Breaking All the Rules: The First Recorded Hard Substrate Sessile Benthic Community Far Beneath an Antarctic Ice Shelf
- 1British Antarctic Survey, Cambridge, United Kingdom
- 2Ryan Institute, National University of Ireland Galway, Galway, Ireland
- 3Geoscience Australia, Canberra, ACT, Australia
- 4National Institute of Water and Atmospheric Research, Wellington, New Zealand
- 5Department of Physics, University of Auckland, Auckland, New Zealand
- 6Earth and Planetary Sciences, University of California, Santa Cruz, Santa Cruz, CA, United States
The seafloor beneath floating ice shelves accounts roughly a third of the Antarctic’s 5 million km2 of continental shelf. Prior to this study, our knowledge of these habitats and the life they support was restricted to what has been observed from eight boreholes drilled for geological and glaciological studies. The established theory of sub-ice shelf biogeography is that both functional and taxonomic diversities decrease along a nutrient gradient with distance from the ice shelf front, resulting in a depauperate fauna, dominated by mobile scavengers and predators toward the grounding line. Mobile macro-benthic life and mega-benthic life have been observed as far as 700 km under an ice shelf. New observations from two boreholes in the Filchner-Ronne Ice Shelf challenge the idea that sessile organisms reduce in prevalence the further under the ice you go. The discovery of an established community consisting of only sessile, probably filter feeding, organisms (sponges and other taxa) on a boulder 260 km from the ice front raises significant questions, especially when the local currents suggest that this community is somewhere between 625 km and 1500 km in the direction of water flow from the nearest region of photosynthesis. This new evidence requires us to rethink our ideas with regard to the diversity of community types found under ice shelves, the key factors which control their distribution and their vulnerability to environmental change and ice shelf collapse.
Introduction
Antarctic continental shelf benthos is often dominated by large, sessile, filter feeding communities (Gutt et al., 2013). These have been shaped by millennia of cold and highly seasonal conditions driven by glacial cycles, annual sea ice formation and melt, and the impacts of iceberg scour. The huge flux of food coming from the plankton above, driven by the summer melt and continuous daylight, allow these communities to thrive and achieve very high levels of biomass (Griffiths, 2010). This contrasts sharply with the areas beneath the floating ice shelves, which are hidden from daylight and often far from areas of primary productivity (Ingels et al., 2021).
Ice shelves cover roughly a third of the Antarctic’s 5 million km2 of continental shelf (Ingels et al., 2018). The Ronne-Filchner Ice Shelf, in the Weddell Sea, is the second largest Antarctic ice shelf, accounting for ∼28% of the total area under ice shelves, covering around 420,000 km2 of seabed. Further north on the East Antarctic Peninsula, the collapses of the Larsen A and Larsen B ice-shelves, in 1995 and 2002, and the recent high-profile calving of the giant iceberg, A-68, from Larsen C, have highlighted how little we know about the habitat beneath these floating ice shelves.
Our knowledge of the biological communities beneath these ice shelves is limited to sparse observations through boreholes and scientific expeditions that investigated the sites of Larsen A and B at least 5 years after their collapse (Ingels et al., 2021). Current theory suggests a gradient in abundance and community type exists under ice shelves with distance from open water. Sessile suspension-feeders are believed to be restricted to areas of inflow, close to the ice shelf front, with deposit-feeders and detritivores, feeding on ever more limited food, further under the ice shelf (Ingels et al., 2018).
These borehole records are the result of images captured as part of geological and glaciological sampling which happened to record images of the seafloor life beneath the ice shelves. To date, the furthest “inland” from the ice shelf front where life has been observed is 700 km from the Ross Ice Shelf front (Table 1). To put this in perspective that is over 64 times the depth of the Mariana Trench in distance from any known primary productivity. The WISSARD Program observed amphipods and fish at the seafloor and pelagic gelatinous organisms in the cavity beneath the Whillans Ice Stream (Kingslake et al., 2018), but there was no evidence of benthic organisms or for bioturbation in the sediment cores. Similarly, the Ross Ice Shelf Project (1977-78) used baited traps and cameras to observe mobile fauna such as numerous amphipods, two fish and an isopod; however, no live sessile organisms were recorded (Bruchhausen et al., 1979; Lipps et al., 1979). Sediment samples obtained from the borehole, ∼475 km from the Ross Ice Shelf front, also contained the dead remains of meiofaunal foraminifera, bivalves, gastropods, ostracods, and possible polychete tubes but did not find any living infauna. The 2017 Aotearoa New Zealand Ross Ice Shelf Program drilled a borehole in the middle of the ice shelf, some 300 km from the shelf front (Stevens et al., 2020). In addition to multiple pelagic organisms, they observed an ophiuroid, a benthic fish, and what appeared to be infaunal burrows.
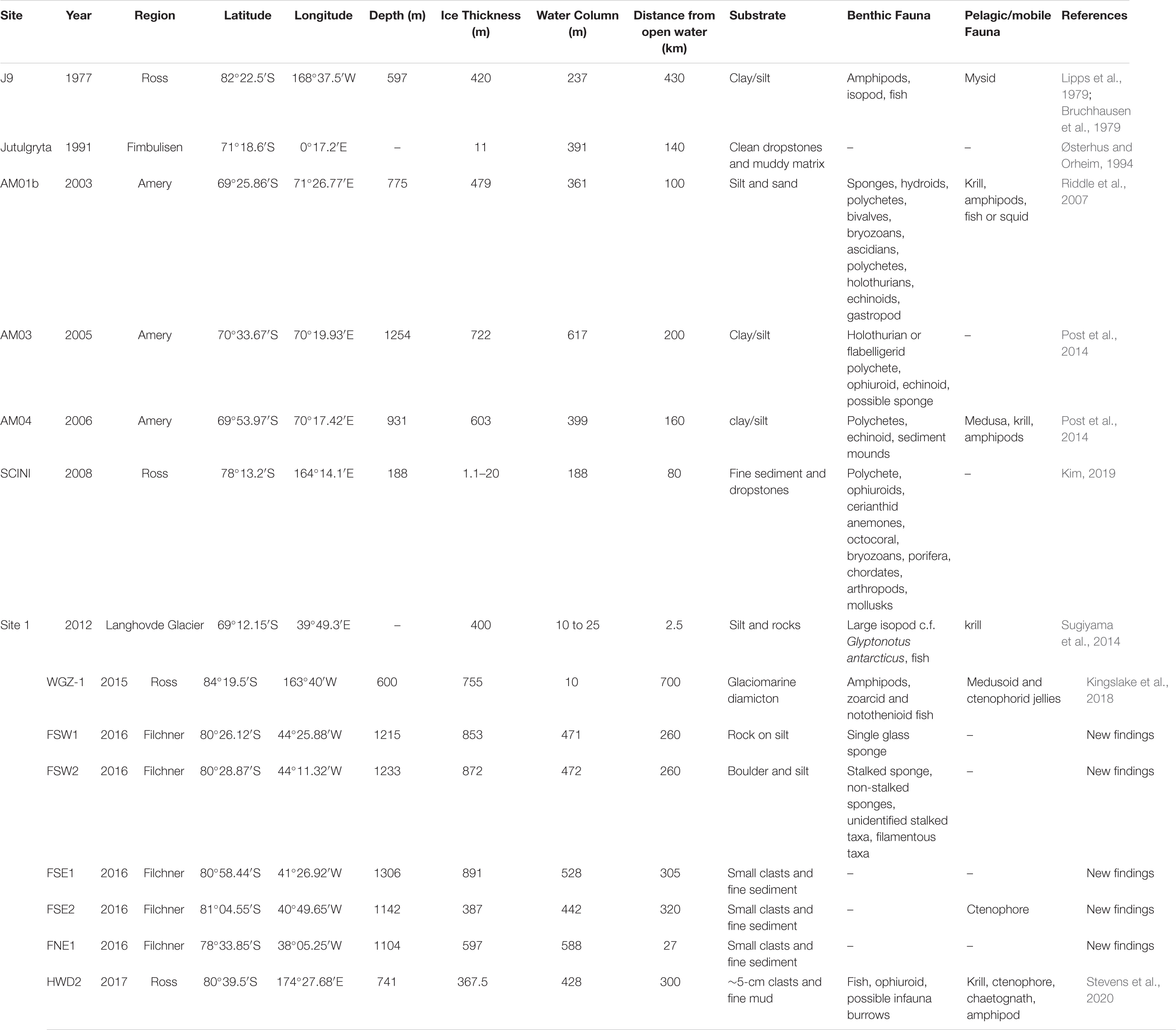
Table 1. Details of Antarctic Ice Shelf boreholes that have seafloor images, including those with living organisms.
