The Role of Paracrine Regulation of Mesenchymal Stem Cells in the Crosstalk With Macrophages in Musculoskeletal Diseases: A Systematic Review
- 1Department of Orthopaedics and Traumatology, Faculty of Medicine, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
- 2Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Hong Kong, China
- 3Developmental and Regenerative Biology TRP, Faculty of Medicine, School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong, China
- 4Faculty of Medicine, Li Ka Shing Institute of Health Sciences, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
- 5Department of Orthopadics, China Medical University Hospital, Taichung, Taiwan
The phenotypic change of macrophages (Mφs) plays a crucial role in the musculoskeletal homeostasis and repair process. Although mesenchymal stem cells (MSCs) have been shown as a novel approach in tissue regeneration, the therapeutic potential of MSCs mediated by the interaction between MSC-derived paracrine mediators and Mφs remains elusive. This review focused on the elucidation of paracrine crosstalk between MSCs and Mφs during musculoskeletal diseases and injury. The search method was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and Cochrane Guidelines. The search strategies included MeSH terms and other related terms of MSC-derived mediators and Mφs. Ten studies formed the basis of this review. The current finding suggested that MSC administration promoted proliferation and activation of CD163+ or CD206+ M2 Mφs in parallel with reduction of proinflammatory cytokines and increase in anti-inflammatory cytokines. During such period, Mφs also induced MSCs into a motile and active phenotype via the influence of proinflammatory cytokines. Such crosstalk between Mφs and MSCs further strengthens the effect of paracrine mediators from MSCs to regulate Mφs phenotypic alteration. In conclusion, MSCs in musculoskeletal system, mediated by the interaction between MSC paracrine and Mφs, have therapeutic potential in musculoskeletal diseases.
Introduction
The inflammatory processes in response to musculoskeletal diseases and injury, such as bone fractures, osteoarthritis (OA), osteoporosis, tendon injuries, and muscle injuries, are essential for the correct restoration of structure and function to the affected area (Bosurgi et al., 2011; Mianehsaz et al., 2019; Pajarinen et al., 2019; Wang et al., 2019; Yang and Yang, 2019). However, the dysregulation of inflammatory reactions can aggravate the tissue healing results (Saldana et al., 2019).
Macrophages (Mφs) are the critical regulators involved in initiation, propagation, and resolution of inflammatory response throughout the tissue regenerative process. Mφs have a broad spectrum of adaptive phenotypes and functional transitions that might exacerbate and resolve inflammation during tissue repair process (Saldana et al., 2019). In 2008, Mosser and Edwards analyzed the phenotypic changes of Mφs and robustly classified Mφs into two types: M1 and M2 (Mosser and Edwards, 2008). Proinflammatory Mφs are identified as the classic M1 Mφs, which are involved in the early stages of tissue repair, whereas the anti-inflammatory Mφs are identified as M2 Mφs, which dominated later stages of tissue repair (Murray et al., 2014; Spiller and Koh, 2017). Upon injury, the early presence of M1 Mφs initiates tissue repair, but the persistent of M1 activity can deteriorate the repair process (Krzyszczyk et al., 2018). On the other hand, the early presence of M2 can prevent cellular and vascular infiltration that impairs tissue development through ectopic secretion of fibrotic chemokines and cytokine (Stahl et al., 2013; Bility et al., 2014; Moore et al., 2015). Moreover, several different M2 subtypes have been identified, including M2a, M2b, M2c, and M2d (Stein et al., 1992; Donnelly et al., 1999; Anderson and Mosser, 2002). The undisciplined regulation of Mφ phenotypic change impairs tissue repair, and each of the subtypes might have specific functions; therefore, further investigation is needed to identify the explicit role of M2 subtypes (O'Brien et al., 2019).
In the 1980's, Arnold Caplan and his colleagues published an isolation method of fibroblast-like stromal cells from bone marrow and first identified them as mesenchymal stem cells (MSCs) because of their multilineage differentiation potential (Caplan, 1991). A rapid expansion in the field of MSC-based therapy in immunomodulation and regenerative medicine has been acknowledged (Kingery et al., 2019). It has been reported that MSCs can switch M1 Mφs or resting Mφs into M2 Mφs (Kim and Hematti, 2009; Nemeth et al., 2009; Melief et al., 2013b). Previous studies showed that prostaglandin E2 (PGE2), interleukin-4 (IL-4), IL-6, and IL-10 released from MSCs can induce the Mφ polarization toward M2 to accelerate tissue regeneration (da Costa Goncalves and Paz, 2019). On the other hand, such immunomodulatory ability of MSCs is adjusted by inflammatory factors released by macrophages (Waterman et al., 2010; Carrero et al., 2012). After the stimulation, MSCs would secrete anti-inflammatory factors, such as transforming growth factor β (TGF-β) and CCL-18, to further suppress activation of lymphocytes and inhibit major histocompatibility complex (MHC) class II and CD86 in lipopolysaccharide (LPS)–stimulated Mφs (Melief et al., 2013b; Cho et al., 2014). This suggested that the interplay between MSCs and Mφs is in control of inflammation, and their crosstalk may be recommended to advocate tissue healing or repair.
Besides versatile soluble proteins, MSC-derived extracellular vesicles (MSC-EVs) have raised worldwide attention in regenerative medicine because of their immunomodulation ability (Raposo and Stoorvogel, 2013). EVs represent a heterogeneous group of cell-derived membranous vesicles, such as microvesicles (MVs) and exosomes, which were first described in the 1970's (Vakhshiteh et al., 2019). MVs, ranging from 40 to 2,000 nm in diameter (Bruno et al., 2012; Kim et al., 2012), and exosomes, ranging from 30 to 150 nm in diameter (Li et al., 2013; Zhao et al., 2015), are critical mediators for intercellular communication via the delivery of the embedded RNAs, DNAs, and cytosolic proteins. Moreover, boosting the therapeutic potential of MSC-EVs by changing the intrinsic bioactive factors or modifying membrane by bioengineering approach is possible (Lu and Huang, 2020). Refining the culture condition of MSCs could significantly increase the production yield and improve the efficacy of MSC-EVs (Luan et al., 2017; Willis et al., 2017; Bagno et al., 2018; Cha et al., 2018; Ferguson et al., 2018). Also, MSC-derived EVs could serve as a promising drug delivery vector, owing to their high biocompatibility, high efficacy of delivery, and low immunogenicity (Clayton et al., 2003; Ridder et al., 2014; Zomer et al., 2015). EVs derived from different origins have preferential targeting cells due to their distinct membrane composition gained from their parental cells that impart differential effect on body systems (Luan et al., 2017; Ferguson et al., 2018), which could be addressed by exploiting the nature of EVs as natural carriers of miRNA or other molecules by considering them as drug delivery vehicles (Cheng et al., 2017). In addition, MSC-derived EVs in conjunction with other materials might provide substantial advances in both immunomodulation and tissue regeneration (Cosenza et al., 2018). And it has been reported that a variety of material's environment could affect cell's downstream response by cell–material interactions (Darnell et al., 2018). Taken together, bioengineered MSC-derived EVs are novel adjustable biomaterials for tissue regeneration.
The therapeutic effect of MSCs showed a clinical benefit in children suffering from osteogenesis imperfecta (Otsuru et al., 2012) and preclinical benefit in bone fracture (Li Y. et al., 2019), OA (Cosenza et al., 2017), tendon injury (Chamberlain et al., 2019), pulmonary hypertension (Lee et al., 2012), cancer (Silva et al., 2013), and infectious diseases (Cheng and Schorey, 2013). The mechanisms of these mentioned therapeutic potentials of MSCs need elucidation. This review aimed to expound the role of MSC paracrine, especially MSC-derived EVs, in the crosstalk with Mφs in musculoskeletal diseases. Moreover, a systemic understanding of MSC-EVs properties and activities will provide a solid foundation to boost MSC-EVs for regenerative medicine and will significantly facilitate the translation value of MSC-based therapy (Zhang et al., 2020).
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used for this article. Meanwhile, the Cochrane handbook was selected as guidelines for the study protocol (Moher et al., 2009).
Search Strategy and Study Selection
Two different investigators (H.-T.X., L.-Y.S.) conducted the customized up-to-date literature search. PubMed database and EMBASE (Excerpta Medica Database) database were selected in this study.
The title and abstract field were selected to search for MeSH terms and other related terms, which pertained to “Mesenchymal Stem Cells,” “Macrophages,” and “Paracrine Regulation,” such as “Exosomes,” “Extracellular Vesicles” and “Culture Media, Conditioned.” The details of selected search terms and searching procedures that were used in the individual database are available in Appendices 1–3 in Supplementary Material. Additional studies were also located by searching papers referenced in listed articles. Those studies identified by the search outcomes were combined, and duplicates were excluded. Then, the screening procedure of titles and abstracts was performed before elaboration on the selected full-text articles. Two investigators (H.-T.X., L.-Y.S.) screened the titles and abstracts of those identified studies individually. In cases of disagreement between the two authors, a consensus was reached by discussion with a third author (C.-W.L.). After that, the full text of screened articles were examined. Corresponding authors of the reviewed articles could be contacted with essential needs to obtain those missing data.
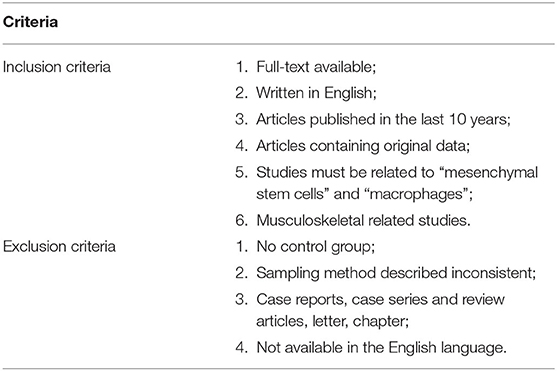
The search yielded 433 studies across all databases (239 studies across PubMed database and 194 studies across EMBASE database). A total of 93 duplicates were removed. According to the inclusion and exclusion criteria, all these studies were screened and reviewed by titles and abstracts first; 322 studies were excluded because they were review articles, case reports, case series, letters, chapters, or studies published more than 10 years ago. Of the 18 remaining studies, which were applied by a filter to include musculoskeletal-related studies, eight studies were excluded via reviewing full text. The 10 remaining studies underwent secondary full-text review and were confirmed as fitting the inclusion criteria. The flowchart of the selected studies selection process, which was based on the PRISMA 2009 Flow Diagram (Moher et al., 2009), is shown in Figure 1, and the details of inclusion and exclusion criteria are shown in Table 1.
