Mining Anti-Inflammation Molecules From Nippostrongylus brasiliensis-Derived Products Through the Metabolomics Approach
- 1Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Key Laboratory of National Health Commission on Parasitic Disease Control and Prevention, Key Laboratory of Jiangsu Province on Parasite and Vector Control Technology, Jiangsu Institute of Parasitic Diseases, Wuxi, China
- 3Department of Cardiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 4Department of Cardiology, The Friendship Hospital of Ili Kazakh Autonomous Prefecture Ili & Jiangsu Joint Institute of Health, Ili, China
Hookworm is one type of soil-transmitted helminth, which could exert an anti-inflammatory effect in human or animal host, which provides a beneficial possibility for the discovery of inflammatory-related disease interventions. The identification of hookworm-derived anti-inflammatory molecules is urgently needed for future translational research. The emergence of metabolomics has become a powerful approach to comprehensively characterize metabolic alterations in recent times. Herein, excretory and secretory products (ESPs) were collected from cultured adult worm, while small intestinal contents were obtained from Nippostrongylus brasiliensis (N. brasiliensis, Nb)-infected mice. Through ultra-high-performance liquid chromatography coupled with mass spectrometry (UHPLC-MS) platform, metabolomics analysis was used to explore the identification of anti-inflammatory molecules. Out of 45 differential metabolites that were discovered from ESPs, 10 of them showed potential anti-inflammatory properties, which could be subclassed into amino acids, furanocoumarins, linear diarylheptanoids, gamma butyrolactones, and alpha-keto acids. In terms of intestinal contents that were derived from N. brasiliensis-infected mice, 14 out of 301 differential metabolites were discovered to demonstrate anti-inflammatory effects, with possible subclassification into amino acids, benzylisoquinolines, quaternary ammonium salts, pyrimidines, pregnane steroids, purines, biphenyls, and glycerophosphocholines. Furthermore, nine of the differential metabolites appeared both in ESPs and infected intestinal contents, wherein four were proven to show anti-inflammation properties, namely, L-glutamine, glutamine (Gln), pyruvate, and alanine-Gln (Ala-Gln). In summary, we have provided a method for the identification and analysis of parasite-derived molecules with potential anti-inflammatory properties in the present study. This array of anti-inflammatory metabolites could provide clues for future evaluation and translational study of these anti-inflammatory molecules.
Introduction
As a soil-transmitted helminth, hookworm has been implicated in the incidence of several conditions, namely, iron deficiency anemia (IDA), malnutrition, and other chronic health problems, which are defined by intensity of infection in human host, wherein they can cause impaired physical and cognitive development as well as adverse outcome of pregnancy and lethargy (Loukas et al., 2016). Humans could be infected by three principal species of hookworm, viz., Ancylostoma ceylanicum, Ancylostoma duodenale, and Necator americanus, which complete their life cycle through skin penetration, pulmonary migration, and small intestine maturity in their host (Brooker et al., 2004). Hookworm infection remains an important health problem in areas with inadequate sanitation (namely rural subtropical and tropical countries), wherein it affects almost 500 million people with approximately 4.1 million annual loss of disability adjusted life years (DALYs) (Bartsch et al., 2016). Meanwhile, with a major burden of hookworm infection in areas described above, epidemiological evidence showed that a negative correlation was observed between hookworm infection and occurrence/frequency of inflammatory diseases such as metabolic disorders, allergic conditions, and inflammatory bowel disease (IBD), which could be defined as “hygiene hypothesis” (Maizels et al., 2004; Briggs et al., 2016; Ryan et al., 2020). Guided by this hypothesis, numerous studies of helminth-based therapy for different inflammatory disease models have been explored in recent years, wherein it was found that derived products of helminths showed drug-like anti-inflammatory activities in human, mice, and other larger animals (Summers et al., 2005; McSorley et al., 2012; Scholmerich et al., 2017). However, different mechanisms were elucidated in various models intervened by different species of helminth or derived molecules, including alternatively activated macrophages, mucus production, and wound repairing as well as regulatory cell population-induced abundance production of IL-10 and powerful drivers of type II immune responses (Harris and Loke, 2018; Lothstein and Gause, 2021). Moreover, microbiome and its metabolite interactions with helminths might also become increasingly important for anti-inflammatory mechanisms (Ramanan et al., 2016; Brosschot and Reynolds, 2018). Thus, identification and evaluation of helminth-derived molecules as anti-inflammatory agents are urgently needed for future translational research.
Considering the ethical concerns of live helminth infection, numerous helminth-derived molecules including proteins, lipids, and enzymes have been identified to potentially exert several functions of immunoregulation in different models of inflammatory disease (Lothstein and Gause, 2021). Ac-AIP-2 and Nb-DNase II, derived from two hookworm experimental models, namely, Ancylostoma caninum (hookworm in dog) and N. brasiliensis (rodent hookworm), could modulate innate immune responses and regulate dendritic cell development and Treg activation in vitro and in vivo (Navarro et al., 2016; Bouchery et al., 2020). As hookworm parasitizes in the small intestine of the host, hookworm-derived excretory and secretory products (ESPs) including soluble proteins, small molecules, and extracellular vesicles could collectively play an important role in host–pathogen interactions. However, previous studies mainly focused on hookworm-derived proteins or enzymes with immune regulation or anti-inflammatory properties. Only two papers focused on the non-protein small metabolites derived from N. brasiliensis, which identified an array of small metabolites with potential anti-inflammatory activities in vitro (Wangchuk et al., 2019; Yeshi et al., 2020). However, it is still unclear what might happen to the metabolic process in vivo during N. brasiliensis infection and/or whether these small molecules could also exist or act within the small intestinal environment of the host.
Metabolomics, as one of the “omics” technologies, has egressed to become a powerful approach to comprehensively characterize metabolic alterations in recent times, wherein among other omics strategies, it is generally recognized as being closer and more representative of the phenotype (Nicholson et al., 2012; Guijas et al., 2018). Mass spectrometry (MS)-based analysis platform makes it possible to identify and quantify small-molecule metabolites from different tissue or body fluid samples, which possibly reflect the health or disease status and provide clues for disease diagnosis and treatment (Shah et al., 2015). For parasite infection, metabolomics analysis has been mostly applied for the discovery of biomarkers and the identification of a new drug target or intervention strategies (Legido-Quigley, 2010; Li et al., 2016; Huang et al., 2020). However, only very few studies focused on the identification of anti-inflammatory molecules during parasite infection. In the present study, we utilized a more sensitive metabolomics platform, ultra-high-performance liquid chromatography coupled with mass spectrometry (UHPLC-MS), to perform analysis of ESPs that were collected from cultured N. brasiliensis adult worm (in vitro) and small intestinal contents from N. brasiliensis-infected mice (in vivo), accordingly. Furthermore, we carried out a comparative analysis to explore common metabolites and reveal the N. brasiliensis-derived anti-inflammatory metabolites (as shown in Figure 1A).
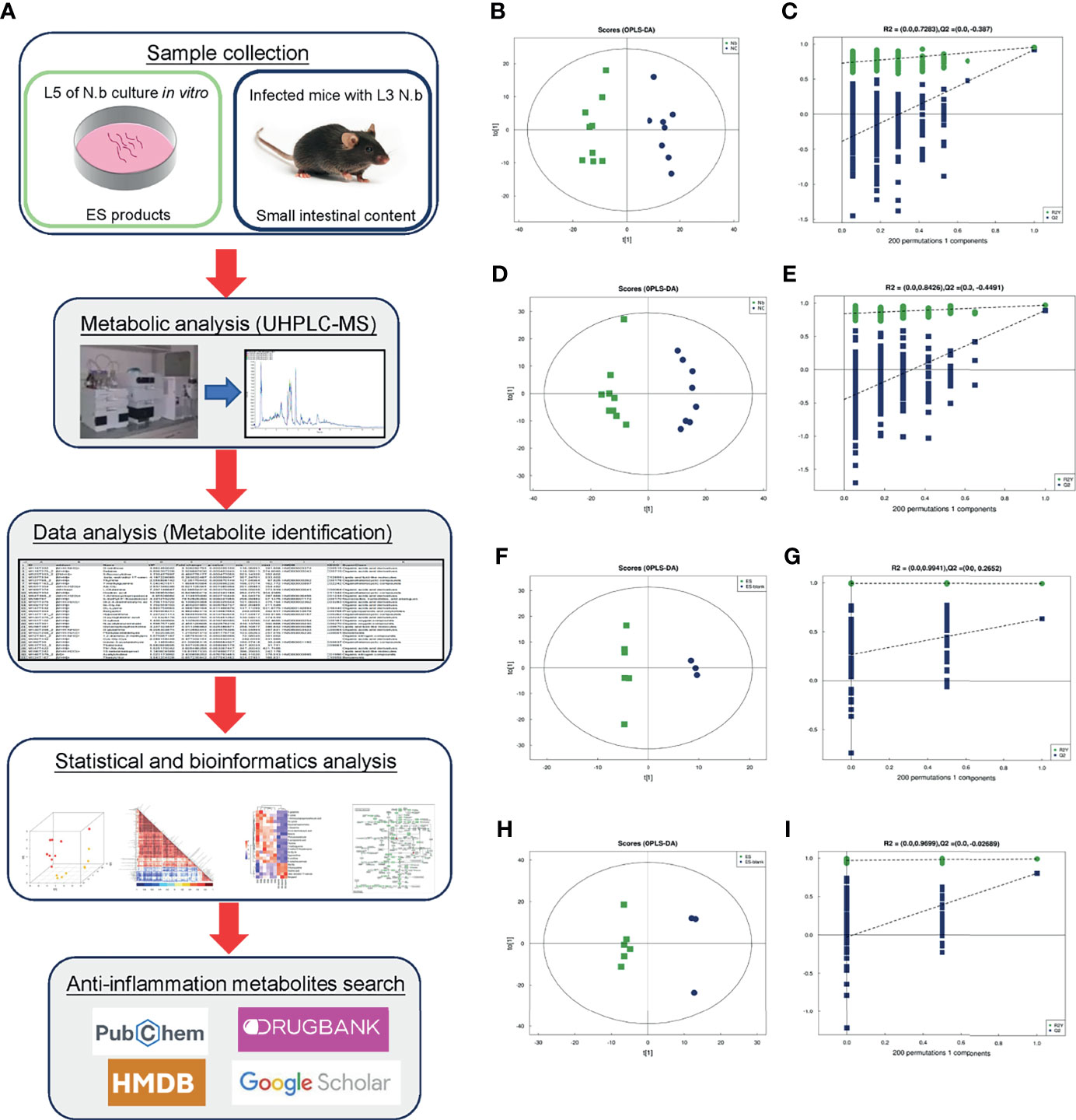
Figure 1 Schematic flowchart, OPLS-DA score plots, and corresponding permutation tests in positive and negative modes of the present study. Schematic flowchart of metabolomic analysis for ESPs and intestinal content from Nb-infected mice (A). The scatter plots of the OPLS-DA score of ion mode: (B, F) positive and (D, H) negative. The results of the permutation test of ion mode: (C, G) positive and (E, I) negative.
