The Fecal Microbiota Transplantation: A Remarkable Clinical Therapy for Slow Transit Constipation in Future
- 1Department of Colorectal Surgery, Tianjin Union Medical Center, Nankai University, Tianjin, China
- 2Department of Pathology, Tianjin Union Medical Center, Nankai University, Tianjin, China
- 3Key Laboratory of Molecular Microbiology and Technology, Ministry of Education, TEDA Institute of Biological Sciences and Biotechnology, Nankai University, Tianjin, China
Slow transit constipation is a common condition that would be difficult to treat in clinical practice with a widespread incidence in the population. Pharmacotherapy and surgery are common treatment modalities. However, the clinical effect is limited, and patients still suffer from it. As the researchers strived in this field for decades, the profound relationship between slow transit constipation and fecal microbiota transplantation has comprehensively been sustained. It is very pivotal to maintain intestinal homeostasis, the structure function and metabolic function of symbiotic bacteria, which can inhibit the engraftment of intestinal pathogens. This mini review explains the treatment effects and possible mechanisms of the fecal microbiota transplantation in treating slow transit constipation. Simultaneously, it is found that there is significant improvement in the disease by adjusting the intestinal microbes like fecal microbiota transplantation. Fecal microbiota transplantation has efficient therapeutic effects in slow transit constipation compared with traditional therapies.
Introduction
Fecal microbiota transplantation (FMT), as the major treatment of an untargeted microbiome modulation, means transplanting the functional flora from the feces of healthy people into the gastrointestinal tract of patients so as to reconstruct the new intestinal flora and realize the treatment of intestinal and extra-intestinal diseases (Gu et al., 2017). The aim of FMT is to correct dysbiosis by implanting feces collected from donors and treat the underlying disease (Matsuoka, 2020). The abundance and composition of intestinal flora are associated with a variety of diseases, and improving the abundance and composition of intestinal flora using FMT is a potential treatment for many diseases such as gastrointestinal, metabolic, nervous system, and autoimmune diseases. Ning Li et al. suggested that FMT was effective and safe for slow transit constipation (STC) (Ding et al., 2018). At short- and long-term follow-up, the Wexner constipation scale, stool consistency, and constipation symptoms got a huge improvement. This demonstrated that FMT combined with soluble dietary fiber (pectin) had efficacy in treating STC both in short term and long term (Zhang et al., 2018). In Ning Li et al.’s clinical trial, the FMT group had a 30% higher cure rate for treatment of STC than conventional treatment (Tian et al., 2017). FMT has been recommended for the treatment of recurrent or refractory Clostridium difficile infection (CDI) by many clinical guidelines and consensus. From Jessica R et al., FMT failed only in 5 of the 49 patients treated, while 45 patients underwent Clostridium difficile decolonization 1 week after FMT, and polymerase chain reaction was negative (Allegretti et al., 2020). In addition, FMT has shown certain efficacy in clinical studies on other diseases such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) and functional constipation, and cirrhosis (McDonald et al., 2018; Mullish et al., 2018).
The Intestinal Microflora in Human Gut
Researchers found that Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria phyla are predominant in the human gut, while Bacteroidetes phyla, Firmicutes, and Proteobacteria are regularly found in the colon tract (Meng et al., 2018). In the human gut, it is the anaerobic bacteria that play a vital role throughout. Pivotal anaerobic bacteria species in the gut include the following: Firmicutes, Bacteroidetes, and Verrucomicrobia (Dupont et al., 2020). Alteration in the proportion of anaerobic bacteria frequently caused chronic disorders associated with dysbiosis. Proteobacteria in excess of 10% caused intestinal flora abnormalities and disorders (Shin et al., 2015). Above all, there are four pivotal anaerobic bacteria (Proteobacteria, Bacteroidetes, Firmicutes, and Verrucomicrobia) in the human gut connected with intestinal diseases. (1) Proteobacteria is one of the largest phyla of bacteria, including many pathogenic bacteria, such as Escherichia coli, Salmonella, Vibrio cholerae, Helicobacter pylori, and other well-known species (Shin et al., 2015). Microbial maladaptation of the intestinal microbiome is identified by Proteobacteria. (2) Bacteroidetes are the largest phylum of gram-negative bacteria inhabiting in the human gut, considered the leading players of the healthy state and sophisticated homeostasis safeguarded by gut microbiota (Gibiino et al., 2018). From the healthy FMT donors, Reetta et al. isolated intestinal commensal bacteria with anti-inflammatory capacity. They isolated Bacteroides and Parabacteroides, which were recognized as Parabacteroides distasonis, Bacteroides caccae, Bacteroides intestinalis, Bacteroides uniformis, Bacteroides fragilis, Bacteroides vulgatus, and Bacteroides ovatus through the whole genome sequencing (Hiippala and Kainulainen, 2020). (3) The Firmicutes/Bacteroidetes ratio was a possible biomarker of gut dysbiosis. In healthy adult humans, the intestinal bacteria Firmicutes were in the range of quantitative data of 20.5% up to 80%, while Bacteroidetes were from 13.85% up to 75.3%. Many studies found that, in humans, many diseases such as obesity were associated with an increased Firmicutes/Bacteroidetes ratio in comparison with lean individuals (Grigor'eva, 2020). Several bacterial species in intestinal diseases, for instance, colorectal cancer, seem to preferentially inhabit either on-tumor or off-tumor sites. However, members of the phylum Firmicutes displayed a disparate distribution. Some species were enriched in the on-tumor tissue, whereas others inhabited the adjacent healthy mucosa in the off-tumor sites. This demonstrated that organisms, even though belonging to the same taxonomic clade, could have different functional roles in an ecosystem. That depended on their interactions between the bacteria and their environment (Tjalsma et al., 2012). (4) Martin et al. used healthy Chilean fecal samples through the V3–V4 region of the 16S rRNA gene of bacterial DNA. They found that Verrucomicrobia (8.5 ± 10.4%) was the third most dominant strain in the gut, followed by Firmicutes (43.6 ± 9.2%) and Bacteroidetes (41.6 ± 13.1%). Besides, the microbiota of the Chilean subjects was rich in Verrucomicrobia. What is more, the mucus-degrading bacterium Akkermansia muciniphila was the only identified member of the Firmicutes phylum. This microorganism was a hallmark of the healthy gut due to its anti-inflammatory and immunostimulant properties and its ability to improve gut barrier function, insulin sensitivity, and endotoxinemia (Fujio-Vejar et al., 2017).