Multiple boreholes have been drilled through the Amery Ice Shelf at varying distances from the ice front. In 2003, a downward facing camera system investigated a borehole (AM01b) 100 km from the ice shelf front (Table 1 and Figure 1) and observed a diverse assemblage comparable with coastal, sea ice-dominated locations or deeper water communities (Riddle et al., 2007). Observed taxa were dominated by sessile suspension feeders such as bryozoans, ascidians, polychetes, hydroids, bivalves, and sponges, as well as mobile fauna such as echinoids, flabelligerid worms, holothurians, and gastropods (Riddle et al., 2007). A less diverse and more sparse community was found in 2005 by cameras in a borehole 200 km in from the Amery Ice Shelf front (AM03), recording mobile deposit feeders and evidence of potential suspension/filter feeders (Post et al., 2014). In 2006, a further borehole (AM04) revealed a surface-living benthic polychete, a heart urchin, and polychete tubes 160 km from the Amery Ice Shelf front (Table 1). All Amery Ice Shelf observations recorded evidence of krill and amphipods in the water column, with multiple observations of medusa at AM04. The overall distribution of life beneath the Amery appears to be strongly controlled by ocean circulation, with richer and more diverse taxa associated with nutrient-rich inflowing currents and more impoverished seafloor coinciding with nutrient-poor outflow (Post et al., 2014).
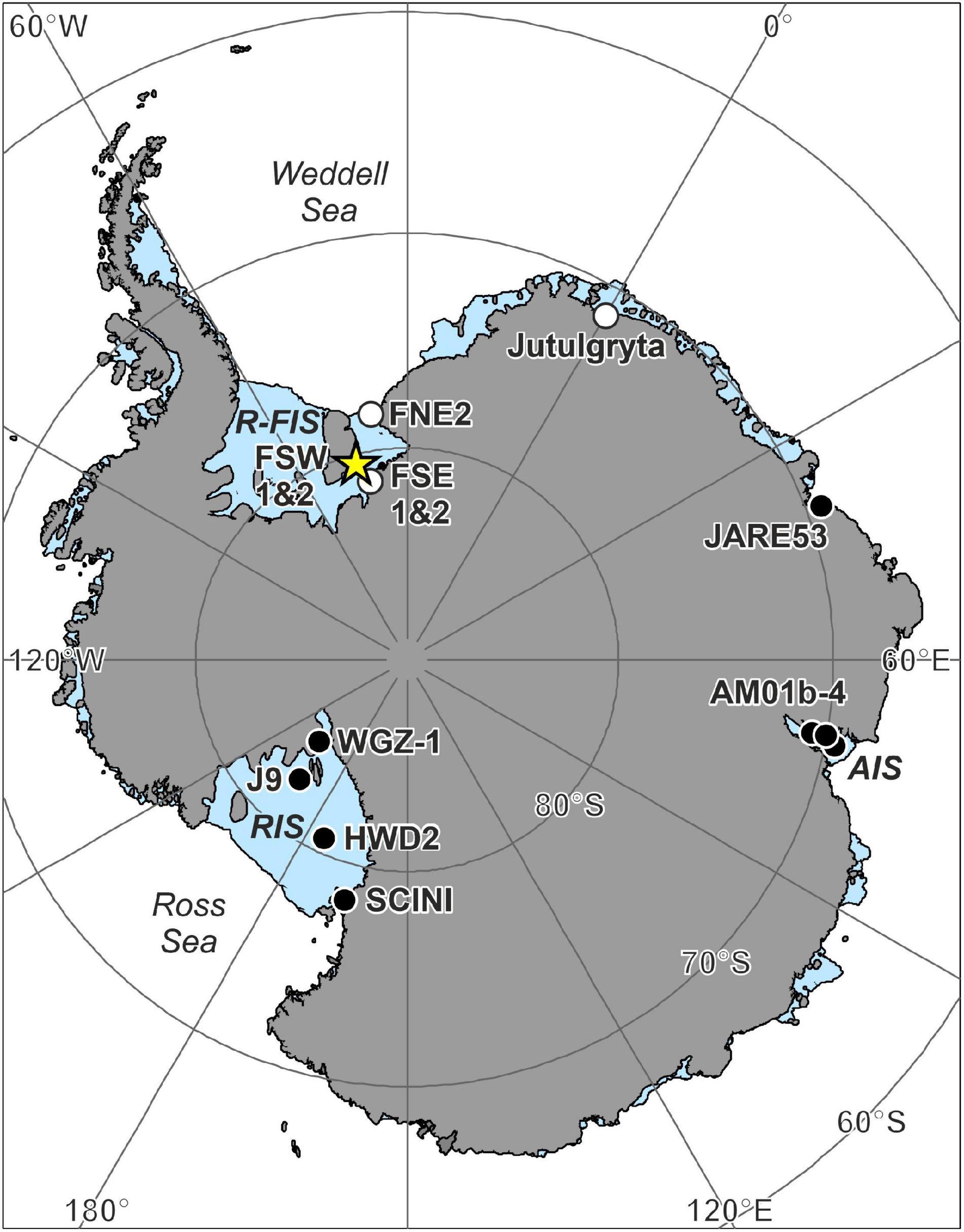
Figure 1. Antarctic ice shelf borehole locations with seafloor images. Details for each location can be found in Table 1. New records with life present from this study are marked with a star, boreholes where life was observed with a black circle and where no life was observed or reported with a white circle. R-FIS, Ronne-Filchner Ice Shelf; RIS, Ross Ice Shelf; AIS, Amery Ice Shelf.
Other studies have sampled narrower ice shelves or regions nearer to the ice shelf front. The most diverse fauna recorded came from the Ross Sea, 80 km inland from the ice front (Kim, 2019). They observed eight different phyla and a high biomass of organisms. Observations from only 2.5 km landward of the Langhovde Glacier ice front yielded only a single large isopod crustacean and a fish (Sugiyama et al., 2014). Not all boreholes have resulted in observations of benthic organisms. An expedition to the Fimbul Ice Shelf in 1991 found a seafloor of mud and dropstones, but no visible life, 140 km from the ice shelf front (Østerhus, 1994).
