- 1Pregnancy Health and Beyond Laboratory, Flinders Health and Medical Research Institute, Flinders University, Adelaide, SA, Australia
- 2South Australian Genomics Centre, South Australian Health & Medical Research Institute, Adelaide, SA, Australia
- 3Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
Parturition signals the end of immune tolerance in pregnancy. Term labour is usually a sterile inflammatory process triggered by damage associated molecular patterns (DAMPs) as a consequence of functional progesterone withdrawal. Activation of DAMPs recruits leukocytes and inflammatory cytokine responses in the myometrium, decidua, cervix and fetal membranes. Emerging evidence shows components of the inflammasome are detectable in both maternal decidua and placenta. However, the activation of the placental inflammasome with respect to mode of delivery has not been profiled. Placental chorionic villus samples from women delivering at term via unassisted vaginal (UV) birth, labouring lower segment caesarean section (LLSCS, emergency caesarean section) and prelabour lower segment caesarean section (PLSCS, elective caesarean section) underwent high throughput RNA sequencing (NextSeq Illumina) and bioinformatic analyses to identify differentially expressed inflammatory (DE) genes. DE genes (IL1RL1, STAT1, STAT2, IL2RB, IL17RE, IL18BP, TNFAIP2, TNFSF10 and TNFRSF8), as well as common inflammasome genes (IL1B, IL1R1, IL1R2, IL6, IL18, IL18R1, IL18R1, IL10, and IL33), were targets for further qPCR analyses and Western blotting to quantify protein expression. There was no specific sensor molecule-activated inflammasome which dominated expression when stratified by mode of delivery, implying that multiple inflammasomes may function synergistically during parturition. Whilst placentae from women who had UV births overall expressed pro-inflammatory mediators, placentae from LLSCS births demonstrated a much greater pro-inflammatory response, with additional interplay of pro- and anti-inflammatory mediators. As expected, inflammasome activation was very low in placentae from women who had PLSCS births. Sex-specific differences were also detected. Placentae from male-bearing pregnancies displayed higher inflammasome activation in LLSCS compared with PLSCS, and placentae from female-bearing pregnancies displayed higher inflammasome activation in LLSCS compared with UV. In conclusion, placental inflammasome activation differs with respect to mode of delivery and neonatal sex. Its assessment may identify babies who have been exposed to aberrant inflammation at birth that may compromise their development and long-term health and wellbeing.
Introduction
Pregnancy is a time of immunological challenge and change. Implantation and placentation are defined as pro-inflammatory phases, followed by an anti-inflammatory stage associated with fetal development and growth. Finally, parturition signals the end of immunological tolerance in pregnancy, indicating another pro-inflammatory stage which mediates labour (1). Although inflammation at the fetomaternal interface is often associated with pregnancy complications (2), inflammation and the expression of pro-inflammatory cytokines in the uterus are critical for cervical ripening and labour initiation for healthy delivery (3–6).
Labour at term follows functional progesterone withdrawal, inducing a normally sterile inflammatory process characterised by signals of cellular stress (damage-associated molecular patterns [DAMPs]). DAMPs, often expressed in response to rupture of membranes, are recognised by pattern recognition receptors (PRRs), leading to innate immune system activation of the inflammasome (7–9). The inflammasome is a cytosolic multi-subunit protein complex that consists of a sensor molecule, typically a PRR, an adaptor protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and pro-CASP-1 (7–9). While there are multiple inflammasome pathways, five have been well-characterised; NLR and pyrin domain-containing protein (NLRP)-1 (10); NLRP3 (11); NLR family caspase activation and recruitment domain (CARD) domain-containing protein-4 (NLRC4) (12, 13); absent in melanoma-2 (AIM2) (14); and pyrin (15).
Activation of the nuclear factor kappa B (NF-κB) pathway leads to assembly of the inflammasome complex, as well as independently leading to increased pro-interleukin (IL)-1β and pro-IL-18 expression (9, 16, 17). Assembly of the inflammasome complex leads to a proteolytic inflammatory cascade mediated by cleavage of procaspase-1 (pro-CASP-1) into its active form, CASP-1, which cleaves pro-IL-1β and pro-IL-18 into the active cytokines, IL-1β and IL-18 (7, 8, 16, 18), contributing to the labour cascade. For this reason, IL-1β and IL-18 can be measured as markers of inflammasome activation.
Key molecules in the inflammasome, plus generalised inflammatory pathways, have been localised to the chorioamniotic membranes, in which spontaneous term labour is associated with greater inflammasome assembly than those without spontaneous labour (7). Significantly increased concentrations of CASP-1 and IL-1β were also identified in amniotic fluid of women who underwent spontaneous labour at term compared to women who did not undergo labour at term (19). Additionally, it has been suggested that inflammasome overactivation can also contribute to the progression of pregnancy complications such as preeclampsia (8, 20–24), premature rupture of membranes (PROM) (25), fetal growth restriction and preterm labour (9).
Whilst characterising the expression of inflammasome-related molecules has mostly been confined to the chorioamniotic membranes and the amniotic fluid, not the placenta, there is evidence to suggest that the placenta can serve as a proxy for uterine inflammation (5, 26) (see Discussion).
Even for term births, delivery can be spontaneous and unassisted, operative vaginal delivery, in labour emergency caesarean section or planned prelabour caesarean section. The latter can be for medical indications but there are increasing numbers planned for non-medical reasons. It is not known whether these different modes of delivery affect the placental inflammasome. In this study, we profiled the expression of these pro-inflammatory cytokines, as well as other inflammatory molecules, in the placentae of women who delivered via unassisted vaginal birth (UV), emergency caesarean section [labouring lower segment caesarean section (LLSCS)] and elective caesarean section [prelabour lower segment caesarean section (PLSCS)]. Elucidating the inflammasome activation in the placentae from women with different modes of delivery would allow inferences to be made regarding potential overactivation of the inflammasome and an exaggerated inflammatory response in certain modes of delivery and, in turn, the potential effects on mothers and their neonates.
Materials and Methods
Tissue Samples
Term placentae were obtained at the Lyell McEwin Hospital in Elizabeth, South Australia, from women recruited as part of the SCreening fOr Pregnancy Endpoints (SCOPE) (2005–2008) and Screening Tests to predict poor Outcomes of Pregnancy (STOP) (2015–2018) cohort studies (27). Both SCOPE and STOP studies were registered with the Australian and New Zealand Clinical Trials Registry (ACTRN 12607000551493 and ACTRN 12614000985684, respectively).
All placentae were associated with uncomplicated pregnancies and defined by the following methods of delivery: UV, LLSCS, and PLSCS. Samples from LLSCS deliveries underwent emergency Caesarean section due to failure to progress in labour, and/or detection of fetal distress (lowered heartrate or presence of meconium). Tissue biopsies from placentae were washed in phosphate-buffered saline (PBS) before being submerged in RNAlater Stabilisation Solution (Thermo Fisher Scientific, Massachusetts, USA) and stored at -80°C. Ethics approvals were obtained from the Queen Elizabeth Hospital Human Research Ethics Committee (TQEH/LMH HREC/1712/5/2008; SCOPE) and Women’s and Children’s Health Network Human Research Ethics Committee (HREC/14/WCHN/90; STOP). All women provided written informed consent.
RNA Extraction
Term placental tissues (0.25g) were weighed and washed with phosphate-buffered saline (PBS). Tissue was disrupted by homogenizing for 3.5 mins at 30 Hz (TissueLyser, QIAGEN) in 600 μL Buffer RLT Plus (RNeasy Plus Mini Kit; QIAGEN, Victoria, Australia). Total RNA was extracted from the supernatant using the RNeasy Plus Mini Kit (QIAGEN) according to the manufacturer’s protocol. The purity and integrity of extracted RNA samples were determined using the Experion™ (BioRad, New South Wales, Australia) and samples used had a RIN ≥ 8.
Sequencing and Bioinformatic Analyses
High Throughput Sequencing was performed at Flinders University using a NextSeq Illumina sequencer. Alignment was performed using BWA version 0.7.17-r1188 (GRCh37) and output generated in FASTQ format (28). Quality control metrics were assessed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to check for per base sequence quality, sequence length distribution and duplication levels. Differential expression analyses were conducted in the R statistical environment (v.4.0.5), using the edgeR (v.3.16.5) (29) and limma (v.3.30.11) (30) packages. Briefly, edgeR was used to filter mRNA with low expression and normalise for library composition bias. All samples were then normalised using the Trimmed Mean of M values (TMM). Sample-weights and log transformation were performed using the limma package with the voom function used to estimate the mean-variance relationship between individual observations and then applied to the normalised log-counts data. Differential expression analyses including moderated F-statistic evaluation, adjusted p-value estimation and log2 fold change analysis were performed using a moderated t-test (31) with Benjamini-Hochberg (BH) multiple hypothesis test corrections (32). After adjustment, gene expression was considered significantly different at FDR ≤ 0.05.
cDNA Synthesis and Quantitative Polymerase Chain Reaction (qPCR)
Synthesis of complementary DNA (cDNA) was conducted beginning with 1μg of total RNA using the QuantiNova Reverse Transcription Kit (QIAGEN) according to the manufacturer’s protocol. qPCR was conducted with SYBR Green (QIAGEN) according to manufacturer’s instructions, with YWHAZ and β-actin as housekeeping genes (primer sequences in Table 1). Denaturation was performed at 95°C for 10 secs, annealing at 53°C for 45 secs, and extension at 72°C for 30 secs, for a total of 50 cycles. qPCR results were analysed using the 2-ΔΔCT method (33). Samples were sorted into groups depending on mode of delivery (see Table 2 for numbers and characteristics of women’s and infants’ samples used).
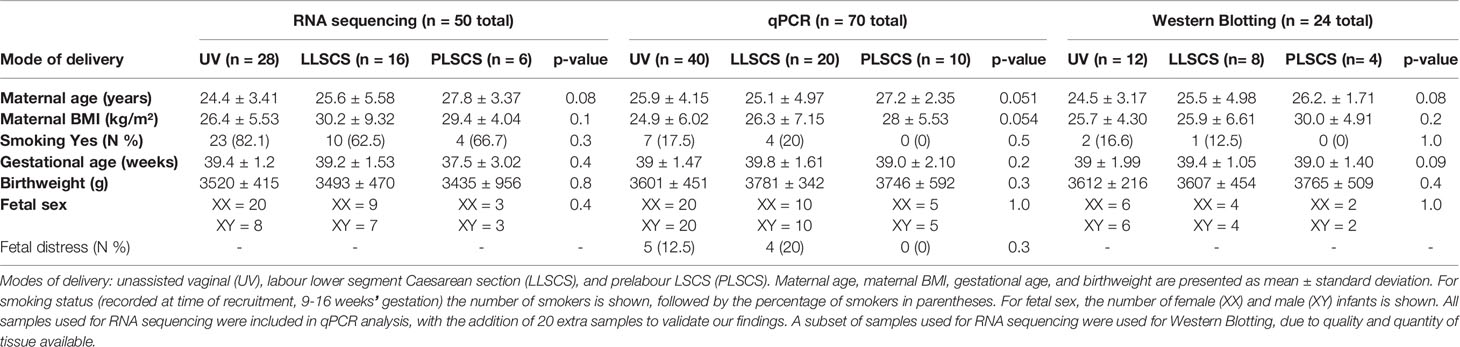
Table 2 Characteristics of women and infants from the study by mode of delivery (UV, LLSCS, PLSCS) and analytic technique (RNA sequencing, qPCR analysis and Western Blotting).