Materials and Methods
Parasite, Animal, and Ethical Statement
Nippostrongylus brasiliensis (a kind gift by Professor Alex Loukas at James Cook University, Australia) was maintained and cycled in the laboratory of Jiangsu Institute of Parasitic Diseases (JIPD) according to a previous published reference (Camberis et al., 2003). C57BL/6 mice (5 weeks old, male) and Sprague–Dawley rats (about 300 g of weight each, male) that were provided by the Animal Center of JIPD (Wuxi, China) were used in the present study. Standard conditions (20–25°C and 12 h light–12 h dark) were maintained in the laboratory where the animals were housed amid unrestricted access to standard chow and water. The Ethical Committee for the Use of Experimental Animals at JIPD (Wuxi, China) gave approval to the protocol of the experiments.
Nippostrongylus brasiliensis Culture and ESP Preparation
Sprague–Dawley rats were used to maintain N. brasiliensis and to collect adult worm as described previously (Camberis et al., 2003). Briefly, L3 stage larvae (3,500 larvae/rat) were used to infect the rats via the subcutaneous route, before they were euthanized with carbon dioxide asphyxiation on day 7 post-infection. Later, the rats were dissected to collect the adult worm from their small intestine. The small intestine was moved into a clean Petri dish containing 5 ml Dulbecco’s phosphate-buffered saline (DPBS) (Gibco-Thermo Fisher, MA, USA), prior to longitudinal slicing and cutting into smaller pieces. Afterwards, pieces of the intestines were placed on a gauze at the bottom of a funnel before it was filled with DPBS. Adult worms migrated out after 2 h of incubation (37°C) to settle at the underneath of the tube but retained debris of the intestinal contents of the host. The suspension containing worms was transferred from the bottom of the tube to a sterile centrifugated tube (50 ml), prior to washing twice with 20 ml of DPBS comprising antibiotic-antimycotic(5×) (Gibco-Thermo Fisher, MA, USA) amid counting under a dissecting microscope. Using 24-well (500 worms/well) plates, the culturing of adult worms was done for 7 days in RPMI-1640 medium (Gibco-Thermo Fisher, MA, USA) containing antibiotic-antimycotic(1×) under 37°C and 5% CO2 conditions. The cultured supernatant was collected every 24 h and replaced with fresh medium. Through centrifugation (at 2,000g and 4°C) for 10 min, the eggs and parasite fragments were removed and filtered with a 0.22-μm filter to obtain the final ESPs before storage at −80°C till it was used in a later experiment.
Animal Infection and Sample Collection In Vivo
The C57BL/6 mice were used for infection and metabolomics analysis in vivo. Briefly, allocation of the mice into two groups (10 mice per group), namely, N. brasiliensis (Nb) and negative control (NC) groups, was done prior to the respective subcutaneous inoculation with L3 larvae of N. brasiliensis (500 larvae/mouse) or sterile saline (both in a 100-μl volume). On day 12 post-inoculation, the entire group of mice was euthanized and their small intestines were taken out under sterile conditions. The small intestinal content from each mouse was scraped into a 1.5-ml sterile tube, weighed, and preserved by storing at −80°C until further experiment.
Analysis of Samples Using the LC-MS/MS Technique
An UHPLC (Agilent Technol-1290 infinity LC) that has been coupled to a time-of-flight (TOF) quadrupole platform (AB Sciex Triple-TOF 6600, Applied Protein Technol. Co. Ltd., Shanghai, China) was applied for further metabolomical analysis of the samples that were collected from ESPs and small intestinal contents of N. brasiliensis-infected mice coupled with negative controls, respectively (ES vs. ES-blank, Nb vs. NC). Also, separation of the samples with the HILIC technique was performed on a specialized column (ACQUIY-UPLC-BEH, Waters, Ireland). Mobile phase A consisted of ammonium acetate (25 mM)/aqueous ammonium hydroxide (25 mM), while mobile phase B consisted of acetonitrile in the positive and negative ESI modes. The mobile phase composition was altered as follows: for 1 min, B was maintained at 85% before linear reduction to 65% (within 11 min) and further reduction to 40% (within 0.1 minute) prior to keeping at this composition for 4 min. Later, the mobile gradient increased to 85% within 0.1 min but allowed a re-equilibration period of 5 min. Separation of the samples via the RPLC system was carried out on the Waters column (ACQUIY-UPLC-HSS T3, Ireland). The composition of the mobile phase for the positive ESI mode (positive) was water comprising formic acid (0.1%)—A and acetonitrile containing formic acid (0.1%)—B, while that of the negative ESI mode was made up of aqueous ammonium fluoride (0.5 mM)—A and acetonitrile—B. Gradient elution was performed by maintaining B at 0.1% for 1.5 min before linear increase to 99% within 11.5 min prior to further keeping the gradient for 3.5 min. Afterwards, the gradient was reduced to 1% within 0.1 min and allowed a re-equilibration period of 3.4 min. The temperature of the column was maintained at 25°C, while 0.3 ml/min was the flow rate of the mobile phases with sample injected in aliquots (2 µl). The following are the conditions of the ESI source: curtain gas (CUR), 30; gas 1, 60; gas 2, 60; source temperature, 600°C; and ion-spray voltage floating (ISVF), ± 5,500 V. The mass/charge (m/z) range for MS (for acquisition only) was set over 60–1,000 Da, while 0.20 s/spectra was the TOF-MS scan accumulation time. Likewise, the m/z range for the MS/MS auto method for acquisition was set over 25–1,000 Da with 0.05 s/spectra being the product ion scan (PIS) accumulation time. Acquisition of PIS was carried out in high sensitive mode through information-dependent acquisition (IDA). The following parameters were fixed accordingly: 35 V with ±15 eV as collision energy (CE) and −60 V (−) and 60 V (+) as declustering potential (DP), with the exclusion of 4 Da isotopes and monitoring of ion candidate per cycle (set at 10).
Processing of Data
Prior to importation to XCMS software (freely available), the conversion of the raw data of MS (from.wiff file to.mzXML file) was carried out with Proteo-Wizard MSConvert. Parameters such as 25 ppm for cent-Wave m/z, c(10, 60) for peak-width, and c(10, 100) for pre-filter were used for picking the peaks. Likewise, grouping of the peaks was done using the accompanying parameters, viz., mzwid (0.025), minfrac (0.5), and bw (5). Isotopic and adduct annotations were performed with the collection of algorithms of metabolite profile annotation (CAMERA). Technically, variables that had more than 50% of the non-zero measurement values (in at least one group) were kept and employed for the extracted ion features. We established a database in our lab using reliable standards there were readily available to identify metabolites via comparison with accurate m/z value (less than 25 ppm) and spectra of MS/MS.
Statistical Analysis
The R package (Ropls) was used to analyze the processed data after sum normalization. Multivariable analysis of data was performed, which was comprised of orthogonal partial least squares discriminant analysis (OPLS-DA) and principal component analysis (PCA) coupled with Pareto scaling. Evaluation of model robustness was done with the seven-fold cross-validation and response permutation testing. Using the OPLS-DA model, the variable importance in projection (VIP) score of each variable was computed to show the contribution of VIP to the classification. Determination of significant differences within two groups of independent measurements was statistically carried out with Student’s t-test. Significantly changed metabolites were screened when p <0.05 and VIP >1. Correlation between two variables was analyzed using Pearson’s correlation analysis.
Differentially expressed metabolites were analyzed through the metabolomics pathway using MetaboAnalyst. Presentation of Nb infection-associated pathways was done based on pathway impact and p-values derived from pathway topology and pathway enrichment analyses, respectively.
Comprehensive Literature Searches and Content Analyses of Metabolites With Pharmacological Effects
Literature searches and database survey were comprehensively conducted (for each metabolite) on the account of the list of differential metabolites that were identified from the Nb and ES groups. Databases that were searched included the Human Metabolome Database (HMDB, comprising metabolite entries of 114,100, including metabolites that are soluble in lipid and water) (Wishart et al., 2013) and PubChem (Kim et al., 2016). Additionally, references that were relevant to the biological properties of each metabolite were identified through Google Scholar. Anti-inflammatory, biological activities, and immune regulation were the specific keywords that ensured a unique search. Performance of database and reference content analyses focused on previously reported biological properties, which was subsequently tabularized and quoted against each substance.
Results
System Stability Assessment
In this experiment, changes in the metabolic profile of the samples were analyzed through metabolomics methods on the account of UHPLC-Q TOF-MS technology. As shown by the chromatographic total ion (TIC) technique of QC samples, overlapping of the intensity and retention time of each chromatographical peak was observed. Analysis of peaks that were excerpted from the entire experimental and QC samples was performed using the PCA method with results shown in Supplementary Figure S1, wherein outliers were obviously seen in the samples of positive and negative ion modes, while those of the QC were clustered closely. The results suggest the stability of the system of instrumental analysis used in this experiment and the reliability of the experimental data obtained. Thus, the observed metabolic spectrum differences during the experiment could reflect the biologic differences between the samples.
