Effects of dietary Galla Chinensis tannin supplementation on immune function and liver health in broiler chickens challenged with lipopolysaccharide
- 1Key Laboratory of Efficient Utilization of Non-grain Feed Resources (Co-construction by Ministry and Province), Ministry of Agriculture and Rural Affairs, Shandong Provincial Key Laboratory of Animal Biotechnology and Disease Control and Prevention, Department of Animal Science and Veterinary Medicine, Shandong Agricultural University, Tai'an, China
- 2Animal Husbandry Development Center of Changyi City, Weifang, China
- 3Agricultural and Rural Comprehensive Service Center of Bincheng District, Binzhou, China
- 4College of Life Sciences, Shandong Agricultural University, Tai'an, China
Herein, Galla Chinensis tannin (GCT) was examined for its influence on preventing lipopolysaccharide (LPS)-induced liver damage in broiler chickens. Approximately 486 one-day-old healthy broilers were randomly allocated to 3 treatment groups (control, LPS, and LPS + GCT). The control and LPS groups were fed a basal diet and the LPS+GCT group was fed the basal diet supplemented with 300 mg/kg GCT. LPS was intraperitoneally injected (1 mg/kg body weight BW) in broilers in the LPS and LPS+GCT groups at 17, 19, and 21 days of age. The results manifested that dietary GCT addition attenuated LPS-induced deleterious effects on serum parameters and significantly increased serum immunoglobulin and complement C3 concentrations relative to the control and LPS groups. Dietary supplementation of GCT inhibited LPS-induced increase in broiler hepatic inflammatory cytokines, caspases activities, and TLR4/NF-κB pathway-related gene mRNA expression. Therefore, 300 mg/kg GCT addition to the diet improved the immune function of broilers and inhibit liver inflammation by blocking the TLR4/NF-κB pathway. Our findings provide support for the application of GCT in poultry production.
Introduction
The liver performs a multitude of functions, including immune activity, detoxification, phagocytizing bacteria, and nutrient metabolism. Its health is pertinent and indispensable to livestock production (1, 2). Contemporarily, in the poultry industry, intensive production has resulted in their confinement to limited spaces, exacerbating the risk of microbial infection. Substances absorbed from the gut are transported to the liver via the portal vein, making it the first barrier against enteric pathogenic microorganisms and their by-products (3–5). Liver inflammatory injury is a common clinical disease due to insensitive poultry production (6–8).
Tannins are naturally occurring polyphenolic compounds found in high concentrations in pasture, shrub, grain, herbal, and fruit species (9, 10). Depending on the chemical structure, tannins can be divided into hydrolyzable tannins (HT) and concentrated tannins (CT) (11). Tannins have exerted positive effects on growth performance, gut morphology, and antioxidant activity in broilers under normal and stressful conditions in previous studies (12–15). Galla Chinensis has long been used as a traditional medicine in China, and its main bioactive component is HT, gallotannins (16). Recently, 300 mg/kg Galla Chinensis tannin (GCT) addition in the broiler diet was shown to improve growth performance and immune functions (17). Moreover, Zhang et al. (18) also showed that 250 and 500 mg/kg GC improved growth performance and antioxidant capacity, along with protecting gut health in AFB1-challenged broilers to an extent. Whether supplementing GCT in the diet can alleviate liver injury under inflammatory conditions in broiler chickens remains unknown.
As a by-product of microbes, lipopolysaccharide (LPS) is a cell wall component of Gram-negative bacteria. LPS activates acute immune responses and induces systemic dysfunction via generating proinflammatory cytokines that can inhibit innate immune receptors expressions and downregulate immunoglobulin synthesis (19). Upon recognition of LPS by the liver immune system, cytokines are generated, which in turn result in liver inflammation (20, 21). Huang et al. (22) have indicated that intraperitoneal injection of LPS can cause acute liver injury and alter hepatic structure and function by regulating toll-like receptor 4 (TLR4) signaling and its downstream molecules along with specific cytokines in broilers.
Therefore, this study aimed to investigate the effect of adding 300 mg/kg GCT in the diet on alleviating systemic and liver inflammatory responses in broiler chickens challenged by LPS. Our findings provide useful insights for the application of GCT in poultry production.
Materials and methods
Experimental design and management
A total of 486 1-day-old Arbor Acres (AA) broiler chickens with uniform body weight (48.35 ± 0.41g) were chosen and randomly allocated to 3 treatment groups, each of which included 6 replicates per treatment (27 chickens in each replicate), for a 21-day study. The three treatment groups were control (CON), LPS, and LPS+GCT. Broilers in CON and LPS groups were fed a basal diet and those in the LPS+GCT group were fed a basal diet supplemented with 300 mg/kg GCT (17). The LPS (E. coli L2880; Sigma-Aldrich, MO, USA) was intraperitoneally injected (1 mg/kg body weight BW) in broilers in the LPS and LPS+GCT groups at 17, 19, and 21 days of age, while an equivalent amount of sterile saline solution was injected in the CON group intraperitoneally (23). The basal diet (Table 1) was formulated to provide nutrients that met or exceeded the needs of the broilers as recommended by the National Research Council (24). Microencapsulated GCT products with a net 40% TA content were produced by Wufeng Chicheng Biotechnology Co., Ltd., Yichang, Hubei, China. During the experimental period, all chickens were raised in standard chicken coops with free access to water and food. The test site and chicken house were cleaned and sanitized daily, and normal disinfection was conducted. The test house was well-ventilated and maintained at the desired temperature. Strict anti-epidemic and disinfection measures were implemented during the trial. On the 7th and 14th days of the experiment, the birds were vaccinated with the Newcastle disease vaccine and infectious bursal disease vaccine, respectively.
Samples collection
Three hours after LPS or saline solution injection at 21 days of age (23), one broiler in each replicate was chosen and 5 mL of wing vein blood samples were collected using a coagulation vacuum container tube. The sera were obtained by centrifugation at 4,000 × g for 15 min at 4°C and stored at −80°C until further analysis. Subsequently, the broilers were euthanized and slaughtered for collecting liver samples. Initially, a part of the liver samples was quickly frozen with liquid nitrogen and stored at −80°C until further analysis, while another portion was fixed for 24 h in a 4% paraformaldehyde solution.
Determination of serum biochemical parameters and inflammatory cytokines concentrations
The commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) were used to detect alanine aminotransferase (ALT), total protein (TP), low-density lipoprotein (LDL), albumin (ALB), total cholesterol (TCHO), urea nitrogen (UREA), triglyceride (TG), high-density lipoprotein (HDL), and glucose (GLU) concentrations in the sera on the Roche Cobus-Mira-Plus platform (Roche Diagnostic System Inc., USA) (25). The levels of inflammatory cytokines including tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-4, IL-6, and IL-10 were examined using chicken-specific ELISA kits (Jiangsu Meimian Industrial Co., Ltd, Jiangsu, China) and all experimental steps were conducted following the corresponding protocols described by Li et al. (23).
