- 1Division of Pharmacogenomics and Personalized Medicine, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: There are 3 classes of HLA molecules; HLA class I, II and III, of which different classes have different functions. HLA-B gene which belongs to HLA class I play an important role predicting drug hypersensitivity.
Materials and Methods: Nine hundred and eighty-six Thai subjects who registered at a pharmacogenomics laboratory were determined for HLA-B genotype using a two-stage sequence-specific oligonucleotide probe system (PCR-SSOP).
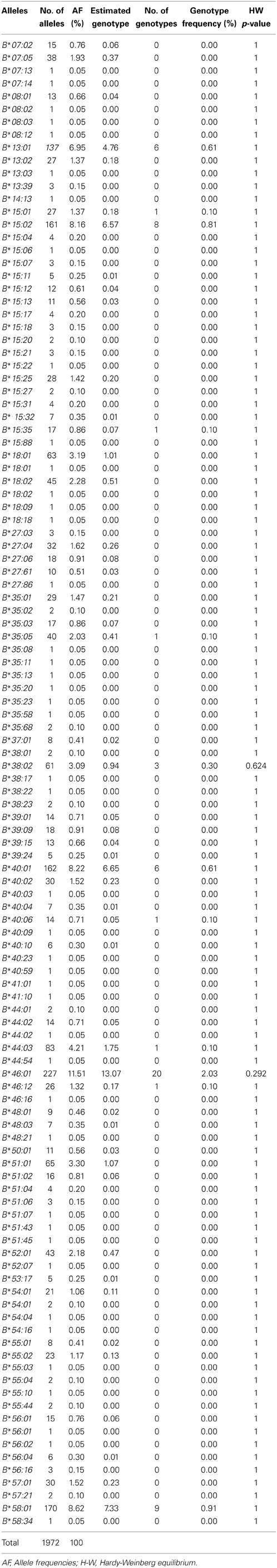
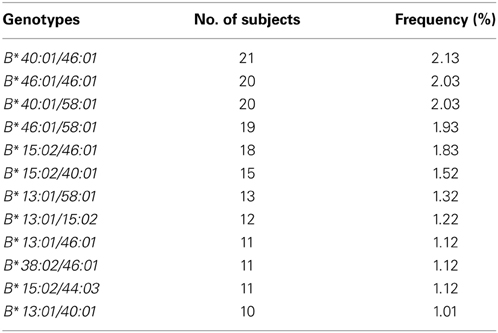
Results: In this study, HLA-B alleles did not deviate from Hardy-Weinberg equilibrium (P > 0.05). The most common HLA-B alleles observed in this population were HLA-B*46:01 (11.51%), HLA-B*58:01 (8.62%), HLA-B*40:01 (8.22%), HLA-B*15:02 (8.16%) and HLA-B*13:01 (6.95%). This finding revealed that HLA-B allele frequency in the Thai population was consistent with the Chinese population (p > 0.05), however, differed from the Malaysian population (p < 0.05). The top five HLA-B genotypes were HLA-B*40:01/46:01 (2.13%), HLA-B*46:01/46:01 (2.03%), HLA-B*40:01/58:01 (2.03%), HLA-B*46:01/58:01 (1.93%) and HLA-B*15:02/46:01 (1.83%). This study found that 15.92% of Thai subjects carry HLA-B*15:02, which has been associated with carbamazepine-induced severe cutaneous adverse drug reactions (SCARs). Moreover, 16.33% of Thai subjects carry the HLA-B*58:01 allele, which has been associated with allopurinol-induced SCARs.
Conclusion: This study demonstrates a high diversity of HLA-B polymorphisms in this Thai population. The high frequency of HLA-B pharmacogenomic markers in the population emphasizes the importance of such screening to predict/avoid drug hypersensitivity.
Introduction
Human leukocyte antigen (HLA) genes are located in the short arm of chromosome 6, are part of a large family of genes, and are inherited from both parents, one of paternal origin and one of maternal origin for each of the loci (Alper et al., 2006). HLA proteins are present at the cellular surface of antigen presenting cells. There are 3 classes of HLA. Class I loci (HLA-A, -B, -C) present endogenous antigens such as viral protein or tumor antigen to CD8+ T cells. Class II loci (HLA-DR, -DQ, -DP) present exogenous antigens to CD4+ T cells. Class III (Bf, C2, C4A) are involved in the complement system. HLA genes are highly polymorphic, which becomes important in associations of susceptibility or resistance to disease, development of tumor processes, molecular vaccine development (Dawson et al., 2001) and determination of organ or tissue transplant survival (Dhaliwal et al., 2003; Avila-Rios et al., 2009). In addition, HLA allele frequency might be useful in anthropological studies. Previous studies have reported HLA allele and haplotype frequencies of Thai populations. The most common HLA haplotypes were A*33-Cw*0302-B*5801-DRB*0301-DQB1*02 (4.6%), A*0207-Cw*01-B*4601-DRB1*09-DQB1*0303 (3.4%) and A*33-Cw*07 (01-03)-B*44-DRB1*07-DQB1*02 (2.6%) (Kupatawintu et al., 2010; Romphruk et al., 2010).
