- 1Illawarra Health and Medical Research Institute, Wollongong, NSW, Australia
- 2Molecular Horizons and School of Chemistry and Molecular Bioscience, University of Wollongong, Wollongong, NSW, Australia
Introduction
Streptococcus pyogenes (Group A Streptococcus; GAS) is a human specific bacterial pathogen, recognised globally as one of the top ten leading causes of infectious disease mortality (1). It is responsible for causing a wide range of pathologies from superficial skin infections to invasive disease including necrotising fasciitis (1). Post-streptococcal sequelae, such as rheumatic heart disease, are severe complications that develop in response to repeated infections and are largely responsible for the global GAS-related death toll surpassing 500,000 fatalities annually (2). GAS expresses various virulence factors to subvert the host innate immune system, which is central to controlling GAS infection (3). Since current treatment strategies are failing (4), there is a need to further examine the (dys)regulation of host immune networks underlying GAS pathophysiology and identify novel therapeutic targets to arrest disease progression.
Purinergic signalling is an important immunomodulatory network in health and disease. In response to bacterial infections, damaged cells release adenosine 5′-triphosphate (ATP) and other nucleotides that can activate ionotropic P2X receptors and metabotropic P2Y receptors to promote a pro-inflammatory phenotype through cytokine production and immune cell recruitment. Cell surface ectonucleotidases, including CD39 and CD73, help arrest this pro-inflammatory response by hydrolysing exogenous ATP and adenosine 5’-diphosphate (ADP) to adenosine to restrict P2 receptor activation. This in turn stimulates metabotropic P1 (adenosine) receptors to promote an anti-inflammatory phenotype in immune and other cell types (5).
Several bacterial species can modulate the purinergic signalling network to enhance infection (6). Furthermore, nucleotides can modulate immune cell functions such as mTOR signalling and mitochondrial activation in neutrophils to regulate the chemotaxis of these cells (7, 8), which have key roles in regulating GAS and other infections (9). Concurrent with purinergic receptor activation, a hyperinflammatory response is characteristic of invasive GAS infection (10), but current understanding of purinergic signalling mechanisms in GAS pathogenesis and host immunity remains limited. This opinion article provides a brief overview of current research data available on purinergic signalling in GAS infection and the potential of this signalling system as a therapeutic target in GAS infection.
GAS Virulence Factors as a Nexus to Purinergic Signalling
A current priority in GAS research is understanding the molecular mechanisms associated with disease progression in order to establish potential therapeutic targets. Underlying invasive GAS pathogenesis is the ability of this bacterium to subvert and exploit the host innate immune response, which is partially attributed to virulence factor expression (3). A major conserved GAS virulence factor is the antigenically diverse M protein with M1-expressing GAS strains reported to have a high global prevalence (11). As explained below, several GAS virulence factors have been associated with purinergic signalling pathways, indirectly supporting a role for purinergic signalling in GAS infection.
Extracellular Nucleotide Metabolism as a Mode of Immune Evasion by GAS
Secreted virulence factors are commonly associated with invasive inflammation and pathology (10). Streptococcal 5′-nucleotidase A (S5nA) is a secreted virulence factor and bacterial ectonucleotidase first identified in M1-expressing GAS (12). Similar to the mammalian ectonucleotidases that aid in immune regulation (5), S5nA hydrolyses extracellular nucleotides to adenosine, with a rank order of deoxygenated adenosine 5’-monophosphate (AMP) > AMP > ADP, and no effect on ATP (12). Although this study provided evidence of increased production of Sn5A during invasive GAS infection through sera analysis from infected patients, a clear role for this virulence factor over the course of disease is yet to be elucidated but may relate to either adenosine production and/or nucleotide reduction. A detailed description of bacterial ectonucleotidases and roles in infection is beyond the scope of this article, but readers are directed to a recent review on this topic (13).