Determination of serum immunoglobulins and complements concentrations
ELISA kits (Jiangsu Meimian) were used to detect serum immunoglobulin (Ig)G, IgM, IgA, complement 3 (C3), and complement 4 (C4) levels following the detection procedures described previously (26).
Examination of hepatic histopathology
Liver samples were fixed in a 4% paraformaldehyde solution for 24 h, dehydrated with graded concentrations of ethyl alcohol, and embedded in liquid paraffin according to the standard histological procedure (17). After embedding the liver tissue in paraffin wax, 5 μm sections were cut and stained with hematoxylin and eosin (H&E). An Olympus digital microscope (Olympus BX51, Tokyo, Japan) was used to observe the liver sections.
Determination of inflammatory cytokines, health biomarkers and caspases levels in liver
ELISA kits (Jiangsu Meimian) were used to detect the levels of TNF-α, IL-1β, IL-6, IL-18, NOD-like receptor family pyrin domain containing 3 (NLRP3), heat shock 70 kDa protein (HSP70), caspase-1, caspase-3, and hepatic 8-hydroxy-2-deoxyguanosine(8-OHdG) following the corresponding protocols described by Chen et al. (27).
Determination of gene expression
Following the manufacturer's instructions, total RNA was extracted from the liver samples using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Subsequently, cDNA was synthesized using TaKaRa reverse transcription kit (TaKaRa, Dalian, China), and amplified by real-time quantitative polymerase chain reaction (RT-PCR) using the specific kit (TaKaRa, Dalian, China) following the protocol described by Li et al. (28). All primer sequences (Table 2) were based on the articles of Niu et al. (17), including those for TLR4, nuclear factor-kappa B (NF-κB), myeloid differentiation primary response 88 (MyD88), NLRP3, B-cell lymphoma-2 (Bcl-2), and Bcl-2-associated X (Bax), were synthesized by Sangon Bioengineering Ltd. (Shanghai, China). The 2−ΔΔCT method was used to evaluate the target mRNA expression relative to the levels of the internal reference gene, β-actin.
Statistical analysis
After the data were evaluated for normality by the Shapiro-Wilk's statistic (W > 0.05), one-way analysis of variance (ANOVA) with the SAS software (Version 9.4, Institute Inc., Cary, NC, US) was used to assess statistically significant differences among the three treatments. Multiple comparison analyses were performed by the least significant difference procedure. Data were presented using the mean ± standard error and plotted. Significant differences were considered at *p < 0.05, **p < 0.01, and ***p < 0.001, and #0.05 < p < 0.10 was regarded as a trend toward significance. “ns” represented non-significant differences.
Results
Effects of GCT supplementation on serum concentrations of biochemical parameters
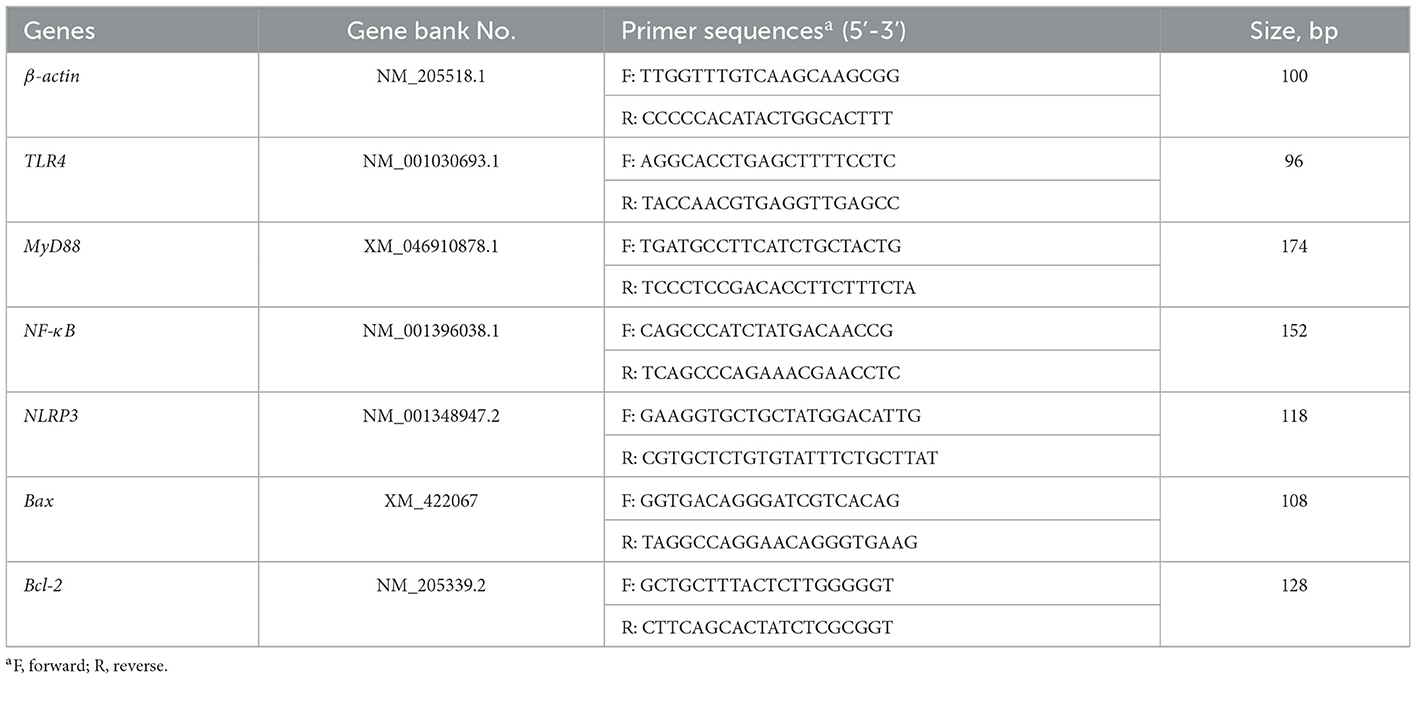
As displayed in Figure 1, compared to CON broilers, LPS broilers showed significantly higher serum LDL and ALT levels (p < 0.05), and 300 mg/kg GCT supplementation suppressed the LPS-induced increase in serum LDL and ALT levels to those observed in the CON group (p < 0.05). However, there were no significant differences in serum TP, ALB, HDL, TCHO, TG, GLU, and UREA contents in broilers among the three treatment groups (p >0.05).
