- 1Center for Infectious Diseases, Beijing You’an Hospital, Capital Medical University, Beijing, China
- 2Infectious Diseases Department, Peking Union Medical College Hospital, Beijing, China
- 3The Aaron Diamond AIDS Research Center, New York, NY, United States
- 4School of Biomedical Engineering, Capital Medical University, Beijing, China
Background: The World Health Organization (WHO) Consolidated antiretroviral therapy (ART) guidelines set the CD4+ T-cell counts threshold to 500 cells/mm3 in 2013, and 2015 guidelines recommend treating all HIV-infected adults regardless of their CD4+ T-cell counts. To inform the decision-making around ART guidelines for people living with HIV, we systematically reviewed the literature to estimate differences in clinical benefits between individuals starting treatment with baseline CD4+ T-cell counts ≥500 cells/mm3 (early initiation) as compared to <500 cells/mm3 (deferred initiation).
Methods: We systematically searched the electronic databases and abstracts for randomized controlled trials (RCT) and observational studies. Outcomes were mortality, AIDS progression, AIDS or death, immunologic recovery, and virologic suppression. We pooled data across studies and performed analyses of effect sizes.
Results: We identified 13 studies comparing early and deferred treatment. The pooled risk ratio (RR) of mortality of 11 observational studies was 0.90 (95% CI 0.82–0.99), with moderate heterogeneity (I2 = 53%). The pooled RR for progression to AIDS from two observational studies was 0.77 (95% CI 0.47–1.24). Five observational studies found a pooled RR of death or AIDS of 0.94 (95% CI 0.93–0.95). For the outcome of immunologic recovery, defined as CD4+ T-cell counts reaching at least 800 cells/mm3 after ART, one observational study found early initiation of ART had an HR (hazard ratio) of 2.39 (95% CI 1.93–2.96). The pooled RR of viral suppression (a viral load <50 copies/ml) after 9 months from one cohort was 1.04 (95% CI 0.99–1.09).
Conclusion: Mortality risk and risk for AIDS appear to be reduced among people living with HIV with early initiation of ART, based on current WHO guidelines, as compared to those with deferred initiation of ART (<500 cells/mm3).
Introduction
Optimal timing of antiretroviral therapy (ART) initiation for people living with HIV and AIDS (PLWHA) is essentially a risk-benefit question (1). In the mid-1990s, with the advent of combined ART, the prognosis of the disease improved tremendously (2, 3). Since then, different thresholds for initiating ART have been proposed over time (CD4 counts of 200, 350, and 500 cells/mm3). There is a consensus that CD4 cell counts measurements have been at the core of understanding disease progression and decisions regarding initiating treatment in AIDS patients. The measurements are used to prevent opportunistic infections as well as to monitor the treatment response (4).
During the first 10 years of ART use, the ART-initiation threshold fluctuated. After a short period in the late 1990 s, Department of Health and Human Services recommended to start treatment for ≤500 cells/mm3; however, this recommendation came in the absence of randomized trial evidence and was not taken up in clinical practice (5). The threshold for treating asymptomatic adults dropped below 200 cells/mm3 at the beginning of the 2000s (5). This shift was mainly due to taking into account the cumulative risk of ART drug-related toxicity. During that period, randomized clinical trials of structured treatment interruption after immune recovery above 350 cells/mm3 were carried out as a way to minimize ARV-related toxicity (6, 7). However, these studies concluded that the damage of HIV is more severe than that of antiretroviral drugs.
Between 2006 and 2009, World Health Organization (WHO) raised the CD4 threshold to 350 cells/mm3 based on research demonstrating that ART initiation at this threshold reduced mortality, disease progression and serious adverse events (8). The 2013 WHO consolidated ART guidelines further set the threshold to 500 cells/mm3 or less. Finally, between 2012 and 2015, all international guidelines took the last step and recommended treating all HIV-infected adults, regardless of CD4 counts (9–11). Of note, in resource-limited countries, health care infrastructure and the availability of antiretroviral drugs may be more limited, and in that case ART remains prioritized for patients with the most advanced disease.
Meanwhile, The Joint United Nations Programme on HIV/AIDS (UNAIDS) set international goals for 2020 termed the “90–90–90 targets” to curb the HIV epidemic: 90% of PLWHA know their HIV status, 90% of people who know their HIV status access treatment and 90% of people on treatment have suppressed viral loads (12). The second “90” (treatment), can realistically only be accomplished with early ART initiation. Furthermore, ART does not just reduce mortality in the treated individuals, it also reduces the number of people with a detectable viral load. That finding, as expected, show that early ART initiation helps to accomplish the third “90” (suppression) of 90–90–90 targets.
