A Review and Meta-Analysis of Potential Impacts of Ocean Acidification on Marine Calcifiers From the Southern Ocean
- 1Institute of Marine Sciences (ICM-CSIC), Barcelona, Spain
- 2Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
- 3National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand
- 4Fenner School of Environment and Society, Australian National University, Canberra, ACT, Australia
- 5British Antarctic Survey, Cambridge, United Kingdom
- 6Geoscience Australia, Canberra, ACT, Australia
- 7Australian Antarctic Division, Kingston, TAS, Australia
Understanding the vulnerability of marine calcifiers to ocean acidification is a critical issue, especially in the Southern Ocean (SO), which is likely to be the one of the first, and most severely affected regions. Since the industrial revolution, ~30% of anthropogenic CO2 has been absorbed by the global oceans. Average surface seawater pH levels have already decreased by 0.1 and are projected to decline by ~0.3 by the year 2100. This process, known as ocean acidification (OA), is shallowing the saturation horizon, which is the depth below which calcium carbonate (CaCO3) dissolves, likely increasing the vulnerability of many resident marine calcifiers to dissolution. The negative impact of OA may be seen first in species depositing more soluble CaCO3 mineral phases such as aragonite and high-Mg calcite (HMC). Ocean warming could further exacerbate the effects of OA in these particular species. Here we combine a review and a quantitative meta-analysis to provide an overview of the current state of knowledge about skeletal mineralogy of major taxonomic groups of SO marine calcifiers and to make projections about how OA might affect a broad range of SO taxa. We consider a species' geographic range, skeletal mineralogy, biological traits, and potential strategies to overcome OA. The meta-analysis of studies investigating the effects of the OA on a range of biological responses such as shell state, development and growth rate illustrates that the response variation is largely dependent on mineralogical composition. Species-specific responses due to mineralogical composition indicate that taxa with calcitic, aragonitic, and HMC skeletons, could be at greater risk to expected future carbonate chemistry alterations, and low-Mg calcite (LMC) species could be mostly resilient to these changes. Environmental and biological control on the calcification process and/or Mg content in calcite, biological traits, and physiological processes are also expected to influence species-specific responses.
Introduction
Since the industrial revolution, the concentration of carbon dioxide (CO2) released to the atmosphere has increased from 280 to above 400 μatm due to human activities such as burning of fossil fuels and deforestation. Approximately 30% of this has been absorbed by the oceans (Feely et al., 2004). This results in changes in seawater CO2 chemistry [e.g., oceanic partial pressure of CO2 (pCO2), pH, bicarbonate concentrations ([]), calcium carbonate (CaCO3) saturation state (Ω)], a process known as ocean acidification (OA) (Figure 1). In particular, OA induces an increase in pCO2 and [] and a decrease in pH and Ω, which is a function of [] and calcium ion concentrations ([Ca2+]) expressed as
where Ksp is the solubility product, which depends on the specific CaCO3 mineral phase, temperature, salinity, and pressure (Zeebe, 2012).
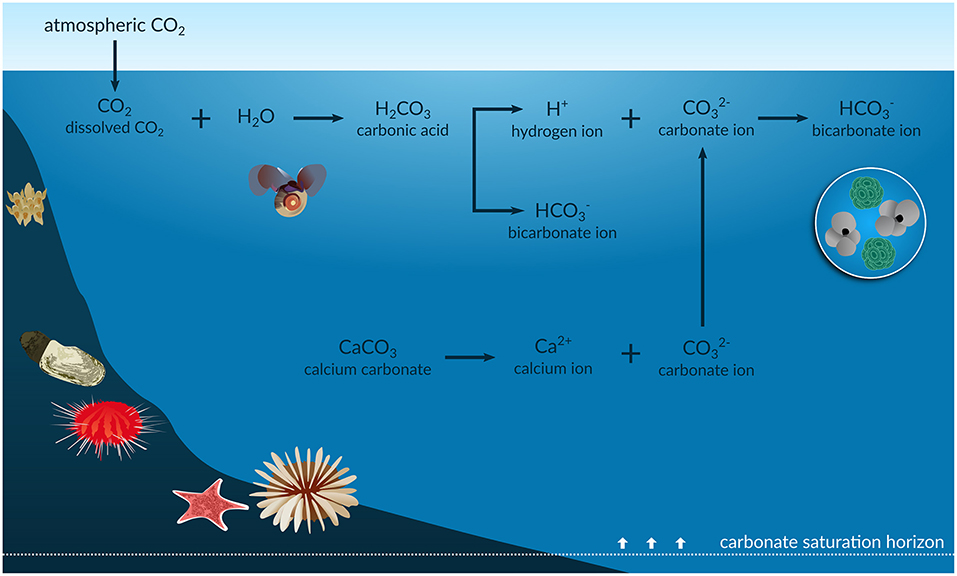
Figure 1. Infographic of the ocean acidification process. The anthropogenic CO2 absorbed by the oceans results in an increase in the concentration of hydrogen ions (H+) and in bicarbonate ions () and a decrease in carbonate ions (). The reduction in is shallowing the carbonate saturation horizons, with potential impacts on shells and skeletons of marine calcifiers such as foraminifera, corals, echinoderms, molluscs, and bryozoans.
Surface waters are naturally supersaturated with respect to carbonate (Ω > 1), where the highest [] is found as a result of surface photosynthesis. The [] decreases, and the solubility of CaCO3 minerals (such as aragonite) increases, with depth due to the increased pressure and lower temperature. Thus, Ω is greater in shallow, warm waters than in cold waters and at depth (Feely et al., 2004). Current aragonite saturation in Southern Ocean (SO) surface waters ranges from ΩAr of 1.22 to 2.42, compared with values of 3.5 to 4 in the tropics (Jiang et al., 2015), and varies spatially, e.g., across ocean basins and with depth, and seasonally. However, OA is both decreasing saturation levels and shallowing the carbonate saturation horizon, which is the depth below which CaCO3 can dissolve (Ω < 1). This is likely to increase the vulnerability of CaCO3 sediments and many marine calcifiers (CaCO3 shell/skeleton-building organisms) to dissolution (Haese et al., 2014). As CaCO3 sediments have important roles in global biogeochemical cycling and provide suitable habitat for many marine calcifying species, undersaturated seawater conditions will lead to unprecedented challenges and alterations to the function, structure, and distribution of carbonate ecosystems exposed to these conditions (Andersson et al., 2008).
The processes underlying organisms' vulnerabilities to OA have been much discussed between biologists and chemists (e.g., Waldbusser et al., 2015, 2016; Cyronak et al., 2016a,b). While some studies highlight how the energetic cost of calcification increases with decreasing Ω (Waldbusser et al., 2016), other studies indicate that Ω per se may not always be a good indicator, and that it is important to consider other components of carbonate chemistry, and the biocalcification process (Roleda et al., 2012; Fassbender et al., 2016). For instance, some studies illustrate that the reduction in seawater pH is the main driver of changes in calcification rates (the production and deposition of CaCO3), rather than the reduction in [] (Roleda et al., 2012; Cyronak et al., 2016b), with a projected drop in calcification rates by 30 to 40% by mid-century due to OA (Kleypas et al., 1999). Future studies should attempt to tease apart the influence of Ω and pH on calcification, which are not mutually exclusive, to improve our predictions of OA long-term effects in marine calcifiers.
A number of marine calcifiers, particularly benthic populations from the tropics and deep waters, are already exposed to undersaturated conditions with respect to their species-specific mineralogies (Lebrato et al., 2016). This suggests these organisms have evolved compensatory physiological mechanisms to maintain their calcified structures (Lebrato et al., 2016). This does not mean that all taxa can adapt to rapid decreases in the Ω, rather it illustrates the complexity of taxon-level responses. For instance, SO marine calcifiers may be particularly sensitive to these changes since the SO is characterized by a much lower natural variability in surface ocean [] (Conrad and Lovenduski, 2015) relative to the rate of projected change (Turley et al., 2006). For species with some biological control over calcification and other physiological processes, we can expect variation in responses depending on local adaptation. Species inhabiting coastal areas, which naturally experience rapid fluctuations in environmental conditions, compared to the open ocean (e.g., from ice melting, phytoplankton blooms, corrosive upwelling events), may have a greater level of resilience and/or adaptation to changes in CO2 exposures (Fassbender et al., 2016; Vargas et al., 2017).
Seawater pH levels have already decreased by 0.1 since ~1850, and are predicted to decline by ~0.3 (851–1,370 μatm) by the year 2100 (IPCC, 2014, 2019). The long-term persistence of calcifying taxa is dependent upon calcification exceeding the loss of CaCO3 that occurs through breakdown, export, and dissolution processes (Andersson and Gledhill, 2013). Therefore, even though species-specific responses to OA are expected, a tendency toward reduction in overall CaCO3 production at the local or community level is anticipated (Andersson and Gledhill, 2013) with potentially adverse implications across SO ecosystems.
Southern Ocean Acidification
The SO, which covers about 34.8 million km2, is widely anticipated to be the one of the first, and most severely affected region from OA due to naturally low levels of CaCO3, the increased solubility of CO2 at low temperatures, and a lower buffering capacity (Orr et al., 2005; Fabry et al., 2009). Thus, understanding the vulnerability of Antarctic marine calcifiers to OA is a critical issue, particularly in Antarctic Peninsula, which is also one of the fastest warming areas on Earth (Vaughan and Marshall, 2003). To establish the potential threat of OA to SO marine calcifiers, it is crucial to consider different factors that may influence the vulnerability of their skeletons to OA. These include interacting factors (e.g., warming), the local seasonal and spatial variability in seawater carbon dioxide chemistry, the distribution of sedimentary CaCO3 (which provides suitable habitat for many benthic calcifiers), and a range of different phyla and species with diverse geographic range, skeletal mineralogies, biological traits, physiological processes, and strategies to compensate for pH changes.
Local Seasonal and Spatial Variability in Seawater Carbon Dioxide Chemistry
Differences in solar irradiance and temperature, which can lead to changes in the duration of primary production, modify seawater CO2 chemistry seasonally, and latitudinally (Takahashi et al., 2002; Racault et al., 2012; Kapsenberg and Cyronak, 2019). For instance, seawater pH and ΩAr increase during phytoplankton blooms in Austral summer (McNeil et al., 2011; Kapsenberg et al., 2015). While SO areas with higher primary productivity could act as spatial refugia, studies are needed to test whether seasonally high pH may benefit marine calcifiers (Kapsenberg and Cyronak, 2019).
The depth of the present-day SO Aragonite Saturation Horizon (ASH) exceeds 1,000 m across most of the basin, while naturally shallower saturation horizons (~400 m) occur in the core of the Antarctic Circumpolar Current due to upwelling of CO2-rich deep water (Negrete-García et al., 2019). The Calcite Saturation Horizon (CSH) is much deeper (>2,000 m in some parts of the SO) than the ASH, since aragonite is more soluble than calcite (Feely et al., 2004; Barnes and Peck, 2008). It is thus projected that the ASH will reach surface waters earlier than the CSH (McNeil and Matear, 2008). In particular, it is anticipated that aragonite in some SO regions (south of 60°S) will be undersaturated by 2050 (Orr et al., 2005; McNeil and Matear, 2008) and that 70% of the SO could be undersaturated by 2100 (Hauri et al., 2016). OA will drive even the least soluble form of calcium carbonate, calcite, to undersaturation by 2095 in some SO waters (McNeil and Matear, 2008). However, the depth and year of emergence of a shallow ASH still vary spatially due to natural variation in the present-day saturation horizon depth and in the physical circulation of the SO (Negrete-García et al., 2019).
Aragonite undersaturation in surface waters is projected initially during the austral winter (Conrad and Lovenduski, 2015; Negrete-García et al., 2019) as the [] of surface waters decrease south of the Polar Front due to the strong persistent winter winds that promote the upwelling of carbonate-depleted deep waters (Orr et al., 2005). In addition, lower temperatures enhance uptake of atmospheric CO2 (Orr et al., 2005). Specifically, several studies (McNeil and Matear, 2008; McNeil et al., 2010; Mattsdotter Björk et al., 2014) have projected that wintertime aragonite undersaturation will occur by 2030 in the latitudinal band between 65 and 70°S where deep-water upwelling occurs. Recently, Negrete-García et al. (2019) considered that the rate of change, and therefore the onset of aragonite undersaturation, was occurring more rapidly than previously projected. These forecasted changes imply there will be a sudden decline in the availability of suitable habitat for diverse SO taxa such as pteropods, foraminifera, cold-water corals, and coralline algae in the near-future (Negrete-García et al., 2019).
Distribution of Carbonate Sediments
The distribution of sedimentary CaCO3 on the Antarctic shelves displays distinct regional and depth patterns (Hauck et al., 2012). This is evident from the deposition and preservation of carbonates in the surface sediments. These are driven by the flux of organic matter to the ocean floor (related to primary production) and its respiration and remineralization in the sediments, transport of carbonate material by currents, and Ω of the water mass above the sediment (Hauck et al., 2012). In particular, an enhanced flux of organic matter to the seafloor increases respiration in sediments and alters carbonate chemistry. CaCO3 content of shelf surface sediments is usually low, however high values (>15%) of CaCO3 occur at shallow water depths (150–200 m) on the narrow shelves of the eastern Weddell Sea and at a depth range of 600–900 m on the broader and deeper shelves of the Amundsen, Bellingshausen, and western Weddell Seas (Hauck et al., 2012).
Regions with high levels of primary production, such as the Ross Sea and the western Antarctic Peninsula, generally have low CaCO3 contents in the surface sediments as carbonate produced by benthic organisms is subsequently dissolved and thus not preserved. Conversely, carbonate contents of sediments in areas of low levels of primary productivity reflect the concentration of planktonic foraminifera that are especially abundant in sea ice (Hauck et al., 2012). As carbonate-rich sediments on the continental shelf can rapidly react to the decreasing Ω with respect to carbonate minerals (Haese et al., 2014), OA will potentially have critical implications to their distribution and, consequently, the habitats of benthic organisms.
Carbonate Mineral Composition of Shells and Skeletons
Ocean acidification will not only impact carbonate mineral composition in surface sediments of coastal and continental shelf environments but also on diverse shelf assemblages of marine calcifiers. Globally, most scleractinian cold-water corals (>70%) will be exposed to undersaturated conditions by 2100 when the ASH is expected to reach surface waters (Guinotte et al., 2006; Turley et al., 2007). Many SO organisms are expected to be unable to adapt to projected rapid changes, as this region is characterized by low interannual variability in surface ocean [] (Orr et al., 2005; Conrad and Lovenduski, 2015). OA may consequently impact biomineralization of their skeletons and promote their dissolution (Andersson et al., 2008; Fabry, 2008). Currently, studies of OA impact on organisms are few, and therefore, further studies are required to better understand the influence of carbonate chemistry on marine calcifiers, as mentioned above.