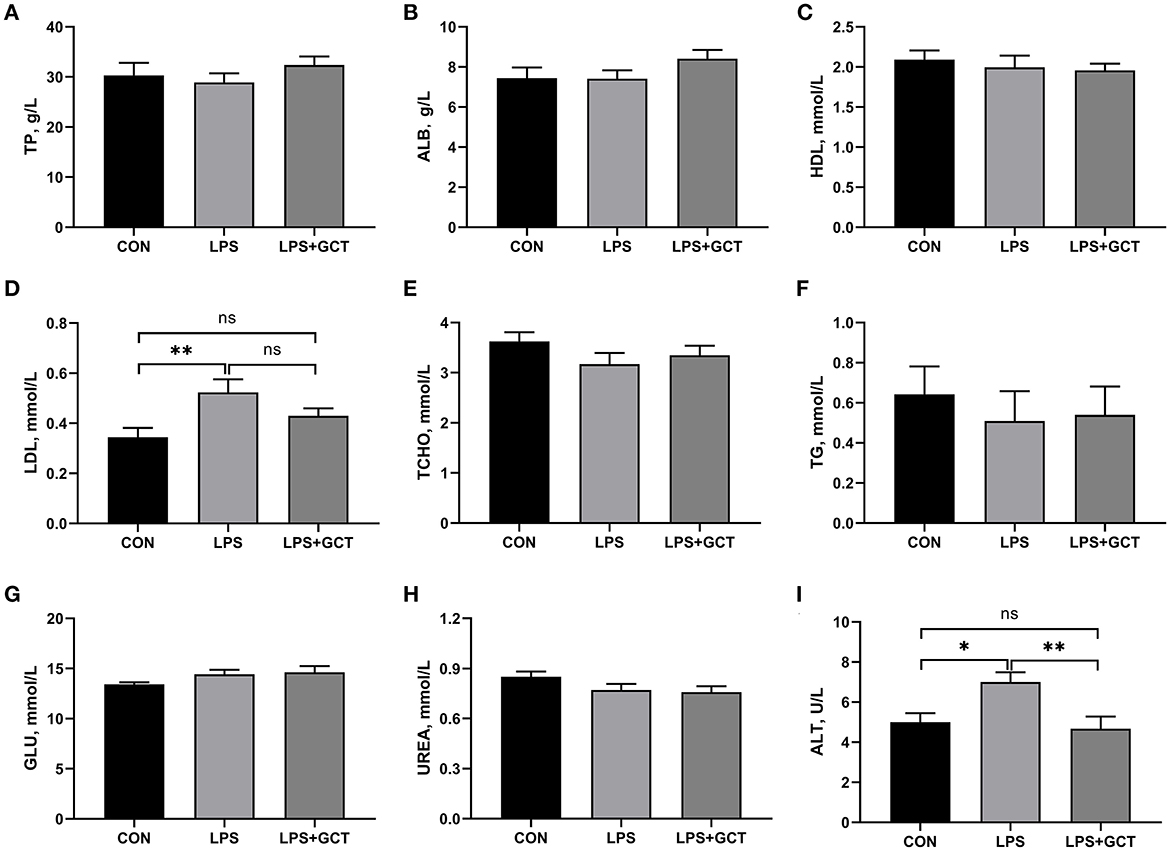
Figure 1. Effects of dietary Galla Chinensis tannin (GCT) supplementation on serum biochemical parameters of broilers under lipopolysaccharide (LPS) challenge. (A) Total protein (TP); (B) Albumin (ALB); (C) High-density lipoprotein (HDL); (D) Low-density lipoprotein (LDL); (E) Total-cholesterol (TCHO); (F) Triglyceride (TG); (G) Glucose (GLU); (H) Urea nitrogen (UREA); (I) Alanine transaminase (ALT). CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01; ns, non-significant.
Effects of GCT supplementation on serum inflammatory cytokines concentrations
Significantly enhanced serum TNF-α, IL-6, IL-1β, and IL-10 concentrations and decreased serum IL-4 levels were observed in LPS broilers relative to CON broilers (p < 0.05; Figure 2). The addition of GCT significantly reduced serum TNF-α, IL-1β, IL-6, and IL-10 concentrations and increased that of IL-4 in the LPS-challenged broilers (p < 0.05). The CON group showed significantly lower IL-1β level and significantly higher IL-10 concentration in serum than the LPS+GCT group (p < 0.05). However, there were no significant differences in serum TNF-α, IL-4, and IL-6 concentrations between CON and LPS+GCT groups (p > 0.05).
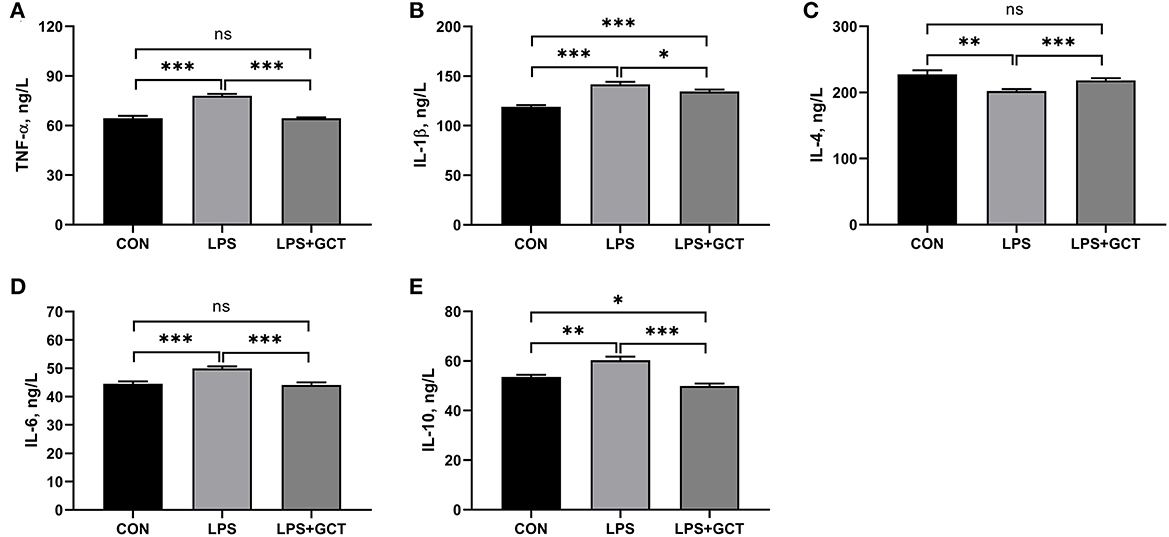
Figure 2. Effects of dietary Galla Chinensis tannin (GCT) supplementation on serum inflammatory factors of broilers under lipopolysaccharide (LPS)-challenge. (A) Tumor necrosis factor-α (TNF-α); (B) Interleukin-1β (IL-1β); (C) Interleukin-4 (IL-4); (D) Interleukin-6 (IL-6); (E) Interleukin-10 (IL-10). CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001; ns, non-significant.
Effects of GCT supplementation on serum levels of immunoglobulins and complements
As displayed in Figure 3, significantly reduced serum IgM, complement C3, complement C4 concentrations were observed in LPS broilers, and GCT supplementation significantly increased serum IgA, IgM, complement C3, and complement C4 concentrations in LPS-challenged broilers (p < 0.05). Besides, the LPS+GCT broilers showed significantly higher serum IgA, IgM, and complement C3 concentrations than the CON broilers (p < 0.05), and there was no significant difference in serum complement C4 between broilers in CON and LPS+GCT groups (p > 0.05).