Extracellular adenosine production is advantageous for bacterial survival as it suppresses the host inflammatory response by modulating leukocyte chemotaxis and inhibiting the production of pro-inflammatory mediators such as cytokines, which in turn may facilitate bacterial immune evasion (14). Mutagenesis of S5nA in three phylogenetically distinct GAS strains however had no effect on host survival in a Galleria mellonella model of infection (15). A major caveat in using insect models to study human disease is the absence of an adaptive immune system, which can be regulated by extracellular adenosine in mammals (5). By stimulating A2A receptors, adenosine can inhibit adaptive immunity and limit inflammation by restricting T cell activation and effector function. It has been recently shown that an ectonucleotidase of Staphylococcus aureus, an invasive bacterial pathogen like GAS, can generate adenosine to stimulate A2A receptors and subsequently restrain T helper 17 cell immunity and promote recurrent infection (16). Thus, it can be hypothesised that a similar strategy may be used by GAS through the secretion of S5nA to evade host adaptive immunity (Figure 1), which could be assessed using more complex systems such as murine models (17) or humanised mouse models (18) of GAS infection.
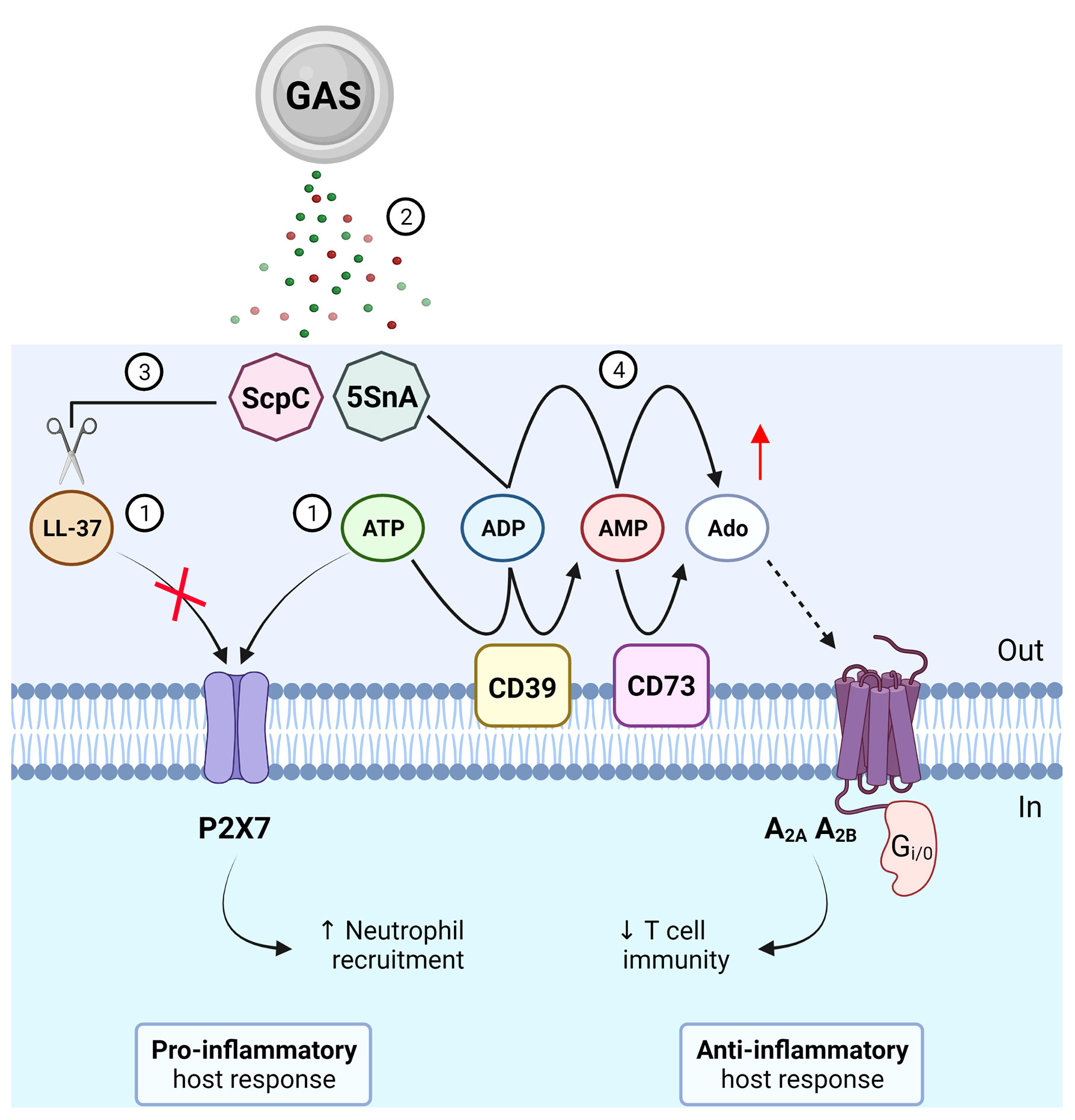
Figure 1 Roles of purinergic signalling in Group A Streptococcus (GAS) immunity and evasion. (1) GAS causes the release of LL-37 and ATP, which can activate P2X7 to promote neutrophil recruitment and protection from GAS infection. (2) GAS secretes virulence factors, ScpC and streptococcal 5’-ectonucleotidase A (5SnA). (3) ScpC can cleave LL-37 to prevent P2X7 activation and promote immune evasion including reduced neutrophil recruitment. (4) 5SnA can hydrolyse ADP and AMP to increase extracellular adenosine (Ado), mimicking the action of host ectonucleotidases (CD39 and CD73), to activate P1 receptors (A2A and A2B) and potentially restrain T cell immunity and promote immune evasion. Created with BioRender.com.