Slow Transit Constipation in Clinical Perspective
Chronic constipation severely compromises the quality of life for long-term duration (Irvine et al., 2002). Constipation includes two types: primary constipation, also called functional constipation, and secondary constipation (Figure 1A) (Tillou and Poylin, 2017). Slow transit constipation (STC) accounts for a significant proportion of constipation cases. STC diagnosis requires evidence of slowed colonic transit. The Rome criteria, which was the most recent iteration (Rome III), was produced in 2006, providing a standardized definition of constipation (Tillou and Poylin, 2017). Patients with functional constipation were classified in three subgroups: normal transit constipation, disorders of defecatory or rectal evacuation (outlet obstruction), and STC (Figure 1A) (Lembo and Camilleri, 2003; Prather, 2004). Primary constipation is frequently due to constipation-predominant IBS, STC, obstructed defecation such as paradoxical contraction, pudendal neuropathy, increased perineal descent, or nonrelaxation of the puborectalis. The following six conditions contributed to the development of secondary constipation: (1) dehydration, (2) poor dietary fiber intake, (3) a variety of medications, (4) numerous medical conditions, (5) low physical activity levels, and (6) mechanical obstruction such as rectal stricture (Tillou and Poylin, 2017). While a variety of medical therapies exist, these are often met with limited success, and many patients have to face the surgery. Subtotal colectomy with ileorectal anastomosis is the most commonly performed surgical procedure (Tillou and Poylin, 2017). Nevertheless, for many patients, the surgery clinical effect is limited. The neuroendocrine system and the autonomic and enteric nervous systems have all been implicated in colonic dysmotility (Tillou and Poylin, 2017). However, the underlying mechanism of colonic dysmotility that leads to STC is ill defined.
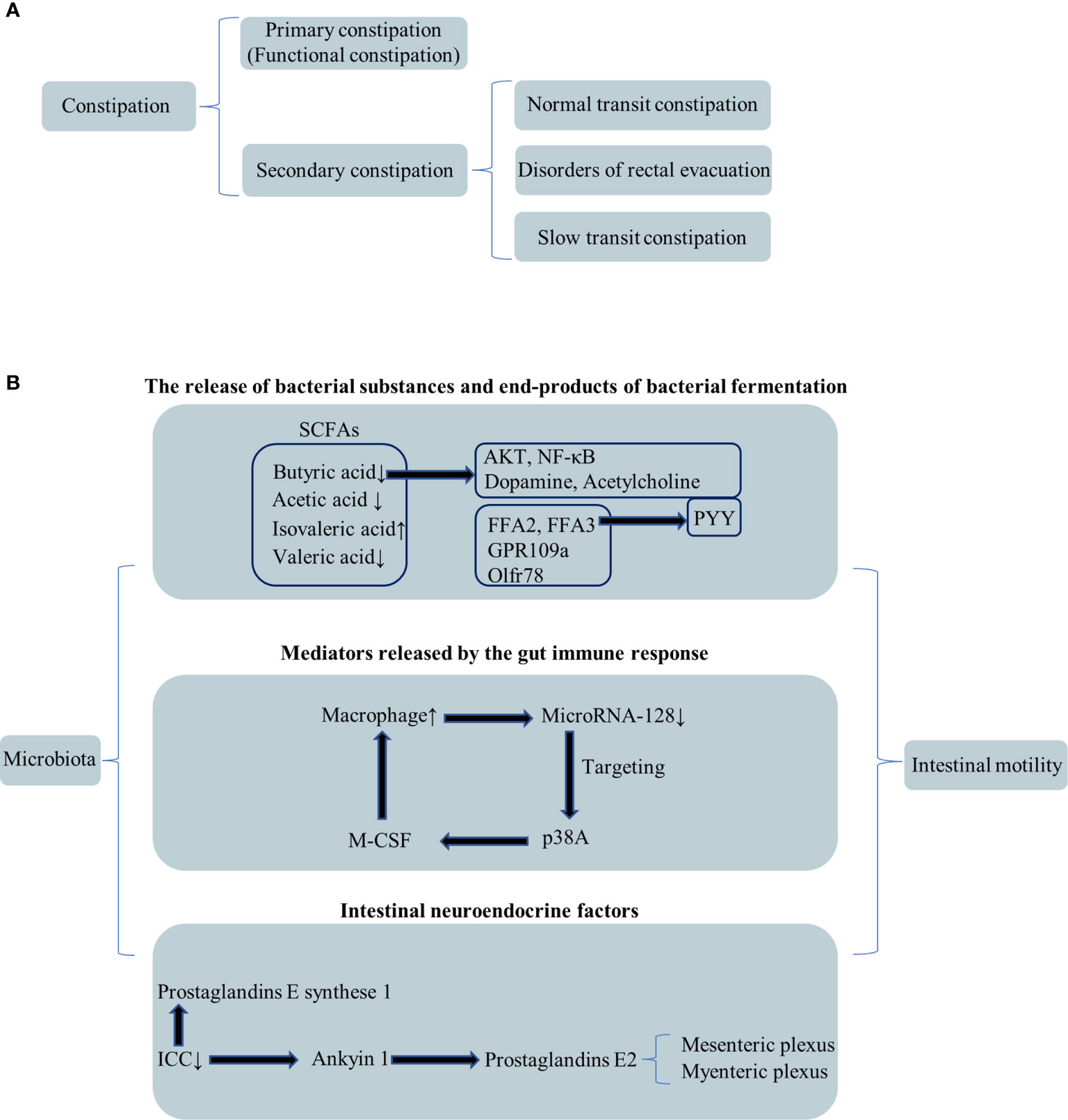
Figure 1 Classification of constipation and relationship among the microbiota and intestinal motility. (A) Secondary classification of constipation. (B) The relationship between the microbiota and intestinal motility through three perspectives.