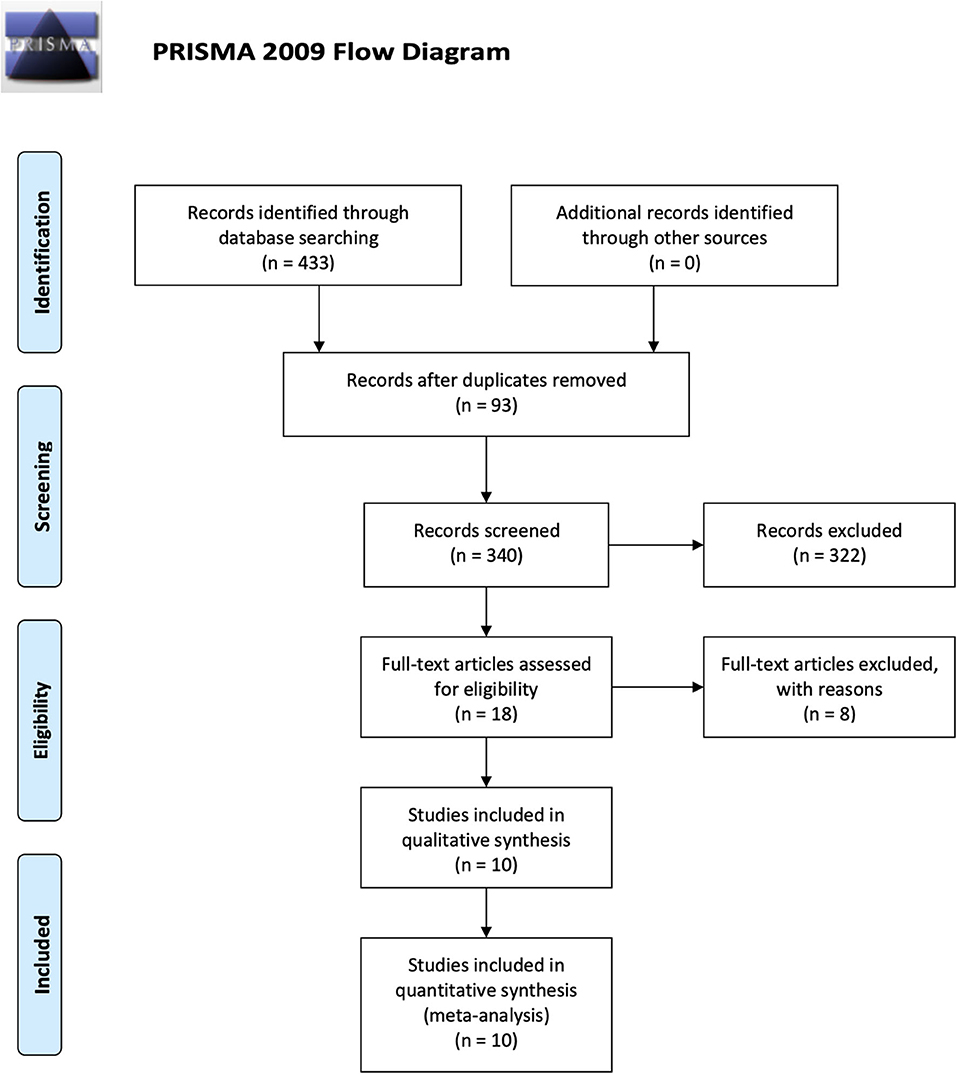
Figure 1. Flowchart presenting the results of the literature search and the strategy used to select studies that relate to the crosstalk between MSCs and Mφs of musculoskeletal diseases. Study selection process. The search revealed 433 records. A total of 93 overlaps were removed between the databases. The remaining 340 records were screened by title and abstract, and 322 records were excluded. The remaining 18 studies were examined using their full texts, and finally ten eligible studies were identified.
Data Extraction
All relevant data were extracted by H.-T.X. and X.-B.Z.: author information, published journal, year of publication, sample source, target disease, study type (in vivo, in vitro, or both), cell management, and measurement instrument. The details of results extraction consisted of variable/control group descriptions, laboratory effects, proposed mechanisms, article conclusions, and research implications. After that, the selected articles were classified according to the type of target disease.
Methodological Quality Assessment
The methodological assessment should be used as an essential procedure, which could exclude articles with a large degree of bias or with a higher degree of potential bias, has been highlighted to readers (McElvany et al., 2015). Identified studies fitting all criteria were reviewed, and all included data were extracted and analyzed based on study heterogeneity and methodological quality. Because of the natural heterogeneity of measurement across studies, a meta-analysis could not be performed. Two independent authors (H.-T.X. and Z.-H.W.) separately assessed and graded the methodological quality of all selected studies. Disagreements between the two independent researchers were identified and resolved by discussion with a third reviewer (C.-W.L.). The selected studies were assessed with a quality scoring system raised by Wells and Julia (2009) (Appendix 4 in Supplementary Material). The quality assessment system was based on the following eight questions: Was the study hypothesis/aim/objective clearly described? Were the animal models for the study well-described? Were the methods well-described? Were the data collection time point clearly defined? Were the main outcome measures clearly defined? Were the experiment group well-compared with the control group? Were the results well-described? Were the articles discussed the limitation? For each question, 1 point was allocated for “yes,” and 0 point was allocated for “no.” The number of “yes” answers was counted for each selected study to give a total score out of 8. A study's rating was considered as excellent with a score ranging from 6 to 8, good with a score ranging from 4 to 6, poor with a score ranging from 2 to 4, and bad with a score ranging from 0 to 2.
Results
Study Methodology Quality Assessment
The scoring system was used to calculate all ten selected studies in Appendix 5 in Supplementary Material. The mean score is 6.6 (range, 5–8), including eight studies exceeding 6 points (Cosenza et al., 2017; Lo Sicco et al., 2017; Hyvarinen et al., 2018; Zhang et al., 2018; Chamberlain et al., 2019; Li Y. et al., 2019; Shi et al., 2019; Shen et al., 2020).
Study Characteristics
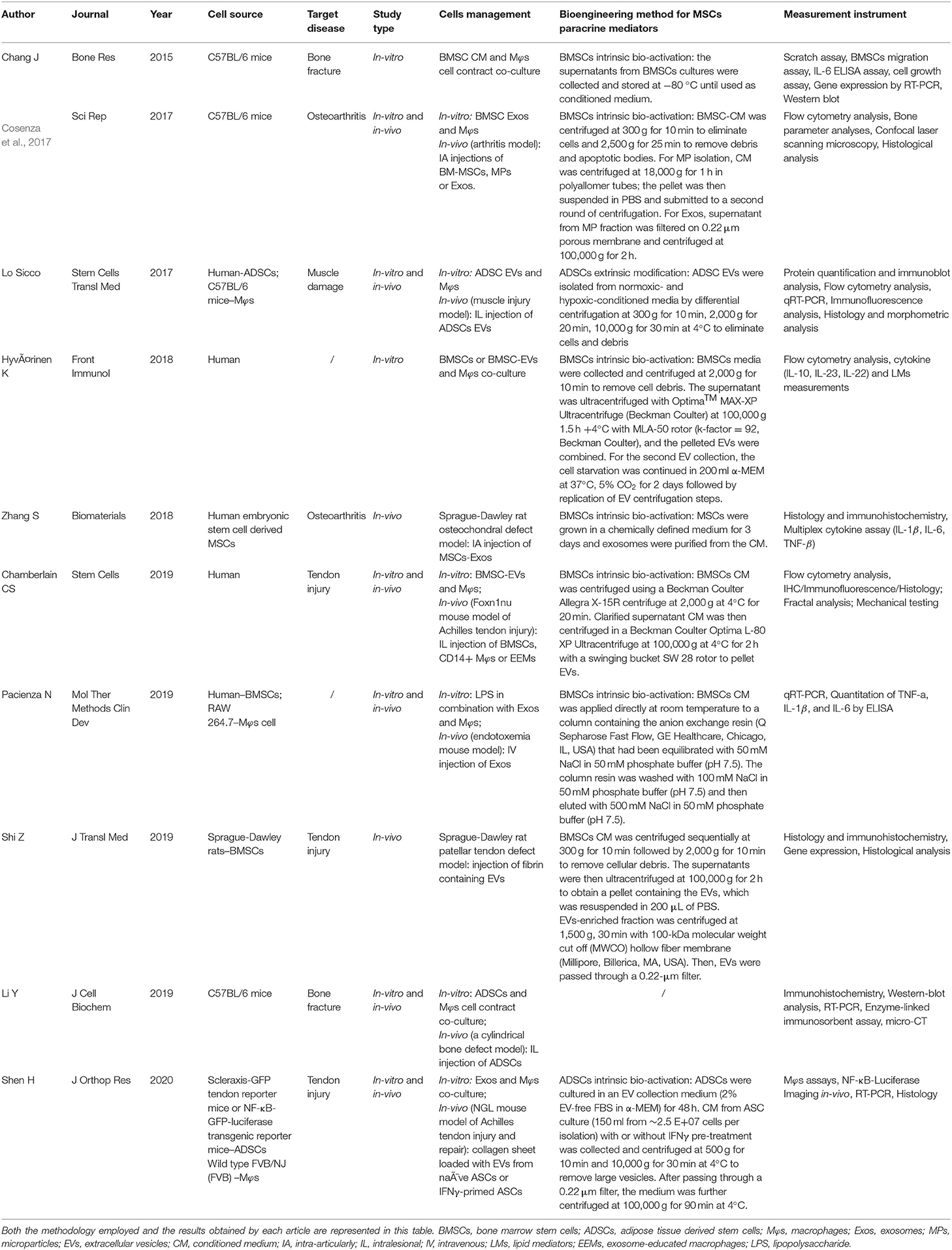
Of the ten selected studies, one study was published in 2015 (Chang et al., 2015), two were published in 2017 (Cosenza et al., 2017; Lo Sicco et al., 2017), two studies were published in 2018 (Hyvarinen et al., 2018; Zhang et al., 2018), four studies were published in 2019 (Chamberlain et al., 2019; Li Y. et al., 2019; Pacienza et al., 2019; Shi et al., 2019), and the final one was published in 2020 (Shen et al., 2020) (Figure 2A). All selected studies were related to the musculoskeletal system. For details of target diseases, two studies were bone fracture–related studies (Chang et al., 2015; Li Y. et al., 2019), two studies were OA-related studies (Cosenza et al., 2017; Zhang et al., 2018), one study was a muscle damage–related study (Lo Sicco et al., 2017), three studies were tendon injury–related studies (Chamberlain et al., 2019; Shi et al., 2019; Shen et al., 2020), and the other two studies were included without targeting disease (Hyvarinen et al., 2018; Pacienza et al., 2019) (Figure 2B). Six studies used a mice model (Cosenza et al., 2017; Lo Sicco et al., 2017; Chamberlain et al., 2019; Li Y. et al., 2019; Pacienza et al., 2019; Shen et al., 2020), two studies used a rat model (Zhang et al., 2018; Shi et al., 2019), and two studies performed in vitro experiments only (Chang et al., 2015; Hyvarinen et al., 2018) (Figure 2C). Three paracrine factors were extracted from the results: MSC-derived exosomes (Cosenza et al., 2017; Zhang et al., 2018; Pacienza et al., 2019; Shen et al., 2020), MSC-derived EVs (Lo Sicco et al., 2017; Hyvarinen et al., 2018; Chamberlain et al., 2019; Shi et al., 2019), and MSC-derived conditioned medium (CM) (Chang et al., 2015; Li Y. et al., 2019) (Figure 2D). Five MSC and Mφs cell sources for EVs isolation were described in these selected studies: human (Lo Sicco et al., 2017; Hyvarinen et al., 2018; Zhang et al., 2018; Chamberlain et al., 2019; Pacienza et al., 2019), cell lines (Pacienza et al., 2019), Sprague–Dawley rats (Shi et al., 2019), C57BL/6 mice (Chang et al., 2015; Cosenza et al., 2017; Lo Sicco et al., 2017; Li Y. et al., 2019), and transgenic mice [scleraxis–green fluorescent protein (GFP) tendon reporter mice, nuclear factor (NF-κB)–GFP–luciferase transgenic reporter mice, and wild-type FVB/NJ (FVB) mice] (Shen et al., 2020). The detailed data are graphically shown in Figure 2E.