In addition to adenosine generation and subsequent adenosine receptor activation, bacterial ectonucleotidases may limit immunity to GAS by reducing the concentration of extracellular nucleotides and limiting the activation of pro-inflammatory P2 receptors. ADP, a substrate of S5nA, is a natural agonist of P2Y1,12,13 with roles in haemostasis and immune cell regulation. Knockout of P2Y1 in mice decreased their survival in a model of Pseudomonas aeruginosa infection (19) while activation of P2Y12 and P2Y13 enhanced macrophage-mediated immunity in a murine model of Escherichia coli infection (20). As such, ADP hydrolysis by S5nA may increase GAS survival by dysregulating P2 receptor-mediated inflammatory processes including platelet activation, macrophage or neutrophil recruitment, or other processes prominent in GAS pathology, such as wound healing (21) in which P2 receptor activation is involved (22, 23). The broader effects of this may include activation or inhibition of intracellular signaling pathways and components downstream of purinergic receptor activation, such as inflammasomes, mTOR and mitochondria.
Emerging Roles of P2X7 in GAS Infection
Due to its role in cytokine production, T cell activation, and cell survival, P2X7 is the most studied P2 receptor in relation to host immunity against bacterial infections (24). P2X7 is abundantly expressed on immune cells including neutrophils and macrophages (24), which are considered the chief regulators of GAS immunity (25). Activation of P2X7 by extracellular ATP triggers the assembly of the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome that activates caspases to stimulate pro-inflammatory interleukin (IL)-1β maturation and release (26). Since excessive cytokine release and hyperinflammation has been associated with both P2X7 activation (24) and invasive GAS pathologies (10), several studies have investigated a role for P2X7 in GAS infection.
Virulence factors of GAS have been shown to evade P2X7 activation and function. The endogenous immune cell peptide, cathelicidin LL-37, is a potent antimicrobial and chemotactic agent (27) and can activate P2X7 in the absence of extracellular ATP (28). LL-37 may therefore represent a non-nucleotide P2X7 agonist but its mechanism of action remains unclear (29). Of note, secretion of a GAS protease, ScpC, cleaves LL-37 into fragments to prevent P2X7 activation and reduce neutrophil chemotaxis and longevity in vitro (30) (Figure 1). Moreover, in a murine model of deep tissue infection, ScpC-knockout GAS increased neutrophil recruitment and prevented dissemination into deeper tissue, a protective phenotype that was abolished using wild-type GAS (30). P2X7 can also promote the internalisation of LL-37 to enhance the killing and clearance of intracellular S. aureus in human macrophages (31) but whether this process also occurs for GAS remains unknown. Similarly, a role for P2X7 in GAS infection has been demonstrated through another secreted virulence factor, β-nicotinamide adenine dinucleotide+-glycohydrolase (NADase). NADase can inhibit P2X7-mediated IL-1β release from human and murine macrophages challenged with M1-expressing GAS (32). Together, these studies suggest important roles for P2X7 in protective host immunity against GAS infection. In contrast to ScpC and NADase, activation of P2X7 protects human mast cells from streptolysin O (SLO)-induced cytotoxicity by initiating membrane blebbing as a mode of cell repair in response to GAS (33). However, SLO can induce human and murine macrophage NLRP3 inflammasome activation and IL-1β release independently of P2X7 activation (34).
