competitive groups in the West Indies. Behaviour 122, 182–194.
Clapham, P. J., Wetmore, S. E., Smith, T. D., and Mead, J. G. (1999). Length at birth and at independence in humpback whales. J. Cetacean Res. Manag. 1, 141–146.
Clapham, P., Mikhalev, Y., Franklin, W., Paton, D., Baker, S., Ivashchenko, Y. V., et al. (2009). Catches of humpback whales, Megaptera novaeangliae, by the soviet union and other nations in the Southern Ocean, 1947-1973. Mar. Fish. Rev. 71, 39–43.
Constantine, R., Steel, D., Allen, J., Anderson, M., Andrews, O., Baker, C. S., et al. (2014). Remote Antarctic feeding ground important for east Australian humpback whales. Mar. Biol. 161, 1087–1093. doi: 10.1007/s00227-014-2401-2
Corkeron, P. (1993). Aerial Survey Methodology for Hervey Bay Marine Park Queensland – A Review. Report to the Queensland Department of Environment and Heritage, Brisbane, QLD: Queensland Department of Environment and Heritage, 1–33.
Corkeron, P. J., Brown, M., Slade, R. W., and Bryden, M. M. (1994). Humpback Whales, Megaptera novaeangliae (Cetacea: balaenopteridae), in Hervey Bay, Queensland. Wildl. Res. 21, 293–305. doi: 10.1071/wr9940293
Craig, A. S., Herman, L. M., and Pack, A. A. (2002). Male mate choice and male-male competition coexist in the humpback whale (Megaptera novaeangliae). Can. J. Zool. Revue Can. Zool. 80, 745–755.
Craig, A. S., Herman, L. M., Pack, A. A., and Waterman, J. O. (2014). Habitat segregation by female humpback whales in Hawaiian waters: avoidance of males? Behaviour 151, 613–631.
Dalla Rosa, L., Félix, F., Stevick, P. T., Secchi, E. R., Allen, J. M., Chater, K., et al. (2012). Feeding grounds of the eastern South Pacific humpback whale population include the South Orkney Islands. Pol. Res. 31:17324. doi: 10.3402/polar.v31i0.17324
Darling, J. D. (2001). Characterization of Behaviour of Humpback Whales in Hawaiian Waters. Honolulu, HI: Hawaiian Islands Humpback Whale National Marine Sanctuary, Division of Aquatic Resources, Department of Land and Natural Resources, 61.
Darling, J. D., and Berube, M. (2001). Interactions of singing humpback whales with other males. Mar. Mamm. Sci. 17, 570–584. doi: 10.1111/j.1748-7692.2001.tb01005.x
Darling, J. D., Gibson, K. M., and Silber, G. K. (1983). “Observations on the abundance and behavior of humpback whales (Megaptera novaeangliae) off West Maui Hawaii 1977-1979,” in Communication and Behavior of Whales, ed. R. Payne (Boulder, CO: Westview Press), 201–222.
Darling, J. D., Jones, M. E., and Nicklin, C. P. (2006). Humpback whale songs: do they organize males during the breeding season? Behaviour 143, 1051–1101.
Dawbin, W. H. (1966). “The seasonal migratory cycle of humpback whales,” in Whales, Dolphins and Porpoises, ed. K. S. Norris (Berkeley, CA: University of California Press), 145–170. doi: 10.1525/9780520321373-011
Dawbin, W. H. (1997). Temporal segregation of humpback whales during migration in southern hemisphere waters. Mem. Qld. Mus. 42, 105–138.
Delmore, K. E., Fox, J. W., and Irwin, D. E. (2012). Dramatic intraspecific differences in migratory routes, stopover sties and wintering areas, revealed using light-level geolocators. Proc. R. Soc. B 279, 4582–4589. doi: 10.1098/rspb.2012.1229
Derville, S., Torres, L. G., Zerbini, A. N., Oremus, M., and Garrigue, C. (2020). Horizontal and vertical movements of humpback whales inform the use of critical pelagic habitats in the western South Pacific. Sci. Rep. 10:4871. doi: 10.1038/s41598-020-61771-z
Dujon, A. M., Schofield, G., Lester, R. E., Esteban, N., and Graeme, C. H. (2017). Fatloc-GPS reveals daytime departure and arrival during long-distance migration and the use of different resting strategies in sea turtles. Mar. Biol. 164:187. doi: 10.1007/s00227-017-3216-8
Franklin, T. (2012). The Social and Ecological Significance of Hervey Bay Queensland for Eastern Australian Humpback Whales (Megaptera novaeangliae). Ph.D. thesis. Lismore, NSW: School of Environmental Science and Management, Southern Cross University, 245.