Whilst the studies beneath the Amery and Ross Ice Shelf have been transformative to our understanding of sub-ice-shelf ecosystems, the paucity of information from other ice shelves undoubtedly means we are missing vital information about the diversity and structure of sub-ice-shelf habitats. Such information is important for our understanding of how ice shelf collapse might affect these communities and our interpretation of ice shelf history from sediment records. Here we present the first observations of the sub-ice shelf fauna of the Filchner-Ronne Ice Shelf (FRIS) and discuss its significance in relation to previous records elsewhere.
Materials and Methods
Access holes were drilled through the 387–890 m thick Filchner Ice Shelf (FIS) during the austral summer of 2015–2016 and 2016–2017 (Figure 2) using the British Antarctic Survey (BAS) hot water drill system (Makinson and Anker, 2014). Water column and seabed imagery was obtained using a GoPro HERO4 video camera, protected within an off-the-shelf pressure housing that was mounted above a BAS-modified UWITEC gravity corer. The GoPro recorded video at 30 frames per second, with a 1080 p resolution and a fixed ISO of 1600. Conductivity-temperature-depth (CTD) profiles were additionally obtained using a Seabird SBE49 with estimated accuracies of 0.004°C and 0.005, for temperature and salinity, respectively (Huhn et al., 2018).
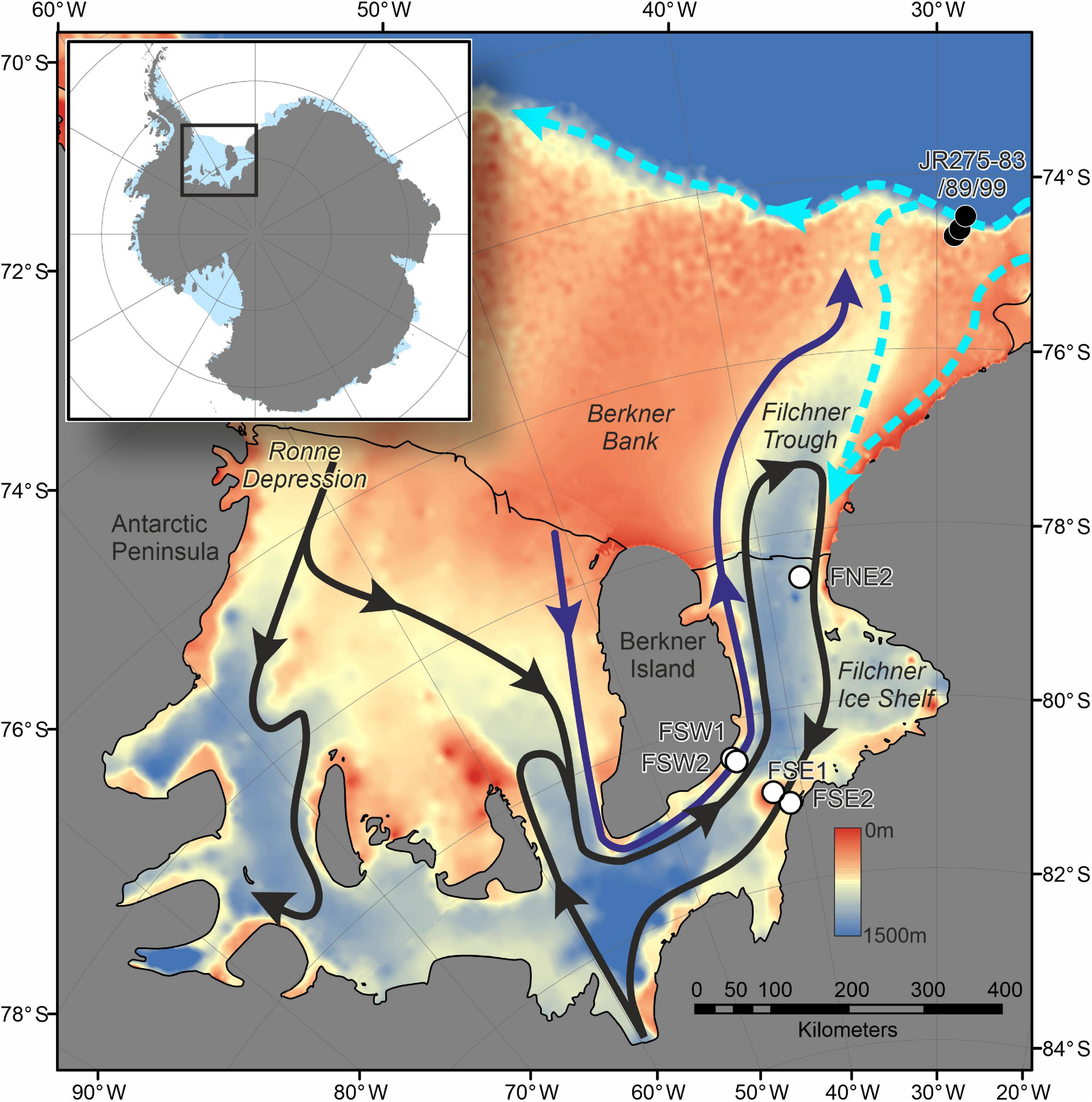
Figure 2. Map showing location of drill sites on Filchner Ice Shelf (FSW1-2, FSE1-2, and FNE2), comparable samples from continental shelf collected during JR275 as well as the major sub-ice shelf circulation. Black arrows show flows derived from High Salinity Shelf Water (HSSW) from the Ronne Depression. Purple arrow shows the flow from HSSW formed over Berkner Bank (Nicholls, 2004). Ice Shelf Water (ISW) exits along the eastern margin of Filchner Trough, with a possible seasonal influx of modified Warm Deep Water (mWDW) (Darelius et al., 2016). Dashed light blue arrows represent the flow of the slope front and coastal currents (Nicholls et al., 2009). Bathymetry is derived from ETOPO1 (NOAA National Geophysical Data Center, 2009).
Drill sites FSW1 and FSW2 (Figures 1, 2 and Table 1) are located on the western margin of FIS close to Berkner Island, 260 km from the ice shelf front, in a region where the ice shelf base is experiencing no significant melting or freezing (Makinson et al., 2011). Sites FSW1 and FSW2 are on the opposite side of the Filchner Trough, over 300 km from the ice shelf front. Drill site FNE2 was located on the northern end of the FIS in an area of inflow, only 27 km from the ice shelf front (Figures 1, 2 and Table 1).
The primary location of our observations is FSW2, where the ice shelf and water column are 872 and 472 m, respectively (Figures 1, 2 and Table 1). Despite multiple attempts to obtain a sediment core at FSW2, the corer hit a large sub-rounded boulder which is black/gray in color that was found to host a diverse benthic fauna (see Supplementary Video). Inspection of available video footage suggests that the boulder is mafic (gabbro?). Possible source regions include the Pensacola Mountains, which is part of an extensive Middle Jurassic igneous province related to and emplaced just prior to Gondwana break-up (Ford, 1976; Ferris et al., 1998). The Dufek Massif, for example, consists of well-layered pyroxene gabbro that contains abundant magnetite in higher levels that is visually similar to the boulder imaged here (Ford, 1976). We assume that the boulder has been transported by the ice shelf, eventually melting out at the drill site. In this context, a source in the Dufek Massif would make sense as the drill site lies directly downstream of this mountain range.