Western Blotting
Protein Extraction
Term placental tissues (0.25g) were weighed into Powerlyzer tubes and disrupted in 400 μL ice cold RIPA (Radio Immuno Precipitation Assay) buffer. Samples (see Table 2 for numbers and characteristics of women’s and infants’ samples used) were homogenised (3.5 mins at 30 Hz) using the TissueLyser (QIAGEN). After homogenisation, samples were diluted with 400 μL RIPA buffer. Protein concentration was measured via a Bradford Assay using the SpectraMax iD5 (Molecular Devices) at 595 nm absorbance, prior to sample storage at -80°C.
SDS-PAGE
Samples (25 μg each) were denatured and reduced in Laemmli 4x buffer (GTX16355, GeneTex) and 8% 2-mercaptoethanol for 5 mins at 95°C. Samples were then loaded onto either a 4–20% Mini-PROTEAN® TGX Stain-Free™ Protein Gel (4568094, Bio-Rad) or 4–20% Mini-PROTEAN® TGX™ Precast Protein Gel (4561094, Bio-Rad), along with a molecular weight marker (GTX49384, GeneTex). SDS-PAGE was carried out for 1 h at 100 V in 1x Running buffer (pH 8.3) using a Mini-PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad). Stain-Free Protein gels were checked for complete protein separation using the ChemiDoc Touch Imaging System (Bio-Rad).
Transfer
Protein was transferred to a PVDF membrane over 16 hours at 27V and 4°C in transfer buffer (25 mM Trizma Base, 190 mM glycine, 20% methanol; pH 8.3) using the Criterion™ blotter (Bio-Rad). Successful transfer of protein was checked by Ponceau S staining of the membrane, as well as either Coomassie Blue staining (for the TGX Precast Protein Gel) or the ChemiDoc Touch Imaging System (Bio-Rad) (for the TGX Stain-Free™ Protein Gel).
Blotting
Membrane was blocked in Tris Buffered Saline Tween20 (TBST) with 5% skim milk for 2 h at room temperature. Membrane was then incubated in blocking buffer for 1 h with either IL-1β Polyclonal Antibody (P420B, Invitrogen) at 1:200 dilution, or IL-18 Polyclonal Antibody (PA5-110679, Invitrogen) at 1:300. Membrane was washed 3-4 times with TBST, then incubated with a goat anti-rabbit Immunoglobulins/HRP secondary antibody (P044801-2, Dako) at 1:5000 in the blocking buffer. Membrane was washed 3-4 times in TBST, then incubated in Clarity Western ECL Substrate (1705060, Bio-Rad) for 5 mins and imaged. The density of each band (determined by the ChemiDoc Touch Imaging System) was determined prior to analysis using ImageLab software from Bio-Rad™. Analysis controlled protein loading for each sample by normalizing using stain-free total protein quantification (34) and was further normalized to an internal control sample (pooled term placentae) on each membrane. Samples were run in duplicate and averaged for final analysis.
Statistical Analysis
Statistical analysis for differences between groups from qPCR and Western Blotting data was undertaken using SPSS Statistics Software. Outliers were removed from the data using a Grubbs’ test. Data were assessed for normality distribution and a two-way ANOVA test was conducted. Differences between groups were considered significant for p ≤ 0.05. All tests of statistical significance are two-sided, and adjustments were made for multiple comparisons.
Results
Cohort Characteristics With Respect to Mode of Delivery (UV, LLSCS or PLSCS)
RNA sequencing data was available for 50 placentae from uncomplicated pregnancies where mode of delivery was known. Mode of delivery and birth characteristics are presented in Table 2.
Genes Are Differentially Expressed in a Sex-Specific Manner in Term Placenta
In a larger study to investigate gene expression in human placentae, we performed double-stranded RNA-Seq on 96 (35 early and 61 term gestation) samples with an average of ~35.8 million paired-end reads per sample. For the purposes of the current study, the 35 early gestation samples were excluded, and 1 term sample was removed due to ambiguous labelling, leaving a total of 50 [26 samples from the SCOPE cohort (11 male, 15 female) and 24 samples from the STOP cohort (9 male, 15 female)] samples for downstream analyses. FASTQ files were aligned to human genome GRCh37 (hs37d5) using STAR (35) and RNA-Seq data was summarised to the gene level using featureCounts (36) from which we detected 20654 genes with an official gene symbol. After removing genes from the X- and Y-Chromosomes, and mitochondrial genes (706 genes removed), and filtering for genes with low expression (<2 counts per million in 6 samples), 12903 genes remained for downstream analyses.
Sex-specific differential expression analysis between male LLSCS and PLSCS showed 33 up- and 5 down-regulated genes (lower expression in LLSCS: FDR < 0.05) including up-regulation of IL18R1, IL1RL1, IL20RA (Figure 1). There were no significantly differentially expressed genes in the female comparison. It is important to note that the RNA-Seq differential expression experiment was performed to guide targets of our PCR experiments. The small number of samples used in this comparison, male LLSCS (n = 7), male PLSCS (n = 3) limit the stand-alone interpretation of these results.
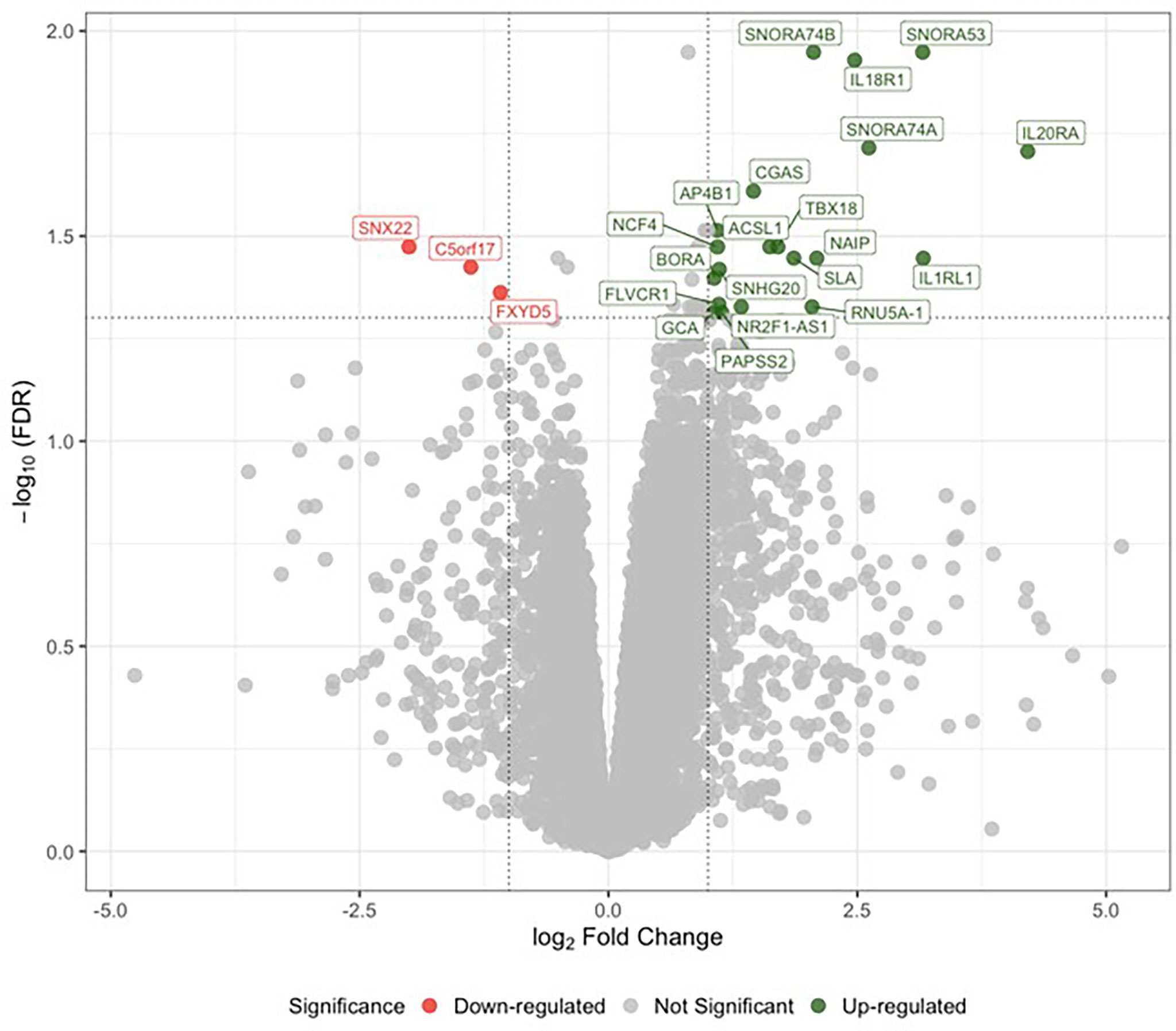
Figure 1 Analysis of differential gene expression determined by RNA sequencing between labouring lower segment Caesarean section (LLSCS) (n = 7) and prelabour lower segment Caesarean section (PLSCS) (n = 3) in male-bearing pregnancies only. Volcano plot shows the level of change (log transformed normalised counts); differentially expressed genes (false discovery rate (FDR) < 0.05 & log fold change (logFC) > |1|) indicated in red and green. Horizontal dotted line corresponds to FDR of 0.05 (-log10 scaled). Vertical dotted lines correspond to logFC > |1|.
Markers of Inflammasome Activation, IL-1β and IL-18, Are Significantly Increased at mRNA Level in LLSCS Births
Activation of any inflammasome complex results in the production of mature IL-1β and IL-18 pro-inflammatory cytokines. Expression of IL1B and IL18 mRNA (Figures 2A, D, respectively) was significantly upregulated in placentae from LLSCS births compared with UV for both female- (pIL1B < 0.0001; pIL18 < 0.0001) and male-bearing (pIL1B < 0.0001; pIL18 < 0.0001) pregnancies, as well as in placentae from LLSCS births compared with PLSCS for both female- (pIL1B < 0.0001; pIL18 < 0.0001) and male-bearing (pIL1B < 0.0001; pIL18 < 0.0001) pregnancies. Expression of IL1B and IL18 mRNA was also upregulated in UV births compared with PLSCS for both female- (pIL1B < 0.0001; pIL18 < 0.0001) and male-bearing (pIL1B < 0.0001; pIL18 < 0.0001) pregnancies.