Multivariate Statistical Analysis of Metabolite Profiling
After multivariate pattern recognition analysis of OPLS-DA, the metabolic profile of Nb was observed to be distinct from that of NC. Thus, the OPLS-DA model (Figure 1) could clearly distinguish between Nb and NC. The heatmap constructed from 17 samples of mice (Figures 3A, B) supported the aforementioned observation. Testing of the robustness of the model showed a good predictive model performance (for the negative ion mode: R2Y = 0.842 and Q2 = 0.608, for the positive ion mode: R2Y = 0.986 and Q2 = 0.956) but was not overfitting (Figures 1C, E), indicating that Nb infection may induce obvious alterations in the metabolism of the mice.
Similarly, a supervised analysis of the ES group was done with OPLS-DA analysis. Observation of the OPLS-DA score plots showed a clear discrimination between the ES and the control groups. The permutation tests verified the validity of the model (for the positive ion mode: R2Y = 0.996 and Q2 = 0.637, for negative ion mode: R2Y = 0.995 and Q2 = 0.807) (Figures 1G, I). Subsequently, heatmaps of ES metabolic characteristics were carried out to view the data more intuitively (Figures 3C, D). The results of heatmaps were consistent with those of OPLS-DA. Overall, the above results indicate that the adult worms secreted several small molecules.
Screening and Analysis of Differential Metabolites
Through fold change (FC) and t-test methods, the volcano plot analysis was used to identify distinctive metabolites. Easy isolation of Nb infection-induced metabolites and identification of excretory secretions of adult worms were carried out with the help of the volcano plots. As shown in Figure 2, points that have the greatest degrees of difference are those found at the two extremes of the plot. Log2(FC > 1.5 or <0.67) was plotted on the x-axis with −log10(p-value, derived from t-test) on the y-axis (Figure 2).
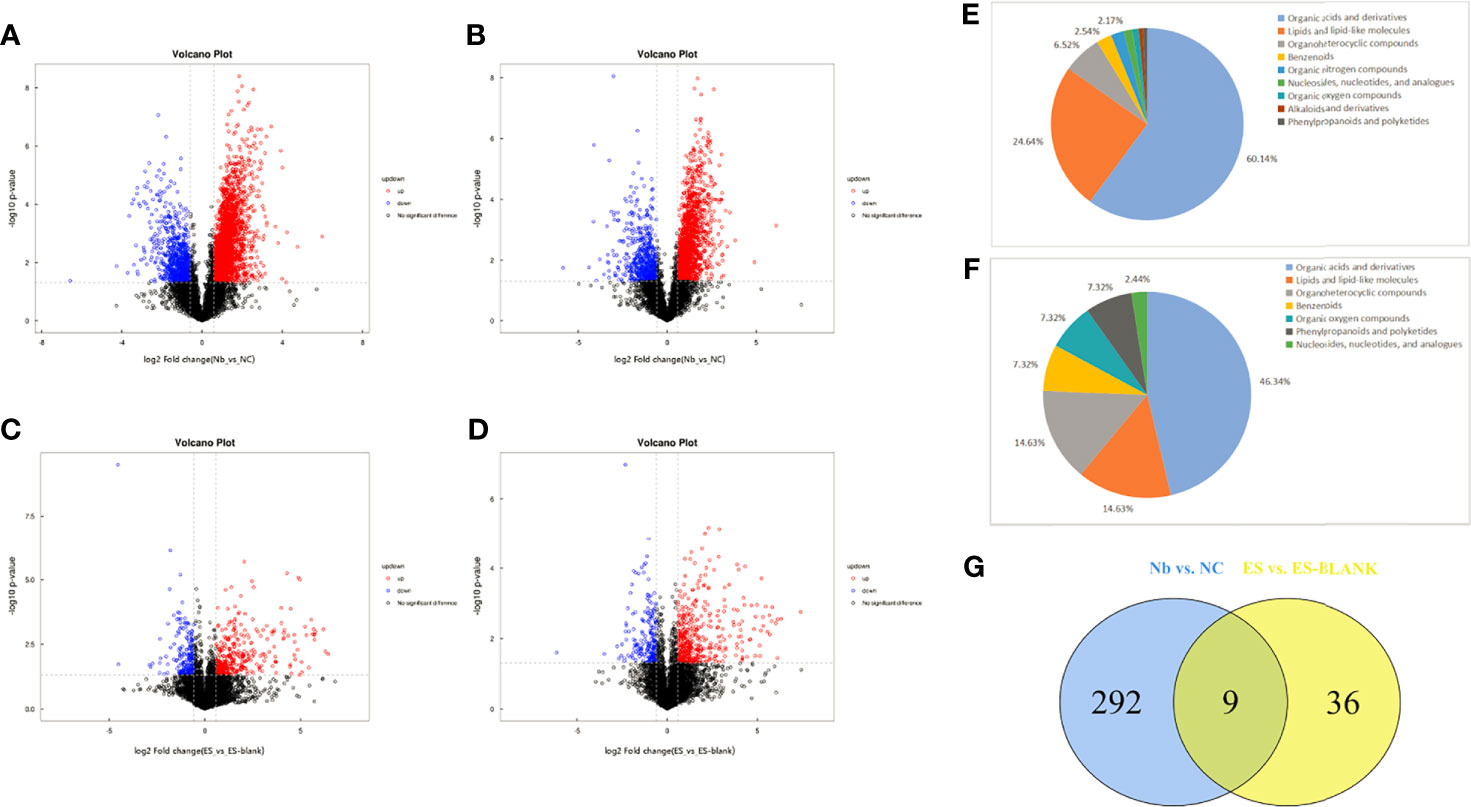
Figure 2 Unique and common differential metabolites in Nb vs. NC and ES vs. ES-blank groups. The volcano maps of differential metabolites of positive (A, C) and negative (B, D) ion modes. Red scatters denote the upward trend in metabolites, blue scatters indicate the downward trend in metabolites, and black scatters show the non-significant trend in metabolites. The chemical classification chart of distinct metabolites in the Nb (E) and ES groups (F) as well as common and unique metabolite distribution within the two sample groups (G). Different colors in the picture indicate distinct chemical classifications. Percentage represents the proportion of the metabolite number in the chemical classification to the total metabolite number. The Venn diagram shows the overlap of the significantly different metabolites. Two circles were used to denote the clustering of the entire distinctly expressed metabolites into two comparison groups. The number of distinctly expressed metabolites in one comparison group was taken as the sum of the entire figures represented in one circle.
Calculation of VIP score for the individual variables in the OPLS-DA model was done to assess the VIP contribution to classification. Obviously, a total of 301 metabolites exhibited abundance shifts after the mice were infected with Nb, including downregulated (71) and upregulated (230) metabolites. In totality, 45 different metabolites were screened among the ES/ES-blank, wherein 16 were downregulated, while 29 were upregulated. Supplementary Table S1 shows detailed information of individual metabolites such as fold change analysis, HMDB-ID, and VIP values.
Different metabolites in the Nb group belong to nine different chemical classification categories/groups with seven in the ES group (Figures 2E, F). Three categories that had the highest differential metabolite content among the Nb and ES groups were mainly organic acids and their derivatives, followed by lipid-like and lipid compounds and organoheterocyclic molecules.
In addition, the difference in the expression of patterns of metabolites in various samples was assessed with hierarchical cluster analysis heatmap (Figure 3). Importantly, clustering heatmaps can more intuitively show the relationship between samples. Usually, the variation patterns of metabolome compositions are clearly shown by the heatmap, wherein they are obviously separated from the control. Exemplarily, alanine glutamine and pyruvate were abundantly present in the ESPs of Nb but absent in the culture medium.
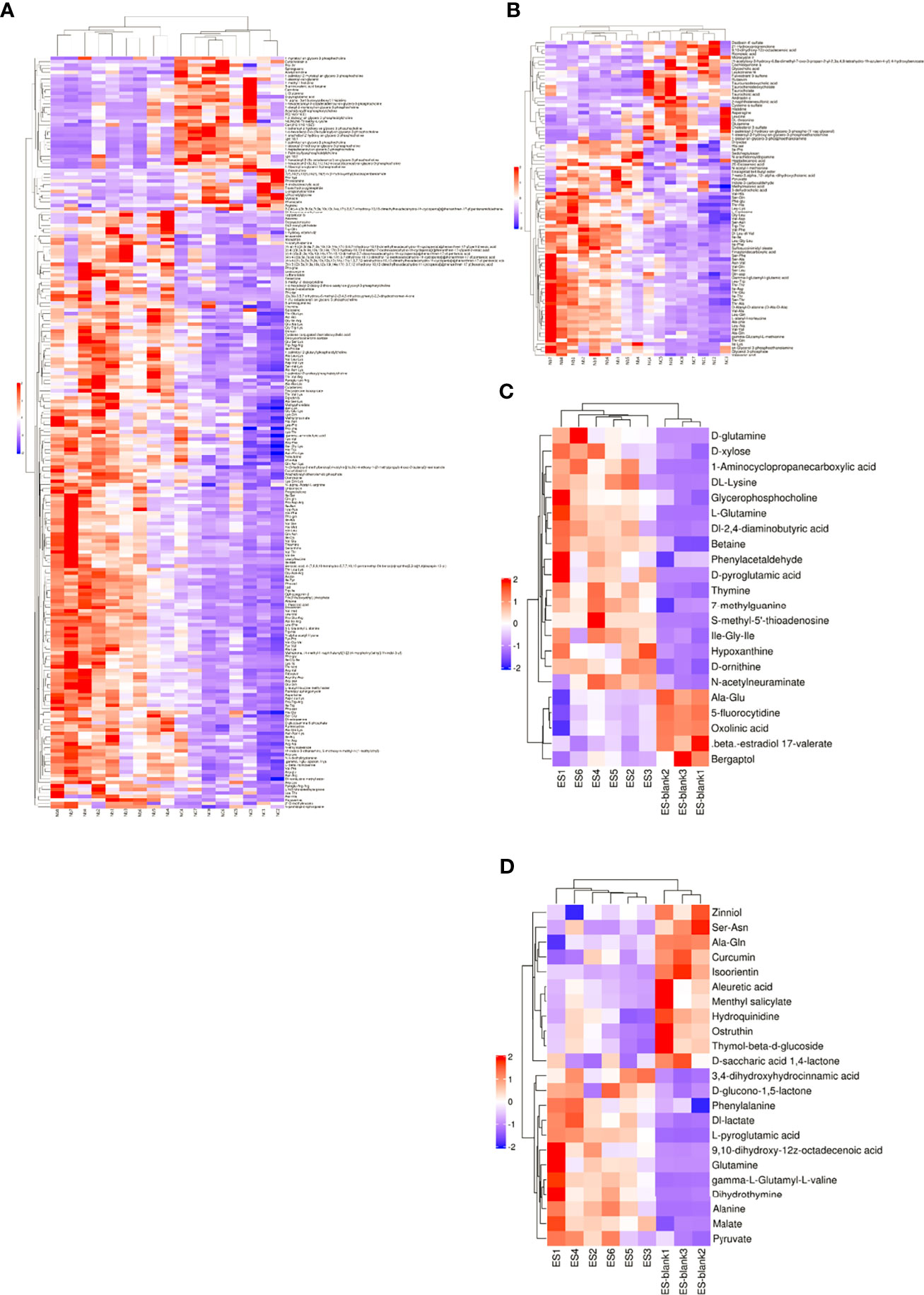
Figure 3 Heatmaps of different metabolites within the Nb and ES groups. (A, C) show the positive ion mode and (B, D) show the negative ion mode. The significance of metabolite change (red denotes upregulated and blue indicates downregulated) was proportional to the color of each section. Rows correspond to metabolites, while columns correspond to samples.