The benefit of treating all HIV-infected adults, rather than deferring treatment until CD4+ T cell counts drops below 500 cells/mm3 or even lower, was initially demonstrated in a large cohort study (13), followed by two randomized controlled trials (RCTs) (14, 15). The goal of this meta-analysis was to comprehensively review the available observational study data for evidence supporting the benefits of early ART. We systematically reviewed the literature to estimate differences in risk of disease progression between people living with HIV-1 whose CD4+ T cell counts at ART initiation was 350–499 cells/mm3 and patients whose CD4+ T cell counts at ART initiation was ≥500 cells/mm3. The primary outcome was mortality, a composite outcome that included AIDS progression, AIDS or death, immunological recovery and virological suppression. We conducted a meta-analysis to evaluate the outcomes of patients initiating ART at different CD4 cell counts.
Methods
The review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO): CRD42017072465.
Data Sources and Search Strategy
We followed the meta-analysis of observational studies in epidemiology guidelines for study procedures (16). We searched PubMed, MEDLINE, Web of Science and Embase for journal articles published between 1 January 2000 and 31 May 2017 which reported CD4 counts at ART initiation among HIV-infected adults. The search strategy included Medical Subject Heading terms and a range of relevant keywords, including “HIV,” “ART,” “early/deferred therapy,” and “CD4.” We included studies and investigations in HIV-1-infected patients who began ART with CD4+ T cell counts less than 500 cells/mm3 and at least 500 cells/mm3. Randomized trials, prospective and retrospective cohorts, and routine clinic cohorts were eligible for inclusion. There were no geographic or age restrictions. The search was limited to peer-reviewed and English-written journal articles.
Study Selection and Data Extraction
We first screened titles and abstracts to identify relevant articles for inclusion. To exclude duplicate references, we imported search results into bibliographic citation management software (EndNote X7). Two authors reviewed the titles, abstracts, and descriptor entries of the remaining citations to identify potentially eligible reports independently. We obtained full-text articles for references that were considered potentially meeting the inclusion criteria. We reviewed these full-text articles and applied the inclusion criteria to determine the eligibility or ineligibility of each study. We planned to resolve any differences of opinion through discussion and, if necessary, we would have a neutral third party arbiter. After determining articles for inclusion, we performed an examination and extracted data from each study.
Information extracted from articles included article author, publication year, type of screening test, sample size, CD4+ T-cell counts, and primary outcomes (mortality and AIDS progression).
Statistical Analysis and Data Synthesis
We combined data across studies and estimated summary treatment effect sizes. We performed meta-analyses using Review Manager 5.3. We used published estimated relative risks (RRs) if these data were provided in study reports. If not available, we calculated RRs for outcomes and the 95% confidence interval (CI). Subgroup analysis was also performed to evaluate the moderate effect of study location on mortality due to sufficient comparisons. Due to the clinical heterogeneity between study designs and populations, we chose fixed-effects or random-effects model based meta-analysis according to I2 for combining data. We used I2 to quantify the degree of heterogeneity (17, 18). Estimates of I2 were defined as the percentage of variability in effect estimates due to heterogeneity rather than chance.
Assessment of Evidence Quality
We used the Newcastle Ottawa Scale to assess quality and risk of bias in the nonrandomized studies (Table 1) (19). This scale judges three general areas: selection of study groups, comparability of groups, and ascertainment of outcomes (in the case of cohort studies). We assessed the quality of evidence from the relevant studies for each outcome. Because of the particularity of the study, the literature we collected are observational studies. Evidence from these studies starts at low, but can be upgraded if the sample size and magnitude of treatment effect are very large, if there is an obvious dose–response relationship or if all possible mixed factors would decrease the magnitude of the treatment effect (20). Evidence from observational studies can also be downgraded. To minimize the problem of overlap, we removed one smaller sample study from the final analysis, thereby avoiding the possibility of double-counting patient populations.
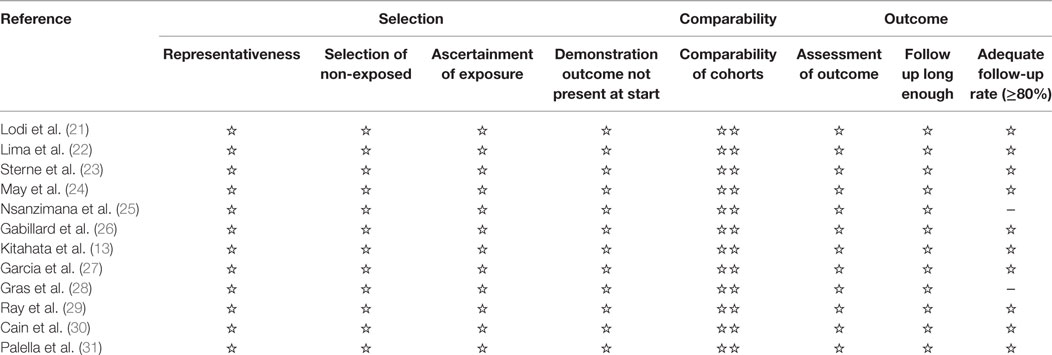
Table 1. Summary of critical appraisal of included studies using the Newcastle–Ottawa Quality Assessment Scale for observational studies.