HLA-B alleles have been used as a marker for predicting drug-induced adverse reactions (Sukasem et al., 2014a) and are a major contributor to hypersensitivity reactions involving direct stimulation of immune effector cells and imitating an allergic reaction. Several studies reported that allopurinol-induced severe cutaneous adverse reactions (SCARs) are strongly associated with the HLA-B*58:01 allele in Han Chinese and Thais (Hung et al., 2005; Tassaneeyakul et al., 2009; Jantararoungtong et al., 2014). The HLA-B*15:02 allele was strongly related to carbamazepine-induced Steven-Johnson syndrome (SJS)-toxic epidermal necrolysis (TEN) in Taiwanese and Thai populations (Tassaneeyakul et al., 2010; Chen et al., 2011; Sukasem et al., 2014b). Importantly, Thais showed cutaneous adverse reactions associated with HLA-B*35:05 (Chantarangsu et al., 2009, 2011). In addition, the HLA-B*57:01 allele is involved in abacavir-induced hypersensitivity reaction (Mallal et al., 2008). Consequently, screening of HLA-B genotyping before prescription of medication may reduce the risk of SCARs and drug hypersensitivity reaction.
Presently, HLA-B genotyping is available in clinical practices before commencing therapy with the aforementioned drugs in Thailand (Sukasem et al., 2014a). This study aimed to determine the distribution of HLA-B haplotypes, and to establish the most common HLA haplotype-associated drug induced hypersensitivity using routine pharmacogenomic laboratory procedures. Over a period of 3 years, HLA-B allele genotype was analyzed in 986 Thai people registered at the Laboratory for Pharmacogenomics, Ramathibodi Hospital, Thailand. The frequency of HLA-B alleles and HLA-B genotypes were determined and used to define the probability associated with a Pharmacogenomic marker indicating adverse drug reaction.
Materials and Methods
Subjects and Characteristics
A descriptive, observational, cross-sectional, retrospective study was conducted in 986 unrelated individuals. Data were collected at the Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center (SDMC), Ramathibodi Hospital, Bangkok, Thailand during August 2011–June 2014. Among these patients, there were 590 (59.83%) males and 396 (40.17%) females. The mean age of patients at recruitment was 41.98 ± 4.40 years. The study was approved by the Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University. The protocol number is ID 04-56-24.
Genomic DNA Extraction
Blood samples were collected into EDTA tubes. DNA was isolated using the MagNA Pure automated extraction system (Roche diagnostics, USA), which uses magnetic-bead technology with a lysis buffer and proteinase K. Nucleic acids bind to the surface of the magnetic glass particles. Cellular debris was removed by several washing steps and the purified nucleic acids were eluted. From the 1 ml input volume of EDTA-whole blood, 200 μL output volume of extracted genomic DNA product was obtained.
The quality of genomic DNA was assessed by using Nano Drop ND-1000. Genomic DNA was detected by measuring absorbance at 260 nm. Purity of the sample was evaluated by calculation of the optical density (OD) ratio, 260/280 nm. The recommended purified genomic DNA template for this study was 20 ng, and the OD ratio greater than 1.7. All DNA was aliquotted and stored at −20°C before analysis.
HLA-B Typing
HLA-B genotyping was carried out using the Luminex™ Multiplex Technology (Luminex®IS 100, USA) based on Polymerase Chain Reaction-sequence specific oligonucleotides probe (PCR-SSOP) principles. Briefly, the PCR products were hybridized against a panel of oligonucleotide probes coated onto polystyrene microspheres. Probe sequences were complementary to stretches of polymorphic sequence within the target HLA-B alleles. The amplicon-probe complex was visualized using a colorimetric reaction and fluorescence detection technology. Data analyses for the HLA-B assays were performed with HLA fusion™2.0 software.