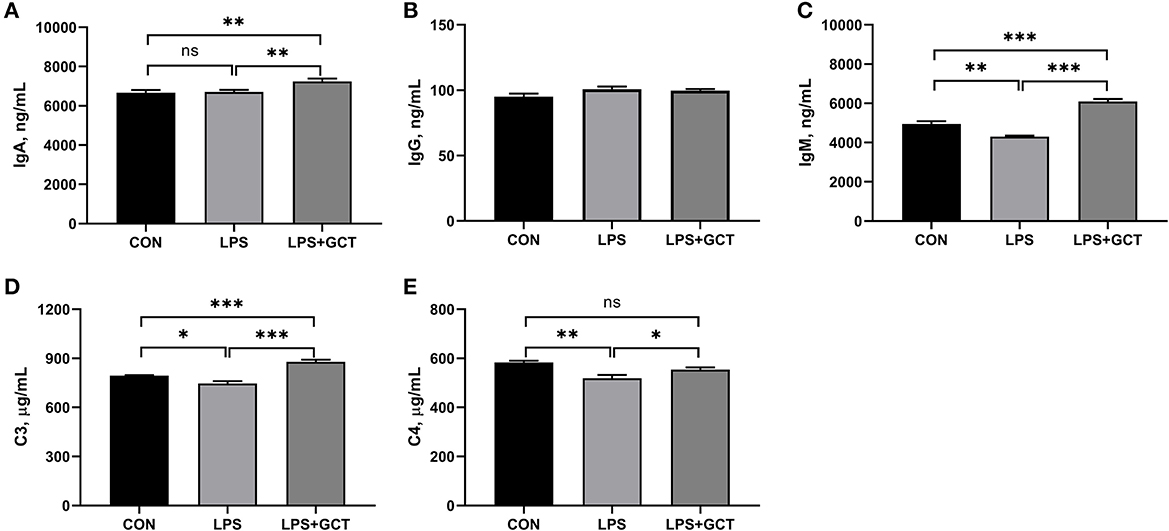
Figure 3. Effects of dietary Galla Chinensis tannin (GCT) supplementation on serum immunoglobulins and complements of broiler chickens under lipopolysaccharide (LPS)- challenge. (A) Immunoglobulin A (IgA); (B) Immunoglobulin G (IgG); (C) Immunoglobulin M (IgM); (D) Complement C3; (E) Complement C4. CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001; ns, non-significant.
Effects of GCT supplementation on hepatic histopathology
As shown in Figure 4, the hepatocytes in CON group were structurally complete and arranged in an orderly manner with minimal hyperchromatism, and showed normal development hepatic sinusoid but macrovesicular fatty change. The hepatocytes in LPS group showed obvious injury, including dissolved nucleus, cellular degeneration, and inflammatory cell infiltration with few lymphocytes. The hepatocytes in LPS+GCT group were structurally complete and arranged in an orderly manner with hyperchromatism, and also showed normal development hepatic sinusoid. However, LPS+GCT group showed fewer inflammatory cell infiltration and obvious cell divide and repair compared with LPS group.
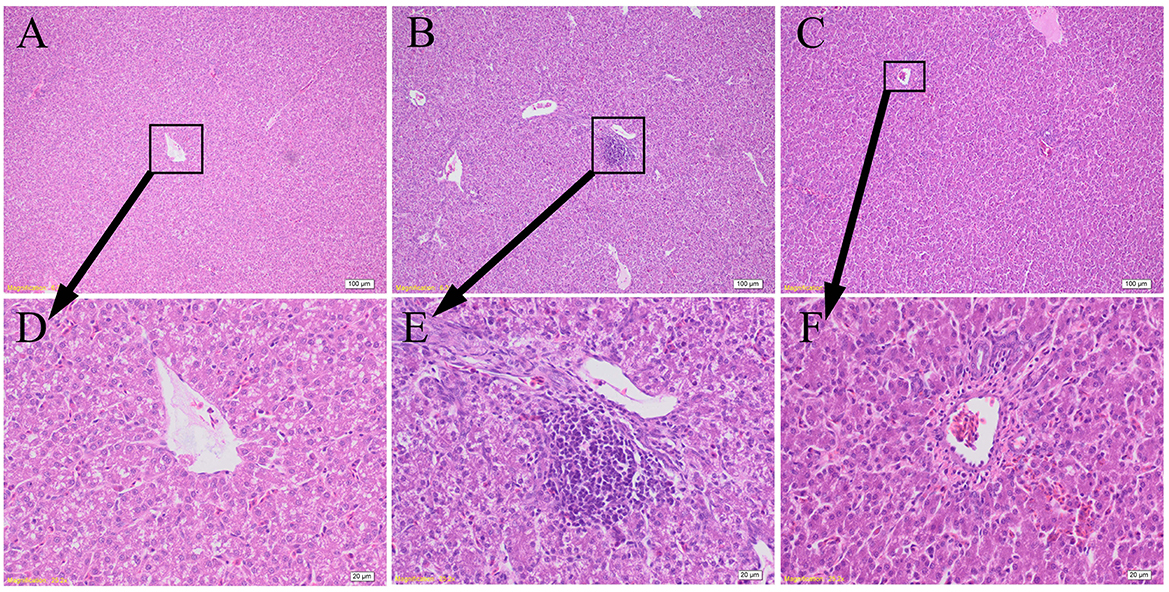
Figure 4. Effects of dietary Galla Chinensis tannin (GCT) supplementation on hepatic histopathology. (A–C) Hematoxylin and eosin photomicrographs of CON, LPS, and LPS+GCT obtained at 100× magnification, respectively; (D–F) Hematoxylin and eosin photomicrographs of CON, LPS, and LPS+GCT obtained at 400× magnification, respectively. CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS.
Effects of GCT supplementation on hepatic levels of inflammatory cytokines and caspases
As displayed in Figure 5, compared to the CON broilers, the LPS broilers showed markedly higher TNF-α, IL-6, and NLRP3 levels (p < 0.05), and tended to increase IL-18 level (p < 0.10) in their livers. However, 300 mg/kg GCT supplementation suppressed the LPS-induced raising in the hepatic levels of TNF-α, IL-6, and NLRP3 to those observed in the CON group (p < 0.05). Moreover, the concentrations of IL-1β and IL-18 in the LPS+GCT group were markedly lower than those in the LPS group (p < 0.05), and IL-18 level was notably lower in the LPS+GCT group than in the CON group (p < 0.05).