While there is great uncertainty in the predictions of “winners” and “losers” under forecasted global-change, the CaCO3 form of shells and skeletons, when combined with other biological traits, could be a key factor in determining their response to change. Carbonate shells and skeletons may be composed of three different phases: aragonite, calcite and Mg-calcite. Calcite and aragonite are polymorphs (different mineral structure) of CaCO3. Mg-calcite has the same mineral structure as calcite but some Ca2+ ions have been replaced by Mg2+ ions. These CaCO3 minerals have different physical and chemical properties. The structure of aragonite is known to be less stable, and thus more soluble, than that of calcite. A high percentage of marine calcifiers incorporate significant amounts of Mg into their skeletons, which additionally reduces mineral stability. Their shells and skeletons are categorized as low-Mg calcite (LMC; 0–4 wt% MgCO3), intermediate-Mg calcite (IMC; 4–8 wt% MgCO3), and high-Mg calcite (HMC; >8 wt% MgCO3) (Rucker and Carver, 1969). Thus, HMC shells or skeletons are also more soluble than those comprised of LMC and pure calcite and even, in some situations, aragonite; consequently, they may be most susceptible to OA impacts (Andersson et al., 2008; Fabry, 2008).
Energetic cost associated with acid-base regulation in response to OA is another important issue to consider OA impacts, which may even have more influence on the species sensitivity to OA than the CaCO3 composition of their shell or skeleton (Collard et al., 2015; Duquette et al., 2018). The increase in energetic investment into acid-base regulation may lead to a decrease allocation to other functions such as reproduction, growth or calcification.
Ocean warming could further exacerbate the effects of OA in species with Mg-calcite shells/skeletons, as Mg content in calcite generally increases with seawater temperature and thereby further accelerates skeletal solubility. Ocean warming, in combination with OA, is thus forecasted to increase the vulnerability of some marine calcifiers, particularly taxa depositing more soluble CaCO3 mineral phases (aragonite or HMC) (Andersson et al., 2008). However, the IMC or LMC cold-water species are potentially less soluble than those from warmer waters, and could also be at increased risk to near-future OA, as Antarctica is acidifying at a faster rate than much of the rest of the global ocean (Fabry et al., 2009). In addition, species with known limited thermotolerance and/or mobility (e.g., some cold-water species) are of particular concern, as they may not be able to shift their distribution to higher latitudes and/or deeper waters, to respond to these changing conditions.
Information on skeletal mineralogy of Antarctic marine calcifiers is currently limited and biased to only a small number of taxa, and to specific geographic and bathymetric ranges (Borisenko and Gontar, 1991; Taylor et al., 2009; McClintock et al., 2011; Figuerola et al., 2015; Krzeminska et al., 2016; IPCC, 2019). For instance, little attention has been paid to Antarctic benthic communities from the deep-sea and important areas such as the Vulnerable Marine Ecosystem (VME) in East Antarctica (Post et al., 2010). These communities are characterized by high levels of species richness in marine calcifiers, in particular habitat-forming bryozoans and hydrocorals (Stylasteridae) (Beaman and Harris, 2005; Post et al., 2010; Bax and Cairns, 2014).
Many studies have focused on the impacts of OA without considering its interactions with other environmental (e.g., warming, food quality, or salinity) and biological (e.g., skeletal growth rate) factors which are involved in mediating the susceptibility of marine calcifiers to OA, for example, through controlling the incorporation of Mg into the skeletal calcite (Chave, 1954; Andersson et al., 2008; Hermans et al., 2010; Sewell and Hofmann, 2011; Swezey et al., 2017). Here we review, and for the first time, provide a quantitative meta-analysis to give an overview of the current state of knowledge about skeletal mineralogy of major taxonomic groups of SO marine calcifiers; and utilize these findings to project future implications of OA impacts on different groups of organisms.
Methods
In order to project how OA could impact SO marine calcifiers, environmental, and biological data for a broad range of organisms were compiled. These included species geographic range, skeletal mineralogy, the calcification responses, and strategies to overcome OA. Their CaCO3 composition was reported as aragonite and/or calcite. Calcite composition was further classified as LMC, IMC and HMC (Rucker and Carver, 1969). Data previously reported as mol% MgCO3 were converted to weight% (wt%) MgCO3 for comparisons. Individual SO maps with species distributions were then produced for two groups (echinoderms and bryozoans) with Mg-calcite skeletons for which there is relatively good data and for benthic species identified here as particularly vulnerable to OA.
With the aim of performing a meta-analysis, a thorough database search was conducted to compile all peer-reviewed journal articles and literature reviews that investigated the effect of altered seawater carbonate chemistry on SO marine calcifiers. We conducted searches using three databases (ISI Web of Science, Scopus and Aquatic Sciences, and Fisheries Abstracts). Keywords used are provided in Supplementary Table 1 (database searches completed May 2020). Articles were screened to include studies that manipulated the carbonate chemistry to investigate the effects of OA on specifically SO marine calcifiers. The CO2 treatment levels were separated into two categories for the meta-analysis: ambient treatment and elevated CO2/lowered pH treatment. The ambient treatment used from each study was the ambient treatment defined by the individual studies with obvious variation between these based on what is ambient at the particular study site/time of year (all ambient treatments were below 500 μatm). Due to the limited number of studies, all elevated CO2/lowered pH treatments were combined for the meta-analysis. If a study had multiple elevated CO2/lowered pH treatments, the highest value of treatment of these was included, and no treatments above 1,500 μatm CO2 were included. After manual screening, 20 studies remained for inclusion in the meta-analysis (Supplementary Figure 1). Data included bivalves (Mollusca; Bivalvia), brachiopods (Brachiopoda), limpets (Mollusca; Gastropoda; Patellogastropoda), pteropods (Mollusca; Gastropoda; Pteropoda), sea snails (Mollusca; Gastropoda; Trochida), sea stars (Echinodermata; Asteroidea), sea urchins (Echinodermata; Echinoidea), and coccolithophores (Haptophyta; Coccolithophyceae). The effects of OA on the following biological responses were investigated: development (bivalves, sea stars, and sea urchins; fertilization rate or normal development), growth rate (brachiopods, pteropods and coccolithophores), shell state (bivalves, limpets, pteropods, and sea snails; adult calcification or dissolution rate), coccolith volume (coccolithophores), and survival (sea urchins). We analyzed the data following previous methods (Kroeker et al., 2010, 2013; Hancock et al., 2020). For each study the ln-transformed response ratio to OA was calculated using the formula
where and are the mean value for the biological responses in the CO2 experimental treatment and the ambient treatment, respectively (Hedges et al., 1999). We used the log response ratio (LnRR) due to its robustness to small sample sizes and its ability to detect true effects (Lajeunesse and Forbes, 2003). For all response ratios, a positive ratio is a positive response to OA, a negative response ratio is a negative response to OA, and a response ratio of zero indicates there is no effect of OA. These response ratios (LnRR) were then weighted by the variance (v), calculated by their associated standard error and sample size using
where SE and SC are the standard error for the experimental treatment mean and ambient (control treatment mean, respectively, and nE and nC are the sample size for the experimental mean and ambient (control) mean, respectively (Hedges and Olkin, 1985). Studies with a lower standard error and higher sample size were weighted more heavily than those with a higher standard error and lower sample size (Hedges and Olkin, 1985). Meta-analyses were conducted with all studies combined and separated based on the mineralogical composition of the study organism (as outline above) using the R package metafor (Viechtbauer, 2010) in R version 3.5.0 (R Core Team, 2018). We used a random-effects model to estimate a summary response ratio for each biological response in each CO2 category, weighted by the inverse-variance weights (v). This model accounts for both random sampling variation in each study and the variation among studies in estimating the effect size. The true variation in effect size is calculated by the between-study variance (using the ln-transformed response ratios, LnRR), with each study weighted by the inverse sum of the individual study variance (v). We used restricted maximum-likelihood estimator (REML) to estimate the amount of (residual) heterogeneity (Q), which tests whether the variability in the effect size is larger than would be expected based on sampling variance alone (Brown and Kempton, 1994). Statistical significance of the mean response ratio was based on the 95% confidence interval on the estimated summary response ratio (Viechtbauer, 2010).
Results
For the Mg calcite-producing species, the calcite composition was classified in a total of 123 species belonging to 89 bryozoan and 34 echinoderm species (Supplementary Tables 2, 3). Individual SO maps showed a general pattern of increase of Mg content in calcite toward lower latitudes in bryozoans and echinoderms (Figure 2).
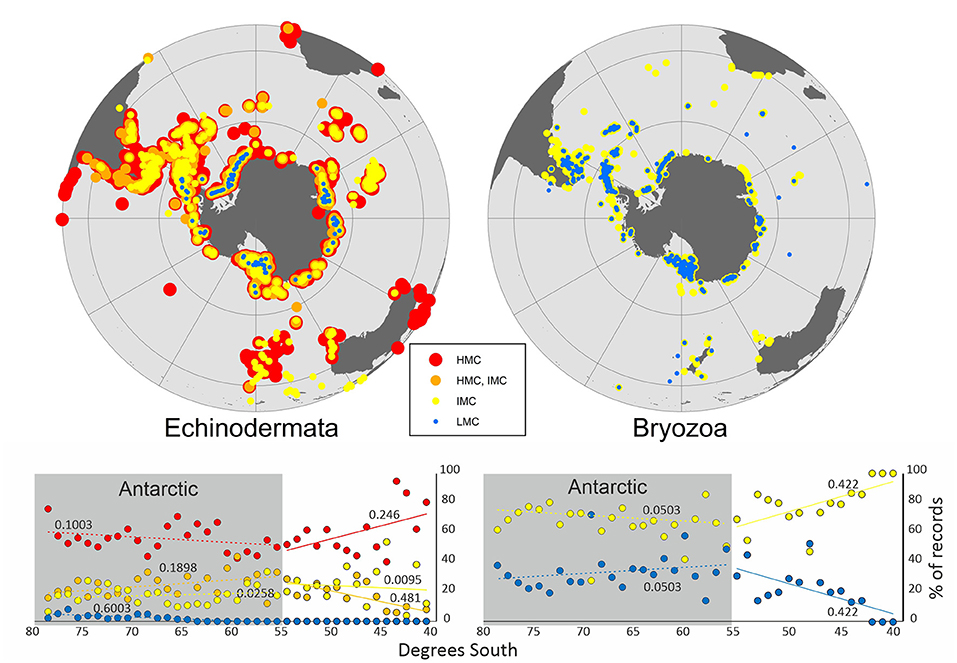
Figure 2. General distribution patterns for Echinodermata (24 HMC, 3 HMC/IMC, 6 IMC, and 1 LMC species) and Bryozoa (59 IMC and 30 LMC species) based on Mg-calcite distribution in the Southern Ocean.
Twenty studies on the effect of altered carbonate chemistry on marine calcifiers south of 60°S were included in the meta-analysis (Supplementary Figure 1 and Supplementary Table 4). A total of 25 response ratios (two for bivalves, two for brachiopods, two for sea stars, nine for sea urchins, two for pteropods, one for a sea snail, one for patellogastropoda, and six for coccolithophores) were calculated from the 20 studies (some studies reported more than one experiment and/or species response). With all response ratios combined, there was a negative response to OA in marine calcifiers (Figure 3). However, significant heterogeneity was found (Q = 48,669.264, df = 24, p < 0.001; Supplementary Table 3), indicating that the variability between the studies is greater than expected by chance alone. The response varied depending on the mineralogical composition of the organism (Figure 3). Whilst there was a negative response to OA in calcitic, aragonitic, and HMC species, LMC species were mostly unaffected [noting there was limited data for this group with only three response ratios calculated from Liothyrella uva (Broderip, 1833)]. Significant heterogeneity was observed for aragonite, calcite and HMC (p < 0.001) but not for LMC (Q = 0.6085, df = 1, p = 0.4354; Supplementary Table 3). The echinoderms, Sterechinus neumayeri (Meissner, 1900) and Odontaster validus (Koehler, 1906), were sensitive to increased CO2 above current levels (>500 μatm), with a negative effect on their development. The shell state and growth of the pteropod Limacina helicina (Phipps, 1774) and the shell state of the sea snail Margarella antarctica (Lamy, 1906) were negatively affected at moderate CO2 levels (>800 μatm). The response was also negative in CO2 treatments exceeding 1,000 μatm in the brachiopod Liothyrella uva (growth), the bivalve Laternula elliptica (P. P. King, 1832) (development and shell state), the coccolithophore Emiliania huxleyi (Lohmann) W.W. Hay & H. P. Mohler, 1967 (growth), and the sea urchin S. neumayeri (survival) (see Supplementary Figure 1 for details).
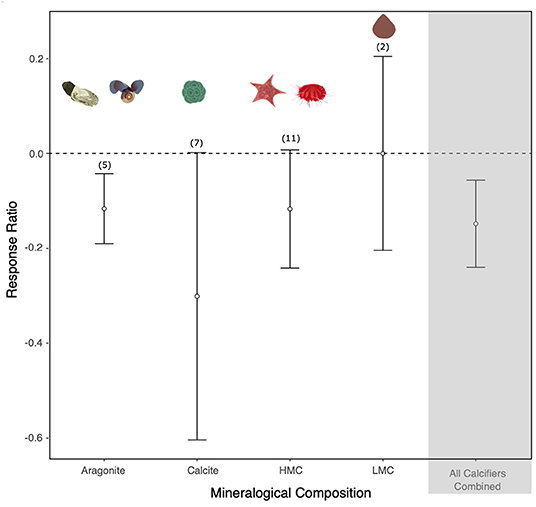
Figure 3. The effect of ocean acidification on aragonitic, calcitic, high-Mg calcite (HMC), and low-Mg calcite (LMC) species. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category given in brackets. A mean response ratio of zero (hashed line) indicates no effect. Background shading indicates response ratios of all the phyla.
Information on the vulnerability of a range of different common Antarctic phyla was assessed based on their mineralogy-related sensitivity and their strategies to overcome OA (Table 1).