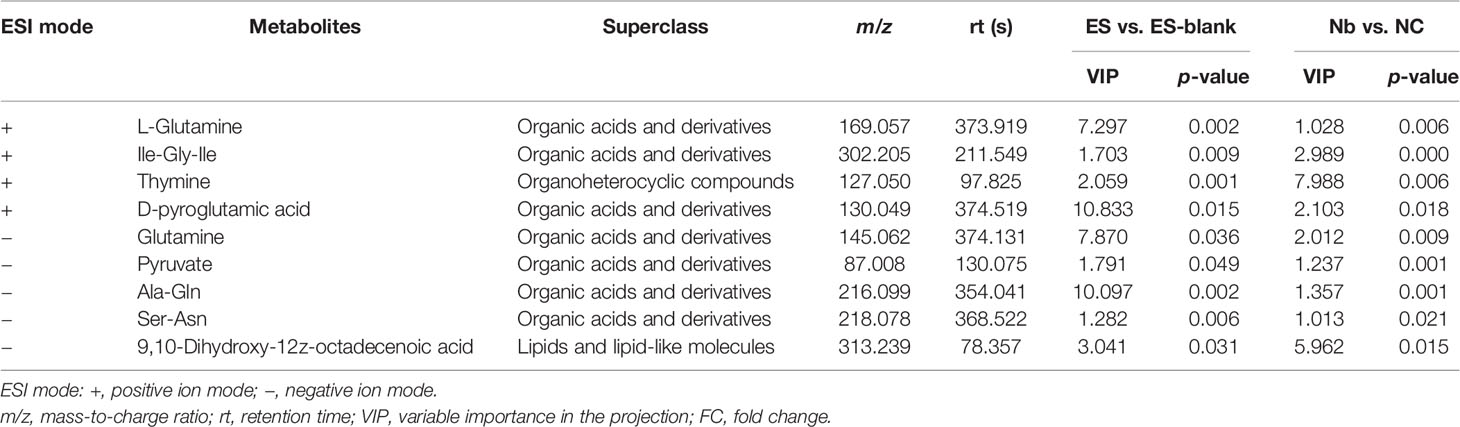
Nine metabolites were found to be common with regard to their differential features when differences in their metabolic profiles were compared, namely, Nb vs. NC and ES vs. ES-blank (Figure 2G), wherein these metabolites comprised mostly products of organic acids and derivatives, followed by organoheterocyclic molecules as well as lipid-like and lipid compounds (Table 1).
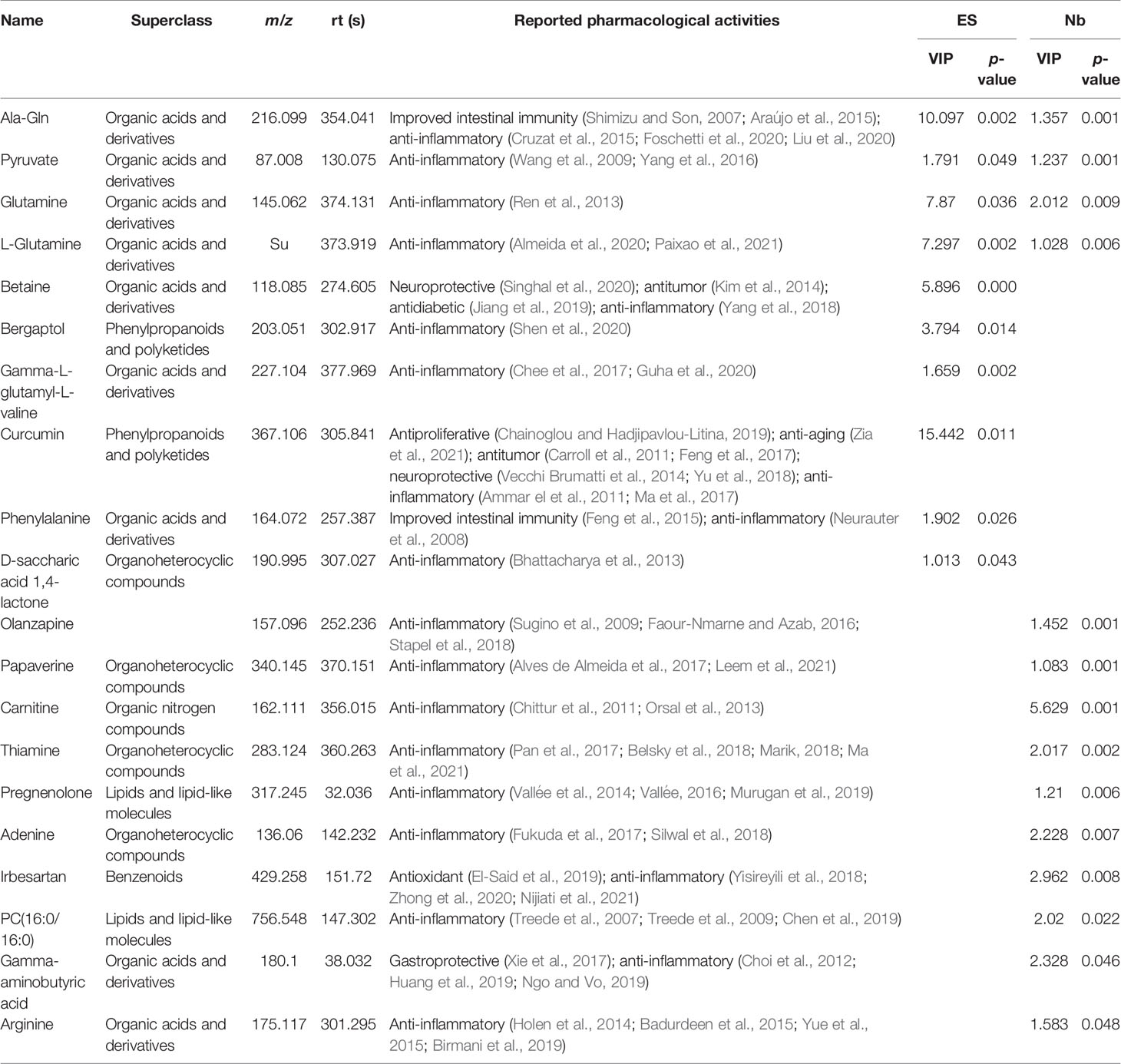
Comprehensive literature search coupled with database survey discovered 20 small molecules with anti-inflammatory activity with Table 2 showing the retention times, m/z, and chemotaxonomy, alongside the described biological activities of the metabolites. Besides anti-inflammatory activity shown in Table 2, the associated activities of metabolites were also discovered including improved intestinal immunity (Ala-Gln, phenylalanine) and neuroprotective (betaine, curcumin), antitumor (betaine, curcumin), antidiabetic (betaine), antiproliferative (curcumin), anti-aging (curcumin), antioxidant (irbesartan), and gastroprotective properties (gamma-aminobutyric acid).
Analyses of the Biosynthetic and Metabolic Pathways of the Identified Compounds
Through KEGG pathway analysis for the related biological effects and pathways of distinctly expressed metabolites, the determined metabolic pathway enrichment diagram (Figure 4) suggests that eight similar metabolic pathways were observed in the Nb and ES groups. These include metabolism of central carbons in tumor; metabolism of glutamate, aspartate, and alanine; digestion and absorption of proteins; absorption of minerals; biosynthesis of amino acids; metabolism of glutamine; and biosynthesis of amino-acyl tRNA and transporters of ATP binding cassette. Figure 4 displays the perturbed metabolic pathways in samples of intestinal content, thereby depicting bile secretion; mTOR signaling pathway; insulin resistance; GABAergic synapse; biosynthesis of isoleucine, leucine, and valine; and metabolism of beta-alanine, cholesterol, pyrimidine, histidine, and glycerophospholipids, which were significantly enriched in the infected mice compared with the healthy control. Meanwhile, metabolism of pyruvate, glutathione, cysteine, methionine, threonine, glycine, and serine as well as bicarbonate reclamation in the proximal tubules; glucagon signaling pathway; type II diabetes mellitus; citrate cycle (TCA cycle); and other metabolic pathways were significantly affected in the ES group.