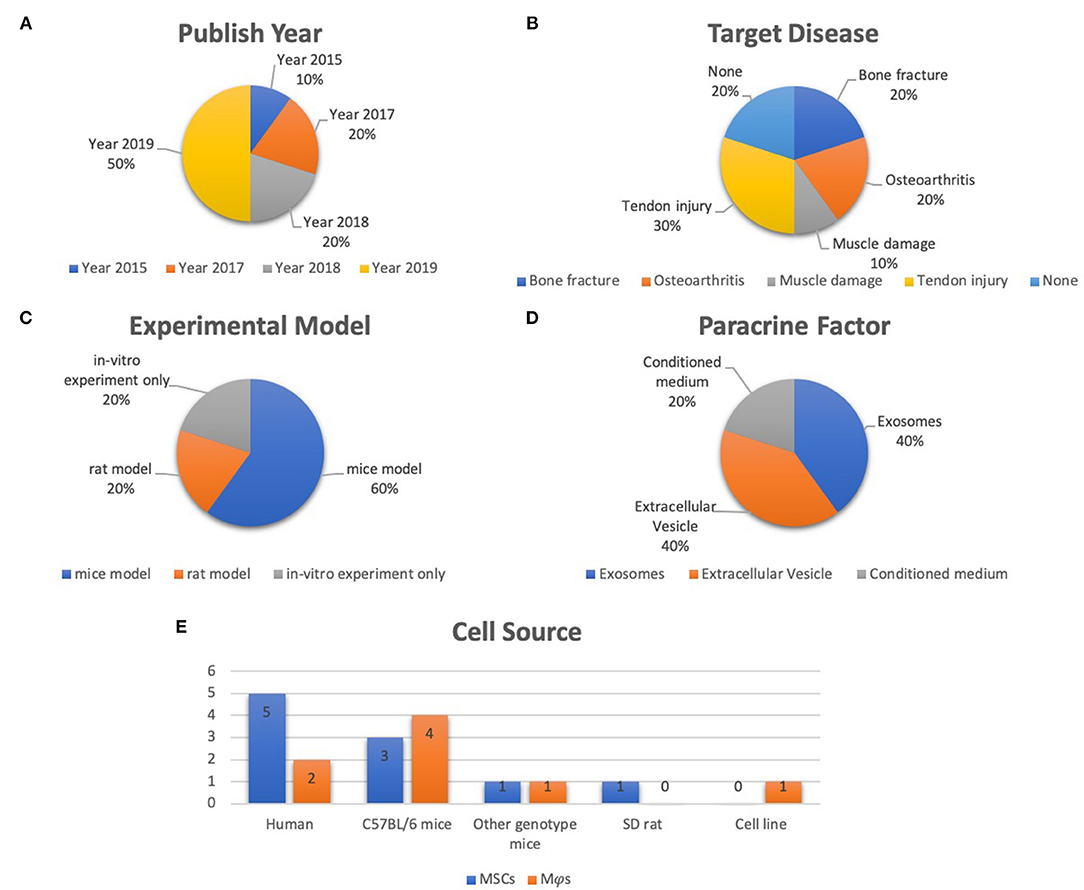
Figure 2. Representative graph of the included studies presented in the articles reviewed. (A) Publication year of included studies (range from 2015 to 2020). (B) Target disease of included studies (include bone fracture, OA, muscle damage, tendon injury). (C) Experimental model of included studies (including mice model, rat model, in vitro experiment only). (D) Paracrine factors (including extracellular vesicle, exosomes, CM). (E) Cell source of included studies (including human, C57Bl/6 mice, other genotype mice, SD rat, cell line).
Target Diseases and Experimental Animal Models
In the bone fracture–related studies (Chang et al., 2015; Li Y. et al., 2019), Li Y. et al. (2019) established a cylindrical bone defect mice model by using an electric drill to make a defect with 1-mm diameter and 1-mm depth at the bone callus of the femur. Chang et al. (2015) studied only in vitro experiments, and they cocultured bone marrow–derived mesenchymal stem cells (BMSCs) with Mφs. Two studies selected OA as the target disease (Cosenza et al., 2017; Zhang et al., 2018). Cosenza et al. (2017) established a collagenase-induced arthritis mouse model by intra-articularly injecting 1U type VII collagenase (in 5 μL saline) into the knee joint of 10-week-old C57BL/6 mice at days 0 and 2. Zhang et al. (2018) generated an osteochondral defect model in an 8-week-old Sprague–Dawley rat. Osteochondral defects, with 1.5-mm diameter and 1-mm depth, were generated on the trochlear grooves of the distal femurs by a drill bit. For muscle injury study, cardiotoxin-induced muscle injury was applied in 8-week-old male C57BL/6 mice by intramuscular administration of cardiotoxin into the Tibialis Anterior (TA) muscle (Lo Sicco et al., 2017). Three tendon injury models were used in the selected studies (Chamberlain et al., 2019; Shi et al., 2019; Shen et al., 2020). Chamberlain et al. (2019) established a surgically transected Achilles tendon mouse model. After superficial digital flexor tendon was removed, the Achilles tendon was completely transected at the midpoint. Then tendon was sutured together by using 5-0 Vicryl suture. In Shen et al. (2020) study, Achilles tendon was two-thirds transected at the midpoint part between calcaneal insertion and the musculotendinous junction and then was sutured with a two-strand modified Kessler technique. Shi et al. (2019) applied the Sprague–Dawley rat patellar tendon defect model. Briefly, the central one-third of the patellar tendon was removed from the distal apex of the patellar to the insertion of the tibial tuberosity to achieve a tendon structural defect condition. In addition, Pacienza et al. (2019) established a mouse endotoxemia model by injecting LPS via tail vein, which was used to study the crosstalk between MSC-derived exosomes and Mφs. Hyvarinen et al. (2018) only cocultured BMSCs and BMSC-derived EVs with Mφs without preforming animal study. The target diseases and experimental models are represented in Table 2 in detail.
Cell Sources and Cell Management
Five of 10 studies used human source cells (Lo Sicco et al., 2017; Hyvarinen et al., 2018; Zhang et al., 2018; Chamberlain et al., 2019; Pacienza et al., 2019). Human adipose-derived mesenchymal stem cells (ADSCs) were used for in vitro experiments in Lo Sicco's research; the EVs derived from ADSCs were cocultured with mouse Mφs and intralesionally injected into the mouse muscle injury model (Lo Sicco et al., 2017). In Hyvarinen et al. (2018) study, they used human BMSCs or BMSC-derived EVs to coculture with human Mφs. Zhang et al. (2018) intra-articularly injected exosomes from human embryonic stem cell–derived MSCs into rat with osteochondral defect. Chamberlain et al. (2019) also used human BMSCs and Mφs for the in vitro experiment. BMSCs, CD14+ Mφs and exosome-educated Mφs were intralesionally injected in the mice with Achilles tendon injury. Pacienza et al. (2019) used human BMSCs and RAW 264.7 for the in vitro experiment. Exosomes derived from BMSCs were intravenously injected into the endotoxemia mouse model. The in vitro experiment cell sources were all C57BL/6 mice in Chang et al. (2015), Cosenza et al. (2017) and Li Y. et al. (2019) studies. Fibrin containing Sprague–Dawley rat BMSC-derived EVs was intralesionally injected into the patellar tendon defect rat in Shi et al. (2019) study. Shen et al. (2020) used an in vitro model whose cell source was isolated from specific transgenic mice. The details of cell management are summarized in Table 2.
