Does Helping-at-the-Nest Help? The Case of the Acorn Woodpecker
- 1Lab of Ornithology, Department of Neurobiology and Behavior, Cornell University, Ithaca, NY, United States
- 2Hastings Reservation, University of California, Berkeley, Berkeley, CA, United States
- 3Department of Biological Sciences, Old Dominion University, Norfolk, VA, United States
Cooperative breeding groups often involve “helpers-at-the-nest”; indeed, such behavior typically defines this intriguing breeding system. In few cases, however, has it been demonstrated that feeding nestlings by helpers, rather than some other behavior associated with helpers' presence, leads to greater reproductive success. One prediction of the hypothesis that feeding behavior per se is responsible for the fitness benefits conferred by helpers is that there should be close congruence between the patterns of helping-at-the-nest and the fitness effects of helpers. Here we look for such a relationship in the cooperatively breeding acorn woodpecker (Melanerpes formicivorus) in order to begin to identify the behaviors of helpers that drive the increased fitness benefits they confer. In terms of young fledged, a helper male confers approximately the same fitness benefits to a group as does a helper female; more dramatically, the effects of helper males increases with increasing food supply, most importantly the prior year's acorn crop on which this species depends, whereas that of helper females does not. These patterns do not match the nest-feeding patterns of helpers, which are greater for females than males and do not increase with a larger acorn crop the prior autumn. In contrast, the proportion of time helpers spend tending acorn-storage facilities (granaries) and are present in or near their home territory is greater for males than females and, at least for males, positively related to the size of the acorn crop. These results fail to support the hypothesis that the primary benefit conferred by helpers is feeding young in the nest; rather, they suggest that behaviors such as territorial defense and predator detection are more important. Understanding exactly what those behaviors are in this, and most other cooperatively breeding systems, remain to be determined.
Introduction
A central problem in the field of evolutionary biology is to understand why helpers help (Pennisi, 2005). Like other evolutionary questions, this problem can be approached at different levels of analysis (Tinbergen, 1963; Sherman, 1988). At the functional level, a classic answer for many cooperative breeding species is that the dispersal of helpers is ecologically constrained because of habitat saturation (Koenig and Pitelka, 1981; Emlen, 1982) and helpers are gaining what inclusive fitness benefits they can—that is, “making the best of a bad job”—by helping to feed and thus raise offspring to which they are genetically related (Emlen, 1991; Koenig, 2017). Among the issues that remain unresolved, however, is the question of what is driving the observed variability in helping behavior. Despite decades of work on dozens of species quantifying helping-at-the-nest, there are few studies demonstrating conclusively that helping behavior—in many cases the trait that defines cooperative breeding—is responsible for the increase in reproductive success observed in such taxa when helpers are present.
Because helpers are typically offspring of the breeders (Emlen, 1991), and since cooperative breeding is generally defined by the presence of more than a pair of individuals feeding at a nest, the assumption is typically that such helping behavior increases the reproductive success of the group, and that the additional offspring that helpers help raise, above and beyond what would be produced in the absence of helpers, confers inclusive fitness benefits. There are, however, several factors that complicate this assumption. One is that there are a sizeable number of cooperative breeding species that are not kin-based (Riehl, 2013), and thus, despite evidence supporting the importance of kin selection in many cooperative breeding systems (Russell and Hatchwell, 2001; Griffin and West, 2003; Browning et al., 2012), helpers are in some cases presumably gaining direct, rather than indirect, fitness benefits. A second problem is that helpers do not always appear to enhance the reproductive success of their group; in other words, increased offspring production is not always the best measure of fitness benefits for helpers. In many such cases, this appears to be due to compensatory care or “load-lightening.” This is the phenomenon whereby the help provided by helpers allows other group members to reduce their own investment and thereby presumably enhance their survival (and thus the helper's future indirect benefits; Mumme et al., 1989) at the cost of the helper's current reproductive success (Crick, 1992; Heinsohn, 2004; Russell et al., 2007; Hammers et al., 2019). There remain, however, cases in which no discernable fitness effects of helping have been identified (Leonard et al., 1989; Magrath and Yezerinac, 1997). A third issue is that offspring in some species delay dispersal but do not provision at nests, demonstrating that feeding at the nest is not an automatic consequence of delayed dispersal, but rather a phenomenon that demands its own explanation (Ekman and Griesser, 2016).
Even when fitness benefits of helpers appear unambiguous—that is, groups produce more young when they have helpers—it is often not obvious what helpers are doing that drives this effect. A classic example is the Florida scrub-jay (Aphelocoma coerulescens), where experimental removals revealed that despite helpers providing considerable food to nestlings (Stallcup and Woolfenden, 1978), a primary fitness benefit of helpers is not increased fledging success, but rather increased survival of young, including fledglings, largely as a result antipredator behavior around nests (Mumme, 1992). Thus, although there appears to be some benefit of feeding nestlings in terms of enhancing nestling condition, much of the benefit conferred by helpers is apparently not due to helping-at-the-nest per se, but rather to other beneficial, antipredator behaviors.
Yet another potential confound is that the presence of helpers may covary with factors associated with higher quality territories, and thus greater resource abundance, rather than any behavior or even the presence of helpers, may be driving the observed increased productivity. Thus, “why do helpers help?” remains an open question in many cooperatively breeding systems.
If feeding behavior per se is driving the fitness benefits conferred by helpers, one prediction is that the observed fitness benefits should mirror the patterns observed in the feeding behavior of helpers. If this is not the case, other behaviors are likely to be more important, especially to the extent that the patterns of variability observed in those behaviors match the observed fitness benefits of helpers. Alternatively, helpers may exhibit “alternative helping tactics.” For example, some individuals may provision offspring while others defend the territory. Analyses lumping helpers into a single category of helping behavior may cloud the interpretation of individual helper effects.
