Introduction: The bilayered skin construct consisting of fibroblast and keratinocytes seems to be the most efficient in healing the skin defect. To construct of bilayered skin substitutes, the nanofibrous membranes can be advantageously used[1]. These types of cell carriers can enable physical and humoral communication between fibroblasts and keratinocytes[2]. Moreover, the nanostructure of material well mimic nanofibrous component of extracellular matrix. The nanostructured materials enhance the cell adhesion by adsorption of cell-adhesion molecules from body fluids or cell culture medium in appropriate spatial conformation well recognized by the cell adhesion receptors. The bioactivity of nanofibrous membranes can be further enhanced by coating of these membranes by fibrin, molecule playing important role during wound healing, or by molecules present in nature skin (collagen, fibronectin)[3].
The aim of the study is to evaluate the adhesion and growth of human dermal fibroblasts and human HaCaT keratinocytes on polylactide (PLA) nanofibrous membranes with cell degradable fibrin and collagen structures. We further investigated the effect of fibrin structures on nanofibrous membranes on synthesis of collagen by fibroblasts, mainly in the form of collagen fibrils deposited on the membranes as ECM.
Materials and Methods: The polylactide (PLA) nanofibrous membranes were prepared using Nanospider needleless electrospinning technology. The membranes were coated with fibrin and collagen I, and subsequently fibronectin was attached. We evaluated the adhesion, proliferation and metabolic activity (determined with