The Clinical Therapeutic Effects and Mechanisms of Fecal Microbiota Transplantation in Slow Transit Constipation
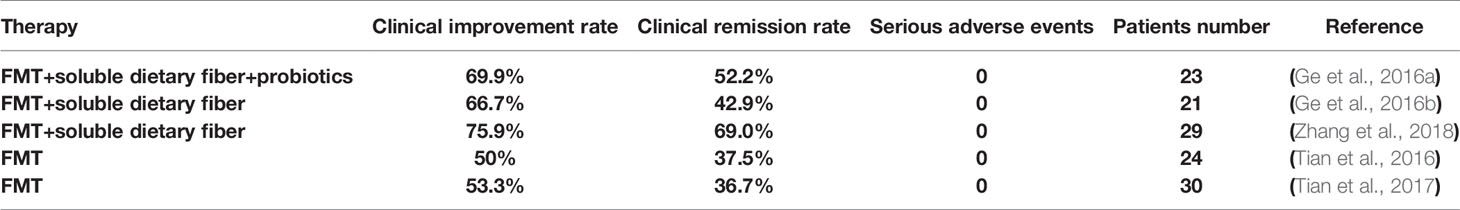
Li Ning’s team is an international authority in clinical trials in FMT treating STC. Recently, there have been seven published clinical trial reports about the FMT clinical applications for STC in Li Ning’s team (Table 1-1). In 2016, they pressed 23 STC patients who received FMT combined with soluble dietary fiber and probiotics [registered in ClinicalTrials.gov (NCT02016469)]. During the researchers’ team follow-up, the STC patient clinical improvement reached 69.6% (16/23), and the remission reached 52.2% (12/23). In the whole clinical research, no serious adverse events were observed (Ge et al., 2016a). As soluble dietary fiber and probiotics are known to have an independent effect on colon transit time, the study design has imperfection.
At the same year, they came out that 21 patients with STC, who received FMT on three consecutive days and soluble dietary fiber for 4 weeks (8 g, twice daily), are responding well to treatment. The clinical improvement reached 66.7%, and remission of constipated reached 42.9% (Ge et al., 2016b). In Ning Li et al.’s clinical trial, 60 patients were randomly assigned to two groups on average. Thirty patients were randomized to conventional treatment alone and another to FMT through a nasointestinal tube. The results showed that, besides not observing any serious adverse events, the FMT group had a 30% higher cure rate for treatment of STC than conventional treatment (Tian et al., 2017). In 2018, they came off the press that the FMT has a remarkable effect in STC clinical application. Li Ning’s study shows that FMT was effective and safe for STC. They compared different therapy times and found that 3 to 4 weeks receive the best benefits (Table 1-2) (Ding et al., 2018). Their results, published in 2018, indicate that FMT with soluble dietary in combination fiber has both short- and long-term efficacy in treating STC (Zhang et al., 2018). Li Ning’s research group compared the three treatment routes of bacterial flora transplantation between nasojejunal tube, oral enterobacterial capsule treatment, and colonoscopy infusion (pressed in 2020). Three months after treatment, they demonstrated the clinical improvement rates: 71.1% (69/97), 53.6% (45/84), and 44.0% (11/25), respectively. The nasojejunal tube route had better clinical efficacy above all (Table 1-3) (Tian H. L. et al., 2020). In this prospective open-label study, 24 patients with STC were enrolled. Through nasojejunal tubes, patients received FMT on three consecutive days. After treatment, there was a 12-week follow-up. Based on clinical activity at week 12, the clinical improvement rate was 50% (12/24), and the clinical remission rate was 37.5% (9/24). In this study, significant overall improvements were seen in the gastrointestinal quality-of-life index score at weeks 1, 2, 4, 8, and 12 of follow-up compared with baseline. The improvements were accompanied by the declining colonic transit time. Except for venting (6/24), abdominal pain (3/24), bloating (2/24), and diarrhea, there were no severe adverse events during the whole FMT procedure follow-up. This study, published in 2016 by Li Ning’s team, demonstrated that FMT was safe and had the potential to improve symptoms in patients with STC (Tian et al., 2016).