Here we address this issue in the cooperatively breeding acorn woodpecker (Melanerpes formicivorus). Our goal is to answer the question of whether helping-at-the-nest is driving the increased reproductive success of groups containing helpers, and if not, identifying other behaviors that may be contributing to those benefits.
Background and Questions
Acorn woodpeckers are cooperative breeders, common in western North America and highlands of Mexico and Central America, that live in polygynandrous family groups containing a variable number of breeders (1–8 breeder males and 1–4 breeder females) and an equally variable number (0–10) of non-breeding helpers, who may be of either sex (Koenig and Mumme, 1987; Koenig et al., 1995). Helpers are offspring of the breeders in the group and do not participate in reproduction either in their own or in other groups (Dickinson et al., 1995; Haydock et al., 2001). Thus, the system is not complicated by extra-group parentage and helpers are always closely related to the nestlings they help feed. Territories are typically focused around “granaries” containing hundreds to thousands of small holes, drilled by the birds over generations, in which acorns harvested directly off oak trees are stored each autumn and subsequently used as food for themselves during the winter and fed to nestlings, along with insects, the following spring (Koenig et al., 2008, 2016). The provisioning rate of helpers is also quite variable but substantial, particularly among older helpers (Koenig and Walters, 2011). Experimental studies have demonstrated that, despite considerable load-lightening (Koenig and Walters, 2012a), the feeding rate of both helpers and breeders is primarily determined by brood size rather than the converse (Koenig and Walters, 2012b).
Prior work has also indicated that there is a clear, positive effect of helper presence on fledgling success in this species. This effect, however, differs importantly between the sexes and, in the case of males, on food availability—with the positive effects of a male helper (but not a female helper) being significantly correlated with the size of the prior autumn's acorn crop (Koenig et al., 2011).
What are helpers doing that drives these results, particularly the dramatic difference in the dependence of the helper effect on the size of the acorn crop? Here we examine three hypotheses as to behavioral differences between male and female helpers that may be contributing to these patterns: (1) differences in nest-feeding rates, (2) differences in the amount of time birds spend storing acorns—important for subsequent nesting success—and tending storage facilities in the autumn, and (3) differences in “group-augmentation effects” (Kingma et al., 2014)—the proportion of time birds are present on or near their home territories and therefore able to contribute to other, non-nest-feeding-related cooperative behaviors benefiting the group such as defending the granary and scanning for predators.
Materials and Methods
Acorn woodpeckers were studied at Hastings Natural History Reservation, a field station run by the University of California, Berkeley located in central coastal California, USA (36.379°N, 121.567°W). Birds have been color-banded and studied continuously at this site since 1972 (MacRoberts and MacRoberts, 1976; Koenig and Mumme, 1987). A recent summary of work in this population is provided by Koenig et al. (2016).
Analyses here include data from 1,427 group-years of reproductive success between 1972 and 2018; 3,645 nest watches for a total of 10,807 h of observation conducted between 1979 and 2018; 63 granary watches for a total of 170 h of observation done during the autumn and winters of 2013, 2014, 2017, and 2018; and 4,042 days of radio-tracking of 55 different individuals between 1 July 2017 and 31 October 2018. The tracking data included 481 days of monitoring 10 different helpers (4 males and 6 females).
Nest watches were conducted from blinds using spotting scopes to identify all individuals feeding at nests. Granary watches were similar, with the number of visits and length of time individuals spent at granaries being recorded. Radio-tracking was performed using an automated telemetry system. Birds were fitted with solar-powered nanotags (Pegan et al., 2018) weighing <1% of body mass with leg loop harnesses adjusted for body size (Rappole and Tipton, 1991). Tagged birds were detected by a permanently installed array of 43 autonomous, solar-powered base stations placed at the center of territories or within the centroid of a cluster of territories when they were <100 m apart.
Tags emitted an encoded 64-bit radio ping every 1.5 s when exposed to sunlight that were detected by nearby base stations. Detections were stored in files created every 15 min and stored on removable memory drives collected once per week. Instances of birds being detected at two base stations simultaneously were resolved by assigning the bird's location based on the greatest signal strength. The data used here were primarily estimates of the proportion of time helpers spent on or in the vicinity of their home territory, calculated for all days when an individual was detected at home during at least one 15-min time period. Additional details regarding this system are provided in Barve et al. (submitted).
In addition to the bird data, analyses involved estimates of the acorn crop at the study site based on annual visual surveys of 250 oaks (Quercus spp.) divided among the five species common in the study area. Surveys were conducted starting in 1980 and involved averaging the ln-transformed number of acorns detected on trees by two observers each counting as many acorns as they could in a 15 s period using binoculars when necessary (Koenig et al., 1994a,b). Because the acorn crop influences the reproductive success of acorn woodpeckers the following year (Koenig et al., 2016), analyses involving the acorn crop were restricted to woodpecker breeding seasons from 1981 to 2018.
Analyses were performed in R 3.5.1 (R Core Team, 2018). Analysis of the statistical effect size of a single helper was estimated by linear regressions of the number of young fledged during the spring breeding season on group composition (number of breeder males, breeder females, helper males, and helper females), territory quality (based on the size of the group's granary; 1: <1,000 storage holes, 2: 1,000–2,500, 3: >2,500), prior breeding experience (whether there had been a change in the breeder composition of either or both males and females since the prior breeding season), and the overall size of the prior autumn's acorn crop. Adding the interactions between the number of helper males and the acorn crop and the number of helper females and the acorn crop to this analysis tested whether the effect of the prior autumn's acorn crop differed significantly between helper males and helper females. Interactions were visualized using the R package interplot (Solt and Hu, 2018). Linear regressions testing the effects of individual helpers on the number of young fledged, controlling for group composition and prior breeder experience, were also conducted for each year to correlate the effect size of helpers in a particular year vs. the prior autumn's acorn crop. This latter analysis updates one presented in Koenig et al. (2011).
