Worldwide Presence and Features of Flea-Borne Rickettsia asembonensis
- 1Viral and Rickettsial Diseases Department, Naval Medical Research Center, Silver Spring, MD, United States
- 2Department of Biology, Shippensburg University, Shippensburg, PA, United States
- 3Department of Preventative Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD, United States
Rickettsia asembonensis, the most well-characterized rickettsia of the Rickettsia felis-like organisms (RFLO), is relatively unknown within the vector-borne diseases research community. The agent was initially identified in peri-domestic fleas from Asembo, Kenya in an area in which R. felis was associated with fever patients. Local fleas collected from domestic animals and within homes were predominately infected with R. asembonensis with <10% infected with R. felis. Since the identification of R. asembonensis in Kenya, it has been reported in other locations within Africa, Asia, the Middle East, Europe, North America, and South America. With the description of R. asembonensis-like genotypes across the globe, a need exists to isolate these R. asembonensis genotypes in cell culture, conduct microscopic, and biological analysis, as well as whole genome sequencing to ascertain whether they are the same species. Additionally, interest has been building on the potential of R. asembonensis in infecting vertebrate hosts including humans, non-human primates, dogs, and other animals. The current knowledge of the presence, prevalence, and distribution of R. asembonensis worldwide, as well as its arthropod hosts and potential as a pathogen are discussed in this manuscript.
Introduction
Rickettsia asembonensis is a Gram negative, obligate intracellular bacteria of the order Rickettsiales and family Rickettsiaceae (1). Among Rickettsia spp. with validly published names, it is most closely related to R. felis (Table 1) (4–6, 8, 9, 11, 13–15, 17–19, 30). However, among incompletely characterize rickettsiae, R. asembonensis genetically groups with other R. felis-like organisms (RFLO). The RFLOs are genetically related to R. felis but consist of a unique group of rickettsiae that are associated with various arthropods including fleas, ticks, mites, and tsetse flies for which limited knowledge of their biology and pathogenicity is available (3, 16, 31). Unfortunately, the genetic information of the majority of RFLOs in the GenBank database is fragmentary. Of the RFLOs described, only R. asembonensis (32) and “Candidatus Rickettsia senegalensis” (3) have been cultured (from C. felis) and characterized.