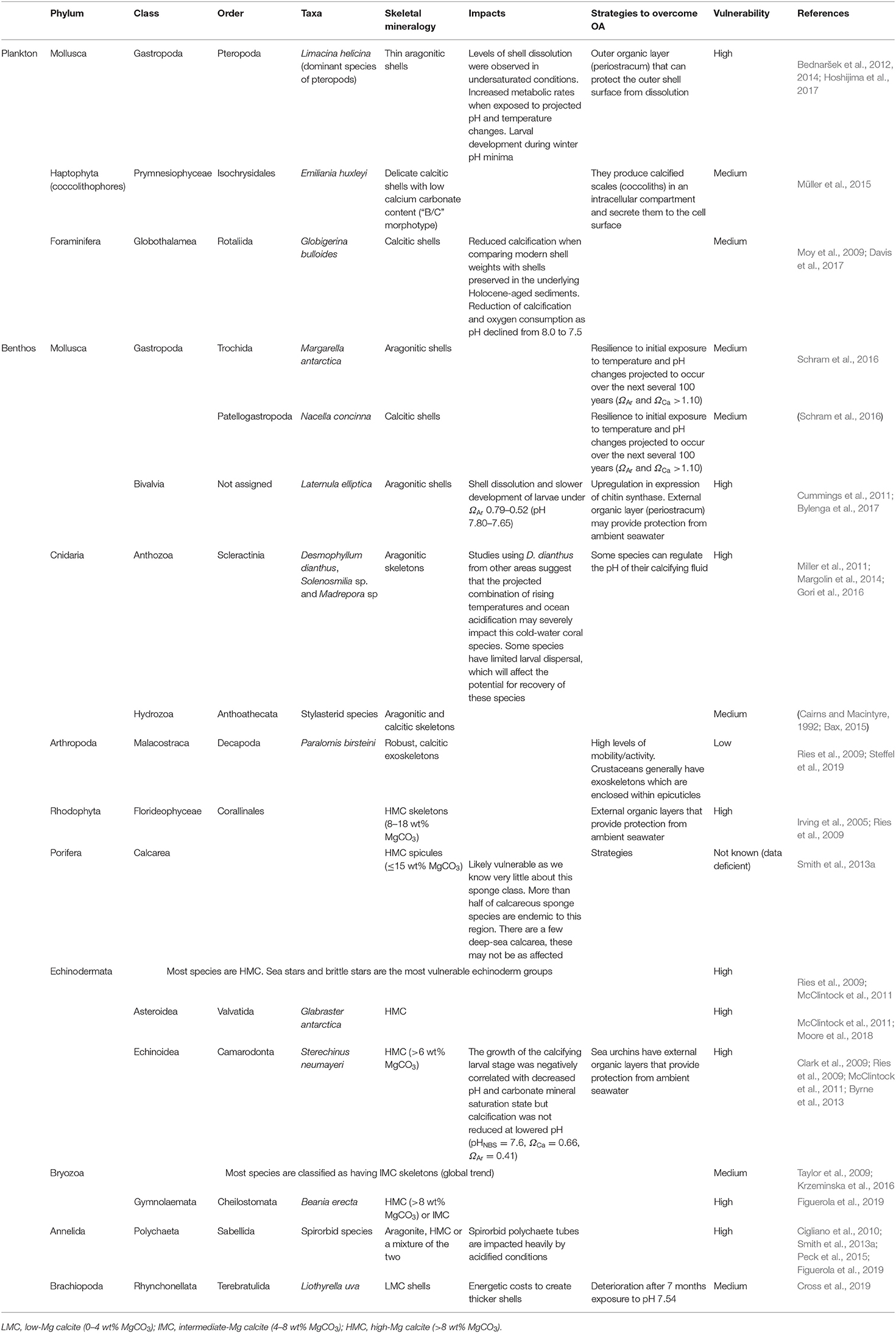
Table 1. Potential vulnerability of a range of different common Antarctic phyla based on their mineralogy-related sensitivity and their strategies to overcome ocean acidification.
Effects of Ocean Acidification on Southern Ocean Calcifying Taxa
It is predicted that marine calcifiers living in SO regions will be exposed to undersaturated seawater conditions with respect to aragonite by 2050 (Orr et al., 2005; McNeil and Matear, 2008) and with respect to calcite by 2095 (McNeil and Matear, 2008). The situation of some regions south of the Polar Front may be aggravated during austral winters (Orr et al., 2005), when wintertime aragonite undersaturation may occur as early as 2030 in the latitudinal band between 65 and 70°S (McNeil and Matear, 2008; McNeil et al., 2010; Mattsdotter Björk et al., 2014), and during the seasonal decline in primary production, when there is less biological drawdown of CO2.
As the saturation horizon shoals, deep water calcifying species that currently live just above the saturation horizon may be amongst the earliest to be affected by OA (Turley et al., 2007). Many benthic organisms could potentially be affected by reductions of their habitat, particularly those on the narrow shallow water shelves of the eastern Weddell Sea and deeper shelves of the Amundsen, Bellingshausen, and western Weddell Seas, which currently have higher levels of CaCO3 in their sediments. As organic matter alters carbonate chemistry of sediments, species inhabiting regions where the flux of organic matter to the seafloor is expected to increase (e.g., new ice-free areas) could also be at risk. Although taxa characterized by broad bathymetric ranges and/or circumpolar distributions [e.g., Laternula elliptica, Glabraster antarctica (E. A. Smith, 1876), and Sterechinus neumayeri; Figure 4] may find temporary refuge in shallower waters, and/or in some SO areas where undersaturation may occur later (e.g., far from latitudinal band between 65 and 70°S), distribution shifts are also likely to be influenced by other environmental factors such as warming. However, further molecular studies are needed to determine if broad circumpolar and/or eurybathic ranges are actually separate cryptic species (Baird et al., 2011; Allcock and Strugnell, 2012).
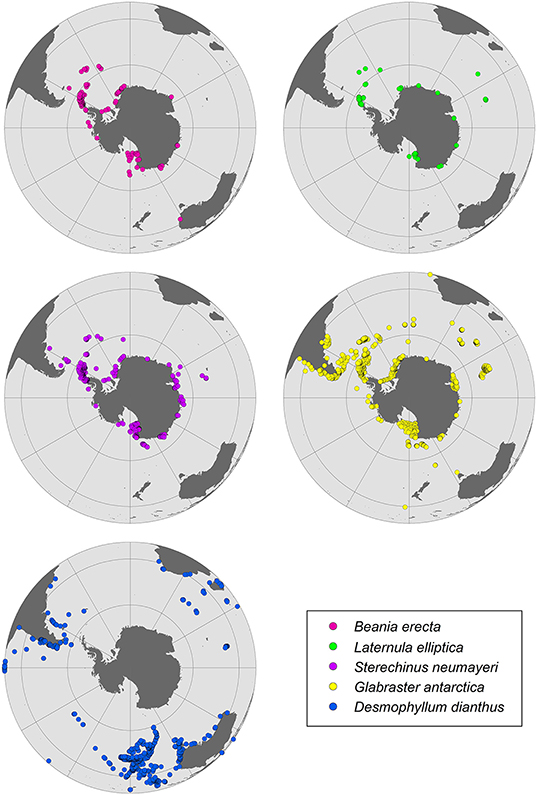
Figure 4. Distribution patterns for benthic species from the Southern Ocean identified here as particularly vulnerable to ocean acidification.
Calcified invertebrates and plants perform important roles in Antarctic ecosystem functioning including: as important food sources (e.g., pteropods) for higher trophic levels such as carnivorous zooplankton, fishes and other predators; creating habitats used as spawning, nursery and feeding areas of higher trophic levels (e.g., corals, spirorbid polychaetes); as grazers or predators influencing the structure of benthic communities (e.g., echinoderms) (Dayton et al., 1974; Prather et al., 2013; Wright and Gribben, 2017); and/or contributing to marine carbon cycling and storage (e.g., foraminifera, coccolithophores, molluscs) (Lebrato et al., 2010). Some Antarctic calcifying species with circumpolar distributions and broad bathymetric ranges could be potential indicators or sentinel species of chemical changes in the SO if they show sensitivity to environmental changes (Dayton et al., 1974; Brandt et al., 2007; Barnes and Kuklinski, 2010; Figuerola et al., 2012). A number of factors determine the influence of OA on a species including its mineralogy, biological traits (including physiology), and environmental constraints (such as variation).
Mineralogy-Related Sensitivity to Ocean Acidification
Our meta-analysis of the known effects of the OA on a range of biological responses (development, growth rate, survival, and/or shell state) in relation to the mineralogy of SO calcifiers found that SO calcitic, aragonitic, and HMC species are likely to be more vulnerable to OA, whereas LMC species were found to be unaffected (Supplementary Table 4). However, the available data is currently limited and the need for further studies was reinforced. A series of earlier comprehensive global meta-analyses of responses to OA have also shown reductions in survival, calcification, growth, and development in a range of marine calcifiers in comparison to non-calcifying taxa (Kroeker et al., 2010, 2013). These meta-analyses concluded that organisms depositing high-Mg calcite could be more resilient to OA than those depositing calcite or aragonite, bringing into question the usefulness of simplified mineralogical composition in predicting organism sensitivities to OA (Kroeker et al., 2010). A subsequent constructive debate led to suggestions to improve the accuracy of predictions, such as including other biological variables as well as the mineralogical composition, and redefining the HMC category as skeletons containing >12 mol% MgCO3 and excluding those with very complex mineralogy (e.g., crustaceans) (Andersson and Mackenzie, 2011; Kroeker et al., 2011). These studies also reinforced the need for more mineralogical studies to characterize a wider range of species. A more recent meta-analysis specifically investigated the effect of altered carbonate chemistry on marine organisms south of 60°S (from 60 studies) and found results consistent with the previous meta-analyses including reduced survival and shell calcification rates, increased dissolution in adult calcifying invertebrates, and a reduction in embryonic survival and larval development rates (Hancock et al., 2020). Some generalizations can be made for taxonomic groups, for example our study indicated that molluscs are one of the groups most sensitive to OA, whereas crustaceans are more resilient (Table 2). However, exceptions to such generalizations can be found within groups.
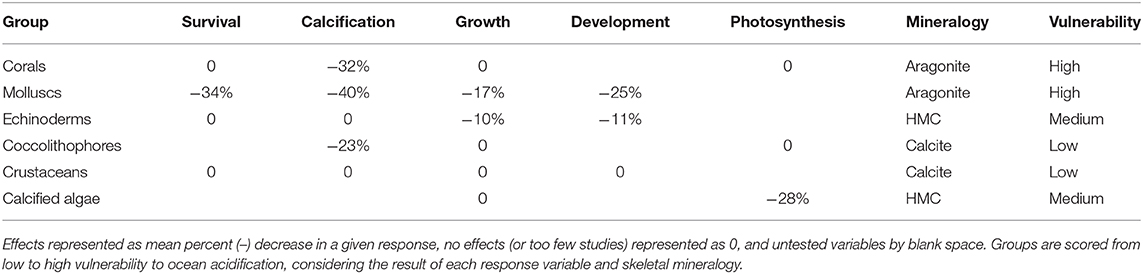
Table 2. Effects of acidification on survival, calcification, growth, development, photosynthesis in marine calcifier groups from Kroeker et al. (2013), including new data of mineralogy-related sensitivity and vulnerability.
Recent studies have examined the relationships between variation in the mineralogical composition of Antarctic and sub-Antarctic marine calcifiers and decreasing seawater pH and greater solubility of HMC and aragonite. A hypothesized decrease of skeletal Mg-calcite along a depth gradient in four Antarctic bryozoan species was not supported (Figuerola et al., 2015), and nor was an hypothesized increase of aragonitic forms toward shallower waters in sub-Antarctic stylasterid populations (Bax and Robinson, unpublished data). These mineralogy findings do not consider other factors influencing species distributions, such as gene flow across depth gradients, which may work against selective pressures influencing mineralogy (Miller et al., 2011). Our meta-analysis highlights the detrimental impact of OA on dispersal stages which could have significant long-term consequences for isolated populations (Miller et al., 2011), or eventually lead to depth related differences in mineralogy. A further step will be to consider a broader array of species and a wider depth range to test the effect of the lower CaCO3 Ω on distributions. Several common Antarctic marine calcifiers secrete more soluble CaCO3 mineral phases: aragonite (e.g., scleractinian cold-water corals, infaunal bivalves, pteropods) or HMC (e.g., bryozoans and echinoderms) (Turley et al., 2007; McClintock et al., 2009, 2011; Figuerola et al., 2019).
There is a need for information on mineralogy of all major groups of calcifying invertebrates over a wider depth range to evaluate patterns related to environmental (e.g., local CSH and ASH) and biological factors (e.g., food availability), with a focus on identifying taxa or communities that may be potentially vulnerable to near-future OA, and that may make suitable indicators (sentinels) to monitor effects of OA in the SO.
Taxonomic Variation in Mineralogy
Cold-water corals form a dominant component of some SO benthic communities and have a wide range of mineralogy. The discovery of large field-like aggregations of deep-sea stylasterid coral reefs in the Antarctic benthos and their classification as a vulnerable marine ecosystem (VME) under the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) highlights their conservation importance, and recognizes the role these reefs play in the maintenance of biodiversity (Bax and Cairns, 2014). VMEs have been described throughout the Antarctic and sub-Antarctic which include: gorgonian corals (Moore et al., 2017), colonial scleractinian corals such as Solenosmilia and Madrepora (Cairns, 1982; Cairns and Polonio, 2013), and stylasterid corals (e.g., Errina sp.) (Bax and Cairns, 2014). These coral groups have been shown to have differential carbonate mineralogy: gorgonians: calcite/aragonite (Thresher et al., 2011); scleractinians: aragonite (Margolin et al., 2014), and stylasterids: calcite/aragonite (Cairns and Macintyre, 1992). Notably, stylasterid corals have the ability to utilize both aragonite and calcite (Cairns and Macintyre, 1992), and therefore, could have a greater capacity to acclimate to changing oceanic pH than purely aragonite calcifiers (Bax and Cairns, 2014). Therefore, it is likely that coral response to OA will be varied, however, there is very little data on Antarctic coral species beyond taxonomic descriptions (Cairns, 1982, 1983), and biogeographic studies (Bax and Cairns, 2014), leaving their calcification responses to OA largely unknown, despite their expected vulnerability (Tittensor et al., 2010). One exception is the cosmopolitan aragonite scleractinian coral Desmophyllum dianthus (Esper, 1794) (Figure 4), for which there is a reasonable literature relating to OA impacts (Miller et al., 2011; Fillinger and Richter, 2013; Jantzen et al., 2013). Most of these studies, however, are based on populations outside of Antarctica, predominantly within the Chilean fjords. The scleractinid coral species D. dianthus, Solenosmilia sp. and Madrepora sp., with aragonitic skeletons, could also be impacted by OA, due to their mineralogy-related sensitivity and their biological traits. D. dianthus has a common depth range between 200 and 2,500 m (Miller et al., 2011; Fillinger and Richter, 2013), and exhibits reduced respiration rates under a combination of rising temperatures and OA scenarios (Gori et al., 2016). Thus, it is likely that this species may be severely impacted by these two key CO2-related stressors. Scleractinid corals comprise dominant components of VMEs on the Antarctic shelf and the effects of OA at the species level could lead to broader and potentially negative ecosystem level effects.