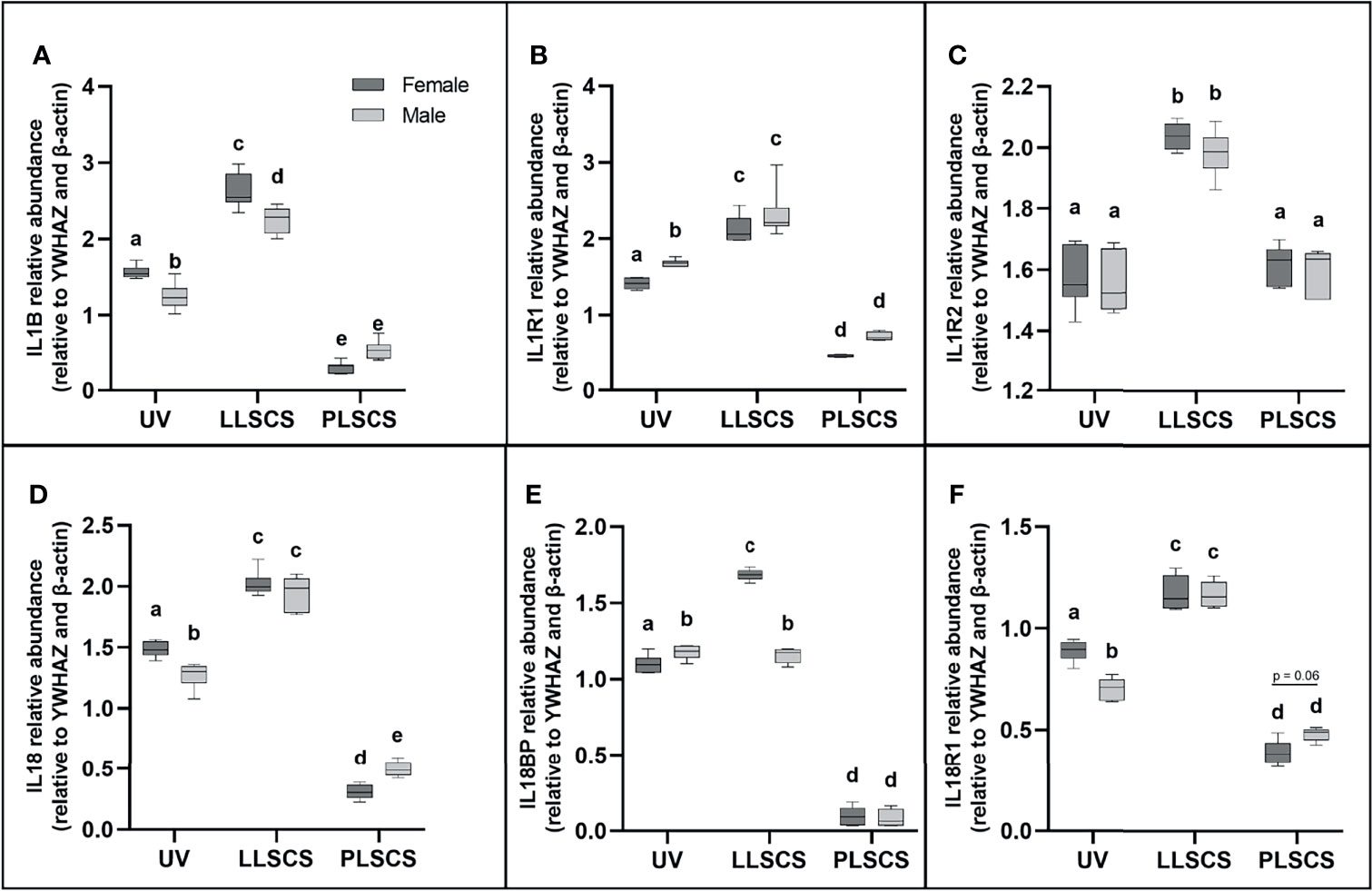
Figure 2 The mRNA expression of (A) Interleukin 1β (IL1B), (B) Interleukin 1 Type 1 Receptor (IL1R1), (C) Interleukin 1 Type 2 Receptor (IL1R2), (D) Interleukin 18 (IL18), (E) Interleukin 18 Binding Protein (IL18BP) and (F) Interleukin 18 Type 1 Receptor (IL18R1) in placentae from female- and male-bearing pregnancies classed by delivery mode (as determined by qPCR). Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (Number of samples: unassisted vaginal (UV) 20/fetal sex; labouring lower segment Caesarean section (LLSCS) = 10/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 5/fetal sex).
Expression of both IL1B and IL18 mRNA was significantly decreased in placentae delivered via UV method in male compared to female-bearing pregnancies (pIL1B = 0.0002; pIL18 = 0.0018). IL1B mRNA expression was significantly decreased in placentae delivered via LLSCS method in male- compared to female-bearing pregnancies (p < 0.0001) and IL18 mRNA expression was significantly decreased in placentae delivered via PLSCS method in female- compared to male-bearing pregnancies (p = 0.0132).
Receptors for IL-1β and IL-18 Are Significantly Increased in LLSCS Births
IL-1β can exert its inflammatory effects by binding to the IL1R1. IL1R1 mRNA expression (Figure 2B) was significantly increased in placentae from LLSCS births compared with UV for both female- and male-bearing pregnancies, as well as from LLSCS births compared with PLSCS for both female- and male-bearing pregnancies and from UV births compared with PLSCS for both female- and male-bearing pregnancies (all p < 0.0001).
IL1R1 mRNA expression was significantly increased in placentae delivered via UV birth in male- compared to female-bearing pregnancies (p = 0.0348).
IL-1β binding to the IL1R2 can have anti-inflammatory effects. IL1R2 mRNA expression (Figure 2C) was significantly increased in placentae from LLSCS births compared with UV for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
IL18BP is a potent inhibitor of IL-18 activity. IL18BP mRNA expression (Figure 2E) was significantly increased in placentae from LLSCS births compared with UV for female-bearing (p < 0.0001) pregnancies only, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies. IL18BP mRNA expression was significantly increased in placentae from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
IL18BP mRNA expression was significantly increased in placentae delivered via LLSCS method in female- compared to male-bearing pregnancies (p < 0.0001) and significantly decreased in placentae delivered via UV method in female- compared to male-bearing pregnancies (p = 0.0408).
Finally, IL18R1 is the receptor by which IL-18 binding exerts inflammatory effects. IL18R1 mRNA expression (Figure 2F) was significantly increased in placentae from LLSCS births compared with UV for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies. IL18R1 mRNA expression was significantly increased in placentae from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
IL18R1 mRNA expression was significantly increased in placentae delivered via UV in female- compared to male-bearing pregnancies (p < 0.0001).
Expression of Pro-Inflammatory IL6, IL2RB, IL17RE, TNFAIP2, TNFSF10, and TNFRSF8 Is Increased in LLSCS Births
IL-6 is commonly used as a marker of inflammation. IL6 mRNA expression (Figure 3A) was significantly increased in placentae from LLSCS births compared with UV for female- (p < 0.0001) and male-bearing (p = 0.0035) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies, and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
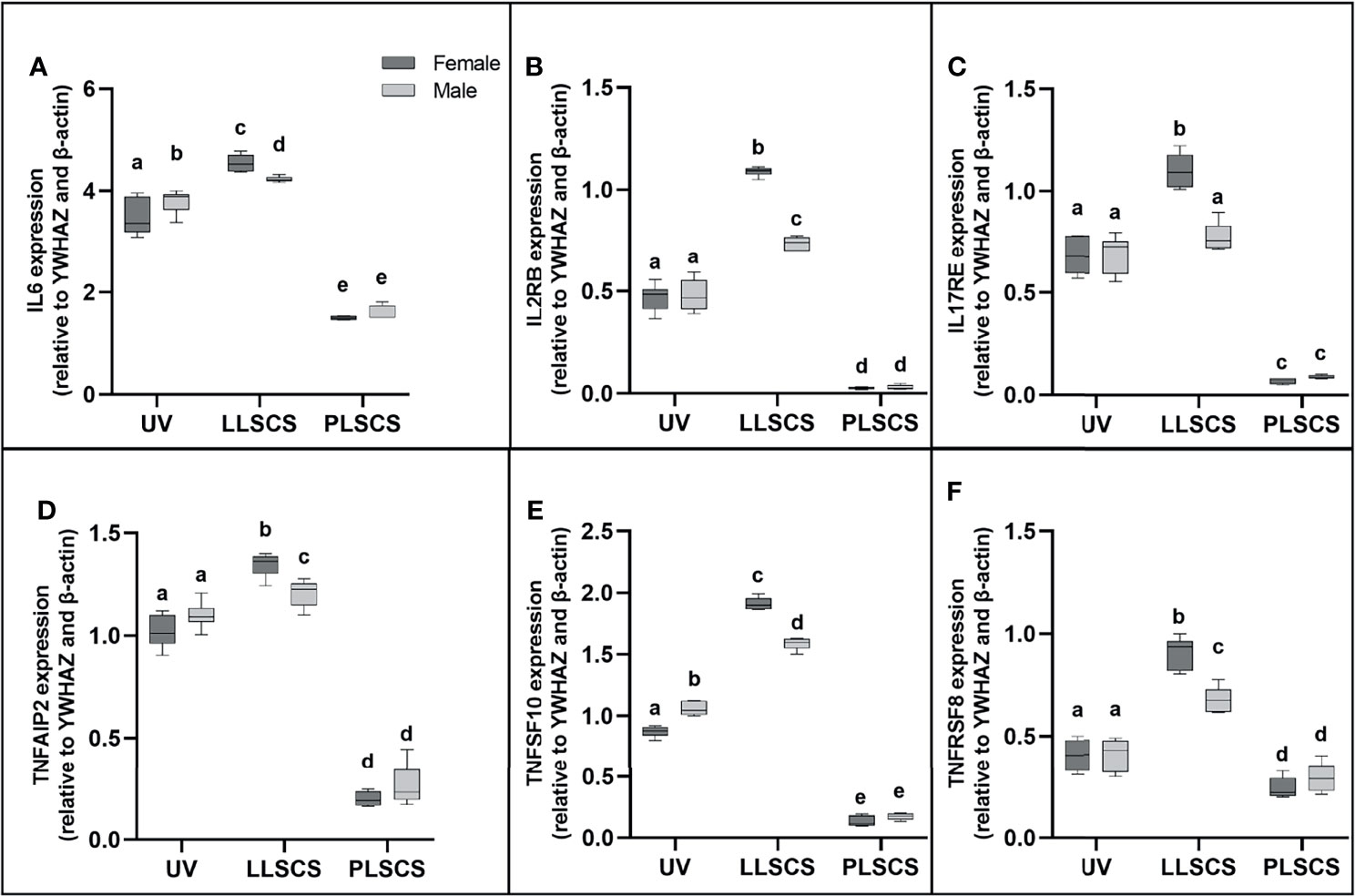
Figure 3 (A) Interleukin 6 (IL6), (B) Interleukin 2 Receptor β (IL2RB), (C) Interleukin 17 Receptor E (IL17RE), (D) Tumour Necrosis Factor Alpha Induced Protein 2 (TNFAIP2), (E) Tumour Necrosis Factor Super Family Member 10 (TNFSF10) and (F) Tumour Necrosis Factor Receptor Super Family Member 8 (TNFRSF8) mRNA expression in placentae from female- and male-bearing pregnancies classed by delivery mode (qPCR). Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (Number of samples: unassisted vaginal (UV) 20/fetal sex; labouring lower segment Caesarean section (LLSCS) = 10/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 5/fetal sex).
IL6 mRNA expression was significantly increased in placentae delivered via LLSCS in female- compared to male-bearing pregnancies (p = 0.0392) and significantly decreased in placentae delivered vaginally in female- compared to male-bearing pregnancies (p = 0.0359).
IL2RB (IL-2Rβ) refers to the β subunit of the interleukin-2 receptor. It is involved in the inflammatory response by activating T and NK cell subsets (37). Specifically, the human IL2RB gene has been shown to use an LTR alternative promoter to drive expression, specifically in the placenta (38). IL2RB mRNA expression (Figure 3B) was significantly increased in placentae from LLSCS births compared with UV for female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
IL2RB mRNA expression was significantly increased in placentae delivered via LLSCS method in female- compared to male-bearing pregnancies (p < 0.0001).
IL17RE is part of the IL-17C pathway and functions as a pivotal regulator of innate immunity (39). Interestingly, IL-17C induces inflammation, but also promotes tissue healing (40). IL17RE mRNA expression (Figure 3C) was significantly increased in placentae from LLSCS births compared with UV for female-bearing (p < 0.0001) pregnancies only, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies; and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
IL17RE mRNA expression was significantly increased in placentae delivered via LLSCS in female- compared to male-bearing pregnancies (p < 0.0001).