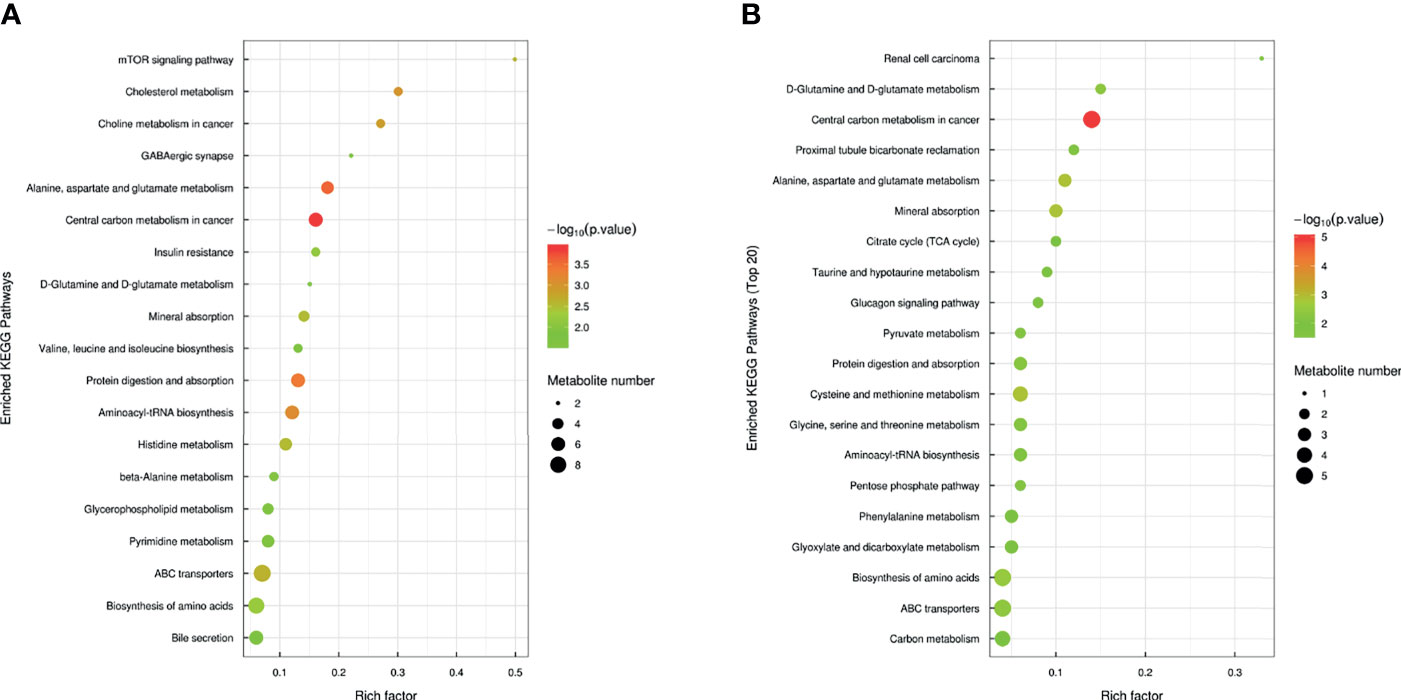
Figure 4 Bubble plot of the pathway analysis of Nb vs. NC (A) and ES vs. ES-blank (B). A metabolic pathway is represented by each bubble in the bubble chart. In the topological analysis, the pathway is influenced by factors such as the size and abscissa of the bubble. The ordinate and color of the bubble with the latter indicating the p-value of the enrichment analysis. The darker the color, the smaller the p-value and the more significant the enrichment degree. The rich factor represents the ratio of the different metabolites in the pathway to the detected metabolites.
Discussion
Available evidence suggests that hookworm could exert an anti-inflammation property for their long-term survival in human or animal host, which may in turn provide a beneficial effect against inflammatory-related diseases (Ferreira et al., 2017; Lothstein and Gause, 2021). Consequently, screening and identification of hookworm-derived molecules with pharmacological activity will be highly investigated in the near future. Besides genomic and proteomic techniques, the application of the metabolomic platform to the pharmaceutical field has begun in recent years, wherein it could screen and identify non-protein small metabolites, thereby reflecting metabolic changes in the host (Wangchuk et al., 2019; Yeshi et al., 2020). The difficulty in obtaining and maintaining human hookworms in the laboratory is a major challenge in human hookworm studies. In view of the similarity of life cycle and pathogenicity to human hookworm, N. brasiliensis has been defined as a model and is widely used in human hookworm research especially in immunobiology, drug screening, etc. (Dominguez et al., 2000; Camberis et al., 2003). Therefore, N. brasiliensis was employed as a model to carry out two independent metabolomics analyses in order to explore anti-inflammatory molecules that were derived from N. brasiliensis through in-vitro and in-vivo pathways in the current study.
Principal molecules of helminths constitute the ESPs and parasite external surface (Hewitson et al., 2009). As a key boundary between the hosts and helminthic parasites, ESPs are usually secreted as a mixture of carbohydrates, lipids, and proteins from the outer surface or oral orifice of the parasite (Pearson et al., 2012). We analyzed ESPs of N. brasiliensis in our study, wherein 10 out of 45 differential metabolites were discovered to demonstrate potential anti-inflammatory properties as described in published works. These metabolites could be subclassed into amino acids, furanocoumarins, linear diarylheptanoids, gamma butyrolactones, and alpha-keto acids. Although glutamine and phenylalanine have also been detected in previous research through GC-MS-based platform (Wangchuk et al., 2019), the other eight metabolites have not been found in other previous published works, which may be ascribable to the application of different metabolomical platforms. Furthermore, through non-targeted liquid chromatography mass spectrometry (LC-MS) platform, metabolites such as betaine and L-glutamine were also found to be in ESPs of N. brasiliensis L3 stage (Yeshi et al., 2020). Therefore, standardization of metabolomics analysis for ESPs of hookworm should be established in the future to increase the credibility of the results.
In addition to in-vitro work on the ESPs of cultured adult worm, the in-vivo study on the N. brasiliensis-parasitized mouse small intestines could provide direct information on host–parasite interaction (Wangchuk et al., 2019). In the present study, we carried out metabolomics analysis of the intestinal contents of N. brasiliensis-infected mouse for the first time, and our results revealed that 301 metabolites were differentially expressed. Through literature searching, we discovered 14 metabolites with anti-inflammatory or antioxidative properties which could be subclassed into amino acids, benzylisoquinolines, quaternary ammonium salts, pyrimidines, pregnane steroids, purines, biphenyls, and glycerophosphocholines. For example, as a candidate for anti-inflammation, glutamine could crucially influence the long-term treatment outcome of inflammatory conditions by regulating inflammation through pathways such as mitogen-activated protein-kinases (MAPK), signal transducer and activator of transcription (STAT), and nuclear factor-kappa B (NF-κB) (Ren et al., 2013). Also, existing evidence has shown that in response to vaccination against the influenza virus, L-glutamine could improve the immunity of the mucosa by modulating the salivary cytokine profile (IL-6 and IL-10) and increasing SIgA levels in the upper airways (Paixao et al., 2021). Irbesartan could ameliorate inflammation and fibrosis in the hypertensive renal injury model through inhibiting Th22 cell chemotaxis and infiltration as well as CCL20, CCL22, and CCL27 expression (Zhong et al., 2020). Papaverine could inhibit the activation of NLRP3 inflammasome by modulating NF-kappa B and CREB signaling pathways, which results in reduced microglial activation and neuronal cell death (Leem et al., 2021). Olanzapine treatment could decrease in expression and secretion of IL-1beta and TNF-alpha significantly in ex vivo stimulation of primary human peripheral blood mononuclear cells (Stapel et al., 2018). All these suggest that a switch to anti-inflammation status of host immune system might have occurred with the production of numerous anti-inflammatory metabolites during N. brasiliensis infection in mice. This could further confirm the inhibitory role of inflammatory responses following infection by the parasites.
To further explore the source of the differential metabolites, especially those with anti-inflammatory properties, we compared the differential metabolites from ESPs and intestinal contents of mice that were infected by N. brasiliensis. We found that nine metabolites co-existed in two different samples which we speculated to originate from N. brasiliensis. Furthermore, four metabolites of the discovered nine common metabolites had anti-inflammatory properties, and they included L-glutamine, glutamine, pyruvate, and Ala-Gln. For instance, pyruvate, a key intermediate in glucose metabolism, could exert an anti-inflammation role in models of experimental stroke and inflammation, as well as systemic inflammation and multiple dysfunctions (Wang et al., 2009; Yang et al., 2016). Ala-Gln, short for the dipeptide alanyl-glutamine, could attenuate inflammation in various experiments including intestinal mucositis, Escherichia coli lipopolysaccharide-induced vascular hyporeactivity, asthma, obesity-associated diabetes, and concomitant inflammatory via sirtuin 1/HUR signaling in β cells (Jing et al., 2007; Araújo et al., 2015; Cruzat et al., 2015; Liu et al., 2020). Besides the N. brasiliensis-derived anti-inflammatory metabolites, the host-derived metabolites might be more important for anti-inflammation roles after N. brasiliensis infection as demonstrated by the other 10 anti-inflammatory metabolites that were also discovered in this work. However, the other metabolites detected in the present study might also have anti-inflammatory effects or other pharmacological activities which require further research. On the other hand, the trilateral relationship among parasite, microbiota, and host cells should also be taken into consideration (Midha et al., 2021), which indicates that intestinal microbiota may also play a crucial role for the alteration of metabolites during N. brasiliensis infection in a mouse model, albeit a comprehensive investigation is needed for a clear understanding of this interaction.
In summary, we have established a method for the analysis of parasite-derived anti-inflammatory molecules and revealed an array of metabolites with anti-inflammatory activities through the UHPLC-MS platform, which could therefore provide clues for the further identification, evaluation, and translation of such metabolites in the not too distant future. However, N. brasiliensis, a model of human hookworm, is different from human hookworm in terms of species taxonomy and parasitism as reported above (Camberis et al., 2003). How to translate N. brasiliensis-based study results to the pharmacological properties of human hookworm will be another question that needs to be interrogated in the future.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary material. Metabolomics data have been deposited to the EMBL-EBI MetaboLights database (doi: 10.1093/nar/gkz1019, PMID:31691833) with the identifier MTBLS3486. The complete dataset can be accessed here https://www.ebi.ac.uk/metabolights/MTBLS3486.
Ethics Statement
The animal study was reviewed and approved by the Ethical Committee for the Use of Experimental Animals at Jiangsu Institute of Parasitic Diseases (Approval Number: JIPD-2020-007).