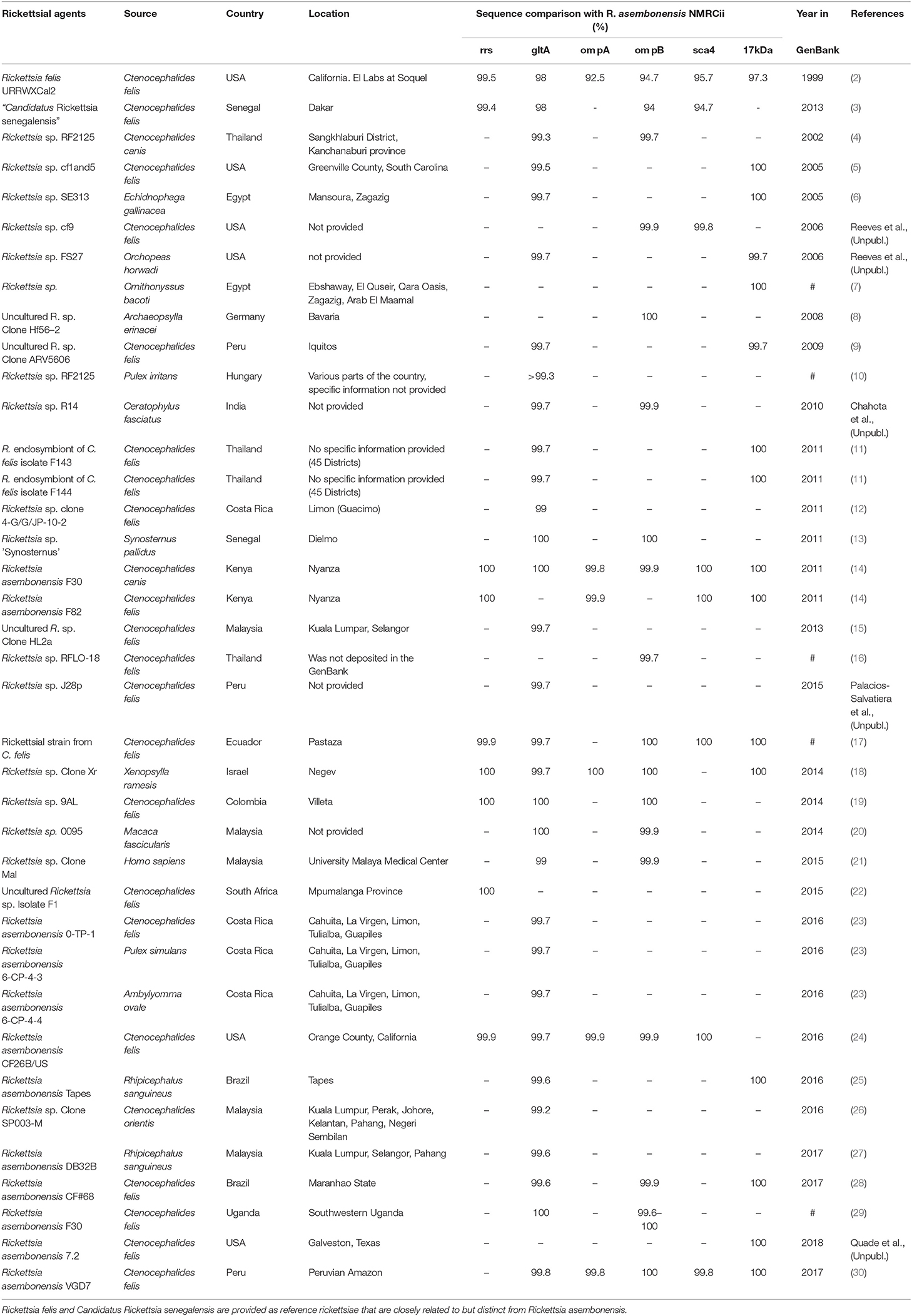
Table 1. Worldwide distribution of Rickettsia asembonensis and closely related, incompletely characterized rickettsiae.
Other flea-borne rickettsiae include, besides the aforementioned R. felis and “Ca. R. senegalensis,” Rickettsia typhi, a member of the typhus group of rickettsiae (TGR). R. typhi is the causative agent of murine typhus, a febrile disease that is found throughout the world. R. typhi is vectored by various flea species-especially X. cheopis, but also other Xenopsylla species such as X. astia and X. brazilliensis (33, 34), Synosternus pallidus, and rarely, but importantly, Ctenocephalides felis the common cat flea that readily parasitizes cats, opossums, and other domestic, peri-domestic, and wild animals. C. felis is believed to be capable of hosting R. typhi and to vector murine typhus in areas outside the traditional range of rat fleas and rats (35, 36).
R. felis, R. asembonensis, and “Ca. R. senegalensis” fall within the spotted fever group rickettsiae (SFGR) that genetically clusters within the transitional group of rickettsiae (37). R. felis is associated with flea-borne spotted fever (38, 39) and the pathogenicity of R. asembonensis and “Ca. R. senegalensis” is currently unknown. These three agents have worldwide distribution, are often sympatric and most often found parasitizing cat and dog fleas (3, 4, 14, 38, 40, 41).
“Candidatus R. senegalensis” was first described in C. felis fleas from Senegal (3) and an agent believed to be “Ca. R. senegalensis”-like (Rickettsia sp. RF31) had been detected previously in C. felis near the Thailand-Myanmar border (4). A very close genetic relationship (99.9% based on gltA gene sequence) between Rickettsia sp. RF31 and the latter is notable (3). “Ca. R. senegalensis” is distinct from, but can be sympatric with, R. felis and R. asembonensis (40). It has worldwide distribution but is not reported as often as R. felis or R. asembonensis. Reports of its molecular presence in cat tissues suggests it may be able to infect vertebrate animals (41).