Aragonite Producing Species
One of the effects of OA predicted to occur in coming years is the shoaling of the ASH, resulting in undersaturation of aragonite in shallow waters. This is of a particular concern for shallow water species with aragonitic shells and/or skeletons such as benthic molluscs and zooplankton species such as pteropods. SO pteropods are the major pelagic producers of aragonite and comprise up to one-quarter of total zooplankton biomass in several Antarctic regions (the Ross Sea, Weddell Sea, and East Antarctica), with Limacina helicina the dominant species (McNeil and Matear, 2008). The thin aragonitic shells of these free-swimming marine gastropods are vulnerable to OA. Shell dissolution was observed when individuals of L. helicina antarctica were exposed to undersaturated conditions (ΩA = 0.8) (Bednaršek et al., 2012, 2014), along with increased metabolic rate when exposed to lowered pH (pH 7.7), and a high temperature (4°C). These responses are likely to be related to the increased energetic costs for shell repair (Hoshijima et al., 2017). L. helicina has a life cycle of 1–2 years and its veliger larva develops during winter, when the projected aragonite undersaturation will first occur. If L. helicina is not able to cope with anticipated OA, it will likely lead to cascading impacts through higher trophic levels (Seibel and Dierssen, 2003). Pteropods may be particularly vulnerable to the projected emergence of a shallow saturation horizon since they generally live in the upper 300 m (Hunt et al., 2008). A recent review and meta-analysis indicated that pteropods are at risk in the ΩAr range from 1.5 to 0.9, and that OA effects on survival and shell dissolution are exacerbated with warming (Bednaršek et al., 2019). Our meta-analysis shows significant effects of OA on growth (Hoshijima et al., 2017) and shell state (Bednaršek et al., 2014) in the pteropod L. helicina (Supplementary Figure 1). This species was already considered an OA sentinel due to its thin aragonitic shell, which is susceptible to dissolution (Figure 5) (Manno et al., 2017). L. helicina may also not have the genetic plasticity required to adapt to projected OA changes in the SO (Johnson and Hofmann, 2017).

Figure 5. Infographic of predictions about how ocean acidification will affect particular groups of marine calcifiers from Antarctica. Information on different mineral composition, functional, and life history traits is provided for each group.
Similarly, the D-larval stage of the common circumpolar Antarctic bivalve Laternula elliptica, which possess aragonitic shells (Figure 4), showed shell dissolution and slower development under OA conditions (ΩAr 0.79–0.52, pH 7.80–7.65) (Bylenga et al., 2015, 2017). Empty adult shells of other SO molluscs (bivalves and limpets), including calcitic species, also show significant dissolution when exposed to reduced pH (pH = 7.4, ΩAr = 0.47, ΩCa = 0.74) (McClintock et al., 2009). Such species may be suitable as sentinels of OA in the SO (Bednaršek et al., 2014). In contrast, aragonitic shells of the gastropod Margarella antarctica were resilient to initial exposure to temperature and pH changes projected to occur over the next several 100 years (ΩAr and ΩCa > 1.10) (Schram et al., 2016). The larvae of the bivalve L. elliptica, which have predominantly aragonite shells, also seem to be sensitive to undersaturated conditions (shell dissolution and slower development) (Supplementary Figure 1; Bylenga et al., 2015, 2017). Given its circumpolar distribution (Figure 4) and broad depth range (<10–~700 m) (Waller et al., 2017), individuals from shallower waters could avoid the initial projected occurrences of aragonite undersaturation events.
Calcite-Producing Species
Coccolithophores are a globally ubiquitous, major phytoplankton calcitic calcifier group, accounting for nearly half of global CaCO3 production (Moheimani et al., 2012). These unicellular flagellate algae play a significant role in the SO carbon cycle, contributing 17% to annually integrated net primary productivity south of 30°S (Nissen et al., 2018). Coccolithophores show taxon-specific and regional variation in response to increased pCO2 and light intensity (Donahue et al., 2019). Emiliania huxleyi, the most abundant species, has different morphotypes that exhibit a clear north-to-south distribution gradient, with the SO “B/C” morphotype forming delicate coccoliths with relatively low CaCO3 (calcite) content compared to other more calcified morphotypes from lower latitudes (Müller et al., 2015). Three morphotypes from different latitudes reduced their rate of calcification under simulated OA scenarios; the B/C morphotype was found to be the most sensitive, although even in the more resilient morphotype A a 3-fold reduction in calcification was observed (Müller et al., 2015, 2017).
In contrast, net calcification in the limpet Nacella concinna (Strebel, 1908), with calcitic shells (Mg-calcite; J.B. Schram, unpublished X-ray diffraction data), was resilient to seawater changes projected to occur over the next several 100 years (temperature and pH changes ΩAr and ΩCa > 1.10) (Schram et al., 2016).
Mg Calcite-Producing Species
High latitude surface seawater is currently undersaturated with respect to Mg-calcite minerals containing >10 wt% MgCO3 (Andersson et al., 2008), yet species with Mg-calcite mineralogy are common and important components of Antarctic benthic communities. Encrusting organisms like spirorbid polychaetes and bryozoans, with r-selected life-history strategies, are amongst the most remarkable early Antarctic colonizers (Stark, 2008). The skeletal Mg-calcite composition has been determined for a considerable number of species from several Antarctic groups (e.g., echinoderms and bryozoans), although there is still a significant gap in knowledge of other taxonomic groups, and their geographic variation in general.
Most genera of foraminifera have calcitic shells with varying amounts of Mg, ranging from LMC to HMC (Blackmon and Todd, 1959). Planktonic foraminifera are ubiquitous in the SO and a major component of calcifiers among marine zooplankton, with ~25% settling on the seafloor and their calcitic shells contributing ~32–80% to the total deep-marine calcite budget (Schiebel, 2002). It is known that reductions in pH and [] generally alter the performance of this group, together with pteropods and coccolithophores (Barker and Elderfield, 2002; Manno et al., 2012). Globigerina bulloides d'Orbigny, 1826, is one of the most commonly used species for environmental paleo-reconstructions at high latitudes (Anand and Elderfield, 2005). The common SO species have shown reduced calcification when comparing modern shell weights with shells preserved in the underlying Holocene-aged sediments (Moy et al., 2009). The authors linked the change to anthropogenic OA. Other lab and field studies using different species of planktonic foraminifera showed the same trend (Manno et al., 2012; Marshall et al., 2013). Consistent with these findings, a recent study showed a reduction of calcification and oxygen consumption of G. bulloides as pH declined from 8.0 to 7.5 (Davis et al., 2017). The lack of studies on the OA effects on foraminifera in the SO, and in general, highlights the significant gap in knowledge, and therefore the importance of further research to aid more accurate predictions.
Crustose coralline algae (CCA) is abundant and diverse worldwide and in a range of habitats on hard substrata of the continental shelves and is an important component of shallow water benthic communities and is a key substrate for benthos in some Antarctic fjords at 10–30 m (Wiencke et al., 2014), and it has been found to colonize most newly ice-free areas (Quartino et al., 2013). The distribution of CCA in Antarctica is not well known and the taxonomy is also not well-resolved (Wiencke et al., 2014). It has been found to cover up to 70–80% of the substrate under macroalgal canopies in Antarctica in shallow water (Irving et al., 2005). The tissue of CCA skeletons typically ranges from 8–18 wt% MgCO3 (Chave, 1954). One Antarctic species, Phymatolithon foecundum L. Düwel & S. Wegeberg (Alongi et al., 2002), has been reported to depths of 70 m (Cormaci et al., 2000) in the Ross Sea, but the mineralogy of this species has not been investigated. A recent investigation of four Phymatolithon spp. in the north Atlantic revealed that, within a single crust, Mg content of the carbonate ranged from 8 to 20 mol% MgCO3 (Nash and Adey, 2017). Furthermore, the proportions of carbonate types varied within the tissues, and also between species (Nash and Adey, 2017), making it difficult to predict OA impacts. Antarctic crustose macroalgae may be resilient to OA, with a study conducted on Clathromorphum obtectulum (Foslie) W. H. Adey, 1970 observing no negative impacts on CaCO3 content and Mg/Ca ratio with OA (Schoenrock et al., 2016).
Sponges are often species-rich, dominant, high biomass, habitat-providers in many Antarctic seafloors, with calcareous sponges representing more than 12% of all species, which is far higher than the global average (McClintock et al., 2005; Soest et al., 2012). Calcareous sponges are also found to be highly endemic, and have two genera found only in this region (Downey et al., 2012). Species belonging to the class Calcarea generally produce CaCO3 spicules with very high Mg contents (≤15 wt% MgCO3), and are likely to be vulnerable to projected lowered pH (Smith et al., 2013a). Close to 90% of calcareous sponges are only found on the shelves of the Antarctic continent and sub-Antarctic islands (Janussen and Downey, 2014), and most of these species have very narrow longitudinal and latitudinal ranges, which likely increases their vulnerability to projected OA in the SO. More research is needed to ascertain the impacts of OA on SO calcareous sponges.
Recent studies have investigated the skeletal Mg contents in calcite of 29 Antarctic echinoderm species to understand how OA may affect different groups (McClintock et al., 2011; Duquette et al., 2018) (Supplementary Table 2). Most species were found to be HMC, although variation of skeletal Mg-calcite existed between taxonomic classes (asteroids, ophiuroids, echinoids, and crinoids), and for the same species from different locations. McClintock et al. (2011) suggested sea stars (e.g., Glabraster antarctica), which can be Antarctic keystone predators (Dayton et al., 1974), and brittle stars, with higher mean Mg values, will likely be the first echinoderms to be affected by OA. The fertilization and early development of the common Antarctic sea urchin Sterechinus neumayeri were not affected by near-future changes and its fertilization success only declined significantly when exposed to one stressor (pH) at reduced levels projected for 2300 (decreases of ~0.7 pH units) (Ericson et al., 2010; Ho et al., 2013). However, a negative interactive effect of projected changes in seawater temperature (3°C) and pH was found in fertilization (11% reduction at pH 7.5) (Ericson et al., 2012) and on cleavage success (pH 7.6) (Foo et al., 2016), although the results may vary depending on sperm concentrations, population or experimental conditions (Sewell et al., 2014). The growth of the calcifying larval stage of this species was also found to be negatively correlated with decreased pH and Ω (Byrne et al., 2013), but calcification was not reduced at lowered pH (pHNBS = 7.6, ΩCa = 0.66, ΩAr = 0.41) (Clark et al., 2009). Additionally, its larvae had shorter arm lengths when exposed to undersaturated conditions (730 μatm, Ω = 0.82) (Yu et al., 2013). Similarly, normal development of the seastar Odontaster validus was reduced at low pH (7.8) (Karelitz et al., 2017), as was survival and growth of the larvae at pHNIST 7.6 (ΩCa = 0.89, ΩAr = 0.57) (Gonzalez-Bernat et al., 2013), indicating that they are unable to adjust to changes in extracellular pH, even though Asteroidea are non-calcifying during their larval development. The latter study illustrates how polar organisms are affected by characteristics of OA other than direct effects on carbonate structures, which could explain their different responses and sensitivities, as well as their lack of relationship to Ω (Ries et al., 2009; Waldbusser et al., 2015). Recent results have also illustrated that Antarctic regular euechinoid and cidaroid species have acid-base regulation systems, suggesting they are not particularly at risk although more experimental studies combining different stressors (e.g., temperature) and using other species are needed (Collard et al., 2015).
Sea stars and brittle stars may also be highly vulnerable to OA due to their HMC skeletons, and the apparent lack of strategies to overcome reduced pH/carbonate conditions (Table 2). One example is the sea star Glabraster antarctica, which has higher mean Mg values (>10 wt% MgCO3) than other SO species. Gonzalez-Bernat et al. (2013) found the growth of the seastar larvae Odontaster validus, is significantly affected when exposed to low pH levels. Prior studies indicate differential sensitivities depending on the biological responses in the common Antarctic sea urchin Sterechinus neumayeri (Ericson et al., 2010; Ho et al., 2013). While this species fertilization success and early development were not affected by near-future changes, the former declined significantly when only exposed to low pH levels (Ericson et al., 2010; Ho et al., 2013). In addition, other studies have revealed significant interactive effects of projected changes in seawater temperature and pH on the percentage of fertilization (Ericson et al., 2012) and on the cleavage success (Foo et al., 2016). The growth of S. neumayeri's calcifying larval stage was also found to be negatively correlated with reduced pH and Ω (Byrne et al., 2013), although its calcification was not reduced in a previous study (Clark et al., 2009). This sensitivity of larvae could cause a key bottleneck in their life-cycle (Dupont et al., 2010). Both species are circumpolar and exhibit a high range of eurybathy, ranging from the shallow subtidal to 2,930 m (O. validus) and to 500 m depth (S. neumayeri) (Pierrat et al., 2012; Moore et al., 2018). The negative impact of OA on echinoderms, especially on some sea star species which are common key predators in Antarctic ecosystems, could alter the structure and biodiversity of these ecosystems, with cascading impacts on multiple trophic levels. Like other SO echinoderm taxa, the distribution of these common species may become limited to shallow waters above the ASH, therefore, they could be affected by warming.
The Mg content from individuals of 90 Antarctic bryozoan species (nearly one-quarter of all Antarctic bryozoan species), has found extreme intraspecific and interspecific variation (Borisenko and Gontar, 1991; Taylor et al., 2009; Loxton et al., 2013, 2014; Figuerola et al., 2015, 2019; Krzeminska et al., 2016) (Supplementary Table 3). These studies showed that most species consisted of IMC. Taylor et al. (2009) previously confirmed the absence of aragonitic bryozoan species at high latitudes (>40°S) and the rarity of bimineralic species. Figuerola et al. (2019) found that the circumpolar cheilostome Beania erecta Waters, 1904 (Figure 4) may be particularly vulnerable to global ocean surface pH reductions of 0.3–0.5 units by the year 2100 as some specimens had HMC skeletons. The Antarctic cyclostome Fasciculipora ramosa d'Orbigny, 1842 also appears to be influenced, to some degree by environmental factors, as shown by the significant variability in branch diameter and Mg levels among depths (Figuerola et al., 2015, 2017). B. erecta, like other Antarctic bryozoan species, can form dense mats that, in some cases, cover large areas of substratum. Potential decreases of some species could indirectly negatively impact other organisms which use their colonies as substrate, food and shelter (Figuerola et al., 2019).
Spirorbid polychaetes are impacted heavily by acidified conditions as their tubes are mainly composed of aragonite, HMC or a mixture of the two (Cigliano et al., 2010; Smith et al., 2013b; Peck et al., 2015). A recent study did not find any temporal variation over 6 yrs in the wt% MgCO3 in calcite in Antarctic bryozoans and spirorbid polychaetes, suggesting that these particular taxa could be good indicator species for the potential effects of environmental change in coastal regions (Figuerola et al., 2019).
Brachiopods have some representatives in Antarctic waters, in particular, the rhynchonelliform brachiopods are locally important members in shallow water communities, and have LMC shells (Barnes and Peck, 1996). The circumpolar rhynchonelliform brachiopod Liothyrella uva showed deterioration after 7 months exposure to pH 7.54 (ΩCa = 0.5, ΩAr = 0.3; Cross et al., 2019) although the projected acidified conditions did not alter shell growth rates and the ability to shell repair (Cross et al., 2014).
The exoskeletons of several crustacean species have also been shown to contain Mg-calcite, although they often have a very complex mineralogy (a combination of calcite, Mg-calcite, calcium phosphate, and chitin), compared to skeletons of other groups, such as bryozoans (Chave, 1954; Andersson and Mackenzie, 2011). King crabs (Lithodidae), inhabiting deep water habitats of the SO that are already undersaturated in aragonite (Steffel et al., 2019), produce calcareous, even robust, exoskeletons (e.g., Paralomis birsteini Macpherson, 1988). As projected global warming removes thermal barriers controlling their distribution, king crabs may expand their bathymetric range to the Antarctic continental shelf, a region currently characterized by the absence of durophagous (skeleton-breaking) predators (Griffiths et al., 2013). Therefore, a range of calcifying invertebrates (e.g., echinoderms and molluscs) with thin and fragile shells and skeletons, could be at real risk of increased predation, were such predators to establish on the Antarctic shelf (Watson et al., 2017).