Results
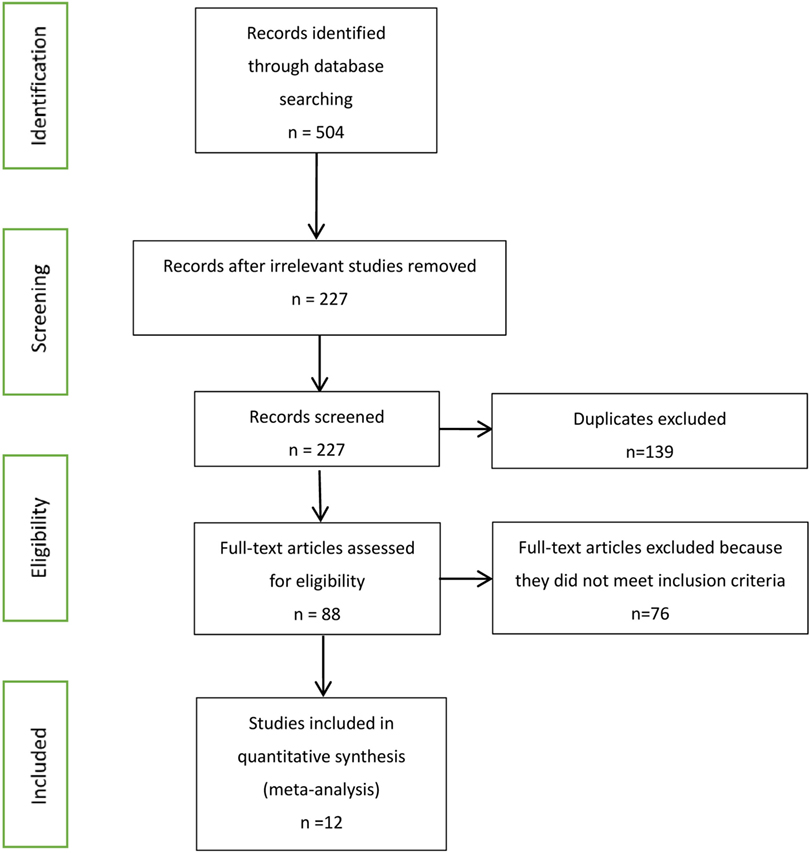
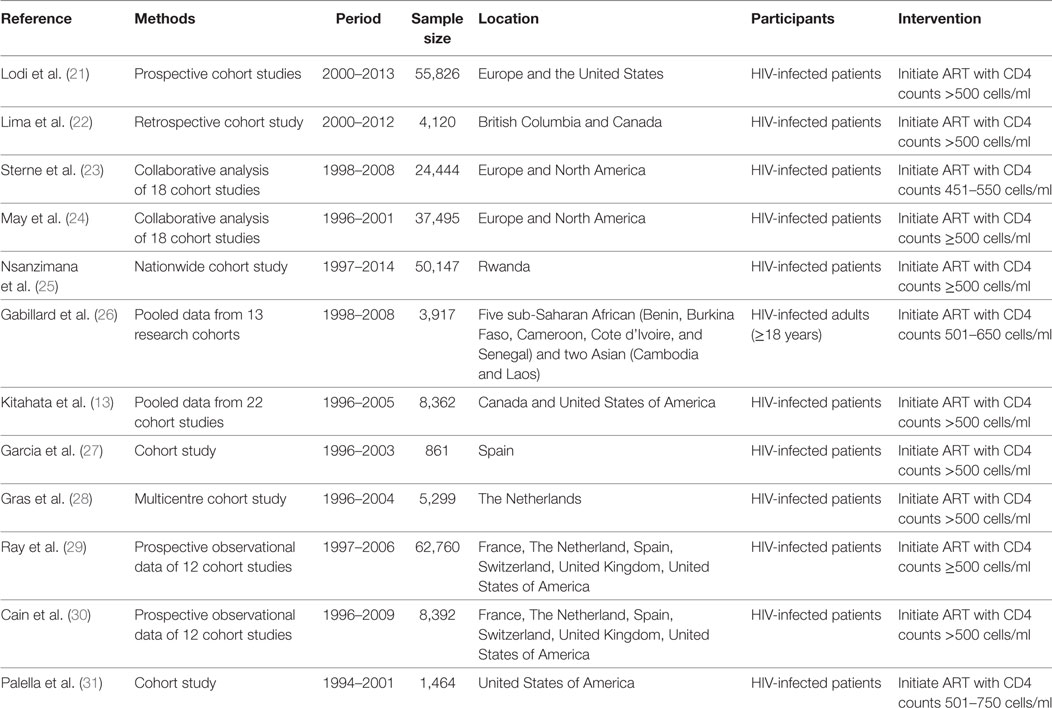
Our searches identified 504 published journal articles. After review of the title and abstract, we selected 12 full-text articles that met our inclusion criteria (Figure 1). Two RCT studies failed to meet all inclusion criteria, because they focused on HIV-infected patients who began ART with CD4+ T cell counts less than 350 cells/mm3 and more than 500 cells/mm3. The remainder were observational studies (13, 21–31) (Table 2) with a variety of outcomes, including mortality, progression to AIDS, progression to AIDS or death, serious non-AIDS events, CD4+ T-cell counts increase, virologic suppression, and virologic failure.
