A major difficulty in the field of cardiac regeneration, alongside several other translational fields, is the successful transition of candidate therapeutics from small- to large- animal pre-clinical models and then into the clinic. This transition, from proof-of-principle studies in mice to demonstrating pre-clinical effectiveness and safety in larger scale animals, such as in pigs, is often mired by failure to recapitulate therapeutic effects up each scale of animal model. Successfully moving from porcine models into humans is the ultimate transition and encompasses several major hurdles, where avoiding the financial burdens of late-state attrition calls for an even more thorough examination and understanding of pre-clinical models. For the heart in particular, it is widely known that fundamental physiological differences exist on the organ level, across the different mammalian pre-clinical models used to study myocardial infarction (M.I). These include differences in organization of chambers, coronary vasculature, electrophysiology and beating dynamics.
In this work, we sought to characterize the differences in the infarct itself, as well as disease progression, across mice, rats and pigs. Our lab has considerable experience studying LAD-ligation induced acute- and chronic- M.I, in the context of mice [3], rats [2],[4],[6] and pigs [1],[4]-[6], and have successfully translated biomaterial-based therapeutics from small to large animals [1],[4]-[6]. We have noticed that the same procedure to induce M.I results in different disease pathology across each model, such as location of infarct and disease progression. Knowledge of these subtleties will be helpful in guiding the design of biomaterial-based therapeutic strategies and their translation into the clinic.
All M.I surgeries, sectioning, staining and analysis were performed as previously described [1]-[6].
Our preliminary results show that the same M.I surgery yields an infarct in different regions of the mice, rat and porcine heart.
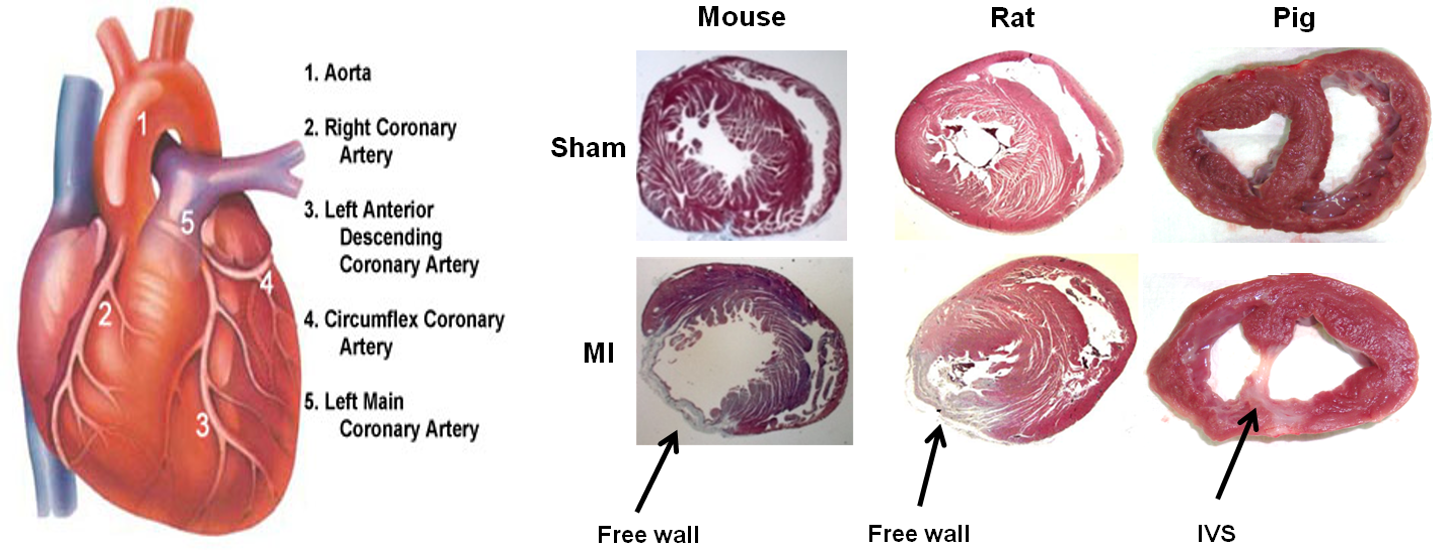
This is likely due to the differences in coronary vasculature and heart structure. In mice and rats, the infarct is localized to the free wall. In contrast, in the porcine heart we observed the infarct region is centralized to the interventricular septum. We also noticed that post-M.I progression of disease, in particular pathological remodelling, is different. These differences may have subtle implications on several aspects of biomaterials research and reinforce previously observed effects. For example, from a biomechanical point of view, it is known that strategies which provide structural support at the infarct area in the murine heart minimzes pathological remodelling and provides therapeutic benefit. On the other hand, in the porcine heart, the infarct being at the interventricular septum represents a more static location; less dependent on this added biomechanical structural support.
In conclusion, upon LAD-ligation induced M.I, differences such as location of the infarct itself and progression of disease can be observed across the commonly studied mammalian pre-clinical models of acute and chronic M.I. Ultimately, these differences may have an impact on the efficacy of biomaterial based therapeutics, such as effect of infarct location on drug diffusion or construct bio-mechanics, and experimental design, such as whether to approach the infarct by epicardial, intramyocardial or even transendocardial injection of therapeutics.
References:
[1] Chen, C.-H., Chang, M.-Y., Wang, S.-S., & Hsieh, P. C. H. (2014). Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. American Journal of Physiology. Heart and Circulatory Physiology, 306(7), H1078–86. doi:10.1152/ajpheart.00801.2013
[2] Chen, C.-H., Wang, S.-S., Wei, E. I., Chu, T.-Y., & Hsieh, P. C. H. (2013). Hyaluronan enhances bone marrow cell therapy for myocardial repair after infarction. Molecular Therapy : The Journal of the American Society of Gene Therapy, 21(3), 670–9. doi:10.1038/mt.2012.268
[3] Chang, M.-Y., Yang, Y.-J., Chang, C.-H., Tang, A. C. L., Liao, W.-Y., Cheng, F.-Y., … Hsieh, P. C. H. (2013). Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. Journal of Controlled Release : Official Journal of the Controlled Release Society, 170(2), 287–94. doi:10.1016/j.jconrel.2013.04.022
[4] Lin, Y.-D., Luo, C.-Y., Hu, Y.-N., Yeh, M.-L., Hsueh, Y.-C., Chang, M.-Y., … Hsieh, P. C. H. (2012). Instructive Nanofiber Scaffolds with VEGF Create a Microenvironment for Arteriogenesis and Cardiac Repair. Science Translational Medicine, 4(146), 146ra109–146ra109. doi:10.1126/scitranslmed.3003841
[5] Lin, Y. D., Yeh, M. L., Yang, Y. J., Tsai, D. C., Chu, T. Y., Shih, Y. Y., … Hsieh, P. C. H. (2010). Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation, 122(11 SUPPL. 1). doi:10.1161/CIRCULATIONAHA.110.939512
[6] Lin, Y.-D., Ko, M.-C., Wu, S.-T., Li, S.-F., Hu, J.-F., Lai, Y.-J., … Hsieh, P. C. H. (2014). A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomaterials Science, 2(4), 567. doi:10.1039/c3bm60289c