Landscape Evolution as a Diversification Driver in Freshwater Fishes
- 1Department of Geology, Federal University of Ouro Preto, Ouro Preto, Brazil
- 2Department of Earth and Environmental Sciences, Tulane University, New Orleans, LA, United States
- 3Department of Earth Sciences, Stanford University, Stanford, CA, United States
- 4Department of Biology, University of Louisiana at Lafayette, Lafayette, CA, United States
The exceptional concentration of vertebrate diversity in continental freshwaters has been termed the “freshwater fish paradox,” with > 15,000 fish species representing more than 20% of all vertebrate species compressed into tiny fractions of the Earth’s land surface area (<0.5%) or total aquatic habitat volume (<0.001%). This study asks if the fish species richness of the world’s river basins is explainable in terms of river captures using topographic metrics as proxies. The River Capture Hypothesis posits that drainage-network rearrangements have accelerated biotic diversification through their combined effects on dispersal, speciation, and extinction. Yet rates of river capture are poorly constrained at the basin scale worldwide. Here we assess correlations between fish species density (data for 14,953 obligate freshwater fish species) and basin-wide metrics of landscape evolution (data for 3,119 river basins), including: topography (elevation, average relief, slope, drainage area) and climate (average rainfall and air temperature). We assess the results in the context of both static landscapes (e.g., species-area and habitat heterogeneity relationships) and transient landscapes (e.g., river capture, tectonic activity, landscape disequilibrium). We also relax assumptions of functional neutrality of basins (tropical vs. extratropical, tectonically stable vs. active terrains). We found a disproportionate number of freshwater species in large, lowland river basins of tropical South America, Africa, and Southeast Asia, under predictable conditions of large geographic area, tropical climate, low topographic relief, and high habitat volume (i.e., high rainfall rates). However, our results show that these conditions are only necessary, but not fully sufficient, to explain the basins with the highest diversity. Basins with highest diversity are all located on tectonically stable regions, places where river capture is predicted to be most conducive to the formation of high fish species richness over evolutionary timescales. Our results are consistent with predictions of several landscape evolution models, including the River Capture Hypothesis, Mega Capture Hypothesis, and Intermediate Capture Rate Hypothesis, and support conclusions of numerical modeling studies indicating landscape transience as a mechanistic driver of net diversification in riverine and riparian organisms with widespread continental distributions.
Introduction
Freshwater or continental fishes constitute one of the most species rich—and species dense—vertebrate faunas on Earth (Tedesco et al., 2017a). With more than 18,167 species freshwater fishes constitute about 26% of all living vertebrate species (Fricke et al., 2021), yet occupy a tiny fraction (less than 0.01%) of Earth’s total surface area, and an even smaller proportion (0.001%) of Earth’s total aquatic habitat volume (Lundberg et al., 2000). Such a high concentration of biodiversity in continental freshwaters has been termed the “freshwater fish paradox” (Tedesco et al., 2017b; McDermott, 2021). Freshwater fishes also exhibit high values of other prominent biodiversity metrics, such as ecological and physiological diversity (Helfman et al., 2009), genetic diversity (Manel et al., 2020) and phylogenetic and taxonomic disparity (Li et al., 2020; Su et al., 2021).
Studies of freshwater fish diversity have focused on salient features of landscape heterogeneity and landscape evolution, focusing on rapid speciation in tectonic lakes (McGee et al., 2020), ancient diversification in the global Greenhouse world of the Paleogene and Upper Cretaceous (Miller and Román-Palacios, 2021), the dendritic habitat architecture of river drainage networks and riverine population structure (Fagan, 2002; Thomaz et al., 2016), and the role of river capture dynamics in fragmenting and merging riverine ecosystems through time and space (Burridge et al., 2006, 2007; Albert et al., 2018a,2020). From a macroevolutionary perspective, the rate of net lineage diversification is a function of the constituent rates of speciation and extinction, clade age, and depending on conditions, the carrying capacity or maximum species-density of a geographic region (Figure 1).
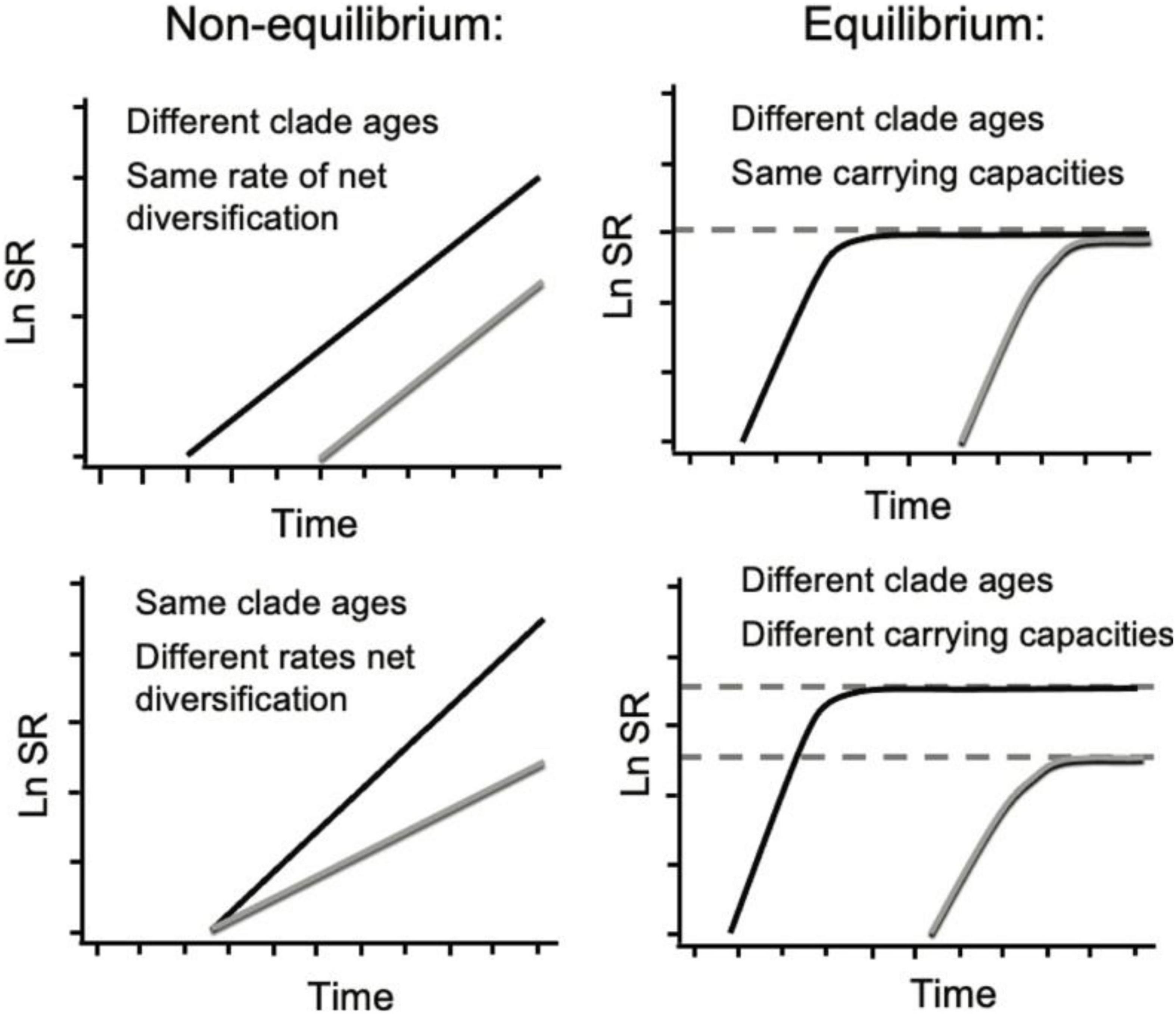
Figure 1. Species richness (SR) through time profiles for two clades (black and gray curves) under the influence of different macroevolutionary processes. Modified from Rabosky (2010). Left: Under exponential diversification, SR is a function of clade age (or time t, x-intercept top right panel) or net rate of diversification (slope bottom right panel): δ = λ–μ, where δ is net diversification rate, λ is speciation rate and μ is extinction rate. Right: Under density-dependent diversification (where μ→λ as SR → SRmax over time t), SR eventually becomes controlled by SRmax (depicted as dashed horizontal lines).
All evolutionary processes involve complex interactions among intrinsic organismal traits and extrinsic environmental factors (Jablonski, 2017; Saupe and Myers, 2021). Organismal traits associated with diversification in freshwater fishes include body size, feeding and locomotory specializations, habitat utilization, reproductive modes, and dispersal capacity (e.g., Davis et al., 2016; Kolmann et al., 2020; Burns, 2021). These functional traits may also affect the ecological processes that govern local species coexistence (alpha diversity) and regional species richness (beta diversity) (Winemiller, 1991; Matthews, 2012; Salgueiro et al., 2021). Yet the combined effects of these biological processes depend critically on physical environmental settings related to climate and geography, which alter the connections, persistence, and quality and configuration of freshwater habitats (Pringle, 2003; Smith et al., 2010; Dias et al., 2014). By altering the geographic range and/or configuration of landscape surfaces geomorphic processes may influence the rates of macroevolutionary diversification (Figure 1; see also Badgley et al., 2017).
The evolution of river drainage networks depends on landscape evolution processes that can be classified into one of two groups based on how they affect landscape equilibrium (Moodie et al., 2018): (1) Internal or autogenic processes (i.e., fluvial incision, aggradation, landsliding) occur continuously on all ice-free continental surfaces, transforming landscape structures like the geometry of fluvial networks and valley spacing toward erosional equilibrium (e.g., Perron et al., 2009; Willett et al., 2014; Scheingross et al., 2020). Such autogenic processes include differential exhumation of rocks with contrasting erodibility (e.g., Gallen, 2018), formation of stepped bedrock morphologies (e.g., Scheingross et al., 2019), and alluvial dynamics (e.g., Hajek and Straub, 2017); (2) external or exogenic perturbations, such as tectonism, precipitation change, and sea level change, modify the boundary conditions of underlying erosional processes, pushing landscapes away from erosional equilibrium (Densmore and Hovius, 2000; Crosby and Whipple, 2006; Kirby and Whipple, 2012; Whittaker, 2012). Erosion rates over landscapes increase with tectonic activity, but the latter possibly lowers the probability of river capture events in high-relief regions (Portenga and Bierman, 2011; Kirby and Whipple, 2012; Lyons et al., 2020).
Both autogenic and exogenic landscape evolution processes can drive changes in river basin base-level, defined as the lowest elevation or mouth of a drainage basin (Goudie, 2004). Base-level changes can promote disequilibrium in erosion rates that propagate upstream as a wave of migrating knickpoints, defined as a change in the channel slope which can be locally convex-up such as waterfalls and rapids, or concave-up such as a lake (Pazzaglia and Brandon, 1996; Tinkler, 2004). For the physical landscape, this imbalance is reflected as transient changes in topography and local relief (e.g., Gallen et al., 2013; Whipple et al., 2017). If the imbalance affects neighboring basins differently, they may create spatially heterogeneous relief across drainage divides (e.g., Gilbert, 1877; Forte and Whipple, 2018) and trigger discrete or continuous river network changes, such as river captures and geometric network disequilibrium, respectively (e.g., Willett et al., 2014; Beeson et al., 2017; Stokes et al., 2018).
River capture is a landscape evolution process in which topographic change alters the river networks and drainage areas of adjacent basins (Bishop, 1995). During this process a river network portion is disconnected from its original larger network and joined to the river network of the adjacent basin, all of which impact fluvial connectivity and resident biotas (Burridge et al., 2006). Over time the spatial configuration of river networks and the drainage area of basins can change as erosion and surface uplift alter topographic structure. A change in local boundary conditions such as tectonic uplift and climatic regime prompts surface processes, potentially including river capture, to adjust topography toward a new equilibrium until boundary conditions change again (e.g., Willett et al., 2014). A change in the spatial configuration of surface uplift or climate regime may accompany drainage area change if persistent at timescales longer than the time it takes for the landscape to respond to the new conditions (e.g., Whipple et al., 2017). While the topographic disequilibrium may eventually trigger river captures (i.e., Beeson et al., 2017; Whipple et al., 2017), it is the river capture process itself and its temporally discrete nature and magnitude that most likely affects the diversity profile of inhabiting aquatic species (e.g., Albert et al., 2017).
By altering the position of drainage divides, the size of basins, and habitat structure, river captures strongly affect the diversity and distribution of freshwater aquatic organisms (Burridge et al., 2006; Albert and Crampton, 2010; Albert et al., 2017). On the other hand, landscape stability (i.e., equilibrium between erosion and uplift rates and spatially homogeneous erosion rates) or the slow migration of drainage divides impedes discrete river network rearrangements, thus allowing the accumulation of frequent and small-scale background erosional processes operating over extensive time periods (Sieben et al., 2018). Importantly, under an ever-changing geological substrate, a plausible and common scenario in continental interiors, equilibrium landscapes and therefore fixed drainage basin sizes may rarely be attained (e.g., Forte et al., 2016), especially when exogenic and autogenic processes interact to form feedback loops (Scheingross et al., 2020). Moreover, river captures accompany base-level fall, which may trigger other river captures both upstream and downstream of the capture point (e.g., Willett et al., 2014; Whipple et al., 2017; Giachetta and Willett, 2018). Therefore, landscape dynamics is understood to have multiple cascading and complex effects on the evolutionary diversification of continentally distributed groups of plants and animals (Ward et al., 2002; Coblentz and Riitters, 2004; Hoorn et al., 2010; Badgley et al., 2014; Rahbek et al., 2019; Igea and Tanentzap, 2021; Roell et al., 2021).
In this paper we ask if the fish species richness of the world’s river basins is explainable in terms of river captures using topographic metrics as proxies. Specifically, we explore at what scale do landscape-species relationships emerge. Do the cumulative effects of smaller-scale river capture events (<10,000 km2) that drive large scale watershed migration fully explain patterns of fish biodiversity, or do we need to understand the influence of rarer and larger-scale processes, tectonically driven or otherwise, like mega-river capture events (> 10,000 km2; Albert et al., 2018a,2021)? We proceed from the expectation that basins with SR values close to that expected by regression against climatic or geographic variables (e.g., precipitation, topographic relief) are closer to species-equilibrium, reflecting feedback between rates of smaller-scale river capture events and rates of macroevolutionary processes (e.g., speciation and extinction). Contrariwise, basins with SR values far from these regressions are expected to be further from species-equilibrium, due to the historical effects of rare and large mega-river captures (Albert et al., 2018a).