History of Rickettsia asembonensis
Incompletely characterized rickettsiae with various identities most closely related to R. asembonensis populated the literature in the early 2000s (Table 1). These agents were detected by molecular techniques [i.e., PCR, nested PCR (nPCR), and/or quantitative real-time PCR (qPCR)] and then characterized by sequencing different size fragments of one or more commonly used gene targets (rrs, gltA, ompA, ompB, sca4, or the 17 kDa antigen gene). The first agent, referred to as Rickettsia sp. RF2125, was detected in Ctenocephalides canis in western Thailand near the Myanmar border (4). The agent was characterized by the sequence of a 1,171 bp fragment of the gltA that showed the rickettsial agent to be unique but most closely related to R. felis (4). The sequence of a 790 bp fragment of ompB (JX183538) from the original Rickettsia sp. RF2125 DNA preparation was obtained at that same time as the gltA but was not reported in the original article (4). It was reported in 2013 (14). We believe that RF2125 may have been the first detection of R. asembonensis or a very similar agent. Additional reports of R. asembonensis or an agent closely related to it continued to occur worldwide (Figure 1) shortly thereafter including: Rickettsia sp. cf1 and 5, USA (5); Rickettsia sp. SE313, Egypt (6); Rickettsia sp. Hf56-2, Germany (8); Rickettsia sp. ARV5606, Peru (9); and Rickettsia sp. Synosternus, Senegal (13). These partially characterized agents were described prior to our complete characterization of R. asembonensis (1). These agents are summarized along with R. asembonensis to include their distribution, vector hosts, and genetic characterization (see Table 1).
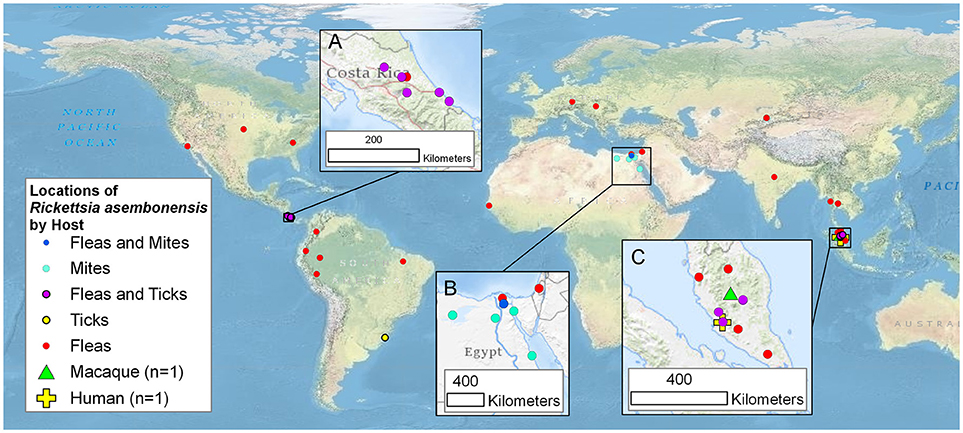
Figure 1. Worldwide mapof the locations of Rickettsia asembonensis, genetically similar rickettsiae, and associated vertebrate and invertebrate hosts. Inset maps are for points in (A): Costa Rica; (B): Egypt and Israel; and (C): Malaysia. This map was created using ArcGIS® software by Esri. ArcGIS® and ArcMap™ which are the intellectual property of Esri and are used herein under license. Copyright © Esri.
R. asembonensis was initially described as an unknown Rickettsia sp. detected in various flea species (i.e., C. felis, C. canis, Echidnophaga gallinacean, X. cheopis, and Pulex irritans) collected from various domestic animals (i.e., dogs, cats, and rodents) and houses (by light traps) in Asembo, Kisumu, in western Kenya during an epidemiologic surveillance study (14). This study was conducted concurrently with a fever study in which the presence of R. felis was identified in 7.2% of febrile patients (42). The initial molecular characterization of the R. asembonensis agent was accomplished utilizing a multilocus sequence typing (MLST) algorithm (43). Prevalence of this new agent (~91.7%) in collected fleas was found to be distinctly different from that of R. felis (8.3%) (14).
Subsequently, additional fleas collected from the same hosts and locations within the livestock-owning compounds in Asembo were processed for rickettsial culture. The new agent, Rickettsia asembonensis NMRCii, was successfully cultured from a pool of five individual flea triturate cultures isolated from C. canis and C. felis fleas obtained from domestic dogs. The cultures were initially grown in S2 and subsequently in C6/36 cell lines at 25°C (32), but not in Vero and L929 cell lines or embryonated chicken eggs incubated at 37°C (1).