Mortality (350–499 vs. ≥500 cells/mm3)
There were 10 studies (13, 21–26, 29–31) reporting a mortality outcome (Figure 2). One study reported a slightly increased risk of death in patients who initiated ART at CD4+ T-cell counts of at least 500 cells/mm3 (31), whereas nine studies reported a decreased risk of death (pooled RR 0.90, 95% CI 0.82–0.99), which was statistically significant for two of these nine studies (13, 21).
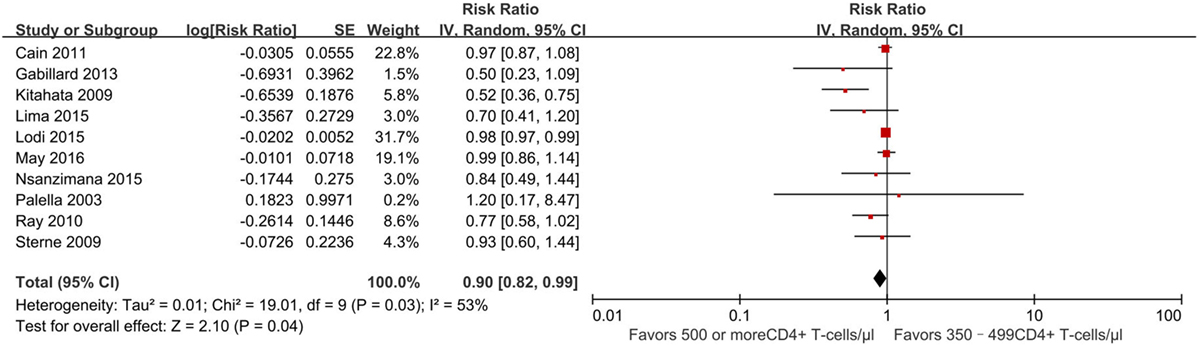
Figure 2. Forest plots of mortality. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
Furthermore, we conducted a subgroup analysis of developed and developing countries separately based on the location of these 10 studies (Figure 3). Further analysis focus on whether developing countries with a higher burden of infectious diseases have different outcomes than developed countries. Of 10 studies, 8 were conducted in developed countries (13, 21–24, 29–31) and 2 in developing countries (25, 26). Subgroup analysis revealed no statistically significant difference between the two groups (RR for developing country, 0.91, 95% CI 0.83–1.01, developed country, 0.70, 95% CI 0.43–1.14, p = 0.29).
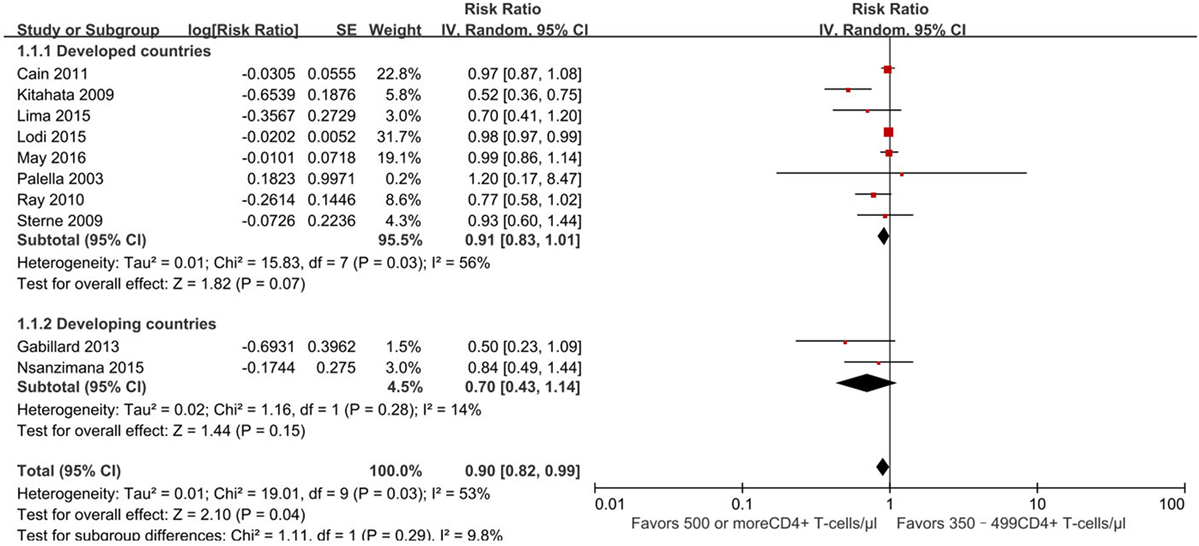
Figure 3. Forest plots of subgroup analysis of mortality. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
The evidence quality of the observational literature which reported mortality was poor due to clinical heterogeneity and inconsistency of findings across the 10 studies. However, the evidence quality of the non-overlapping studies was moderate because of a large effect size.