The hydrography of site FSW2 is dominated by inflow of cold High Salinity Shelf Water (HSSW; Figure 1) which forms along Berkner Bank or is re-circulated, originally entering the cavity via Ronne Depression (Ronne Trough) (Nicholls et al., 2009). The water column is characterized by two well-mixed zones, the upper 100 m below the ice shelf base and in the 250 m thick layer above the bottom (Huhn et al., 2018). Potential temperature (θ) close to the ice shelf base is cold (−2.49°C), although this is still warmer than the in situ freezing temperature at this depth. The bottom layer is warmer (−2.2°C) and more saline (34.61) (Huhn et al., 2018). Ice Shelf Water exits Filchner Trough along the eastern margin, which is also characterized by the re-circulation of HSSW and seasonal input of modified Warm Deep Water (Figure 2) (Darelius et al., 2016; Nachtsheim et al., 2019). Currents at FSW2 are likely to be strong with model estimates of up to 0.25 m s–1 (Daae et al., 2020) and carry with them a visible particulate load of silt-sized detritus. It is unclear if this is entirely terrigenous or whether it also includes a biogenic component.
The dimensions of the boulder and associated fauna were estimated by comparison with those of the corer which had a maximum radius of 11 cm and a height of 75 cm.
Results
The boulder below FSW2 is located at a depth of 1,233 m and approximately 260 km from the modern calving margin of FIS (measured as a straight line through water). However, this is in the opposite direction of the main flow of HSSW (Nicholls, 2004). Following the two main sources of HSSW would put the boulder at > 1500 km from FIS front (following HSSW from the Ronne Depression) or > 625 km from Ronnie Ice Shelf front (following HSSW formed over Berkner Bank) (Figure 2).
The boulder itself is approximately 96 cm long by 69 cm wide and around 75 cm high. Fauna is largely concentrated on the sides of the boulder (Figure 3). The upper surface of the boulder seems to have a patchy coating of sediment of a similar color to the surrounding substrate The surrounding sediments show ripples formed by currents but there is no visible evidence of infauna or mobile epifauna.
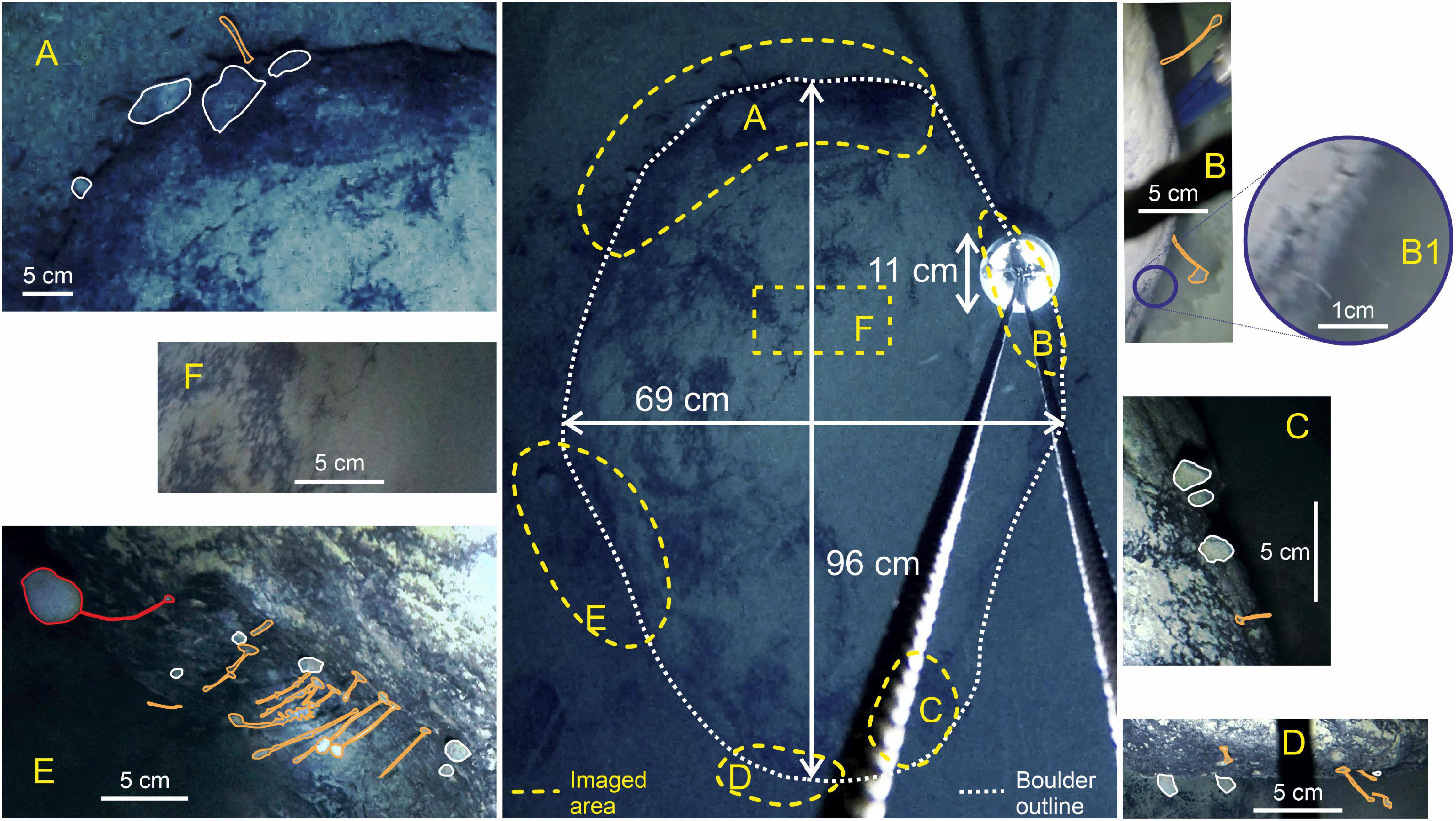
Figure 3. Dimensions and close-ups of the boulder, highlighting where life is clearly visible (A–E) and the top of the boulder where no obvious life is visible (F). The taxa visible on the boulder: Red, large stalked sponge; White, sponge; Orange, stalked taxa [possible sponge, ascidians, hydroid, barnacles, cnidaria (e.g., tubularia), and polychetes].
The fauna associated with the boulder can be categorized into three main types of suspension feeders: a stalked sponge, non-stalked sponges, and unidentifiable stalked taxa (possible sponges, ascidians, hydroids, barnacles, cnidarian, or polychetes). It is also possible that the stalked sponge and/or stalked taxa might be carnivorous sponges, similar to Cladorhizidae. Only one confirmed stalked sponge (Figure 3E) was observed at a length of approximately 8.9 cm; 15 non-stalked sponges were observed around the edges of the boulder, the largest of which was 6.64 cm wide by 4 cm tall. Unidentifiable stalked taxa were the most numerous group, accounting for 58% of all observed individuals (22 individuals), the longest of which was estimated to be ∼6.6 cm long (Table 2). Figure 3B shows evidence of filamentous organisms of around 1 cm in length which could not be identified further but are possibly bacterial mats or hydroids. The upper surface of the boulder (Figure 3F) may also have a covering of filamentous organisms coated by the sediment layer but none of the images available had high enough resolution to investigate this.