Franklin, T., Franklin, W., Brooks, L., and Harrison, P. (2018). Site-specific female-biased sex ratio of humpback whales (Megaptera novaeangliae) during a stopover early in the southern migration. Can. J. Zool. 96, 533–544. doi: 10.1139/cjz-2017-0086
Franklin, T., Franklin, W., Brooks, L., Harrison, P. L., Burns, D., Holmberg, J., et al. (2020). Photo-identification of individual Southern Hemisphere humpback whales (Megaptera novaeangliae) using all available natural marks: implications for misidentification & automated algorithm matching technology. J. Cetacean Res. Manag. 21, 71–83.
Franklin, T., Franklin, W., Brooks, L., Harrison, P., Baverstock, P., and Clapham, P. (2011). Seasonal changes in pod characteristics of eastern Australian humpback whales (Megaptera novaeangliae), Hervey Bay 1992-2005. Mar. Mamm. Sci. 27, E134–E152. doi: 10.1111/j.1748-7692.2010.00430.x
Franklin, W. (2014). Abundance, Population Dynamics, Reproduction, Rates of Population Increase and Migration Linkages of Eastern Australian Humpback Whales (Megaptera novaeangliae) Utilising Hervey Bay, Queensland. Ph.D. thesis. Lismore, NSW: School of Environmental Science and Management, Southern Cross University, 396.
Franklin, W., Franklin, T., Andrews-Goff, V., Paton, D. A., and Double, M. (2017). Movement of two Humpback whales (Megaptera novaeangliae) satellite-radio tagged off Eden, NSW and matched by photo-identification with the Hervey Bay catalogue. J. Cetacean Res. Manag. 17, 29–33.
Franklin, W., Franklin, T., Brooks, L., Gibbs, N., Childerhouse, S., Smith, F., et al. (2012). Antarctic waters (Area V) near the Balleny Islands are a summer feeding area for some Eastern Australian (E (i) breeding group) Humpback whales (Megaptera novaeangliae). J. Cetacean Res. Manag. 12, 321–327.
Garrigue, C., Clapham, P. J., Geyer, Y., Kennedy, A. S., and Zerbini, A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc. Open Sci. 2:150489. doi: 10.1098/rsos.150489
Gill, P. C., Evans, K. J., and Wapstra, H. (1998). Feeding by Humpback Whales in Tasmanian Waters. Records of the Queen Victoria Museum Launceston 107. Launceston, TAS: Queen Victoria Museum and Art Gallery, 1–5.
Glockner, D. (1983). “Determining the sex of humpback whales (Megaptera novaeangliae) in their natural environment,” in Communication and Behaviour of Whales, ed. R. Payne (Boulder, CO: Westview Press Inc), 447–464.
Glockner, D. A., and Venus, S. C. (1983). “Identification, growth rate and behavior of humpback whale (Megaptera novaeangliae) cows and calves in the waters off Maui, Hawaii, 1977-1979,” in Communication and Behavior of Whales, ed. R. S. Payne (Boulder, CO: Westview Press), 223–258.
Harrison, P. L., and Woinarski, J. C. Z. (2018). “Recovery of Australian subpopulations of humpback whale. Chapter 2,” in Recovering Australian Threatened Species, eds S. Garnett, P. Latch, D. Lindemeyer, and J. Woinarski (Clayton, VIC: CSIRO Publishing), 5–12.
Herman, L. M. (2017). The multiple functions of male song within the humpback whale (Megaptera novaeangliae) mating system: review, evaluation, and synthesis. Biol. Rev. 92, 1795–1818. doi: 10.1111/brv.12309
Herman, L. M., and Antinoja, R. C. (1977). Humpback whales in Hawaiian breeding waters: population and pod characteristics. Sci. Rep. Whales Res. Inst. 29, 59–85.