The culture of R. asembonensis NMRCii was analyzed by microscopy, including Diff-Quik/acridine orange staining and transmission electron microscopy (32). The R. asembonensis were observed in the Drosophila S2 and Aedes albopictus C6/36 cells lines as early as 3 days post-infection, and could be observed at multiple time points throughout the average culture time of 40–45 days (32). Rickettsiae were observed both intra- and extracellularly at time points ranging from 15 to 30 days throughout the course of the continuous culture (32). The new agent was observed by acridine orange staining in singlets, doublets, and during heavy parasitization of host cells, in long chains (32). Transmission electron microscopy of the R. asembonensis revealed multiple free rickettsiae (round to elongated morphology) in the cytoplasm of the host cells, with normal rickettsial size [diameter 0.375–0.5 μm (round morphology), length 0.5–0.625 μm, width/diameter 0.25–0.375 μm (elongated morphology)]. A cell wall membrane, defined periplasmic space, and cytoplasmic membrane were observed, as well as the electron lucent “halo” (rickettsial slime layer) (32). Intranuclear localization/growth of the agent was not detected by acridine orange or by transmission electron microscopy (32).
Genetic characterization of the cultured R. asembonensis NMRCii by MLST using rickettsial genes rrs, gltA, ompA, ompB, and sca4; plasmid analysis; and whole genome sequencing confirmed that the new agent was indeed a unique Rickettsia species (1, 44). R. asembonensis NMRCii was shown to have an estimated genome size of 1.40 Mb, possessed a 21,692 bp circular plasmid and had a G+C content of 32.2%. The R. asembonensis plasmid, pRAS01, was discovered to be unique as it only shared 89% homology with that of R. africae ESF5 and only 84% homology with that of R. felis. The R. asembonensis genome has 1,147 predicted protein-coding genes, 33 tRNA genes, and three rrn operons. These characteristics are similar with those found within the genome of R. felis (NC_007109), which is 1.49 Mb in size and contains 1,400 protein-coding genes, 33 tRNA genes, and three rrn operons. Of the R. felis proteins, 1,157 (83%) have homologs in R. asembonensis (1, 44).
The sequences of R. asembonensis NMRCii, were 100% identical to those previously described for “Ca. R. asemboensis” isolates F30 and F82 for the following genes: rrs, gltA, sca4, and the 17kD antigen gene. For the ompA and ompB genes, the R. asembonensis NMRCii shared 99.86 and 99.98% similarity respectively, with the “Ca. R. asemboensis” isolates F30 and F82. The differences observed were as a result of nucleotide substitutions in two positions for the ompA gene and in one position for the ompB gene. A molecular phylogenetic analysis using 4,130 bp sequence of the variable gene-ompB open reading frame was conducted and the phylogenetic relationship between Rickettsia asembonensis NMRCii with R. felis, Rickettsia sp. PU01-02 (“Ca. R. senegalensis”) and other recognized Rickettsia species was determined (Figure 2).
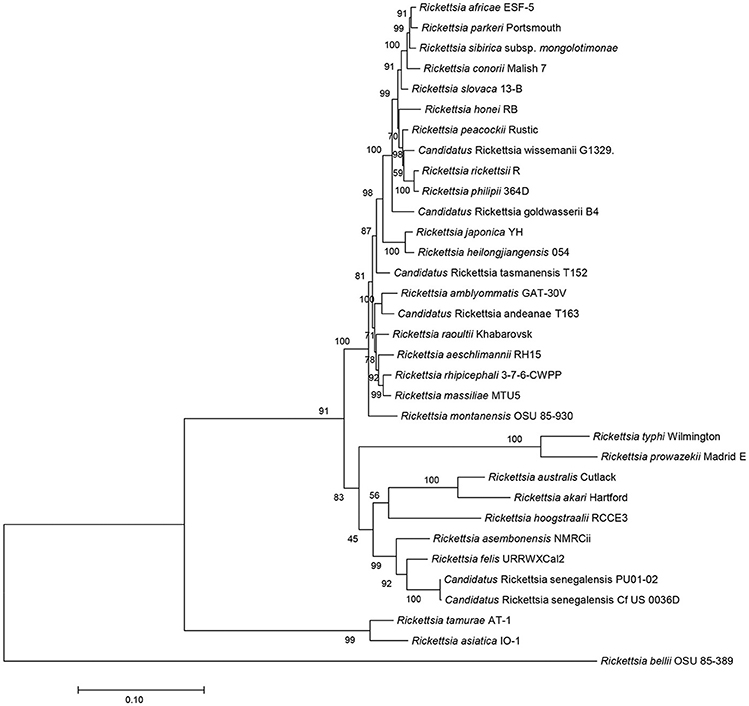
Figure 2. Molecular phylogenetic analysis using ompB open reading frame (4,130 bp). The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Evolutionary analyses were conducted with MEGA7.