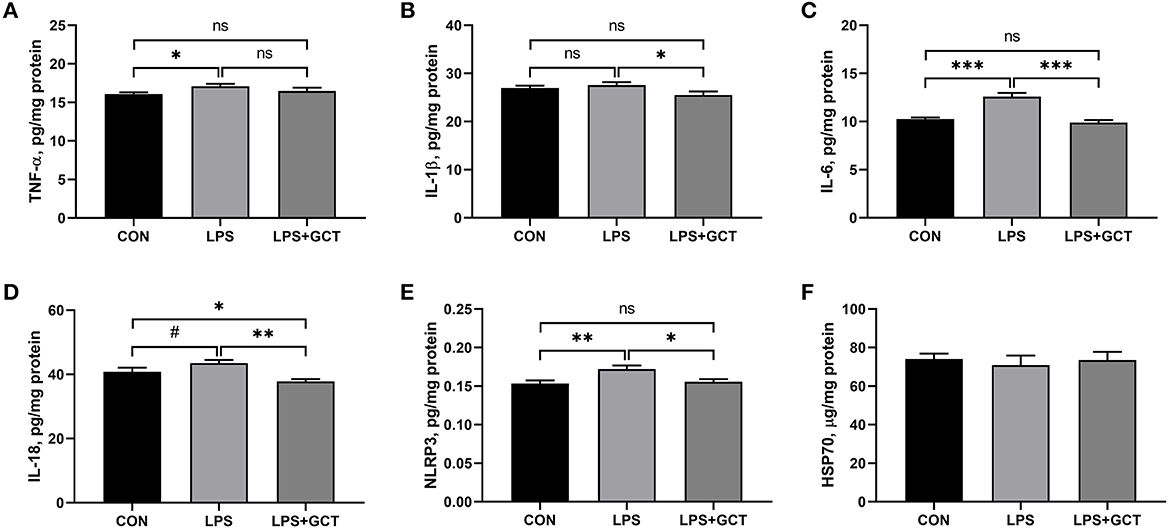
Figure 5. Effects of dietary Galla Chinensis tannin (GCT) supplementation on hepatic inflammatory cytokines of broilers under lipopolysaccharide (LPS)-challenge. (A) Tumor necrosis factor-α (TNF-α); (B) Interleukin-1β (IL-1β); (C) Interleukin-6 (IL-6); (D) Interleukin-18 (IL-18); (E) NLRs family pyrin domain containing 3 (NLRP3); (F) Heat shock 70 kDa protein (HSP70). CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, #0.05 < p < 0.10; ns, non-significant.
Effects of GCT supplementation on the hepatic caspases and 8-OHDG levels
Hepatic caspases and 8-OHDG levels are displayed in Figure 6. Compared to the CON group, LPS administration markedly elevated the caspase-1, caspase-3, and 8-OHDG levels in the livers of broilers (p < 0.05). Dietary 300 mg/kg GCT addition significantly decreased hepatic caspase-1 and caspase-3 activities in the liver compared to the LPS broilers (p < 0.05). Moreover, the LPS+GCT broilers showed significantly lower caspase-3 activity (p < 0.05), and tended to have higher caspase-1 activity in the liver compared to the CON broilers (p < 0.10).
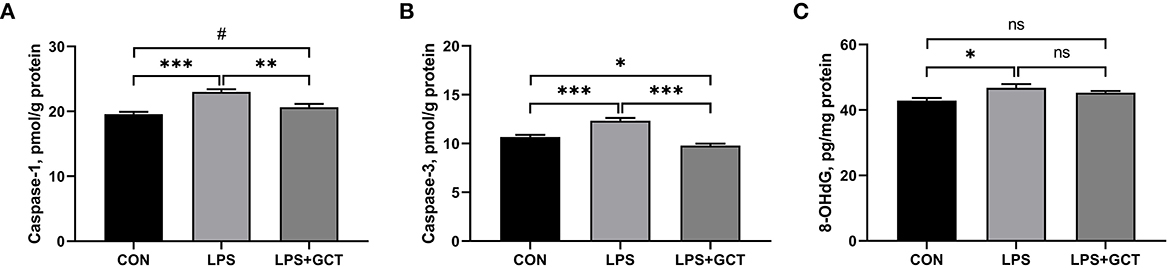
Figure 6. Effects of dietary Galla Chinensis tannin (GCT) supplementation on hepatic caspases levels of broilers under lipopolysaccharide (LPS)-challenge. (A) Caspase-1; (B) Caspase-3; (C) 8-hydroxy-2-deoxyguanosine (8-OHdG). CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, #0.05 < p < 0.10; ns, non-significant.
Effects of GCT supplementation on hepatic genes expressions
Inflammatory pathway gene expressions in the liver are displayed in Figure 7. Compared to the CON broilers, the LPS broilers showed significantly up-regulated mRNA expressions of TLR4, NF-κB, and NLRP3 (p < 0.05), and tended to have an increased MyD88 mRNA expression (p < 0.10) in the liver. However, 300 mg/kg GCT supplementation suppressed LPS-induced increase in hepatic MyD88, NF-κB, and NLRP3 mRNA expressions to those observed in the CON group (p < 0.05). Moreover, hepatic TLR4 expression of LPS+GCT broilers tended to be lower than that of LPS broilers and showed no statistically significant difference compared with the CON broilers (p > 0.05).
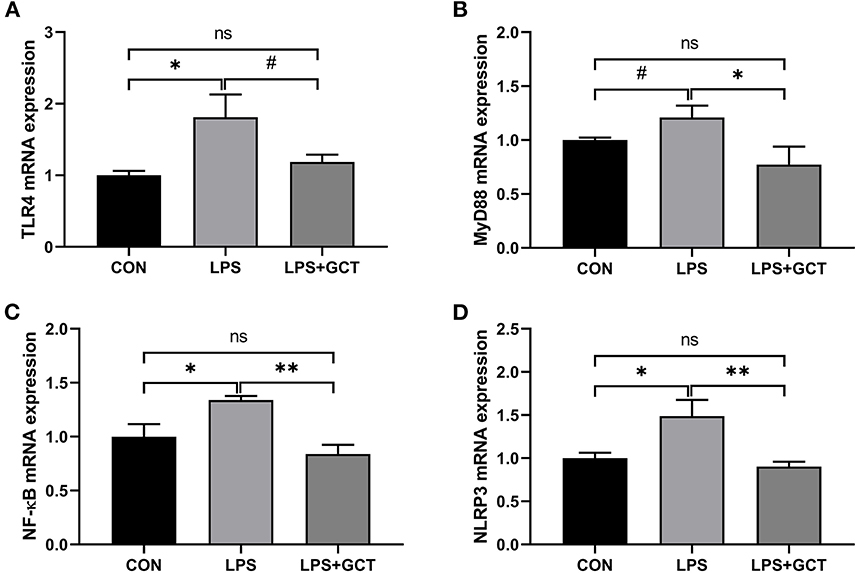
Figure 7. Effects of dietary Galla Chinensis tannin (GCT) supplementation on expressions of inflammatory pathway genes in liver of broilers under lipopolysaccharide (LPS)-challenge. (A) Toll-like Receptor 4 (TLR4); (B) Myeloid differentiation primary response 88 (MyD88); (C) Nuclear factor-kappa B (NF-κB); (D) NLRs family pyrin domain containing 3 (NLRP3). CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). *p < 0.05, **p < 0.01, #0.05 < p < 0.10; ns, non-significant.