Author Contributions
YD and JW conceived and designed the study. YYC, XD, MZ, YY, and YJC performed the experiments and collected the data. YYC, QZ, MZ, and YF analyzed the data. YD, YYC, and JW wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2020YFC1200100), the Jiangsu Provincial Department of Science and Technology (BM2018020), the National Natural Science Foundation of China (NSFC 82170269), the Jiangsu Province’s Key Provincial Talents Program (ZDRCB2016005), the Foundation of Clinical Medical Research Center of Yili Autonomous Prefecture (YL2020ms09), and Jiangsu Provincial Project of Invigorating Health Care through Science, Technology and Education and the Jiangsu Provincial Commission of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.781132/full#supplementary-material
Supplementary Figure 1 | Evaluation of the quality of the experiment. The total ion chromatography (TIC) of the QC sample in modes of positive ion (A) and negative ion (B). Retention time is denoted by x-axis bars, while total ionic strength is represented by y-axis bars. PCA score plots of all experimental samples in the modes of positive ion (C) and negative ion (D).
References
Almeida, E. B., Santos, J. M. B., Paixao, V., Amaral, J. B., Foster, R., Sperandio, A., et al. (2020). L-Glutamine Supplementation Improves the Benefits of Combined-Exercise Training on Oral Redox Balance and Inflammatory Status in Elderly Individuals. Oxid. Med. Cell Longev 2020, 2852181. doi: 10.1155/2020/2852181
Alves de Almeida, A. C., de-Faria, F. M., Dunder, R. J., Manzo, L. P., Souza-Brito, A. R., Luiz-Ferreira, A. (2017). Recent Trends in Pharmacological Activity of Alkaloids in Animal Colitis: Potential Use for Inflammatory Bowel Disease. Evid Based Complement Alternat Med. 2017, 8528210. doi: 10.1155/2017/8528210
Ammar el, S. M., Gameil, N. M., Shawky, N. M., Nader, M. A. (2011). Comparative Evaluation of Anti-Inflammatory Properties of Thymoquinone and Curcumin Using an Asthmatic Murine Model. Int. Immunopharmacol 11 (12), 2232–2236. doi: 10.1016/j.intimp.2011.10.013
Araújo, C. V., Lazzarotto, C. R., Aquino, C. C., Figueiredo, I. L., Costa, T. B., Alves, L. A., et al. (2015). Alanyl-Glutamine Attenuates 5-Fluorouracil-Induced Intestinal Mucositis in Apolipoprotein E-Deficient Mice. Braz. J. Med. Biol. Res. 48 (6), 493–501. doi: 10.1590/1414-431x20144360
Badurdeen, S., Mulongo, M., Berkley, J. A. (2015). Arginine Depletion Increases Susceptibility to Serious Infections in Preterm Newborns. Pediatr. Res. 77 (2), 290–297. doi: 10.1038/pr.2014.177
Bartsch, S. M., Hotez, P. J., Asti, L., Zapf, K. M., Bottazzi, M. E., Diemert, D. J., et al. (2016). The Global Economic and Health Burden of Human Hookworm Infection. PLoS Negl. Trop. Dis. 10 (9), e0004922. doi: 10.1371/journal.pntd.0004922
Belsky, J. B., Wira, C. R., Jacob, V., Sather, J. E., Lee, P. J. (2018). A Review of Micronutrients in Sepsis: The Role of Thiamine, L-Carnitine, Vitamin C, Selenium and Vitamin D. Nutr. Res. Rev. 31 (2), 281–290. doi: 10.1017/S0954422418000124
Bhattacharya, S., Manna, P., Gachhui, R., Sil, P. C. (2013). D-Saccharic Acid 1,4-Lactone Protects Diabetic Rat Kidney by Ameliorating Hyperglycemia-Mediated Oxidative Stress and Renal Inflammatory Cytokines via NF-kappaB and PKC Signaling. Toxicol. Appl. Pharmacol. 267 (1), 16–29. doi: 10.1016/j.taap.2012.12.005
Birmani, M. W., Raza, A., Nawab, A., Tang, S., Ghani, M. W., Li, G., et al. (2019). Importance of Arginine as Immune Regulator in Animal Nutrition. Int. J. Vet Sci. Res. 5 (1), 1–10. doi: 10.18488/journal.110.2019.51.1.10
Bouchery, T., Moyat, M., Sotillo, J., Silverstein, S., Volpe, B., Coakley, G., et al. (2020). Hookworms Evade Host Immunity by Secreting a Deoxyribonuclease to Degrade Neutrophil Extracellular Traps. Cell Host Microbe 27 (2), 277–289 e276. doi: 10.1016/j.chom.2020.01.011
Briggs, N., Weatherhead, J., Sastry, K. J., Hotez, P. J. (2016). The Hygiene Hypothesis and Its Inconvenient Truths About Helminth Infections. PLoS Negl. Trop. Dis. 10 (9), e0004944. doi: 10.1371/journal.pntd.0004944
Brooker, S., Bethony, J., Hotez, P. J. (2004). Human Hookworm Infection in the 21st Century. Adv. Parasitol 58, 197–288. doi: 10.1016/S0065-308X(04)58004-1
Brosschot, T. P., Reynolds, L. A. (2018). The Impact of a Helminth-Modified Microbiome on Host Immunity. Mucosal Immunol. 11 (4), 1039–1046. doi: 10.1038/s41385-018-0008-5
Camberis, M., Le Gros, G., Urban, J., Jr. (2003). Animal Model of Nippostrongylus Brasiliensis and Heligmosomoides Polygyrus. Curr. Protoc. Immunol. 19, 12. doi: 10.1002/0471142735.im1912s55
Carroll, R. E., Benya, R. V., Turgeon, D. K., Vareed, S., Neuman, M., Rodriguez, L., et al. (2011). Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. (Phila) 4 (3), 354–364. doi: 10.1158/1940-6207.Capr-10-0098
Chainoglou, E., Hadjipavlou-Litina, D. (2019). Curcumin Analogues and Derivatives With Anti-Proliferative and Anti-Inflammatory Activity: Structural Characteristics and Molecular Targets. Expert Opin. Drug Discov. 14 (8), 821–842. doi: 10.1080/17460441.2019.1614560
Chee, M. E., Majumder, K., Mine, Y. (2017). Intervention of Dietary Dipeptide Gamma-L-Glutamyl-L-Valine (Gamma-EV) Ameliorates Inflammatory Response in a Mouse Model of LPS-Induced Sepsis. J. Agric. Food Chem. 65 (29), 5953–5960. doi: 10.1021/acs.jafc.7b02109
Chen, M., Huang, H., Zhou, P., Zhang, J., Dai, Y., Yang, D., et al. (2019). Oral Phosphatidylcholine Improves Intestinal Barrier Function in Drug-Induced Liver Injury in Rats. Gastroenterol. Res. Pract. 2019, 8723460. doi: 10.1155/2019/8723460
Chittur, S., Parr, B., Marcovici, G. (2011). Inhibition of Inflammatory Gene Expression in Keratinocytes Using a Composition Containing Carnitine, Thioctic Acid and Saw Palmetto Extract. Evid. Based Complementary Altern. Med. 2011, 985345. doi: 10.1093/ecam/nep102
Choi, J.-I., Yun, I.-H., Jung, Y., Lee, E.-H., Nam, T.-J., Kim, Y.-M. (2012). Effects of γ-Aminobutyric Acid (GABA)-Anriched Aea Tangle Laminaria Japonica Extract on Lipopolysaccharide-Induced Inflammation in Mouse Macrophage (RAW 264.7) Cells. Fish Aquat. Sci. 15 (4), 293–297. doi: 10.5657/fas.2012.0293
Cruzat, V. F., Keane, K. N., Scheinpflug, A. L., Cordeiro, R., Soares, M. J., Newsholme, P. (2015). Alanyl-Glutamine Improves Pancreatic β-Cell Function Following Ex Vivo Inflammatory Challenge. J. Endocrinol. 224 (3), 261–271. doi: 10.1530/joe-14-0677
Dominguez, L., Saldana, J., Chernin, J. (2000). Use of L4 Larvae of Nippostrongylus Brasiliensis for the In Vivo Screening of Anthelmintic Drugs. Can. J. Vet. Res. 64 (3), 160–163.