Whether helper males feed more than helper females was tested in two ways. First, using only helpers, we performed a general mixed-effects linear model [lme in package nlme (Pinheiro et al., 2019)] with feeds hr−1 as the dependent variable; sex, age of the helper (1: first year; 2: second year; 3: third year and older), group size, number of nestlings, age of nestlings, and the prior autumn's acorn crop as fixed factors; and bird within nest within group as a random factor. The variable of interest in this analysis was the effect size of sex on feeding rates.
Second, we selected nest watches for which there were only one helper male and one helper female and performed a matched-pairs Wilcoxon test comparing feeding rates of the two helpers. This analysis had the advantage of controlling for not only the factors considered in the prior analysis but any other factors that may have affected feeding rates at individual nests. Tests were conducted including all watches in which at least one of the helpers (either the male or the female) fed, and including only watches at which both helpers fed.
To test whether the feeding rate of male helpers varied with the size of the prior autumn's acorn crop, we performed the same general mixed-effects linear model of feeding rates described above, but included the interaction term between the size of the acorn crop and sex of the helper. The main variable of interest in this analysis was the interaction term.
Feeding rates are only a partial measure of the amount of effort being expended on feeding nestlings. We also recorded data on the number of feeding visits in which birds fed insects and acorns, and the size of the bolus being fed (1 = small bolus—no obvious food seen; 2 = medium-size bolus—food seen but causing the bill to expand only 1–2 mm; 3 = large bolus—food expanding the bill >2 mm). We tested whether the proportion of feeds consisting of insects and acorns differed between male and female helpers using the glmer procedure of package lme4 (Bates et al., 2015). The mean size of boluses being fed to nestlings by male vs. female helpers was tested using procedure lme. Mean size of boluses was estimated as (1 × boluses of size 1 + 2 × boluses of size 2 + 3 × boluses of size 3) divided by the total number of feeds for which bolus size was recorded. In both analyses, age category of helpers, the size of the prior autumn's acorn crop, and the acorn crop × sex of helper interaction term were included as factors, and group was included as a random variable.
To test whether helper males spent more time tending granaries than helper females, we performed a generalized model with a binomial error distribution using the proportion of time during the watch the bird was observed in the granary as the dependent variable and sex, age of the helper, group size, and the size of the current acorn crop as main factors. To test whether helpers spent more time tending the granary when the acorn crop was greater, we performed a parallel analysis including the interaction between size of the acorn crop and helper sex.
To test whether male and female helpers spent a greater proportion of their time off their home territory, where they are presumably engaging in forays in search of reproductive vacancies (Hannon et al., 1985; Barve et al., submitted), we performed a generalized linear mixed-effects model with a binomial error distribution using the proportion of time during each day that birds were present on their home territory as the dependent variable; sex of helper, age of helper, group size, territory quality, and the acorn crop as the main factors; and bird within year as a random factor. The proportion of time during the day birds were present was estimated by dividing each day (between 05:00 and 20:00 h PST) into 15-min intervals and determining how many intervals the bird was detected at either their home base station or a base station <250 m away.
The telemetry data encompassed 1.5 years; during the first year (July 2017–June 2018) the acorn crop was relatively small (mean number of acorns counted per 30 s = 7.53), whereas during the second year (July 2018–October 2018) the acorn crop was relatively large (mean number of acorns counted per 30 s = 19.59). Thus, to test for an effect of the acorn crop on the proportion of time helpers spent on their home territory, the acorn crop was coded “1” for samples between July 2017 and June 2018, inclusive, and “2” starting in July 2018 as the current year's acorn crop matured. As in the prior analyses, two models were run, the first including “sex of helper” and “acorn crop” as main factors and the second including both factors and their interaction.
In all analyses, males were coded as “1” and females as “2”; thus, positive effect size of “sex of helper” indicate that female values were greater than male values, while negative effect sizes indicate the reverse. Unless otherwise indicated, values presented are means ± 1 standard error.
Results
Effects of the Acorn Crop on Helpers
Overall, both male and female helpers enhanced reproductive success by about 0.2 additional offspring per helper, although the overall benefit of a male helper was somewhat greater than that of a female helper (Table 1A). The more striking difference between the two was the dependence of the effect of a helper male, but not a helper female, on the size of the acorn crop (Table 1B). These differences are illustrated by the interaction terms between the number of helpers and the size of the prior autumn's acorn crop (Figure 1). On average, the effect of a helper on the reproductive success of a group is more or less the same regardless of the sex of the helper. However, the positive effect of a male helper, but not a female helper, increases with the size of the prior autumn's acorn crop.
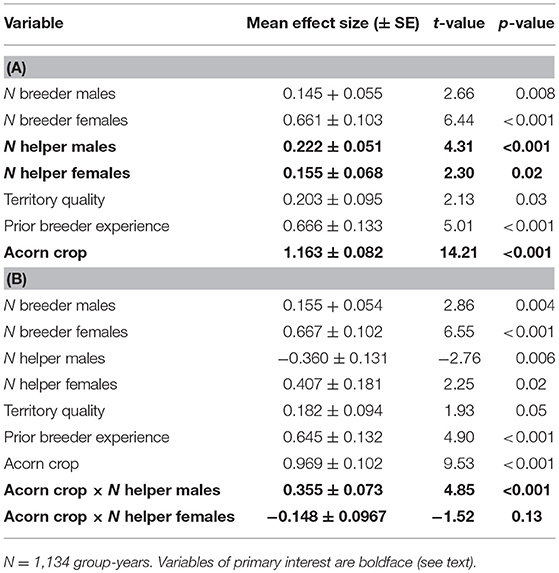
Table 1. Linear regressions of factors influencing reproductive success of groups (A) without interactions between number of helpers and the acorn crop and (B) including these interactions.