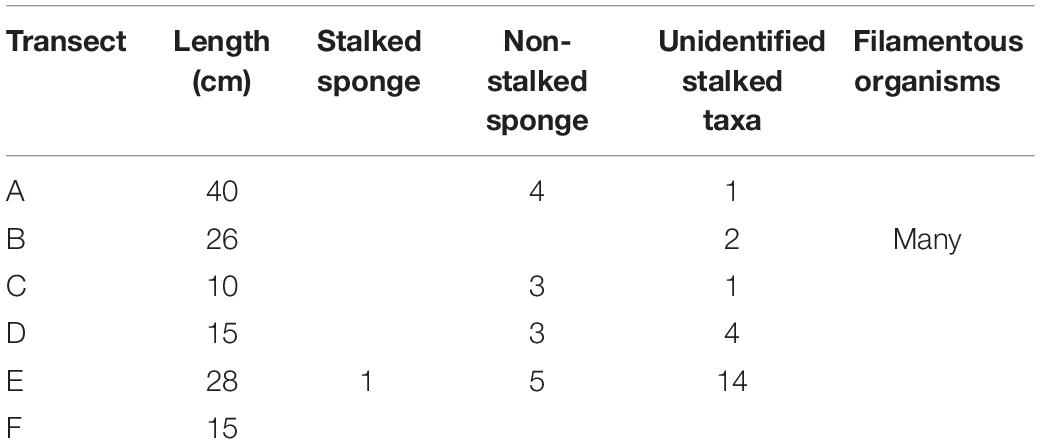
Table 2. Counts of taxa observed on the boulder found at FSW2 (for location of transects refer to Figure 3).
A single, smaller rock with one non-stalked sponge on the surface was observed from under the nearby FSW1 borehole. No other benthic or pelagic life was seen, but the camera did not get as near to this rock as it did to the boulder in FSW2 so the images were not of comparable detail or scale. No benthic animals were observed on the seafloor at FNE2, FSE1 or FSE2. There was a single pelagic ctenophore observed in the borehole water at FSE2. The substrate was similar at each of these locations and consisted of fine-grained sediments and scattered pebble-sized clasts. From the video footage, the sediment, and bubbles from the corer at FNE2 are seen to be moved rapidly by a strong horizontal current.
Discussion
Under ice shelf assemblages are generally believed to resemble the communities of the oligotrophic deep sea, subsisting on advected food particles (Ingels et al., 2018). The few previous examples of these communities from boreholes have all been from soft substrates or glacial sediments (Bruchhausen et al., 1979; Lipps et al., 1979; Riddle et al., 2007; Post et al., 2014; Sugiyama et al., 2014; Kingslake et al., 2018; Kim, 2019; Stevens et al., 2020). This is the first recorded observation of an in situ hard substrate sessile community beneath an ice shelf. The discovery of this suspension feeding community 260 km under a floating ice shelf in an area of outflow is remarkable in itself and goes against the existing paradigm (Ingels et al., 2018). Even though the prevailing current at FSW2 was strong, it was flowing in the wrong direction to connect the location directly to the nearest open water at the point of measurement (Figure 2). Instead, the currents suggest that this community is somewhere between 625 and 1500 km from the nearest region of photosynthesis. The effect of seasonality and pulses in the currents of the region (Darelius and Sallée, 2018) is unknown given that we do not yet know where the food comes from or how often they feed, but may significantly impact these communities, their feeding, and their recruitment.
Previous borehole records, excluding the new records from Filchner Ice Shelf (FSW1 and 2), show a general decrease in overall diversity and in the prevalence of sessile organisms with increasing distance from the ice shelf front (Figure 4). These data support the theory that in these oligotrophic environments mobile taxa are dominant (Ingels et al., 2018), with no sessile fauna previously observed from greater than 200 km under an ice shelf. The new findings from FSW1 and 2 go against this trend, with only sessile organisms present, but agree with the general trend of lower diversity at phylum level. The absence of any observed deposit feeding infauna or mobile epifauna on the sediments surrounding the boulder contrasts with existing theory (Ingels et al., 2018) and the dominance of these groups in the Amery and Ross Sea bore holes (Bruchhausen et al., 1979; Lipps et al., 1979; Riddle et al., 2007; Post et al., 2014; Kingslake et al., 2018; Kim, 2019; Stevens et al., 2020).
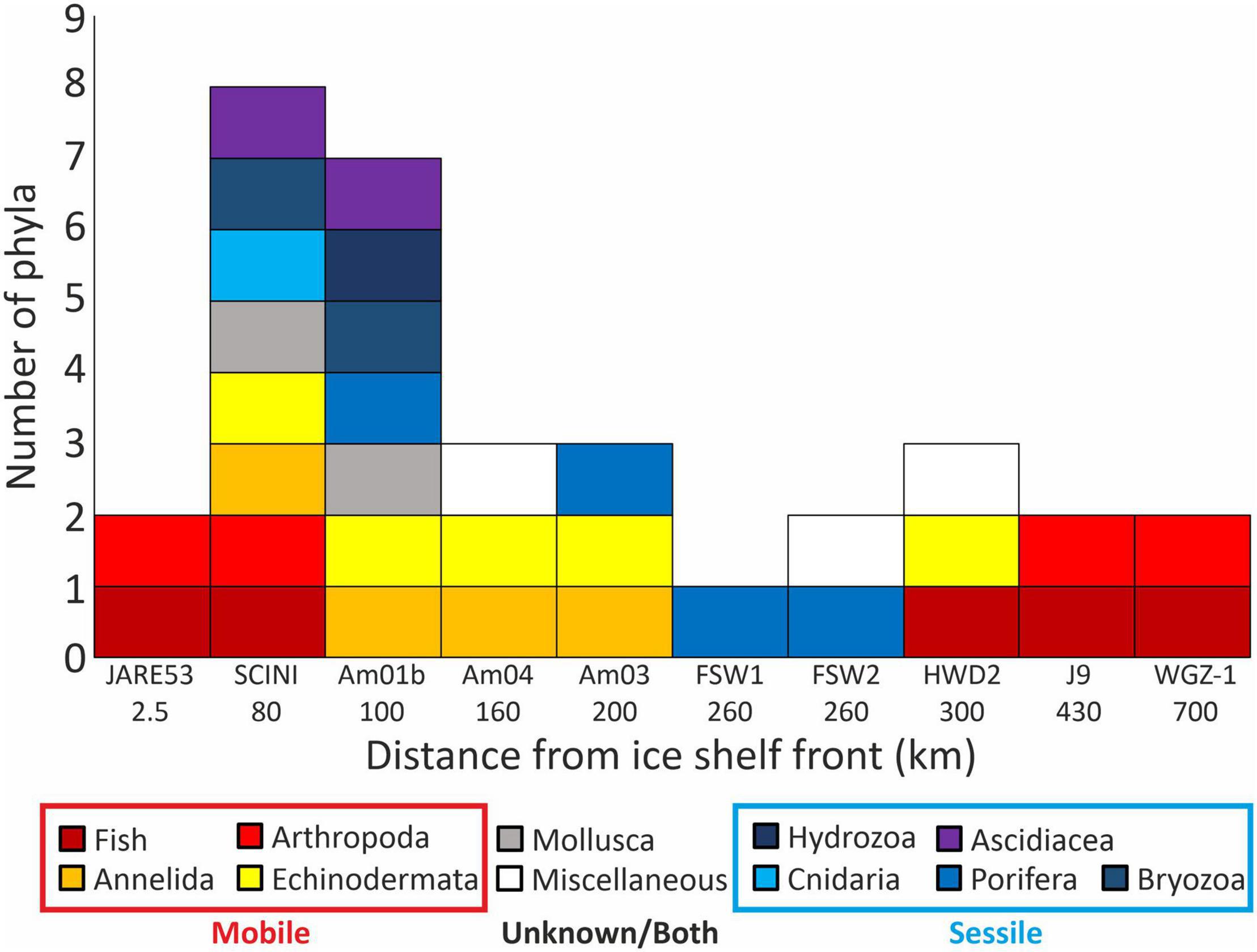
Figure 4. Counts of phyla observed at each borehole with increasing distance from the ice shelf front, boreholes with no observed life are excluded. Miscellaneous, unidentifiable living organisms or evidence of infaunal activity.