El-Said, N. T., Mohamed, E. A., Taha, R. A. (2019). Irbesartan Suppresses Cardiac Toxicity Induced by Doxorubicin via Regulating the P38-MAPK/NF-kappaB and TGF-Beta1 Pathways. Naunyn Schmiedebergs Arch. Pharmacol. 392 (6), 647–658. doi: 10.1007/s00210-019-01624-3
Faour-Nmarne, C., Azab, A. N. (2016). Effects of Olanzapine on LPS-Induced Inflammation in Rat Primary Glia Cells. Innate Immun. 22 (1), 40–50. doi: 10.1177/1753425915613425
Feng, L., Li, W., Liu, Y., Jiang, W. D., Kuang, S. Y., Jiang, J., et al. (2015). Dietary Phenylalanine-Improved Intestinal Barrier Health in Young Grass Carp (Ctenopharyngodon Idella) Is Associated With Increased Immune Status and Regulated Gene Expression of Cytokines, Tight Junction Proteins, Antioxidant Enzymes and Related Signalling Molecules. Fish Shellfish Immunol. 45 (2), 495–509. doi: 10.1016/j.fsi.2015.05.001
Feng, S., Wang, Y., Zhang, R., Yang, G., Liang, Z., Wang, Z., et al. (2017). Curcumin Exerts Its Antitumor Activity Through Regulation of miR-7/Skp2/p21 in Nasopharyngeal Carcinoma Cells. Onco Targets Ther. 10, 2377–2388. doi: 10.2147/ott.S130055
Ferreira, I. B., Pickering, D. A., Troy, S., Croese, J., Loukas, A., Navarro, S. (2017). Suppression of Inflammation and Tissue Damage by a Hookworm Recombinant Protein in Experimental Colitis. Clin. Transl. Immunol. 6 (10), e157. doi: 10.1038/cti.2017.42
Foschetti, D. A., Braga-Neto, M. B., Bolick, D., Moore, J., Alves, L. A., Martins, C. S., et al. (2020). Clostridium Difficile Toxins or Infection Induce Upregulation of Adenosine Receptors and IL-6 With Early Pro-Inflammatory and Late Anti-Inflammatory Pattern. Braz. J. Med. Biol. Res. 53 (9), e9877. doi: 10.1590/1414-431x20209877
Fukuda, T., Majumder, K., Zhang, H., Matsui, T., Mine, Y. (2017). Adenine has an Anti-Inflammatory Effect Through the Activation of Adenine Receptor Signaling in Mouse Macrophage. J. Funct. Foods 28, 235–239. doi: 10.1016/j.jff.2016.11.013
Guha, S., Paul, C., Alvarez, S., Mine, Y., Majumder, K. (2020). Dietary Gamma-Glutamyl Valine Ameliorates TNF-Alpha-Induced Vascular Inflammation via Endothelial Calcium-Sensing Receptors. J. Agric. Food Chem. 68 (34), 9139–9149. doi: 10.1021/acs.jafc.0c04526
Guijas, C., Montenegro-Burke, J. R., Warth, B., Spilker, M. E., Siuzdak, G. (2018). Metabolomics Activity Screening for Identifying Metabolites That Modulate Phenotype. Nat. Biotechnol. 36 (4), 316–320. doi: 10.1038/nbt.4101
Harris, N. L., Loke, P. (2018). Recent Advances in Type-2-Cell-Mediated Immunity: Insights From Helminth Infection. Immunity 48 (2), 396. doi: 10.1016/j.immuni.2018.02.011
Hewitson, J. P., Grainger, J. R., Maizels, R. M. (2009). Helminth Immunoregulation: The Role of Parasite Secreted Proteins in Modulating Host Immunity. Mol. Biochem. Parasitol 167 (1), 1–11. doi: 10.1016/j.molbiopara.2009.04.008
Holen, E., Espe, M., Andersen, S. M., Taylor, R., Aksnes, A., Mengesha, Z., et al. (2014). A Co Culture Approach Show That Polyamine Turnover Is Affected During Inflammation in Atlantic Salmon Immune and Liver Cells and That Arginine and LPS Exerts Opposite Effects on P38mapk Signaling. Fish Shellfish Immunol. 37 (2), 286–298. doi: 10.1016/j.fsi.2014.02.004
Huang, H.-Y., Hsu, T., Lin, B.-F. (2019). Gamma-Aminobutyric Acid Decreases Macrophages Infiltration and Suppresses Inflammatory Responses in Renal Injury. J. Funct. Foods 60, 103419. doi: 10.1016/j.jff.2019.103419
Huang, Y., Wu, Q., Zhao, L., Xiong, C., Xu, Y., Dong, X., et al. (2020). UHPLC-MS-Based Metabolomics Analysis Reveals the Process of Schistosomiasis in Mice. Front. Microbiol. 11, 1517. doi: 10.3389/fmicb.2020.01517
Jiang, Y. P., Yang, J. M., Ye, R. J., Liu, N., Zhang, W. J., Ma, L., et al. (2019). Protective Effects of Betaine on Diabetic Induced Disruption of the Male Mice Blood-Testis Barrier by Regulating Oxidative Stress-Mediated P38 MAPK Pathways. BioMed. Pharmacother. 120, 109474. doi: 10.1016/j.biopha.2019.109474
Jing, L., Wu, Q., Wang, F. (2007). Glutamine Induces Heat-Shock Protein and Protects Against Escherichia Coli Lipopolysaccharide-Induced Vascular Hyporeactivity in Rats. Crit. Care 11 (2), R34. doi: 10.1186/cc5717
Kim, D. H., Sung, B., Kang, Y. J., Jang, J. Y., Hwang, S. Y., Lee, Y., et al. (2014). Anti-Inflammatory Effects of Betaine on AOM/DSSinduced Colon Tumorigenesis in ICR Male Mice. Int. J. Oncol. 45 (3), 1250–1256. doi: 10.3892/ijo.2014.2515
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., et al. (2016). PubChem Substance and Compound Databases. Nucleic Acids Res. 44 (D1), D1202–D1213. doi: 10.1093/nar/gkv951
Leem, Y. H., Park, J. S., Park, J. E., Kim, D. Y., Kim, H. S. (2021). Papaverine Exerts Neuroprotective Effect by Inhibiting NLRP3 Inflammasome Activation in an MPTP-Induced Microglial Priming Mouse Model Challenged With LPS. Biomol Ther. (Seoul) 29 (3), 295–302. doi: 10.4062/biomolther.2021.039
Legido-Quigley, C. (2010). Metabolite-Biomarker Investigations in the Life Cycle of and Infection With Schistosoma. Parasitology 137 (9), 1425–1435. doi: 10.1017/S0031182010000545
Li, Y., Mei, L., Qiang, J., Ju, S., Zhao, S. (2016). Magnetic Resonance Spectroscopy for Evaluating Portal-Systemic Encephalopathy in Patients With Chronic Hepatic Schistosomiasis Japonicum. PLoS Negl. Trop. Dis. 10 (12), e0005232. doi: 10.1371/journal.pntd.0005232
Liu, S. K., Ma, L. B., Yuan, Y., Ji, X. Y., Sun, W. J., Duan, J. X., et al. (2020). Alanylglutamine Relieved Asthma Symptoms by Regulating Gut Microbiota and the Derived Metabolites in Mice. Oxid. Med. Cell Longev 2020, 7101407. doi: 10.1155/2020/7101407
Lothstein, K. E., Gause, W. C. (2021). Mining Helminths for Novel Therapeutics. Trends Mol. Med. 27 (4), 345–364. doi: 10.1016/j.molmed.2020.12.010
Loukas, A., Hotez, P. J., Diemert, D., Yazdanbakhsh, M., McCarthy, J. S., Correa-Oliveira, R., et al. (2016). Hookworm Infection. Nat. Rev. Dis. Primers 2, 16088. doi: 10.1038/nrdp.2016.88
Maizels, R. M., Balic, A., Gomez-Escobar, N., Nair, M., Taylor, M. D., Allen, J. E. (2004). Helminth Parasites–Masters of Regulation. Immunol. Rev. 201, 89–116. doi: 10.1111/j.0105-2896.2004.00191.x
Ma, F., Liu, F., Ding, L., You, M., Yue, H., Zhou, Y., et al. (2017). Anti-Inflammatory Effects of Curcumin Are Associated With Down Regulating microRNA-155 in LPS-Treated Macrophages and Mice. Pharm. Biol. 55 (1), 1263–1273. doi: 10.1080/13880209.2017.1297838
Marik, P. E. (2018). Hydrocortisone, Ascorbic Acid and Thiamine (HAT Therapy) for the Treatment of Sepsis. Focus Ascorbic Acid. Nutrients 10 (11), 1762. doi: 10.3390/nu10111762
Ma, Y., Zhang, Y., Zhang, H., Wang, H. (2021). Thiamine Alleviates High-Concentrate-Diet-Induced Oxidative Stress, Apoptosis, and Protects the Rumen Epithelial Barrier Function in Goats. Front. Vet. Sci. 8, 663698. doi: 10.3389/fvets.2021.663698
McSorley, H. J., O'Gorman, M. T., Blair, N., Sutherland, T. E., Filbey, K. J., Maizels, R. M. (2012). Suppression of Type 2 Immunity and Allergic Airway Inflammation by Secreted Products of the Helminth Heligmosomoides Polygyrus. Eur. J. Immunol. 42 (10), 2667–2682. doi: 10.1002/eji.201142161
Midha, A., Ebner, F., Schlosser-Brandenburg, J., Rausch, S., Hartmann, S. (2021). Trilateral Relationship: Ascaris, Microbiota, and Host Cells. Trends Parasitol 37 (3), 251–262. doi: 10.1016/j.pt.2020.09.002
Murugan, S., Jakka, P., Namani, S., Mujumdar, V., Radhakrishnan, G. (2019). The Neurosteroid Pregnenolone Promotes Degradation of Key Proteins in the Innate Immune Signaling to Suppress Inflammation. J. Biol. Chem. 294 (12), 4596–4607. doi: 10.1074/jbc.RA118.005543
Navarro, S., Pickering, D. A., Ferreira, I. B., Jones, L., Ryan, S., Troy, S., et al. (2016). Hookworm Recombinant Protein Promotes Regulatory T Cell Responses That Suppress Experimental Asthma. Sci. Transl. Med. 8 (362), 362ra143. doi: 10.1126/scitranslmed.aaf8807
Neurauter, G., Schröcksnadel, K., Scholl-Bürgi, S., Sperner-Unterweger, B., Schubert, C., Ledochowski, M., et al. (2008). Chronic Immune Stimulation Correlates With Reduced Phenylalanine Turnover. Curr. Drug Metab. 9 (7), 622–627. doi: 10.2174/138920008785821738
Ngo, D. H., Vo, T. S. (2019). An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 24 (15), 2678. doi: 10.3390/molecules24152678
Nicholson, J. K., Holmes, E., Kinross, J. M., Darzi, A. W., Takats, Z., Lindon, J. C. (2012). Metabolic Phenotyping in Clinical and Surgical Environments. Nature 491(7424), 384–392. doi: 10.1038/nature11708
Nijiati, Y., Maimaitiyiming, D., Yang, T., Li, H., Aikemu, A. (2021). Research on the Improvement of Oxidative Stress in Rats With High-Altitude Pulmonary Hypertension Through the Participation of Irbesartan in Regulating Intestinal Flora. Eur. Rev. Med. Pharmacol. Sci. 25 (13), 4540–4553. doi: 10.26355/eurrev_202107_26247