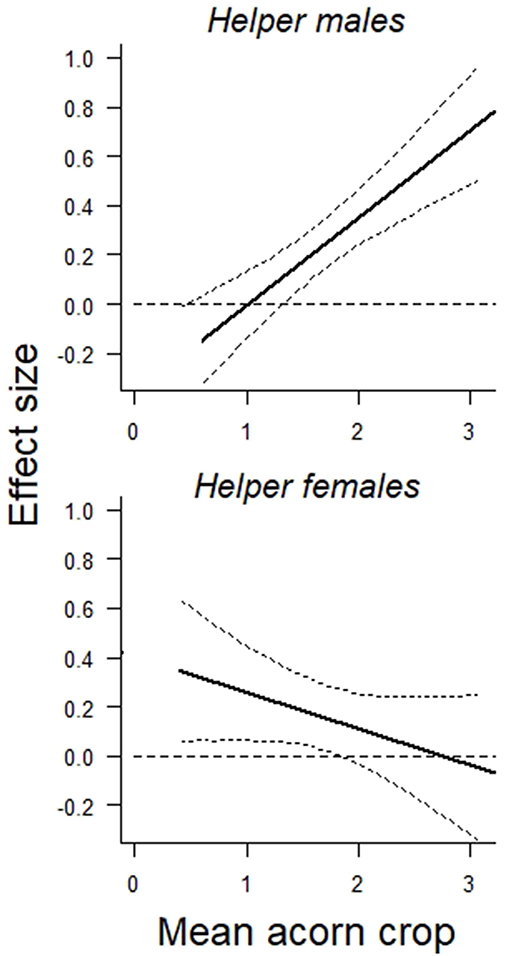
Figure 1. Graphical representation of the interaction between (Top) the prior autumn's acorn crop and the effect size of helper males on reproductive success of the group and (Bottom) the prior autumn's acorn crop and the effect size of helper females on reproductive success of the group. Both are based on linear regressions including group composition, territory quality, prior group history as main factors. Curved lines depict 95% confidence intervals. N = 38 years.
What drives these differences between the effects of male and female helpers on reproductive success? We addressed three hypotheses, testing for sex differences overall and for differences vis-à-vis the acorn crop.
Feeding of Nestlings
In a mixed-effects model of helper feeding rates, sex of the helper is highly significant, with female helpers feeding more frequently than male helpers (Table 2A). As a more tightly controlled comparison, we identified 213 feeding watches involving groups with one helper of each sex. Excluding cases where neither helper fed during the watch, mean feeding rate per hour per nestling (feeds hr−1 nestling−1) was 0.59 ± 0.06 for male helpers and 0.79 ± 0.06 for female helpers (matched-pairs Wilcoxon test, p = 0.03). Including only the 120 cases in which both helpers fed, the difference was even more pronounced (male helpers: 0.77 ± 0.08 feeds hr−1 nestling−1; female helpers: 1.16 ± 0.09 feeds hr−1 nestling−1; matched-pairs Wilcoxon test, p < 0.001).
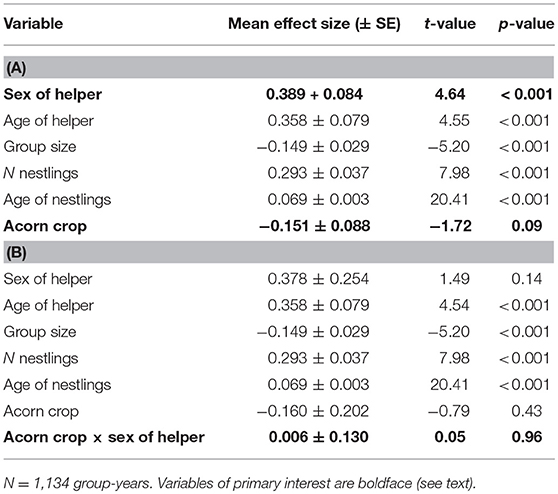
Table 2. Linear regressions of factors influencing feeding rate of helpers (A) without the interaction between number of helpers and the acorn crop and (B) including this interaction.
In the mixed-effects model without the interaction between sex of helper and the acorn crop, there was no significant relationship between the size of the acorn crop and feeding rates of helpers (Table 2A). In the parallel model including the interaction between the acorn crop and sex of helper the interaction was not significant (Table 2B).
The proportion of feeding visits in which helpers fed acorns increased significantly when the prior autumn's acorn crop was larger (Table 3A), and the mean bolus sizes fed to nestlings increased both with the size of the prior autumn's acorn crop and the age of helpers (Table 3B). In neither case, however, was there a significant difference between male and female helpers, nor was there a significant interaction between size of the prior autumn's acorn crop and sex of the helper (Table 3).
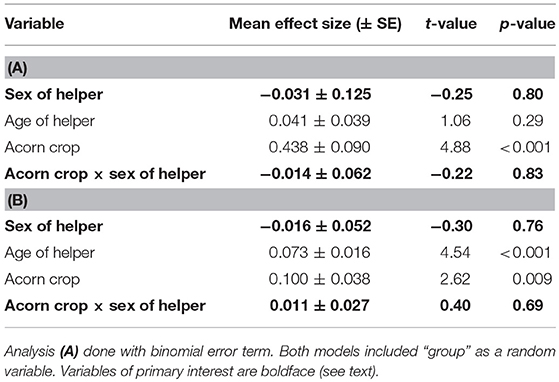
Table 3. General linear models of (A) the proportion of feeding visits in which helpers fed acorns vs. insects and (B) the mean bolus size of food fed to nestlings by helpers.