Statistical Analysis
The HLA-B allele frequencies of the samples were assayed by direct counting and, subsequently, by dividing the total number of occurrences of that allele by the total number of alleles at that locus in the population. The samples were also evaluated for Hardy-Weinberg equilibrium using the Chi-square or Fisher's exact test if the number in any cell of the 2 × 2 contingency tables was less than five.
Results
HLA-B Allele Frequency
The study population comprised 986 samples which were obtained from Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center (SDMC), Ramathibodi Hospital, Thailand. The frequency of HLA-B alleles in 986 Thai patients is shown in Table 1. Overall, 116 different HLA-B alleles were identified in this study, and HLA-B*46:01 (11.51%) was the predominant allele in this population. The most frequent alleles were HLA-B*46:01 (11.51%), HLA-B*58:01 (8.62%), HLA-B*40:01 (8.22%), HLA-B*15:02 (8.16%), HLA-B*13:01 (6.95%) and HLA-B*44:03 (4.21%). The frequencies of HLA-B alleles do not significantly deviate from Hardy-Weinberg equilibrium.
Frequency of HLA-B Genotype Occurrence
Overall, there were 448 different HLA-B genotypes in the 986 Thai individuals analyzed. The most frequently observed HLA-B genotype was HLA-B*40:01/46:01. The 10 most common genotype frequencies in this Thai population were HLA-B*40:01/46:01 (2.13%), HLA-B*46:01/46:01 (2.03%), HLA-B*40:01/58:01 (2.03%), HLA-B*46:01/58:01 (1.93%), HLA-B*15:02/46:01 (1.83%), HLA-B*15:02/40:01 (1.52%), HLA-B*13:01/58:01 (1.32%), HLA-B*13:01/15:02 (1.22%), HLA-B*13:01/46:01 (1.12%), HLA-B*38:02/46:01 (1.12%), HLA-B*15:02/44:03 (1.12%), and HLA-B*13:01/40:01 (1.01%). None of these genotypes were present in more than 5% of the population. Table 2 shows the frequency of the top 10 genotypes the Thai population in this study.
Frequency of the Strong Prediction of HLA-B Pharmacogenomics Markers in Thai Population