TNFAIP2 expression is induced by other cytokines, including IL-1β, and plays essential roles in inflammation as a primary response gene (41), as well as angiogenesis and cell migration (42). TNFAIP2 mRNA expression (Figure 3D) was significantly increased in placentae from LLSCS births compared with UV for female- (p < 0.0001) and male-bearing (p = 0.0443) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
TNFAIP2 mRNA expression was significantly increased in placentae delivered via LLSCS method in female- compared to male-bearing pregnancies (p = 0.0071).
TNFSF10 is a cytokine belonging to the TNF-ligand family. The main role for TNFSF10 is in apoptosis of mutated cells, as it does not kill non-malignant cells (43). However, it is also expressed as an inflammatory marker in most healthy tissues. TNFSF10 mRNA expression (Figure 3E) was significantly increased in placentae from LLSCS births compared with UV for female- (p < 0.0001) and male-bearing (p = 0.0035) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
TNFSF10 mRNA expression was significantly increased in placentae delivered via LLSCS in female- compared to male-bearing pregnancies (p < 0.0001) and significantly decreased in placentae delivered via UV method in female- compared to male-bearing pregnancies (p < 0.0001).
Finally, TNFRSF8 (another member of the TNF-receptor superfamily) is a marker of inflammation as it is thought to be expressed only on activated T and B cells, not on resting cells. Activation of this receptor leads to signaling via the NF-κB pathway. TNFRSF8 mRNA expression (Figure 3F) was significantly increased in placentae from LLSCS births compared with UV for female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies; and from UV births compared with PLSCS for both female- (p = 0.0020) and male-bearing (p = 0.0316) pregnancies.
TNFRSF8 mRNA expression was significantly increased in placentae delivered via LLSCS in female- compared to male-bearing pregnancies (p < 0.0001).
Expression of Transcription Factors STAT1 and STAT2 Is Increased in Female UV Births Only
STAT1 and STAT2 are transcription factors which mediate type I and III interferon signalling via the JAK-STAT pathway; as such they play essential roles in the adaptive immune response (44). STAT1 and STAT2 mRNA expression (Figures 4A, B, respectively) was significantly increased in placentae from UV births compared with LLSCS for female-bearing (pSTAT1 < 0.0001; pSTAT2 < 0.0001) pregnancies only, as well as from LLSCS births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p < 0.0001) pregnancies.
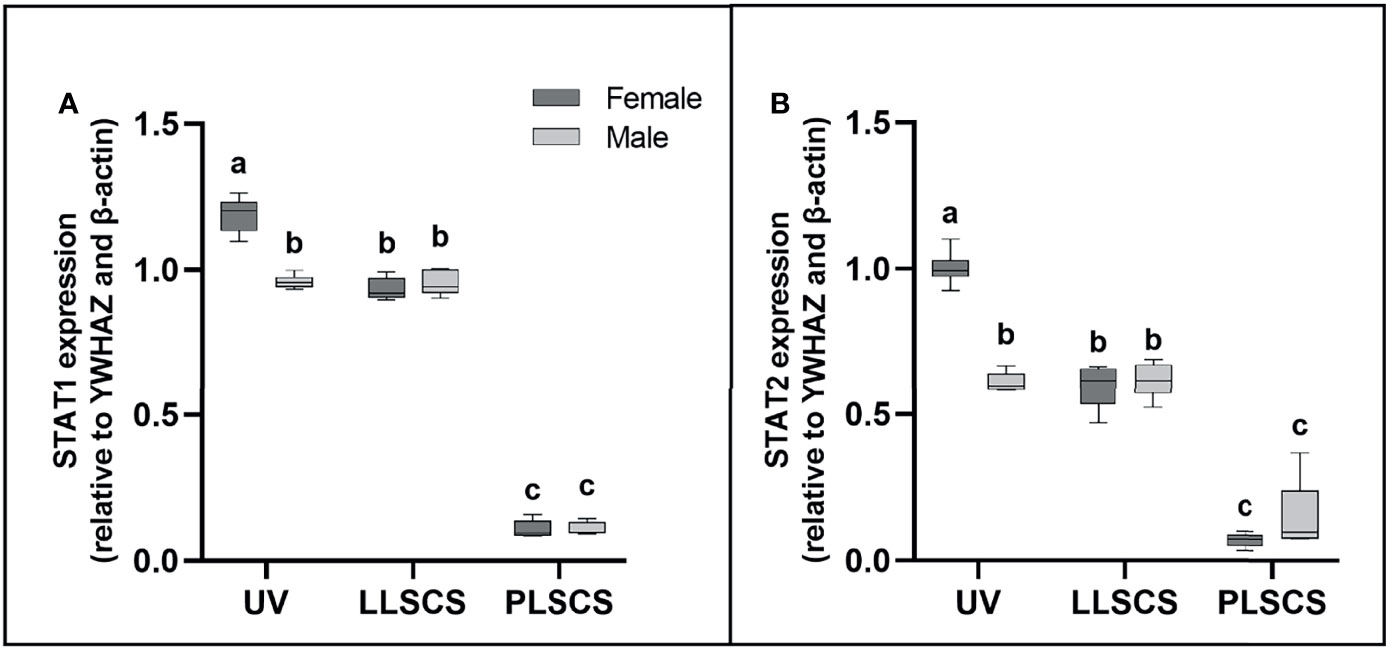
Figure 4 (A) Signal transducer and activator of transcription 1 (STAT1) and (B) 2 (STAT2) mRNA expression (qPCR) in placentae from female- and male-bearing pregnancies classed by delivery mode. Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (Number of samples: unassisted vaginal (UV) 20/fetal sex; labouring lower segment Caesarean section (LLSCS) = 10/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 5/fetal sex).
STAT1 and STAT2 mRNA expression was significantly increased in placentae delivered via UV delivery in female- compared to male-bearing pregnancies (pSTAT1 < 0.0001; pSTAT2 < 0.0001).
Expression of Anti-Inflammatory Interleukin IL-10 Is Increased in Male LLSCS Births Only
IL-10 is a cytokine with potent anti-inflammatory roles; indeed, absence of IL-10 leads to immunopathologies that are detrimental to the host, without any effect on pathogen load (45–47). IL10 mRNA expression (Figure 5) was significantly increased in placentae from LLSCS births compared with UV for male-bearing (p = 0.0063) pregnancies only, as well as from LLSCS births compared with PLSCS for female-bearing (p = 0.0003) pregnancies only, and from UV births compared with PLSCS for both female- (p < 0.0001) and male-bearing (p = 0.0001) pregnancies.
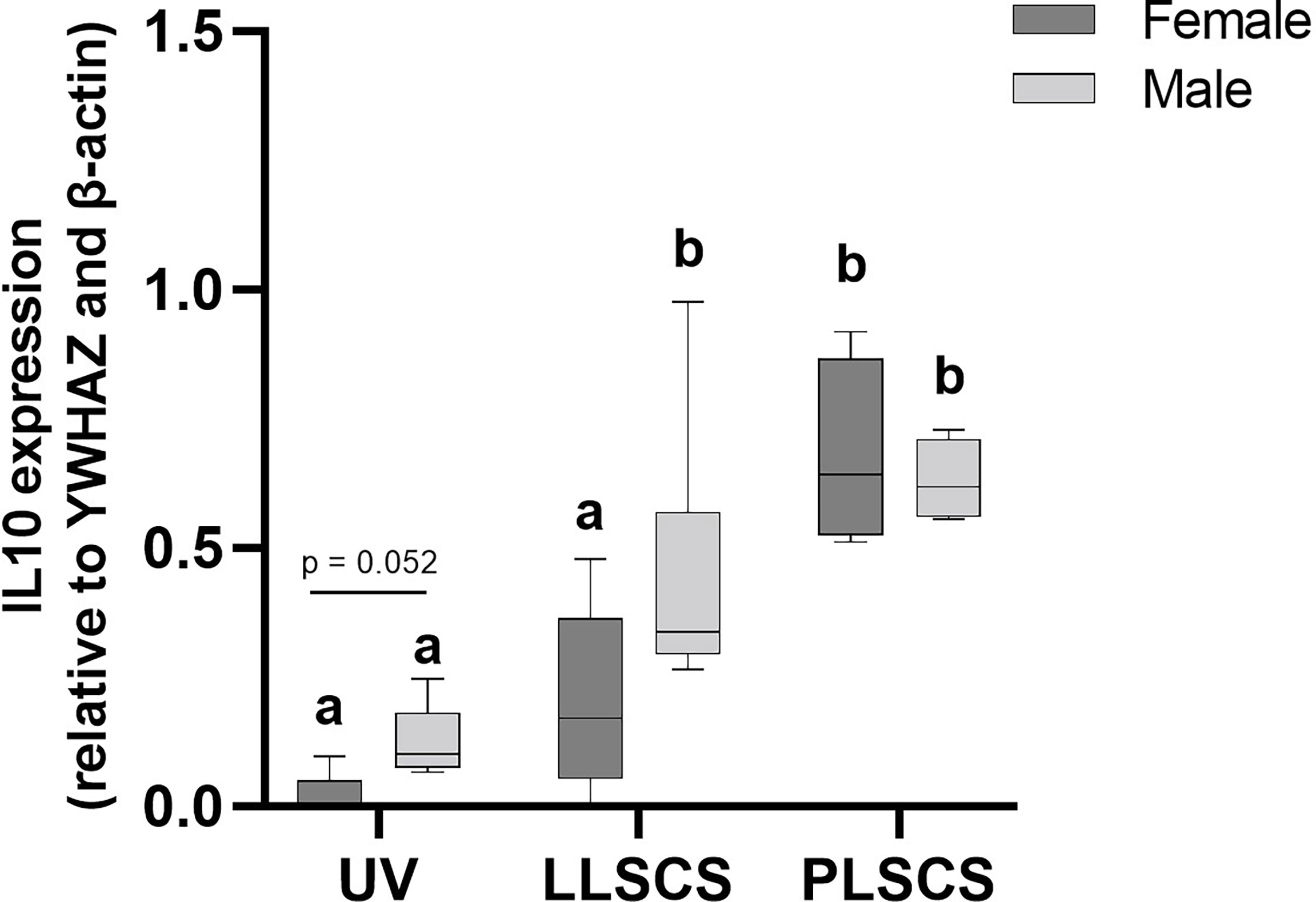
Figure 5 The mRNA expression (as determined by qPCR) of Interleukin 10 (IL10) in placentae from female- and male-bearing pregnancies classed by delivery mode. Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (Number of samples: unassisted vaginal (UV) 20/fetal sex; labouring lower segment Caesarean section (LLSCS) = 10/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 5/fetal sex).
IL10 mRNA expression was significantly increased in placentae delivered via LLSCS method in male- compared to female-bearing pregnancies (p = 0.0474).
Expression of Alarmin IL-33, and Receptor IL1RL1, Is Increased in Male LLSCS Births Only
IL-33 (a member of the IL1 family) is mainly secreted by damaged barrier cells as an alarmin cytokine (48); that is, it is used as an alarm to signal cellular damage or stress. Its receptor, IL1RL1, can be membrane-bound or soluble, and acts as an inhibitor of IL-33 functioning as a decoy receptor (49). IL33 and IL1RL1 mRNA expression (Figures 6A, B, respectively) was significantly increased in placentae from LLSCS births compared with UV for female- (pIL33 < 0.0001; pIL1RL1 = 0.0112) and male-bearing (pIL33 < 0.0001; pIL1RL1 < 0.0001) pregnancies, as well as from LLSCS births compared with PLSCS for female- (pIL33 < 0.0001; pIL1RL1 < 0.0001) and male-bearing (pIL33 < 0.0001; pIL1RL1 < 0.0001) pregnancies, and from UV births compared with PLSCS for both female- (pIL33 < 0.0001; pIL1RL1 < 0.0001) and male-bearing (pIL33 < 0.0001; pIL1RL1 = 0.0004) pregnancies.