Time Spent Tending Granaries
Male helpers spent a significantly greater proportion of time tending granaries in the autumn than helper females (Table 4A). Mean proportion of time spent in the granary during watches was 16.8 ± 14.2% for male helpers and 5.9 ± 9.7% for female helpers.
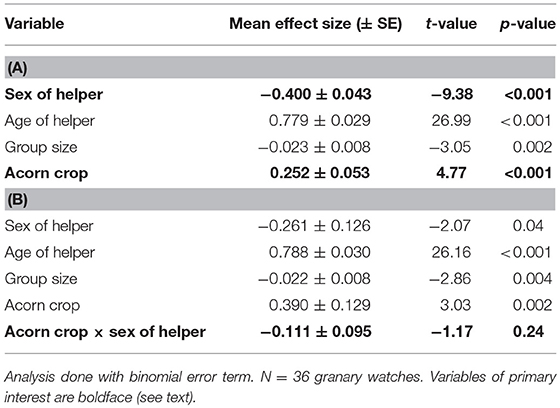
Table 4. General linear model of the proportion of time helpers spend tending the granary (A) without the interaction between the size of the acorn crop and sex of the helper and (B) including this interaction.
Helpers of both sexes spent more time tending granaries when the acorn crop was larger (Table 4A). Based on the interaction between the size of the acorn crop and sex of helper there was no significant sex difference in this relationship (Table 4B).
Time Spent on and Near the Home Territory
Tagged male helpers spent twice the proportion of time on or near their home territory as did helper females (males: 46.8 ± 2.1% of the day; helper females: 23.3 ± 1.3% of the day). This difference was statistically significant (Table 5).
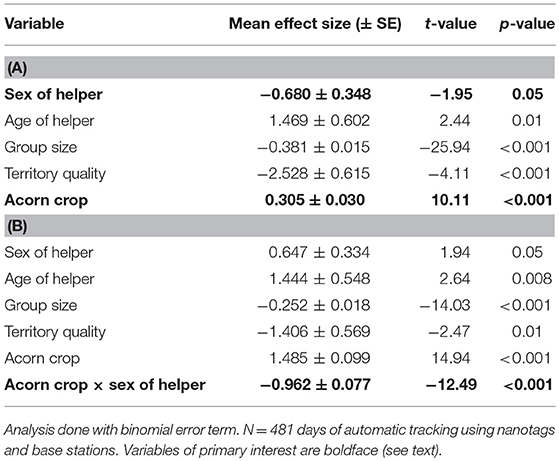
Table 5. Generalized linear mixed-effects model of the proportion of time helpers spend in the vicinity of their home territory (A) without the interaction between the size of the acorn crop and sex of the helper and (B) including this interaction.
Overall, helpers spent a significantly higher proportion of time on or near their home territory the year the acorn crop was greater (Table 5). There was, however, a highly significant sex difference, with the effect of the acorn crop on the proportion of time helpers spent on their home territory. Specifically, this effect was strongly positive for helper males (conditional coefficient from program interplot = 0.524 [95% confidence interval = 0.454 – 0.594]) but strongly negative for helper females (conditional coefficient = −0.439 [95% confidence interval = −0.568 to −0.308]).
Discussion
A key question in cooperative breeding has historically been, “Do helpers help?” (Emlen, 1991; Dickinson and Hatchwell, 2004; Cockburn et al., 2008). Although not resolved in all cases, and despite the fitness benefits of helping in some cases being hard to detect (Russell et al., 2007), decades of empirical and experimental studies have generally supported the conclusion that the answer is “yes.” Here we focus on the behavioral mechanism by which helpers help. Careful studies of a few species, including long-tailed tits (Aegithalos caudatus, Hatchwell et al., 2004) and chestnut-crowned babblers (Pomatostomus ruficeps, Liebl et al., 2016), provide good evidence that the behavior of feeding nestlings yields fitness benefits to helpers, but in many other taxa, including the acorn woodpeckers studied here, it is unclear whether the fitness benefits of helpers is derived from alloparental care or some other behavior associated with group augmentation (Koenig and Mumme, 1990).
As a result, we know little about the mechanisms by which the presence of helpers lead to increased success of the groups to which they belong. We know that helpers feed nestlings—indeed, this is how cooperative breeding is typically defined—but whether the fitness benefits of helpers is due to feeding at the nest, behavior of helpers subsequent to when nests fledge (as found in Florida scrub-jays; Mumme, 1992), or behaviors unrelated to breeding per se, such as predator defense, facilitating social foraging, or helping to stave off competitors, is often unknown.
Here we used the cooperative breeding acorn woodpecker system to test whether the increased reproductive success of groups containing helpers was correlated with the extent to which helpers fed nestlings, or whether the fitness benefits were likely a consequence of non-nesting-related behaviors. Helpers in this system are offspring of the breeders in the group and do not participate in reproduction either in their own or in other groups (Dickinson et al., 1995; Haydock et al., 2001); thus, patterns of helping behavior are not confounded by differences in either genetic relatedness of helpers to the offspring or alternative reproductive tactics. This allows for a test of the concordance between different activities of helpers and the observed fitness benefits they confer on the groups to which they belong.
In terms of their fitness effects: (1) helpers of both sexes conferred a modest reproductive benefit of ~0.2 additional offspring per helper, with the overall effect not differing between helper males and females; and (2) the effects of helper males, but not helper females, was strongly and positively correlated with the size of the prior autumn's acorn crop.