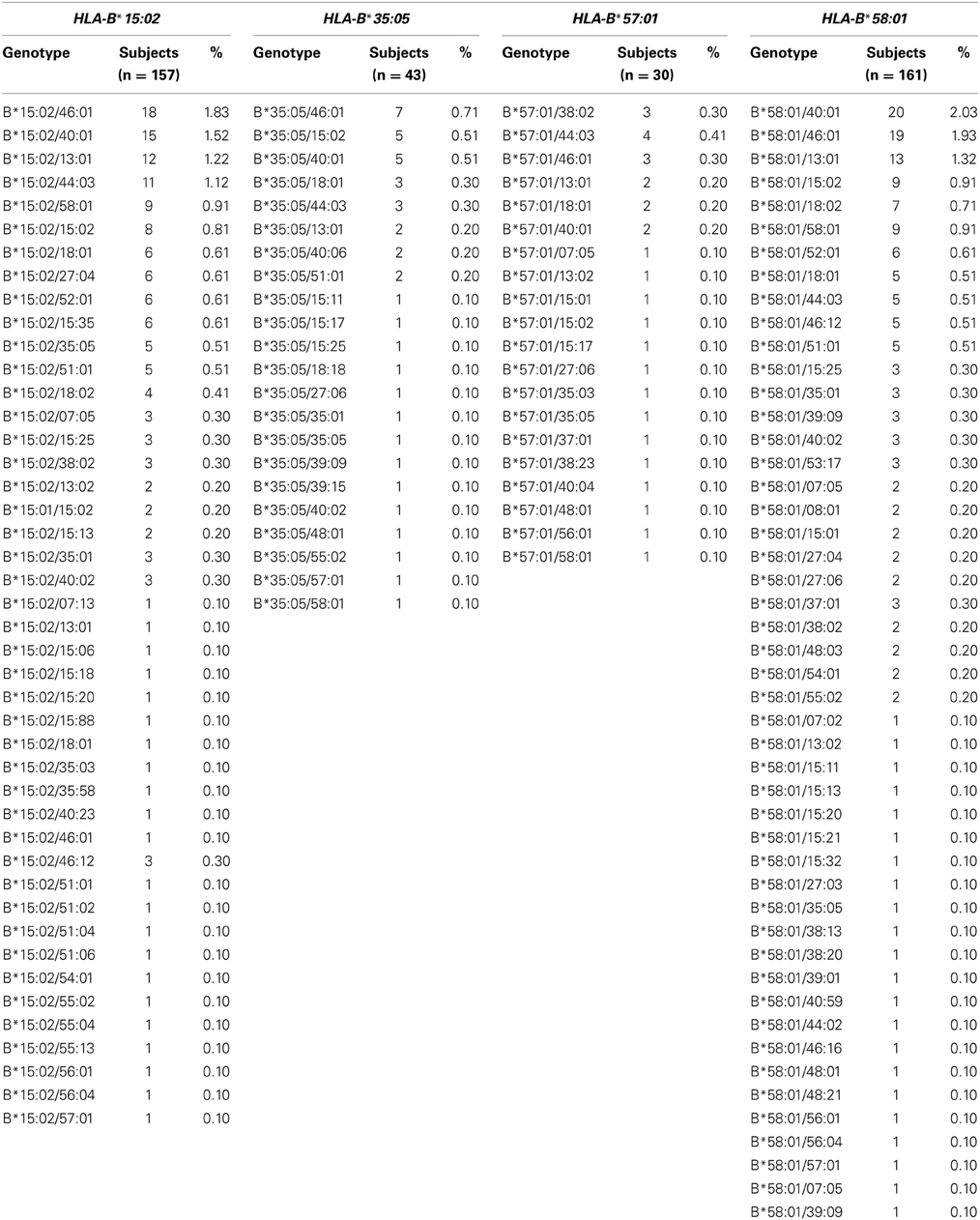
With regard to HLA-B pharmacogenomic markers in the Thai population, high frequencies of HLA-B* 58:01 (16.33%) were observed, followed by HLA-B*15:02 (15.92%), HLA-B* 35:05 (4.36%), and HLA-B*57:01 (3.04%). Table 3 shows the frequencies of the genotypes of HLA-B pharmacogenomic markers in this Thai population. Of the 986 subjects who were included in the study, 161 subjects (16.43%) were found to carry the HLA-B*58:01 allele. One hundred and fifty-two subjects carry the heterozygous HLA-B*58:01 genotype and 9 subjects carry the homozygous HLA-B*58:01 genotype. Among these, 56.02% were males and 43.98% were female.
HLA-B*15:02 is a genetic marker previously related to induction of SJS/TEN in carbamazepine treated patients. One hundred and fifty-seven subjects (15.92%) were found to carry HLA-B 15:02; 151 subjects (15.31%) were heterozygous for the HLA-B*15:02 allele and 8 subjects (0.81%) carry homozygous HLA-B*15:02. Males accounted for 60.23% and females 39.77%. In addition, HLA-B*15:02 belong to the HLA-B75 family, which consists of HLA-B*15:08, HLA-B*15:11, HLA-B*15:18 and HLA-B*15:21, and is associated with carbamazepine-induced SJS/TEN. Five subjects with HLA-B*15:11 heterozygous genotype and 3 subjects with HLA-B*15:18, HLA-B*15:21 heterozygous genotype were found, whereas HLA-B*15:08 was not found in either heterozygous and homozygous genotypes in this population.
Likewise, the HLA-B*3505 allele was associated with nevirapine-induced hypersensitivity reactions in HIV-infected Thai patients. Forty-three subjects (4.36%) carried the HLA-B*35:05 allele, one subject and 42 subjects (4.26%) carry homozygous and heterozygous HLA-B*35:05 genotypes, respectively, Thirty subjects (3.04%) carry heterozygous HLA-B*57:01, which is known to be associated with abacavir-induced hypersensitivity syndrome (AHS). No homozygous HLA-B*57:01 genotype was identified in this study.