We pursue this inquiry using correlations between species richness and topographic metrics of landscape structures known to be associated with landscape disequilibrium (e.g., Beeson et al., 2017; Sassolas-Serrayet et al., 2019), referred to in the field of biogeography as the River Capture Hypothesis (RCH; Albert et al., 2018b; Lyons et al., 2020). For this study, we employ a newly compiled dataset of fish species richness for most (> 3,000) of the river basins located on ice-free continents (Figures 2A,B). We assess the quantitative influences of multiple landscape (e.g., latitude, elevation, topographic relief, tectonic activity) and climatic (e.g., precipitation, temperature) variables on patterns of global freshwater fish diversity, adjusted for measures of habitat volume (e.g., basin area, river discharge). We find wide variation in biological responses among taxa and regions, presumably due to the effects of historically rare but geologically impactful events (e.g., tectonic uplifts) and other evolutionary contingencies (Losos et al., 1998). We also find that the spatial scale at which we look for the interaction between landscape evolution processes and biodiversity matters. For basins larger than 10,000 km2, we find relationships with topographic metrics that are consistent with the RCH, albeit at smaller river capture scales. Based on our results, we propose that the biodiversity profiles of freshwater fishes bear predictable mechanistic relationships with the rates and scales of river captures under different ecological conditions and in different geological settings.
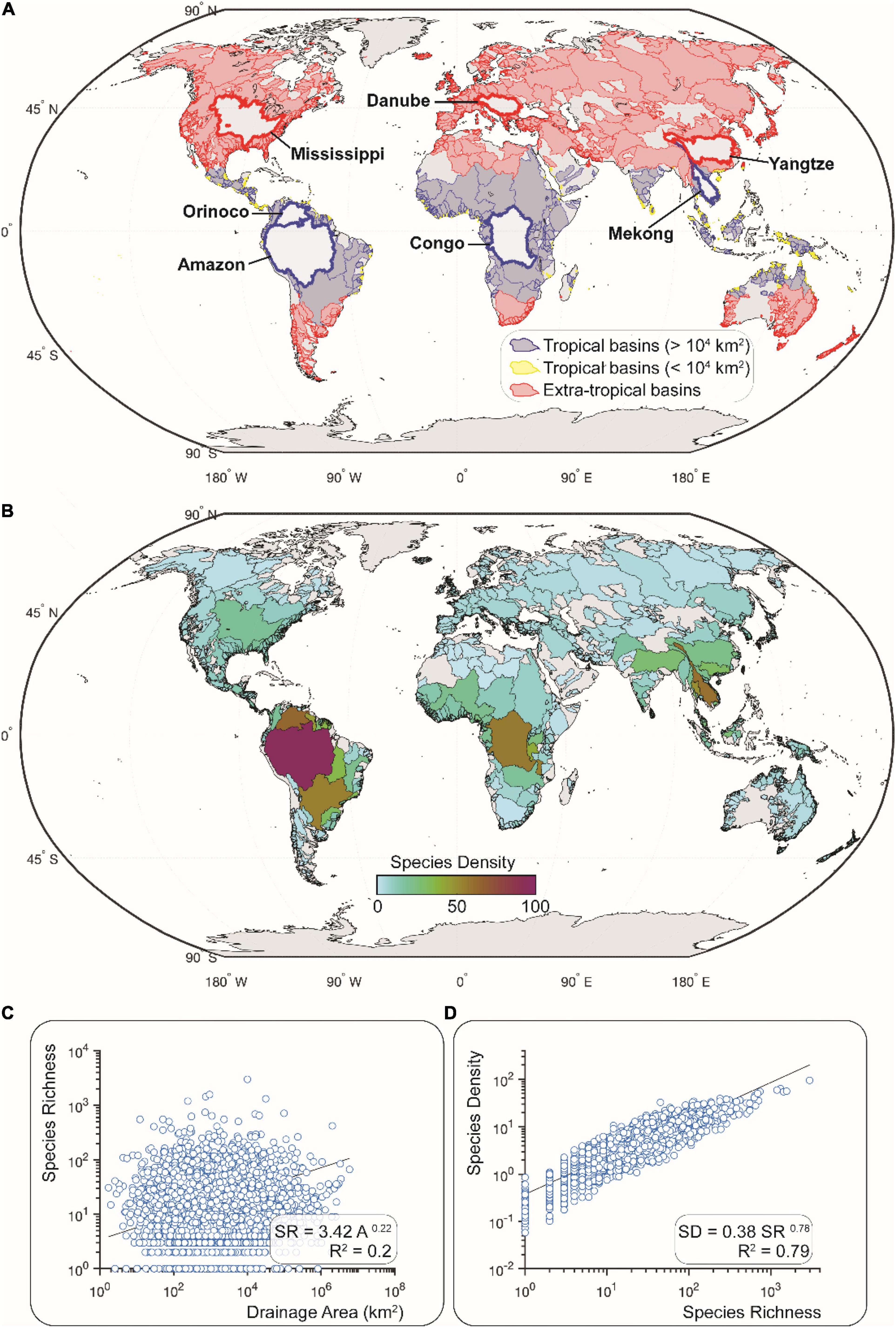
Figure 2. River basins of the world (HydroSHEDS of Lehner et al., 2008; shapefiles provided by Tedesco et al., 2017a). (A) Basins grouped as either tropical (blues; n = 1,058) or extra-tropical (reds; n = 2,061) based on centroid latitude less than or greater than 23 degrees, respectively. Tropical basins with blue and yellow outlines have a surface area greater and lower than 10,000 km2, respectively. Thicker outlines delineate large basins discussed in the text. (B) Species density (SD) values for all basins. SD calculated as SR/Az, where SR is species richness, A is area (km2), and z is the power-function exponent from a power-function regression of SR and A for the global dataset, not distinguishing between latitudinal, seismic, or elevation categories. (C) Species-Area Relationship for the global dataset used to produce the SD map in (B). (D) Relationship between SD and SR values for all 3,119 basins in this dataset (see section “Calculation of Species Richness and Density” for a description of this calculation).
Materials and Methods
Datasets
We used a global dataset of fish occurrence in discrete drainage basins to obtain species richness metrics (Leprieur et al., 2017; Tedesco et al., 2017a). These species richness values are point-estimates for fish “species inhabiting permanently or occasionally freshwater systems.” This dataset is subject to expected errors associated with biodiversity sampling and taxonomic knowledge. Given the size of the dataset, we do not expect these potential errors to bias the results.
Using shapefiles in Figure 2A we obtained basin-wide average topographic and climatic metrics (see Supplementary Dataset 1). For topography, we used the 90 m resolution, Shuttle Radar Topographic Mission (SRTM) digital elevation model downloaded from OpenTopography (Farr et al., 2007) to compute basin-wide average topographic metrics (see section “Basin-Wide Topographic Metrics”). For climatic metrics, we used the monthly mean, 30-year reanalysis dataset for the period of 1961–1990 with 0.5° spatial resolution (New et al., 2002) and the 29-year monthly mean air temperature data (UDel_AirT_Precip v4.01 product) for the period of 1981–2010 with 0.5° spatial resolution (Willmott and Matsuura, 2001) provided by the Physical Sciences Laboratory (National Oceanic and Atmospheric Administration, NOAA).
Given that tectonic activity impacts biodiversity, we created two data partitions such as tectonically active or tectonically stable, which we identified based on seismic activity. Seismic activity was identified through Peak Ground Acceleration (PGA) data obtained from the Global Earthquake Model (GEM) (Pagani et al., 2018). GEM uses several methods to obtain PGA, including compilation of hazard maps and reduction of seismic data. In this dataset, PGA is the 10% probability of exceeding the 50-year reference shear wave velocities (see Pagani et al., 2018). We use a threshold of 3.2 to distinguish between tectonically active vs. stable regions based on the observation that the Amazon basin contains an average PGA of 3.2 (no-data grid cells are not averaged) and is a tectonically stable region.
Calculation of Species Richness and Density
Tedesco et al. (2017a) provides a list of fish species occurring in freshwater for 3,119 basins globally out of which we were able to acquire topographic data for 3,038. For every basin in the dataset, we calculated species richness (SR) as the total number of valid fish species (Figures 2C,D). Species density (SD) was then calculated via a regression of SR on drainage area. The Species-Area Relationship (SAR) assumes the form: SR = Areaz (MacArthur and Wilson, 1966) and, therefore, SD was calculated as SR/Areaz, where the exponent z is obtained from the power-function regression of SR on Area (Rosenzweig, 2004; Albert et al., 2011). We then created data partitions based on seismic activity (active and stable) and latitude (tropics and extra-tropics) to assess differences in SAR based on geologic and climatic settings. Lastly, we focused on tropical regions as basins between 23.5 degrees latitude north and south, where most fish species live. We examined the effects of topography by separating basins into uplands and lowlands defined as median elevation above and below 500 m above sea level, respectively. We then assessed the importance of climatic and topographic predictor variables such as precipitation and relief, respectively, in individually predicting SD in the tropics. We create four sub-groups: (1) tectonically active highlands; (2) tectonically stable highlands; (3) tectonically active lowlands; (4) tectonically stable lowlands.
Basin-Wide Topographic Metrics
We used TopoToolbox to extract topographic metrics for each drainage basin (Schwanghart and Scherler, 2014). We obtained the average values for elevation above sea level as well as topographic relief. The latter was computed as the absolute range of topography over a 2,500 m moving window at every cell within a given basin.
Relief is a topographic metric that describes the local amplitude of topography. In tectonically active regions, relief scales directly with uplift rates and is often used as a proxy to identify relative differences in tectonic uplift (i.e., the rate of advective motion of rock) (e.g., Montgomery and Brandon, 2002; Kirby and Whipple, 2012). Relief also scales with a rock’s resistance to erosion; harder (or softer) rocks promote steeper (or gentler) rivers (e.g., Hack, 1973; Duvall et al., 2004; Gallen, 2018). Thus, actively uplifting/eroding regions with highly variable lithology promote complex transient evolution and topography (e.g., Forte et al., 2016). Importantly, landscapes that are actively changing due to some past perturbation (i.e., base-level fall, river capture, or tectonic uplift) will have a positive correlation between relief, river steepness, and erosion rates within a given basin and, therefore, the amount of sediments actively fluxed through rivers (Portenga and Bierman, 2011; Kirby and Whipple, 2012; Gallen et al., 2013). Based on a previous modeling study, the ratio of the magnitude of the perturbation to the initial landscape relief dictates the degree of drainage reorganization, which in turn affects the placement or removal of dispersal barriers for aquatic organisms (Lyons et al., 2020; Stokes and Perron, 2020). Thus, average basin relief is a good metric for overall topographic steepness of a river basin and for linking topographic responses to autogenic and exogenic forcings. Also, it has been directly assessed with riverine species evolution in modeling studies (e.g., Lyons et al., 2020). Nonetheless, we emphasize relief is the time-integrated outcome of climate, tectonics, and surface and groundwater processes, and not strictly the outcome of landscape transience.
Results
Distribution of Species Density Based on Tectonic Setting and Latitude
The SD values of freshwater fishes among river basins of the world vary systematically by seismic setting and latitude. We found a significant relationship (R2 = 0.79; p < 0.01) between species richness (SR) and species density (SD) among all river basins worldwide. We found a relatively weak although significant relationship (R2 = 0.29; p < 0.01) between SD and area among the largest basins (> 100,000 km2; n = 133), but not for the medium (> 10,000–100,000 km2; n = 504) or smaller basins (< 10,000 km2, n = 2,482).
For both tropical and extra-tropical regions we observe that tectonically stable settings have a higher baseline species density value (i.e., y-intercepts of the SAR) but a lower slope (Figures 3, 4). Moreover, the set of tropical basins exhibits a higher slope value than does the set of extra-tropical basins. Estimated this way, there is no significant relationship between fish species density and drainage area for all of the world’s freshwater basins, although there is a modest relationship between these variables among the 133 basins larger than 10,000 km2 (R2 = 0.12; p < 0.01).
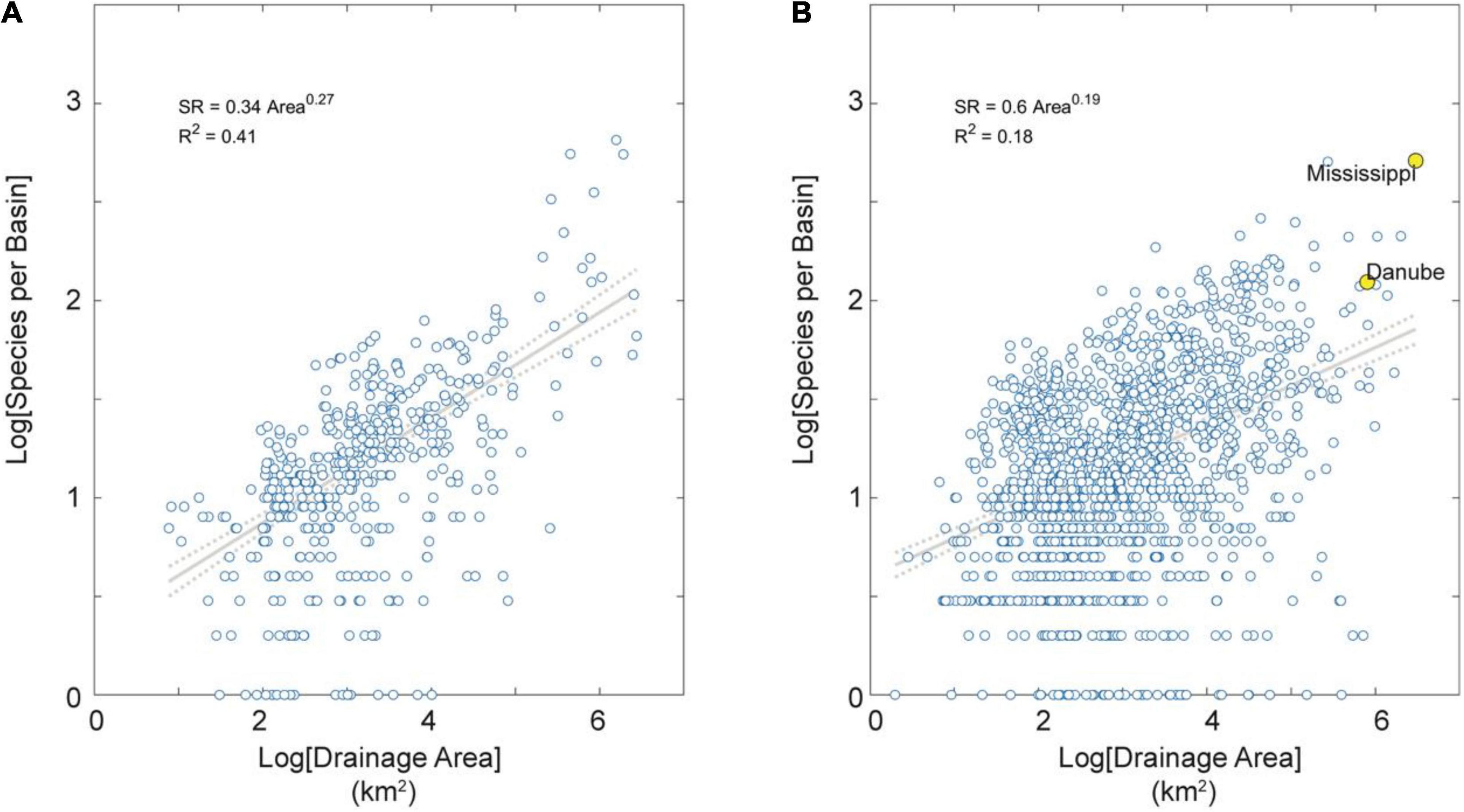
Figure 3. SAR for extra-tropical basins partitioned by tectonic activity. (A) Tectonically active basins in the dataset. (B) Tectonically stable basins in the dataset. Note these SARs extend over about six orders of magnitude in area and are all highly significant (p < 0.01). Note also these SARs have relatively low exponent values (z < 0.30), indicating relatively high shared species (and dispersal) among basins (Rosenzweig, 2004). Note further the lower exponent value in tectonically stable than active regions (z = 0.19 vs. 0.27, respectively) indicating on average more dispersal among basins of low-relief tectonically passive margins. Note finally in (B) the large and species-rich Mississippi and Danube river basins highlighted in yellow for reference.