Herman, L. M., Forestell, P. H., and Antinoja, R. C. (1980). “The 1976/77 migration of humpback whales into Hawaiian waters: composite description,” in Final Report to the U.S. Marine Mammal Commission, Report # MMC-77/19. National Technical Information Service PBSO-162332. Arlington, VA: United States National Technical Information Services, 55.
Herman, L. M., Pack, A. A., Rose, K., Craig, A., Herman, E. Y. K., Hakala, S., et al. (2011). Resightings of humpback whales in Hawaiian waters over spans of 10-32 years: site fidelity, sex ratios, calving rates, female demographics, and the dynamics of social and behavioral roles of individuals. Mar. Mamm. Sci. 27, 736–768.
IBM Corporation (2019). IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corporation.
Ivashchenko, Y. V., and Clapham, P. J. (2014). Too much is never enough: the cautionary tale of soviet illegal whaling. Mar. Fish. Rev. 76, 1–21. doi: 10.7755/mfr.76.1_2.1
Jones, M. E. (2010). Female Humpback Whale (Megaptera novaeangliae) Reproductive Class and Male–Female Interactions During the Breeding Season. Ph.D. thesis. Keene, NH: Antioch University, 202.
Katona, S. K., and Beard, J. A. (1990). Population size, migrations and feeding aggregations of the Humpback whale (Megaptera novaeangliae) in the Western North Atlantic Ocean. Rep. Int. Whaling Comm. Spec. Issue 12, 295–305.
Kennedy, J. S. (1951). The migration of the Desert Locust (Schistocerca gregaria Forsk.) I. The behaviour of swarms. II. A theory of long-range migrations. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 235, 163–290. doi: 10.1098/rstb.1951.0003
Lockyer, C. (1981). Growth and energy budgets of large baleen whales from the Southern Hemisphere. FAO Fish. Ser. 5, 379–487.
Lockyer, C. (1984). Review of baleen whale reproduction and implications for management. Rep. Int. Whaling Comm. Spec. Issue 6, 27–50. doi: 10.1016/0048-9697(94)90087-6
MacKay, M. M., Würsig, B., Bacon, C. E., and Selwyn, J. D. (2016). North Atlantic humpback whale (Megaptera novaeangliae) hotspots defined by bathymetric features off western Puerto Rico. Can. J. Zool. 94, 517–527. doi: 10.1139/cjz-2015-0198
Martin, A. R., Katona, S. K., Matilla, D., Hembree, D., and Waters, T. D. (1984). Migration of humpback whales between the Caribbean and Iceland. J. Mammal. 65, 330–333.
McCabe, J. D., and Olsen, B. J. (2015). Landscape-scale habitat availability, and not local geography, predicts migratory landbird stopover across the Gulf of Maine. J. Avian Biol. 46, 395–405. doi: 10.1111/jav.00598
McCord, J., and Davis, A. (2012). Characteristics of monarch butterflies (Danaus plexippus) that stopover at a site in coastal South Carolina during fall migration. J. Res. Lepid. 45, 1–8.
Meynecke, J.-O., Vindenes, S., and Teixeira, D. (2013). “Monitoring humpback whale (Megaptera novaeangliae) behaviour in a highly urbanised coastline: gold Coast, Australia,” in Global Challenges in Integrated Coastal Zone Management, 2nd Edn, eds E. Moksness, E. Dahl, and J. Støttrup (Chichester: Wiley-Blackwell Ltd), 101–113. doi: 10.1002/9781118496480.ch8
Mikhalev, Y. A. (1997). Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar. Ecol. Prog. Ser. 149, 13–21.
Mobley, J. R., and Herman, L. M. (1985). Transience of social affiliations among humpback whales in Hawaiian breeding waters. Can. J. Zool. 63, 762–772. doi: 10.1139/z85-111
Nivière, M., Chambault, P., Pérez, T., Etienne, D., Bonola, M., Martin, J., et al. (2018). Identification of marine key areas across the Caribbean to ensure the conservation of the critically endangered hawksbill turtle. Biol. Conserv. 223, 170–180. doi: 10.1016/j.biocon.2018.05.002
Noad, M. J., Kniest, E., and Dunlop, R. A. (2019). Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery. Popul. Ecol. 61, 198–209. doi: 10.1002/1438-390X.1014
Owen, K., Warren, J. D., Noad, M. J., Donnelly, D., Goldizen, A. W., and Dunlop, R. A. (2015). Effect of prey type on the fine-scale feeding behaviour of migrating east Australian humpback whales. Mar. Ecol. Prog. Ser. 541, 231–244. doi: 10.3354/meps11551
Pack, A. A., Herman, L. M., Craig, A. S., Spitz, S. S., and Deakos, M. H. (2002). Penis extrusions by humpback whales (Megaptera novaeangliae). Aquat. Mamm. 28, 131–146.