Hepatic apoptosis-related gene expressions are shown in Figure 8. No significant differences were observed in mRNA expression of pro-apoptosis Bax and anti-apoptosis Bcl-2 among all the groups (p > 0.05). However, Bax/Bcl-2 ratio in the LPS group tended to be higher than that in the CON group (p < 0.10), and was markedly higher than that in the LPS+GCT (p < 0.05) group; there was no significant difference in Bax/Bcl-2 ratio between the CON group and LPS+GCT group (p > 0.05).
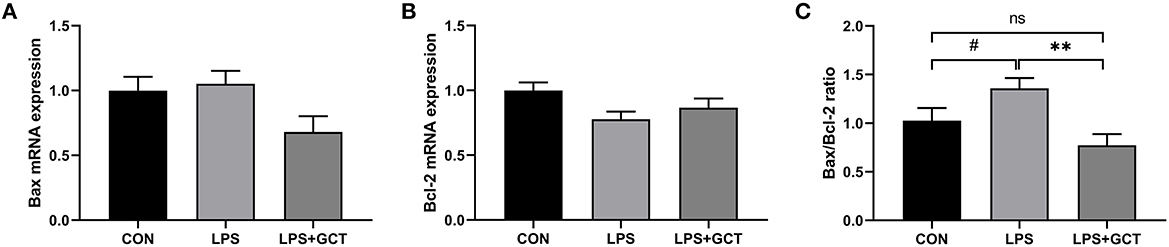
Figure 8. Effects of dietary Galla Chinensis tannin (GCT) supplementation on apoptosis genes in livers of broilers under lipopolysaccharide (LPS)-challenge. (A) BCL2-Associated X (Bax); (B) B-cell lymphoma-2 (Bcl-2); (C) Bax/Bcl-2 ratio. CON, fed on a basal diet and intraperitoneally injected with saline solution; LPS, fed on a basal diet and intraperitoneally injected with LPS; LPS+GCT, fed on a basal diet supplemented with 300 mg/kg GCT and intraperitoneally injected with LPS. Values are presented as mean ± standard error (n = 6). **p < 0.01, #0.05 < p < 0.10; ns, non-significant.
Discussion
Previous studies have shown that LPS injection can cause liver injury and dysfunction in broilers (22). When a liver injury occurs, ALT in hepatocytes enters the blood circulation, increasing serum ALT levels (29). Therefore, ALT is usually regarded as a specific indicator of liver injury in clinical settings (30). In the present study, the results showed that the ALT activity of the GCT group was similar to the serum of broilers in the CON group but significantly higher in the LPS group. The liver is one of the main sites of complement synthesis. Complement is an enzymatically active glycoprotein that plays a key role in the body's immune regulation, and liver damage is usually associated with decreased serum levels of complements (31). Complement C3 deficiency results in decreased liver regeneration (32). We found that GCT supplementation significantly increased serum C3 concentration compared to the CON and LPS groups, and inhibited the decrease in serum C4 concentration induced by the LPS challenge. LPS administration significantly increased hepatic 8-OHdG concentration compared to the CON group. 8-OHdG is considered as the most critical indicator of DNA damage because it can result in the formation of 8-OHdG in the nucleotide pool during DNA replication (33). The above results demonstrated that the animal model of LPS-induced liver injury was successfully established, as evidenced by the hepatic histopathology. Supplementation with GCT in the diet suppressed the adverse changes in serum ALT and complement levels along with hepatic 8-OHdG levels, showing that GCT supplementation benefited the amelioration of LPS-induced liver injury in broilers.
Immune function decline is associated with the development of liver diseases (34). In modern breeding, intensive farming systems cause drastic metabolism in broiler chickens during growth and development, resulting in decreased immune function (25). Our findings showed that the LPS challenge decreased serum IgM concentration but GCT addition increased serum IgA and IgM concentrations in these broilers compared to the other groups. Immunoglobulin acts as a major anti-infective component in the blood and serves as the first line of host defense against infections (35). Previous study in IPEC-J2 cells also indicated that LPS decreased IgA, IgG, and IgM levels (36). Niu et al. (17) showed that GCT addition in the broiler diet elevated the serum levels of IgG, IgM, and complements. Therefore, enhanced immune function by GCT supplementation might contribute to alleviating LPS-induced liver injury.
Increasing evidence shows that inflammatory response is a major important inducement for liver injury (37). LPS challenge can increase liver contents of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6, and result in inflammatory injuries to broilers (22, 38). In the present study, LPS administration induced an increase in the concentrations of TNF-α, IL-6, IL-1β, and IL-10, while reducing the serum IL-4 content. Moreover, the concentrations of TNF-α, IL-6, and IL-18 in the liver were elevated. TNF-α, IL-1β, IL-6, and IL-18 are often used as markers of inflammatory responses and are involved in the induction of liver diseases (39, 40). The main biological roles of IL-4 and IL-10 are inhibition of the production and release of pro-inflammatory cytokines, promoting the proliferation and differentiation of B cells, and enhancing the activity of macrophages (41, 42). However, IL-10 production can be also increased under low-grade inflammatory conditions for inducing antibody generation (26). Therefore, the LPS challenge resulted in a systemic and hepatic inflammatory response in this study. Our findings showed that GCT addition suppressed LPS-induced detrimental changes in inflammatory cytokines concentrations. A previous study in vitro also indicated that tannin addition could inhibit the production of pro-inflammatory cytokines, IL-1β and IL-6, in BV2 microglial cells (43). In vivo experiments in mice have shown that tannic acid promotes the reduction in TNF-α, IL-1β, and IL-6 contents (44). Furthermore, caspases play a key role in mediating inflammatory responses and cellular apoptosis (45). Dietary GCT supplementation inhibits LPS-induced increase in hepatic caspase-1 and caspase-3 activities of broilers. Caspase 1, an initiating caspase or the IL-1β converting enzyme, is required for apoptosis (46). Caspase-3 is an executioner caspase that causes apoptosis-related nuclear changes, including chromatin condensation (47). A high Bax/Bcl-2 ratio usually results in the activation of caspase-1 and caspase-3 in initiating pyroptosis and apoptosis, respectively, ultimately leading to organ damage (48). In this study, dietary GCT addition alleviated the LPS-induced increases in the Bax/Bcl-2 ratio. Importantly, dietary GCT could resist LPS-induced liver injury by inhibiting systemic and hepatic inflammatory responses.