MTS assay) of human dermal fibroblasts and HaCaT keratinocytes in several intervals during cell cultivation. We also studied collagen production by fibroblasts stimulated by fibrin structures on PLA membranes, i.e. formation of extracellular collagen fibers (by immunofluorescence protein staining), relative collagen m-RNA expression (by real-time PCR) and total amount of produced collagen (by Sircol soluble assay). The collagen production was stimulated by adding ascorbic acid into cell culture medium.
Results and Discussion: The results showed that protein structures were stable under standard cell culture conditions. Their degradation during cell cultivation correlated with the degree of cell proliferation (Fig. 1).
The fibrin structures on the membranes supported particularly the adhesion and proliferation of dermal fibroblasts. This could be explained by different requirements of fibroblasts and keratinocytes for the fibrin concentration and stiffness[4]. In addition, fibrin stimulated the fibroblasts to synthetize collagen and to form ECM collagen fibrils (Fig 2).
The collagen structures on the membranes promoted particularly the adhesion and proliferation of human HaCaT keratinocytes. Fibronectin further enhanced the cell attachment to the membrane.
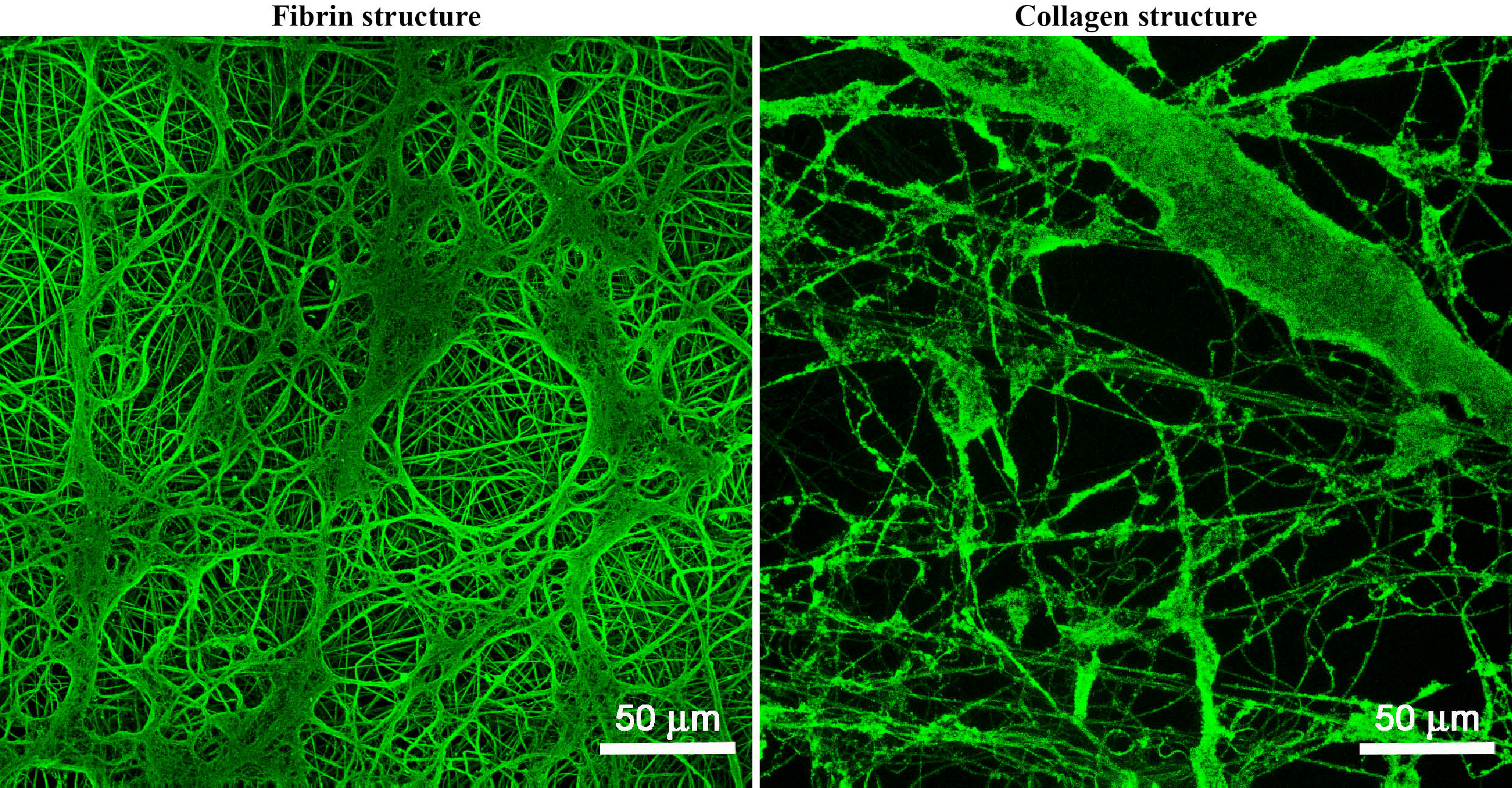
Fig.1: Fibrin and collagen structures on PLA membranes. Stained by immunofluorescence. Leica TCS SPE DM2500 confocal microscope, obj. 40x/1.15 NA oil, bar 50 μm.
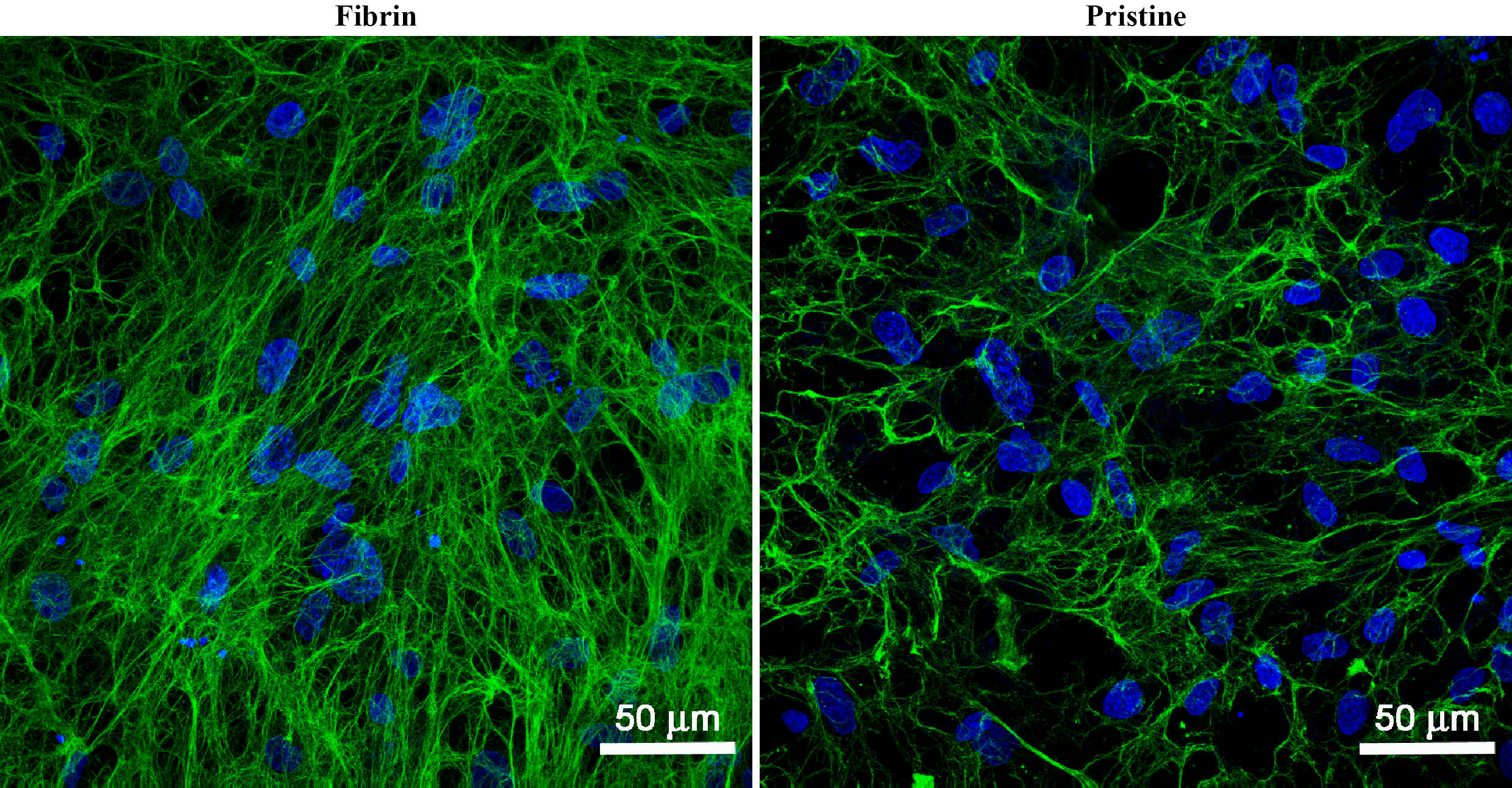