Pack, A. A., Herman, L. M., Craig, A. S., Spitz, S. S., Waterman, J. O., Herman, E. Y. K., et al. (2017). Habitat preferences by individual humpback whale mothers in the Hawaiian breeding grounds vary with the age and size of their calves. Anim. Behav. 133, 131–144. doi: 10.1016/j.anbehav.2017.09.012
Pack, A. A., Herman, L. M., Spitz, S. S., Craig, A. S., Hakala, S., Deakos, M. H., et al. (2012). Size-assortative pairing and discrimination of potential mates by humpback whales in the Hawaiian breeding grounds. Anim. Behav. 84, 983–993.
Pack, A. A., Herman, L. M., Spitz, S. S., Hakala, S., Deakos, M. H., and Herman, E. Y. K. (2009). Male humpback whales in the Hawaiian breeding grounds preferentially associate with larger females. Anim. Behav. 77, 653–662. doi: 10.1016/j.anbehav.2008.11.015
Pallin, L. J., Baker, C. S., Steel, D., Kellar, N. M., Robbins, J., Johnston, D. W., et al. (2018). High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic Peninsula, evidence of a rapidly growing population. R. Soc. Open Sci. 5:180017. doi: 10.1098/rsos.180017
Palsbøll, P. J., Allen, J., Berube, M., Clapham, P. J., Feddersen, T. P., Hammond, P. S., et al. (1997). Genetic tagging of humpback whales. Nature 388, 767–769.
Paterson, R. A. (1991). The migration of Humpback Whales Megaptera novaeangliae in east Australian waters. Mem. Qld. Museum 30, 333–341.
Payne, R., and McVay, S. (1971). Songs of humpback whales. Science 173, 585–597. doi: 10.1126/science.173.3997.585
Ribbe, J. (2014). “Hervey bay and its estuaries,” in Estuaries of Australia in 2050 and Beyond, Estuaries of the World, ed. E. Wolanski (Dordrecht: Springer Science+Business Media),Google Scholar
Rice, M. R., and Balazs, G. H. (2008). Diving behavior of the Hawaiian green turtle (Chelonia mydas) during oceanic migrations. J. Exp. Mar. Biol. Ecol. 356, 121–127. doi: 10.1016/j.jembe.2007.12.010
Sawyer, H., and Kauffman, M. J. (2011). Stopover ecology of a migratory ungulate. J. Anim. Ecol. 80, 1078–1087. doi: 10.1111/j.1365-2656.2011.01845.x
Schaub, M., Jenni, L., and Bairlein, F. (2008). Fuel stores, fuel accumulation, and the decision to depart from a migration stopover site. Behav. Ecol. 19, 657–666. doi: 10.1093/beheco/arn023
Schaub, M., Pradel, R., Jenni, L., and Lebreton, J.-D. (2001). Migrating birds stopover longer than usually thought: an improved capture-recapture analysis. Ecology 82, 852–859. doi: 10.2307/2680203
Sharpe, F. A. (2001). Social Foraging of the Southeast Alaskan Humpback Whale (Megaptera novaeangliae). Ph.D. dissertation. Vancouver, BC: Simon Fraser University, 129.
Sharpe, F., Szabo, A., Pack, A. A., and Nahmens, J. (2013). “The social structure of bubble net feeding whales in SE Alaska. Oral presentation,” in Proceedings of the 20th Biennial Conference on the Biology of Marine Mammals, Dunedin.