Boosting Approaches for MSC-Paracrine Mediators
Lo Sicco et al. (2017) isolated ADSC-derived EVs from normoxic and hypoxic culture condition. Li Y. et al. (2019) used a Transwell coculture system of ADSCs and Mφs without extra management of MSCs paracrine mediators. The other eight studies collected and purified EVs or other paracrine mediators from MSC-derived CM by differential centrifugation approaches (Chang et al., 2015; Cosenza et al., 2017; Lo Sicco et al., 2017; Hyvarinen et al., 2018; Zhang et al., 2018; Chamberlain et al., 2019; Pacienza et al., 2019; Shi et al., 2019; Shen et al., 2020). The detailed methods are described in Table 2.
Measurement Instruments
For classifications on the results, the measurement instruments, including immunohistochemistry, reverse transcriptase–polymerase chain reaction analysis, Western blot analysis, enzyme-linked immunosorbent assay, flow cytometry analysis, multiplex cytokine assay, confocal laser scanning, micro–computed tomography, and mechanical testing, were used. Also, fractal analysis (Chamberlain et al., 2019) and bone parameter analysis (Cosenza et al., 2017) were applied. Detailed results are listed in Table 2.
Experimental Variables and Controls
For two bone fracture–related studies, Li Y. et al. (2019) locally injected a total of 1 × 106 ADSCs at the injury site of their cylindrical bone defect model; the therapeutic efficacy of ADSCs was compared to the non-injected group. In the in vitro experiment, the bone marrow–derived Mφs were cocultured with 1 × 105 ADSCs in the upper chamber of Transwell under with high-glucose Dulbecco modified eagle medium containing 10% fetal bovine serum (FBS). Chang et al. (2015) planted 1 × 105 bone marrow–derived Mφs into six-well plates and cultured 24 h. Then, 1 × 105 BMSCs or apoptotic BMSCs (exposed to UV light treatment for 30 min) were placed on the Mφs in α-minimum essential medium containing 10% FBS. For each time point, Mφs cultured alone, apoptotic BMSCs cultured alone, and BMSCs cultured alone served as control groups.
For the OA-related studies, Cosenza et al. (2017) applied the in vitro OA-like chondrocytes model to investigate the effect of microparticles, BMSC-derived exosomes, BMSC-derived CM, and BMSCs on Mφs in a Transwell system. For their in vivo experiment, BMSCs, microparticles, and exosomes were intra-articularly injected into the arthritis model. Those OA mice without treatment served as a control group. In Zhang et al. (2018) study, exosomes and phosphate-buffered saline (PBS) (used as control) were intra-articularly injected into the rat osteochondral defect model after surgery, respectively.
For three tendon injury–related studies, two of them (Chamberlain et al., 2019; Shen et al., 2020) reported the coculture results of EVs and Mφs, and the another one study reported in vivo experiment (Shi et al., 2019). Chamberlain et al. (2019) reported that exosome-educated Mφs could be generated by MSC-derived EVs, and Mφs treated with PBS served as control groups in their in vitro experiment. To compare the therapeutic potential on tendon healing, the authors intralesionally injected 1 × 106 human BMSCs, 1 × 106 CD14+ Mφs, 1 × 106 exosome-educated Mφs, or Hanks balanced saline solution (used as the injured control) to the surgical sites; the contralateral intact Achilles tendon without any treatment was used as a control (Chamberlain et al., 2019). In Shen et al. (2020) study, EVs produced by IFN-γ-primed and non-primed mouse ADSC were used to treat with Mφs to evaluate the impact of EVs on the Mφs inflammatory response. And EV collection medium and EV-free CM were used as controls in the in vitro experiment. In Shen et al. (2020) in vivo experiment, collagen sheet loaded with EVs was applied around the repair sites, and the collagen sheet only was set as a control group. Shi et al. (2019) placed fibrin glue containing BMSC-EVs in the window defect of patellar tendon. The fibrin glue alone and non-surgical rats were designed as control groups.
In Lo Sicco et al. (2017) study, ADSC-derived EVs and PBS were intramuscularly injected into an injured TA muscle as experimental and control groups, respectively.
No target disease was mentioned in Hyvarinen et al. (2018) and Pacienza et al. (2019) studies. Mφs cocultured with MSCs and MSC-EVs served as experimental variables in Hyvarinen et al. (2018) study, and Mφs served as the control group (Hyvarinen et al., 2018). In Pacienza et al. (2019) study, LPS-stimulated Mφs treated with BMSC-derived exosomes served as the experimental group, and the three control groups were Mφs treated with complete medium alone, LPS, and LPS plus dexamethasone. In Pacienza et al. (2019) in vivo experiment, LPS alone or in combination with exosomes (~5 × 109 vesicles) was injected into mice through tail vein, respectively. Control group was injected with saline (Pacienza et al., 2019). Detailed results are listed in Table 3.
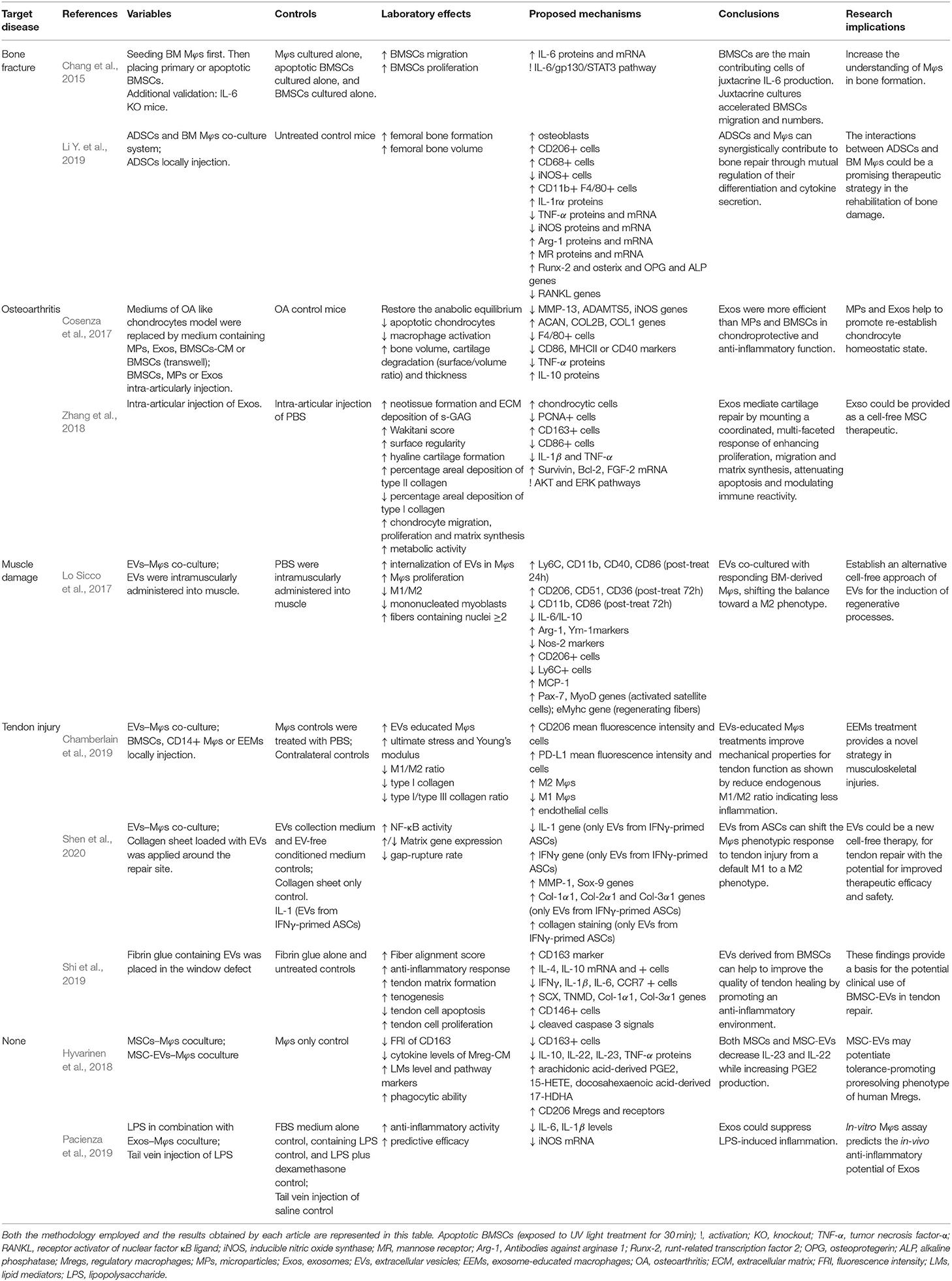
Table 3. Evaluations and results list of selected studies in which the therapeutic potential of the administration of MSCs for the treatment of musculoskeletal diseases.
Laboratory Effects and Proposed Mechanisms
The bone fracture–related studies showed that femoral bone formation and bone volume all increased after ADSC injection. The increase in osteoblasts after ADSC injection was accompanied by increases in CD206+, CD68+, CD11b+, and F4/80+ cells, which represented a typical M2 surface phenotype. Meanwhile, the transcript and protein expression of Arg-1, M2 marker genes, were also up-regulated (Pacienza et al., 2019). Two OA-related studies reported that bone volume, cartilage thickness, hyaline cartilage formation, migration and proliferation of chondrocytes, and matrix synthesis were increased by BMSC-derived exosomes (Cosenza et al., 2017; Zhang et al., 2018). The therapeutic effect of anti-inflammation on OA was in line with a decrease in M1 Mφs (F4/80+, CD86+, MHCII+, CD40+), IL-1β, and tumor necrosis factor α (TNF-α), as well as increases in chondrocytes, M2 Mφs (CD163+), and IL-10 (Cosenza et al., 2017; Zhang et al., 2018). Moreover, BMSC-derived exosomes increased type II collagen deposition, and decreased type I collagen in cartilage was reported by Zhang et al. (2018) Such protective effects on chondrocytes might be attributed to the increase in Survivin, Bcl-2, and FGF-2 by BMSC-derived exosomes administration.