We tested three different behaviors for their concordance with these patterns, the goal being to identify which behaviors were likely contributing to the pattern of increased number of young fledged as a result of helpers being present. Results are summarized in Table 6.
First was the feeding rate of helpers at nests, for which the relationship with the observed patterns of reproductive success were poor. In contrast to the equal or slightly greater overall fitness effect of a helper male, feeding rates of helper females were greater than that of helper males. Moreover, there was no relationship between the size of the prior autumn's acorn crop and feeding rates, possibly in part because acorns constitute an relatively small, albeit important, proportion of the diet of nestlings (Koenig et al., 2008). We also found no differences between helper males and females in the proportion of feeding visits in which they fed acorns vs. insects, the mean bolus size of food fed to nestlings, or in patterns of compensatory care (load-lightening; Koenig and Walters, 2012a).
Concordance between granary tending and the reproductive benefits provided by helpers was better, as males spent more time tending granaries than females and males, but also females, spent somewhat more time tending granaries when the acorn crop was larger. The best of the three factors tested, however, was that between reproduction and the proportion of time helpers spent on their home territory, based on the automated telemetry data. Not only did helper males spend a considerably greater proportion of time on or near their home territory than helper females, but they did so significantly more during the year when the acorn crop was larger, in contrast to helper females, who spent a significantly smaller proportion of their time on their home territory during the better acorn year.
We conclude that the primary fitness benefit of helpers in this species is unlikely to be a consequence of their behavior of feeding at nests. Rather, the increased number of young fledged by groups containing helpers is apparently the result of other activities of helpers related to their presence on or near their home territory. In the case of acorn woodpeckers, such activities may include protecting and feeding young after fledging, helping store acorns and defending the granary, helping to look for and warn other group members when predators or larder thieves are spotted, and other coordinated and/or cooperative behaviors, many of which have been described in other family-living and cooperatively breeding species. The failure of “helping at the nest” to provide the primary fitness benefit of helpers in this population is consistent with the hypothesis that family living plays an essential role as a stepping stone to the more advanced altruistic behaviors exhibited by cooperative breeders (Griesser et al., 2017).
Quantifying the effects of these other beneficial behaviors is a challenge for the future. In the case of acorn woodpeckers, the positive relationship between the effects of male helpers and the acorn crop is likely due to behaviors they engage in during the higher proportion of time they spend on their natal territory when the acorn crop is good. The lack of such a relationship for helper females is possibly due to a tradeoff between the higher proportion of time they spend tending the granary and the smaller proportion of time they are present on or near their natal territory when the acorn crop is good (Table 6).
Besides focusing attention on the need for detailed behavioral data to understand what behaviors helpers are engaging in outside of nesting, that enhances the success of the group, our results highlight our failure to convincingly answer the question of why helpers feed nestlings when this behavior does not necessarily appear to be conferring increased reproductive success to the group. One possibility is that helping-at-the-nest is part of a strategy designed not to maximize young fledged but to decrease the probability of nest failure (Rubenstein, 2011), but prior work has not supported such a “bet-hedging” strategy in acorn woodpeckers (Koenig and Walters, 2015). Alternatively, patterns of helping-at-the-nest may be condition dependent (Russell et al., 2003) or complicated by feeding only when circumstances require additional help (Baglione et al., 2010), both of which would potentially obscure the relationship between feeding rates and fitness effects of helpers. These possibilities remain to be tested critically in acorn woodpeckers.
Our results are preliminary in that the data focusing on behaviors other than feeding at the nest are based on relatively small numbers of individuals. Nonetheless, they focus attention on the importance of gathering behavioral data on activities other than feeding at the nest to understand the fitness consequences of helping behavior and cooperative breeding in general. Developments in tracking technology such as used here will help to quantify where individuals spend their time, but will require integration of such data with detailed behavioral observations to understand exactly what individuals are doing that yields fitness benefits for themselves and the group as a whole. Only with such data will it eventually be possible to determine why helpers help, and what activities helpers engage in that drive the fitness effects they confer.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Field work was conducted under the auspices of the Animal Care and Use Committees of the University of California, Berkeley (protocol R010–0412), Cornell University (protocol 2008–0185) and Old Dominion University (protocol 12–001).
Author Contributions
WK conceptualized the idea, performed the statistical analyses, and wrote the initial draft of the paper. EW and WK provided funding. All authors contributed to field work and commented on the paper.
Funding
Funding was provided by National Science Foundation grants IOS-1455881, IOS-1455900, and DEB-1256394. Nanotag and base station development was funded in part by NSF grant DBI-1556138 to D.W. Winkler.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The manuscript was greatly improved by the comments of the reviewers. We thank our colleagues, the 150+ field and lab assistants that have assisted with the project since 1975, and David Winkler and Rich Gabrielson, who helped arrange for and design the radiotags and base stations. Special thanks to the staff of Hastings Natural History Reservation and the Museum of Vertebrate Zoology, University of California, Berkeley, for logistical support.