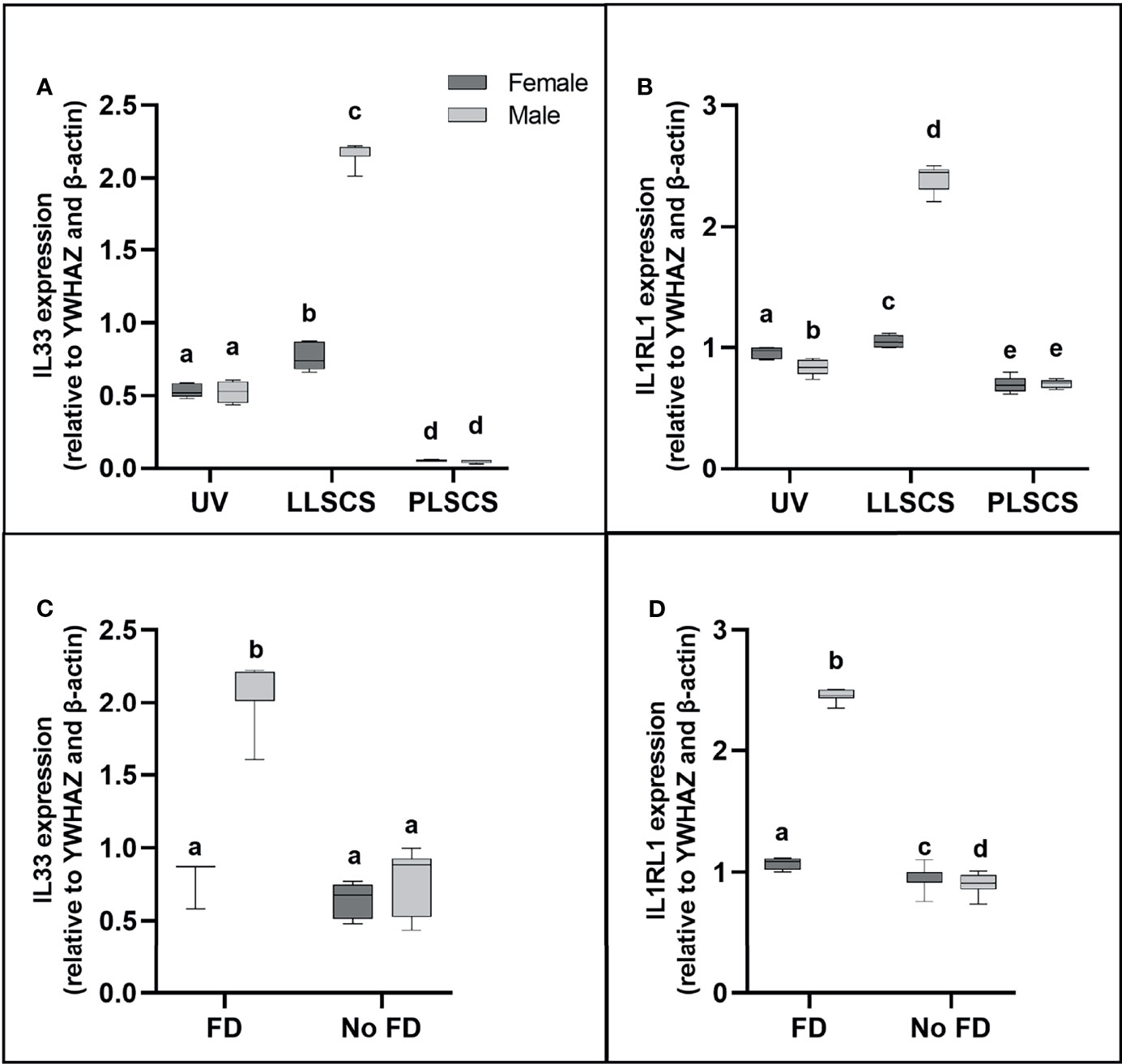
Figure 6 (A) Interleukin 33 (IL33) and (B) Interleukin 1 Receptor Like 1 (IL1RL1) mRNA expression in placentae from female- and male-bearing pregnancies classed by delivery mode, and by indication of fetal distress (FD) [(C) IL33 in FD, (D) IL1RL1 in FD)] during labour (qPCR). Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (Number of samples: unassisted vaginal (UV) 20/fetal sex; labouring lower segment Caesarean section (LLSCS) = 10/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 5/fetal sex. Samples with FD = 9, samples with no FD = 51).
IL33 and IL1RL1 mRNA expression was significantly increased in placentae delivered via LLSCS in male- compared to female-bearing pregnancies (pIL33 < 0.0001; pIL1RL1 < 0.0001). IL1RL1 mRNA expression was also significantly decreased in placentae delivered via UV delivery in male- compared to female-bearing pregnancies (p = 0.0007).
Expression of Alarmin IL33, and Receptor IL1RL1 Is Only Increased in Male LLSCS Births With Fetal Distress During Labour
Fetal distress (FD) was ascertained from clinical notes indicating meconium presence in the amniotic fluid, and/or obstetrician’s decision to use a Ventouse cap or forceps, or proceed to LLSCS due to failure to progress. Interestingly, IL33 expression (Figure 6C) was only significantly increased in placentae from male babies with FD compared with births where no FD was detected (p < 0.0001), regardless of delivery mode. IL1RL1 mRNA expression (Figure 6D) was significantly increased in placentae from both female- (p = 0.0329) and male-bearing (p < 0.0001) births with FD compared with births where no FD was detected.
IL33 and IL1RL1 mRNA expression was significantly increased in placentae from male- compared to female-bearing deliveries where FD was detected (pIL33 < 0.0001; pIL1RL1 < 0.0001). IL1RL1 mRNA expression was also significantly decreased in placentae from male- compared to female-bearing deliveries where no FD was detected (p = 0.0129).
Expression of IL-1β Protein Is Significantly Increased in LLSCS Females Only
IL-1β protein expression (Figures 7A, C) was significantly increased in placentae from LLSCS births compared with UV for female-bearing pregnancies only (p = 0.0031).
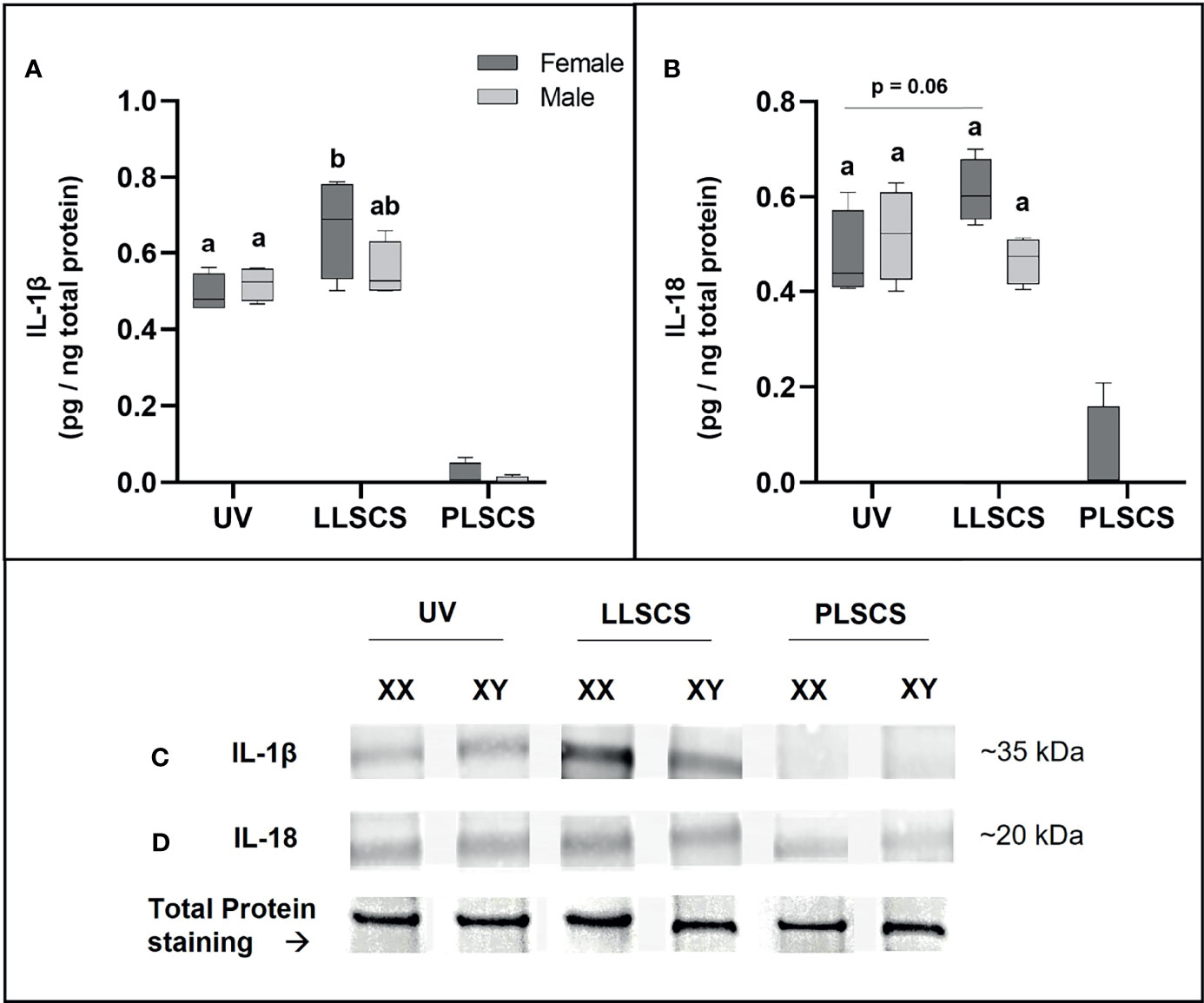
Figure 7 The mRNA expression of Interleukin 1β (IL-1β) (A, C) and Interleukin 18 (IL-18) (B, D) protein in placentae from female- and male-bearing pregnancies classed by delivery mode. For (A, B), Data are presented as a 10-90 percentile interleaved box-and-whisker plot. The same letter above bars indicates that groups are not different from each other. A different letter above bars indicates that groups are different (all p < 0.05). Dark grey colour denotes placenta samples from female fetal sex; light grey colour denotes placenta samples from male fetal sex. (C, D) show representative images of staining with an IL-1β antibody (molecular weight ~35 kDa) and an IL-18 antibody (molecular weight ~20 kDa), respectively. Below this is a representative image of stain-free Total Protein staining as a control. (Number of samples: unassisted vaginal (UV) = 6/fetal sex; labouring lower segment Caesarean section (LLSCS) = 4/fetal sex; prelabour lower segment Caesarean section (PLSCS) = 2/fetal sex).
IL-18 protein expression (Figures 7B, D) was higher in placentae from LLSCS births compared with UV for female-bearing pregnancies only but was not statistically significant (p = 0.0602).
IL-1β and IL-18 protein expression in placentae from PLSCS births is illustrated in Figure 7, however statistical analyses could not be performed due to low sample numbers (n = 2/fetal sex).
Discussion
This study has shown that there is a general increase in inflammasome activation (as evidenced by the expression of genes encoding IL-1β and IL-18), and inflammatory molecule expression, in placentae from LLSCS births compared to UV and PLSCS births. This result was consistent with our hypothesis that, as emergency caesarean sections in labour are conducted due to maternal or fetal distress, placental gene expression in LLSCS births would reflect increased inflammation.