Orsal, E., Halici, Z., Bayir, Y., Cadirci, E., Bilen, H., Ferah, I., et al. (2013). The Role of Carnitine on Ovariectomy and Inflammation-Induced Osteoporosis in Rats. Exp. Biol. Med. 238 (12), 1406–1412. doi: 10.1177/1535370213502614
Paixao, V., Almeida, E. B., Amaral, J. B., Roseira, T., Monteiro, F. R., Foster, R., et al. (2021). Elderly Subjects Supplemented With L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination. Vaccines (Basel) 9 (2), 107. doi: 10.3390/vaccines9020107
Pan, X. H., Yang, L., Beckers, Y., Xue, F. G., Tang, Z. W., Jiang, L. S., et al. (2017). Thiamine Supplementation Facilitates Thiamine Transporter Expression in the Rumen Epithelium and Attenuates High-Grain-Induced Inflammation in Low-Yielding Dairy Cows. J. Dairy Sci. 100 (7), 5329–5342. doi: 10.3168/jds.2016-11966
Pearson, M. S., Tribolet, L., Cantacessi, C., Periago, M. V., Valero, M. A., Jariwala, A. R., et al. (2012). Molecular Mechanisms of Hookworm Disease: Stealth, Virulence, and Vaccines. J. Allergy Clin. Immunol. 130 (1), 13–21. doi: 10.1016/j.jaci.2012.05.029
Ramanan, D., Bowcutt, R., Lee, S. C., Tang, M. S., Kurtz, Z. D., Ding, Y., et al. (2016). Helminth Infection Promotes Colonization Resistance via Type 2 Immunity. Science 352 (6285), 608–612. doi: 10.1126/science.aaf3229
Ren, W. K., Yin, J., Zhu, X. P., Liu, G., Li, N. Z., Peng, Y. Y., et al. (2013). Glutamine on Intestinal Inflammation: A Mechanistic Perspective. Eur. J. Inflamm. 11 (2), 315–326. doi: 10.1177/1721727x1301100201
Ryan, S. M., Eichenberger, R. M., Ruscher, R., Giacomin, P. R., Loukas, A. (2020). Harnessing Helminth-Driven Immunoregulation in the Search for Novel Therapeutic Modalities. PLoS Pathog. 16 (5), e1008508. doi: 10.1371/journal.ppat.1008508
Scholmerich, J., Fellermann, K., Seibold, F. W., Rogler, G., Langhorst, J., Howaldt, S., et al. (2017). A Randomised, Double-Blind, Placebo-Controlled Trial of Trichuris Suis Ova in Active Crohn's Disease. J. Crohns Colitis 11 (4), 390–399. doi: 10.1093/ecco-jcc/jjw184
Shah, N. J., Sureshkumar, S., Shewade, D. G. (2015). Metabolomics: A Tool Ahead for Understanding Molecular Mechanisms of Drugs and Diseases. Indian J. Clin. Biochem. 30 (3), 247–254. doi: 10.1007/s12291-014-0455-z
Shen, C. Y., Wang, T. X., Jiang, J. G., Huang, C. L., Zhu, W. (2020). Bergaptol From Blossoms of Citrus Aurantium L. Var. Amara Engl Inhibits LPS-Induced Inflammatory Responses and Ox-LDL-Induced Lipid Deposition. Food Funct. 11 (6), 4915–4926. doi: 10.1039/c9fo00255c
Shimizu, M., Son, D. O. (2007). Food-Derived Peptides and Intestinal Functions. Curr. Pharm. Des. 13 (9), 885–895. doi: 10.2174/138161207780414287
Silwal, P., Lim, K., Heo, J.-Y., Park, J. I. L., Namgung, U., Park, S.-K. (2018). Adenine Attenuates Lipopolysaccharide-Induced Inflammatory Reactions. kjpp 22 (4), 379–389. doi: 10.4196/kjpp.2018.22.4.379
Singhal, N. K., Sternbach, S., Fleming, S., Alkhayer, K., Shelestak, J., Popescu, D., et al. (2020). Betaine Restores Epigenetic Control and Supports Neuronal Mitochondria in the Cuprizone Mouse Model of Multiple Sclerosis. Epigenetics 15 (8), 871–886. doi: 10.1080/15592294.2020.1735075
Stapel, B., Sieve, I., Falk, C. S., Bleich, S., Hilfiker-Kleiner, D., Kahl, K. G. (2018). Second Generation Atypical Antipsychotics Olanzapine and Aripiprazole Reduce Expression and Secretion of Inflammatory Cytokines in Human Immune Cells. J. Psychiatr. Res. 105, 95–102. doi: 10.1016/j.jpsychires.2018.08.017
Sugino, H., Futamura, T., Mitsumoto, Y., Maeda, K., Marunaka, Y. (2009). Atypical Antipsychotics Suppress Production of Proinflammatory Cytokines and Up-Regulate Interleukin-10 in Lipopolysaccharide-Treated Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 33 (2), 303–307. doi: 10.1016/j.pnpbp.2008.12.006
Summers, R. W., Elliott, D. E., Urban, J. F., Jr., Thompson, R. A., Weinstock, J. V. (2005). Trichuris Suis Therapy for Active Ulcerative Colitis: A Randomized Controlled Trial. Gastroenterology 128 (4), 825–832. doi: 10.1053/j.gastro.2005.01.005
Treede, I., Braun, A., Jeliaskova, P., Giese, T., Füllekrug, J., Griffiths, G., et al. (2009). TNF-Alpha-Induced Up-Regulation of Pro-Inflammatory Cytokines Is Reduced by Phosphatidylcholine in Intestinal Epithelial Cells. BMC Gastroenterol. 9, 53. doi: 10.1186/1471-230x-9-53
Treede, I., Braun, A., Sparla, R., Kühnel, M., Giese, T., Turner, J. R., et al. (2007). Anti-Inflammatory Effects of Phosphatidylcholine. J. Biol. Chem. 282 (37), 27155–27164. doi: 10.1074/jbc.M704408200
Vallée, M. (2016). Neurosteroids and Potential Therapeutics: Focus on Pregnenolone. J. Steroid Biochem. Mol. Biol. 160, 78–87. doi: 10.1016/j.jsbmb.2015.09.030
Vallée, M., Vitiello, S., Bellocchio, L., Hébert-Chatelain, E., Monlezun, S., Martin-Garcia, E., et al. (2014). Pregnenolone can Protect the Brain From Cannabis Intoxication. Science 343 (6166), 94–98. doi: 10.1126/science.1243985
Vecchi Brumatti, L., Marcuzzi, A., Tricarico, P. M., Zanin, V., Girardelli, M. (2014). Curcumin and Inflammatory Bowel Disease: Potential and Limits of Innovative Treatments. Molecules 19 (12), 21127–21153. doi: 10.3390/molecules191221127
Wangchuk, P., Kouremenos, K., Eichenberger, R. M., Pearson, M., Susianto, A., Wishart, D. S., et al. (2019). Metabolomic Profiling of the Excretory-Secretory Products of Hookworm and Whipworm. Metabolomics 15 (7), 101. doi: 10.1007/s11306-019-1561-y
Wang, Q., van Hoecke, M., Tang, X. N., Lee, H., Zheng, Z., Swanson, R. A., et al. (2009). Pyruvate Protects Against Experimental Stroke via an Anti-Inflammatory Mechanism. Neurobiol. Dis. 36 (1), 223–231. doi: 10.1016/j.nbd.2009.07.018
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2013). HMDB 3.0–The Human Metabolome Database in 2013. Nucleic Acids Res. 41 (Database issue), D801–D807. doi: 10.1093/nar/gks1065
Xie, M., Chen, H., Nie, S., Tong, W., Yin, J., Xie, M. (2017). Gastroprotective Effect of Gamma-Aminobutyric Acid Against Ethanol-Induced Gastric Mucosal Injury. Chemico-Biol Interact. 272, 125–134. doi: 10.1016/j.cbi.2017.04.022
Yang, J. M., Zhou, R., Zhang, M., Tan, H. R., Yu, J. Q. (2018). Betaine Attenuates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats via Inhibiting Inflammatory Response. Molecules 23 (6), 1274. doi: 10.3390/molecules23061274
Yang, R., Zhu, S., Tonnessen, T. I. (2016). Ethyl Pyruvate Is a Novel Anti-Inflammatory Agent to Treat Multiple Inflammatory Organ Injuries. J. Inflamm. 13 (1), 37. doi: 10.1186/s12950-016-0144-1
Yeshi, K., Creek, D. J., Anderson, D., Ritmejeryte, E., Becker, L., Loukas, A., et al. (2020). Metabolomes and Lipidomes of the Infective Stages of the Gastrointestinal Nematodes, Nippostrongylus Brasiliensis and Trichuris Muris. Metabolites 10 (11), 446. doi: 10.3390/metabo10110446
Yisireyili, M., Uchida, Y., Yamamoto, K., Nakayama, T., Cheng, X. W., Matsushita, T., et al. (2018). Angiotensin Receptor Blocker Irbesartan Reduces Stress-Induced Intestinal Inflammation via AT1a Signaling and ACE2-Dependent Mechanism in Mice. Brain Behav. Immun. 69, 167–179. doi: 10.1016/j.bbi.2017.11.010
Yue, Y., Huang, W., Liang, J., Guo, J., Ji, J., Yao, Y., et al. (2015). IL4I1 Is a Novel Regulator of M2 Macrophage Polarization That can Inhibit T Cell Activation via L-Tryptophan and Arginine Depletion and IL-10 Production. PLoS One 10 (11), e0142979. doi: 10.1371/journal.pone.0142979
Yu, Y., Shen, Q., Lai, Y., Park, S. Y., Ou, X., Lin, D., et al. (2018). Anti-Inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 9, 386. doi: 10.3389/fphar.2018.00386
Zhong, Y., Tang, R., Lu, Y., Wang, W., Xiao, C., Meng, T., et al. (2020). Irbesartan may Relieve Renal Injury by Suppressing Th22 Cells Chemotaxis and Infiltration in Ang II-Induced Hypertension. Int. Immunopharmacol 87, 106789. doi: 10.1016/j.intimp.2020.106789
Keywords: hookworm, Nippostrongylus brasiliensis, anti-inflammatory activity, host–pathogen interactions, metabolomics approach
Citation: Chen Y, Zhang M, Ding X, Yang Y, Chen Y, Zhang Q, Fan Y, Dai Y and Wang J (2021) Mining Anti-Inflammation Molecules From Nippostrongylus brasiliensis-Derived Products Through the Metabolomics Approach. Front. Cell. Infect. Microbiol. 11:781132. doi: 10.3389/fcimb.2021.781132
Received: 22 September 2021; Accepted: 18 October 2021;
Published: 11 November 2021.
Edited by:
Gaoqian Feng, Burnet Institute, AustraliaReviewed by:
Amir Abdoli, Jahrom University of Medical Sciences, IranKong Qingming, Hangzhou Medical College, China
Copyright © 2021 Chen, Zhang, Ding, Yang, Chen, Zhang, Fan, Dai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Dai, daiyang@jipd.com; Junhong Wang, wangjunhong@jsph.org.cn
†These authors have contributed equally to this work
 Yuying Chen1,2†
Yuying Chen1,2†  Yang Dai
Yang Dai Junhong Wang
Junhong Wang