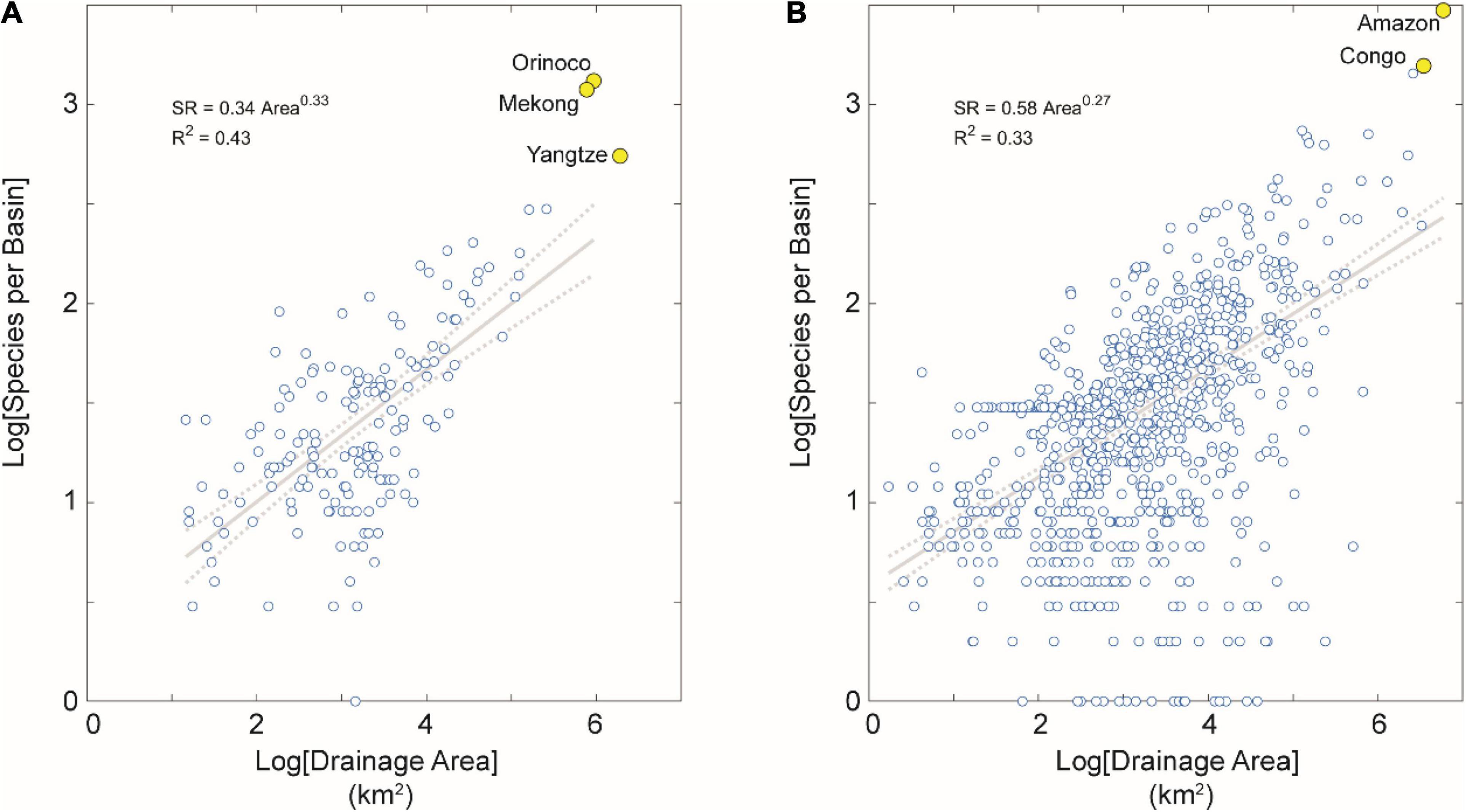
Figure 4. SAR for tropical basins partitioned by tectonic activity. (A) Tectonically active basins in the dataset. (B) Tectonically stable basins in the dataset. Note the higher correlation between areal extent and SR for tectonically stable basins in the tropics than in the extra-tropics, but no such difference in these correlations between tectonically active basins in the tropics than the extra-tropics. We interpret this result as due to the more heterogeneous geographical conditions and geological history of the many river basins in extra-tropical and tectonically stable regions contained in this dataset (n = 1,478 basins, or 47% of the total). Note also several large-area and species-rich river basins highlighted in yellow for reference, including the Amazon and Congo basins with highest fish SR on Earth.
The most species-dense drainage basins are substantial outliers in each of the geographic sets of basins by tectonic activity and latitude (Figures 3, 4). The statistical distributions of species density within each set reinforce the differences between tectonically stable and active regions (Figure 5). In all cases, the distributions are approximately log-normal, with the highest species densities with heavier tails in the tectonically stable group (Figures 5A,B). The average species density is higher in the tectonically stable regions compared to active areas irrespective of latitude (Figures 5C,D).
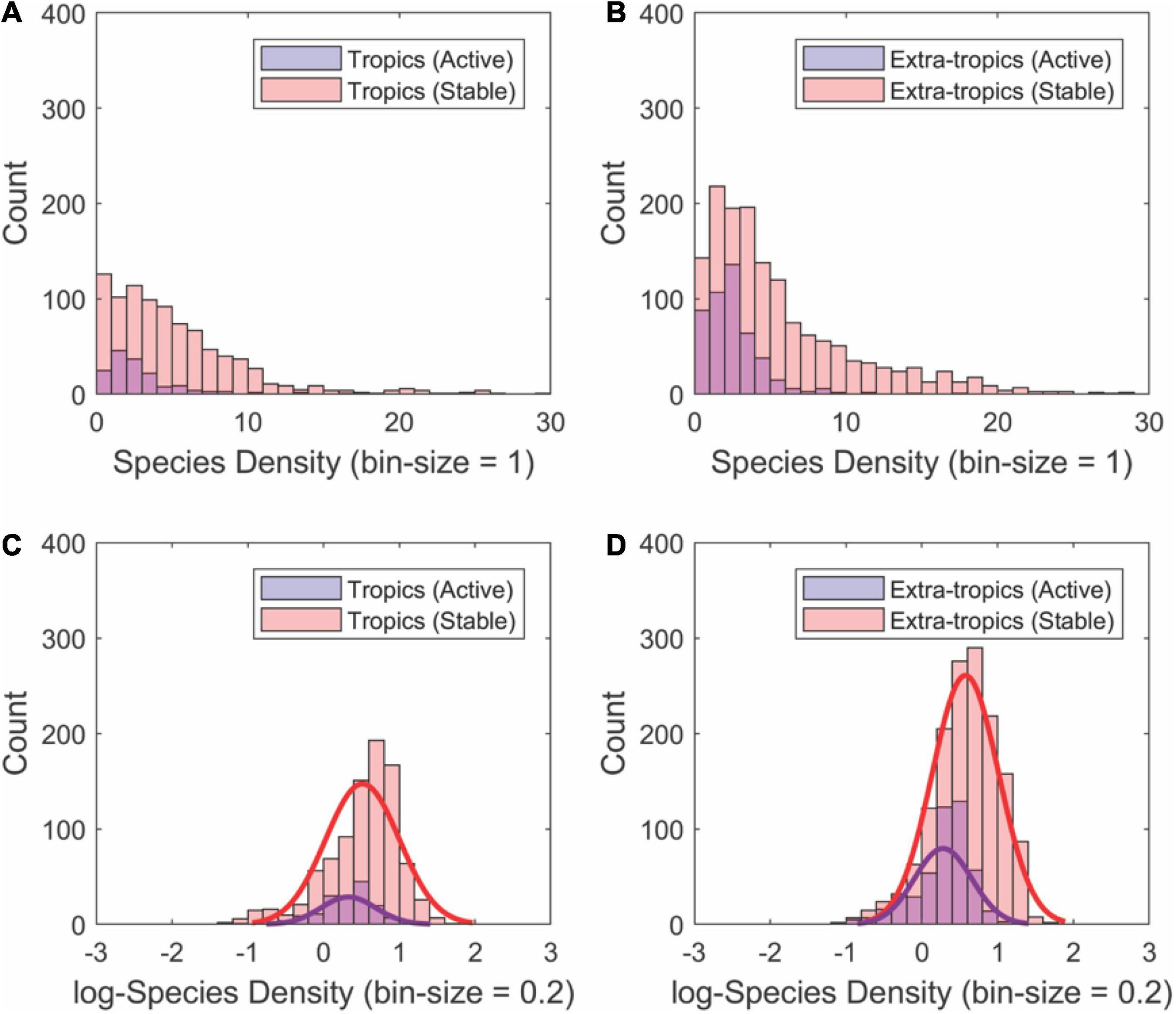
Figure 5. Histograms showing the approximately log-normal distributions of fish SD values for river basins (n = 3,038) partitioned by geographic region [tropical (A,C) and extra-tropical latitudes (B,D)] and tectonic activity (active vs. stable). Species density for each subgroup was calculated using the regressions shown in Figures 4, 5. In all cases, tectonically stable regions contain the higher mean species density as well as a greater amount of extremely high species density. Note the log-transforming these data distributions closely approximate normal distributions (see fits to bottom panels).
Species Richness in the Tropics—Upland vs. Lowland
Elevation (i.e., uplands and lowlands) is a well-known parameter controlling the distribution of aquatic species (Albert et al., 2018a). As expected, lowlands contain a 10-fold higher baseline species density (i.e., intercept of the SAR) but a lower slope. Basins affected by Neogene mega river captures (i.e., Amazon, Congo, Mekong) are exceptionally diverse and outliers in the SAR (Figures 2B, 6).
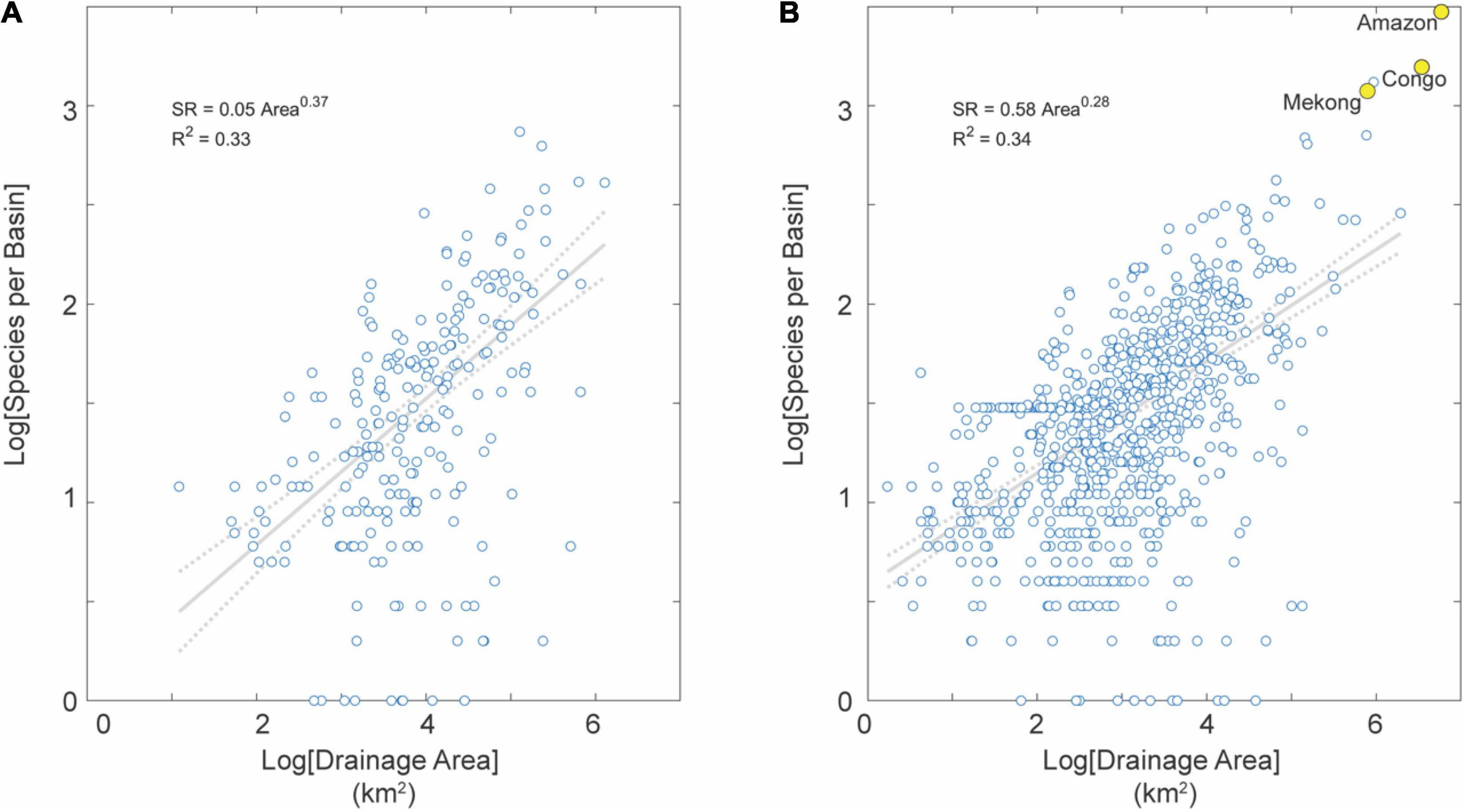
Figure 6. SAR for basins in uplands (A) and lowlands (B) in the tropics (not distinguished by tectonic activity). Note the much larger number of basins in lowlands than uplands, reflecting the fact that the Earth is (mostly) flat with most basins in this dataset (78%) characterized by low average topographic relief (Willenbring et al., 2013). Note also several large-area and species-rich river basins highlighted in yellow for reference, including the Amazon and Congo basins with highest fish SR on Earth.