Discussion
The HLA-B allele frequencies of 968 Thai subjects were investigated. This is the first report of HLA-B genotyping in the Thai population using the Luminex HLA-SSOP method. Our data showed that the Thai population has extensive diversity at the HLA-B locus. One hundred and sixteen allele types were identified and the frequencies of HLA-B alleles did not significantly deviate from Hardy-Weinberg equilibrium. The top five alleles with frequencies over 5% include HLA-B*46:01 (11.51%), HLA-B*58:01 (8.62%), HLA-B*40:01 (8.22%), HLA-B*15:02 (8.16%), and HLA-B*13:01 (6.95%). These frequencies were similar to those observed in the Singaporean Chinese: HLA-B*40:01 (17.2%), HLA-B*46:01 (13.2%), HLA-B*58:01 (10.4%), HLA-B*13:01 (8.40%), HLA-B*15:02 (5.70%) and Hong Kong Chinese: HLA-B*46:01 (16.3%), HLA-B*40:01 (15.2%), HLA-B*58:01 (7.30%), HLA-B*15:02 (10.2%) and HLA-B*13:01 (7.80%) (Williams et al., 2001; Middleton et al., 2004). These data differed, however, from the Jehai population in Malaysia nearest the south of Thailand: HLA-B*46:01 (2%), HLA-B*58:01 (6%), HLA-B*40:01 (2%), HLA-B*15:02 (2%), HLA-B*13:01 (8%) (Jinam et al., 2010; Kupatawintu et al., 2010; Romphruk et al., 2010). This may indicate that Thais have a closer relationship with their Chinese neighbors than with Malaysia. Previous studies of HLA alleles in the Thai population had larger subject numbers, but reported no pharmacogenomic data, which we present in the current study. HLA-B*15:02 allele is found in high prevalence among people in South-East Asian countries. The frequency of the HLA-B allele varies among different populations suggesting that different alleles may also function in drug hypersensitivity. Currently, there has been an increase of publications associated with HLA-B alleles and drug hypersensitivity (Sukasem et al., 2014a).
In our study, 15.92% (n = 157) of Thai subjects carry HLA-B*15:02 genotype, which has been associated with severe adverse drug reaction in response to carbamazepine during epileptic treatment (Tassaneeyakul et al., 2010; Kulkantrakorn et al., 2012; Tangamornsuksan et al., 2013) or other aromatic amine anticonvulsants such as oxcarbazepine, phenytoin, and lamotrigine (Man et al., 2007; Hung et al., 2010; Koomdee et al., 2014). Further, the serotype HLA-B75 family, such as HLA-B*15:08, HLA-B*15:11 (0.25%, n = 5), HLA-B*15:18 (0.15%, n = 3) and HLA-B*15:21 (0.15%, n = 3), is associated with carbamazepine-induced SJS/TEN (Daly and Day, 2009; Lin et al., 2009). In this study, 168 subjects (17.03%) carrying members of serotype HLA-B75 family alleles may be susceptible to carbamazepine-induced SJS/TEN.
The genetic predisposition to nevirapine and abacavir-induced hypersensitivity reaction (HSR) has been reported for HLA-B*35:05 and HLA-B*57:01 alleles, respectively (Mallal et al., 2008; Chantarangsu et al., 2009, 2011). The data showed that 4.36% of Thai people carry the HLA-B*35:05 genotype and 3.04% carry the HLA-B*57:01 genotype (Chantarangsu et al., 2009). Furthermore, HLA allotypes closely related to abacavir response are HLA-B*57:03, HLA-B*57:02, and HLA-B*58:01 (Illing et al., 2012). Consequently, 23.73% of Thai people might be at risk of drug hypersensitivity from nevirapine or abacavir treatment.
In the present study, we found that 16.33% of Thai people carry the HLA-B*58:01 allele, which is associated with allopurinol hypersensitivity in gout treatment (Tassaneeyakul et al., 2009; Saokaew et al., 2014). Beside drug hypersensitivity, other studies of HLA-B alleles, such as HLA-B*46:01, report a significant increase in severity of cerebral malaria compared with patients with mild CNS symptoms (Hirayama et al., 1998). Moreover, HLA-B*40:01 is associated with lipodystrophy in Thai HIV-1-positive patients who received antiretroviral therapy containg stavudine (Wangsomboonsiri et al., 2010). HLA-B*13:01 has been suggested as a genetic marker for dermal hypersensitivity associated with Trichloroethylene, an industrial solvent (Li et al., 2007).
Our findings demonstrated the diversity of HLA-B alleles in the Thai population, and many more HLA associated with ADR. Other classes of HLA may be observed in upcoming studies. The subjects enrolled in the study were mostly from central Thailand. This narrow sample may restrict our calculations of HLA-B allele distribution in Thai populations. Future studies should consider this limitation of our survey.
HLA-associated drug hypersensitivity is dependent upon the different populations studied, and variation in genetic backgrounds. Recently, HLA-B genotyping has become accessible in clinical practice, providing appropriate clinical monitoring, patient counseling and recommendations for treatment. Interestingly, “pharmacogenetic tests” and “pharmacogenomic cards” (Figure 1) have been successfully implemented in clinical practice in Thailand at the Laboratory for Pharmacogenomics, Somdech Phra Debaratana Medical Center, Ramathibodi Hospital (Sukasem et al., 2014a). Recently, HLA-B*15:02 screening has been preliminarily introduced by the National Health Security Office (NHSO), Thailand, to screen patients who may be at risk of carbamazepine-induced SJS/TEN.