Lo Sicco et al. (2017) reported that ADSC-derived EVs were internalized in Mφs at the muscle injury site, which could promote myofiber regeneration. Interestingly, a dynamic change of Mφs subpopulation was observed after transplantation of ADSC-derived EVs. ADSC-derived EVs increased M1 Mφs (Ly6C+, CD11b+, CD40+, CD86+) at 24 h post-treatment and increased M2 Mφs (CD206+, CD51+, CD36+) at 72 h post-treatment.
Extended results could be concluded from three tendon injury–related studies (Chamberlain et al., 2019; Shi et al., 2019; Shen et al., 2020). The shift of Mφs from M1 to M2 showed consistent variation to the increase in ultimate stress, Young's modulus (Chamberlain et al., 2019), NF-κB activity (Shen et al., 2020), fiber alignment score, tendon matrix formation, tenogenesis, and tendon cell proliferation (Shi et al., 2019) and the decrease in type I/type III collagen ratio (Chamberlain et al., 2019), gap-rupture rate (Shen et al., 2020), and tendon cell apoptosis (Shi et al., 2019) by BMSC-derived or ADSC-derived exosome administration.
Discussion
Nowadays, it has been emphatically contended that all newly presented clinical interventions should start with and end with a systematic review, or even meta-analysis (Clarke et al., 2007). Although the therapeutic effects of MSCs via paracrine factors have been demonstrated in many pre-clinical investigations, the substantial clinical evidence in beneficial effects of MSCs in musculoskeletal diseases is not adequately investigated (Regenberg et al., 2009; Lukomska et al., 2019). This systematic review aims to analyze the impact of MSC and MSC-derived EVs on Mφs in the treatment of musculoskeletal diseases. According to the outcomes of the included studies, MSCs could promote musculoskeletal tissue repair or healing via their paracrine regulation on Mφs.
Interactions of Predominant Mφs With MSC-EVs
Mφs are predominant myeloid cells that chronologically accumulate in musculoskeletal tissue at the onset of injury-induced inflammation and exhibit regulatory activity at all stages of the healing process (Tidball, 2011; Varol et al., 2015). Therefore, Mφs are potent triggers for tissue healing processes, including cell recruitment, proliferation, and remodeling (Artlett, 2013).
Growing evidence has demonstrated that the phenotypic switch of Mφs is critical in MSC-mediated tissue regeneration, which is presented in Figure 3. Moreover, M1 Mφ-released proinflammatory cytokines enhanced the migratory capacity of MSCs that facilitates the accessibility of exogenous MSCs toward the injured site. Then, the attracted MSCs would regulate Mφ phenotypes into M2 via paracrine effect to facilitate the tissue remodeling at a later stage (Le Blanc and Mougiakakos, 2012; Maxson et al., 2012; Shi et al., 2012; Bernardo and Fibbe, 2013; Ma et al., 2014). Interestingly, the secretomes of MSCs, such as EVs or exosomes, were also altered by M1 Mφ that boosts the therapeutic effect of MSCs, so-called primed MSCs (Aktas et al., 2017; Saldana et al., 2019).
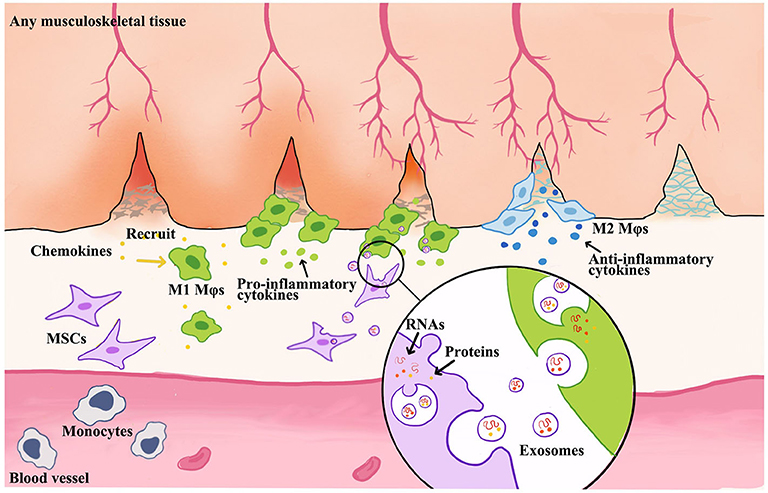
Figure 3. Schematic illustration of MSC-derived exosome-guided macrophage reprogramming. MSC-derived exosomes can induce a conversion of M1 to M2 Mφs and accelerate musculoskeletal tissue healing. Mφs could be activated by inflammatory chemokines and then to produce proinflammatory factors. This creates a feedback loop whereby proinflammatory cytokines produced by Mφs stimulate MSC to produce immune modulators, such as exosomes or EVs. Therefore, the formation of exosomes begins with membrane invagination in the form of endosomes, leading to the development of the early endosomes. Upon maturation, the endosomes become multivesicular endosomes, which release their contents in the form of exosomes.
The cross-talk between Mφs and MSC-derived EVs regulates a shift in Mφs subtypes from M1 into M2 (Galli et al., 2011). An increasing number of musculoskeletal tissue injury models showed that Mφs phenotypic alteration mediated MSC-based therapy (Lo Sicco et al., 2017; Chu et al., 2019). M1 Mφs promote recruitment of inflammatory immune cells and release extracellular matrix (ECM) degrading proteins to allow quick migration to inflamed sites. As the Mφs shift to M2 subtypes, the release of proinflammatory cytokines is inhibited, angiogenesis is stimulated, and fibroblasts are activated to produce and restore more ECM. Also, Mφs induced MSCs into a motile phenotype with increased secretion of IL-6 and IL-10, which benefit MSCs to migrate to injury site (Anton et al., 2012; Wolfe et al., 2016). Typically, MSC-EVs impact the maturation of Mφs by decreasing the expression levels of IL-12 and TNF-α and increasing IL-6 and IL-10 in Mφs (Kim and Hematti, 2009; Cosenza et al., 2017; Lo Sicco et al., 2017; Hyvarinen et al., 2018; Shi et al., 2019). PGE2 and TNF-α inducible gene 6 (TSG-6) embedded in MSC-EVs can promote M2 polarization in inflammatory microenvironment (Nemeth et al., 2009; Maggini et al., 2010; Melief et al., 2013b). EVs, which were released from proinflammatory cytokines–activated MSCs, could enhance anti-inflammatory properties to suppress MHC class II and CD86 signaling in LPS-stimulated Mφs (Melief et al., 2013a,b; Cho et al., 2014). Lo Sicco et al. (2017) and Chamberlain et al. (2019) reported the MSC-EVs change the M1-to-M2 Mφs ratio. And Lo Sicco et al. (2017) demonstrated that MSC-EVs could be efficiently internalized by responding cells, inducing an increase in their proliferation rate, and shifting the balance toward an alternatively anti-inflammatory M2 phenotype. M2 Mφs phenotype could be commonly associated with the secretion of the anti-inflammatory cytokine IL-10 and scavenger receptors CD206 and CD163 (Sica and Mantovani, 2012; Mantovani et al., 2013).
In this review, we found consistent results suggesting that an M2 phenotype could be induced from M1 Mφs upon coculture with MSCs, MSC-EVs, and CM. The enrichment of M1 Mφs appears at early phases (1–3 days) during bone remodeling (Chang et al., 2015; Li Y. et al., 2019), muscle regeneration (Lo Sicco et al., 2017), and tendon healing (Chamberlain et al., 2019; Harvey et al., 2019; Shi et al., 2019; Shen et al., 2020) and is later replaced by M2 Mφs (4–7 days). As Mφ polarization and tissue repair by MSC-EVs are highly associated, MSC-EVs–mediated Mφs phenotypic transformation must play a significant role during tissue healing. However, the molecular action of MSC-EVs in such Mφ polarization and tissue regeneration needs further investigation (Chen et al., 2008; Rodero and Khosrotehrani, 2010).
Although studies have provided evidence of immunosuppressive effects of MSCs in clinical trials for graft-vs.-host disease and Crohn disease (Godoy et al., 2019), the transplanted MSCs have not been proven in the persistence after injection and the contribution in tissue regeneration (Pittenger, 2009; Parekkadan and Milwid, 2010; Caplan and Correa, 2011). It is likely that the main immunosuppressive effects of MSCs resulted from paracrine regulation through secreted mediators, including EVs (Raposo and Stoorvogel, 2013). The immune modulation effect of MSC has been generalized as the inhibition of both innate and adaptive immunity and also derives inflammatory, autoimmune, and infectious disease pathology (Le Blanc and Mougiakakos, 2012; English, 2013). However, increasing analysis in animal models of inflammation demonstrated that MSC-EVs suppressed immune response through the transfer of RNAs and protein (Cantaluppi et al., 2012; Arslan et al., 2013). The inflammatory signals are essential to initiate and maintain the MSC- or EV-mediated tissue repair process; afterward, MSC-derived EVs could manipulate the niche by switching Mφ phenotype to facilitate tissue repair.
Functional MSC-EVs Cargo and Boosting Approaches
Recently, the use of MSC-based therapies has emerged. The therapeutic benefits of MSC transplantation have been attributed into two types, EVs and soluble factors. Soluble components include a wide variety of secreted chemokines, growth factors, and hormones with immunomodulatory activity. For example, PGE2 and TGF-β are vital mediators of anti-inflammatory in therapeutic therapy of MSCs (Yoo et al., 2013). And lots of anti-inflammatory proteins also represent tissue protection, such as TSG-6, which has been reported with healing ability by reducing the influx of neutrophils to the tissue injury site (Oh et al., 2010). The studies regarding MSC-derived EVs have grown exponentially since it has been recognized that EVs containing mRNA, miRNA, and protein could exchange intracellular information and act as sophisticated mediators of recipient cell behavior, particularly in immunomodulation (Wolfers et al., 2001; Valadi et al., 2007; Skog et al., 2008). MSC-derived EVs contain not only more than 200 mRNA and 60 miRNA, but also more than 800 proteins (Bruno et al., 2009; Chen et al., 2010; Lai et al., 2012). These EV-derived proteins have been reported with an integral role in activating anti-inflammatory responses and regulating the cascade of tissue healing process in various injury models (Lee et al., 2012; Zhang et al., 2018; Li et al., 2019).