Inflammasome activation, and inflammatory molecule expression, is also increased in placentae from LLSCS compared with PLSCS births, and in UV compared with PLSCS deliveries. This was also consistent with our hypothesis that placentae from PLSCS births would show very low levels of inflammation compared with other modes of delivery, given that they do not experience the inflammatory response associated with labour. However, whilst we did not see the degree of inflammatory molecule expression that was observed in labouring births, there was some inflammatory molecule expression in placentae from PLSCS births as would be expected. Although all of the women with PLSCS in our study had uncomplicated pregnancies, these deliveries are often scheduled due to a pregnancy complication such as preeclampsia or gestational diabetes.
The initiation of labour is reliant on the functional withdrawal of progesterone and a significant increase in myometrial progesterone receptor A (PR-A), leading to an overall rise in the PR-A/PR-B expression ratio (50). This functional withdrawal allows estrogen signalling to dominate inducing upregulation of contraction-associated proteins (CAPs), prostaglandins, oxytocin receptors in the myometrium, and collagenases and metalloproteinases in the cervix that cause its ripening. These uterotonins lead to coordinated uterine contractions. Whilst the exact mechanism inducing progesterone withdrawal is not completely understood, progesterone has long been known to be anti-inflammatory and maintains myometrial quiescence. Upon progesterone withdrawal, prostaglandins have also been implicated in labour (51), potentially by establishing a positive-feedback loop in the uterus with prostaglandins and inflammatory cytokines released by infiltrating leukocytes in the myometrium (3, 52). Furthermore, an association has long been established between inflammatory cell infiltration of the fetal membranes and decidua in labour and release of increased prostaglandins and leukotrienes (53, 54). Indeed, the fetal membranes are essential for allowing glucocorticoid signalling, leading to stimulation of prostaglandin production (55).
Whilst parturition is associated with an influx of leukocytes into the myometrium (26), this same phenomenon has been observed in the decidua (56) as well as in the cervix, where they potentially play a role in cervical ripening (57). Previous studies have shown that these leukocytes express pro-inflammatory cytokines IL-1β, IL-18 and IL-6 (58), and while inflammatory cells have not been detected in the placenta during parturition, this increase in pro-inflammatory cytokines that is observed in the myometrium and uterus is also in the chorio-decidua (26), as well as in amniotic fluid (59). Our data imply that expression of inflammatory cytokines and inflammation observed in the placenta following delivery could serve as an indication of molecular changes occurring within the uterus.
Not only did this study find that pro-inflammatory cytokines are increased in the placenta in labour compared to no labour, but patterns of expression were also noted which were fetal sex specific (Table 3). The expression of genes encoding inflammatory cytokines IL-1β and IL-18 was higher in placentae from female bearing pregnancies compared to male, indicating increased inflammasome activation in females.
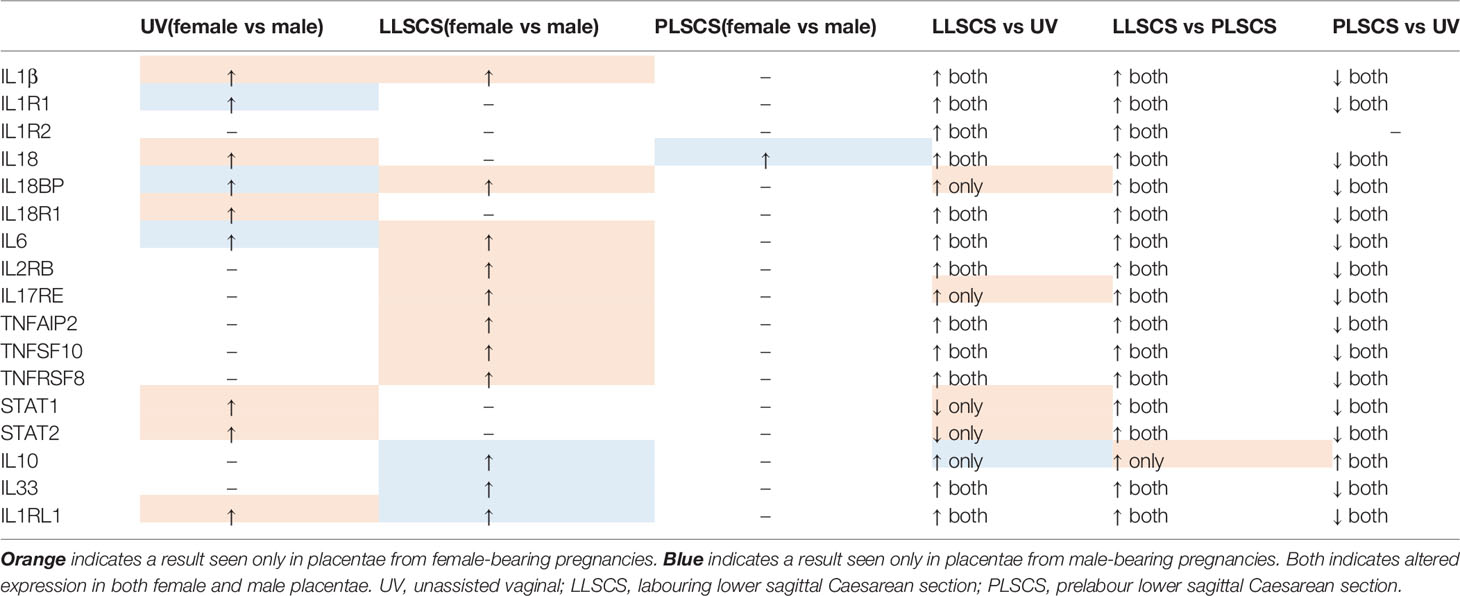
Table 3 Expression patterns of inflammasome and inflammatory molecules in placentae from female and male-bearing pregnancies, classified by mode of delivery.
Many inflammatory genes and/or receptors (IL1B, IL6, IL2RB, IL17RE, TNFAIP2, TNFSF10 and TNFRSF8; for interactions see Figure 8) also followed a pattern of upregulation in placentae from LLSCS compared with UV and PLSCS births, but also were significantly upregulated in placentae from female compared to male bearing LLSCS births. These clearly indicate heightened inflammation in placentae from female-bearing LLSCS births. Interestingly, IL18BP mRNA expression was also significantly increased in placentae from female-bearing LLSCS births compared with UV births, where this was not observed in males. As IL18BP functions as an inhibitor of IL-18 activity by competitively binding to IL-18, impeding IL-18/IL18R1 binding, it is classified as an anti-inflammatory molecule. The upregulation of IL18BP in female but not male-bearing placentae could indicate a strategy to minimize damage from a large inflammatory response, given the clearly larger inflammatory reaction observed in placentae from female compared to male-bearing LLSCS births. This is important as excess inflammasome activation is associated with extensive pyroptosis (60). However, in the placenta it is not known what impact this may have for these female babies.
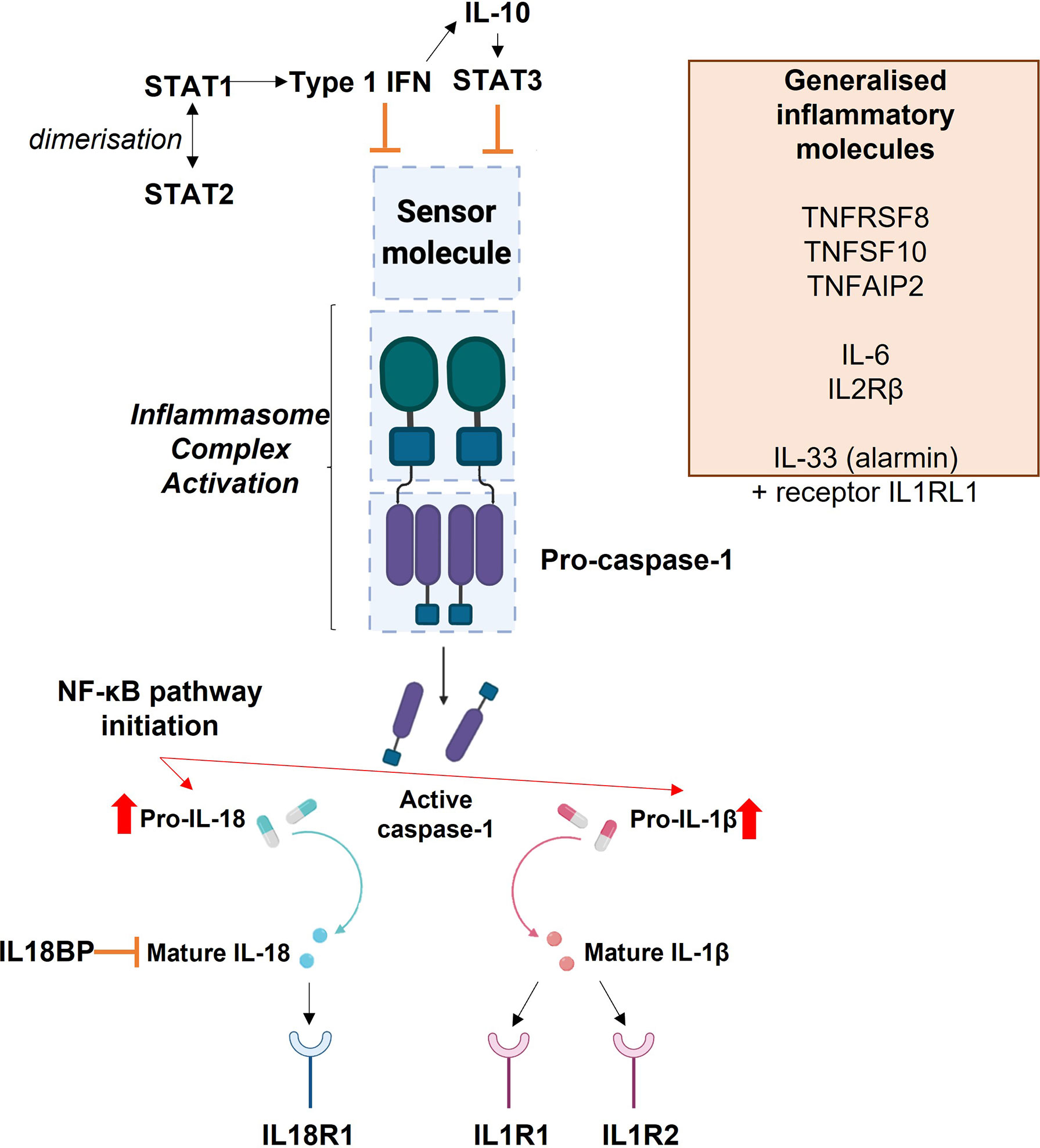
Figure 8 Diagrammatic representation of the interactions of inflammasome and inflammatory molecules explored in this study.
In addition to the relatively lower expression of inflammasome and inflammatory molecules in placentae from male compared with female-bearing LLSCS births, anti-inflammatory cytokine IL10 mRNA expression was significantly increased. IL-10 plays an essential role in preventing pathological inflammation (45), as well as preventing chronic inflammatory conditions (61). In fact, the levels of IL10 expression in placentae from male-bearing LLSCS births was no different to that in PLSCS births, potentially controlling the inflammatory response in LLSCS births to ensure there is not overactivation of the inflammasome. This was not observed in female-bearing placentae, which had the same level of IL10 expression in LLSCS and UV births.
Furthermore, IL33 and IL1RL1 mRNA were significantly upregulated by approximately 2.5-fold in placentae from male compared with female-bearing LLSCS births. As briefly mentioned above, IL-33 does not function as a typical inflammatory cytokine but instead as an alarmin, providing an indication to surrounding tissue of damage or stress.