STC and constipation-predominant irritable bowel syndrome (IBS-C) have similar clinical symptoms. Long-term follow-up results showed the IBS-C patients’ stool frequency up to 2.68 ± 1.15 times per week from 1.5 ± 1.38 times after FMT treatment within 1 month (Cui et al., 2021). In response to FMT, there was no sex difference (El-Salhy et al., 2021). Although no serious adverse events were detected, one-third of the patients had mild adverse reactions during follow-up, such as abdominal distension, nausea, vomiting, headache, and fever (Cui et al., 2021). Alexander C. Ford and his research team via meta-analysis demonstrated that there was no benefit according to the IBS subtype from FMT, although they grouped patients with irritable bowel syndrome with diarrhea (IBS-D), irritable bowel syndrome with mixed (IBS-M), and IBS-C together. Since only 20 patients with IBS-C enrolled, a meaningful estimate of efficacy in IBS-C could not be provided (Ianiro et al., 2019). The conflicting data of FMT in IBS-C suggested the limitation of FMT in clinical applications. It opens up new ideas for future research on pathological differences and intestinal microflora characteristics of STC and IBS-C.
Using a mice animal model to determine the mechanism underlying delayed gut motility, researchers found that short-chain fatty acids (SCFAs) and secondary bile acids were decreased in mice, which received microbiota from constipated donors (Ge et al., 2017). Compared to the STC patients, the gut microbiota compositional changes in species richness and α diversity were much higher than those in healthy volunteers. Alterations of the microbiome via altered microbial-derived metabolites could affect gut motility in the development of constipation, and the clinical phenotype can improve after the restoration of disturbed microbiota. That means for STC, regulating the intestinal environment will be a novel therapy strategy (Ge et al., 2017).
However, the exact molecular mechanism of FMT’s treatment of STC has not been found. The pathogenesis of STC remains largely unknown; it is a motility disorder characterized by markedly increased total bowel transit time measured by radioactive markers with a normal radiologically assessed bowel diameter (Wang, 2015). Intestinal movement disorders cause STC. The gut microbiota and bacterial fermentation products may play a prominent role in intestinal dysmotility (Ikee et al., 2020). Barbara et al. came up with a hypothesis that there are three mechanisms responsible for the effects of microbiota on intestinal motility: (1) the release of bacterial substances or end products of bacterial fermentation; (2) intestinal neuroendocrine factors; and (3) mediators released by the gut immune response (Figure 1B) (Barbara et al., 2005).
Fatty Acids, Lipid Metabolism, and Metabolites
The alterations in fatty acid and lipid metabolism might be the reason causing functional constipation (Mancabelli et al., 2017). According to the number of carbon atoms in the carbon chain, the organic fatty acids with less than six carbon atoms are called short-chain fatty acids (SCFAs), which mainly include acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid. SCFAs might aggravate the symptoms of STC via enhancing colonic fluid and sodium absorption. Besides, the acetate concentration in the STC patient group was significantly reduced compared with the controls (Tian H. et al., 2020). In the human gut, SCFAs are generated by bacterial fermentation sensed by specific membrane-bound receptors: FFA2, FFA3, GPR109a, and Olfr78 (Priyadarshini et al., 2018). Researchers got the consensus that the shorter the carbon chain length of the SCFA, the higher the potency at FFA2 and vice versa for FFA3 (Hudson et al., 2012).
In STC mouse stools, Qiulan He et al. found that butyric and valeric acid declined while isovaleric acid content increased. By regulating AKT and NF-κB (nuclear factor-kappaB) signaling, butyrate could improve defecation and intestinal mobility (He et al., 2020). After supplementation with butyrate and deoxycholic acid, some symptoms in mice from STC donors were reversed (Ge et al., 2017). Above all, the decrease of butyrate was closely related to the formation of STC. The SCFA receptors FFA2 and FFA3 affect peptide YY(PYY) secretion, and PYY lowers the intestinal transit rate (Beglinger and Degen, 2006).
The effect of probiotics in the gut is also achieved through short-chain fatty acids. A significant association between dysregulation of gut flora and STC has been identified, suggesting that probiotics may be an important potential treatment option for constipation in the future. Hiroshi Ohno’s team found that Bifidobacterium bifidum G9-1 (BBG9-1) has an effect on loperamide-induced STC in the rat model. BBG9-1 improved constipation parameters (fecal quantity, fecal water content, and fecal hardness) in constipated rats. It was found that BBG9-1 improved intestinal flora imbalance, prevented the decrease in intestinal butyric acid concentration, increased serum serotonin, and inhibited the increase in serum dopamine and the decrease in acetylcholine. In addition, increased expression of tryptophan hydroxylase 1 (5-HT synthase) was observed. These results suggested that BBG9-1 ameliorates ecological dysregulation, which leads to an increase in organic acids, further increasing intestinal fluidity, and ultimately to the relief of constipation. Therefore, the probiotics BBG9-1 might be a potential treatment option for STC (Makizaki et al., 2021).
Intestinal Neuron
Enteric neuron could regulate gut motility by the microbiota. Vassilis et al.’s study showed that the transcription factor aryl hydrocarbon receptor (AHR) acts as a biosensor linking the intestinal environment to intestinal motility programs of the enteric nervous system (ENS) (Hindson, 2020). The transcriptional profiling in this study demonstrated that in colonic neurons, microbiota-dependent induction regulates AHR signaling or enteric excitability specific genes (Obata et al., 2020). The mice with deletion of the AHR gene had a longer intestinal transit time than the control mice. Enteric-neuron-specific deletion of the AHR gene reduced colonic peristaltic activity.