The abundance of organisms on the boulder is higher than would be expected so far from a source of primary production (Ingels et al., 2018) and is comparable with large dropstones in the seasonal sea ice regimes of the fjords of the West Antarctic Peninsula (Ziegler et al., 2017) or the Filchner Trough (∼450 km north of the ice shelf front, see Figure 2 and Supplementary Figure 1). However, the taxonomic and functional diversity is much lower than for the dropstones of the Peninsula (Ziegler et al., 2017) or the outer Filchner Trough (Supplementary Figure 1).
The existence of this sessile and probable suspension feeding community so far under the ice shelf raises many ecological questions that cannot be fully answered given our current state of knowledge:
What Species Are Present and Are They Endemic to This Environment?
While it is reasonable to assume that many of the organisms visible are sponges, it is impossible to tell if they are glass sponges, demosponges, or calcareous. Antarctica has high percentages of endemic species from all groups of sponges (Downey et al., 2012), and species level identification would require physical specimens and genetic material. The uncertainty around the identity the other stalked and filamentous taxa lies in the lack of detail obtainable from the video. However, they are sessile and have not been observed at any other previous borehole locations. Given the inherent complexity of obtaining physical samples (except for mobile fauna caught in baited traps) future studies could use environmental DNA (eDNA) techniques on water and sediment samples to identify taxa. The origin of these communities is unknown and the advection of larvae might well play a role, it is also possible that given the huge physical extent of these regions that a specialist endemic fauna, similar in function to that of the oligotrophic deep sea, may have evolved in situ.
How Old Is This Community?
The time frame for survival of this community is unlikely to be limited by biology, assuming a sufficient food supply, with some living sponges estimated to be thousands of years old (Folkers and Rombouts, 2020). However, exposed dropstones are ephemeral in nature, especially in regions of high sedimentation. FSW2 is in a region of strong currents and no significant melt, meaning low sedimentation compared with regions nearer the grounding line or with slower currents. This could mean that the boulder would remain exposed for a long time, as evidenced by the very thin and patchy layer of sediment on the surface with areas of exposed rock, especially on the left-hand side of the image (Figure 3). If this is indeed an endemic and specialized community, for long-term survival it could “island hop” between dropstones, like hydrothermal vent communities between active vents (Tyler and Young, 2003) or between whale falls (Smith et al., 2017). It could also be continuously recruited from a more stable hard substrate region upstream, such as the flanks of Berkner Island or sheltered parts of the edge of the continent.
How Often Does the Community Feed and What Is the Source of Its Food?
Although these organisms observed on the boulder at FSW2 were all sessile, without physical specimens it is impossible to know their true mode of nutrition. At least some of these animals might be carnivorous sponges. Southern Ocean species represent ∼20% of all known carnivorous sponges and they are often found in oligotrophic bathyal regions or on isolated seamounts (Goodwin et al., 2017). Chemosynthesis is believed to play a role in some sub-ice shelf trophic pathways. Methane and hydrogen sulfide associated with cold seeps are suggested as a source of energy beneath the former Larsen B Ice Shelf (Domack et al., 2005), although no typically chemotrophic organisms have been observed through any of the boreholes to date. Nutrients and organic matter can also come from beneath the grounded ice sheet with subglacial water discharge (Gerringa et al., 2012; Death et al., 2014; Vick-Majors et al., 2020) or from sediment melting out of basal ice (Neuhaus et al., 2020).
How Common Are These Hard Substrate Sub-Ice Shelf Communities?
Given that this is the first hard sub-ice shelf substrate habitat observed, we have no estimate for the density, distribution, longevity, or size range of sub-ice shelf dropstones and boulders. Such a census would require the use of autonomous technology with downward facing sensors surveying the under-ice shelf environment. Large boulders, ca. 1 m in diameter and larger, were found to account for about 0.1 volume% of Alpine glacial till (Felletti and Pietro Beretta, 2009). This likely represents an overestimate for glaciomarine sub-ice-shelf sediments in Antarctica which can be reasonably expected to be more fine-grained than Alpine glacial till. But it does suggest that large boulders can be spaced at the bottom of sub-ice shelf cavities at intervals as short as 1 km, or greater.
This study shows that there are regions where suitably stable hard substrate (suited for sessile fauna) coincides with currents that are sufficiently powerful to advect food from open waters. However, in other localities, such as FNE2, that are far closer (27 km) to the ice shelf front and experience significant currents showed no sign of life at all. To really understand sub-ice shelf communities, we need to combine information on both suitable oceanography and substrate. Other locations might provide one of these factors but not the other. The grounding line, for instance, may have a higher number of rocks, but may provide a poor food source or a higher sedimentation rate, burying the rocks more rapidly. These results demonstrate the potential for finding other similar communities elsewhere under large Antarctic ice shelves. We cannot currently pinpoint the exact location of similar habitats, future high-resolution modeling of sub-ice shelf oceanography and topography will enable us to target further investigation.
In the case of WGZ-1 (Kingslake et al., 2018), being very close to the grounding line the factor that might prevent sessile fauna from becoming established is the rainout of debris from the melting ice base. Sediment deposition rates may be as high as a few centimeters per year. The water column is also heavily loaded with suspended fine sediment which would inhibit filter feeding organisms which tend to favor regions of low inorganic turbidity (Turner, 2009). Near to grounding lines, these factors may be as important as the distance from the open ocean, if not more so. This is supported by the fact that mobile predators, scavengers, and detritivores (amphipods and fish) were observed 700 km from the ice front, suggesting that the absence of sessile macroscopic benthic may not be from a lack of food but potentially because of the high sediment flux. The sedimentary material raining out of the ice base does contain organic matter (at a level of per mils by weight), and there may be organic material coming from beneath the ice sheet (Vick-Majors et al., 2020).
How Does the Existence of This Community Inform Our Knowledge of the Physical Environment and Regime Under the Ronne-Filchner Ice Shelf and Other Ice Shelves?
Our findings also suggest that sub-ice shelf oceanographic conditions that are capable of providing a food source may be more widespread than previously thought. The presence of a sessile community 260 km from the ice shelf front supports the possibility that diatoms or other advected organic material are traveling far beneath the ice shelf. This has major implications for the study of glaciology and Antarctic marine geology, as the presence and composition of these marine microfossils in the sedimentary record have traditionally been used to determine presence/absence of paleo-ice shelves, as well as the proximity to the open-ocean (Smith et al., 2019).
What Would Become of These Communities in the Event of Ice Shelf Collapse?
These findings may be evidence of an Antarctic sub-ice shelf hard substrate benthic community that is well adapted to a low food supply, which makes it particularly vulnerable to the effects of ice shelf collapse and associated changes in productivity regimes. However, if this community turns out to be a restricted subset of the more general Weddell Sea hard substrate community, then it would be logical to assume that ice shelf loss would allow the community to thrive and succession would result in a community resembling that of the sea ice zone (Supplementary Figure 1). In the smaller Larsen A and B regions, the shift to more open water conditions with high local primary productivity was rapid (Ingels et al., 2018). The Larsen A and B communities are believed to be derived from the nearby shelf communities restricted by limited food availability. This is also reflected in many of the boreholes, with mobile fish, echinoderms, and arthropods, recognizable from the sea ice zone, being the only fauna observed on soft or glacial sediments (Bruchhausen et al., 1979; Kingslake et al., 2018; Stevens et al., 2020).