Previous studies have identified that male-bearing pregnancies are more likely to end in LLSCS compared with female-bearing (62); however, this is not due to failure to progress through labour but instead due to signs of fetal distress (63). In conjunction with our data, this could indicate that placentae from male-bearing LLSCS births upregulate IL-33 as a signal of fetal distress, inducing IL1RL1 expression and mediating inflammation. This is not seen in placentae from female-bearing LLSCS births, where the magnitude of the IL33 expression is significantly reduced, and inflammatory markers are more highly expressed compared with males. Indeed, when samples were separated by clinical notes indicating fetal distress, a large increase in IL33 and IL1RL1 expression was observed in placentae from male, but not female-bearing births, suggesting enhanced signalling for damage and stress in problematic male-bearing labours compared with female-bearing.
A major limitation of this study is its opportunistic nature that relied on data from a larger unrelated study that profiled the placental transcriptome across gestation. Data for uncomplicated pregnancies at term for whom mode of delivery was known were selected. Therefore, the data may not represent the whole population. Furthermore, data was available for a relatively small number of placentae from PLSCS births. Information about intra-partum medications were not available so data may be influenced by these for both emergency and planned caesarean delivery. Moreover, all data are from analysis of chorionic villus samples, and the specific cell types present in these samples were not quantified. Future studies should be larger and designed specifically to determine differences in the inflammasome between different modes of delivery with attention to all details of the labour and delivery including medications, analgesia and anaesthesia.
Overall, increased activation of the placental inflammasome in LLSCS, which is often a life-saving intervention, likely also indicates greater exposure of the fetus during labour and delivery to inflammation. Perinatal inflammation and caesarean delivery are known to impact post-natal health of the infant. The former associates with brain injury in preterm labour and subsequent neurological disorders the best known of which is cerebral palsy (2).
Caesarean section not only has implications for maternal mortality, morbidity, recovery and future pregnancy but it also has been implicated in long term health and wellbeing for children [reviewed in (64)]. Specifically, children who were delivered by caesarean section are at greater risk of immune disorders such as asthma, atopy, allergy with evidence for an altered gut microbiome and potentially they are at risk of childhood obesity and metabolic disorders (64). A recent systematic review and meta-analysis of over 20 million births has demonstrated increased risk for autism spectrum disorders (ASD) and attention deficit hyperactivity disorder (ADHD) in children delivered by caesarean section with similar effects for both in labour and planned (65). Compression of the fetus as it traverses the birth canal causes a surge in glucocorticoids that is believed to be beneficial to the quick transition to extrauterine life that the neonate must make. Previously it was thought that negative effects of caesarean delivery are due to the lack of the big squeeze experienced during vaginal delivery. However, more recent thinking on the mechanisms of adverse effects of caesarean delivery on child development implicate altered epigenetic state and changes to the gut microbiome (66). Inflammation could be a factor in these. Here we have identified changes in placental expression of inflammatory mediators in both LLSCS and PLSCS deliveries. With further studies and follow up of children, these could potentially be used as indicators of perinatal exposure to inflammation that may impact child development, even for term deliveries where future risks to the child are generally not suspected.
To summarise our findings, placentae from female-bearing LLSCS deliveries had the highest inflammasome activation, followed by male-bearing LLSCS deliveries and UV deliveries. Placentae from PLSCS deliveries had the lowest inflammasome activation, though some expression of inflammatory genes was detected. Compared to babies delivered UV, the most common method of delivery, those delivered by LLSCS may be more at risk of inflammatory insult. The placental inflammasome may be useful for non-invasively identifying those babies most at risk. Conversely, PLSCS placentae reflect low level inflammation that may also disadvantage the baby who may not be optimally primed for their transition to extrauterine life, but this requires further investigation.
Our study shows that inflammation is indeed evident in the placenta in term uncomplicated UV births, accompanying the loss of immune tolerance at parturition. Not only is this necessary to mediate the labour cascade but inflammasome expression in the placenta without significant infiltration by maternal leukocytes may aid in placental separation, blood clotting and closure of maternal spiral arteries which are further compressed by uterine contractions following delivery of the placenta. We have certainly demonstrated that placental inflammasome activation at delivery reflects the end of tolerance. However, clearly the end of tolerance is not the same for all pregnancies and mode of delivery and its impact on the placental inflammasome are clear indicators of that. Placental inflammation associated with UV delivery may help not only labour but also the rapid transition to extrauterine life that babies must make. Reductions in the placental inflammasome in PLSCS may be associated with the reduced ease with which these babies, so delivered, make that transition.
Signal Transducer and Activator of Transcription 1 and 2 (STAT1 and STAT2) dimerise in response to Type 1 Interferon (IFN) signalling. This signalling can itself inhibit inflammasome activation or can inhibit inflammasome activation via Interleukin-10 (IL-10), which then leads to the production of STAT3. The blue boxes in the centre of the image depict the basic inflammasome activation pathway: a sensor molecule is stimulated which leads to activation of the inflammasome complex by pro-caspase-1. This leads to the production of active caspase-1, which promotes the conversion of pro-IL-1β and pro-IL-18 into their mature forms. The release of mature IL-1β and IL-18 signal activation of the inflammasome. Their release also initiates the NF-κB pathway. Mature IL-18 acts upon the IL-18 Receptor 1 (IL18R1), where its binding stimulates a pro-inflammatory response. Interleukin 18 Binding Protein (IL18BP) acts as an inhibitor of IL-18. Mature IL-1β can bind to the Interleukin 1 Type 1 Receptor (IL1R1) to initiate a pro-inflammatory response, or to the Interleukin 1 Type 2 Receptor (IL1R2) to induce anti-inflammatory activity. The orange box lists other inflammatory molecules explored within this study which are not specifically associated with this inflammasome activation pathway: Tumour Necrosis Factor Receptor Superfamily Member 8 (TNFRSF8), Tumour Necrosis Factor Superfamily Member 10 (TNFSF10), Tumour Necrosis Factor Alpha Induced Protein 2), Interleukin 6 (IL-6), Interleukin 2 Receptor β (IL2RB), Interleukin 33 (IL-33) and Interleukin 1 Receptor Like 1 (IL1RL1).
Data Availability Statement
The data presented in the study are deposited in the Short reads Archive (SRA) repository, accession number PRJNA816267.
Ethics Statement
The studies involving human participants were reviewed and approved by The Queen Elizabeth Hospital and Lyell McEwin Hospital Human Research Ethics Committee TQEH/LMH HREC/1712/5/2008 (SCOPE Study) Women’s and Children’s Network Human Research Ethics Committee HREC/14/WCHN/90 (STOP Study). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AA conceptualised this work, performed experimental work and formal analysis of data, original draft preparation, and review and editing. MS optimised the methodology for, and performed, bioinformatic analysis of data, prepared visualisations, and reviewed and edited the manuscript. MH contributed to experimental work and review and editing. JB contributed to methodology optimisation of bioinformatic analysis. DM optimised the methodology for, and performed, experimental work. FT contributed to experimental work. SL contributed to statistical methodologies used in the paper, as well as review and editing. GD provided clinical expertise and edited the manuscript. TJ-K reviewed and edited the manuscript. CR provided supervision, funding acquisition, interpretation of data, review and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
RNA sequencing data used in this study was funded by NIH NICHD R01 HD089685-01 Maternal molecular profiles reflect placental function and development across gestation PI Roberts, a National Health and Medical Research Council Investigator Grant (GNT1174971) awarded to CTR and a Matthew Flinders Professorial Fellowship awarded to CTR and funded by Flinders University. JB is supported by the James & Diana Ramsay Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the women who consented to their placental tissue being used for research. We thank Dr Sara Tommasi for help with Western blotting.
References
1. Mor G, Cardenas I. The Immune System in Pregnancy: A Unique Complexity. Am J Reprod Immunol (2010) 63(6):425–33. doi: 10.1111/j.1600-0897.2010.00836.x
2. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in Pregnancy: Its Roles in Reproductive Physiology, Obstetrical Complications, and Fetal Injury. Nutr Rev (2007) 65(suppl_3):S194–202. doi: 10.1301/nr.2007.dec.S194-S202
3. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory Processes in Preterm and Term Parturition. J Reprod Immunol (2008) 79(1):50–7. doi: 10.1016/j.jri.2008.04.002
4. Hansen VL, Faber LS, Salehpoor AA. Miller RD. A Pronounced Uterine Pro-Inflammatory Response at Parturition Is an Ancient Feature in Mammals. Proc R Soc B: Biol Sci (2017) 284(1865):20171694. doi: 10.1098/rspb.2017.1694
5. Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, et al. Leukocyte Density and Pro-Inflammatory Cytokine Expression in Human Fetal Membranes, Decidua, Cervix and Myometrium Before and During Labour at Term. MHR: Basic Sci Reprod Med (2003) 9(1):41–5. doi: 10.1093/molehr/gag001
6. Nagamatsu T, Schust DJ. The Contribution of Macrophages to Normal and Pathological Pregnancies. Am J Reprod Immunol (2010) 63(6):460–71. doi: 10.1111/j.1600-0897.2010.00813.x
7. Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol (2018) 79(6):e12440. doi: 10.1111/aji.12440
8. Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J Immunol (2019) 203(11):2757–69. doi: 10.4049/jimmunol.1900901
9. Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, et al. Inhibition of the Nlrp3 Inflammasome Can Prevent Sterile Intra-Amniotic Inflammation, Preterm Labor/Birth, and Adverse Neonatal Outcomes. Biol Reprod (2019) 100(5):1306–18. doi: 10.1093/biolre/ioy264
10. Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of Proil-B. Mol Cell (2002) 10(2):417–26. doi: 10.1016/S1097-2765(02)00599-3
11. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. Nalp3 Forms an Il-1β-Processing Inflammasome With Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity (2004) 20(3):319–25. doi: 10.1016/S1074-7613(04)00046-9
12. Poyet J-L, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a Human Caspase-1-Activating Protein Related to Apaf-1. J Biol Chem (2001) 276(30):28309–13. doi: 10.1074/jbc.C100250200
13. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential Activation of the Inflammasome by Caspase-1 Adaptors Asc and Ipaf. Nature (2004) 430(6996):213–8. doi: 10.1038/nature02664
14. Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, et al. An Orthogonal Proteomic-Genomic Screen Identifies Aim2 as a Cytoplasmic DNA Sensor for the Inflammasome. Nat Immunol (2009) 10(3):266. doi: 10.1038/ni.1702
15. Gavrilin MA, Abdelaziz DH, Mostafa M, Abdulrahman BA, Grandhi J, Akhter A, et al. Activation of the Pyrin Inflammasome by Intracellular Burkholderia Cenocepacia. J Immunol (2012) 188(7):3469–77. doi: 10.4049/jimmunol.1102272
16. Guo H, Callaway JB, Ting JP. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat Med (2015) 21(7):677–87. doi: 10.1038/nm.3893
17. Liu T, Zhang L, Joo D, Sun S-C. Nf-Kb Signaling in Inflammation. Signal Transduct targeted Ther (2017) 2(1):1–9. doi: 10.1038/sigtrans.2017.23
18. Nunes PR, Peracoli MTS, Romao-Veiga M, Matias ML, Ribeiro VR, Fernandes CJDC, et al. Hydrogen Peroxide-Mediated Oxidative Stress Induces Inflammasome Activation in Term Human Placental Explants. Pregnancy Hypertens (2018) 14:29–36. doi: 10.1016/j.preghy.2018.07.006
19. Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, et al. In Vivo Evidence of Inflammasome Activation During Spontaneous Labor at Term. J Matern-Fetal Neonat Med (2019) 32(12):1978–91. doi: 10.1080/14767058.2017.1422714
20. Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, et al. Uric Acid Induces Trophoblast Il-1β Production Via the Inflammasome: Implications for the Pathogenesis of Preeclampsia. Am J Reprod Immunol (2011) 65(6):542–8. doi: 10.1111/j.1600-0897.2010.00960.x
21. Shirasuna K, Karasawa T, Takahashi M. Role of the Nlrp3 Inflammasome in Preeclampsia. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.00080
22. Stødle G, Silva G, Tangerås LH, Gierman L, Nervik I, Dahlberg U, et al. Placental Inflammation in Pre-Eclampsia by Nod-Like Receptor Protein (Nlrp) 3 Inflammasome Activation in Trophoblasts. Clin Exp Immunol (2018) 193(1):84–94. doi: 10.1111/cei.13130
23. Weel IC, Romão-Veiga M, Matias ML, Fioratti EG, Peraçoli JC, Borges VT, et al. Increased Expression of Nlrp3 Inflammasome in Placentas From Pregnant Women With Severe Preeclampsia. J Reprod Immunol (2017) 123:40–7. doi: 10.1016/j.jri.2017.09.002
24. Matias ML, Romão M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, et al. Endogenous and Uric Acid-Induced Activation of Nlrp3 Inflammasome in Pregnant Women With Preeclampsia. PloS One (2015) 10(6):e0129095. doi: 10.1371/journal.pone.0129095
25. Zhu J, Ma C, Zhu L, Li J, Peng F, Huang L, et al. A Role for the Nlrc4 Inflammasome in Premature Rupture of Membrane. PloS One (2020) 15(8):e0237847. doi: 10.1371/journal.pone.0237847
26. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes Infiltrate the Myometrium During Human Parturition: Further Evidence That Labour Is an Inflammatory Process. Hum Reprod (1999) 14(1):229–36. doi: 10.1093/humrep/14.1.229
27. Mohammed H, Roberts CT, Grzeskowiak LE, Giles LC, Dekker GA, Marshall HS. Safety and Protective Effects of Maternal Influenza Vaccination on Pregnancy and Birth Outcomes: A Prospective Cohort Study. EClinicalMedicine (2020) 26:100522. doi: 10.1016/j.eclinm.2020.100522
28. Li H, Durbin R. Fast and Accurate Long-Read Alignment With Burrows–Wheeler Transform. Bioinformatics (2010) 26(5):589–95. doi: 10.1093/bioinformatics/btp698
29. Robinson MD, McCarthy DJ, Smyth GK. Edger: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics (2010) 26(1):139–40. doi: 10.1093/bioinformatics/btp616
30. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma Powers Differential Expression Analyses for Rna-Sequencing and Microarray Studies. Nucleic Acids Res (2015) 43(7):e47–e. doi: 10.1093/nar/gkv007
31. Smyth GK. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat Appl Genet Mol Biol (2004) 3(1):7–18. doi: 10.2202/1544-6115.1027
32. Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B (Methodol) (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
33. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2– Δδct Method. Methods (2001) 25(4):402–8. doi: 10.1006/meth.2001.1262
34. Hammond M, Kohn J, Oh K, Piatti P, Liu N. A Method for Greater Reliability in Western Blot Loading Controls—Stain-Free Total Protein Quantitation. Bio-Rad Bull (2013) 6360:1–4.
35. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. Star: Ultrafast Universal Rna-Seq Aligner. Bioinformatics (2013) 29(1):15–21. doi: 10.1093/bioinformatics/bts635
36. Liao Y, Smyth GK, Shi W. Featurecounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics (2014) 30(7):923–30. doi: 10.1093/bioinformatics/btt656
37. Ma A, Koka R, Burkett P. Diverse Functions of Il-2, Il-15, and Il-7 in Lymphoid Homeostasis. Annu Rev Immunol (2006) 24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727
38. Cohen CJ, Rebollo R, Babovic S, Dai EL, Robinson WP, Mager DL. Placenta-Specific Expression of the Interleukin-2 (Il-2) Receptor B Subunit From an Endogenous Retroviral Promoter. J Biol Chem (2011) 286(41):35543–52. doi: 10.1074/jbc.M111.227637
39. Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, et al. Il-17re Is the Functional Receptor for Il-17c and Mediates Mucosal Immunity to Infection With Intestinal Pathogens. Nat Immunol (2011) 12(12):1151. doi: 10.1038/ni.2155
40. Brevi A, Cogrossi LL, Grazia G, Masciovecchio D, Impellizzieri D, Lacanfora L, et al. Much More Than Il-17a: Cytokines of the Il-17 Family Between Microbiota and Cancer. Front Immunol (2020) 11:2914. doi: 10.3389/fimmu.2020.565470
41. Sarma V, Wolf F, Marks R, Shows T, Dixit V. Cloning of a Novel Tumor Necrosis Factor-Alpha-Inducible Primary Response Gene That Is Differentially Expressed in Development and Capillary Tube-Like Formation in Vitro. J Immunol (1992) 148(10):3302–12.
42. Jia L, Shi Y, Wen Y, Li W, Feng J, Chen C. The Roles of Tnfaip 2 in Cancers and Infectious Diseases. J Cell Mol Med (2018) 22(11):5188–95. doi: 10.1111/jcmm.13822
43. Collison A, Foster PS, Mattes J. Emerging Role of Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand (Trail) as a Key Regulator of Inflammatory Responses. Clin Exp Pharmacol Physiol (2009) 36(11):1049–53. doi: 10.1111/j.1440-1681.2009.05258.x
44. Au-Yeung N, Mandhana R, Horvath CM. Transcriptional Regulation by Stat1 and Stat2 in the Interferon Jak-Stat Pathway. Jak-stat (2013) 2(3):e23931. doi: 10.4161/jkst.23931
45. Saraiva M, O'garra A. The Regulation of Il-10 Production by Immune Cells. Nat Rev Immunol (2010) 10(3):170–81. doi: 10.1038/nri2711
46. O'Garra A, Vieira P. Th 1 Cells Control Themselves by Producing Interleukin-10. Nat Rev Immunol (2007) 7(6):425–8. doi: 10.1038/nri2097
47. Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, et al. In the Absence of Endogenous Il-10, Mice Acutely Infected With Toxoplasma Gondii Succumb to a Lethal Immune Response Dependent on Cd4+ T Cells and Accompanied by Overproduction of Il-12, Ifn-Gamma and Tnf-Alpha. J Immunol (1996) 157(2):798–805.
48. Cayrol C, Girard J-P. Il-33: An Alarmin Cytokine With Crucial Roles in Innate Immunity, Inflammation and Allergy. Curr Opin Immunol (2014) 31:31–7. doi: 10.1016/j.coi.2014.09.004
49. Kakkar R, Lee RT. The Il-33/St2 Pathway: Therapeutic Target and Novel Biomarker. Nat Rev Drug Discovery (2008) 7(10):827–40. doi: 10.1038/nrd2660
51. Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, et al. Prostaglandins and Mechanisms of Preterm Birth. Reproduction-Cambridge- (2002) 124(1):1–17. doi: 10.1530/rep.0.1240001
52. Phillips RJ, Fortier MA, Bernal AL. Prostaglandin Pathway Gene Expression in Human Placenta, Amnion and Choriodecidua Is Differentially Affected by Preterm and Term Labour and by Uterine Inflammation. BMC pregnancy childbirth (2014) 14(1):1–14. doi: 10.1186/1471-2393-14-241
53. Lopez Bernal A, Hansell D, Khong T, Keeling J, Turnbull A. Prostaglandin E Production by the Fetal Membranes in Unexplained Preterm Labour and Preterm Labour Associated With Chorioamnionitis. Int J Gynecol Obstetrics (1990) 32(2):190–. doi: 10.1016/0020-7292(90)90509-J
54. Bernal AL, Hansell D, Khong T, Keeling J, Turnbull A. Placental Leukotriene B4 Release in Early Pregnancy and in Term and Preterm Labour. Early Hum Dev (1990) 23(2):93–9. doi: 10.1016/0378-3782(90)90132-3
55. Gibb W. The Role of Prostaglandins in Human Parturition. Ann Med (1998) 30(3):235–41. doi: 10.3109/07853899809005850
56. Keski-Nisula L, Aalto M-L, Katila M-L, Kirkinen P. Intrauterine Inflammation at Term: A Histopathologic Study. Hum Pathol (2000) 31(7):841–6. doi: 10.1053/hupa.2000.8449
57. Bokström H, Brännström M, Alexandersson M, Norström A. Leukocyte Subpopulations in the Human Uterine Cervical Stroma at Early and Term Pregnancy. Hum Reprod (Oxf Engl) (1997) 12(3):586–90. doi: 10.1093/humrep/12.3.586
58. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of Proinflammatory Cytokines in Myometrium, Cervix, and Fetal Membranes During Human Parturition at Term. Biol Reprod (2002) 66(2):445–9. doi: 10.1095/biolreprod66.2.445
59. Romero R, Parvizi S, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic Fluid Interleukin-1 in Spontaneous Labor at Term. J Reprod Med (1990) 35(3):235–8.
60. Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of Inflammasome Signaling. Nat Immunol (2012) 13(4):333. doi: 10.1038/ni.2237
61. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-Deficient Mice Develop Chronic Enterocolitis. Cell (1993) 75(2):263–74. doi: 10.1016/0092-8674(93)80068-P
62. Antonakou A, Papoutsis D. The Effect of Fetal Gender on the Delivery Outcome in Primigravidae Women With Induced Labours for All Indications. J Clin Diagn Res: JCDR (2016) 10(12):QC22. doi: 10.7860/JCDR/2016/22099.9104
63. Lieberman E, Lang JM, Cohen AP, Frigoletto FD Jr, Acker D, Rao R. The Association of Fetal Sex With the Rate of Caesarean Section. Am J Obstet Gynecol (1997) 176(3):667–71. doi: 10.1016/S0002-9378(97)70567-2
64. Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-Term and Long-Term Effects of Caesarean Section on the Health of Women and Children. Lancet (2018) 392(10155):1349–57. doi: 10.1016/S0140-6736(18)31930-5
65. Zhang T, Sidorchuk A, Sevilla-Cermeño L, Vilaplana-Pérez A, Chang Z, Larsson H, et al. Association of Caesarean Delivery With Risk of Neurodevelopmental and Psychiatric Disorders in the Offspring: A Systematic Review and Meta-Analysis. JAMA Net Open (2019) 2(8):e1910236–e. doi: 10.1001/jamanetworkopen.2019.10236
Keywords: placenta, inflammation, pregnancy, labour, parturition, inflammasome
Citation: Arthurs AL, Smith MD, Hintural MD, Breen J, McCullough D, Thornton FI, Leemaqz SY, Dekker GA, Jankovic-Karasoulos T and Roberts CT (2022) Placental Inflammasome mRNA Levels Differ by Mode of Delivery and Fetal Sex . Front. Immunol. 13:807750. doi: 10.3389/fimmu.2022.807750
Received: 02 November 2021; Accepted: 21 February 2022;
Published: 25 March 2022.
Edited by:
Surendra Sharma, Women & Infants Hospital of Rhode Island, United StatesCopyright © 2022 Arthurs, Smith, Hintural, Breen, McCullough, Thornton, Leemaqz, Dekker, Jankovic-Karasoulos and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anya L. Arthurs, anya.arthurs@flinders.edu.au; Claire T. Roberts, claire.roberts@flinders.edu.au
 Anya L. Arthurs
Anya L. Arthurs Melanie D. Smith1
Melanie D. Smith1 Mhyles D. Hintural
Mhyles D. Hintural James Breen
James Breen Tanja Jankovic-Karasoulos
Tanja Jankovic-Karasoulos Claire T. Roberts
Claire T. Roberts