It has been demonstrated that genetically modified MSCs possess great prosurvival, proangiogenic, and anti-inflammatory properties not only by the altering release of soluble proteins, but also EVs (Huang et al., 2013; Wang et al., 2016; Lou et al., 2017; Ma et al., 2017; Qu et al., 2017; Tao et al., 2017). MSC-derived EVs are engineered at the cellular level under natural conditions, and it further highlighted the unique advantage of EV-based nanoplatforms for cargo delivery (Luan et al., 2017). For clinical applications, some advantages of MSC-derived EVs include easier injection, reduced Mφs phagocytosis and vascular occlusion (EL Andaloussi et al., 2013), innate biocompatibility, high physicochemical stability, high penetrability, and long-distance communication (Clayton et al., 2003; Ridder et al., 2014; Zomer et al., 2015).
Many strategies have been applied to modify EVs, including cell modification and direct EV modification (Armstrong and Perriman, 2016). Because EVs are secreted from cells, they can intrinsically express some lipids or cell adhesion molecules and ligands that naturally target certain types of recipient cells (Luan et al., 2017). Undoubtedly, it was inevitable that genetic engineering has been used to modify EVs. Overexpression of mRNA or miRNA in cells could be assembled into EVs, which could be fused to target cells to introduce or inhibit gene expression (Kosaka et al., 2010; Akao et al., 2011; Ridder et al., 2014; Zomer et al., 2015). For a significant amount of time, researchers have explored non-native biomaterials to cells to augment therapeutic function (Armstrong et al., 2015; Correia Carreira et al., 2016). Therefore, biomaterials delivered to the membrane could also naturally be incorporated into budding EVs, while internalized material may be packaged into exosomes for secretion (Armstrong et al., 2017). Taking advantage of these methods allows cellular processes and cell engineering techniques to be specifically adapted to EV functionalization.
Because only a small fraction of material could be packaged into EVs, the efficiency issue results in a low-yielding ending. And besides the widely explored cancer cells, the yield of exosomes derived from MSCs is one of the major factors that limits the expansion of cell-free therapeutic productions (Phan et al., 2018). Functional EVs could be derived from native ECM (Huleihel et al., 2016; Du et al., 2017), and three-dimensional environment for cell attachment and growth has been particularly attracted because it can mimic the ECM structure and function (Phan et al., 2018). Such structure has been reported with the effects of influencing EV secretion. For example, Tao et al. reported that Avitene Ultrafoam collagen hemostat caused the BMSCs to release 2-fold of exosomes compared to the plastic surface culture based on protein assay (Tao et al., 2017). Besides, a bioactive artificial ECM that was modified by adding molecules has been used to imitate native ECM mimicking structures and to improve yield of MSC exosomes by presenting specific functional ligands (Hao et al., 2017).
Moreover, an essential and urgent solution can provide an approach to improve the yield without sacrificing the functionality or with enhancing the efficacy simultaneously. Therefore, researchers tried to purify EVs, which could ensure that all modified sites or encapsulated species could be localized at the vesicle (Armstrong et al., 2017). As non-living entities, EVs have a major advantage over cells when they received membrane surface modification. It has been reported that excessive pressures, temperature, chemical induction, or hypoxia environment exposure could cause membrane disruption, vesicle aggregation, and surface protein denaturation (Smyth et al., 2014; Armstrong et al., 2017; Lo Sicco et al., 2017). Also, multivalent electrostatic interactions, receptor–ligand binding, and hydrophobic insertion have been commonly applied as methods of biological membrane modifications (Nakase and Futaki, 2015; Correia Carreira et al., 2016; Lee et al., 2016). In addition, electroporation, which is an alternative approach to EV active loading strategies, has been reported to transiently permeabilize the EV membrane to enhance the absorptivity of small molecules (Tian et al., 2014; Fuhrmann et al., 2015). Taken together, the engineered EVs will open up exciting opportunities in EV-based therapies by boosting therapeutic capability, which is beyond their native functions.
Strengths and Limitations
This systematic review poses several advantages compared to other attempts to summarize the experimental results of MSC-derived paracrine mediators for musculoskeletal diseases. First, the up-to-date literature search has been yielded by two widely used databases: PubMed and EMBASE. The search strategies included MeSH terms and other related terms. Therefore, we could identify a number of eligible studies, which might remain relatively unnoticed. Second, the quality of included studies and risk of bias, including publication bias, were assessed. Third, study heterogeneity had been explored to point out potential explanatory variables. Fourth, to standardize the spontaneous recovery in the control groups, we analyzed the variables and controls specifically. And studies without scientific controls have been excluded during full-text screening procedure.
There were several limitations to this study. The first limitation is the small number of included studies. The second limitation is that we could not standardize the effectiveness of Mφs depletion among different studies. Moreover, the depletion is not permanent and, once subsided, could result in a reactive increase in Mφs numbers with unknown consequences. Third, these studies utilized different cell sources and delivery methods. Different types of animals were selected for in vivo studies. Fourth, the assessment methods also widely varied among studies; therefore, it was impossible to perform a quantitative analysis or a meta-analysis with the included studies.
Conclusion
This review demonstrated that MSC and MSC-EVs are authentic biomaterials to treat musculoskeletal problems. The broad therapeutic effect of MSC and MSC-EVs attribute to the management of Mφ polarization, at least in part. A further understanding in the molecular mechanism of how MSCs regulate Mφ polarization will facilitate the development of bioengineering approach to boost the therapeutic capacity of MSCs and their clinical application. However, more pre-clinical studies are needed to understand how EVs and their subcomponent play a role in musculoskeletal tissue healing process.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
HX: conception and design, manuscript writing, and data analysis and interpretation. C-WL: conception and design, financial support, and manuscript writing. Y-FW: manuscript writing. L-YS, Y-HW, ZW, and XZ: data analysis and interpretation. PY and OL: administrative support and final approval of manuscript. SH: revisions to scientific content of manuscripts. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by MWLC Associate Member Programme, Ming Wai Lau Centre for Reparative Medicine of Karolinska Institute to OL; CUHK Research Committee Direct Grant for Research (Reference no. 2018.020) and Hong Kong Government Research Grant Council, General Research Fund (Reference no. 14104620) to CW-L.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.587052/full#supplementary-material
References
Akao, Y., Iio, A., Itoh, T., Noguchi, S., Itoh, Y., Ohtsuki, Y., et al. (2011). Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol. Ther. 19, 395–399. doi: 10.1038/mt.2010.254
Aktas, E., Chamberlain, C. S., Saether, E. E., Duenwald-Kuehl, S. E., Kondratko-Mittnacht, J., Stitgen, M., et al. (2017). Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an achilles tendon segmental defect. J. Orthop. Res. 35, 269–280. doi: 10.1002/jor.23258
Anderson, C. F., and Mosser, D. M. (2002). A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 72, 101–106.