Climatic and Topographic Drivers
In this study, we focus on river basins situated in the tropics from where a majority (66%) of freshwater fish species are known. Based on the observation that uplands and lowlands have differing relationships with topographic metrics depending on tectonic activity, we grouped basins based on drainage area, mean elevation, and tectonic activity and assessed four groups: tectonically active and stable lowlands and uplands.
Absent rainfall rates, we find no systematic relationships between species density and temperature, elevation, and relief when considering all basin sizes in each data subcategory. However, we find statistically significant correlations with these metrics for basins greater than 10,000 km2 which is an approximate threshold at which landscape evolution processes might impact biodiversity (Albert et al., 2018a,2021). Given this observation, we describe the following results for basins larger than this drainage area threshold.
Upland Basins
Of the four regressors used (rainfall rate, air temperature, average elevation, average relief), upland basins in tectonically active regions have statistically stronger relationships with topographic metrics than with climatic metrics (Figure 7). Tectonically active uplands have positive relationships with air temperature and negative relationships with elevation and relief (Figure 7). Of the climatic metrics, air temperature is a better predictor than rainfall rates and describes a positive relationship with species density (Figures 7A,B). Consistent with temperature gradients, basins at higher average elevations harbor lower species densities (Figures 7B,C). Similarly, basins with lower average relief are also more species dense (Figure 7D). Upland basins in tectonically stable regions do not have the same relationships as those in the tectonically active regions (Figure 8). Here, the most significant regressor for species density is the rainfall rate (R2 = 0.57, p < 0.01). We observe no relationship with air temperature, elevation, or relief (Figure 8).
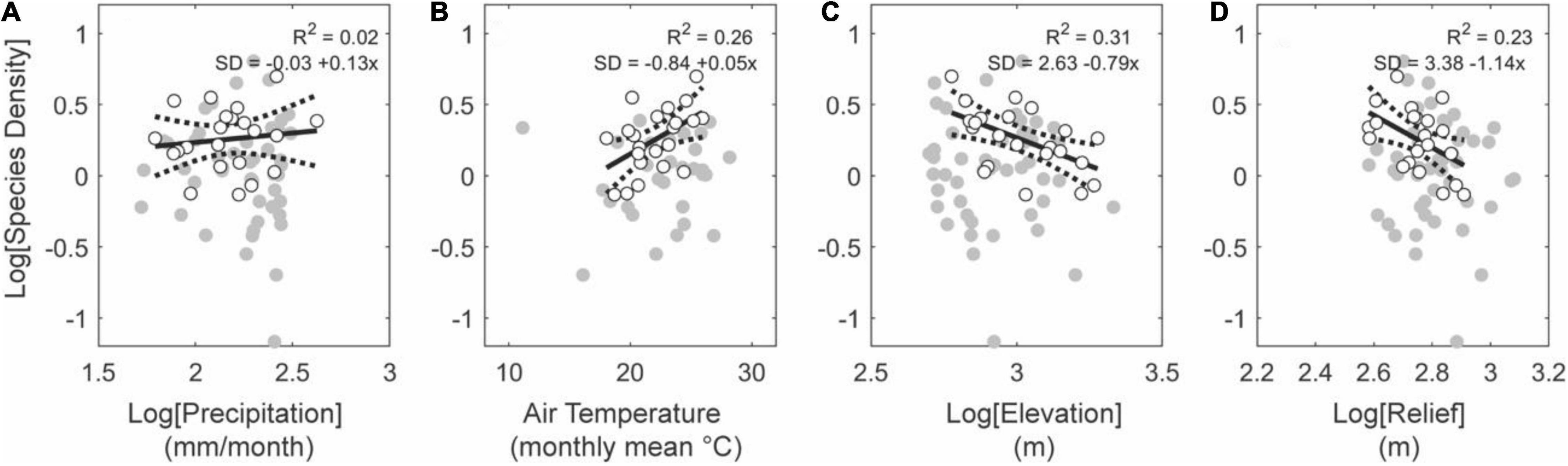
Figure 7. Effects of climate and topography on fish species density for upland basins (average elevation above 500 m) in active tectonic settings. Note monotonic positive relationships between precipitation (A) and temperature (B), and monotonic negative relationships with elevation (C) and relief (D). The relationships reported in (C,D) contrasts with biodiversity patterns observed in mammals, birds, and vascular plants, which exhibit a mid-elevation richness peak in most regions of the world (McCain and Grytnes, 2010). Gray circles show all basins falling in this subset (i.e., upland, tectonically active regions) from which basins larger than 10,000 km2 are shown with white circles.
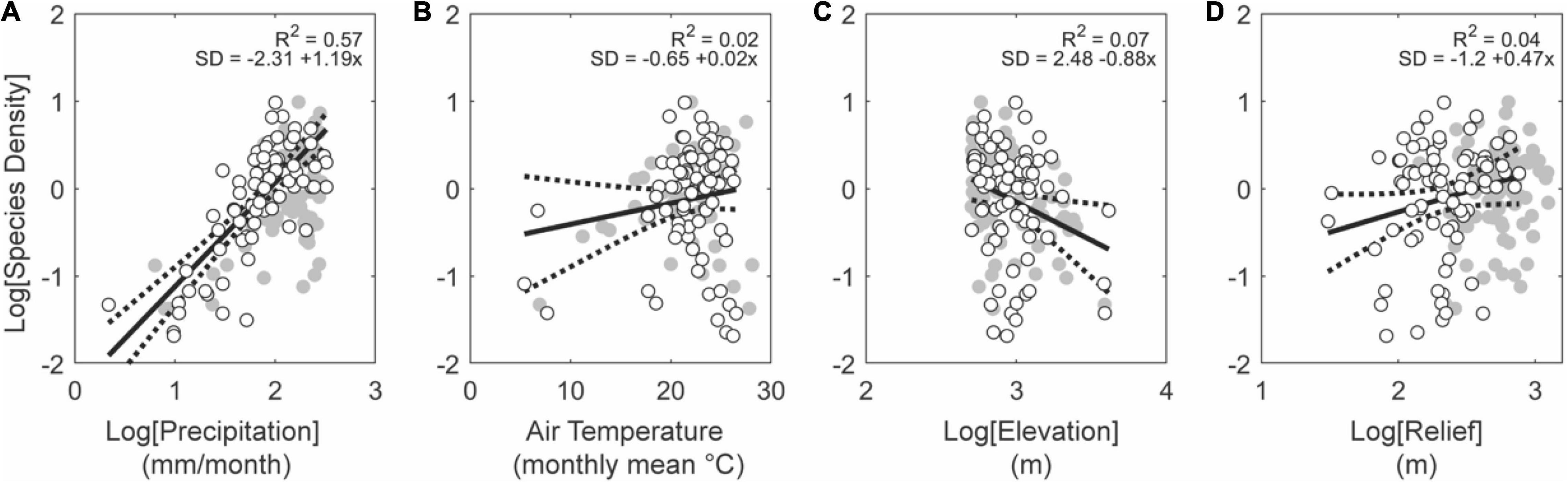
Figure 8. Effects of climate and topography on fish species density for upland basins (average elevation above 500 m) in stable tectonic settings. Note monotonic positive relationships between precipitation (A) and temperature (B), and relief (D), and monotonic negative relationships with elevation (C). Gray and white circles show all basins and white circles the basins larger than 10,000 km2, both within this subset of data.
Lowland Basins
We observe no relationship between the metrics assessed here and species density (Figure 9). There are very few basins in lowland regions that are also tectonically active and even fewer basins in this subgroup that are larger than 10,000 km2. Conversely, considering tectonically stable regions, basins in low elevations describe statistically significant relationships (Figure 10). Rainfall rate is the strongest predictor in this case (R2 = 0.52, p < 0.01; Figure 10A) while relief is half as significant (R2 = 0.26, p < 0.01; Figure 10D). Air temperature and elevation are insignificant in this subgroup (Figures 10B,C), which likely arise from the low range of average temperatures and elevations among tropical lowland basins.
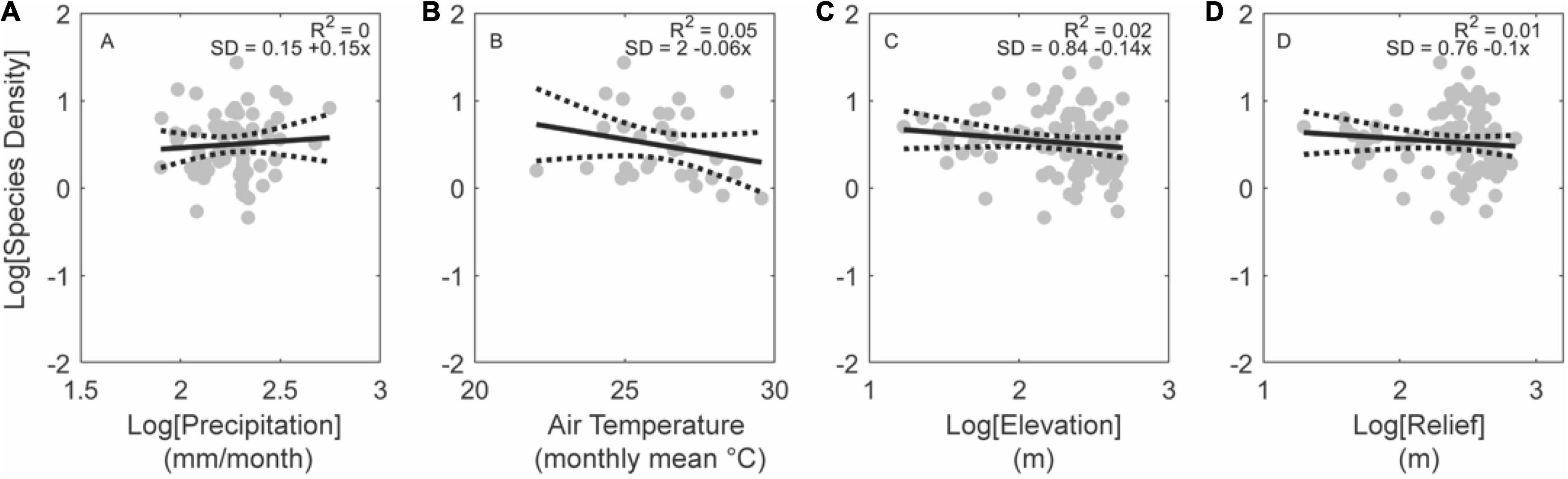
Figure 9. Effects of climate and topography on fish species density for low-elevation basins in active tectonic settings. Note the lack of a significant relationship with precipitation (A), temperature (B), elevation (C), or relief (D). Regressions plotted for basins larger than 10,000 km2 (gray circles; n = 92). Gray circles show all basins within this subset of data. No white circles are shown as very few basins in this subset are larger than 10,000 km2.

Figure 10. Effects of climate and topography on fish species density for basins low elevation in stable tectonic settings. Note significant positive relationships between fish species density with precipitation (A) and topographic relief (D), consistent with the hypotheses that total aquatic habitat volume (McGarvey and Terra, 2016), static riverscape heterogeneity (Thomaz et al., 2016), and dynamic riverscape landscape transience (Lyons et al., 2020; Stokes and Perron, 2020), promote net lineage diversification under these conditions. Gray and white circles show all basins and white circles the basins larger than 10,000 km2, both within this subset of data.
In summary, total precipitation (as a proxy for total aquatic habitat volume) is a strong predictor of fish SD in tectonically stable areas, irrespective of upland or lowland areas, but not in tectonically active regions. Neither temperature nor elevation are strong predictors of SD among large lowland tropical basins. Topographic relief is a strong predictor of SD among large lowland but not large upland topical basins. We suspect this is because the absolute relief values represent a much larger proportion of elevational differences in flat (low relief) lowlands.
Discussion
General Macroecological Expectations
The results of regressions of freshwater fish SD values against two climatic (i.e., precipitation and temperature) metrics among river basins of the world are consistent with general macroecological expectations of highest diversity in wetter, warmer regions (Worm and Tittensor, 2018). Among freshwater basins globally, a disproportionate number of fish species inhabit the lowlands of large tropical river basins in South America, Africa, and Southeast Asia, under predictable ecological conditions of large geographic area, warm, humid tropical climate, relatively flat topography (i.e., low average relief), and high habitat volume (i.e., high precipitation and run-off) (Lundberg et al., 2000; Oberdorff et al., 2011; McGarvey and Terra, 2016; Leprieur et al., 2017; Antonelli et al., 2018b). The six river basins with greatest fish SR values (i.e., Amazon, Congo, La Plata, Orinoco, Mekong, and Tocantins basins) have a combined total of more than 9,200 species in an area of 13.7 million km2, thus accounting for 51% of all freshwater fishes globally, in an area of just 14% of the 98.7 million km2 of all river basins on Earth combined.
Among both tropical and extra-tropical basins, the species-area exponent is higher for tectonically (seismically) active than stable regions (Figures 3, 4). This result suggests higher rates of dispersal among basins of low-relief tectonically passive margins, as indicated by previous studies showing higher SR in tectonically stable settings (Badgley et al., 2017; Griffiths, 2018). The higher correlation between area and SD for stable basins in tropics than extra-tropics indicates more heterogeneous geographical conditions and geological history of the many (n = 1,478 or 47% of the total) river basins in extra-tropical and tectonically stable regions (Figure 4).