Thus, modulating AHR signaling could have potential application for the treatment of STC. In Gu et al.’s study, there were three groups of patients: healthy control group, normal transit constipation group, and STC group. Compared with the healthy control group, concentrations of neurotensin and motilin were both significantly reduced in the normal transit constipation group and the STC group. They found that the miR-19a level was the highest, whereas the circORC2 level was the lowest in the slow transit constipation group. Their study established a relationship web among circORC2, miR-19a, and neurotensin/motilin. Those indicated that the overexpression of circORC2 could upregulate the levels of neurotensin and motilin exerting a beneficial treatment for STC (Wang et al., 2021).
Immune System
Liu et al. found a significant increase in the number of macrophages in 20 (80%) of the 25 colonic specimens from patients with STC compared with patients without a history of constipation, which was associated with downregulating the expression of microRNA-128. They further demonstrated that microRNA-128 might directly target p38A in intestinal epithelial cells to regulate the expression of the macrophage colony stimulating factor (M-CSF). Researchers hypothesized that more macrophages were recruited to the colon to activate the immune response, leading to the onset of STC. In addition, in specimens from patients with STC, the authors observed a reduction in the number of interstitial cells of Cajal (ICCs) in colon, further supporting that ICC is the marker of intestinal peristalsis (Liu et al., 2015). Treg is specialized by T cells noted for immune-suppressive effects against autoantigens. SCFAs’ receptor FFA2 could affect immune homeostasis through the regulation of the number and function of Tregs (Sakaguchi et al., 2008). Besides, SCFAs regulated their expansion in the intestine through FFA2 signaling (Priyadarshini et al., 2018).
Interstitial Cells of Cajal and Others
Other investigations found that the quantitative alternations of the ICC are the significant biomarker of the gastrointestinal tract motility. Researchers used antibodies of CD117 or DOG1 as the ICC’s biomarker. They demonstrated a complete absence or significant reduction in the number of ICC in colon specimens resected from patients with STC compared with normal controls (He et al., 2000; Geramizadeh et al., 2009). Prostaglandins have been reported to play an indispensable role in the mechanism of intestinal motility (Staumont et al., 1988; Dey et al., 2006) through regulating the function of intestinal smooth muscle (Tokita et al., 2015). Prostaglandin E2 acted on individual myocytes and coordinated peristalsis via the mesenteric and myenteric plexus (Haupt et al., 2000). Mesenchymal cells promoted colorectal contraction by two ways: (1) synthesizing the vital enzymes needed in prostaglandin synthesis, such as prostaglandin E synthase 1, and (2) expressing transient receptor potential ankyrin 1 to activating Prostaglandin E2 release (Yang et al., 2019). Lubiprostone, a Prostaglandin E2 derivative, alleviated small bowel bacterial overgrowth in patients with chronic constipation (Sarosiek et al., 2016) and modulated the pacemaker activity of ICC in mice (Jiao et al., 2014). Above all, the researchers’ findings demonstrate the dynamic interaction between the gut microbiome, epithelial function, and intestinal motility.
Future Perspective for FMT
FMT is a promising treatment at present. However, only one randomized controlled trial has been performed yet; more evidence is needed to certify FMT as an available clinical therapy for STC. Furthermore, FMT to be considered as a first choice is unlikely due to the challenges in identifying donors as well as the complexity of the procedure. FMT might be more suitable in patients who are refractory to conventional therapeutic strategies. In most studies, donors were recruited without checking the microbial profile (Ohkusa et al., 2019). It is not nearly enough to say what one person’s gut microbiota does to another. In addition to safety issues, the lack of standards of behavior is currently a barrier to the spread of FMT. Strict implementation standards are still incomplete, and systematic data on the efficacy and long-term and short-term adverse reactions of FMT are still lacking, including the need to determine and monitor the improvement or deterioration of each patient’s response to FMT. With the uncertain pathogenesis and the complexity of microorganisms in the human gut, there is a long way to unraveling the veil of STC pathogenesis and the mechanism of FMT in STC. FMT is the first and most natural method to change the intestinal microecology, especially in the treatment of STC, which highlights the effects that both doctors and patients agree on. However, in clinical practice, the treatment effect declines over time, and repetitive FMT is required to acquire a sustained effect. Besides, its long-term prognosis and overall advantages and disadvantages are not fully understood. In the future, more animal and clinical trials will be needed to further validate this treatment. Even if the current evidence is not sufficient, we still have high expectations and full confidence about the future therapeutic effects of FMT.
Author Contributions
JL, LG, YL, and XZ designed the paper, contributed to manuscript writing, and approved the manuscript before submission. JL and LG collected literatures and approved the manuscript before submission. SZ, MZ, and MW gave constructive comments on the manuscript and approved the manuscript before submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Foundation of the committee on science and technology of Tianjin (20JCQNJC01870) and Joint Scientific Research Project between Nankai University and Tianjin Union Medical Center (2016rmnk002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHR, aryl hydrocarbon receptor; BBG9-1, Bifidobacterium bifidum G9-1; CDI, Clostridium difficile infection; ENS, enteric nervous system; FMT, Fecal microbiota transplantation; IBD, Inflammatory bowel disease; IBS, Irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with mixed; ICC, interstitial cells of Cajal; M-CSF, macrophage colony stimulating factor; NF-κB, nuclear factor-kappaB; PYY, peptide YY; SCFA, Short-chain fatty acid; STC, Slow transit constipation.