References
Baglione, V., Canestrari, D., Chiarati, E., Vera, R., and Marcos, J. M. (2010). Lazy group members are substitute helpers in carrion crows. Proc. R. Soc. B 277, 3275–3282. doi: 10.1098/rspb.2010.0745
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Browning, L. E., Patrick, S. C., Rollins, L. A., Griffith, S. C., and Russell, A. F. (2012). Kin selection, not group augmentation, predicts helping in an obligate cooperatively breeding bird. Proc. R. Soc. B 279, 3861–3869. doi: 10.1098/rspb.2012.1080
Cockburn, A., Sims, R. A., Osmond, H. L., Green, D. J., Double, M. C., and Mulder, R. A. (2008). Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438. doi: 10.1111/j.1365-2656.2007.01351.x
Crick, H. Q. P. (1992). Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis 134, 56–61. doi: 10.1111/j.1474-919X.1992.tb07230.x
Dickinson, J. L., and Hatchwell, B. J. (2004). “Fitness consequences of helping,” In: Ecology and Evolution of Cooperative Breeding in Birds, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 48–66. doi: 10.1017/CBO9780511606816.004
Dickinson, J. L., Haydock, J., Koenig, W. D., Stanback, M. T., and Pitelka, F. A. (1995). Genetic monogamy in single-male groups of acorn woodpeckers, Melanerpes formicivorus. Mol. Ecol. 4, 765–769. doi: 10.1111/j.1365-294X.1995.tb00277.x
Ekman, J., and Griesser, M. (2016). “Siberian jays: delayed dispersal in the absence of cooperative breeding,” in Cooperative Breeding in Vertebrates: Studies in Ecology, Evolution and Behavior, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 6–18. doi: 10.1017/CBO9781107338357.002
Emlen, S. T. (1982). The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39. doi: 10.1086/283888
Emlen, S. T. (1991). “Evolution of cooperative breeding in birds and mammals,” in Behavioural Ecology: An Evolutionary Approach, 3rd Edn, eds J. R. Krebs and N. B. Davies (Oxford: Blackwell Scientific Publications, 301–337.
Griesser, M., Drobniak, S. M., Nakagawa, S., and Botero, C. A. (2017). Family living sets the stage for cooperative breeding and ecological resilience in birds. PLoS Biol. 15:e2000483. doi: 10.1371/journal.pbio.2000483
Griffin, A. S., and West, S. A. (2003). Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636. doi: 10.1126/science.1089402
Hammers, M., Kingma, S. A., Spurgin, L. G., Bebbington, K., Dugdale, H. L., Komdeur, J., et al. (2019). Breeders that receive help age more slowly in a cooperatively breeding bird. Nat. Commun. 10:1301. doi: 10.1038/s41467-019-09229-3
Hannon, S. J., Mumme, R. L., Koenig, W. D., and Pitelka, F. A. (1985). Replacement of breeders and within-group conflict in the cooperatively breeding acorn woodpecker. Behav. Ecol. Sociobiol. 17, 303–312. doi: 10.1007/BF00293208
Hatchwell, B. J., Russell, A. F., MacColl, A. D. C., Ross, D. J., Fowlie, M. K., and McGowan, A. (2004). Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10. doi: 10.1093/beheco/arg091
Haydock, J., Koenig, W. D., and Stanback, M. T. (2001). Shared parentage and incest avoidance in the cooperatively breeding acorn woodpecker. Mol. Ecol. 10, 1515–1525. doi: 10.1046/j.1365-294X.2001.01286.x
Heinsohn, R. G. (2004). “Parental care, load-lightening, and costs,” in Ecology and Evolution of Cooperative Breeding in Birds, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 67–80. doi: 10.1017/CBO9780511606816.005
Kingma, S. A., Santema, P., Taborsky, M., and Komdeur, J. (2014). Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476–484. doi: 10.1016/j.tree.2014.05.013
Koenig, W. D. (2017). What drives cooperative breeding? PLoS Biol. 15:e2002965. doi: 10.1371/journal.pbio.2002965
Koenig, W. D., Knops, J. M. H., Carmen, W. J., Stanback, M. T., and Mumme, R. L. (1994a). Estimating acorn crops using visual surveys. Can. J. For. Res. 24, 2105–2112. doi: 10.1139/x94-270
Koenig, W. D., and Mumme, R. L. (1987). Population Ecology of the Cooperatively Breeding Acorn Woodpecker. Princeton, NJ: Princeton University Press.
Koenig, W. D., and Mumme, R. L. (1990). “Levels of analysis and the functional significance of helping behavior,” in Interpretation and Explanation in the Study of Animal Behavior, Vol. 2, eds M. Bekoff and D. Jamieson (Boulder, CO: Westview Press, 268–303.
Koenig, W. D., Mumme, R. L., Carmen, W. J., and Stanback, M. T. (1994b). Acorn production by oaks in central coastal California: variation in and among years. Ecology 75, 99–109. doi: 10.2307/1939386
Koenig, W. D., and Pitelka, F. A. (1981). “Ecological factors and kin selection in the evolution of cooperative breeding in birds,” in Natural Selection and Social Behavior: Recent Research and New Theory, eds R. D. Alexander and D. W. Tinkle (New York, NY: Chiron Press, 261–280.