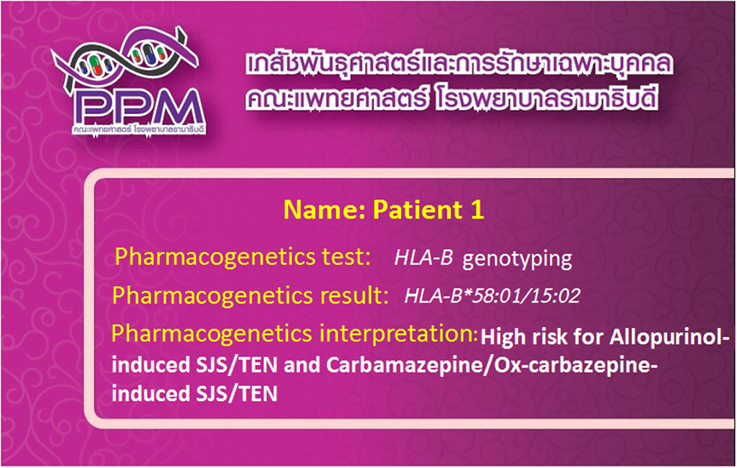
Figure 1. “Pharmacogenomics cards” have been successfully implemented in clinical practice in Thailand. The patients are tested for the HLA-B alleles which are associated with the ADRs related with the use of concerned drugs.
In conclusion, our data suggest that HLA-B genotype should be screened before medication is prescribed to reduce the incidence of drug hypersensitivity. These findings provided useful information in the study of pharmacogenomics and HLA-B polymorphisms in the Thai population. The frequency of the HLA-B alleles associated with drug hypersensitivity will help in designing alternative treatment regimens or better therapy for affected individuals. Understanding of the mechanism underlying drug hypersensitivity reactions requires further investigation.
Conflict of Interest Statement
The reviewer Wichittra Tassaneeyakul declares that, despite having collaborated with authors Apichaya Puangpetch and Chonlaphat Sukasem, the review process was handled objectively and no conflict of interest exists. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Acknowledgments
This study was supported by grants from the (1) Khun Poom Foundation, The Project of Her Royal Highness Princess Ubonratana Rajakanya Siriwatana Bhanawadee, (2) Pharmacogenomics for Autistic Children, Office of National Research Council of Thailand (3) Faculty of Medicine, Ramathibodi Hospital, Mahidol University (4) Pharmacogenomics Project under Thailand Center of Excellence in Life Sciences.
References
Alper, C. A., Larsen, C. E., Dubey, D. P., Awdeh, Z. L., Fici, D. A., and Yunis, E. J. (2006). The haplotype structure of the human major histocompatibility complex. Hum. Immunol. 67, 73–84. doi: 10.1016/j.humimm.2005.11.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Avila-Rios, S., Ormsby, C. E., Carlson, J. M., Valenzuela-Ponce, H., Blanco-Heredia, J., Garrido-Rodriguez, D., et al. (2009). Unique features of HLA-mediated HIV evolution in a Mexican cohort: a comparative study. Retrovirology 6:72. doi: 10.1186/1742-4690-6-72
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chantarangsu, S., Mushiroda, T., Mahasirimongkol, S., Kiertiburanakul, S., Sungkanuparph, S., Manosuthi, W., et al. (2009). HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet. Genomics 19, 139–146. doi: 10.1097/FPC.0b013e32831d0faf
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chantarangsu, S., Mushiroda, T., Mahasirimongkol, S., Kiertiburanakul, S., Sungkanuparph, S., Manosuthi, W., et al. (2011). Genome-wide association study identifies variations in 6p21.3 associated with nevirapine-induced rash. Clin. Infect. Dis. 53, 341–348. doi: 10.1093/cid/cir403
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, P., Lin, J. J., Lu, C. S., Ong, C. T., Hsieh, P. F., Yang, C. C., et al. (2011). Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N. Engl. J. Med. 364, 1126–1133. doi: 10.1056/NEJMoa1009717
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Daly, A. K., and Day, C. P. (2009). Genetic association studies in drug-induced liver injury. Semin. Liver Dis. 29, 400–411. doi: 10.1055/s-0029-1240009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dawson, D. V., Ozgur, M., Sari, K., Ghanayem, M., and Kostyu, D. D. (2001). Ramifications of HLA class I polymorphism and population genetics for vaccine development. Genet. Epidemiol. 20, 87–106. doi: 10.1002/1098-2272(200101)20:1<87::AID-GEPI8>3.0.CO;2-R
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dhaliwal, J. S., Too, C. L., Lisut, M., Lee, Y. Y., and Murad, S. (2003). HLA-B27 polymorphism in the Malays. Tissue Antigens 62, 330–332. doi: 10.1034/j.1399-0039.2003.00107.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hirayama, K., Kikuchi, M., Oumaporn, T., Yupaporn, W., Na-Bangchang, K., Karbwang, J., et al. (1998). HLA-B*4601 Increased in the adult patients with severe malaria at mae sod hospital in Thailand. Tokai J. Exp. Clin. Med. 23, 81–83.