References
Allegretti, J. R., Kelly, C. R., Grinspan, A., Mullish, B. H., Kassam, Z., Fischer, M. (2020). Outcomes of Fecal Microbiota Transplantation in Patients With Inflammatory Bowel Diseases and Recurrent Clostridioides Difficile Infection. Gastroenterology 159 (5), 1982–1984. doi: 10.1053/j.gastro.2020.07.045
Barbara, G., Stanghellini, V., Brandi, G., Cremon, C., Di Nardo, G., De Giorgio, R., et al. (2005). Interactions Between Commensal Bacteria and Gut Sensorimotor Function in Health and Disease. Am. J. Gastroenterol. 100 (11), 2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x
Beglinger, C., Degen, L. (2006). Gastrointestinal Satiety Signals in Humans–Physiologic Roles for GLP-1 and PYY? Physiol. Behav. 89 (4), 460–464. doi: 10.1016/j.physbeh.2006.05.048
Cui, J., Lin, Z., Tian, H., Yang, B., Zhao, D., Ye, C., et al. (2021). Long-Term Follow-Up Results of Fecal Microbiota Transplantation for Irritable Bowel Syndrome: A Single-Center, Retrospective Study. Front. Med. (Lausanne) 8, 710452. doi: 10.3389/fmed.2021.710452
Dey, I., Lejeune, M., Chadee, K. (2006). Prostaglandin E2 Receptor Distribution and Function in the Gastrointestinal Tract. Br. J. Pharmacol. 149 (6), 611–623. doi: 10.1038/sj.bjp.0706923
Ding, C., Fan, W., Gu, L., Tian, H., Ge, X., Gong, J., et al. (2018). Outcomes and Prognostic Factors of Fecal Microbiota Transplantation in Patients With Slow Transit Constipation: Results From a Prospective Study With Long-Term Follow-Up. Gastroenterol. Rep. (Oxf) 6 (2), 101–107. doi: 10.1093/gastro/gox036
Dupont, H. L., Jiang, Z. D., Dupont, A. W., Utay, N. S. (2020). THE INTESTINAL MICROBIOME IN HUMAN HEALTH AND DISEASE. Trans. Am. Clin. Climatol Assoc. 131, 178–197.
El-Salhy, M., Casen, C., Valeur, J., Hausken, T., Hatlebakk, J. G. (2021). Responses to Faecal Microbiota Transplantation in Female and Male Patients With Irritable Bowel Syndrome. World J. Gastroenterol. 27 (18), 2219–2237. doi: 10.3748/wjg.v27.i18.2219
Fujio-Vejar, S., Vasquez, Y., Morales, P., Magne, F., Vera-Wolf, P., Ugalde, J. A., et al. (2017). The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 8, 1221. doi: 10.3389/fmicb.2017.01221
Ge, X., Ding, C., Gong, J., Tian, H., Wei, Y., Chen, Q., et al. (2016a). [Short-Term Efficacy on Fecal Microbiota Transplantation Combined With Soluble Dietary Fiber and Probiotics in the Treatment of Slow Transit Constipation]. Zhonghua Wei Chang Wai Ke Za Zhi 19 (12), 1355–1359.
Geramizadeh, B., Hayati, K., Rahsaz, M., Hosseini, S. V. (2009). Assessing the Interstitial Cells of Cajal, Cells of Enteric Nervous System and Neurotransmitters in Slow Transit Constipation, Using Immunohistochemistry for CD117, PGP9.5 and Serotonin. Hepatogastroenterology 56 (96), 1670–1674.
Ge, X., Tian, H., Ding, C., Gu, L., Wei, Y., Gong, J., et al. (2016b). Fecal Microbiota Transplantation in Combination With Soluble Dietary Fiber for Treatment of Slow Transit Constipation: A Pilot Study. Arch. Med. Res. 47 (3), 236–242. doi: 10.1016/j.arcmed.2016.06.005
Ge, X., Zhao, W., Ding, C., Tian, H., Xu, L., Wang, H., et al. (2017). Potential Role of Fecal Microbiota From Patients With Slow Transit Constipation in the Regulation of Gastrointestinal Motility. Sci. Rep. 7 (1), 441. doi: 10.1038/s41598-017-00612-y
Gibiino, G., Lopetuso, L. R., Scaldaferri, F., Rizzatti, G., Binda, C., Gasbarrini, A. (2018). Exploring Bacteroidetes: Metabolic Key Points and Immunological Tricks of Our Gut Commensals. Dig Liver Dis. 50 (7), 635–639. doi: 10.1016/j.dld.2018.03.016
Grigor'eva, I. N. (2020). Gallstone Disease, Obesity and the Firmicutes/Bacteroidetes Ratio as a Possible Biomarker of Gut Dysbiosis. J. Pers. Med. 11 (1), 20. doi: 10.3390/jpm11010013
Gu, L., Ding, C., Tian, H., Yang, B., Zhang, X., Hua, Y., et al. (2017). Serial Frozen Fecal Microbiota Transplantation in the Treatment of Chronic Intestinal Pseudo-Obstruction: A Preliminary Study. J. Neurogastroenterol. Motil. 23 (2), 289–297. doi: 10.5056/jnm16074
Haupt, W., Jiang, W., Kreis, M. E., Grundy, D. (2000). Prostaglandin EP Receptor Subtypes Have Distinctive Effects on Jejunal Afferent Sensitivity in the Rat. Gastroenterology 119 (6), 1580–1589. doi: 10.1053/gast.2000.20337
He, C. L., Burgart, L., Wang, L., Pemberton, J., Young-Fadok, T., Szurszewski, J., et al. (2000). Decreased Interstitial Cell of Cajal Volume in Patients With Slow-Transit Constipation. Gastroenterology 118 (1), 14–21. doi: 10.1016/s0016-5085(00)70409-4