Collectively, the above studies indicate that the role of P2X7 in GAS infection varies between host cell types. Moreover, other GAS virulence factors, such as M protein and SpyA, can also induce NLRP3 inflammasome activation and IL-1β release in macrophages (35, 36), and GAS infection can induce IL-1β release from neutrophils (37, 38) but a role for P2X7 in these settings has not been investigated.
Future Directions and Therapeutic Potential of Purinergic Signalling in the Resolution of GAS Infections
Current treatments to resolve GAS infections are limited to antibiotics, intravenous immunoglobulin therapy or surgical intervention (3). The number of antibiotics approved by Food and Drug Administration has dramatically declined since the 1980’s with β-lactam antibiotics such as penicillin remaining the primary course of treatment for GAS clearance (39). While there is no report of penicillin resistance in GAS to date, antibiotic resistance has become one of the most significant health issues of the 21st century (40). Coupled with GAS treatment failure (41), this has focused research on non-antibiotic therapeutic targets such as purinergic receptors and their ligands.
Understanding the molecular mechanisms underlying invasive GAS pathogenesis and host persistence are a prerequisite for novel vaccine and therapeutic developments that can prevent recurrent infection and disease progression. Although a vaccine has yet to be approved, the GAS virulence factors S5nA and ScpC, which are highly conserved, have been proposed as potential vaccine candidates (42). Immunisation of mice using S5nA and ScpC antigens increased host survival across different GAS serotypes, inclusive of the clinically relevant M1-expressing GAS infection (42) supporting their potential as vaccine targets. Notably as described above, both virulence factors are linked to host purinergic signalling pathways, suggesting that such vaccines may promote antibodies that can neutralise S5nA and ScpC to prevent unwanted modulation of purinergic signalling.
The notion of purinergic receptors as therapeutic targets has been demonstrated in a variety of pre-clinical applications to date (43), however limited progress has been made in the context of GAS infection. Patients receiving Anakinra, an IL-1 receptor antagonist, were documented to have an alarmingly increased susceptibility to developing necrotising fasciitis, a severe soft tissue infection of GAS, with an associated high mortality rate (44). This finding supports a vital role for IL-1β release in controlling GAS infection. Given that the contribution of P2X7 may be minor compared to other mechanisms of IL-1β release in GAS infection (32, 34), coupled with the protective roles of P2X7 in GAS immunity (30, 32, 33), potentiating P2X7 activation with therapeutic agonists may safely reduce GAS hyperinflammation and disease severity without exacerbating pro-inflammatory cytokine production.
Finally, other purinergic receptors, particularly P2X4, may also be important in regulating GAS infection. A recent study observed that P2X4 activation was integral for macrophage-mediated immunity in a cecal ligation and puncture sepsis model (45). Specifically, activation of P2X4 by ATP decreased bacterial load and increased inflammatory cytokines to enhance murine survival. Notably, selective treatment with ivermectin, a positive modulator of P2X4, showed analogous protective effects in mice but without increasing inflammatory cytokine production (45). Given that GAS-induced sepsis is often accompanied by aberrant cytokine release (10), selective modulation of P2X4 may improve host survival and offer novel therapeutic direction.
Given the current knowledge of GAS pathogenesis, and the diverse functions of the purinergic signalling axis, harnessing purinergic ligands or receptors as therapeutic targets may lead to new developments in the clinical space to limit the severity of GAS infections.
Author Contributions
All authors have read and approved the final manuscript. TM (Conceptualisation, visualisation, writing – original draft); MS-S (Conceptualisation, validation, supervision, writing – review and editing); RS (Conceptualisation, validation, supervision, writing – review and editing).
Funding
TM is supported through an Australian Government Research Training Program Scholarship. MS-S and RS are supported by Molecular Horizons and a “UOW Near Miss Grant” from the University of Wollongong.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADP, adenosine 5’-diphosphate; AMP, adenosine 5’-monophosphate; ATP, adenosine 5′-triphosphate; GAS, Group A Streptococcus; IL, interleukin; NADase, β-nicotinamide adenine dinucleotide+ glycohydrolase; NLRP3, NOD-like receptor family pyrin domain-containing 3; S5nA, streptococcal 5′-nucleotidase A; SLO, streptolysin O.