AIDS Progression (350–499 vs. ≥500 cells/mm3)
Compared to ART initiation at 350–499 CD4+ T cells/mm3, initiation of ART at ≥500 cells/mm3 was found to be associated with decreased risk of developing AIDS-defining opportunistic infections in a pooled analysis of two non-overlapping studies: RR = 0.77 (95% CI 0.47–1.24) (22, 26) (Figure 4), there are no statistically significant effects (p = 0.28). The evidence quality of the observational literature was very poor due to small number of events. None of these two studies reported adjusted estimates, so that the risk of bias from unadjusted estimates has been increased.
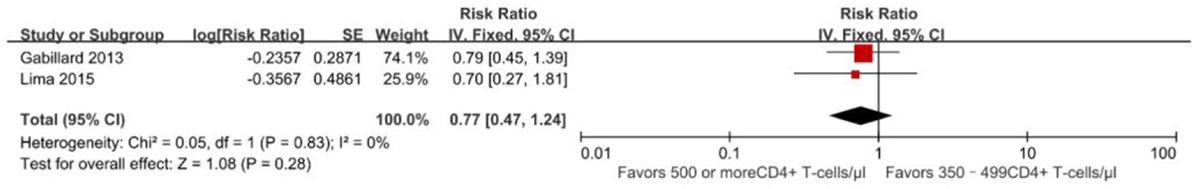
Figure 4. Forest plots of AIDS progression. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
Mortality and/or AIDS-free Survival (350–499 vs. ≥500 cells/mm3)
Four studies (21, 23, 27, 30) evaluated the effect of early vs. deferred ART initiation. All of these found a decreased risk of progression to AIDS and/or death among persons who initiated ART early as compared to those who deferred treatment. The RR was 0.94 (95% CI 0.93–0.95) (Figure 5). Two studies found a statistically significant effect (21, 30). Both were based on data from the HIV-CAUSAL collaboration (21) of cohorts in Europe and the United States, for which sample size is large; they found a slightly lower risk of AIDS progression among patients beginning treatment at CD4+ T-cell counts of ≥500 cells/mm3 compared to those with deferred treatment.
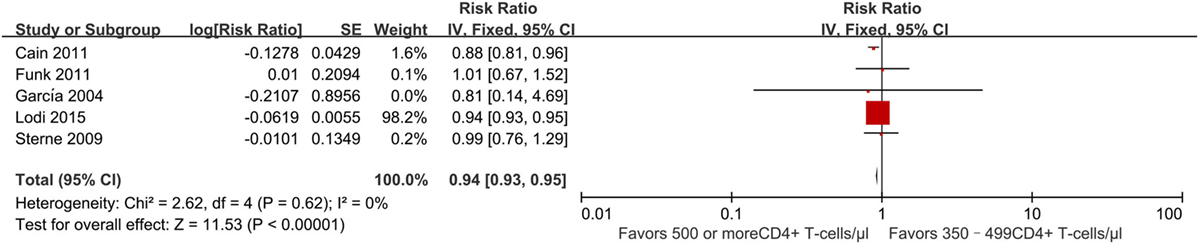
Figure 5. Forest plots of AIDS progression or death. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
These observational studies were consistent in the identification of treatment effect. However, the quality of the literature for this outcome was poor due to imprecision owing to its observational status and the lack of strength.
Immunologic Recovery
Outcome used to determine immune recovery in this study included CD4+ T-cell counts reaching at least 800 cells/mm3 after initiation of ART. One study from the AIDS Therapy Evaluation Project and Netherlands (ATHENA) national observational HIV cohort (28) estimated the effect of early vs. deferred treatment on CD4+ T-cell counts reaching 800 cells/mm3 or more after 7 years of ART. It found that compared to patients who began ART at 350–499 CD4+ T cell/mm3, those who began ART at ≥500 CD4+ T cells/mm3 had an HR of 2.39 (95% CI 1.93–2.96) (Figure 6). This outcome was statistically significant (p < 0.00001), although evidence quality of this literature was rated as poor owing to its observational nature and overlap of patient populations between studies.