Changes in seawater temperature, which strongly influence growth rate and also Ω, are also thought to drive changes in skeletal Mg content, although other environmental factors such as salinity could be influential (Chave, 1954; Mackenzie et al., 1983). There is a general trend of increasing aragonite and in Mg content in calcite, toward lower latitudes, in different marine calcifying taxa (Lowenstam, 1954; Taylor et al., 2009; McClintock et al., 2011). A similar trend was found in different life stages (e.g. juveniles and larvae vs. adults) and nine skeletal elements (e.g., spines and tooth) of echinoids (Smith et al., 2016). This general pattern was also observed in individuals of echinoderms and bryozoans with LMC skeletons only reported in Antarctica and in sub-Antarctic and Antarctic regions, respectively (Figure 2).
Other Biological Traits
As suggested in previous work, it is important to consider other aspects of the biology of organisms when assessing their sensitivity to OA. Some biological traits increase sensitivity to changes in pH and CaCO3 Ω, whereas other traits provide either resistance or resilience to OA. Such biological traits, along with factors such as nutritional status and source population, enhance the variability of responses to OA (Kroeker et al., 2013). Furthermore, a range of indirect effects and variation arise when considering species response within multi-species assemblages (Kroeker et al., 2013).
Mechanisms likely to provide marine calcifiers with greater resilience to OA include high levels of mobility/activity and consequently higher metabolic rates (e.g., crustaceans), greater larval dispersal, capacity to regulate the pH of their calcifying fluid; and protection of shells or skeletons from the surrounding water (protective external organic layers) (Melzner et al., 2009; Ries et al., 2009). Marine calcifiers such as CCA, some sea urchin and molluscan taxa, possess skeletons/shells covered by external organic layers that may provide protection from ambient seawater, making them less vulnerable to OA (Ries et al., 2009). Crustaceans generally have exoskeletons which are enclosed within epicuticles, which are waxy protective outer layers (Ries et al., 2009). Some brachiopods and molluscs, such as the pteropod L. helicina antarctica, have an outer organic layer (periostracum) that can protect the outer shell surface from dissolution (Bednaršek et al., 2012, 2014). However, this periostracum can be easily damaged, exposing the underlying shell to surrounding seawater. Similarly, the level of epidermis protection in tests and spines of regular sea urchins is questionable due to its permeability and thinness, respectively (Holtmann et al., 2013; Dubois, 2014). Whilst spines usually have LMC compared to the tests, they are often damaged (Dubois, 2014). Mature spines of Cidaroids (a group well-represented in the SO), possess special outer polycrystalline layers (cortex) instead of epidermis, which appear to make them more resistant to acidification (Dery et al., 2014). Additionally, the biofilm and epibionts covering the cortex may also reduce its direct exposure to seawater. Some taxa lack a protective external organic sheet, such as spirorbid polychaetes, and may be highly vulnerable to OA due to their HMC and/or aragonitic skeletons (Cigliano et al., 2010; Smith et al., 2013b; Peck et al., 2015).
Biological traits such as adult sessile lifestyle and limited larval dispersal will act to increase the potential impacts of OA (Thatje, 2012) as the distribution of such organisms is less likely to shift to more favorable habitat. Fauna such as corals, bryozoans and spirorbid polychaetes are ecosystem engineers, creating habitat complexity and refugia for many associated macro- and micro-invertebrates. Dissolution effects could thus lead to the loss of habitat complexity with serious consequences to Antarctic ecosystems.
Acclimation and Adaptation
Responses and mechanisms for adaptation (permanent evolutionary modifications induced by response to repeated stressors) or acclimation (potential for an organism to adjust to changes in an environment) to the forecast changes are anticipated to be species-specific due to the phylogenetic control on biological processes such as calcification and the Mg content in calcite (Chave, 1954; Cairns and Macintyre, 1992). For example, an evolutionary study shows that calcification in the coccolithophore Emiliania huxleyi can be partly restored under OA conditions over ~1 year or 500-generations (Lohbeck et al., 2012). Given the shorter turn-over time of phytoplankton compared to higher-level organisms (Peck, 2018), the ability of coccolithophores to rapidly adapt to changing environmental conditions could help maintain their functionality within the ecosystem. The occurrence of heavily calcified morphotypes in higher latitudes/lower pH waters, though rare, highlights that some coccolithophores have already adapted to lower CaCO3 conditions (Beaufort et al., 2011).
Capacity to Regulate Calcification
Biocalcification (a biologically facilitated process) is either extracellular through deposition of CaCO3 on the exterior of an organism; or intracellular and controlled from within the organism (Weiner and Dove, 2003). Extracellular mineralization of CaCO3 requires the Ω to be of a high level in order for calcification to occur; and for the organism to control the [H+] in the surrounding area to prevent it bonding with , which reduces the availability of carbonate for shell building. Through the active pumping of out of a cell, an organism increases the amount of free for calcification. Alternatively, can be pumped into a vesicle within a cell and then the vesicle containing the CaCO3 is secreted to the outside the cell (Weiner and Dove, 2003). Molluscs and corals generally utilize extracellular calcification secreting their shells and skeletons, respectively, from a calcifying fluid (Ries, 2011). Further studies on the capacity of SO calcifiers to control their acid-base balance, are particularly needed to estimate their capacity to regulate their extracellular pH (Collard et al., 2015).
Intracellular mineralization of CaCO3 is achieved within an organism and retained internally to form a skeleton or internal structure, or is secreted to the outside of the organism where it is protected by the covering cell membrane (Weiner and Dove, 2003). Examples of organisms that utilize intracellular calcification include echinoderms and coccolithophores. In particular, coccolithophores control calcification through production of calcified scales (coccoliths) in an intracellular compartment and secrete them to the cell surface (Marsh, 2003). The capacity to regulate calcification via regulation of the pH of intracellular calcifying fluids used to secrete CaCO3 may provide some resistance to OA effects and resilience to recover from periods of low pH such as in winter. Some species (e.g., some corals) can regulate the pH of their calcifying fluid and thus they could buffer OA effects (Ries et al., 2009). While there have been no studies of SO CCA and OA (either distributions, or potential responses), the Arctic CCA Phymatolithon spp. exhibits strong biological control over its surface chemistry, with efficient carbon concentrating mechanisms that may prevent net dissolution by elevating pH and at their surface (Hofmann et al., 2018). In contrast, molluscs are generally not capable of regulating the pH and carbonate chemistry of their calcifying fluid (Ries, 2012; Bednaršek et al., 2014).
Energetic Costs of Acclimation to Ocean Acidification
Maintaining calcified skeletons, especially those with high Mg content, is more energetically demanding in cold waters, as the solubility of CaCO3 increases with decreasing temperature (Weyl, 1959). This is supported by a clear signal of decreasing skeletal investment with latitude and decreasing temperature, although substantial flexibility in some Antarctic species exists, e.g., laternulid clams have thicker shells than lower latitude congenerics (Watson et al., 2012). Under a global warming scenario, a general increase in skeletal Mg content is expected, likely making the skeletons even more vulnerable to dissolution (Chave, 1954; Andersson et al., 2008; Hermans et al., 2010; Sewell and Hofmann, 2011). Hence, energetic costs to counteract dissolution will likely have implications for many taxa.
For example, the congeneric deep-sea species Solenosmilia variabilis from New Zealand (3.5°C), maintained for 12 months under reduced pH conditions (pH = 7.65, ΩAr = 0.69 ± 0.01), only showed colony tissue loss (Gammon et al., 2018). The authors hypothesized that this response could indicate a reallocation of energy, with physiological processes (e.g., growth and respiration) being maintained at the expense of coenenchyme production (the common tissue that surrounds and links the polyps in octocorals) (Gammon et al., 2018).
Plasticity in, and control over, biomineralization is a likely mechanism by which calcifiers can adapt to OA. For example, Laternula ellipctica has shown upregulation in expression of chitin synthase, a gene involved in the shell formation process in response to reduced pH conditions (Cummings et al., 2011). Potentially higher energetic costs of this upregulation were indicated by elevated respiration rates which, when modeled at the population level, projected significant declines in abundance (Guy et al., 2014). A study of Mytilus spp. from temperate to high Arctic latitudes revealed mussels producing thinner shells with a higher organic content in the polar region (Telesca et al., 2019). The periostracum was thicker, and the shells contained more calcite in their prismatic layer than aragonite, which potentially provide greater protection against dissolution (Telesca et al., 2019). The brachiopod Liothyrella uva also has the ability to form thicker shells as a compensatory mechanism to dissolution under acidified conditions (Cross et al., 2019). However, the energetic cost of calcification in cold waters may also lead to other compensatory responses. For example, the Antarctic king crab Paralomis birsteini invest more resources in building robust, predatory chelae than in protective carapaces, suggesting a greater investment in predation than in defense as its predation pressure is limited in Antarctic waters (Steffel et al., 2019).
Local Adaptation to Ocean Acidification
The potential role of local adaptation through phenotypic plasticity in the resilience of species to OA has been investigated by examining the variability in responses between populations from different areas with contrasting physical conditions (Vargas et al., 2017). Populations from areas naturally exposed to high pCO2 levels and high levels of pCO2 variation show resistance and resilience to OA effects, with less impact on physiological traits, for plankton and molluscs (Vargas et al., 2017). In contrast, populations from areas with lower pCO2 and lower variability showed significant negative effects when exposed to elevated pCO2. Similarly, in a species of coralline algae it has been demonstrated that elevated pCO2 more strongly negatively impacted populations that experienced both lower pCO2 and lower variability in carbonate chemistry, including effects on physiology and growth rates (Padilla-Gamiño et al., 2016). Thus, the role of natural variability in carbonate chemistry of seawater on moderating the effects of OA on species needs to be considered when assessing species-specific responses and suitability as potential biomonitors.
The effects of such local variation on responses to OA in Antarctic species has not been investigated, however considerable variation has been found to occur in carbonate chemistry of SO waters, particularly in coastal areas (Kapsenberg et al., 2015; Stark et al., 2018). Further work is required to understand how SO carbonate systems vary on regional and local scales.
Future Directions
The SO could serve as a sentinel for evaluating the impacts of OA on marine calcifiers (Fabry et al., 2009) and aid in making predictions of the future impacts at tropical and temperate lower latitudes, where OA is expected to occur later. Some SO regions are also warming faster than other global oceans, along with other stressors such as nutrient inputs. Key marine calcifiers may not be able to cope with these multiple simultaneous projected changes, which could ultimately affect food webs and have cascading impacts from low to higher trophic levels. Combined temperature and pH studies of several Antarctic taxa indicate that temperature may have a stronger impact on survival and growth, but that the effects of OA could be severe (e.g., increased rates of abnormal development, sublethal effects). Such studies of multiple stressors are essential to explore the capacity of different species to adapt to future environmental changes. However, our understanding on the potential impact of OA on Antarctic marine calcifiers, including keystone organisms, is still constrained by the limited mineralogical and biological data available for most species. Furthermore, broader understanding is also constrained by the limited geographical coverage of studies, lack of reliable long-term monitoring, and the overall lack of multidisciplinary studies over longer times scales integrating interactions between stressors and an array of species with different mineral composition, functional, and life history traits. In particular, other environmental and biological factors, which may influence mineral susceptibility (such as organic content, protective periostracum, physiological constraints in cold waters), need further evaluation. These studies should also incorporate natural variability to better understand the limits to plasticity across organismal traits, populations and species (Vargas et al., 2017). Understanding of ecosystem effects can be improved through the use of in-situ OA studies over long times scales, such as by using free-ocean CO2 enrichment (FOCE) systems (Stark et al., 2018). Being semi-open systems, these not only allow manipulation of pH, but also incorporate natural variation in physicochemical variables, such as temperature and salinity, thus creating realistic near-future OA conditions. Recently, FOCE technology has successfully been implemented in Antarctica (Stark et al., 2018) and other in situ OA experiments have been conducted (Barr et al., 2017; Cummings et al., 2019), opening new possibilities for OA research in polar regions. Finally, special attention should be paid to the use of standardized protocols for experimental approaches (Cornwall and Hurd, 2016), the manipulation and reporting carbonate chemistry conditions (Riebesell et al., 2010), in order to compare results from different studies, improve reproducibility in experiments (Williamson et al., 2020), and make predictions about how OA may impact different groups. Building interdisciplinary collaborations via projects such as the Census of Antarctic Marine Life (CAML) is essential (Gutt et al., 2018) to successfully perform OA research on and in remote and at risk regions like Antarctica.
Author Contributions
BF conceived the initial idea for the review, organized the database and made tables for the mineralogical data, wrote the first draft of the manuscript, and edited the final version. BF and JSS organized the sections. BF, AH, and NB participated in the design advice for the figures. HG reviewed and analyzed the data available to produce species distribution maps. AH and BF screened papers and AH performed the meta-analysis and its figures. AH and BF interpreted the results. Authors reviewed the first draft with substantial contributions by section as follows: AH (2, 3, and 4: meta-analysis), NB (4.1: corals, 6), VC (4.2: algae and echinoderms, 5.2), RD (4.5: sponges), JS (1, 1.1.2: carbonate sediments), and JSS (1, 5.1, 5.3). All authors provided comments and suggestions and approved the final manuscript.
Funding
We thank the MEASO Support Group for their assistance. We are grateful for support to publish this paper from The Pew Charitable Trusts. We acknowledged support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI). BF was supported by a postdoctoral contract Juan de la Cierva-Incorporación (IJCI-2017-31478) of Ministerio de Ciencia, Innovación y Universidades.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with one of the authors JSS at time of review.