Koenig, W. D., Schaefer, D. J., Mambelli, S., and Dawson, T. E. (2008). Acorns, insects, and the diet of adult versus nestling acorn woodpeckers. J. Field Ornithol. 79, 280–285. doi: 10.1111/j.1557-9263.2008.00174.x
Koenig, W. D., Stacey, P. B., Stanback, M. T., and Mumme, R. L. (1995). “Acorn woodpecker (Melanerpes formicivorus),” in The Birds of North America, No. 194, eds A. Poole and F. Gill (Washington, DC: Academy of Natural Sciences, Philadelphia, and The American Ornithologists' Union), 1–24. doi: 10.2173/bna.194
Koenig, W. D., and Walters, E. L. (2011). Age-related provisioning behaviour in the cooperatively breeding acorn woodpecker: testing the skills and the pay-to-stay hypotheses. Anim. Behav. 82, 437–444. doi: 10.1016/j.anbehav.2011.05.028
Koenig, W. D., and Walters, E. L. (2012a). Brooding, provisioning, and compensatory care in the cooperatively breeding acorn woodpecker. Behav. Ecol. 23, 181–190. doi: 10.1093/beheco/arr172
Koenig, W. D., and Walters, E. L. (2012b). An experimental study of chick provisioning in the cooperatively breeding acorn woodpecker. Ethology 118, 566–574. doi: 10.1111/j.1439-0310.2012.02043.x
Koenig, W. D., and Walters, E. L. (2015). Temporal variability and cooperative breeding: testing the bet-hedging hypothesis in the acorn woodpecker. Proc. R. Soc. B 282:20151742. doi: 10.1098/rspb.2015.1742
Koenig, W. D., Walters, E. L., and Haydock, J. (2011). Variable helpers effects, ecological conditions, and the evolution of cooperative breeding in the acorn woodpecker. Am. Nat. 178, 145–158. doi: 10.1086/660832
Koenig, W. D., Walters, E. L., and Haydock, J. (2016). “Acorn woodpeckers: helping at the nest, polygynandry, and dependence on a variable acorn crop,” in Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution and Behavior, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 217–236. doi: 10.1017/CBO9781107338357.014
Leonard, M. L., Horn, A. G., and Eden, S. F. (1989). Does juvenile helping enhance breeder reproductive success? A removal experiment on moorhens. Behav. Ecol. Sociobiol. 25, 357–361. doi: 10.1007/BF00302993
Liebl, A. L., Nomano, F. Y., Browning, L. E., and Russell, A. F. (2016). Experimental evidence for fully additive care among male carers in the cooperatively breeding chestnut-crowned babbler. Anim. Behav. 115, 47–53. doi: 10.1016/j.anbehav.2016.02.024
MacRoberts, M. H., and MacRoberts, B. R. (1976). Social organization and behavior of the acorn woodpecker in central coastal California. Ornithol. Monogr. 21, 1–115. doi: 10.2307/40166738
Magrath, R. D., and Yezerinac, S. M. (1997). Facultative helping does not influence reproductive success or survival in cooperatively breeding white-browed scrubwrens. J. Anim. Ecol. 66, 658–670. doi: 10.2307/5919
Mumme, R. L. (1992). Do helpers increase reproductive success? Behav. Ecol. Sociobiol. 31, 319–328. doi: 10.1007/BF00177772
Mumme, R. L., Koenig, W. D., and Ratnieks, F. L. W. (1989). Helping behaviour, reproductive value, and the future component of indirect fitness. Anim. Behav. 38, 331–343. doi: 10.1016/S0003-3472(89)80094-6
Pegan, T. M., Craig, D. P., Gulson-Castillo, E. R., Gabrielson, R. M., Bezner Kerr, W., MacCurdy, R., et al. (2018). Solar-powered radio tags reveal patterns of post-fledging site visitation in adult and juvenile tree swallows Tachycineta bicolor. PLoS ONE 13:e0206258. doi: 10.1371/journal.pone.0206258
Pennisi, E. (2005). How did cooperative behavior evolve? Science 309:93. doi: 10.1126/science.309.5731.93
Pinheiro, J., Bates, D., Debroy, S., and Sarkar, D. (2019). Linear and Nonlinear Mixed Effects Models, Version 3.1-140. Available online at: https://CRAN.R-project.org/package=nlme
R Core Team (2018). R 3.5.1: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org
Rappole, J. H., and Tipton, A. R. (1991). New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337.
Riehl, C. (2013). Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B 280:20132245. doi: 10.1098/rspb.2013.2245
Rubenstein, D. R. (2011). Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl. Acad. Sci. U.S.A. 108, 10816–10822. doi: 10.1073/pnas.1100303108
Russell, A. F., and Hatchwell, B. J. (2001). Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. R. Soc. B 268, 2169–2174. doi: 10.1098/rspb.2001.1790
Russell, A. F., Langmore, N. E., Cockburn, A., Astheimer, L. B., and Kilner, R. M. (2007). Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944. doi: 10.1126/science.1146037
Russell, A. F., Sharpe, L. L., Brotherton, P. N., and Clutton-Brock, T. H. (2003). Cost minimization by helpers in cooperative vertebrates. Proc. Natl. Acad. Sci. U.S.A. 100, 3333–3338. doi: 10.1073/pnas.0636503100
Sherman, P. W. (1988). The levels of analysis. Anim. Behav. 36, 616–619. doi: 10.1016/S0003-3472(88)80039-3
Solt, F., and Hu, Y. (2018). Interplot: Plot the Effects of Variables in Interaction Terms. Available online at: https://cran.r-project.org/web/packages/interplot/vignettes/interplot-vignette.html
Stallcup, J. A., and Woolfenden, G. E. (1978). Family status and contributions to breeding by Florida scrub jays. Anim. Behav. 26, 1144–1156. doi: 10.1016/0003-3472(78)90104-5
Keywords: acorn woodpecker, automated telemetry, cooperative breeding, helpers-at-the-nest, helping behavior, Melanerpes formicivorus
Citation: Koenig WD, Walters EL and Barve S (2019) Does Helping-at-the-Nest Help? The Case of the Acorn Woodpecker. Front. Ecol. Evol. 7:272. doi: 10.3389/fevo.2019.00272
Received: 30 April 2019; Accepted: 02 July 2019;
Published: 17 July 2019.
Edited by:
James Luke Savage, University of Sheffield, United KingdomReviewed by:
Michael Griesser, University of Zurich, SwitzerlandVittorio Baglione, Universidad de León, Spain
Andy Russell, University of Exeter, United Kingdom
Copyright © 2019 Koenig, Walters and Barve. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walter D. Koenig, wdk4@cornell.edu
 Walter D. Koenig
Walter D. Koenig Eric L. Walters
Eric L. Walters Sahas Barve
Sahas Barve