To further investigate the possible mechanisms underlying the inhibition of LPS-induced broiler liver inflammatory injury by GCT, the gene expression levels of the TLR4/NF-κB pathway were quantified herein. TLR4, belonging to the pattern recognition receptor family of genes, plays an important role in activating the innate immune response, participating in the body's response to infection due to various pathogens (49). TLR4 can quickly trigger the activation and generation of several cytokines involved in regulating immunological and inflammatory responses by activating the NF-κB signaling pathway through the MyD88 protein (50, 51). Previous studies have shown that intraperitoneal injection of LPS can mediate acute liver injury by up-regulating TLR4 and its downstream molecules including MyD88, NF-κB, IL-1β, and TNF-α (22). Gastric administration of 20 and 40 mg/kg of pure tannic acid down-regulates the expression of NF-κB, IL-6, and TNF-α induced by arsenic trioxide in rats (52). In the present study, the LPS challenge up-regulated the expressions of TLR4/NF-κB pathway-related genes and dietary GCT supplementation alleviated LPS-induced increase in the hepatic mRNA expressions of TLR4, MyD88, NF-κB, and NLRP3. Taken together, GCT could suppress hepatic inflammatory responses likely by inhibiting the TLR4/NF-κB signaling pathway.
Conclusion
To sum up, our findings demonstrated that addition with 300 mg/kg GCT could alleviate the systemic and hepatic inflammatory responses in broilers challenged by LPS. These findings can facilitate the mechanistic understanding by which dietary GCT can promote liver development and enhance immune regulation, supporting the application of GCT in poultry production.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Shandong Agricultural University. The protocol for this experiment involving animals was in accordance with the guidelines of the Animal Care and Use Committee of Shandong Agricultural University (Ethics approval code: SDAUA-2021-019).
Author contributions
Conceptualization: PY, WY, and YLi. Methodology, data curation, and project administration: PY, JN, and YLiu. Software: WY, HX, YM, LH, and YLi. Validation, visualization, and supervision: LH and YLi. Formal analysis: PY, HX, YM, and LH. Investigation and resources: NJ, SJ, and XY. Writing—original draft preparation: PY. Writing—review and editing: YLi. Funding acquisition: WY and YLi. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong science and technology-based small and medium-sized enterprises innovation capacity improvement project (grant number: 2022TSGC12) and the Starting Research Fund from the Shandong Agricultural University (040/76598).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. (2009) 31:333–43. doi: 10.1007/s00281-009-0173-4
2. Knolle PA, Wohlleber D. Immunological functions of liver sinusoidal endothelial cells. Cell Mol Immunol. (2016) 13:347–53. doi: 10.1038/cmi.2016.5
3. Crispe IN. The liver as a lymphoid organ. AnnuRev Immunol. (2009) 27:147–63. doi: 10.1146/annurev.immunol.021908.132629
5. Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Carteni M, et al. Gut–liver axis: The impact of gut microbiota on nonalcoholic fatty liver disease. Nutr Metab Cardiovas. (2012) 22:471–6. doi: 10.1016/j.numecd.2012.02.007
6. Gao X, Xu J, Jiang L, Liu W, Hong H, Qian Y, et al. Morin alleviates aflatoxin b1-induced liver and kidney injury by inhibiting heterophil extracellular traps release, oxidative stress and inflammatory responses in chicks. Poult Sci. (2021) 100:101513. doi: 10.1016/j.psj.2021.101513
7. Wu Y, Li Q, Liu J, Liu Y, Xu Y, Zhang R, et al. Integrating serum metabolome and gut microbiome to evaluate the benefits of lauric acid on lipopolysaccharide- challenged broilers. Front Immunol. (2021) 12:759323. doi: 10.3389/fimmu.2021.759323
8. Yu Z, Guo F, Zhang Z, Luo X, Tian J, Li H. Protective effects of glycyrrhizin on LPS and amoxicillin/potassium clavulanate-induced liver injury in chicken. Pak Vet J. (2017) 37:13–8.
9. Jezierny D, Mosenthin R, Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Animal Feed Sci Tech. (2010) 157:111–28. doi: 10.1016/j.anifeedsci.2010.03.001
10. Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. (2018) 4:137–50. doi: 10.1016/j.aninu.2017.09.004
11. Buyukcapar H, Atalay A, Kamalak A. Growth performance of nile tilapia (oreochromis niloticus) fed with diets containing different levels of hydrolysable and condensed tannin. J Agr Sci Tech-Iran. (2011) 13:1045–51.
12. Schiavone A, Guo K, Tassone S, Gasco L, Hernandez E, Denti R, et al. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult Sci. (2008) 87:521–27. doi: 10.3382/ps.2007-00113
13. Xiong Y, Dong S, Zhao X, Guo K J, Gasco L, Zoccarato I. Gene expressions and metabolomic research on the effects of polyphenols from the involucres of castanea mollissima blume on heat-stressed broilers chicks. Poult Sci. (2016) 95:1869–80. doi: 10.3382/ps/pew170
14. Liu H W, Li K, Zhao J S, Deng W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J Anim Physiol Anim Nutr. (2018) 102:717–26. doi: 10.1111/jpn.12839
15. Liu HS, Mahfuz SU, Wu D, Shang QH, Piao XS. Effect of chestnut wood extract on performance, meat quality, antioxidant status, immune function, and cholesterol metabolism in broilers. Poult Sci. (2020) 99:4488–95. doi: 10.1016/j.psj.2020.05.053
16. Ren YY, Zhang XR, Li TN, Zeng YJ, Wang J, Huang QW. Galla Chinensis, a traditional chinese medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J Ethnopharmacol. (2021) 278,114247. doi: 10.1016/j.jep.2021.114247
17. Niu J, Wang Q, Jing C, Liu Y, Liu H, Jiao N, et al. Dietary Galla Chinensis tannic acid supplementation in the diets improves growth performance, immune function and liver health status of broiler chicken. Front Vet Sci. (2022) 9:1024430. doi: 10.3389/fvets.2022.1024430
18. Zhang ZF, Xi Y, Wang ST, Zheng LY, Qi Y, Guo SS, et al. Effects of Chinese gallnut tannic acid on growth performance, blood parameters, antioxidative status, intestinal histomorphology, and cecal microbial shedding in broilers challenged with aflatoxin b1. J Anim Sci. (2022) 100:skac099. doi: 10.1093/jas/skac099
19. Wu QJ, Zhou YM, Wu YN, Zhang LL, Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to lps in broiler chickens. Vet Immunol Immunop. (2013) 153:70–6. doi: 10.1016/j.vetimm.2013.02.006
20. Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, et al. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, egf and tlr4 signaling. Amino Acids. (2013) 45:513–22. doi: 10.1007/s00726-012-1295-x