The observed negative relationship between SD and average basin elevation among tectonically active uplands basins is consistent with previous observations of species occurrence along elevation gradients in the Amazon (Lujan et al., 2013), and across the South and North American continents (Smith et al., 2010Griffiths, 2018). Relationships of SD with air temperature and precipitation in these regions (Figures 7A,B) are consistent with contributing roles of both contemporary ecological and historical (time-integrated) macroevolutionary effects driving down aquatic species diversity at higher elevations (e.g., Lujan et al., 2013; Hazzi et al., 2018). Similarly, the history of topographic growth, as opposed to present-day topographic or climatic conditions, is thought to be more important to the evolution and enrichment of many terrestrial (Castroviejo-Fisher et al., 2014; Antonelli et al., 2018a; Azevedo et al., 2020; Réjaud et al., 2020; Igea and Tanentzap, 2021) and aquatic (Smith et al., 2010; Badgley et al., 2017) vertebrate faunas.
General Macroevolutionary Expectations
Macroevolutionary theory predicts that regional SR values arise from interactions among three fundamental parameters: rate of net diversification (δ), time of net diversification (t), and regional carrying capacity (Smax) (Rabosky, 2010). The per-species net diversification rate (δ) is: δ = λ + d—μ, where per-species speciation (λ) and dispersal (d) rates add species, and per-species extinction rate (μ) removes species from a region. When diversity is unbounded (i.e., δ is independent of Smax), δ and t provide limits to diversity; i.e., δ = δ0, where δ0 is the intrinsic diversification rate. Under these non-equilibrium conditions, the number of species at time t is: SRt = eδ0⋅t (Cornell, 2013). Such a model applies when speciation and dispersal rates are low relative to total available niche space and/or geological age of a region, or when μ > λ + d for sufficient time that a diversity limit (i.e., SRmax) is not approached. When diversity is bounded, SRt depends on SRmax and t; i.e., δ = δ0—aSR, where a is the strength of diversity-dependent feedback on δ, the carrying capacity (Smax = δ0/a) depends on the time-integrated δ or δt = ∫ [λ(t) + d(t) – μ(t)] dt, and expected equilibrium species richness is: SRt = eδ t (Rabosky, 2013; Cornell and Harrison, 2014; Harmon and Harrison, 2015; Rabosky and Hurlbert, 2015).
Under this theory, the smaller difference in correlation values between tectonically active basins (5%) among tropical vs. extra-tropical (9%) basins is unexpected (Stanley, 2014; Albert et al., 2017), because there are many more tectonically stable basins (Figure 4). This result is partly due to the much wider range of SD values among large basins on stable terrains, in particular from the many large basins from cold boreal regions (e.g., northern Canada and Russia) and arid tropical regions (e.g., northern Africa, central Australia) with low SD values (Figure 1 lower panels). There are fewer counterparts of these large low SD basins on active terrains.
Both macroecological and macroevolutionary models predict higher SD values in the dendritic geometry of river drainage networks (Fagan, 2002; Thomaz et al., 2016). River networks are a more spatially fragmented substrate than an equivalent Euclidean landscape with the same surface area (Rodriguez-Iturbe and Rinaldo, 2001; Dias et al., 2013). The hierarchical-branching of drainage networks is a more effective geometry for breaking up a geographically widespread species into daughter species (Wiens, 2002; Muneepeerakul et al., 2007; Tonkin et al., 2018). However, even more effective than a 3D dendritic surface for fragmenting and merging populations is a 4D dendritic surface changing in time; i.e., river capture (Albert and Crampton, 2010; Albert et al., 2017, 2018a).
The River Capture Hypothesis
The results of this study are consistent with several predictions particular of the RCH (Figure 11; Albert et al., 2018a). By merging geographic areas (geodispersal), river capture facilitates organismal dispersal and gene flow, and therefore acts to slow rates of speciation and extinction, i.e., lower species turnover (Albert and Crampton, 2010). However, by subdividing areas (vicariance) river capture also acts to increase rates of speciation and extinction. The results of this study suggest that genial ecological conditions are necessary, but not fully sufficient, to explain the basins with the highest diversity, consistent with the prediction of the RCH that dispersal across the watershed margins of adjacent lowland basins increases basin-wide SR and SD values. These increases occur both by adding individuals of different species, and adding individuals of existing species, thereby lowering the within-basin extinction rate (i.e., rescue effect; Brown and Kodric-Brown, 1977).
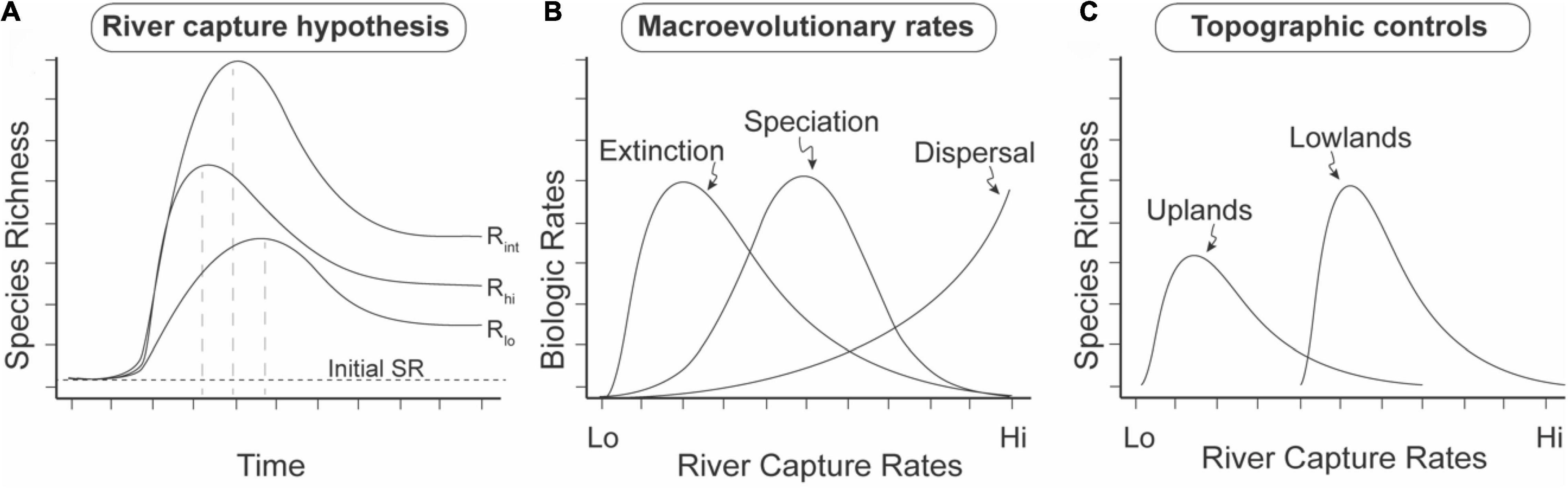
Figure 11. Salient predictions of the River Capture Hypothesis (RCH) on macroevolutionary diversification in riverine and riparian taxa. Predictions from empirical (Albert et al., 2018a) and landscape modeling studies (Lyons et al., 2020; Stokes and Perron, 2020). SR = Species Richness. River capture rate is measured as the number of river capture events per unit time interval per unit area. (A) Intermediate river-capture rate hypothesis (IRH). Prediction 1: Transient SRmax values (dashed vertical lines), with subsequent decay to a higher post-capture than initial SR baseline. Prediction 2: Highest SR values at intermediate rates of river capture. (B) Macroevolutionary rates. Prediction 3: Relative order of modal rate values in lowland, stable regions: extinction < speciation < dispersal. Rates integrated over the whole time interval of (A). (C) Topographic controls. Prediction 4. Higher modal rates of river capture on low-relief, lowland landscapes, than on high-relief, upland landscapes based on landscape evolution modeling (Lyons et al., 2020). Prediction 5. Higher equilibrium SR on low-relief lowland landscapes. Log-normal river capture curves follow data from Goldberg et al. (2021).
Dispersal of freshwater fishes among adjacent river basins may occur by multiple abiotic and biotic mechanisms (e.g., Tagliacollo et al., 2017). In some cases, the effects of geodispersal by river capture and biotic dispersal by organismal movements can be difficult to separate, for examples in seasonally flooded wetlands that straddle low-elevation drainage divides; e.g., Rupununi and Izozog swamps at the margins of the Amazon and adjacent basins which are sites of longer-term river captures and seasonal dispersal (Albert et al., 2011). In other cases, riverine corridors serve as ecological filters in which organismal trait values (adult body size, habitat utilization, tropic specializations) influence the species richness and composition of the biotic interchanges due to river capture; e.g., Casiquiare River.
The Mega Capture Hypothesis
The Mega Capture Hypothesis (MCH) predicts that large river captures (> 10,000 km2) leave a disproportionately enhanced signature on the accumulation of basin-wide SR values in riverine and riparian taxa (Tagliacollo et al., 2015; Albert et al., 2018a,2021). The basins with highest fish SD values (i.e., Amazon, Orinoco, La Plata, Congo, Mekong) have all been exposed to the effects of mega river captures within the past 20 Ma, which merged portions of the whole biotas of riverine and riparian taxa among adjacent basins (e.g., Bragança and Costa, 2019; Musher et al., 2019; Van Steenberge et al., 2020; Albert et al., 2021; Chen et al., 2021; Sun et al., 2021; van der Merwe et al., 2021).
Although SR and SD are significantly correlated among basins worldwide (R2 = 0.8, n = 3,038, p < 0.01), basins with highest SD values drain primarily tectonically stable regions and these basins were assembled during the Neogene (c. 23–2.6 Ma) and Quaternary (2.6–0 Ma) through the action of mega river capture events (Albert et al., 2018a). Among the largest basins worldwide (i.e., those > 100,000 km2), basins with the top 10 SD values are (in descending order): Amazon, Orinoco, Chao Phraya (Thailand), Mekong, Essequibo, Paraná-Paraguay, Congo, Tocantins, Uruguay, and Zhujiang (Pearl) basins. Under the RCH, the unexpectedly high SD values of these river basins, as assessed by their positive deviations from the regression in Figures 3–6, arose from the merging of multiple older and smaller basins through mega river capture events (Albert et al., 2018b,2021; Sun et al., 2021). All these basins have undergone substantial changes to their watershed margins over the last 20 million years, with significant portions, sometimes>50% (e.g., Hoorn et al., 2010) of their modern areas having been affected by river capture during this time (e.g., Clark et al., 2004; Goudie, 2005; Hoorn et al., 2010).
The Intermediate Capture Rate Hypothesis
The Intermediate Capture Rate Hypothesis (ICH) predicts highest SD values on landscape with “just right” rates of river captures through time and at appropriate spatial scales (Albert et al., 2018a). The results of SD regressions against two landscape metrics (i.e., elevation and relief) are consistent with several predictions of the ICH. Maximum SD values are obtained in tectonically stable lowland basins (Figure 10), where river capture dynamics are expected to drive an excess of speciation and dispersal events as compared with extinction events per unit time, and therefore a net accumulation of SR through time. By contrast, SD values are lower, and not correlated with relief, among seismically active lowland basins (Figure 9) and stable upland basins (Figure 8). Finally, SD values are lowest, and are negatively correlated with relief, among the set of active uplands basins (Figure 7).
Basins with highest fish SD values are located within the tropics and on stable terrains, although not all basins in the tropics or on stable terrains have high fish species density (Figure 2). This is similar to the results of Albert et al. (2018a) who show differing river capture rates on stable vs. active tectonic platforms. Rates of river capture are poorly constrained on most landscapes worldwide, but preliminary evidence from South America indicates they may be several orders of magnitude faster on alluvial lowland sedimentary basins of continental interior than on upland cratonic regions (Ruokolainen et al., 2019; Goldberg et al., 2021).
Landscape evolution modeling results arrive at similar conclusions and suggest that river captures are likely more frequent and larger in low-relief landscapes (Lyons et al., 2020), which exhibit highest SD values worldwide (Figure 2B). Conversely, high relief in tectonically active landscapes acts to fragment the species range and increase extinction rates at smaller spatial scales as compared to low-relief landscapes (e.g., Smith et al., 2002; Albert et al., 2006, 2018a; Borregaard et al., 2012; Griffiths, 2018). Binned by relief, our dataset supports these directional relationships and reveals that SD values peak at an intermediate relief value when comparing tectonically stable lowlands with tectonically active uplands (Figure 12). The probability of river captures increases where neighboring basins erode laterally at different rates, which especially true near topographic escarpments with uniform rock types (Salgado et al., 2014; Willett et al., 2018; Calegari et al., 2021; Wang and Willett, 2021). The across-divide differences in mean relief, elevation, slope, and other topographic metrics that measure steepness, dictate the direction and rate at which divides migrate (Whipple et al., 2017). Importantly, in lower relief settings, landscape perturbations by long-wavelength (100 s of km) and low-amplitude (<1 km) uplift (i.e., dynamic topography; e.g., Bicudo et al., 2019), local uplift (i.e., faults), and base-level fall are likely to reach or surpass the observed ranges of relief, which more easily prompts drainage reorganization (Lyons et al., 2020). To the extent that the value of topographic relief affects the frequency of river captures across these landscape settings (e.g., Lyons et al., 2020), our findings are consistent with the expected effects of barrier displacement caused by landscape transience and support the ICH (Albert et al., 2017).