The first observation of a hard substrate community far under an ice shelf demonstrates that dropstones and boulders must play a similarly significant role in these regions as they do in the rest of the Southern Ocean acting as islands of hard substrate in a sea of mud (Ziegler et al., 2017). The biological and physical attributes that allow this community to survive, despite our current theories, suggest that these communities are either better connected to the outside world than we can currently explain or that the organisms themselves represent highly specialized extreme oligotrophic adaptation.
Given that our combined knowledge of in situ under ice shelf habitats (more than 1.5 million km2) is drawn from 10 discrete observations covering a total area comparable to that of a tennis court, it should not come as a surprise that we are still discovering previously unseen types of sub-ice shelf communities far from open water. These findings raise more questions than they answer, highlighting the need for a concerted international effort to systematically observe, sample, and quantify these communities; their wider role in the Southern Ocean; and their physiological adaptations to this extreme environment. These observations challenge our understanding of what types of organisms can survive so far from daylight and have wider implications with regard to the evolution of the first complex organisms on earth, in particular through the “snowball earth” period, astrobiology, and the survival of polar organisms during more recent glacial maxima.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JS and PA collected the seabed imagery in Antarctica. HG analyzed the images and compared with other studies. CS and ST provided images and data for comparison. HG wrote the manuscript with contributions from JS, PA, KL, AP, JM, CS, and ST. All authors have contributed to previous versions and approved the final, submitted version.
Funding
HG, PA, KL, and JS are part of the British Antarctic Survey’s Polar Science for Planet Earth Program (NC-Science). Images from Filchner were obtained using the UK Natural Environment Research Council grant number NE/L013770/1, Ice shelves in a warming world: Filchner Ice Shelf system, Antarctica. JM is funded by the Irish Research Council GOIPG/2019/4020. AP is funded by Geoscience Australia. CS is funded by the New Zealand Antarctic Research Institute Ross Ice Shelf Program and the New Zealand Antarctic Science Platform (ASP). ST was funded by NSF-OPP award 0838947 as part of the Whillans Ice Stream Subglacial Access Drilling (WISSARD) project supported by the ANDRILL drilling team at the University of Nebraska, Lincoln, and by logistics provided by the United States Antarctic Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The fieldwork on the Filchner Ice Shelf in 2015–2016 was undertaken under the permit 45/2015 issued by the Foreign and Commonwealth Office, London, to section 3 of the Antarctic Act 1994. We would like to thank everyone at the Alfred Wegener Institute (AWI) and the British Antarctic Survey (BAS) who supported the FISP and FISS drilling campaigns 2015/2016 and 2016/2017 by logistics and funding. The 2017 Ross Ice Shelf borehole was made possible by Antarctica New Zealand and the Victoria of University of Wellington Antarctic Research Centre.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.642040/full#supplementary-material
Supplementary Figure 1 | Comparable images of JR275 from open water (seasonal sea ice) sites for comparison with the fauna observed on the boulder.
Supplementary Video | Video taken at the seafloor beneath borehole FSW2 in the Weddell Sea beneath the Ronne-Filchner Ice Shelf. Visible in the video are multiple sessile organisms attached to a large boulder.
References
Bruchhausen, P. M., Raymond, J. A., Jacobs, S. S., Devries, A. L., Thorndike, E. M., and Dewitt, H. H. (1979). Fish, crustaceans, and the sea floor under the ross ice shelf. Science 203, 449–451. doi: 10.1126/science.203.4379.449
Daae, K., Hattermann, T., Darelius, E., Mueller, R. D., Naughten, K. A., Timmermann, R., et al. (2020). Necessary conditions for warm inflow toward the Filchner Ice Shelf, Weddell Sea. Geophy. Res. Lett. 47, 1–11. doi: 10.1029/2020gl089237
Darelius, E., Fer, I., and Nicholls, K. W. (2016). Observed vulnerability of Filchner-Ronne Ice Shelf to wind-driven inflow of warm deep water. Nat. Commun. 7:12300.
Darelius, E., and Sallée, J. B. (2018). Seasonal outflow of ice shelf water across the front of the Filchner Ice Shelf, Weddell Sea, Antarctica. Geophys. Res. Lett. 45, 3577–3585. doi: 10.1002/2017gl076320
Death, R., Wadham, J. L., Monteiro, F., Le Brocq, A. M., Tranter, M., Ridgwell, A., et al. (2014). Antarctic ice sheet fertilises the Southern Ocean. Biogeosciences 11, 2635–2643. doi: 10.5194/bg-11-2635-2014
Domack, E., Ishman, S., Leventer, A., Sylva, S., Willmott, V., and Huber, B. (2005). A chemotrophic ecosystem found beneath Antarctic Ice Shelf. EOS Transact. Am. Geophys. Union 86:269. doi: 10.1029/2005eo290001
Downey, R. V., Griffiths, H. J., Linse, K., and Janussen, D. (2012). Diversity and distribution patterns in high southern latitude sponges. PLoS One 7:e41672. doi: 10.1371/journal.pone.0041672
Felletti, F., and Pietro Beretta, G. (2009). Expectation of boulder frequency when tunneling in glacial till: a statistical approach based on transition probability. Eng. Geol. 108, 43–53. doi: 10.1016/j.enggeo.2009.06.006
Ferris, J., Johnson, A., and Storey, B. (1998). Form and extent of the Dufek intrusion, Antarctica, from newly compiled aeromagnetic data. Earth Planet Sci. Lett. 154, 185–202. doi: 10.1016/s0012-821x(97)00165-9
Folkers, M., and Rombouts, T. (2020). Sponges Revealed: A Synthesis of Their Overlooked Ecological Functions Within Aquatic Ecosystems. YOUMARES 9 – The Oceans: Our Research, Our Future. Cham: Springer, 181–193. doi: 10.1007/978-3-030-20389-4-9
Ford, A. B. (1976). Stratigraphy of the Layered Gabbroic Dufek Intrusion, Antarctica. Washington, D.C.: US Government Printing Office, 36. doi: 10.3133/b1405d
Gerringa, L. J. A., Alderkamp, A. C., Laan, P., Thuróczy, C. E., De Baar, H. J. W., Mills, M. M., et al. (2012). Iron from melting glaciers fuels the phytoplankton blooms in Amundsen Sea (Southern Ocean): iron biogeochemistry. Deep Sea Res. 2 Top. Stud. Oceanogr. 7, 16–31. doi: 10.1016/j.dsr2.2012.03.007
Goodwin, C. E., Berman, J., Downey, R. V., and Hendry, K. R. (2017). Carnivorous sponges (Porifera : Demospongiae : Poecilosclerida : Cladorhizidae) from the drake passage (Southern Ocean) with a description of eight new species and a review of the family Cladorhizidae in the Southern Ocean. Inverteb. Syst. 31, 37–64. doi: 10.1071/is16020