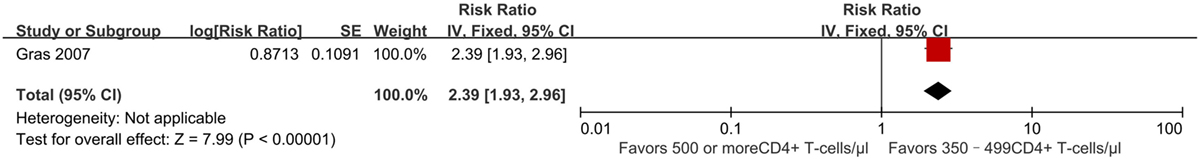
Figure 6. Forest plots of immunologic recovery. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
Virologic Suppression
Successful treatment requires a prolonged and profound virological response, so a key consideration in deciding when to initiate ART is whether it can achieve viral suppression. We modeled the probability of maintaining viral suppression (a viral load <50 copies/ml) over time. In a retrospective cohort study performed between 2000 and 2012, initiating ART at CD4+ T-cell counts of ≥500 cells/mm3 was associated with a better effect of achieving viral suppression after 9 months (RR 1.04, 95% CI 0.99–1.09) (Figure 7). The percentage of individuals displaying viral suppression was 89% in those initiating ART ≥500 cells/mm3, compared to 86% in those initiating ART at 350–499 cells/mm3 (22). The quality of this literature for outcomes was high with no observed study limitations.
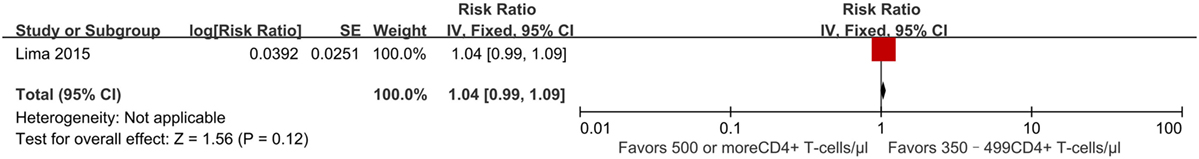
Figure 7. Forest plot of virologic suppression. CI, confidence interval; DF, degrees of freedom; IV, inverse variance.
Discussion
World Health Organization recommendations to initiate ART for persons switching from CD4 counts ≤500 cells/mm3 (32) to treating all HIV-infected adults regardless of their CD4 counts (9–11) have spurred debate about the benefits of adopting this guide as well as discussion about the extent to which existing health systems can accommodate the expansion of treatment availability (33–35).
There is a strong association between CD4+ T-cell counts at ART initiation and all outcomes investigated in this study. We found evidence to suggest immediate ART initiation (with baseline CD4 ≥500 cells/mm3) can reduce the risk of mortality and progression to AIDS, can improve the likelihood of immunologic recovery (CD4+ T-cell counts reaching 800 cells/mm3 or more after ART) and can increase viral suppression at 9 months. There was generally consistent agreement between the large observational literature although few individual studies did show opposite results (31).
Our findings indicate that, compared with delayed treatment strategies, immediate universal ART slightly prolongs survival, decreases risk of progression to AIDS/death, and we tentatively put forward it also increases the percentage of individuals who achieve virological suppression. They render further support to the evidence from three major published trials, HPTN 052, Temprano ANRS 12136 and START, which demonstrated that there is a health benefit from immediate initiation of ART, and that this is all the more true for those living in low resource settings. In fact, HIV-infected persons are now recommended to initiate ART regardless of CD4 counts all over the world, and the objective of ART clearly turns to suppressing viral replication and preventing inflammation and immune deficiency (1). Observational studies will continue to play a role in understanding the long-term effects of initiating ART at high CD4 counts in conventional clinical settings. Moreover, our study indicates a similar decreased mortality in developing and developed countries. However, the reality is that treatment roll-out to all those in need of ART remains far from complete, especially in countries with a high HIV burden (36). Broadening treatment eligibility directly leads to an increase in the number of individuals treated, while the vast majority of the 35 million PLWHA are in resource-limited settings (37). Improving the timeliness of ART initiation will also require significant additional financial investment (38). This is a challenge for developing countries. One question is whether in HIV elite controllers, the benefits of early treatment actually do outweigh the risks (39, 40). In this respect, one needs to explore the relevant question of “when not to start ART immediately” (40).
The achievement of the goals of “90-90-90 targets” requires immediate treatment linked to viral suppression. According to this recommendation of starting ART immediately in HIV-infected adults, countries should adopt and implement this policy to accelerate progress toward the goals and eventually reach epidemic control (41). However, to achieve early ART and eliminate AIDS globally will require constant effort and substantially higher levels of treatment coverage, a near doubling of the number of people on treatment today (42).