Fig. 2: Collagen I fibers (green) deposited on PLA membranes with fibrin structures and in pristine state by dermal fibroblasts on day 14 after cell seeding. Cells cultivated in the cell culture medium with ascorbic acid. Cell nuclei stained with Hoechst #33258 (blue). Leica TCS SPE DM2500 confocal microscope, obj. 40x/1.15 NA oil, bar 50 μm.
Conclusion: The PLA membranes coated mainly with fibrin are promising for construction of temporary carriers for skin cells. They promoted the adhesion, growth and collagen ECM formation by dermal fibroblasts.
This study was supported by the Grant Agency of the Charles University in Prague (Grant No. 38214) and the Grant Agency of the Czech Republic (Grant No. P108/12/G108).
References:
[1] Bacakova, L., E. Filova, M. Parizek, T. Ruml, and V. Svorcik. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv 29 (6): 739-767, 2011
[2] Idrus, R. B., M. A. Rameli, K. C. Low, J. X. Law, K. H. Chua, M. B. Latiff, and A. B. Saim. Full-thickness skin wound healing using autologous keratinocytes and dermal fibroblasts with fibrin: bilayered versus single-layered substitute. Adv Skin Wound Care 27 (4): 171-180, 2014.
[3] McMillan, J. R., M. Akiyama, M. Tanaka, S. Yamamoto, M. Goto, R. Abe, D. Sawamura, M. Shimomura, and H. Shimizu. Small-diameter porous poly (epsilon-caprolactone) films enhance adhesion and growth of human cultured epidermal keratinocyte and dermal fibroblast cells. Tissue Eng 13 (4): 789-798, 2007.
[4] Reinertsen, E., M. Skinner, B. Wu, and B. Tawil. Concentration of fibrin and presence of plasminogen affect proliferation, fibrinolytic activity, and morphology of human fibroblasts and keratinocytes in 3D fibrin constructs. Tissue Eng Part A 20 (21-22): 2860-2869, 2014.