Acknowledgments
We thank the MEASO Support Group and Steering Committee for assisting with figures, Stacey McCormack for developing Figures 1, 5 and Dr Andrew Constable and two reviewers for their input and constructive suggestions. JS publishes with the permission of the CEO, Geoscience Australia. This manuscript has been released as a pre-print at bioRxiv (Figuerola et al., 2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.584445/full#supplementary-material
References
Allcock, A. L., and Strugnell, J. M. (2012). Southern Ocean diversity: new paradigms from molecular ecology. Trends Ecol. Evol. 27, 520–528. doi: 10.1016/j.tree.2012.05.009
Alongi, G., Cormaci, M., and Furnari, G. (2002). The Corallinaceae (rhodophyta) from the Ross sea (Antarctica): a taxonomic revision rejects all records except Phymatolithon foecundum. Phycologia 41, 140–146. doi: 10.2216/i0031-8884-41-2-140.1
Anand, P., and Elderfield, H. (2005). Variability of Mg/Ca and Sr/Ca between and within the planktonic foraminifers Globigerina bulloides and Globorotalia truncatulinoides. Geochem. Geophys. Geosystems 6:Q11D15. doi: 10.1029/2004GC000811
Andersson, A. J., and Gledhill, D. (2013). Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar. Sci. 5, 321–348. doi: 10.1146/annurev-marine-121211-172241
Andersson, A. J., and Mackenzie, F. T. (2011). Technical comment on Kroeker et al. (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters, 13, 1419–1434. Ecol. Lett. 14, E1–E2. doi: 10.1111/j.1461-0248.2011.01646.x
Andersson, A. J., Mackenzie, F. T., and Bates, N. R. (2008). Life on the margin: Implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 373, 265–273. doi: 10.3354/meps07639
Baird, H. P., Miller, K. J., and Stark, J. S. (2011). Evidence of hidden biodiversity, ongoing speciation and diverse patterns of genetic structure in giant Antarctic amphipods. Mol. Ecol. 20, 3439–3454. doi: 10.1111/j.1365-294X.2011.05173.x
Barker, S., and Elderfield, H. (2002). Foraminiferal calcification response to glacial-interglacial changes in atmospheric CO2. Science 297, 833–836. doi: 10.1126/science.1072815
Barnes, D. K. A., and Kuklinski, P. (2010). Bryozoans of the Weddell Sea continental shelf, slope and abyss: did marine life colonize the Antarctic shelf from deep water, outlying islands or in situ refugia following glaciations? J. Biogeogr. 37, 1648–1656. doi: 10.1111/j.1365-2699.2010.02320.x
Barnes, D. K. A., and Peck, L. S. (1996). Epibiota and attachment substrata of deep-water brachiopods from Antarctica and New Zealand. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 677–687. doi: 10.1098/rstb.1996.0064
Barnes, D. K. A., and Peck, L. S. (2008). Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim. Res. 37, 149–163. doi: 10.3354/cr00760
Barr, N. G., Lohrer, A. M., and Cummings, V. J. (2017). An in situ incubation method for measuring the productivity and responses of under-ice algae to ocean acidification and warming in polar marine habitats. Limnol. Oceanogr. Methods 15, 264–275. doi: 10.1002/lom3.10154
Bax, N. (2015). Deep-Sea Stylasterid Corals in the Antarctic, SubAntarctic and Patagonian Benthos: Biogeography, Phylogenetics, Connectivity and Conservation. Available online at: https://eprints.utas.edu.au/22768/2/whole_Bax_thesis_ex_pub_mat.pdf
Bax, N. N., and Cairns, S. D. (2014). “Stylasteridae (Cnidaria; Hydrozoa),” in Biogeographic Atlas of the Southern Ocean, ed C. DeBroyer C (Cambridge: SCAR). Available online at: http://repository.si.edu/xmlui/handle/10088/22593 (accessed June 11, 2020).
Beaman, R. J., and Harris, P. T. (2005). Bioregionalisation of the George V Shelf, East Antarctica. Cont. Shelf Res. 25, 1657–1691. doi: 10.1016/j.csr.2005.04.013
Beaufort, L., Probert, I., de Garidel-Thoron, T., Bendif, E. M., Ruiz-Pino, D., Metzl, N., et al. (2011). Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature 476, 80–83. doi: 10.1038/nature10295
Bednaršek, N., Feely, R. A., Howes, E. L., Hunt, B. P. V., Kessouri, F., León, P., et al. (2019). Systematic review and meta-analysis toward synthesis of thresholds of ocean acidification impacts on calcifying pteropods and interactions with warming. Front. Mar. Sci. 6:227. doi: 10.3389/fmars.2019.00227
Bednaršek, N., Tarling, G. A., Bakker, D. C. E., Fielding, S., and Feely, R. A. (2014). Dissolution dominating calcification process in polar pteropods close to the point of aragonite undersaturation. PLoS ONE 9:e109183. doi: 10.1371/journal.pone.0109183
Bednaršek, N., Tarling, G. A., Bakker, D. C. E., Fielding, S., Jones, E. M., Venables, H. J., et al. (2012). Extensive dissolution of live pteropods in the Southern ocean. Nat. Geosci. 5, 881–885. doi: 10.1038/ngeo1635
Blackmon, P. D., and Todd, R. (1959). Mineralogy of some foraminifera as related to their classification and ecology. J. Paleontol. 33, 1–15.
Borisenko, Y., and Gontar, V. I. (1991). Biogeochemistry of skeletons of coldwater Bryozoa. Biol. Morya 1, 80–90.
Brandt, A., Gooday, A. J., Brandão, S. N., Brix, S., Brökeland, W., Cedhagen, T., et al. (2007). First insights into the biodiversity and biogeography of the Southern ocean deep sea. Nature 447, 307–11. doi: 10.1038/nature05827
Brown, H. K., and Kempton, R. A. (1994). The application of REML in clinical trials. Stat. Med. 13, 1601–1617. doi: 10.1002/sim.4780131602
Bylenga, C. H., Cummings, V. J., and Ryan, K. G. (2015). Fertilisation and larval development in an Antarctic bivalve, Laternula elliptica, under reduced pH and elevated temperatures. Mar. Ecol. Prog. Ser. 536, 187–201. doi: 10.3354/meps11436
Bylenga, C. H., Cummings, V. J., and Ryan, K. G. (2017). High resolution microscopy reveals significant impacts of ocean acidification and warming on larval shell development in Laternula elliptica. PLoS ONE 12:e0175706. doi: 10.1371/journal.pone.0175706
Byrne, M., Ho, M. A., Koleits, L., Price, C., King, C. K., Virtue, P., et al. (2013). Vulnerability of the calcifying larval stage of the Antarctic sea urchin Sterechinus neumayeri to near-future ocean acidification and warming. Glob. Change Biol. 19, 2264–2275. doi: 10.1111/gcb.12190
Cairns, S. D. (1982). Antarctic and subantarctic scleractinia. Antarct. Res. Ser. 34, 1–74. doi: 10.1029/AR034p0001
Cairns, S. D. (1983). A generic revision of the Stylasterina (Coelenterata: Hydrozoa). Part 1. Description of the Genera. Bull. Mar. Sci. 33, 427–508.
Cairns, S. D., and Macintyre, I. G. (1992). Phylogenetic implications of calcium carbonate mineralogy in the Stylasteridae (Cnidaria: Hydrozoa). Palaios 7, 96–107. doi: 10.2307/3514799
Cairns, S. D., and Polonio, V. (2013). New records of deep-water scleractinia off Argentina and the Falkland islands. Zootaxa 3691, 58–86. doi: 10.11646/zootaxa.3691.1.2
Chave, K. E. (1954). Aspects of the biogeochemistry of magnesium 1. Calcareous marine organisms. J. Geol. 62, 266–283. doi: 10.1086/626162
Cigliano, M., Gambi, M. C., Rodolfo-Metalpa, R., Patti, F. P., and Hall-Spencer, J. M. (2010). Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar. Biol. 157, 2489–2502. doi: 10.1007/s00227-010-1513-6
Clark, D., Lamare, M., and Barker, M. (2009). Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar. Biol. 156, 1125–1137. doi: 10.1007/s00227-009-1155-8
Collard, M., Ridder, C. D., David, B., Dehairs, F., and Dubois, P. (2015). Could the acid–base status of Antarctic sea urchins indicate a better-than-expected resilience to near-future ocean acidification? Glob. Change Biol. 21, 605–617. doi: 10.1111/gcb.12735
Conrad, C. J., and Lovenduski, N. S. (2015). Climate-driven variability in the Southern ocean carbonate system. J. Clim. 28, 5335–5350. doi: 10.1175/JCLI-D-14-00481.1
Cormaci, M., Furnari, G., and Scammacca, B. (2000). “The Macrophytobenthos of Terra Nova Bay,” in Ross Sea Ecology: Italiantartide Expeditions (1987–1995), eds. F. M. Faranda, L. Guglielmo, and A. Ianora (Berlin: Springer), 493–502.
Cornwall, C. E., and Hurd, C. L. (2016). Experimental design in ocean acidification research: problems and solutions. ICES J. Mar. Sci. 73, 572–581. doi: 10.1093/icesjms/fsv118
Cross, E. L., Harper, E. M., and Peck, L. S. (2019). thicker shells compensate extensive dissolution in brachiopods under future ocean acidification. Environ. Sci. Technol. 53, 5016–5026. doi: 10.1021/acs.est.9b00714
Cross, E. L., Peck, L. S., and Harper, L. (2014). Ocean acidification does not impact shell growth or repair of the Antarctic brachiopod Liothyrella uva (Broderip, 1833). J. Exp. Marine Biol. Ecol. 462, 29–35. doi: 10.1016/j.jembe.2014.10.013
Cummings, V., Hewitt, J., Rooyen, A. V., Currie, K., Beard, S., Thrush, S., et al. (2011). Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS ONE 6:e16069. doi: 10.1371/journal.pone.0016069
Cummings, V. J., Barr, N. G., Budd, R. G., Marriott, P. M., Safi, K. A., and Lohrer, A. M. (2019). In situ response of Antarctic under-ice primary producers to experimentally altered pH. Sci. Rep. 9:6069. doi: 10.1038/s41598-019-42329-0
Cyronak, T., Schulz, K. G., and Jokiel, P. L. (2016a). Response to Waldbusser et al. (2016): “Calcium carbonate saturation state: on myths and this or that stories.” ICES J. Mar. Sci. 73, 569–571. doi: 10.1093/icesjms/fsv224
Cyronak, T., Schulz, K. G., and Jokiel, P. L. (2016b). The Omega myth: what really drives lower calcification rates in an acidifying ocean. ICES J. Mar. Sci. 73, 558–562. doi: 10.1093/icesjms/fsv075
Davis, C. V., Rivest, E. B., Hill, T. M., Gaylord, B., Russell, A. D., and Sanford, E. (2017). Ocean acidification compromises a planktic calcifier with implications for global carbon cycling. Sci. Rep. 7:2225. doi: 10.1038/s41598-017-01530-9
Dayton, P. K., Robilliard, G. A., Paine, R. T., and Dayton, L. B. (1974). Biological accommodation in the benthic community at Mcmurdo sound, Antarctica. Ecol. Monogr. 44, 105–128. doi: 10.2307/1942321
Dery, A., Guibourt, V., Catarino, A. I., Compère, P., and Dubois, P. (2014). Properties, morphogenesis, and effect of acidification on spines of the cidaroid sea urchin Phyllacanthus imperialis. Invertebr. Biol. 133, 188–199. doi: 10.1111/ivb.12054
Donahue, K., Klaas, C., Dillingham, P. W., and Hoffmann, L. J. (2019). Combined effects of ocean acidification and increased light intensity on natural phytoplankton communities from two Southern ocean water masses. J. Plankton Res. 41, 30–45. doi: 10.1093/plankt/fby048
Downey, R. V., Griffiths, H. J., Linse, K., and Janussen, D. (2012). Diversity and distribution patterns in high southern latitude sponges. PLoS ONE 7:e41672. doi: 10.1371/journal.pone.0041672
Dubois, P. (2014). The Skeleton of postmetamorphic echinoderms in a changing world. Biol. Bull. 226, 223–236. doi: 10.1086/BBLv226n3p223
Dupont, S., Dorey, N., and Thorndyke, M. (2010). What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast. Shelf Sci. 89, 182–185. doi: 10.1016/j.ecss.2010.06.013
Duquette, A., Halanych, K. M., Angus, R. A., and McClintock, J. B. (2018). Inter and intraspecific comparisons of the skeletal Mg/Ca ratios of high latitude Antarctic echinoderms. Antarct. Sci. 30, 160–169. doi: 10.1017/S0954102017000566
Ericson, J. A., Ho, M. A., Miskelly, A., King, C. K., Virtue, P., Tilbrook, B., et al. (2012). Combined effects of two ocean change stressors, warming and acidification, on fertilization and early development of the Antarctic echinoid Sterechinus neumayeri. Polar Biol. 35, 1027–1034. doi: 10.1007/s00300-011-1150-7
Ericson, J. A., Lamare, M. D., Morley, S. A., and Barker, M. F. (2010). The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH: effects on fertilisation and embryonic development. Mar. Biol. 157, 2689–2702. doi: 10.1007/s00227-010-1529-y
Fabry, V., McClintock, J., Mathis, J., and Grebmeier, J. (2009). Ocean acidification at high latitudes: the bellwether. Oceanography 22, 160–171. doi: 10.5670/oceanog.2009.105
Fabry, V. J. (2008). Marine Calcifiers in a High-CO2 Ocean. Science 320, 1020–1022. doi: 10.1126/science.1157130
Fassbender, A. J., Sabine, C. L., and Feifel, K. M. (2016). Consideration of coastal carbonate chemistry in understanding biological calcification. Geophys. Res. Lett. 43, 4467–4476. doi: 10.1002/2016GL068860
Feely, R. A., Sabine, C. L., Lee, K., Berelson, W., Kleypas, J., Fabry, V. J., et al. (2004). Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366. doi: 10.1126/science.1097329
Figuerola, B., Ballesteros, M., Monleón-Getino, T., and Avila, C. (2012). Spatial patterns and diversity of bryozoan communities from the Southern ocean: South Shetland islands, Bouvet island and Eastern Weddell sea. Syst. Biodivers. 10, 109–123. doi: 10.1080/14772000.2012.668972
Figuerola, B., Gore, D. B., Johnstone, G., and Stark, J. S. (2019). Spatio-temporal variation of skeletal Mg-calcite in Antarctic marine calcifiers. PLoS ONE 14:e0210231. doi: 10.1371/journal.pone.0210231
Figuerola, B., Hancock, A. M., Bax, N., Cummings, V., Downey, R., Griffiths, H. J., et al. (2020). Predicting potential impacts of ocean acidification on marine calcifiers from the Southern ocean. bioRxiv [Preprint]. doi: 10.1101/2020.11.15.384131
Figuerola, B., Kuklinski, P., Carmona, F., and Taylor, P. D. (2017). Evaluating potential factors influencing branch diameter and skeletal Mg-calcite using an Antarctic cyclostome bryozoan species. Hydrobiologia 799, 101–110. doi: 10.1007/s10750-017-3213-4
Figuerola, B., Kuklinski, P., and Taylor, P. D. (2015). Depth patterns in Antarctic bryozoan skeletal Mg-calcite: can they provide an analogue for future environmental changes? Mar. Ecol. Prog. Ser. 540, 109–120. doi: 10.3354/meps11515
Fillinger, L., and Richter, C. (2013). Vertical and horizontal distribution of Desmophyllum dianthus in Comau Fjord, Chile: a cold-water coral thriving at low pH. PeerJ 1:e194. doi: 10.7717/peerj.194
Foo, S. A., Sparks, K. M., Uthicke, S., Karelitz, S., Barker, M., Byrne, M., et al. (2016). Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Mar. Biol. 163:130. doi: 10.1007/s00227-016-2903-1
Gammon, M. J., Tracey, D. M., Marriott, P. M., Cummings, V. J., and Davy, S. K. (2018). The physiological response of the deep-sea coral Solenosmilia variabilis to ocean acidification. PeerJ. 6:e5236. doi: 10.7717/peerj.5236
Gonzalez-Bernat, M. J., Lamare, M., and Barker, M. (2013). Effects of reduced seawater pH on fertilisation, embryogenesis and larval development in the Antarctic seastar Odontaster validus. Polar Biol. 36, 235–247. doi: 10.1007/s00300-012-1255-7
Gori, A., Ferrier-Pagès, C., Hennige, S. J., Murray, F., Rottier, C., Wicks, L. C., et al. (2016). Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ. 4:e1606. doi: 10.7717/peerj.1606
Griffiths, H. J., Whittle, R. J., Roberts, S. J., Belchier, M., and Linse, K. (2013). Antarctic crabs: invasion or endurance? PLoS ONE 8:e66981. doi: 10.1371/journal.pone.0066981
Guinotte, J. M., Orr, J., Cairns, S., Freiwald, A., Morgan, L., and George, R. (2006). Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 4, 141–146. doi: 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2
Gutt, J., Isla, E., Bertler, A. N., Bodeker, G. E., Bracegirdle, T. J., Cavanagh, R. D., et al. (2018). Cross-disciplinarity in the advance of Antarctic ecosystem research. Mar. Genomics 37, 1–17. doi: 10.1016/j.margen.2017.09.006
Guy, C. I., Cummings, V. J., Lohrer, A. M., Gamito, S., and Thrush, S. F. (2014). Population trajectories for the Antarctic bivalve Laternula elliptica: identifying demographic bottlenecks in differing environmental futures. Polar Biol. 37, 541–553. doi: 10.1007/s00300-014-1456-3
Haese, R. R., Smith, J., Weber, R., and Trafford, J. (2014). High-magnesium calcite dissolution in tropical continental shelf sediments controlled by ocean acidification. Environ. Sci. Technol. 48, 8522–8528. doi: 10.1021/es501564q
Hancock, A. M., King, C. K., Stark, J. S., McMinn, A., and Davidson, A. T. (2020). Effects of ocean acidification on Antarctic marine organisms: a meta-analysis. Ecol. Evol. 10, 4495–4514. doi: 10.1002/ece3.6205
Hauck, J., Gerdes, D., Hillenbrand, C.-D., Hoppema, M., Kuhn, G., Nehrke, G., et al. (2012). Distribution and mineralogy of carbonate sediments on Antarctic shelves. J. Mar. Syst. 90, 77–87. doi: 10.1016/j.jmarsys.2011.09.005
Hauri, C., Friedrich, T., and Timmermann, A. (2016). Abrupt onset and prolongation of aragonite undersaturation events in the Southern ocean. Nat. Clim. Change 6, 172–176. doi: 10.1038/nclimate2844
Hedges, L., and Olkin, I. (1985). Statistical Methods for Meta-Analysis. New York, NY: Academic Press.