References
1. Ralph AP, Carapetis JR. Group a Streptococcal Diseases and Their Global Burden. Curr Top Microbiol Immunol (2013) 368:1–27. doi: 10.1007/82_2012_280
2. Carapetis JR, Steer AC, Mulholland EK, Weber M. The Global Burden of Group A Streptococcal Diseases. Lancet Infect Dis (2005) 5(11):685–94. doi: 10.1016/s1473-3099(05)70267-x
3. Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin Microbiol Rev (2014) 27(2):264–301. doi: 10.1128/cmr.00101-13
4. Johnson AF, LaRock CN. Antibiotic Treatment, Mechanisms for Failure, and Adjunctive Therapies for Infections by Group A Streptococcus. Front Microbiol (2021) 12:760255. doi: 10.3389/fmicb.2021.760255
5. Linden J, Koch-Nolte F, Dahl G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol (2019) 37:325–47. doi: 10.1146/annurev-immunol-051116-052406
6. Coutinho-Silva R, Savio LEB. Purinergic Signalling in Host Innate Immune Defence Against Intracellular Pathogens. Biochem Pharmacol (2021) 187:114405. doi: 10.1016/j.bcp.2021.114405
7. Bao Y, Ledderose C, Graf AF, Brix B, Birsak T, Lee A, et al. mTOR and Differential Activation of Mitochondria Orchestrate Neutrophil Chemotaxis. J Cell Biol (2015) 210(7):1153–64. doi: 10.1083/jcb.201503066
8. Kondo Y, Ledderose C, Slubowski CJ, Fakhari M, Sumi Y, Sueyoshi K, et al. Frontline Science: Escherichia Coli Use LPS as Decoy to Impair Neutrophil Chemotaxis and Defeat Antimicrobial Host Defense. J Leukoc Biol (2019) 106(6):1211–9. doi: 10.1002/jlb.4hi0319-109r
9. Tsatsaronis JA, Ly D, Pupovac A, Goldmann O, Rohde M, Taylor JM, et al. Group A Streptococcus Modulates Host Inflammation by Manipulating Polymorphonuclear Leukocyte Cell Death Responses. J Innate Immun (2015) 7(6):612–22. doi: 10.1159/000430498
10. Wilde S, Johnson AF, LaRock CN. Playing With Fire: Proinflammatory Virulence Mechanisms of Group A Streptococcus. Front Cell Infect Microbiol (2021) 11:704099. doi: 10.3389/fcimb.2021.704099
11. Gherardi G, Vitali LA, Creti R. Prevalent Emm Types Among Invasive GAS in Europe and North America Since Year 2000. Front Public Health (2018) 6:59. doi: 10.3389/fpubh.2018.00059
12. Zheng L, Khemlani A, Lorenz N, Loh JM, Langley RJ, Proft T. Streptococcal 5’-Nucleotidase A (S5nA), a Novel Streptococcus pyogenes Virulence Factor That Facilitates Immune Evasion. J Biol Chem (2015) 290(52):31126–37. doi: 10.1074/jbc.M115.677443
13. Zakataeva NP. Microbial 5’-Nucleotidases: Their Characteristics, Roles in Cellular Metabolism, and Possible Practical Applications. Appl Microbiol Biotechnol (2021) 105(20):7661–81. doi: 10.1007/s00253-021-11547-w
14. Lee JS, Yilmaz Ö. Unfolding Role of a Danger Molecule Adenosine Signaling in Modulation of Microbial Infection and Host Cell Response. Int J Mol Sci (2018) 19(1):199. doi: 10.3390/ijms19010199
15. Dangel ML, Dettmann JC, Haßelbarth S, Krogull M, Schakat M, Kreikemeyer B, et al. The 5’-Nucleotidase S5nA is Dispensable for Evasion of Phagocytosis and Biofilm Formation in Streptococcus pyogenes. PloS One (2019) 14(1):e0211074. doi: 10.1371/journal.pone.0211074
16. Deng J, Zhang BZ, Chu H, Wang XL, Wang Y, Gong HR, et al. Adenosine Synthase A Contributes to Recurrent Staphylococcus Aureus Infection by Dampening Protective Immunity. EBioMedicine (2021) 70:103505. doi: 10.1016/j.ebiom.2021.103505
17. Medina E, Lengeling A. Genetic Regulation of Host Responses to Group A Streptococcus in Mice. Brief Funct Genomic Proteomic (2005) 4(3):248–57. doi: 10.1093/bfgp/4.3.248