Anton, K., Banerjee, D., and Glod, J. (2012). Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS ONE 7:e35036. doi: 10.1371/journal.pone.0035036
Armstrong, J. P., Holme, M. N., and Stevens, M. M. (2017). Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11, 69–83. doi: 10.1021/acsnano.6b07607
Armstrong, J. P., and Perriman, A. W. (2016). Strategies for cell membrane functionalization. Exp. Biol. Med. 241, 1098–1106. doi: 10.1177/1535370216650291
Armstrong, J. P. K., Shakur, R., Horne, J. P., Dickinson, S. C., Armstrong, C. T., Lau, K., et al. (2015). Artificial membrane-binding proteins stimulate oxygenation of stem cells during engineering of large cartilage tissue. Nat. Commun. 6:7405. doi: 10.1038/ncomms8405
Arslan, F., Lai, R. C., Smeets, M. B., Akeroyd, L., Choo, A., Aguor, E. N., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312. doi: 10.1016/j.scr.2013.01.002
Artlett, C. M. (2013). Inflammasomes in wound healing and fibrosis. J. Pathol. 229, 157–167. doi: 10.1002/path.4116
Bagno, L., Hatzistergos, K. E., Balkan, W., and Hare, J. M. (2018). Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol. Ther. 26, 1610–1623. doi: 10.1016/j.ymthe.2018.05.009
Bernardo, M. E., and Fibbe, W. E. (2013). Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402. doi: 10.1016/j.stem.2013.09.006
Bility, M. T., Cheng, L., Zhang, Z., Luan, Y., Li, F., Chi, L., et al. (2014). Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 10:e1004032. doi: 10.1371/journal.ppat.1004032
Bosurgi, L., Manfredi, A. A., and Rovere-Querini, P. (2011). Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front. Immunol. 2:62. doi: 10.3389/fimmu.2011.00062
Bruno, S., Grange, C., Collino, F., Deregibus, M. C., Cantaluppi, V., Biancone, L., et al. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 7:e33115. doi: 10.1371/journal.pone.0033115
Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067. doi: 10.1681/ASN.2008070798
Cantaluppi, V., Gatti, S., Medica, D., Figliolini, F., Bruno, S., Deregibus, M. C., et al. (2012). Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427. doi: 10.1038/ki.2012.105
Caplan, A. I. (1991). Mesenchymal stem cells. J. Orthop. Res. 9, 641–650. doi: 10.1002/jor.1100090504
Caplan, A. I., and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9, 11–15. doi: 10.1016/j.stem.2011.06.008
Carrero, R., Cerrada, I., Lledo, E., Dopazo, J., Garcia-Garcia, F., Rubio, M. P., et al. (2012). IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev. Rep. 8, 905–916. doi: 10.1007/s12015-012-9364-9
Cha, J. M., Shin, E. K., Sung, J. H., Moon, G. J., Kim, E. H., Cho, Y. H., et al. (2018). Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 8:1171. doi: 10.1038/s41598-018-19211-6
Chamberlain, C. S., Clements, A. E. B., Kink, J. A., Choi, U., Baer, G. S., Halanski, M. A., et al. (2019). Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cells 37, 652–662. doi: 10.1002/stem.2988
Chang, J., Koh, A. J., Roca, H., and McCauley, L. K. (2015). Juxtacrine interaction of macrophages and bone marrow stromal cells induce interleukin-6 signals and promote cell migration. Bone Res. 3:15014. doi: 10.1038/boneres.2015.14
Chen, L., Tredget, E. E., Wu, P. Y., and Wu, Y. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3:e1886. doi: 10.1371/journal.pone.0001886
Chen, T. S., Lai, R. C., Lee, M. M., Choo, A. B., Lee, C. N., and Lim, S. K. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38, 215–224. doi: 10.1093/nar/gkp857
Cheng, L., Zhang, K., Wu, S., Cui, M., and Xu, T. (2017). Focus on mesenchymal stem cell-derived exosomes: opportunities and challenges in cell-free therapy. Stem Cells Int. 2017:6305295. doi: 10.1155/2017/6305295
Cheng, Y., and Schorey, J. S. (2013). Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 43, 3279–3290. doi: 10.1002/eji.201343727
Cho, D. I., Kim, M. R., Jeong, H. Y., Jeong, H. C., Jeong, M. H., Yoon, S. H., et al. (2014). Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow–derived macrophages. Exp. Mol. Med. 46:e70. doi: 10.1038/emm.2013.135
Chu, S. Y., Chou, C. H., Huang, H. D., Yen, M. H., Hong, H. C., Chao, P. H., et al. (2019). Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 10:1524. doi: 10.1038/s41467-019-09402-8
Clarke, M., Hopewell, S., and Chalmers, I. (2007). Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J. R. Soc. Med. 100, 187–190. doi: 10.1177/014107680710011415
Clayton, A., Harris, C. L., Court, J., Mason, M. D., and Morgan, B. P. (2003). Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 33, 522–531. doi: 10.1002/immu.200310028
Correia Carreira, S., Armstrong, J. P., Seddon, A. M., Perriman, A. W., Hartley-Davies, R., and Schwarzacher, W. (2016). Ultra-fast stem cell labelling using cationised magnetoferritin. Nanoscale 8, 7474–7483. doi: 10.1039/C5NR07144E
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C., and Noel, D. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 7:16214. doi: 10.1038/s41598-017-15376-8
Cosenza, S., Toupet, K., Maumus, M., Luz-Crawford, P., Blanc-Brude, O., Jorgensen, C., et al. (2018). Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8, 1399–1410. doi: 10.7150/thno.21072
da Costa Goncalves, F., and Paz, A. H. (2019). Cell membrane and bioactive factors derived from mesenchymal stromal cells: cell-free based therapy for inflammatory bowel diseases. World J. Stem Cells 11, 618–633. doi: 10.4252/wjsc.v11.i9.618
Darnell, M., O'Neil, A., Mao, A., Gu, L., Rubin, L. L., and Mooney, D. J. (2018). Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. U.S.A. 115, E8368–E8377. doi: 10.1073/pnas.1802568115
Donnelly, R. P., Dickensheets, H., and Finbloom, D. S. (1999). The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19, 563–573. doi: 10.1089/107999099313695
Du, W., Zhang, K., Zhang, S., Wang, R., Nie, Y., Tao, H., et al. (2017). Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 133, 70–81. doi: 10.1016/j.biomaterials.2017.04.030
EL Andaloussi, S., Mager, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357. doi: 10.1038/nrd3978
English, K. (2013). Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 91, 19–26. doi: 10.1038/icb.2012.56
Ferguson, S. W., Wang, J., Lee, C. J., Liu, M., Neelamegham, S., Canty, J. M., et al. (2018). The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci. Rep. 8:1419. doi: 10.1038/s41598-018-19581-x
Fuhrmann, G., Serio, A., Mazo, M., Nair, R., and Stevens, M. M. (2015). Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 205, 35–44. doi: 10.1016/j.jconrel.2014.11.029
Galli, S. J., Borregaard, N., and Wynn, T. A. (2011). Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 12, 1035–1044. doi: 10.1038/ni.2109
Godoy, J. A. P., Paiva, R. M. A., Souza, A. M., Kondo, A. T., Kutner, J. M., and Okamoto, O. K. (2019). Clinical translation of mesenchymal stromal cell therapy for graft versus host disease. Front Cell Dev Biol. 7:255. doi: 10.3389/fcell.2019.00255
Hao, D., Xiao, W., Liu, R., Kumar, P., Li, Y., Zhou, P., et al. (2017). Discovery and characterization of a potent and specific peptide ligand targeting endothelial progenitor cells and endothelial cells for tissue regeneration. ACS Chem. Biol. 12, 1075–1086. doi: 10.1021/acschembio.7b00118
Harvey, T., Flamenco, S., and Fan, C. M. (2019). A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 21, 1490–1503. doi: 10.1038/s41556-019-0417-z
Huang, F., Zhu, X., Hu, X. Q., Fang, Z. F., Tang, L., Lu, X. L., et al. (2013). Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 31, 484–492. doi: 10.3892/ijmm.2012.1200
Huleihel, L., Hussey, G. S., Naranjo, J. D., Zhang, L., Dziki, J. L., Turner, N. J., et al. (2016). Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2:e1600502. doi: 10.1126/sciadv.1600502
Hyvarinen, K., Holopainen, M., Skirdenko, V., Ruhanen, H., Lehenkari, P., Korhonen, M., et al. (2018). Mesenchymal stromal cells and their extracellular vesicles enhance the anti-inflammatory phenotype of regulatory macrophages by downregulating the production of interleukin (IL)-23 and IL-22. Front. Immunol. 9:771. doi: 10.3389/fimmu.2018.00771
Kim, H. S., Choi, D. Y., Yun, S. J., Choi, S. M., Kang, J. W., Jung, J. W., et al. (2012). Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 11, 839–849. doi: 10.1021/pr200682z
Kim, J., and Hematti, P. (2009). Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp. Hematol. 37, 1445–1453. doi: 10.1016/j.exphem.2009.09.004
Kingery, M. T., Manjunath, A. K., Anil, U., and Strauss, E. J. (2019). Bone marrow mesenchymal stem cell therapy and related bone marrow-derived orthobiologic therapeutics. Curr. Rev. Musculoskelet. Med. 12, 451–459. doi: 10.1007/s12178-019-09583-1
Kosaka, N., Iguchi, H., Yoshioka, Y., Takeshita, F., Matsuki, Y., and Ochiya, T. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452. doi: 10.1074/jbc.M110.107821
Krzyszczyk, P., Schloss, R., Palmer, A., and Berthiaume, F. (2018). The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 9:419. doi: 10.3389/fphys.2018.00419
Lai, R. C., Tan, S. S., Teh, B. J., Sze, S. K., Arslan, F., de Kleijn, D. P., et al. (2012). Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics 2012:971907. doi: 10.1155/2012/971907
Le Blanc, K., and Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396. doi: 10.1038/nri3209
Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126, 2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173
Lee, J., Lee, H., Goh, U., Kim, J., Jeong, M., Lee, J., et al. (2016). Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl. Mater. Interfaces 8, 6790–6795. doi: 10.1021/acsami.6b01315
Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. doi: 10.1089/scd.2012.0395
Li, Y., Kong, N., Li, Z., Tian, R., Liu, X., Liu, G., et al. (2019). Bone marrow macrophage M2 polarization and adipose-derived stem cells osteogenic differentiation synergistically promote rehabilitation of bone damage. J. Cell. Biochem. 120, 19891–19901. doi: 10.1002/jcb.29297
Li, Z., Liu, F., He, X., Yang, X., Shan, F., and Feng, J. (2019). Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 67, 268–280. doi: 10.1016/j.intimp.2018.12.001
Lo Sicco, C., Reverberi, D., Balbi, C., Ulivi, V., Principi, E., Pascucci, L., et al. (2017). Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl. Med. 6, 1018–1028. doi: 10.1002/sctm.16-0363
Lou, G., Yang, Y., Liu, F., Ye, B., Chen, Z., Zheng, M., et al. (2017). MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 21, 2963–2973. doi: 10.1111/jcmm.13208
Lu, M., and Huang, Y. (2020). Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 242:119925. doi: 10.1016/j.biomaterials.2020.119925
Luan, X., Sansanaphongpricha, K., Myers, I., Chen, H., Yuan, H., and Sun, D. (2017). Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763. doi: 10.1038/aps.2017.12