Hedges, L. V., Gurevitch, J., and Curtis, P. S. (1999). The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. doi: 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2
Hermans, J., Borremans, C., Willenz, P., André, L., and Dubois, P. (2010). Temperature, salinity and growth rate dependences of Mg/Ca and Sr/Ca ratios of the skeleton of the sea urchin Paracentrotus lividus (Lamarck): an experimental approach. Mar. Biol. 157, 1293–1300. doi: 10.1007/s00227-010-1409-5
Ho, M. A., Price, C., King, C. K., Virtue, P., and Byrne, M. (2013). Effects of ocean warming and acidification on fertilization in the Antarctic echinoid Sterechinus neumayeri across a range of sperm concentrations. Mar. Environ. Res. 90, 136–141. doi: 10.1016/j.marenvres.2013.07.007
Hofmann, L. C., Schoenrock, K., and de Beer, D. (2018). Arctic coralline algae elevate surface pH and carbonate in the dark. Front. Plant Sci. 9:1416. doi: 10.3389/fpls.2018.01416
Holtmann, W. C., Stumpp, M., Gutowska, M. A., Syré, S., Himmerkus, N., Melzner, F., et al. (2013). Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: the role of body cavity epithelia and stereom dissolution. Mar. Biol. 160, 2631–2645. doi: 10.1007/s00227-013-2257-x
Hoshijima, U., Wong, J. M., and Hofmann, G. E. (2017). Additive effects of pCO2 and temperature on respiration rates of the Antarctic pteropod Limacina helicina antarctica. Conserv. Physiol. 5:cox064. doi: 10.1093/conphys/cox064
Hunt, B. P. V., Pakhomov, E. A., Hosie, G. W., Siegel, V., Ward, P., and Bernard, K. (2008). Pteropods in Southern ocean ecosystems. Prog. Oceanogr. 78, 193–221. doi: 10.1016/j.pocean.2008.06.001
IPCC (2014). Synthesis Report in Climate Change 2014: Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC.
IPCC (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Geneva: IPCC. Available online at: http://www.unenvironment.org/resources/report/ipcc-special-report-ocean-and-cryosphere-changing-climate
Irving, A. D., Connell, S. D., Johnston, E. L., Pile, A. J., and Gillanders, B. M. (2005). The response of encrusting coralline algae to canopy loss: an independent test of predictions on an Antarctic coast. Mar. Biol. 147, 1075–1083. doi: 10.1007/s00227-005-0007-4
Jantzen, C., Häussermann, V., Försterra, G., Laudien, J., Ardelan, M., Maier, S., et al. (2013). Occurrence of a cold-water coral along natural pH gradients (Patagonia, Chile). Mar. Biol. 160, 2597–2607. doi: 10.1007/s00227-013-2254-0
Janussen, D., and Downey, R. (2014). “Porifera,” in DeBroyer C et al. Biogeographic Atlas of the Southern Ocean. Cambridge: SCAR, 94–102.
Jiang, L.-Q., Feely, R. A., Carter, B. R., Greeley, D. J., Gledhill, D. K., and Arzayus, K. M. (2015). Climatological distribution of aragonite saturation state in the global oceans. Glob. Biogeochem. Cycles 29, 1656–1673. doi: 10.1002/2015GB005198
Johnson, K. M., and Hofmann, G. E. (2017). Transcriptomic response of the Antarctic pteropod Limacina helicina antarctica to ocean acidification. BMC Genomics 18:812. doi: 10.1186/s12864-017-4161-0
Kapsenberg, L., and Cyronak, T. (2019). Ocean acidification refugia in variable environments. Glob. Change Biol. 25, 3201–3214. doi: 10.1111/gcb.14730
Kapsenberg, L., Kelley, A. L., Shaw, E. C., Martz, T. R., and Hofmann, G. E. (2015). Near-shore Antarctic pH variability has implications for the design of oceanacidification experiments. Sci. Rep. 5:9638. doi: 10.1038/srep09638
Karelitz, S. E., Uthicke, S., Foo, S. A., Barker, M. F., Byrne, M., Pecorino, D., et al. (2017). Ocean acidification has little effect on developmental thermal windows of echinoderms from Antarctica to the tropics. Glob. Change Biol. 23, 657–672. doi: 10.1111/gcb.13452
Kleypas, J. A., Buddemeier, R. W., Archer, D., Gattuso, J.-P., Langdon, C., and Opdyke, B. N. (1999). Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120. doi: 10.1126/science.284.5411.118
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Kroeker, K. J., Kordas, R. L., Crim, R. N., and Singh, G. G. (2010). Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x
Kroeker, K. J., Kordas, R. L., Crim, R. N., and Singh, G. G. (2011). Response to technical comment on ‘meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms’. Ecol. Lett. 14, E1–E2. doi: 10.1111/j.1461-0248.2011.01665.x
Krzeminska, M., Kuklinski, P., Najorka, J., and Iglikowska, A. (2016). Skeletal mineralogy patterns of Antarctic Bryozoa. J. Geol. 124, 411–422. doi: 10.1086/685507
Lajeunesse, M. J., and Forbes, M. R. (2003). Variable reporting and quantitative reviews: a comparison of three meta-analytical techniques. Ecol. Lett. 6, 448–454. doi: 10.1046/j.1461-0248.2003.00448.x
Lebrato, M., Andersson, A. J., Ries, J. B., Aronson, R. B., Lamare, M. D., Koeve, W., et al. (2016). Benthic marine calcifiers coexist with CaCO3-undersaturated seawater worldwide. Glob. Biogeochem. Cycles 30, 1038–1053. doi: 10.1002/2015GB005260
Lebrato, M., Feely, R. A., Greeley, D., Jones, O. B., Suarez-bosche, N., Lampitt, R. S., et al. (2010). Global contribution of echinoderms to the marine carbon cycle: a re- assessment of the oceanic CaCO3 budget and the benthic compartments. Ecol. Monogr. 80, 441–467. doi: 10.1890/09-0553.1
Lohbeck, K. T., Riebesell, U., and Reusch, T. B. H. (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351. doi: 10.1038/ngeo1441
Lowenstam, H. A. (1954). Environmental relations of modification compositions of certain carbonate secreting marine invertebrates. Proc. Natl. Acad. Sci. U.S.A. 40, 39–48. doi: 10.1073/pnas.40.1.39
Loxton, J., Kuklinski, P., Barnes, D. K. A., Najorka, J., Jones, M. S., and Porter, J. S. (2014). Variability of Mg-calcite in Antarctic bryozoan skeletons across spatial scales. Mar. Ecol. Prog. Ser. 507, 169–180. doi: 10.3354/meps10826
Loxton, J., Kuklinski, P., Mair, J. M., Jones, M. S., and Porter, J. S. (2013). Patterns of magnesium-calcite distribution in the skeleton of some polar bryozoan species mineralogy of polar bryozoan skeletons. Bryozoan Stud. 143, 169–185. doi: 10.1007/978-3-642-16411-8_12
Mackenzie, F. T., Bischoff, W. D., Bishop, F. C., Loijens, M., Schoonmaker, J., and Wollast, R. (1983). “Magnesian calcites: low-temperature occurrence, solubility and solid-solution behavior,” in Carbonates: Mineralogy and Chemistry. Reviews in Mineralogy, ed. R. Reeder (Washington, DC: Mineralogical Society of America), 97–143.
Manno, C., Bednaršek, N., Tarling, G. A., Peck, V. L., Comeau, S., Adhikari, D., et al. (2017). Shelled pteropods in peril: assessing vulnerability in a high CO2 ocean. Earth-Sci. Rev. 169, 132–145. doi: 10.1016/j.earscirev.2017.04.005
Manno, C., Morata, N., and Bellerby, R. (2012). Effect of ocean acidification and temperature increase on the planktonic foraminifer Neogloboquadrina pachyderma(sinistral). Polar Biol. 35, 1311–1319. doi: 10.1007/s00300-012-1174-7
Margolin, A. R., Robinson, L. F., Burke, A., Waller, R. G., Scanlon, K. M., Roberts, M. L., et al. (2014). Temporal and spatial distributions of cold-water corals in the Drake Passage: Insights from the last 35,000 years. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 237–248. doi: 10.1016/j.dsr2.2013.06.008
Marsh, M. E. (2003). Regulation of CaCO3 formation in coccolithophores. Comp. Biochem. Physiol. B,. Biochem. Mol. Biol. 136, 743–754. doi: 10.1016/S1096-4959(03)00180-5
Marshall, B. J., Thunell, R. C., Henehan, M. J., Astor, Y., and Wejnert, K. E. (2013). Planktonic foraminiferal area density as a proxy for carbonate ion concentration: A calibration study using the cariaco basin ocean time series. Paleoceanography 28, 363–376. doi: 10.1002/palo.20034
Mattsdotter Björk, M., Fransson, A., Torstensson, A., and Chierici, M. (2014). Ocean acidification state in western Antarctic surface waters: controls and interannual variability. Biogeosciences 11, 57–73. doi: 10.5194/bg-11-57-2014
McClintock, J. B., Amsler, C. D., Baker, B. J., and van Soest, R. W. M. (2005). Ecology of antarctic marine sponges: an overview. Integr. Comp. Biol. 45, 359–368. doi: 10.1093/icb/45.2.359
McClintock, J. B., Amsler, M. O., Angus, R. A., Challener, R. C., Schram, J. B., Amsler, C. D., et al. (2011). The Mg-Calcite composition of Antarctic echinoderms: important implications for predicting the impacts of ocean acidification. J. Geol. 119, 457–466. doi: 10.1086/660890
McClintock, J. B., Angus, R. A., Mcdonald, M. R., Amsler, C. D., Catledge, S. A., and Vohra, Y. K. (2009). Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarct. Sci. 21, 449–456. doi: 10.1017/S0954102009990198
McNeil, B. I., and Matear, R. J. (2008). Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. U.S.A. 105, 18860–18864. doi: 10.1073/pnas.0806318105
McNeil, B. I., Sweeney, C., and Gibson, J. A. E. (2011). Short Note: Natural seasonal variability of aragonite saturation state within two Antarctic coastal ocean sites. Antarct. Sci. 23, 411–412. doi: 10.1017/S0954102011000204
McNeil, B. I., Tagliabue, A., and Sweeney, C. (2010). A multi-decadal delay in the onset of corrosive ‘acidified’ waters in the Ross sea of Antarctica due to strong air-sea CO2 disequilibrium. Geophys. Res. Lett. 37:L19607. doi: 10.1029/2010GL044597
Melzner, F., Gutowska, M. A., Langenbuch, M., Dupont, S., Lucassen, M., Thorndyke, M. C., et al. (2009). Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331. doi: 10.5194/bg-6-2313-2009
Miller, K. J., Rowden, A. A., Williams, A., and Häussermann, V. (2011). Out of their depth? Isolated deep populations of the cosmopolitan coral desmophyllum dianthus may be highly vulnerable to environmental change. PLOS ONE 6:e19004. doi: 10.1371/journal.pone.0019004
Moheimani, N. R., Webb, J. P., and Borowitzka, M. A. (2012). Bioremediation and other potential applications of coccolithophorid algae: a review. Algal Res. 1, 120–133. doi: 10.1016/j.algal.2012.06.002
Moore, J. M., Carvajal, J. I., Rouse, G. W., and Wilson, N. G. (2018). The Antarctic circumpolar current isolates and connects: structured circumpolarity in the sea star Glabraster antarctica. Ecol. Evol. 8, 10621–10633. doi: 10.1002/ece3.4551
Moore, K. M., Alderslade, P., and Miller, K. J. (2017). A taxonomic revision of Anthothela (Octocorallia: Scleraxonia: Anthothelidae) and related genera, with the addition of new taxa, using morphological and molecular data. Zootaxa 4304, 1–212. doi: 10.11646/zootaxa.4304.1.1
Moy, A. D., Howard, W. R., Bray, S. G., and Trull, T. W. (2009). Reduced calcification in modern Southern ocean planktonic foraminifera. Nat. Geosci. 2, 276–280. doi: 10.1038/ngeo460
Müller, M., Trull, T., and Hallegraeff, G. (2015). Differing responses of three Southern ocean Emiliania huxleyi ecotypes to changing seawater carbonate chemistry. Mar. Ecol. Prog. Ser. 531, 81–90. doi: 10.3354/meps11309
Müller, M. N., Trull, T. W., and Hallegraeff, G. M. (2017). Independence of nutrient limitation and carbon dioxide impacts on the Southern ocean coccolithophore Emiliania huxleyi. ISME J. 11, 1777–1787. doi: 10.1038/ismej.2017.53
Nash, M. C., and Adey, W. (2017). Multiple phases of mg-calcite in crustose coralline algae suggest caution for temperature proxy and ocean acidification assessment: lessons from the ultrastructure and biomineralization in Phymatolithon (Rhodophyta, Corallinales). J. Phycol. 53, 970–984. doi: 10.1111/jpy.12559
Negrete-García, G., Lovenduski, N. S., Hauri, C., Krumhardt, K. M., and Lauvset, S. K. (2019). Sudden emergence of a shallow aragonite saturation horizon in the Southern Ocean. Nat. Clim. Change 9, 313–317. doi: 10.1038/s41558-019-0418-8
Nissen, C., Vogt, M., Münnich, M., Gruber, N., and Haumann, F. A. (2018). Factors controlling coccolithophore biogeography in the Southern ocean. Biogeosciences 15, 6997–7024. doi: 10.5194/bg-15-6997-2018
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. a, et al. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. doi: 10.1038/nature04095
Padilla-Gamiño, J. L., Gaitán-Espitia, J. D., Kelly, M. W., and Hofmann, G. E. (2016). Physiological plasticity and local adaptation to elevated pCO2 in calcareous algae: an ontogenetic and geographic approach. Evol. Appl. 9, 1043–1053. doi: 10.1111/eva.12411
Peck, L. (2018). “Antarctic marine biodiversity: adaptations, environments and responses to change,” in Oceanography and Marine Biology: An Annual Review, eds. S. J. Hawkins, A. J. Evans, A. C. Dale, L. B. Firth, and I. P. Smith (Taylor and Francis), 105–236. doi: 10.1201/9780429454455-3
Peck, L. S., Clark, M. S., Power, D., Reis, J., Batista, F. M., and Harper, E. M. (2015). Acidification effects on biofouling communities: winners and losers. Glob. Change Biol. 21, 1907–1913. doi: 10.1111/gcb.12841
Pierrat, B., Saucède, T., Laffont, R., Ridder, C. D., Festeau, A., and David, B. (2012). Large-scale distribution analysis of Antarctic echinoids using ecological niche modelling. Mar. Ecol. Prog. Ser. 463, 215–230. doi: 10.3354/meps09842
Post, A. L., O'Brien, P. E., Beaman, R. J., Riddle, M. J., and Santis, L. D. (2010). Physical controls on deep water coral communities on the George V Land slope, East Antarctica. Antarct. Sci. 22, 371–378. doi: 10.1017/S0954102010000180
Prather, C. M., Pelini, S. L., Laws, A., Rivest, E., Woltz, M., Bloch, C. P., et al. (2013). Invertebrates, ecosystem services and climate change. Biol. Rev. 88, 327–348. doi: 10.1111/brv.12002
Quartino, M. L., Deregibus, D., Campana, G. L., Latorre, G. E. J., and Momo, F. R. (2013). Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in potter cove (South Shetland Islands), Antarctica. PLoS ONE 8:e58223. doi: 10.1371/journal.pone.0058223
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna. Available online at: https://www.R-project.org/
Racault, M.-F., Le Quéré, C., Buitenhuis, E., Sathyendranath, S., and Platt, T. (2012). Phytoplankton phenology in the global ocean. Ecol. Indic. 14, 152–163. doi: 10.1016/j.ecolind.2011.07.010
Riebesell, U., Fabry, V., Hansson, L., and Gattuso, J. (2010). Guide to Best Practices for Ocean Acidification Research and Data Reporting. Luxembourg: Publications Office of the European Union. Available online at: https://op.europa.eu/en/publication-detail/-/publication/51887496-10b6-4b42-9876-1c2a1346fac5 (accessed July 15, 2020).