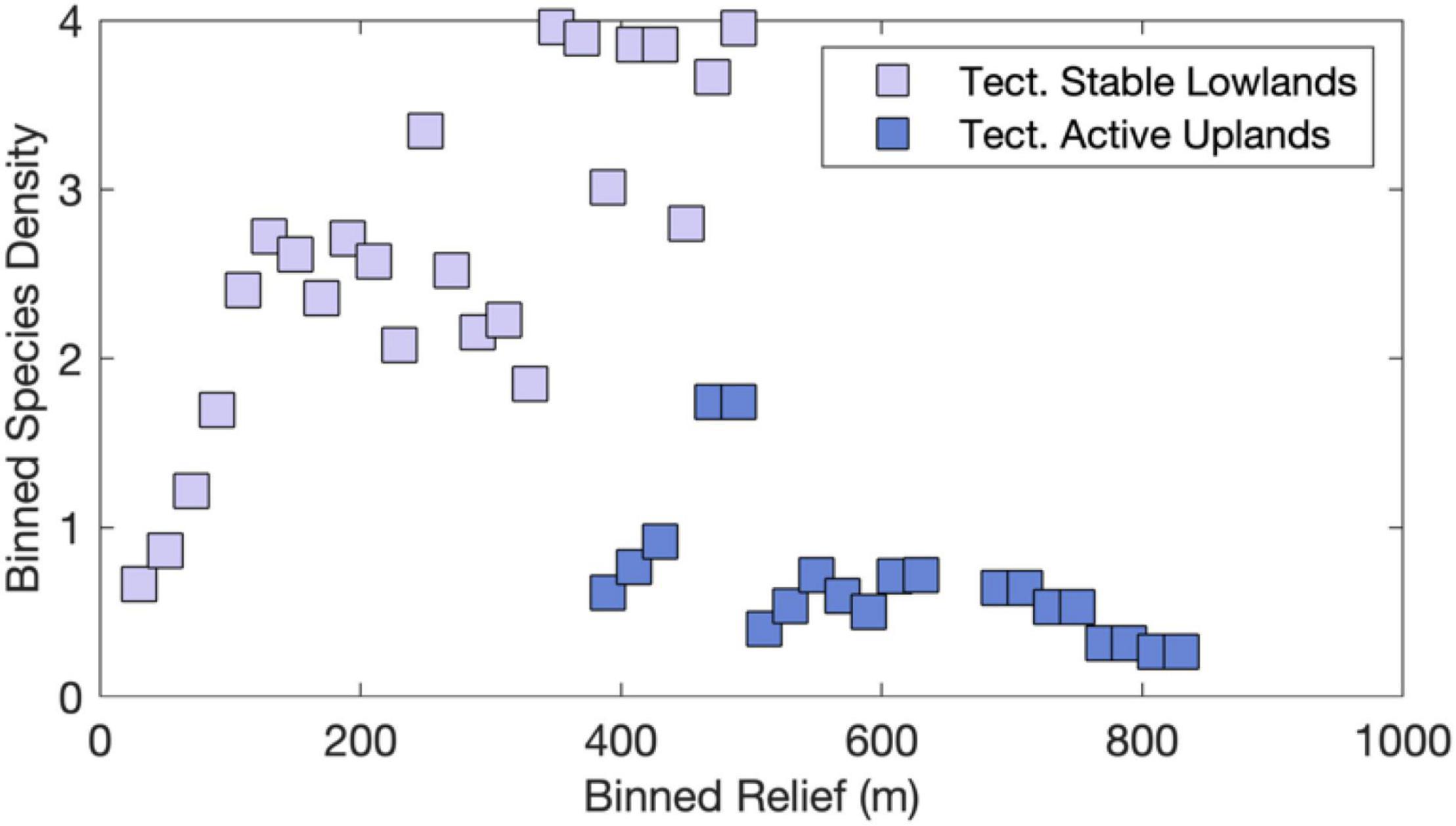
Figure 12. Empirical evidence consistent with Intermediate Capture Rate Hypothesis. SD and relief data were binned in 20-m relief intervals for both sets of tectonically stable lowlands and tectonically active highlands as shown in Figures 7, 10. Note highest SD at intermediate values of relief.
According to the macroevolutionary model outlined above, we may expect to see SRmax values at intermediate rates of river capture under non-equilibrium conditions, when basin-wide species richness (SR) values are growing because the rate of speciation (λ) exceeds extinction (μ). However, under more equilibrium conditions when the rates of speciation (λ) and extinction (μ) are similar, SR may be more strongly controlled by the regional carrying capacity (SRmax; Rosenzweig, 2004; Albert et al., 2017). Results of this study indicate that rates of river capture on stable lowland continental platforms are sufficiently slow enough to allow speciation to occur among isolated portions of river networks through time (t sensu Figure 1), while also being sufficiently fast enough to allow dispersal to populate adjacent basins. This combination of parameter values allows SR values to increase in lowland sedimentary basins which experience higher rates of river capture and reduce the extinction risk of already resident species (Fagan, 2002; Tedesco et al., 2012; Douglas et al., 2013).
By contrast, slower river capture rates on tectonically stable and more erosion resistant upland regions (e.g., continental cratons) are expected to inhibit dispersal among adjacent basins, and thereby lower the rate of increase of diversity through time (lower δ sensu Figure 1). By the same logic, faster rates of river capture within alluvial lowland sedimentary basins of continental interior or coastal plains retard fish diversification, because populations do not become isolated for long enough to allow genetic divergence. In positing that lineage diversification dynamics arises from the multiple effects of dispersal and gene flow on speciation and extinction, the ICH resembles the Shifting Balance Theory (Wright, 1982) and Effect Hypothesis (Vrba, 1983), with the notable differences that, under the ICH, speciation may occur due to genetic drift alone rather than requiring natural selection, and species may therefore not necessarily be adapted to different ecological niches (Harvey et al., 2019). The hypothesis that highest SR values are obtained at intermediate capture rates reflects a larger perspective that all possible evolutionary drivers impose trade-offs on organismal diversification, achieving maximal effectiveness over a limited domain of parameter values; e.g., the intermediate disturbance and productivity hypotheses (Huston, 1994; Fraser et al., 2015).
The log-normal SD frequency distributions observed in this study often characterize biodiversity profiles and other biological systems that grow over time from the multiplicative interactions of many independent random variables (Crow and Shimizu, 1987; Rozenfeld et al., 2008; Magurran, 2013). As numerous studies suggest that landscape transience, river captures, and escarpment migration are common characteristics of intracontinental lowland regions (e.g., Harbor et al., 2005; Gallen et al., 2013; Val et al., 2014; Beeson et al., 2017; Gallen, 2018; Willett et al., 2018; Wang and Willett, 2021), landscape evolution processes might be a common underlying mechanism of diversification in continental regions.
Limitations of This Study
This study examines relationships of fish species richness with possible drivers among basins assigned to broad latitudinal categories (i.e., tropical and extratropical), but does not examine possible effects of latitude on habitat heterogeneity within river basins. Such an analysis would be complicated by many additional factors, with possible expectations for greater habitat heterogeneity in tropical than extra-tropical basins, for basins with N-S than W-E main-stem axis orientations, for upland than lowland basins, and for stable than active terrains. Many other potentially important factors could also be examined, including especially distance from continental geographical centroid or center of connectivity (Smith et al., 2010), and mean or maximum phylogenetic clade age (Miller and Román-Palacios, 2021).
This study also uses topographic metrics such as relief as proxy for landscape transience. Spatial variability in relief is not a unique outcome of exclusively landscape transience. The erosive susceptibility inherent to lithologic types, for example, also influences relief. The primary control of relief and its strong correlation with erosion has been recognized since the early days of geomorphology (Gilbert, 1877), and this correlation continues to be identified using state of the science techniques to measure erosion rates (e.g., von Blanckenburg, 2005). The cross-divide difference in erosion rates can be especially indicative of transient river network reorganization (e.g., Willett et al., 2014; Whipple et al., 2017). Future studies can incorporate cross-divide relief and erosion differences with computational tools such as Forte and Whipple (2018) along with our approach to further investigate links among river captures and species richness.
Conclusion
Results of this study are consistent with the predictions of several widely known macroecological and macroevolutionary models regarding the effects of landscape evolution on freshwater biodiversity; e.g., that the most diverse river basins are all located within tropical latitudes, on stable geological platforms, at lowland elevations, and in areas with high regional precipitation. However not all basins with these features exhibit high SR, due to local historical and geographic conditions, especially proximity and connectedness to species-rich basins that lie near the continental cores, e.g., Amazon, Congo, Danube, Mississippi and Yangtze basins. These discrepancies can be explained, and are predicted, by several models of species and landscape evolution, described here as the following non-mutually exclusive mechanistic hypotheses: the River Capture Hypothesis, Mega Capture Hypothesis, and the Intermediate Capture Hypothesis.
All the most diverse river basins are outliers in SARs (Figures 4, 6) with SD values falling well above that predicted from smaller basins with similar properties. This result means that the predictors of SR based on analysis of many small rivers do not predict the SR of the most diverse basins. The largest basins are different from the others; they are evolutionary arenas with high rates of speciation (i.e., evolutionary cradles) and low rates of extinction (i.e., evolutionary museums) where lineage diversity has accumulated over many tens of millions of years. These results indicate contrasting effects of discrete (higher stream order) river capture events as compared with continuous (headwater or first-level stream order) watershed migration on fish diversity. Moreover, this study also suggests that mega-river captures at the lower and intermediate spatial scales are important drivers of tropical biodiversity. This study is the first to provide empirical support from freshwater fishes worldwide for the conclusions of numerical modeling and empirical studies indicating river capture and landscape transience as mechanistic drivers of net diversification in riverine and riparian organisms that have widespread continental distributions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
PV compiled existing data and collected topographic data for all basins analyzed and produced figures. PV and JA wrote the manuscript, which was revised by NL, NG, and JW. All authors contributed to the article and approved the submitted version.
Funding
PV was supported by the Instituto Serrapilheira (G-1811-25837) and the Brazilian National Council for Scientific and Technological Development (438735/2018-8). NL and NG were supported by the Tulane Oliver Fund grant and NSF (award 1450338) to NG. JW was supported by the National Science Foundation (GEO 1641243). JA was supported by the United States National Science Foundation (DEB 0614334, 0741450, and 1354511).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge William Eschmeyer’s Catalogue of Fishes without which no study on global fish diversity is possible.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2021.788328/full#supplementary-material
References
Albert, J. S., and Crampton, W. G. R. (2010). The geography and ecology of diversification in Neotropical freshwaters. Nat. Educ. Knowl. 1, 13–19. doi: 10.1525/california/9780520268685.003.0001
Albert, J. S., Bernt, M. J., Fronk, A. H., Fontenelle, J. P., Kuznar, S. L., and Lovejoy, N. R. (2021). Late neogene megariver captures and the Great Amazonian biotic interchange. Glob. Planet. Change 205:103554. doi: 10.1016/j.gloplacha.2021.103554
Albert, J. S., Craig, J. M., Tagliacollo, V. A., and Petry, P. (2018a). Upland and lowland fishes: a test of the river capture hypothesis. Mt. Clim. Biodiv. 2018, 273–294.