Simmons, M. L., and Marsh, H. E. (1986). Sightings of humpback whales in Great Barrier Reef waters. Sci. Rep. Whales Res. Inst. 37, 31–46. doi: 10.1002/eap.2214
Smith, J. N., Grantham, H. S., Gales, N., Double, M. C., Noad, M. J., and Paton, D. (2012). Identification of humpback whale breeding and calving habitat in the Great Barrier Reef. Mar. Ecol. Prog. Ser. 447, 259–272. doi: 10.3354/meps09462
Stack, S. H., Currie, J. J., McCordic, J. A., Machernis, A. F., and Olson, G. L. (2020). Distribution patterns of east Australian humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland: a historical perspective. Aust. Mammal. 42, 16–27. doi: 10.1071/am18029
Stamation, K. A., Croft, D. B., Shaughnessy, P. D., and Waples, K. A. (2007). Observations of humpback whales (Megaptera novaeangliae) feeding during their southward migration along the coast of south-eastern New South Wales, Australia: identification of a possible supplemental feeding ground. Aquat. Mamm. 33, 165–174.
Stockin, K. A., and Burgess, E. A. (2005). Opportunistic feeding of an adult humpback whale (Megaptera novaeangliae) migrating along the coast of Southeastern Queensland, Australia. Aquat. Mamm. 31, 120–123. doi: 10.1578/am.31.1.2005.120
True, F. W. (1904). The Whalebone Whales of the Western North Atlantic Compared with Those Occurring in European Waters; With Some Observations on the Species of the North Pacific, Vol. 33. Washington, DC: Smithsonian Institution Press, 1–318.
Tyack, P. (1981). Interactions between singing Hawaiian humpback whales and conspecifics nearby. Behav. Ecol. Sociobiol. 8, 105–116. doi: 10.1007/bf00300822
Tyack, P., and Whitehead, H. (1983). Male competition in large groups of wintering humpback whales. Behaviour 83, 132–154. doi: 10.1163/156853982x00067
Weber, T. P., Alerstam, T., and Hedenström, A. (1998). Stopover decisions under wind influence. J. Avian Biol. 29, 552–560. doi: 10.2307/3677175
Wedderburn, R. W. M. (1974). Quasi-likelihood functions, generalized linear models, and the Gauss—Newton method. Biometrika 61, 439–447. doi: 10.2307/2334725
Weinrich, M. T., and Kuhlberg, A. K. (1991). Short-term association patterns of humpback whale (Megaptera novaeangliae) groups on their feeding grounds in the southern Gulf of Maine. Can. J. Zool. 69, 3005–3011. doi: 10.1139/z91-424
Whitehead, H. (1983). Structure and stability of humpback whale groups off Newfoundland. Can. J. Zool. 61, 1391–1397. doi: 10.1139/z83-186
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2014). The Action Plan for Australian Mammals 2012. Clayton, VIC: CSIRO Publishing, 1038.
Zaynagutdinova, E. M., Kouzov, S. A., Batova, P. R., Mikhailov, Y. M., and Kravchuk, A. V. (2019). Spring migration stopovers of swans Cygnus sp. In the Russian part of the Gulf of Finland. Wildfowl 5, 123–138.
Zoidis, A. M., Lomac-Macnair, K. S., Chomos-Betz, A. E., Day, A. J., and MacFarland, A. S. (2014). Effects of sex, seasonal period, and sea state on calf behavior in Hawaiian humpback whales (Megaptera novaeangliae). Aquat. Mamm. 40, 44–58.
Keywords: humpback whale, Megaptera novaeangliae, Hervey Bay, agonistic and non-agonistic social behaviour, pod associations, mate competition, migratory stopovers
Citation: Franklin T, Franklin W, Brooks L, Harrison P, Pack AA and Clapham PJ (2021) Social Behaviour of Humpback Whales (Megaptera novaeangliae) in Hervey Bay, Eastern Australia, a Preferential Female Stopover During the Southern Migration. Front. Mar. Sci. 8:652147. doi: 10.3389/fmars.2021.652147
Received: 11 January 2021; Accepted: 03 November 2021;
Published: 03 December 2021.
Edited by:
Janet Mann, Georgetown University, United StatesReviewed by:
Ari Friedlaender, University of California, Santa Cruz, United StatesRobert McCauley, Curtin University, Australia
Copyright © 2021 Franklin, Franklin, Brooks, Harrison, Pack and Clapham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trish Franklin, trish@oceania.org.au
 Trish Franklin
Trish Franklin Wally Franklin
Wally Franklin Lyndon Brooks
Lyndon Brooks Peter Harrison
Peter Harrison Adam A. Pack
Adam A. Pack Phillip J. Clapham
Phillip J. Clapham