Hung, S. I., Chung, W. H., Liou, L. B., Chu, C. C., Lin, M., Huang, H. P., et al. (2005). HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. U.S.A. 102, 4134–4139. doi: 10.1073/pnas.0409500102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hung, S. I., Chung, W. H., Liu, Z. S., Chen, C. H., Hsih, M. S., Hui, R. C., et al. (2010). Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 11, 349–356. doi: 10.2217/pgs.09.162
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Illing, P. T., Vivian, J. P., Dudek, N. L., Kostenko, L., Chen, Z., Bharadwaj, M., et al. (2012). Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 486, 554–558. doi: 10.1038/nature11147
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jantararoungtong, T., Rerkpattanapipat, T., Prommas, S., Koomdee, N., Santon, S., Montri, C., et al. (2014). HLA-B*58:01 allele is strongly associated with allopurinol–induced severe cutaneous adverse reactions in a Thai population. Clin. Transl. Allergy 4(Suppl. 3), P120. doi: 10.1186/2045-7022-4-S3-P120
Jinam, T. A., Saitou, N., Edo, J., Mahmood, A., and Phipps, M. E. (2010). Molecular analysis of HLA Class I and Class II genes in four indigenous Malaysian populations. Tissue Antigens 75, 151–158. doi: 10.1111/j.1399-0039.2009.01417.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Koomdee, N., Jantararoungtong, T., Rerkpattanapipat, T., Prommas, S., Santon, S., Chamnanphol, M., et al. (2014). HLA-B*15:02 genotype associated with hypersensitivity syndrome to Lamotrigine in Thai population. Clin. Transl. Allergy 4(Suppl. 3), P121. doi: 10.1186/2045-7022-4-S3-P121
Kulkantrakorn, K., Tassaneeyakul, W., Tiamkao, S., Jantararoungtong, T., Prabmechai, N., et al. (2012). HLA-B*1502 strongly predicts carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Thai patients with neuropathic pain. Pain Pract. 12, 202–208. doi: 10.1111/j.1533-2500.2011.00479.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kupatawintu, P., Pheancharoen, S., Srisuddee, A., Tanaka, H., Tadokoro, K., and Nathalang, O. (2010). HLA-A, -B, -DR haplotype frequencies in the Thai Stem Cell Donor Registry. Tissue Antigens 75, 730–736. doi: 10.1111/j.1399-0039.2010.01450.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, H., Dai, Y., Huang, H., Li, L., Leng, S., Cheng, J., et al. (2007). HLA-B*1301 as a biomarker for genetic susceptibility to hypersensitivity dermatitis induced by trichloroethylene among workers in China. Environ. Health Perspect. 115, 1553–1556. doi: 10.1289/ehp.10325
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lin, L. C., Lai, P. C., Yang, S. F., and Yang, R. C. (2009). Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. Kaohsiung J. Med. Sci. 25, 82–86. doi: 10.1016/S1607-551X(09)70045-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mallal, S., Phillips, E., Carosi, G., Molina, J. M., Workman, C., Tomazic, J., et al. (2008). HLA-B*5701 screening for hypersensitivity to abacavir. N.Engl. J. Med. 358, 568–579. doi: 10.1056/NEJMoa0706135
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Man, C. B., Kwan, P., Baum, L., Yu, E., Lau, K. M., Cheng, A. S., et al. (2007). Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 48, 1015–1018. doi: 10.1111/j.1528-1167.2007.01022.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Middleton, D., Hawkins, B. R., Williams, F., Meenagh, A., Moscoso, J., Zamora, J., et al. (2004). HLA class I allele distribution of a Hong Kong Chinese population based on high-resolution PCR-SSOP typing. Tissue Antigens 63, 555–561. doi: 10.1111/j.0001-2815.2004.00234.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Romphruk, A. V., Romphruk, A., Kongmaroeng, C., Klumkrathok, K., Paupairoj, C., and Leelayuwat, C. (2010). HLA class I and II alleles and haplotypes in ethnic Northeast Thais. Tissue Antigens 75, 701–711. doi: 10.1111/j.1399-0039.2010.01448.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Saokaew, S., Tassaneeyakul, W., Maenthaisong, R., and Chaiyakunapruk, N. (2014). Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PLoS ONE 9:e94294. doi: 10.1371/journal.pone.0094294