Albert, J. S., Lovejoy, N. R., and Crampton, W. G. (2006). Miocene tectonism and the separation of cis-and trans-Andean river basins: evidence from neotropical fishes. J. South Am. Earth Sci. 21, 14–27. doi: 10.1016/j.jsames.2005.07.010
Albert, J. S., Petry, P., and Reis, R. E. (2011). “Major biogeographic and phylogenetic patterns,” in Historical Biogeography of Neotropical Freshwater Fishes, eds J. S. Albert and R. E. Reis (Berkeley, CA: UC Press), 21–57. doi: 10.1525/california/9780520268685.003.0002
Albert, J. S., Schoolmaster, D. R. Jr., Tagliacollo, V., and Duke-Sylvester, S. M. (2017). Barrier displacement on a neutral landscape: toward a theory of continental biogeography. Syst. Biol. 66, 167–182. doi: 10.1093/sysbio/syw080
Albert, J. S., Tagliacollo, V. A., and Dagosta, F. (2020). Diversification of Neotropical freshwater fishes. Annu. Rev. Ecol. Evol. Syst. 51, 27–53. doi: 10.1146/annurev-ecolsys-011620-031032
Albert, J. S., Val, P., and Hoorn, C. (2018b). The changing course of the Amazon River in the Neogene: center stage for neotropical diversification. Neotrop. Ichthyol. 16:e180033. doi: 10.1590/1982-0224-20180033
Antonelli, A., Kissling, W. D., Flantua, S. G., Bermúdez, M. A., Mulch, A., Muellner-Riehl, A. N., et al. (2018a). Geological and climatic influences on mountain biodiversity. Nat. Geosci. 11, 718–725. doi: 10.1038/s41561-018-0236-z
Antonelli, A., Zizka, A., Carvalho, F. A., Scharn, R., Bacon, C. D., Silvestro, D., et al. (2018b). Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. U.S.A. 115, 6034–6039. doi: 10.1073/pnas.1713819115
Azevedo, J. A., Guedes, T. B., and Nogueira, C. C. (2020). Museums and cradles of diversity are geographically coincident for narrow distributed Neotropical snakes. Ecography 43, 328–339. doi: 10.1111/ecog.04815
Badgley, C., Smiley, T. M., and Finarelli, J. A. (2014). Great Basin mammal diversity in relation to landscape history. J. Mammal. 95, 1090–1106. doi: 10.1644/13-MAMM-S-088
Badgley, C., Smiley, T. M., Terry, R., Davis, E. B., DeSantis, L. R., Fox, D. L., et al. (2017). Biodiversity and topographic complexity: modern and geohistorical perspectives. Trends Ecol. Evol. 32, 211–226. doi: 10.1016/j.tree.2016.12.010
Beeson, H. W., McCoy, S. W., and Keen-Zebert, A. (2017). Geometric disequilibrium of river basins produces long-lived transient landscapes. Earth Planet. Sci. Lett. 475, 34–43. doi: 10.1016/j.epsl.2017.07.010
Bicudo, T. C., Sacek, V., de Almeida, R. P., Bates, J. M., and Ribas, C. C. (2019). Andean tectonics and mantle dynamics as a pervasive influence on Amazonian ecosystem. Sci. Rep. 9:16879. doi: 10.1038/s41598-019-53465-y
Bishop, P. (1995). Drainage rearrangement by river capture, beheading and diversion. Prog. Phys. Geogr. 19, 449–473. doi: 10.1177/030913339501900402
Borregaard, M. K., Gotelli, N. J., and Rahbek, C. (2012). Are range-size distributions consistent with species-level heritability? Evolution 66, 2216–2226. doi: 10.1111/j.1558-5646.2012.01581.x
Bragança, P. H., and Costa, W. J. (2019). Multigene fossil-calibrated analysis of the African lampeyes (Cyprinodontoidei: Procatopodidae) reveals an early Oligocene origin and Neogene diversification driven by palaeogeographic and palaeoclimatic events. Organ. Divers. Evol. 19, 303–320. doi: 10.1007/s13127-019-00396-1
Brown, J. H., and Kodric-Brown, A. (1977). Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58, 445–449. doi: 10.2307/1935620
Burns, M. D. (2021). Adaptation to herbivory and detritivory drives the convergent evolution of large abdominal cavities in a diverse freshwater fish radiation (Otophysi: Characiformes). Evolution 75, 688–705. doi: 10.1111/evo.14178
Burridge, C. P., Craw, D., and Waters, J. M. (2006). River capture, range expansion, and cladogenesis: the genetic signature of freshwater vicariance. Evolution 60, 1038–1049. doi: 10.1111/j.0014-3820.2006.tb01181.x
Burridge, C. P., Craw, D., and Waters, J. M. (2007). An empirical test of freshwater vicariance via river capture. Mol. Ecol. 16, 1883–1895. doi: 10.1111/j.1365-294X.2006.03196.x
Calegari, S. S., Peifer, D., Neves, M. A., and de Andrade Caxito, F. (2021). Post-Miocene topographic rejuvenation in an elevated passive continental margin not characterized by a sharp escarpment (northern end of the Mantiqueira Range, Brazil). Geomorphology 393:107946. doi: 10.1016/j.geomorph.2021.107946
Castroviejo-Fisher, S., Guayasamin, J. M., Gonzalez-Voyer, A., and Vilà, C. (2014). Neotropical diversification seen through glassfrogs. J. Biogeogr. 41, 66–80. doi: 10.1111/jbi.12208
Chen, F., Xue, G., Wang, Y., Zhang, H., He, J., Chen, J., et al. (2021). Evolution of the Yangtze River Reconstructed by the Largest Molecular Phylogeny of Cyprinidae. Kerala: Research Square. doi: 10.21203/rs.3.rs-145035/v1
Clark, M. K., Schoenbohm, L. M., Royden, L. H., Whipple, K. X., Burchfiel, B. C., Zhang, X., et al. (2004). Surface uplift, tectonics, and erosion of eastern Tibet from large-scale drainage patterns. Tectonics 23:TC1006. doi: 10.1029/2002TC001402
Coblentz, D. D., and Riitters, K. H. (2004). Topographic controls on the regional-scale biodiversity of the south-western USA. J. Biogeogr. 31, 1125–1138. doi: 10.1111/j.1365-2699.2004.00981.x
Cornell, H. V. (2013). Is regional species diversity bounded or unbounded? Biol. Rev. 88, 140–165. doi: 10.1111/j.1469-185X.2012.00245.x
Cornell, H. V., and Harrison, S. P. (2014). What are species pools and when are they important? Annu. Rev. Ecol. Evol. Syst. 45, 45–67. doi: 10.1146/annurev-ecolsys-120213-091759
Crosby, B. T., and Whipple, K. X. (2006). Knickpoint initiation and distribution within fluvial networks: 236 waterfalls in the Waipaoa River, North Island, New Zealand. Geomorphology 82, 16–38. doi: 10.1016/j.geomorph.2005.08.023
Davis, A. M., Unmack, P. J., Vari, R. P., and Betancur-R, R. (2016). Herbivory promotes dental disparification and macroevolutionary dynamics in grunters (Teleostei: Terapontidae), a freshwater adaptive radiation. Am. Nat. 187, 320–333. doi: 10.1086/684747
Densmore, A. L., and Hovius, N. (2000). Topographic fingerprints of bedrock landslides. Geology 28, 371–374. doi: 10.1130/0091-7613(2000)28<371:TFOBL>2.0.CO;2
Dias, M. S., Cornu, J. F., Oberdorff, T., Lasso, C. A., and Tedesco, P. A. (2013). Natural fragmentation in river networks as a driver of speciation for freshwater fishes. Ecography 36, 683–689. doi: 10.1111/j.1600-0587.2012.07724.x
Dias, M. S., Oberdorff, T., Hugueny, B., Leprieur, F., Jézéquel, C., Cornu, J. F., et al. (2014). Global imprint of historical connectivity on freshwater fish biodiversity. Ecol. Lett. 17, 1130–1140. doi: 10.1111/ele.12319
Douglas, M., Keck, B. P., Ruble, C., Petty, M., Shute, J. R., Rakes, P., et al. (2013). Pelagic larval duration predicts extinction risk in a freshwater fish clade. Biol. Lett. 9:20130672. doi: 10.1098/rsbl.2013.0672
Duvall, A., Kirby, E., and Burbank, D. (2004). Tectonic and lithologic controls on bedrock channel profiles and processes in coastal California. J. Geophys. Res. 109:F3. doi: 10.1029/2003JF000086
Fagan, W. F. (2002). Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83, 3243–3249. doi: 10.1890/0012-9658(2002)083[3243:CFAERI]2.0.CO;2
Farr, T. G., Rosen, P. A., Caro, E., Crippen, R., Duren, R., Hensley, S., et al. (2007). The shuttle radar topography mission. Rev. Geophys. 45:RG2004. doi: 10.1029/2005RG000183
Forte, A. M., Yanites, B. J., and Whipple, K. X. (2016). Complexities of landscape evolution during incision through layered stratigraphy with contrasts in rock strength. Earth Surf. Process. Landf. 41, 1736–1757. doi: 10.1002/esp.3947
Forte, A. M., and Whipple, K. X. (2018). Criteria and tools for determining drainage divide stability. Earth Planet. Sci. Lett. 493, 102–117. doi: 10.1016/j.epsl.2018.04.026
Fraser, L. H., Pither, J., Jentsch, A., Sternberg, M., Zobel, M., Askarizadeh, D., et al. (2015). Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science 349, 302–305. doi: 10.1126/science.aab3916
Fricke, R., Eschmeyer, W. N., and Van der Laan, R. (eds) (2021). Eschmeyer’s Catalog of Fishes: Genera, species, references. Available online at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed August 01, 2021).
Gallen, S. F. (2018). Lithologic controls on landscape dynamics and aquatic species evolution in post-orogenic mountains. Earth Planet. Sci. Lett. 493, 150–160. doi: 10.1016/j.epsl.2018.04.029
Gallen, S. F., Wegmann, K. W., and Bohnenstiehl, D. R. (2013). Miocene rejuvenation of topographic relief in the southern Appalachians. GSA Today 23, 4–10. doi: 10.1016/j.epsl.2018.04.029
Giachetta, E., and Willett, S. D. (2018). Effects of river capture and sediment flux on the evolution of plateaus: insights from numerical modeling and river profile analysis in the upper Blue Nile catchment. J. Geophys. Res. 123, 1187–1217. doi: 10.1029/2017JF004252
Gilbert, G. K. (1877). Geology of the Henry Mountains. Washington, DC: Government Printing Office. doi: 10.3133/70039916
Goldberg, S. L., Schmidt, M. J., and Perron, J. T. (2021). Fast response of Amazon rivers to quaternary climate cycles. JGR Earth Surf. 126:e2021JF006416. doi: 10.1029/2021JF006416
Goudie, A. S. (2004). “Base level,” in Encyclopedia of Geomorphology, ed. A. S. Goudie (Milton Park: Routledge), 62.
Goudie, A. S. (2005). The drainage of Africa since the Cretaceous. Geomorphology 67, 437–456. doi: 10.1016/j.geomorph.2004.11.008
Griffiths, D. (2018). Why does freshwater fish species richness differ between Pacific and Atlantic drainages of the Americas? J. Biogeogr. 45, 784–792. doi: 10.1111/jbi.13167
Hack, J. T. (1973). Stream-profile analysis and stream-gradient index. J. Res. US Geol. Survey 1, 421–429.
Hajek, E. A., and Straub, K. M. (2017). Autogenic sedimentation in clastic stratigraphy. Annu. Rev. Earth Planet. Sci. 45, 681–709. doi: 10.1146/annurev-earth-063016-015935
Harbor, D., Bacastow, A., Heath, A., and Rogers, J. (2005). Capturing variable knickpoint retreat in the Central Appalachians, USA. Geogr. Fis. Din. Quat. 28, 23–36.
Harmon, L. J., and Harrison, S. (2015). Species diversity is dynamic and unbounded at local and continental scales. Am. Nat. 185, 584–593. doi: 10.1086/680859
Harvey, M. G., Singhal, S., and Rabosky, D. L. (2019). Beyond reproductive isolation: demographic controls on the speciation process. Annu. Rev. Ecol. Evol. Syst. 50, 75–95. doi: 10.1146/annurev-ecolsys-110218-024701
Hazzi, N. A., Moreno, J. S., Ortiz-Movliav, C., and Palacio, R. D. (2018). Biogeographic regions and events of isolation and diversification of the endemic biota of the tropical Andes. Proc. Natl. Acad. Sci. U.S.A. 115, 7985–7990. doi: 10.1073/pnas.1803908115
Helfman, G., Collette, B. B., Facey, D. E., and Bowen, B. W. (2009). The Diversity of Fishes: Biology, Evolution, and Ecology. Hoboken, NJ: John Wiley & Sons.
Hoorn, C., Wesselingh, F. P., Ter Steege, H., Bermudez, M. A., Mora, A., Sevink, J., et al. (2010). Amazonia through time: andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931. doi: 10.1126/science.1194585
Huston, M. A. (1994). Biological Diversity: The Coexistence of Species. Cambridge: Cambridge University Press.
Igea, J., and Tanentzap, A. J. (2021). Global topographic uplift has elevated speciation in mammals and birds over the last 3 million years. Nat. Ecol. Evol. 5, 1530–1535. doi: 10.1038/s41559-021-01545-6
Jablonski, D. (2017). Approaches to macroevolution: 1. General concepts and origin of variation. Evol. Biol. 44, 427–450. doi: 10.1007/s11692-017-9420-0
Kirby, E., and Whipple, K. X. (2012). Expression of active tectonics in erosional landscapes. J. Struct. Geol. 44, 54–75. doi: 10.1016/j.jsg.2012.07.009
Kolmann, M. A., Burns, M. D., Ng, J. Y., Lovejoy, N. R., and Bloom, D. D. (2020). Habitat transitions alter the adaptive landscape and shape phenotypic evolution in needlefishes (Belonidae). Ecol. Evol. 10, 3769–3783. doi: 10.1002/ece3.6172
Lehner, B., Verdin, K., and Jarvis, A. (2008). New global hydrography derived from spaceborne elevation data. Eos Trans. Am. Geophys. Union 89, 93–94. doi: 10.1029/2008EO100001
Leprieur, F., Brosse, S., Grenouillet, G., Tedesco, P. A., Beauchard, O., Bigorne, R., et al. (2017). A global database on freshwater fish species occurrences in drainage basins. Sci. Data 4:170141. doi: 10.1038/sdata.2017.141
Li, D., Olden, J. D., Lockwood, J. L., Record, S., McKinney, M. L., and Baiser, B. (2020). Changes in taxonomic and phylogenetic diversity in the Anthropocene. Proc. R. Soc. B 287:20200777. doi: 10.1098/rspb.2020.0777
Losos, J. B., Jackman, T. R., Larson, A., de Queiroz, K., and Rodrıìguez-Schettino, L. (1998). Contingency and determinism in replicated adaptive radiations of island lizards. Science 279, 2115–2118. doi: 10.1126/science.279.5359.2115
Lujan, N. K., Roach, K. A., Jacobsen, D., Winemiller, K. O., Vargas, V. M., Ching, V. R., et al. (2013). Aquatic community structure across an Andes-to-Amazon fluvial gradient. J. Biogeogr. 40, 1715–1728. doi: 10.1111/jbi.12131
Lundberg, J. G., Kottelat, M., Smith, G. R., Stiassny, M. L., and Gill, A. C. (2000). So many fishes, so little time: an overview of recent ichthyological discovery in continental waters. Ann. Mo. Bot. Gard. 87, 26–62. doi: 10.2307/2666207
Lyons, N. J., Val, P., Albert, J. S., Willenbring, J. K., and Gasparini, N. M. (2020). Topographic controls on divide migration, stream capture, and diversification in riverine life. Earth Surf. Dyn. 8, 893–912. doi: 10.5194/esurf-8-893-2020
MacArthur, R. H., and Wilson, E. O. (1966). The Theory of Island Biogeography. Princeton, NJ: Princeton University Press, 203.
Manel, S., Guerin, P. E., Mouillot, D., Blanchet, S., Velez, L., Albouy, C., et al. (2020). Global determinants of freshwater and marine fish genetic diversity. Nat. Commun. 11:692. doi: 10.1038/s41467-020-14409-7
Matthews, W. J. (2012). Patterns in Freshwater Fish Ecology. Berlin: Springer Science & Business Media.
McCain, C. M., and Grytnes, J. A. (2010). “Elevational gradients in species richness,” in Encyclopedia of Life Sciences, (Chichester: John Wiley & Sons Ltd). doi: 10.1002/9780470015902.a0022548
McDermott, A. (2021). Inner workings: reeling in answers to the “freshwater fish paradox. Proc. Natl. Acad. Sci. U.S.A. 118:e2113780118. doi: 10.1073/pnas.2113780118
McGarvey, D. J., and Terra, B. D. F. (2016). Using river discharge to model and deconstruct the latitudinal diversity gradient for fishes of the Western Hemisphere. J. Biogeogr. 43, 1436–1449. doi: 10.1111/jbi.12618
McGee, M. D., Borstein, S. R., Meier, J. I., Marques, D. A., Mwaiko, S., Taabu, A., et al. (2020). The ecological and genomic basis of explosive adaptive radiation. Nature 586, 75–79. doi: 10.1038/s41586-020-2652-7
Miller, E. C., and Román-Palacios, C. (2021). Evolutionary time best explains the latitudinal diversity gradient of living freshwater fish diversity. Glob. Ecol. Biogeogr. 30, 749–763. doi: 10.1111/geb.13253
Montgomery, D. R., and Brandon, M. T. (2002). Topographic controls on erosion rates in tectonically active mountain ranges. Earth Planet. Sci. Lett. 201, 481–489. doi: 10.1038/nature02150
Moodie, A. J., Pazzaglia, F. J., and Berti, C. (2018). Exogenic forcing and autogenic processes on continental divide location and mobility. Basin Res. 30, 344–369. doi: 10.1111/bre.12256
Muneepeerakul, R., Weitz, J. S., Levin, S. A., Rinaldo, A., and Rodriguez-Iturbe, I. (2007). A neutral metapopulation model of biodiversity in river networks. J. Theor. Biol. 245, 351–363. doi: 10.1016/j.jtbi.2006.10.005
Musher, L. J., Ferreira, M., Auerbach, A. L., McKay, J., and Cracraft, J. (2019). Why is Amazonia a ‘source’ of biodiversity? Climate-mediated dispersal and synchronous speciation across the Andes in an avian group (Tityrinae). Proc. R. Soc. B 286:20182343. doi: 10.1098/rspb.2018.2343
New, M., Lister, D., Hulme, M., and Makin, I. (2002). A high-resolution data set of surface climate over global land areas. Clim. Res. 21, 1–25. doi: 10.3354/cr021001
Oberdorff, T., Tedesco, P. A., Hugueny, B., Leprieur, F., Beauchard, O., Brosse, S., et al. (2011). Global and regional patterns in riverine fish species richness: a review. Int. J. Ecol. 2011:967631. doi: 10.1155/2011/967631
Pagani, M., Garcia-Pelaez, J., Gee, R., Johnson, K., Poggi, V., Styron, R., et al. (2018). Global Earthquake Model (GEM) Seismic Hazard Map (version 2018.1 - December 2018). Pavia: Global Earthquake Model Foundation.