Ries, J. B. (2011). A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064. doi: 10.1016/j.gca.2011.04.025
Ries, J. B., Cohen, A. L., and McCorkle, D. C. (2009). Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. doi: 10.1130/G30210A.1
Roleda, M. Y., Boyd, P. W., and Hurd, C. L. (2012). Before ocean acidification: calcifier chemistry lessons1. J. Phycol. 48, 840–843. doi: 10.1111/j.1529-8817.2012.01195.x
Rucker, J. B., and Carver, R. E. (1969). A survey of the carbonate mineralogy of cheilostome bryozoa. J. Paleontol. 43, 791–799.
Schiebel, R. (2002). Planktic foraminiferal sedimentation and the marine calcite budget. Glob. Biogeochem. Cycles 16, 1–21. doi: 10.1029/2001GB001459
Schoenrock, K. M., Schram, J. B., Amsler, C. D., McClintock, J. B., Angus, R. A., and Vohra, Y. K. (2016). Climate change confers a potential advantage to fleshy Antarctic crustose macroalgae over calcified species. J. Exp. Mar. Biol. Ecol. 474, 58–66. doi: 10.1016/j.jembe.2015.09.009
Schram, J. B., Schoenrock, K. M., McClintock, J. B., Amsler, C. D., and Angus, R. A. (2016). Testing Antarctic resilience: the effects of elevated seawater temperature and decreased pH on two gastropod species. ICES J. Mar. Sci. 73, 739–752. doi: 10.1093/icesjms/fsv233
Seibel, B. A., and Dierssen, H. M. (2003). Cascading trophic impacts of reduced biomass in the ross sea, antarctica: just the tip of the iceberg? Biol. Bull. 205, 93–97. doi: 10.2307/1543229
Sewell, M. A., and Hofmann, G. E. (2011). Antarctic echinoids and climate change: a major impact on the brooding forms. Glob. Change Biol. 17, 734–744. doi: 10.1111/j.1365-2486.2010.02288.x
Sewell, M. A., Millar, R. B., Yu, P. C., Kapsenberg, L., and Hofmann, G. E. (2014). Ocean acidification and fertilization in the Antarctic sea Urchin Sterechinus neumayeri: the importance of polyspermy. Environ. Sci. Technol. 48, 713–722. doi: 10.1021/es402815s
Smith, A. M., Berman, J., Key, M. M., and Winter, D. J. (2013a). Not all sponges will thrive in a high-CO2 ocean: review of the mineralogy of calcifying sponges. Palaeogeogr. Palaeoclimatol. Palaeoecol. 392, 463–472. doi: 10.1016/j.palaeo.2013.10.004
Smith, A. M., Clark, D. E., Lamare, M. D., Winter, D. J., and Byrne, M. (2016). Risk and resilience: variations in magnesium in echinoid skeletal calcite. Mar. Ecol. Prog. Ser. 561, 1–16. doi: 10.3354/meps11908
Smith, A. M., Riedi, M. A., and Winter, D. J. (2013b). Temperate reefs in a changing ocean: Skeletal carbonate mineralogy of serpulids. Mar. Biol. 160, 2281–2294. doi: 10.1007/s00227-013-2210-z
Soest, R. W. M. V., Boury-Esnault, N., Vacelet, J., Dohrmann, M., Erpenbeck, D., Voogd, N. J. D., et al. (2012). Global diversity of sponges (porifera). PLoS ONE 7:e35105. doi: 10.1371/journal.pone.0035105
Stark, J. S. (2008). Patterns of higher taxon colonisation and development in sessile marine benthic assemblages at casey station, Antarctica, and their use in environmental monitoring. Mar. Ecol. Prog. Ser. 365, 77–89. doi: 10.3354/meps07559
Stark, J. S., Roden, N. P., Johnstone, G. J., Milnes, M., Black, J. G., Whiteside, S., et al. (2018). Carbonate chemistry of an in-situ free-ocean CO2 enrichment experiment (antFOCE) in comparison to short term variation in Antarctic coastal waters. Sci. Rep. 8:2816. doi: 10.1038/s41598-018-21029-1
Steffel, B. V., Smith, K. E., Dickinson, G. H., Flannery, J. A., Baran, K. A., Rosen, M. N., et al. (2019). Characterization of the exoskeleton of the Antarctic king crab Paralomis birsteini. Invertebr. Biol. 138:e12246. doi: 10.1111/ivb.12246
Swezey, D. S., Bean, J. R., Ninokawa, A. T., Hill, T. M., Gaylord, B., and Sanford, E. (2017). Interactive effects of temperature, food and skeletal mineralogy mediate biological responses to ocean acidification in a widely distributed bryozoan. Proc. R. Soc. B Biol. Sci. 284:20162349. doi: 10.1098/rspb.2016.2349
Takahashi, T., Sutherland, S. C., Sweeney, C., Poisson, A., Metzl, N., Tilbrook, B., et al. (2002). Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Res. Part II Top. Stud. Oceanogr. 49, 1601–1622. doi: 10.1016/S0967-0645(02)00003-6
Taylor, P. D., James, N. P., Bone, Y., Kuklinski, P., and Kyser, T. K. (2009). Evolving mineralogy of cheilostome bryozoans. Palaios 24, 440–452. doi: 10.2110/palo.2008.p08-124r
Telesca, L., Peck, L. S., Sanders, T., Thyrring, J., Sejr, M. K., and Harper, E. M. (2019). Biomineralization plasticity and environmental heterogeneity predict geographical resilience patterns of foundation species to future change. Glob. Change Biol. 25, 4179–4193. doi: 10.1111/gcb.14758
Thatje, S. (2012). Effects of capability for dispersal on the evolution of diversity in Antarctic benthos. Integr. Comp. Biol. 52, 470–482. doi: 10.1093/icb/ics105
Thresher, R. E., Tilbrook, B., Fallon, S., Wilson, N. C., and Adkins, J. (2011). Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep-sea corals and other seamount megabenthos. Mar. Ecol. Prog. Ser. 442, 87–99. doi: 10.3354/meps09400
Tittensor, D. P., Baco, A. R., Hall-Spencer, J. M., Orr, J. C., and Rogers, A. D. (2010). Seamounts as refugia from ocean acidification for cold-water stony corals. Mar. Ecol. 31, 212–225. doi: 10.1111/j.1439-0485.2010.00393.x
Turley, C., Blackford, J., Widdicombe, S., Lowe, D., Nightingale, P., and Rees, A. (2006). “Reviewing the impact of increased atmospheric CO2 on oceanic pH and the marine ecosystem.,” in Avoiding Dangerous Climate Change, eds H. J. Schellnhuber, W. Cramer, N, Nakicenovic, T. Wigley, G. Yohe (Cambridge: Cambridge University Press), 65–70. Available online at: http://necan.org/reviewing-impact-increased-atmospheric-co2-oceanic-ph-and-marine-ecosystem (accessed December 5, 2020).
Turley, C. M., Roberts, J. M., and Guinotte, J. M. (2007). Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448. doi: 10.1007/s00338-007-0247-5
Vargas, C. A., Lagos, N. A., Lardies, M. A., Duarte, C., Manríquez, P. H., Aguilera, V. M., et al. (2017). Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 1, 1–7. doi: 10.1038/s41559-017-0084
Vaughan, D., and Marshall, G. (2003). Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change 243–274. doi: 10.1023/A:1026021217991
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Waldbusser, G. G., Hales, B., and Haley, B. A. (2016). Calcium carbonate saturation state: on myths and this or that stories. ICES J. Mar. Sci. 73, 563–568. doi: 10.1093/icesjms/fsv174
Waldbusser, G. G., Hales, B., Langdon, C. J., Haley, B. A., Schrader, P., Brunner, E. L., et al. (2015). Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat. Clim. Change 5, 273–280. doi: 10.1038/nclimate2479
Waller, C. L., Overall, A., Fitzcharles, E. M., and Griffiths, H. (2017). First report of Laternula elliptica in the Antarctic intertidal zone. Polar Biol. 40, 227–230. doi: 10.1007/s00300-016-1941-y
Watson, S.-A., Morley, S. A., and Peck, L. S. (2017). Latitudinal trends in shell production cost from the tropics to the poles. Sci. Adv. 3:e1701362. doi: 10.1126/sciadv.1701362
Watson, S.-A., Peck, L. S., Tyler, P. A., Southgate, P. C., Tan, K. S., Day, R. W., et al. (2012). Marine invertebrate skeleton size varies with latitude, temperature and carbonate saturation: implications for global change and ocean acidification. Glob. Change Biol. 18, 3026–3038. doi: 10.1111/j.1365-2486.2012.02755.x
Weiner, S., and Dove, P. M. (2003). An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29. doi: 10.2113/0540001
Weyl, P. K. (1959). The change in solubility of calcium carbonate with temperature and carbon dioxide content. Geochim. Cosmochim. Acta 17, 214–225. doi: 10.1016/0016-7037(59)90096-1
Wiencke, C., Amsler, C. D., and Clayton, M. N. (2014). Macroalgae Biogeography Atlas South Ocean. Available online at: http://data.biodiversity.aq/ (accessed February 8, 2020).
Williamson, P., Pörtner, H.-O., Widdicombe, S., and Gattuso, J.-P. (2020). Ideas and perspectives: when ocean acidification experiments are not the same, reproducibility is not tested. Biogeosci. Disc. [Preprint]. doi: 10.5194/bg-2020-394
Wright, J. T., and Gribben, P. E. (2017). Disturbance-mediated facilitation by an intertidal ecosystem engineer. Ecology 98, 2425–2436. doi: 10.1002/ecy.1932
Yu, P. C., Sewell, M. A., Matson, P. G., Rivest, E. B., Kapsenberg, L., and Hofmann, G. E. (2013). Growth attenuation with developmental schedule progression in embryos and early larvae of Sterechinus neumayeri raised under elevated CO2. PLoS ONE 8:e52448. doi: 10.1371/journal.pone.0052448
Keywords: carbonate mineralogy, magnesium, aragonite, climate change, vulnerability, Antarctica, saturation horizon
Citation: Figuerola B, Hancock AM, Bax N, Cummings VJ, Downey R, Griffiths HJ, Smith J and Stark JS (2021) A Review and Meta-Analysis of Potential Impacts of Ocean Acidification on Marine Calcifiers From the Southern Ocean. Front. Mar. Sci. 8:584445. doi: 10.3389/fmars.2021.584445
Received: 17 July 2020; Accepted: 08 January 2021;
Published: 29 January 2021.
Edited by:
Andrew John Constable, University of Tasmania, AustraliaReviewed by:
James McClintock, University of Alabama at Birmingham, United StatesSam Dupont, University of Gothenburg, Sweden
Copyright © 2021 Figuerola, Hancock, Bax, Cummings, Downey, Griffiths, Smith and Stark. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Blanca Figuerola, figuerola@icm.csic.es; bfiguerola@gmail.com
 Blanca Figuerola
Blanca Figuerola Alyce M. Hancock
Alyce M. Hancock Narissa Bax2
Narissa Bax2  Vonda J. Cummings
Vonda J. Cummings Rachel Downey
Rachel Downey Huw J. Griffiths
Huw J. Griffiths Jodie Smith
Jodie Smith Jonathan S. Stark
Jonathan S. Stark