Lukomska, B., Stanaszek, L., Zuba-Surma, E., Legosz, P., Sarzynska, S., and Drela, K. (2019). Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019:9628536. doi: 10.1155/2019/9628536
Ma, J., Zhao, Y., Sun, L., Sun, X., Zhao, X., Sun, X., et al. (2017). Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl. Med. 6, 51–59. doi: 10.5966/sctm.2016-0038
Ma, S., Xie, N., Li, W., Yuan, B., Shi, Y., and Wang, Y. (2014). Immunobiology of mesenchymal stem cells. Cell Death Differ. 21, 216–225. doi: 10.1038/cdd.2013.158
Maggini, J., Mirkin, G., Bognanni, I., Holmberg, J., Piazzon, I. M., Nepomnaschy, I., et al. (2010). Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 5:e9252. doi: 10.1371/journal.pone.0009252
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A., and Locati, M. (2013). Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185. doi: 10.1002/path.4133
Maxson, S., Lopez, E. A., Yoo, D., Danilkovitch-Miagkova, A., and Leroux, M. A. (2012). Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 1, 142–149. doi: 10.5966/sctm.2011-0018
McElvany, M. D., McGoldrick, E., Gee, A. O., Neradilek, M. B., and Matsen, F. A. 3rd (2015). Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am. J. Sports Med. 43, 491–500. doi: 10.1177/0363546514529644
Melief, S. M., Geutskens, S. B., Fibbe, W. E., and Roelofs, H. (2013a). Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica 98, 888–895. doi: 10.3324/haematol.2012.078055
Melief, S. M., Schrama, E., Brugman, M. H., Tiemessen, M. M., Hoogduijn, M. J., Fibbe, W. E., et al. (2013b). Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 31, 1980–1991. doi: 10.1002/stem.1432
Mianehsaz, E., Mirzaei, H. R., Mahjoubin-Tehran, M., Rezaee, A., Sahebnasagh, R., Pourhanifeh, M. H., et al. (2019). Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 10:340. doi: 10.1186/s13287-019-1445-0
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi: 10.1371/journal.pmed.1000097
Moore, J. P., Vinh, A., Tuck, K. L., Sakkal, S., Krishnan, S. M., Chan, C. T., et al. (2015). M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H906–917. doi: 10.1152/ajpheart.00821.2014
Mosser, D. M., and Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969. doi: 10.1038/nri2448
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. doi: 10.1016/j.immuni.2014.06.008
Nakase, I., and Futaki, S. (2015). Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 5:10112. doi: 10.1038/srep10112
Nemeth, K., Leelahavanichkul, A., Yuen, P. S., Mayer, B., Parmelee, A., Doi, K., et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49. doi: 10.1038/nm.1905
O'Brien, E. M., Risser, G. E., and Spiller, K. L. (2019). Sequential drug delivery to modulate macrophage behavior and enhance implant integration. Adv. Drug Deliv. Rev. 149–150, 85–94. doi: 10.1016/j.addr.2019.05.005
Oh, J. Y., Roddy, G. W., Choi, H., Lee, R. H., Ylostalo, J. H., Rosa, R. H. Jr., et al. (2010). Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. U.S.A. 107, 16875–16880. doi: 10.1073/pnas.1012451107
Otsuru, S., Gordon, P. L., Shimono, K., Jethva, R., Marino, R., Phillips, C. L., et al. (2012). Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 120, 1933–1941. doi: 10.1182/blood-2011-12-400085
Pacienza, N., Lee, R. H., Bae, E. H., Kim, D. K., Liu, Q., Prockop, D. J., et al. (2019). In vitro macrophage assay predicts the in vivo anti-inflammatory potential of exosomes from human mesenchymal stromal cells. Mol. Ther. Methods Clin. Dev. 13, 67–76. doi: 10.1016/j.omtm.2018.12.003
Pajarinen, J., Lin, T., Gibon, E., Kohno, Y., Maruyama, M., Nathan, K., et al. (2019). Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 196, 80–89. doi: 10.1016/j.biomaterials.2017.12.025
Parekkadan, B., and Milwid, J. M. (2010). Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117. doi: 10.1146/annurev-bioeng-070909-105309
Phan, J., Kumar, P., Hao, D., Gao, K., Farmer, D., and Wang, A. (2018). Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 7:1522236. doi: 10.1080/20013078.2018.1522236
Pittenger, M. (2009). Sleuthing the source of regeneration by MSCs. Cell Stem Cell 5, 8–10. doi: 10.1016/j.stem.2009.06.013
Qu, Y., Zhang, Q., Cai, X., Li, F., Ma, Z., Xu, M., et al. (2017). Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 21, 2491–2502. doi: 10.1111/jcmm.13170
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. doi: 10.1083/jcb.201211138
Regenberg, A. C., Hutchinson, L. A., Schanker, B., and Mathews, D. J. (2009). Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells 27, 2312–2319. doi: 10.1002/stem.132
Ridder, K., Keller, S., Dams, M., Rupp, A. K., Schlaudraff, J., Del Turco, D., et al. (2014). Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 12:e1001874. doi: 10.1371/journal.pbio.1001874
Rodero, M. P., and Khosrotehrani, K. (2010). Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 3, 643–653.
Saldana, L., Bensiamar, F., Valles, G., Mancebo, F. J., Garcia-Rey, E., and Vilaboa, N. (2019). Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res. Ther. 10:58. doi: 10.1186/s13287-019-1156-6
Shen, H., Yoneda, S., Abu-Amer, Y., Guilak, F., and Gelberman, R. H. (2020). Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J. Orthop. Res. 38, 117–127. doi: 10.1002/jor.24406
Shi, Y., Su, J., Roberts, A. I., Shou, P., Rabson, A. B., and Ren, G. (2012). How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 33, 136–143. doi: 10.1016/j.it.2011.11.004
Shi, Z., Wang, Q., and Jiang, D. (2019). Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 17:211. doi: 10.1186/s12967-019-1960-x
Sica, A., and Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. doi: 10.1172/JCI59643
Silva, A. K., Kolosnjaj-Tabi, J., Bonneau, S., Marangon, I., Boggetto, N., Aubertin, K., et al. (2013). Magnetic and photoresponsive theranosomes: translating cell-released vesicles into smart nanovectors for cancer therapy. ACS Nano 7, 4954–4966. doi: 10.1021/nn400269x
Skog, J., Wurdinger, T., van Rijn, S., Meijer, D. H., Gainche, L., Sena-Esteves, M., et al. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. doi: 10.1038/ncb1800
Smyth, T., Petrova, K., Payton, N. M., Persaud, I., Redzic, J. S., Graner, M. W., et al. (2014). Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 25, 1777–1784. doi: 10.1021/bc500291r
Spiller, K. L., and Koh, T. J. (2017). Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 122, 74–83. doi: 10.1016/j.addr.2017.05.010
Stahl, M., Schupp, J., Jager, B., Schmid, M., Zissel, G., Muller-Quernheim, J., et al. (2013). Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE 8:e81382. doi: 10.1371/journal.pone.0081382
Stein, M., Keshav, S., Harris, N., and Gordon, S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292. doi: 10.1084/jem.176.1.287
Tao, S. C., Guo, S. C., Li, M., Ke, Q. F., Guo, Y. P., and Zhang, C. Q. (2017). Chitosan wound dressings incorporating exosomes derived from MicroRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl. Med. 6, 736–747. doi: 10.5966/sctm.2016-0275
Tian, Y., Li, S., Song, J., Ji, T., Zhu, M., Anderson, G. J., et al. (2014). A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390. doi: 10.1016/j.biomaterials.2013.11.083
Tidball, J. G. (2011). Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 1, 2029–2062. doi: 10.1002/cphy.c100092
Vakhshiteh, F., Atyabi, F., and Ostad, S. N. (2019). Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int. J. Nanomedicine 14, 2847–2859. doi: 10.2147/IJN.S200036
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. doi: 10.1038/ncb1596
Varol, C., Mildner, A., and Jung, S. (2015). Macrophages: development and tissue specialization. Annu. Rev. Immunol. 33, 643–675. doi: 10.1146/annurev-immunol-032414-112220
Wang, B., Yao, K., Huuskes, B. M., Shen, H. H., Zhuang, J., Godson, C., et al. (2016). Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 24, 1290–1301. doi: 10.1038/mt.2016.90
Wang, Y., He, G., Guo, Y., Tang, H., Shi, Y., Bian, X., et al. (2019). Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med. 23, 5475–5485. doi: 10.1111/jcmm.14430
Waterman, R. S., Tomchuck, S. L., Henkle, S. L., and Betancourt, A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 5:e10088. doi: 10.1371/journal.pone.0010088
Wells, K., and Julia, H. L. (2009). Study quality assessment in systematic reviews of research on intervention effects. Res. Soc. Work Pract. 19, 52–62. doi: 10.1177/1049731508317278
Willis, G. R., Kourembanas, S., and Mitsialis, S. A. (2017). Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4:63. doi: 10.3389/fcvm.2017.00063
Wolfe, A. R., Trenton, N. J., Debeb, B. G., Larson, R., Ruffell, B., Chu, K., et al. (2016). Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget 7, 82482–82492. doi: 10.18632/oncotarget.12694
Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Thery, C., Masurier, C., et al. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7, 297–303. doi: 10.1038/85438
Yang, D. H., and Yang, M. Y. (2019). The Role of Macrophage in the pathogenesis of osteoporosis. Int. J. Mol. Sci. 20:2093. doi: 10.3390/ijms20092093
Yoo, S. W., Chang, D. Y., Lee, H. S., Kim, G. H., Park, J. S., Ryu, B. Y., et al. (2013). Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-beta. Neurobiol. Dis. 58, 249–257. doi: 10.1016/j.nbd.2013.06.001
Zhang, S., Chuah, S. J., Lai, R. C., Hui, J. H. P., Lim, S. K., and Toh, W. S. (2018). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27. doi: 10.1016/j.biomaterials.2017.11.028
Zhang, Z., Dombroski, J. A., and King, M. R. (2020). Engineering of exosomes to target cancer metastasis. Cell. Mol. Bioeng. 13, 1–16. doi: 10.1007/s12195-019-00607-x
Zhao, Y., Sun, X., Cao, W., Ma, J., Sun, L., Qian, H., et al. (2015). Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015:761643. doi: 10.1155/2015/761643
Keywords: mesenchymal stem cells (MeSH ID D059630), extracellular vesicles (EVs), exosomes, macrophages, musculoskeletal
Citation: Xu H, Lee C-W, Wang Y-F, Huang S, Shin L-Y, Wang Y-H, Wan Z, Zhu X, Yung PSH and Lee OK-S (2020) The Role of Paracrine Regulation of Mesenchymal Stem Cells in the Crosstalk With Macrophages in Musculoskeletal Diseases: A Systematic Review. Front. Bioeng. Biotechnol. 8:587052. doi: 10.3389/fbioe.2020.587052
Received: 24 July 2020; Accepted: 28 October 2020;
Published: 26 November 2020.
Edited by:
Hae-Won Kim, Institute of Tissue Regeneration Engineering (ITREN), South KoreaReviewed by:
Kunyu Zhang, Johns Hopkins University, United StatesJung-Hwan Lee, Institute of Tissue Regeneration Engineering (ITREN), South Korea
Copyright © 2020 Xu, Lee, Wang, Huang, Shin, Wang, Wan, Zhu, Yung and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oscar Kuang-Sheng Lee, oscarlee9203@gmail.com
†These authors have contributed equally to this work
 Hongtao Xu
Hongtao Xu Chien-Wei Lee
Chien-Wei Lee Yu-Fan Wang1
Yu-Fan Wang1  Oscar Kuang-Sheng Lee
Oscar Kuang-Sheng Lee