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sukasem, C., Ngamsamut, N., Rerkpattanapipat, T., Sinrachatanant, A., Chamkrachchangpada, B., Tan-kam, T., et al. (2014b). Association between HLA-B*1502 allele and aromatic antiepileptic drugs-induced cutaneous adverse drug reactions and the HLA-B*15:02 pharmacogenetics screening in autistic spectrum disorder. Clin. Transl. Allergy 4(Suppl. 3), P124. doi: 10.1186/2045-7022-4-S3-P124
Sukasem, C., Puangpetch, A., Medhasi, S., and Tassaneeyakul, W. (2014a). Pharmacogenomics of drug-induced hypersensitivity reactions: challenges, opportunities and clinical implementation. Asian Pac. J. Allergy Immunol. 32, 111–123.
Tangamornsuksan, W., Chaiyakunapruk, N., Somkrua, R., Lohitnavy, M., and Tassaneeyakul, W. (2013). Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 149, 1025–1032. doi: 10.1001/jamadermatol.2013.4114
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tassaneeyakul, W., Jantararoungtong, T., Chen, P., Lin, P. Y., Tiamkao, S., Khunarkornsiri, U., et al. (2009). Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet. Genomics 19, 704–709. doi: 10.1097/FPC.0b013e328330a3b8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tassaneeyakul, W., Tiamkao, S., Jantararoungtong, T., Chen, P., Lin, S. Y., Chen, W. H., et al. (2010). Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 51, 926–930. doi: 10.1111/j.1528-1167.2010.02533.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wangsomboonsiri, W., Mahasirimongkol, S., Chantarangsu, S., Kiertiburanakul, S., Charoenyingwattana, A., Komindr, S., et al. (2010). Association between HLA-B*4001 and lipodystrophy among HIV-infected patients from Thailand who received a stavudine-containing antiretroviral regimen. Clin. Infect. Dis. 50, 597–604 doi: 10.1086/650003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Williams, F., Meenagh, A., Darke, C., Acosta, A., Daar, A. S., Gorodezky, C., et al. (2001). Analysis of the distribution of HLA-B alleles in populations from five continents. Hum. Immunol. 62, 645–650. doi: 10.1016/S0198-8859(01)00247-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: HLA-B, pharmacogenomic markers, Thai, PCR-SSOP, SCARs, drug hypersensitivity
Citation: Puangpetch A, Koomdee N, Chamnanphol M, Jantararoungtong T, Santon S, Prommas S, Hongkaew Y and Sukasem C (2015) HLA-B allele and haplotype diversity among Thai patients identified by PCR-SSOP: evidence for high risk of drug-induced hypersensitivity. Front. Genet. 5:478. doi: 10.3389/fgene.2014.00478
Received: 30 October 2014; Accepted: 30 December 2014;
Published online: 22 January 2015.
Edited by:
Marcelo Rizzatti Luizon, University of California, San Francisco, USAReviewed by:
Wichittra Tassaneeyakul, Khon Kaen University, ThailandMelih O. Babaoglu, Hacettepe University, Turkey
Copyright © 2015 Puangpetch, Koomdee, Chamnanphol, Jantararoungtong, Santon, Prommas, Hongkaew and Sukasem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chonlaphat Sukasem, Division of Pharmacogenomics and Personalized Medicine, Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Rama VI Road, Phaya Thai, Bangkok 10400, Thailand e-mail: chonlaphat.suk@mahidol.ac.th
 Apichaya Puangpetch
Apichaya Puangpetch Napatrupron Koomdee1,2
Napatrupron Koomdee1,2 Thawinee Jantararoungtong
Thawinee Jantararoungtong Chonlaphat Sukasem
Chonlaphat Sukasem