18. Rivera-Hernandez T, Walker MJ. Humanized Plasminogen Mouse Model to Study Group A Streptococcus Invasive Disease. Methods Mol Biol (2020) 2136:309–16. doi: 10.1007/978-1-0716-0467-0_24
19. Geary C, Akinbi H, Korfhagen T, Fabre JE, Boucher R, Rice W. Increased Susceptibility of Purinergic Receptor-Deficient Mice to Lung Infection With Pseudomonas Aeruginosa. Am J Physiol Lung Cell Mol Physiol (2005) 289(5):L890–895. doi: 10.1152/ajplung.00428.2004
20. Zhang X, Qin J, Zou J, Lv Z, Tan B, Shi J, et al. Extracellular ADP Facilitates Monocyte Recruitment in Bacterial Infection via ERK Signaling. Cell Mol Immunol (2018) 15(1):58–73. doi: 10.1038/cmi.2016.56
21. Vu HM, Hammers DE, Liang Z, Nguyen GL, Benz ME, Moran TE, et al. Group A Streptococcus-Induced Activation of Human Plasminogen Is Required for Keratinocyte Wound Retraction and Rapid Clot Dissolution. Front Cardiovasc Med (2021) 8:667554. doi: 10.3389/fcvm.2021.667554
22. Pfalzgraff A, Bárcena-Varela S, Heinbockel L, Gutsmann T, Brandenburg K, Martinez-de-Tejada G, et al. Antimicrobial Endotoxin-Neutralizing Peptides Promote Keratinocyte Migration via P2X7 Receptor Activation and Accelerate Wound Healing In Vivo. Br J Pharmacol (2018) 175(17):3581–93. doi: 10.1111/bph.14425
23. McEwan TB, Sophocleous RA, Cuthbertson P, Mansfield KJ, Sanderson-Smith ML, Sluyter R. Autocrine Regulation of Wound Healing by ATP Release and P2Y(2) Receptor Activation. Life Sci (2021) 283:119850. doi: 10.1016/j.lfs.2021.119850
24. Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol (2018) 9:52. doi: 10.3389/fphar.2018.00052
25. Fieber C, Kovarik P. Responses of Innate Immune Cells to Group A Streptococcus. Front Cell Infect Microbiol (2014) 4:140. doi: 10.3389/fcimb.2014.00140
26. Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ Efflux is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity (2013) 38(6):1142–53. doi: 10.1016/j.immuni.2013.05.016
27. Yang B, Good D, Mosaiab T, Liu W, Ni G, Kaur J, et al. Significance of LL-37 on Immunomodulation and Disease Outcome. BioMed Res Int (2020) 2020:8349712. doi: 10.1155/2020/8349712
28. Elssner A, Duncan M, Gavrilin M, Wewers MD. A Novel P2X7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1 Beta Processing and Release. J Immunol (2004) 172(8):4987–94. doi: 10.4049/jimmunol.172.8.4987
29. Di Virgilio F, Giuliani AL, Vultaggio-Poma V, Falzoni S, Sarti AC. Non-Nucleotide Agonists Triggering P2X7 Receptor Activation and Pore Formation. Front Pharmacol (2018) 9:39. doi: 10.3389/fphar.2018.00039
30. Biswas D, Ambalavanan P, Ravins M, Anand A, Sharma A, Lim KXZ, et al. LL-37-Mediated Activation of Host Receptors is Critical for Defense Against Group A Streptococcal Infection. Cell Rep (2021) 34(9):108766. doi: 10.1016/j.celrep.2021.108766
31. Tang X, Basavarajappa D, Haeggström JZ, Wan M. P2X7 Receptor Regulates Internalization of Antimicrobial Peptide LL-37 by Human Macrophages That Promotes Intracellular Pathogen Clearance. J Immunol (2015) 195(3):1191–201. doi: 10.4049/jimmunol.1402845
32. Westerlund E, Valfridsson C, Yi DX, Persson JJ. The Secreted Virulence Factor NADase of Group A Streptococcus Inhibits P2X7 Receptor-Mediated Release of IL-1β. Front Immunol (2019) 10:1385. doi: 10.3389/fimmu.2019.01385