He, Q., Han, C., Huang, L., Yang, H., Hu, J., Chen, H., et al. (2020). Astragaloside IV Alleviates Mouse Slow Transit Constipation by Modulating Gut Microbiota Profile and Promoting Butyric Acid Generation. J. Cell Mol. Med. 24 (16), 9349–9361. doi: 10.1111/jcmm.15586
Hiippala, K., Kainulainen, V. (2020). Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides Spp. From A Healthy Fecal Donor. Nutrients 12 (4), 935. doi: 10.3390/nu12040935
Hindson, J. (2020). Enteric Neuron Regulation of Gut Motility by the Microbiota. Nat. Rev. Gastroenterol. Hepatol. 17 (4), 194–195. doi: 10.1038/s41575-020-0283-y
Hudson, B. D., Tikhonova, I. G., Pandey, S. K., Ulven, T., Milligan, G. (2012). Extracellular Ionic Locks Determine Variation in Constitutive Activity and Ligand Potency Between Species Orthologs of the Free Fatty Acid Receptors FFA2 and FFA3. J. Biol. Chem. 287 (49), 41195–41209. doi: 10.1074/jbc.M112.396259
Ianiro, G., Eusebi, L. H., Black, C. J., Gasbarrini, A., Cammarota, G., Ford, A. C. (2019). Systematic Review With Meta-Analysis: Efficacy of Faecal Microbiota Transplantation for the Treatment of Irritable Bowel Syndrome. Aliment Pharmacol. Ther. 50 (3), 240–248. doi: 10.1111/apt.15330
Ikee, R., Sasaki, N., Yasuda, T., Fukazawa, S. (2020). Chronic Kidney Disease, Gut Dysbiosis, and Constipation: A Burdensome Triplet. Microorganisms 8 (12), 1862. doi: 10.3390/microorganisms8121862
Irvine, E. J., Ferrazzi, S., Pare, P., Thompson, W. G., Rance, L. (2002). Health-Related Quality of Life in Functional GI Disorders: Focus on Constipation and Resource Utilization. Am. J. Gastroenterol. 97 (8), 1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x
Jiao, H. Y., Kim, D. H., Ki, J. S., Ryu, K. H., Choi, S., Jun, J. Y. (2014). Effects of Lubiprostone on Pacemaker Activity of Interstitial Cells of Cajal From the Mouse Colon. Korean J. Physiol. Pharmacol. 18 (4), 341–346. doi: 10.4196/kjpp.2014.18.4.341
Lembo, A., Camilleri, M. (2003). Chronic Constipation. N Engl. J. Med. 349 (14), 1360–1368. doi: 10.1056/NEJMra020995
Liu, W., Zhang, Q., Li, S., Li, L., Ding, Z., Qian, Q., et al. (2015). The Relationship Between Colonic Macrophages and MicroRNA-128 in the Pathogenesis of Slow Transit Constipation. Dig Dis. Sci. 60 (8), 2304–2315. doi: 10.1007/s10620-015-3612-1
Makizaki, Y., Uemoto, T., Yokota, H., Yamamoto, M., Tanaka, Y. (2021). Improvement of Loperamide-Induced Slow Transit Constipation by Bifidobacterium Bifidum G9-1 Is Mediated by the Correction of Butyrate Production and Neurotransmitter Profile Due to Improvement in Dysbiosis. PloS One 16 (3), e0248584. doi: 10.1371/journal.pone.0248584
Mancabelli, L., Milani, C., Lugli, G. A., Turroni, F., Mangifesta, M., Viappiani, A., et al. (2017). Unveiling the Gut Microbiota Composition and Functionality Associated With Constipation Through Metagenomic Analyses. Sci. Rep. 7 (1), 9879. doi: 10.1038/s41598-017-10663-w
Matsuoka, K. (2020). Fecal Microbiota Transplantation for Ulcerative Colitis. Immunol. Med. 44 (1), 30–34. doi: 10.1080/25785826.2020.1792040
McDonald, L. C., Gerding, D. N., Johnson, S., Bakken, J. S., Carroll, K. C., Coffin, S. E., et al. (2018). Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66 (7), 987–994. doi: 10.1093/cid/ciy149
Meng, C., Bai, C., Brown, T. D., Hood, L. E., Tian, Q. (2018). Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinf. 16 (1), 33–49. doi: 10.1016/j.gpb.2017.06.002
Mullish, B. H., Quraishi, M. N., Segal, J. P., McCune, V. L., Baxter, M., Marsden, G. L., et al. (2018). The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridium Difficile Infection and Other Potential Indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. Gut 67 (11), 1920–1941. doi: 10.1136/gutjnl-2018-316818
Obata, Y., Castaño, Á., Boeing, S., Bon-Frauches, A. C., Fung, C., Fallesen, T., et al. (2020). Neuronal Programming by Microbiota Regulates Intestinal Physiology. Nature 578 (7794), 284–289. doi: 10.1038/s41586-020-1975-8
Ohkusa, T., Koido, S., Nishikawa, Y., Sato, N. (2019). Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. (Lausanne) 6, 19. doi: 10.3389/fmed.2019.00019
Prather, C. M. (2004). Subtypes of Constipation: Sorting Out the Confusion. Rev. Gastroenterol. Disord. 4 (Suppl 2), S11–S16.