Pazzaglia, F. J., and Brandon, M. T. (1996). Macrogeomorphic evolution of the post-triassic appalachian mountains determined by deconvolution of the offshore basin sedimentary record. Basin Res. 8, 255–278. doi: 10.1046/j.1365-2117.1996.00274.x
Perron, J. T., Kirchner, J. W., and Dietrich, W. E. (2009). Formation of evenly spaced ridges and valleys. Nature 460, 502–505. doi: 10.1002/mgg3.194
Portenga, E. W., and Bierman, P. R. (2011). Understanding Earth’s eroding surface with 10Be. GSA Today 21, 4–10. doi: 10.1130/G111A.1
Pringle, C. (2003). What is hydrologic connectivity and why is it ecologically important? Hydrol. Process. 17, 2685–2689. doi: 10.1126/sciadv.1600026
Rabosky, D. L. (2010). Primary controls on species richness in higher taxa. Syst. Biol. 59, 634–645. doi: 10.1093/sysbio/syq060
Rabosky, D. L. (2013). Diversity-dependence, ecological speciation, and the role of competition in macroevolution. Annu. Rev. Ecol. Evol. Syst. 44, 481–502. doi: 10.1111/ele.13382
Rabosky, D. L., and Hurlbert, A. H. (2015). Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583. doi: 10.1086/680850
Rahbek, C., Borregaard, M. K., Colwell, R. K., Dalsgaard, B. O., Holt, B. G., Morueta-Holme, N., et al. (2019). Humboldt’s enigma: what causes global patterns of mountain biodiversity? Science 365, 1108–1113. doi: 10.1126/science.aax0149
Réjaud, A., Rodrigues, M. T., Crawford, A. J., Castroviejo-Fisher, S., Jaramillo, A. F., Chaparro, J. C., et al. (2020). Historical biogeography identifies a possible role of Miocene wetlands in the diversification of the Amazonian rocket frogs (Aromobatidae: Allobates). J. Biogeogr. 47, 2472–2482. doi: 10.1111/jbi.13937
Rodriguez-Iturbe, I., and Rinaldo, A. (2001). Fractal River Basins: Chance and Self-Organization. Cambridge: Cambridge University Press.
Roell, Y. E., Phillips, J. G., and Parent, C. E. (2021). Effect of topographic complexity on species richness in the Galápagos Islands. J. Biogeogr. 48, 2645–2655. doi: 10.1111/jbi.14230
Rosenzweig, M. L. (2004). “Applying species-area relationships to the conservation of species diversity,” in Frontiers of Biogeography: New Directions in the Geography of Nature, eds M. V. Lomolino and L. R. Heaney (Sunderland: Sinauer Associates), 325–343.
Rozenfeld, H., Rybski, D., Andrade, J. S., Batty, M., Stanley, H. E., and Makse, H. A. (2008). Laws of population growth. Proc. Natl. Acad. Sci. U.S.A. 105, 18702–18707. doi: 10.1073/pnas.0807435105
Ruokolainen, K., Moulatlet, G. M., Zuquim, G., Hoorn, C., and Tuomisto, H. (2019). Geologically recent rearrangements in central Amazonian river network and their importance for the riverine barrier hypothesis. Front. Biogeogr. 11:e45046. doi: 10.21425/F5FBG45046
Salgado, A. A., Marent, B. R., Cherem, L. F., Bourlès, D., Santos, L. J., Braucher, R., et al. (2014). Denudation and retreat of the serra do mar escarpment in Southern Brazil derived from in situ-produced 10Be concentration in river sediment. Earth Surf. Process. Landf. 39, 311–319. doi: 10.1002/esp.3448
Salgueiro, L., Cassemiro, F., Albert, J. S., Frederico, R. G., Hidalgo, M., Hugueny, B., et al. (2021). Drivers of phylogenetic structure in Amazonian freshwater fish assemblages. bioRxiv [Preprint] doi: 10.1101/2021.07.29.454320
Sassolas-Serrayet, T., Cattin, R., Ferry, M., Godard, V., and Simoes, M. (2019). Estimating the disequilibrium in denudation rates due to divide migration at the scale of river basins. Earth Surf. Dyn. 7, 1041–1057. doi: 10.5194/esurf-7-1041-2019
Saupe, E. E., and Myers, C. E. (2021). “Macroevolution,” in Evolutionary Developmental Biology: A Reference Guide, eds L. N. de la Rosa and G. Müller (Basingstoke: Springer Nature), 149–167. doi: 10.1007/978-3-319-32979-6_126
Scheingross, J. S., Lamb, M. P., and Fuller, B. M. (2019). Self-formed bedrock waterfalls. Nature 567, 229–233. doi: 10.1038/s41586-019-0991-z
Scheingross, J. S., Limaye, A. B., McCoy, S. W., and Whittaker, A. C. (2020). The shaping of erosional landscapes by internal dynamics. Nat. Rev. Earth Environ. 1, 661–676. doi: 10.1038/s43017-020-0096-0
Schwanghart, W., and Scherler, D. (2014). TopoToolbox 2–MATLAB-based software for topographic analysis and modeling in Earth surface sciences. Earth Surf. Dyn. 2, 1–7. doi: 10.5194/esurf-2-1-2014
Sieben, E. J., Khubeka, S. P., Sithole, S., Job, N. M., and Kotze, D. C. (2018). The classification of wetlands: integration of top-down and bottom-up approaches and their significance for ecosystem service determination. Wetl. Ecol. Manag. 26, 441–458. doi: 10.1007/s11273-017-9585-4
Smith, G. R., Badgley, C., Eiting, T. P., and Larson, P. S. (2010). Species diversity gradients in relation to geological history in North American freshwater fishes. Evol. Ecol. Res. 12, 693–726.
Smith, G. R., Dowling, T. E., Gobalet, K. W., Lugaski, T. S. D. K., Shiozawa, D. A., and Evans, R. P. (2002). Biogeography and timing of evolutionary events among Great Basin fishes. Great Basin Aquat. Syst. His. 33, 175–234.
Stanley, S. M. (2014). “Fossils, macroevolution, and theoretical ecology,” in Perspectives in Ecological Theory, eds J. Roughgarden, R. M. May, and S. A. Levin (Princeton, NJ: Princeton University Press), 125–134. doi: 10.1515/9781400860180.125
Stokes, M. F., and Perron, J. T. (2020). Modeling the evolution of aquatic organisms in dynamic river basins. J. Geophys. Res. 125:e2020JF005652. doi: 10.1029/2020JF005652
Stokes, M. F., Goldberg, S. L., and Perron, J. T. (2018). Ongoing river capture in the Amazon. Geophys. Res. Lett. 45, 5545–5552. doi: 10.1029/2018GL078129
Su, G., Logez, M., Xu, J., Tao, S., Villéger, S., and Brosse, S. (2021). Human impacts on global freshwater fish biodiversity. Science 371, 835–838. doi: 10.1126/science.abd3369
Sun, H., Li, Z., Landis, J. B., Qian, L., Zhang, T., and Deng, T. (2021). Effects of drainage reorganization on phytogeographic pattern in Sino-Himalaya. Alp. Bot. 1–11. doi: 10.1007/s00035-021-00269-4
Tagliacollo, V. A. Duke-Sylvester, S. M., Matamoros, W. A., Chakrabarty, P., and Albert, J. S. (2017). Coordinated dispersal and pre-isthmian assembly of the Central American ichthyofauna. Syst. Biol. 66, 183–196. doi: 10.1093/sysbio/syv064
Tagliacollo, V. A., Roxo, F. F., Duke-Sylvester, S. M., Oliveira, C., and Albert, J. S. (2015). Biogeographical signature of river capture events in Amazonian lowlands. J. Biogeogr. 42 2349–2362. doi: 10.1111/jbi.12594
Tedesco, P. A., Beauchard, O., Bigorne, R., Blanchet, S., Buisson, L., Conti, L., et al. (2017a). A global database on freshwater fish species occurrence in drainage basins. Sci. Data 4:170141. doi: 10.1038/sdata.2017.141
Tedesco, P. A., Leprieur, F., Hugueny, B., Brosse, S., Dürr, H. H., Beauchard, O., et al. (2012). Patterns and processes of global riverine fish endemism. Glob. Ecol. Biogeogr. 21, 977–987. doi: 10.1111/j.1466-8238.2011.00749.x
Tedesco, P. A., Paradis, E., Lévêque, C., and Hugueny, B. (2017b). Explaining global-scale diversification patterns in actinopterygian fishes. J. Biogeogr. 44, 773–783. doi: 10.1111/jbi.12905
Thomaz, A. T., Christie, M. R., and Knowles, L. L. (2016). The architecture of river networks can drive the evolutionary dynamics of aquatic populations. Evolution 70, 731–739. doi: 10.1111/evo.12883
Tinkler, K. J. (2004). “Knickpoint,” in Encyclopedia of Geomorphology, ed. A. S. Goudie (Milton Park: Routledge), 595–596.
Tonkin, J. D., Heino, J., and Altermatt, F. (2018). Metacommunities in river networks: the importance of network structure and connectivity on patterns and processes. Freshw. Biol. 63, 1–5. doi: 10.1111/fwb.13045
Val, P., Silva, C., Harbor, D., Morales, N., Amaral, F., and Maia, T. (2014). Erosion of an active fault scarp leads to drainage capture in the Amazon region, Brazil. Earth Surf. Process. Landf. 39, 1062–1074. doi: 10.1002/esp.3507
van der Merwe, P. D. W., Cotterill, F. P., Kandziora, M., Watters, B. R., Nagy, B., Genade, T., et al. (2021). Genomic fingerprints of palaeogeographic history: the tempo and mode of Rift tectonics across tropical Africa has shaped the diversification of the killifish genus Nothobranchius (Teleostei: Cyprinodontiformes). Mol. Phylogenet. Evol. 158:106988. doi: 10.1016/j.ympev.2020.106988
Van Steenberge, M. W., Vanhove, M. P., Chocha Manda, A., Larmuseau, M. H., Swart, B. L., Khang’Mate, F., et al. (2020). Unravelling the evolution of Africa’s drainage basins through a widespread freshwater fish, the African sharptooth catfish Clarias gariepinus. J. Biogeogr. 47, 1739–1754. doi: 10.1111/jbi.13858
von Blanckenburg, F. (2005). The control mechanisms of erosion and weathering at basin scale from cosmogenic nuclides in river sediment. Earth Planet. Sci. Lett. 237, 462–479. doi: 10.1016/j.epsl.2005.06.030
Vrba, E. S. (1983). Macroevolutionary trends: new perspectives on the roles of adaptation and incidental effect. Science 221, 387–389. doi: 10.1126/science.221.4608.387
Wang, Y., and Willett, S. (2021). Escarpment retreat rates derived from detrital cosmogenic nuclide concentrations. Earth Surf. Dyn. 9, 1301–1322. doi: 10.1126/science.221.4608.387
Ward, J. V., Tockner, K., Arscott, D. B., and Claret, C. (2002). Riverine landscape diversity. Freshw. Biol. 47, 517–539. doi: 10.1046/j.1365-2427.2002.00893.x
Whipple, K. X., Forte, A. M., DiBiase, R. A., Gasparini, N. M., and Ouimet, W. B. (2017). Timescales of landscape response to divide migration and drainage capture: implications for the role of divide mobility in landscape evolution. J. Geophys. Res. 122, 248–273. doi: 10.1002/2016JF003973
Whittaker, A. C. (2012). How do landscapes record tectonics and climate? Lithosphere 4, 160–164. doi: 10.1130/RF.L003.1
Wiens, J. A. (2002). Riverine landscapes: taking landscape ecology into the water. Freshw. Biol. 47, 501–515. doi: 10.1016/j.scitotenv.2008.03.044
Willenbring, J. K., Codilean, A. T., and McElroy, B. (2013). Earth is (mostly) flat: apportionment of the flux of continental sediment over millennial time scales. Geology 41, 343–346. doi: 10.1130/G33918.1
Willett, S. D., McCoy, S. W., and Beeson, H. W. (2018). Transience of the North American high plains landscape and its impact on surface water. Nature 561, 528–532. doi: 10.1038/s41586-018-0532-1
Willett, S. D., McCoy, S. W., Perron, J. T., Goren, L., and Chen, C. Y. (2014). Dynamic reorganization of river basins. Science 343:1248765. doi: 10.1126/science.1248765
Willmott, C. J., and Matsuura, K. (2001). Terrestrial Air Temperature and Precipitation: Monthly and Annual Time Series (1950 - 1999). Newark, NJ: Center for Climatic Research.
Winemiller, K. O. (1991). Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 61, 343–365. doi: 10.2307/2937046
Worm, B., and Tittensor, D. P. (2018). A Theory of Global Biodiversity (MPB-60). Princeton, NJ: Princeton University Press. doi: 10.23943/princeton/9780691154831.001.0001
Keywords: landscape evolution, tropical biodiversity, river capture, macroecology and macroevolution, biogeography, geobiology
Citation: Val P, Lyons NJ, Gasparini N, Willenbring JK and Albert JS (2022) Landscape Evolution as a Diversification Driver in Freshwater Fishes. Front. Ecol. Evol. 9:788328. doi: 10.3389/fevo.2021.788328
Received: 02 October 2021; Accepted: 09 December 2021;
Published: 11 January 2022.
Edited by:
Jean Boubli, University of Salford, United KingdomReviewed by:
Peter John Unmack, University of Canberra, AustraliaBrian I. Crother, Southeastern Louisiana University, United States
Copyright © 2022 Val, Lyons, Gasparini, Willenbring and Albert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Val, pval@ufop.edu.br
 Pedro Val
Pedro Val Nathan J. Lyons2
Nathan J. Lyons2  James S. Albert
James S. Albert