Several limitations of this meta-analysis should be addressed. First, as with all systematic reviews, we are limited by the sensitivity of our search and our ability to identify relevant studies. Second, our estimates come from high-income countries in Europe and the USA, with only a small contribution from Africa, Asia, and South America, which may somewhat limit the outcome effects. Third, a meta-analysis of observational studies is inherently limited because observational cohort studies may provide a relative lower quality of evidence than randomized controlled clinical trials. Furthermore, it is possible that the data density such as frequency of follow-up visits and clinical assessment between these periods may have affected our results. There might be additional potential limitations given the English language bias.
In conclusion, our findings lead us to argue that more resources should be dedicated to both facilitating an earlier diagnosis of HIV and expediting ART initiation as well as retaining in care of those newly diagnosed (43). This does not only mean more tests and more drugs, but it also requires more caretakers to support those living with HIV and AIDS and to help them remain in care once they have started treatment (38).
Author Contributions
AS, XCL, XH, and HW conceived and designed the protocol and study. JH, WX, NW, HX, and XY identified studies to be screened. XH, KM, D-YO, BS, and XFL identified studies for eligibility, extracted data and assessed the methodologic quality of included studies. AS performed the analysis with assistance from HC and HW. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Chinese Government 13th Five-Year Plan (2017ZX10201101), Major Project of Beijing Municipal Science and Technology Committee (D161100000416003, D171100000517003), the National Natural Science Foundation of China (No. 81701984), the NSFC-NIH Biomedical collaborative research program (81761128001), the Capital Health Research and Development of Special Fund (2016-1-2182), and Beijing Key Laboratory (No. BZ0089).
References
1. Eholie SP, Badje A, Kouame GM, N’Takpe JB, Moh R, Danel C, et al. Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther (2016) 13:27. doi:10.1186/s12981-016-0111-1
2. Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS clinical trials group 320 study team. N Engl J Med (1997) 337(11):725–33. doi:10.1056/NEJM199709113371101
3. Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med (1997) 337(11):734–9. doi:10.1056/NEJM199709113371102
4. Ford N, Meintjes G, Pozniak A, Bygrave H, Hill A, Peter T, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis (2015) 15(2):241–7. doi:10.1016/S1473-3099(14)70896-5
5. Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, Gatell JM, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA (2002) 288(2):222–35. doi:10.1001/jama.288.2.222
6. Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet (2006) 367(9527):1981–9. doi:10.1016/S0140-6736(06)68887-9
7. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med (2006) 355(22):2283–96. doi:10.1056/NEJMoa062360
8. Anglemyer A, Rutherford GW, Easterbrook PJ, Horvath T, Vitoria M, Jan M, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS (2014) 28(Suppl 2):S105–18. doi:10.1097/QAD.0000000000000232
9. Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA (2014) 312(4):410–25. doi:10.1001/jama.2014.8722
10. WHO. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV (2015). Available from: http://www.hoint/hiv/pub/guidelines/earlyrelease–arv/en/
11. Adolescents. DPoAGfAa. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health-and-Human-Services. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
12. UNAIDS. 90-90-90—An Ambitious Treatment Target to Help End the AIDS Epidemic. Available from: http://www.unaidsorg/en/resources/documents/2014/90-90-90
13. Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med (2009) 360(18):1815–26. doi:10.1056/NEJMoa0807252
14. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med (2015) 373(9):795–807. doi:10.1056/NEJMoa1506816
15. Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med (2015) 373(9):808–22. doi:10.1056/NEJMoa1507198
16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi:10.1001/jama.283.15.2008
17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi:10.1002/sim.1186
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi:10.1136/bmj.327.7414.557
19. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalyses. (2013). Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
20. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64(4):383–94. doi:10.1016/j.jclinepi.2010.04.026
21. Lodi S, Phillips A, Logan R, Olson A, Costagliola D, Abgrall S, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV (2015) 2(8):e335–43. doi:10.1016/S2352-3018(15)00108-3
22. Lima VD, Reuter A, Harrigan PR, Lourenco L, Chau W, Hull M, et al. Initiation of antiretroviral therapy at high CD4+ cell counts is associated with positive treatment outcomes. AIDS (2015) 29(14):1871–82. doi:10.1097/QAD.0000000000000790
23. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet (2009) 373(9672):1352–63. doi:10.1016/S0140-6736(09)60612-7
24. May MT, Vehreschild JJ, Trickey A, Obel N, Reiss P, Bonnet F, et al. Mortality according to CD4 count at start of combination antiretroviral therapy among HIV-infected patients followed for up to 15 years after start of treatment: collaborative cohort study. Clin Infect Dis (2016) 62(12):1571–7. doi:10.1093/cid/ciw183
25. Nsanzimana S, Remera E, Kanters S, Forrest JI, Ford N, Condo J, et al. Effect of baseline CD4 cell count at linkage to HIV care and at initiation of antiretroviral therapy on mortality in HIV-positive adult patients in Rwanda: a nationwide cohort study. Lancet HIV (2015) 2(9):e376–84. doi:10.1016/S2352-3018(15)00112-5
26. Gabillard D, Lewden C, Ndoye I, Moh R, Segeral O, Tonwe-Gold B, et al. Mortality, AIDS-morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. J Acquir Immune Defic Syndr (2013) 62(5):555–61. doi:10.1097/QAI.0b013e3182821821
27. Garcia F, de Lazzari E, Plana M, Castro P, Mestre G, Nomdedeu M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr (2004) 36(2):702–13. doi:10.1097/00126334-200406010-00007
28. Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr (2007) 45(2):183–92. doi:10.1097/QAI.0b013e31804d685b
29. Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS (2010) 24(1):123–37. doi:10.1097/QAD.0b013e3283324283
30. Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med (2011) 154(8):509–15. doi:10.7326/0003-4819-154-8-201104190-00001
31. Palella FJ Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med (2003) 138(8):620–6. doi:10.7326/0003-4819-138-8-200304150-00007
32. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO (2013).