Priyadarshini, M., Kotlo, K. U., Dudeja, P. K., Layden, B. T. (2018). Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 8 (3), 1091–1115. doi: 10.1002/cphy.c170050
Sakaguchi, S., Yamaguchi, T., Nomura, T., Ono, M. (2008). Regulatory T Cells and Immune Tolerance. Cell 133 (5), 775–787. doi: 10.1016/j.cell.2008.05.009
Sarosiek, I., Bashashati, M., Alvarez, A., Hall, M., Shankar, N., Gomez, Y., et al. (2016). Lubiprostone Accelerates Intestinal Transit and Alleviates Small Intestinal Bacterial Overgrowth in Patients With Chronic Constipation. Am. J. Med. Sci. 352 (3), 231–238. doi: 10.1016/j.amjms.2016.05.012
Shin, N. R., Whon, T. W., Bae, J. W. (2015). Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 33 (9), 496–503. doi: 10.1016/j.tibtech.2015.06.011
Staumont, G., Fioramonti, J., Frexinos, J., Bueno, L. (1988). Changes in Colonic Motility Induced by Sennosides in Dogs: Evidence of a Prostaglandin Mediation. Gut 29 (9), 1180–1187. doi: 10.1136/gut.29.9.1180
Tian, H. L., Chen, Q. Y., Yang, B., Ma, C. L., Lin, Z. L., Zhang, X. Y., et al. (2020). Effects of Fecal Microbiota Transplantation in Different Routes on the Clinical Efficacy of Slow Transit Constipation. Zhonghua Wei Chang Wai Ke Za Zhi 23 (Z1), 63–68. doi: 10.3760/cma.j.cn.441530-20200415-00212
Tian, H., Chen, Q., Yang, B., Qin, H., Li, N. (2020). Analysis of Gut Microbiome and Metabolite Characteristics in Patients With Slow Transit Constipation. Dig Dis. Sci. 66 (9), 3026–3035. doi: 10.1007/s10620-020-06500-2
Tian, H., Ding, C., Gong, J., Ge, X., McFarland, L. V., Gu, L., et al. (2016). Treatment of Slow Transit Constipation With Fecal Microbiota Transplantation: A Pilot Study. J. Clin. Gastroenterol. 50 (10), 865–870. doi: 10.1097/mcg.0000000000000472
Tian, H., Ge, X., Nie, Y., Yang, L., Ding, C., McFarland, L. V., et al. (2017). Fecal Microbiota Transplantation in Patients With Slow-Transit Constipation: A Randomized, Clinical Trial. PloS One 12 (2), e0171308. doi: 10.1371/journal.pone.0171308
Tillou, J., Poylin, V. (2017). Functional Disorders: Slow-Transit Constipation. Clin. Colon Rectal Surg. 30 (1), 76–86. doi: 10.1055/s-0036-1593436
Tjalsma, H., Boleij, A., Marchesi, J. R., Dutilh, B. E. (2012). A Bacterial Driver-Passenger Model for Colorectal Cancer: Beyond the Usual Suspects. Nat. Rev. Microbiol. 10 (8), 575–582. doi: 10.1038/nrmicro2819
Tokita, Y., Akiho, H., Nakamura, K., Ihara, E., Yamamoto, M. (2015). Contraction of Gut Smooth Muscle Cells Assessed by Fluorescence Imaging. J. Pharmacol. Sci. 127 (3), 344–351. doi: 10.1016/j.jphs.2015.02.002
Wang, H. L. (2015). Understanding the Pathogenesis of Slow-Transit Constipation: One Step Forward. Dig Dis. Sci. 60 (8), 2216–2218. doi: 10.1007/s10620-015-3754-1
Wang, Y. Y., Lu, R. Y., Shi, J., Zhao, S., Jiang, X., Gu, X. (2021). CircORC2 Is Involved in the Pathogenesis of Slow Transit Constipation via Modulating the Signalling of miR-19a and Neurotensin/Motilin. J. Cell Mol. Med. 25 (8), 3754–3764. doi: 10.1111/jcmm.16211
Yang, Y., Wang, S., Kobayashi, K., Hao, Y., Kanda, H., Kondo, T., et al. (2019). TRPA1-Expressing Lamina Propria Mesenchymal Cells Regulate Colonic Motility. JCI Insight 4 (9), e122402. doi: 10.1172/jci.insight.122402
Zhang, X., Tian, H., Gu, L., Nie, Y., Ding, C., Ge, X., et al. (2018). Long-Term Follow-Up of the Effects of Fecal Microbiota Transplantation in Combination With Soluble Dietary Fiber as a Therapeutic Regimen in Slow Transit Constipation. Sci. China Life Sci. 61 (7), 779–786. doi: 10.1007/s11427-017-9229-1
Keywords: slow transit constipation, fecal microbiota transplantation, intestinal neuron, short-chain fatty acids, intestinal motility
Citation: Liu J, Gu L, Zhang M, Zhang S, Wang M, Long Y and Zhang X (2021) The Fecal Microbiota Transplantation: A Remarkable Clinical Therapy for Slow Transit Constipation in Future. Front. Cell. Infect. Microbiol. 11:732474. doi: 10.3389/fcimb.2021.732474
Received: 29 June 2021; Accepted: 04 October 2021;
Published: 22 October 2021.
Edited by:
Stefano Bibbò, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Jayanta Kumar Patra, Dongguk University Seoul, South KoreaPaolo Usai-Satta, G. Brotzu Hospital, Italy
Copyright © 2021 Liu, Gu, Zhang, Zhang, Wang, Long and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xipeng Zhang, liujiafeigarfield@163.com; Yu Long, 1411110405@pku.edu.cn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Jiafei Liu
Jiafei Liu Liqiang Gu
Liqiang Gu Mingqing Zhang1
Mingqing Zhang1  Shiwu Zhang
Shiwu Zhang Min Wang
Min Wang