Griffiths, H. J. (2010). Antarctic marine biodiversity–what do we know about the distribution of life in the Southern Ocean? PLoS One 5:e11683. doi: 10.1371/journal.pone.0011683
Gutt, J., Griffiths, H. J., and Jones, C. D. (2013). Circumpolar overview and spatial heterogeneity of Antarctic macrobenthic communities. Mar. Biodivers. 43, 481–487. doi: 10.1007/s12526-013-0152-9
Huhn, O., Hattermann, T., Davis, P. E. D., Dunker, E., Hellmer, H. H., Nicholls, K. W., et al. (2018). Basal melt and freezing rates from first noble gas samples beneath an ice shelf. Geophy. Res. Lett. 45, 8455–8461. doi: 10.1029/2018gl079706
Ingels, J., Aronson, R. B., and Smith, C. R. (2018). The scientific response to Antarctic ice-shelf loss. Nat. Clim. Chang. 8, 848–851. doi: 10.1038/s41558-018-0290-y
Ingels, J., Aronson, R. B., Smith, C. R., Baco, A., Bik, H. M., Blake, J. A., et al. (2021). Antarctic ecosystem responses following ice-shelf collapse and iceberg calving: science review and future research. Wiley Interdiscip. Rev. 12:e682. doi: 10.1002/wcc.682
Kim, S. (2019). Complex life under the McMurdo Ice Shelf, and some speculations on food webs. Antarct. Sci. 31, 80–88. doi: 10.1017/s0954102018000561
Kingslake, J., Scherer, R. P., Albrecht, T., Coenen, J., Powell, R. D., Reese, R., et al. (2018). Extensive retreat and re-advance of the West Antarctic ice sheet during the holocene. Nature 558, 430–434. doi: 10.1038/s41586-018-0208-x
Lipps, J. H., Ronan, T. E. Jr., and Delaca, T. E. (1979). Life below the ross ice shelf, antarctica. Science 203, 447–449. doi: 10.1126/science.203.4379.447
Makinson, K., and Anker, P. G. D. (2014). The BAS ice-shelf hot-water drill: design, methods and tools. Ann. Glaciol. 55, 44–52. doi: 10.3189/2014aog68a030
Makinson, K., Holland, P. R., Jenkins, A., Nicholls, K. W., and Holland, D. M. (2011). Influence of tides on melting and freezing beneath Filchner-Ronne Ice Shelf, Antarctica. Geophys. Res. Lett. 38:L06601. doi: 10.1029/2010gl046462
Nachtsheim, D. A., Ryan, S., Schröder, M., Jensen, L., Chris Oosthuizen, W., Bester, M. N., et al. (2019). Foraging behaviour of Weddell seals (Leptonychotes weddellii) in connection to oceanographic conditions in the southern Weddell Sea. Prog. Oceanogr. 173, 165–179. doi: 10.1016/j.pocean.2019.02.013
Neuhaus, S. U., Tulaczyk, S. M., Stansell, N. D., Coenen, J. J., Scherer, R. P., Mikucki, J. A., et al. (2020). Did Holocene climate changes drive West Antarctic grounding line retreat and re-advance? Cryosph. Discuss [Preprint]. doi: 10.5194/tc-2020-308
Nicholls, K. W. (2004). Interannual variability and ventilation timescales in the ocean cavity beneath Filchner-Ronne Ice Shelf, Antarctica. J. Geophy. Res. 109:C04014. doi: 10.1029/2003jc002149
Nicholls, K. W., Østerhus, S., Makinson, K., Gammelsrød, T., and Fahrbach, E. (2009). Ice-ocean processes over the continental shelf of the southern Weddell Sea, Antarctica: a review. Rev. Geophy. 47:RG3003. doi: 10.1029/2007rg000250
NOAA National Geophysical Data Center (2009). ETOPO1 1 Arc-Minute Global Relief Model. NOAA National Centers for Environmental Information (accessed March 4, 2019).
Østerhus, S. (1994). Report of the Norwegian Antarctic Research Expedition 1991/1992 Tromsø: Norsk polarinstitutt.
Østerhus, S., and Orheim, O. (1994). Oceanographic and glaciologic investigations through Jutulgryta, Fimbulisen in the 1991/92 season. Norsk Polarinst. Meddel. 124, 21–28.
Post, A. L., Galton-Fenzi, B. K., Riddle, M. J., Herraiz-Borreguero, L., O’Brien, P. E., Hemer, M. A., et al. (2014). Modern sedimentation, circulation and life beneath the Amery Ice Shelf, East Antarctica. Cont. Shelf Res. 74, 77–87. doi: 10.1016/j.csr.2013.10.010
Riddle, M. J., Craven, M., Goldsworthy, P. M., and Carsey, F. (2007). A diverse benthic assemblage 100 km from open water under the Amery Ice Shelf, Antarctica. Paleoceanography 22:PA1204. doi: 10.1029/2006pa001327
Smith, C. R., Amon, D. J., Higgs, N. D., Glover, A. G., and Young, E. L. (2017). Data are inadequate to test whale falls as chemosynthetic stepping-stones using network analysis: faunal overlaps do support a stepping-stone role. Proc. Biol. Sci. 284, 1–16. doi: 10.1098/rspb.2017.1281
Smith, J. A., Graham, A. G. C., Post, A. L., Hillenbrand, C.-D., Bart, P. J., and Powell, R. D. (2019). The marine geological imprint of Antarctic ice shelves. Nat. Commun. 10:5635.
Stevens, C., Hulbe, C., Brewer, M., Stewart, C., Robinson, N., Ohneiser, C., et al. (2020). Ocean mixing and heat transport processes observed under the ross ice shelf control its basal melting. Proc. Natl. Acad. Sci. U.S.A. 117, 16799–16804. doi: 10.1073/pnas.1910760117
Sugiyama, S., Sawagaki, T., Fukuda, T., and Aoki, S. (2014). Active water exchange and life near the grounding line of an Antarctic outlet glacier. Earth Planet. Sci. Lett. 399, 52–60. doi: 10.1016/j.epsl.2014.05.001
Turner, J. (2009). Antarctic Climate Change and the Environment: A Contribution to the International Polar Year 2007-2008. Cambridge: Scientific Committee on Antarctic Research.
Tyler, P. A., and Young, C. M. (2003). Dispersal at hydrothermal vents: a summary of recent progress. Hydrobiologia 503, 9–19. doi: 10.1023/b:hydr.0000008492.53394.6b
Vick-Majors, T., Achberger, A., Michaud, A., and Priscu, J. (2020). “Metabolic and taxonomic diversity in antarctic subglacial environments,” in Life in Extreme Environments: Insights in Biological Capability: Ecological Reviews, eds G. Di Prisco, H. Edwards, J. Elster, and A. Huiskes (Cambridge: Cambridge University Press), 279–296. doi: 10.1017/9781108683319.016
Keywords: dropstone, oligotrophic, borehole, sponge (Porifera), Filchner-Ronne Ice Shelf, Weddell Sea
Citation: Griffiths HJ, Anker P, Linse K, Maxwell J, Post AL, Stevens C, Tulaczyk S and Smith JA (2021) Breaking All the Rules: The First Recorded Hard Substrate Sessile Benthic Community Far Beneath an Antarctic Ice Shelf. Front. Mar. Sci. 8:642040. doi: 10.3389/fmars.2021.642040
Received: 15 December 2020; Accepted: 18 January 2021;
Published: 15 February 2021.
Edited by:
Christian Marcelo Ibáñez, Andres Bello University, ChileReviewed by:
Sebastian Rosenfeld, University of Magallanes, ChileJavier Sellanes, Catholic University of the North, Chile
Copyright © 2021 Griffiths, Anker, Linse, Maxwell, Post, Stevens, Tulaczyk and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huw J. Griffiths, hjg@bas.ac.uk
 Huw J. Griffiths
Huw J. Griffiths Paul Anker1
Paul Anker1  Katrin Linse
Katrin Linse Jamie Maxwell
Jamie Maxwell Alexandra L. Post
Alexandra L. Post Craig Stevens
Craig Stevens Slawek Tulaczyk
Slawek Tulaczyk James A. Smith
James A. Smith