21. Jiang J, Qi L, Lv Z, Jin S, Wei X, Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. (2019) 8:575. doi: 10.3390/antiox8120575
22. Huang XY, Ansari AR, Huang HB, Zhao X, Li NY, Sun ZJ, et al. Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling. BMC Immunol. (2017) 18:1–9. doi: 10.1186/s12865-017-0199-7
23. Li J, Liu Y, Niu J, Jing C, Jiao N, Huang L, et al. Supplementation with paraformic acid in the diet improved intestinal development through modulating intestinal inflammation and microbiota in broiler chickens. Front Microbiol. (2022) 13:975056. doi: 10.3389/fmicb.2022.975056
24. National Research Council. Nutrient Requirements of Poultry. Ninth Revised Edition. Washington, DC: The National Academies Press (1994). doi: 10.17226/2114
25. Liu Y, Li Y, Niu J, Liu H, Jiao N, Huang L, et al. Effects of dietary macleaya cordata extract containing isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth performance and liver health of broiler chickens. Front Vet Sci. (2022) 9:950174. doi: 10.3389/fvets.2022.950174
26. Chen J, Li F, Yang W, Jiang S, Li Y. Comparison of gut microbiota and metabolic status of sows with different litter sizes during pregnancy. Front Vet Sci. (2021) 8:793174. doi: 10.3389/fvets.2021.793174
27. Chen J, Li F, Yang W, Jiang S, Li Y. Supplementation with exogenous catalase from penicillium notatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol Spectrum. (2021) 9:e00654–00621. doi: 10.1128/Spectrum.00654-21
28. Li Y, Yang M, Zhang L, Mao Z, Lin Y, Xu S, et al. Dietary fiber supplementation in gestating sow diet improved fetal growth and placental development and function through serotonin signaling pathway. Front Vet Sci. (2022) 9:831703. doi: 10.3389/fvets.2022.831703
29. Gallego S, Esbri JM, Campos JA, Peco JD, Martin-Laurent F, Higueras P. Microbial diversity and activity assessment in a 100-year-old lead mine. J Hazard Mater. (2021) 410:124618. doi: 10.1016/j.jhazmat.2020.124618
30. Li Y, Zhao X, Jiang X, Chen L, Hong L, Zhuo Y, et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J Anim Sci. (2020) 98:skaa067. doi: 10.1093/jas/skaa067
31. Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The role of complement in liver injury, regeneration, and transplantation. Hepatology. (2019) 70:725–36. doi: 10.1002/hep.30508
33. Cadet J. Oxidative degradation pathways of cellular DNA: product formation and mechanistic insights. Free Radic Bio Med. (2014) 75:S2. doi: 10.1016/j.freeradbiomed.2014.10.598
34. Campisano S, La Colla A, Echarte SM, Chisari AN. Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases. Nutr Res Rev. (2019) 32:128–45. doi: 10.1017/S0954422418000239
35. Balan P, Sik-Han K, Moughan PJ. Impact of oral immunoglobulins on animal health-a review. Anim Sci J. (2019) 90:1099–110. doi: 10.1111/asj.13258
36. Zhao L, Li M, Sun K, Su S, Geng T, Sun H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from lps-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int J Biol Macromol. (2020) 155:1202–15. doi: 10.1016/j.ijbiomac.2019.11.088
37. Ramadori G, Moriconi F, Malik I, Dudas J. Physiology and pathophysiology of liver inflammation, damage and repair. J Physiol Pharmacol. (2008) 59:107–17.
38. Han H, Zhang J, Chen Y, Shen M, Yan E, Wei C, et al. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J Anim Sci. (2020) 98:skaa311. doi: 10.1093/jas/skaa311
39. Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun X, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. (2017) 8:547. doi: 10.3389/fphar.2017.00547
40. Qu J, Wang W, Zhang Q, Li S. Inhibition of lipopolysaccharide-induced inflammation of chicken liver tissue by selenomethionine via tlr4-nf-kappab-nlrp3 signaling pathway. Biol Trace Elem Res. (2020) 195:205–14. doi: 10.1007/s12011-019-01841-0
41. Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth F R. (2010) 21:331–44. doi: 10.1016/j.cytogfr.2010.09.002
42. Li X, Liu X, Tian L, Chen Y. Cytokine-mediated immunopathogenesis of hepatitis b virus infections. Clin Rev Allerg Immul. (2016) 50:41–54. doi: 10.1007/s12016-014-8465-4
43. Wu Y, Zhong L, Yu Z, Qi J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced bv2 microglial cells via inhibition of nf-kappab activation. Drug Dev Res. (2019) 80:262–8. doi: 10.1002/ddr.21490
44. de Veras BO, do Silva MV, Cabral Ribeiro PPC. Tannic acid is a gastroprotective that regulates inflammation and oxidative stress. Food Chem Toxicol. (2021) 156:112482. doi: 10.1016/j.fct.2021.112482
45. El-Hack MEA, El-Saadony MT, Salem HM, El-Tahan AM, Soliman MM, Youssef GBA, et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult Sci. (2022) 101:101696. doi: 10.1016/j.psj.2022.101696
46. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. (1992) 356:768–74. doi: 10.1038/356768a0
47. Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kägi D, et al. Essential contribution of caspase 3/cpp32 to apoptosis and its associated nuclear changes. Gene Dev. (1998) 12:806–19. doi: 10.1101/gad.12.6.806
48. Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. (1999) 59:1693s−700s.
49. Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S. Toll like receptor 4 and hepatocellular carcinoma; a systematic review. Life Sci. (2017) 179:80–7. doi: 10.1016/j.lfs.2017.04.025
50. Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. Tlr4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines. (2017) 5:34. doi: 10.3390/vaccines5040034
51. Kong F, Ye B, Lin L, Cai X, Huang W, Huang Z. Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB signaling in PMA-stimulated THP-1 monocytes. Biomed Pharmacother. (2016) 82:167–72. doi: 10.1016/j.biopha.2016.04.043
Keywords: Galla Chinensis, tannic acid, broiler, liver health, lipopolysaccharide
Citation: Yuan P, Xu H, Ma Y, Niu J, Liu Y, Huang L, Jiang S, Jiao N, Yuan X, Yang W and Li Y (2023) Effects of dietary Galla Chinensis tannin supplementation on immune function and liver health in broiler chickens challenged with lipopolysaccharide. Front. Vet. Sci. 10:1126911. doi: 10.3389/fvets.2023.1126911
Received: 18 December 2022; Accepted: 30 January 2023;
Published: 14 February 2023.
Edited by:
Bruno Solis-Cruz, National Autonomous University of Mexico, MexicoReviewed by:
Zhenqiang You, Zhejiang Academy of Medical Sciences, ChinaAdelijiang Wusiman, Xinjiang Agricultural University, China
Copyright © 2023 Yuan, Xu, Ma, Niu, Liu, Huang, Jiang, Jiao, Yuan, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiren Yang,  wryang@sdau.edu.cn; Yang Li,
wryang@sdau.edu.cn; Yang Li,  li_yang@sdau.edu.cn
li_yang@sdau.edu.cn
 Peng Yuan
Peng Yuan Haitao Xu2
Haitao Xu2  Libo Huang
Libo Huang Ning Jiao
Ning Jiao Weiren Yang
Weiren Yang Yang Li
Yang Li