33. Schoenauer R, Atanassoff AP, Wolfmeier H, Pelegrin P, Babiychuk EB, Draeger A. P2X7 Receptors Mediate Resistance to Toxin-Induced Cell Lysis. Biochim Biophys Acta (2014) 1843(5):915–22. doi: 10.1016/j.bbamcr.2014.01.024
34. Harder J, Franchi L, Muñoz-Planillo R, Park JH, Reimer T, Núñez G. Activation of the Nlrp3 Inflammasome by Streptococcus Pyogenes Requires Streptolysin O and NF-Kappa B Activation But Proceeds Independently of TLR Signaling and P2X7 Receptor. J Immunol (2009) 183(9):5823–9. doi: 10.4049/jimmunol.0900444
35. Lin AE, Beasley FC, Keller N, Hollands A, Urbano R, Troemel ER, et al. A Group A Streptococcus ADP-Ribosyltransferase Toxin Stimulates a Protective Interleukin 1β-Dependent Macrophage Immune Response. mBio (2015) 6(2):e00133. doi: 10.1128/mBio.00133-15
36. Valderrama JA, Riestra AM, Gao NJ, LaRock CN, Gupta N, Ali SR, et al. Group A Streptococcal M Protein Activates the NLRP3 Inflammasome. Nat Microbiol (2017) 2(10):1425–34. doi: 10.1038/s41564-017-0005-6
37. LaRock DL, Russell R, Johnson AF, Wilde S, LaRock CN. Group A Streptococcus Infection of the Nasopharynx Requires Proinflammatory Signaling Through the Interleukin-1 Receptor. Infect Immun (2020) 88(10):e00356–20. doi: 10.1128/iai.00356-20
38. Williams JG, Ly D, Geraghty NJ, McArthur JD, Vyas HKN, Gorman J, et al. Streptococcus Pyogenes M1T1 Variants Induce an Inflammatory Neutrophil Phenotype Including Activation of Inflammatory Caspases. Front Cell Infect Microbiol (2020) 10:596023. doi: 10.3389/fcimb.2020.596023
39. Ventola CL. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T (2015) 40(4):277–83.
40. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog Glob Health (2015) 109(7):309–18. doi: 10.1179/2047773215y.0000000030
41. Bonofiglio L, Gagetti P, García Gabarrot G, Kaufman S, Mollerach M, Toresani I, et al. Susceptibility to β-Lactams in β-Hemolytic Streptococci. Rev Argent Microbiol (2018) 50(4):431–5. doi: 10.1016/j.ram.2017.11.002
42. Fritzer A, Senn BM, Minh DB, Hanner M, Gelbmann D, Noiges B, et al. Novel Conserved Group A Streptococcal Proteins Identified by the Antigenome Technology as Vaccine Candidates for a Non-M Protein-Based Vaccine. Infect Immun (2010) 78(9):4051–67. doi: 10.1128/iai.00295-10
43. Burnstock G. Purinergic Signalling: Therapeutic Developments. Front Pharmacol (2017) 8:661. doi: 10.3389/fphar.2017.00661
44. LaRock CN, Todd J, LaRock DL, Olson J, O’Donoghue AJ, Robertson AA, et al. IL-1β is an Innate Immune Sensor of Microbial Proteolysis. Sci Immunol (2016) 1(2):eaah3539. doi: 10.1126/sciimmunol.aah3539
Keywords: Streptococcus pyogenes, purinergic receptor, A2A receptor, ectonucleotidase, P2X7 receptor, LL-37, innate immunity, virulence factor
Citation: McEwan TB-D, Sanderson-Smith ML and Sluyter R (2022) Purinergic Signalling in Group A Streptococcus Pathogenesis. Front. Immunol. 13:872053. doi: 10.3389/fimmu.2022.872053
Received: 09 February 2022; Accepted: 09 March 2022;
Published: 29 March 2022.
Edited by:
Robson Coutinho-Silva, Federal University of Rio de Janeiro, BrazilReviewed by:
Francisco G. Vázquez-Cuevas, Instituto de Neurobiología, Universidad Nacional Autónoma de México, MexicoWolfgang Junger, Beth Israel Deaconess Medical Center and Harvard Medical School, United States
Copyright © 2022 McEwan, Sanderson-Smith and Sluyter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Sluyter, rsluyter@uow.edu.au
 T. B-D McEwan
T. B-D McEwan M. L. Sanderson-Smith
M. L. Sanderson-Smith R. Sluyter
R. Sluyter