33. Maman D, Pujades-Rodriguez M, Nicholas S, McGuire M, Szumilin E, Ecochard R, et al. Response to antiretroviral therapy: improved survival associated with CD4 above 500 cells/mul. AIDS (2012) 26(11):1393–8. doi:10.1097/QAD.0b013e328352d054
34. De Cock KM, El-Sadr WM. When to start ART in Africa – an urgent research priority. N Engl J Med (2013) 368(10):886–9. doi:10.1056/NEJMp1300458
35. Gallant JE, Mehta SH, Sugarman J. Universal antiretroviral therapy for HIV infection: should US treatment guidelines be applied to resource-limited settings? Clin Infect Dis (2013) 57(6):884–7. doi:10.1093/cid/cit382
36. Rodger AJ, Sabin CA. How have guidelines on when to start antiretroviral therapy affected survival of people living with HIV infection? Curr Opin HIV AIDS (2016) 11(5):487–91. doi:10.1097/COH.0000000000000307
37. Haberer JE, Sabin L, Amico KR, Orrell C, Galarraga O, Tsai AC, et al. Improving antiretroviral therapy adherence in resource-limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc (2017) 20(1):21371. doi:10.7448/IAS.20.1.21371
38. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: a meta-analysis. Clin Infect Dis (2015) 60(7):1120–7. doi:10.1093/cid/ciu1137
39. Hatano H, Yukl SA, Ferre AL, Graf EH, Somsouk M, Sinclair E, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog (2013) 9(10):e1003691. doi:10.1371/journal.ppat.1003691
40. Eholie SP, Vella S, Anglaret X. Commentary: antiretroviral therapy initiation criteria in low resource settings – from ‘when to start’ to ‘when not to start’. AIDS (2014) 28(Suppl 2):S101–4. doi:10.1097/QAD.0000000000000237
41. Alemnji G, Chase M, Branch S, Guevara G, Nkengasong J, Albalak R. Improving laboratory efficiency in the Caribbean to attain the World Health Organization HIV treat all recommendations. AIDS Res Hum Retroviruses (2017). doi:10.1089/AID.2017.0158
42. Granich R, Williams B, Montaner J, Zuniga JM. 90-90-90 and ending AIDS: necessary and feasible. Lancet (2017) 390(10092):341–3. doi:10.1016/S0140-6736(17)31872-X
Keywords: HIV-infected adults, CD4+ T cell, early therapy, mortality, meta
Citation: Song A, Liu X, Huang X, Meyers K, Oh D-Y, Hou J, Xia W, Su B, Wang N, Lu X, Xia H, Yang X, Chen H and Wu H (2018) From CD4-Based Initiation to Treating All HIV-Infected Adults Immediately: An Evidence-Based Meta-analysis. Front. Immunol. 9:212. doi: 10.3389/fimmu.2018.00212
Received: 23 September 2017; Accepted: 25 January 2018;
Published: 13 February 2018
Edited by:
Aurelio Cafaro, Istituto Superiore di Sanità, ItalyReviewed by:
Paul Urquhart Cameron, University of Melbourne, AustraliaSeema N. Desai, Rush University, United States
Copyright: © 2018 Song, Liu, Huang, Meyers, Oh, Hou, Xia, Su, Wang, Lu, Xia, Yang, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Chen, chenhui@ccmu.edu.cn;
Hao Wu, whdoc@sina.com
†These authors have contributed equally to this work.
 Aixin Song
Aixin Song Xinchao Liu
Xinchao Liu Xiaojie Huang
Xiaojie Huang Kathrine Meyers3
Kathrine Meyers3 Jianhua Hou
Jianhua Hou Bin Su
Bin Su Ni Wang
Ni Wang Xiaofan Lu
Xiaofan Lu Huan Xia
Huan Xia Xiaodong Yang
Xiaodong Yang Hui Chen
Hui Chen Hao Wu
Hao Wu