Rickettsia asembonensis NMRCii was deposited in two separate culture collections (= DSM 100172T and = CDC CRIRC RAS001T) and the name officially changed (according to the rules of the International Journal of Systematics and Evolutionary Biology) from “Candidatus Rickettsia asemboensis” to Rickettsia asembonensis (1).
Arthropods Associated With Rickettsia asembonensis
R. asembonensis DNA has been detected in various arthropods, but most commonly in fleas (Table 1). It has been identified in fleas from three families namely the Pulicidae, Ceratophyllidae and Coptopsyllidae. In the cosmopolitan Pulicidae family it has been associated with seven genera: Ctenocephalides (C. felis, C. canis, and C. orientis); Xenopsylla (X. cheopis, X. ramesis, and X. gerbilli); Archaeopsylla (A. erinacei); Echidnophaga (E. gallinacea); Pulex (P. irritans); and Synosternus (S. pallidus). In the family Ceratophyllidae, R. asembonensis has been detected in three genera: Ceratopsyllus (C. fasciatus); Orchopeas (O. howardi); and Nosopsyllus (N. laeviceps) and in one genus in the family Coptopsyllidae: Coptopsylla (C. lamellifer) (45).
High prevalence rates of R. asembonensis have been reported in C. felis and C. canis (sympatric species), S. pallidus, X. ramesis, and X. gerbilli with up to 95, 95, 91.4, 100, and 33.3% of the fleas positive for R. asembonensis, respectively (13, 14, 18, 40, 46). Similar results in Costa Rica and Brazil confirm the high prevalence of R. asembonensis in C. felis (23, 28). In addition, R. asembonensis has been associated with other fleas, usually in much lower prevalence than in the aforementioned fleas. These include E. gallinacea, P. irritans, C. lamellifer, X. hirtipes, and N. laeviceps. Often these fleas are positive for R. asembonensis in the same areas as fleas highly infected with R. asembonensis (14, 46). The presence of the R. asembonensis in minimally infected flea species may be due to co-feeding and not that these fleas are reservoir hosts for R. asembonensis. Other arthropods in which evidence of R. asembonensis has been found include the tropical rat mites (Ornithonysus bacoti) in Egypt (7) and ticks (Amblyomma ovale and Rhipicephalus sanguineus) (23, 25–27).
Pathogenicity
In limited laboratory studies no marked cytopathic effects were observed in S2 and C6/36 cells, beyond lysis of overly parasitized host cells (32). Additionally, no growth was observed in embyronated chicken eggs (1). Moreover, in two febrile studies conducted in Kenya no molecular evidence of this agent in patients' blood was seen whereas R. felis DNA was detected in 3.7 and 7.2% of fever patients' blood (42, 47). However, there is molecular evidence of R. asembonensis in a patient from Malaysia with fever, myalgia, arthralgia, mild headache, conjunctival suffusion, and the presence of petechiae noted on his limbs. Molecular analysis (gltA and ompB sequences) of the patient's blood identified R. sp. RF2125 (21). In addition, in the blood from a healthy free range domestic dog from Mnisi community situated in the northeastern corner of the Bushbuckridge Municipal Area, Mpumalanga Province, South Africa R. asembonensis was detected by NGS (22). Lastly, 12 of 50 healthy monkeys from Peninsular Malaysia had molecular evidence (100% gltA sequence similarity) of R. sp. RF2125/”Ca. R. asemboensis” (20). Thus, from the mixed results presented, the question of pathogenicity for humans and other animals is not yet resolved and requires more investigation.
Future Research Direction
R. asembonensis-genotypes have been described in various biting and non-biting arthropods. Apart from R. asembonensis NMRCii that has been isolated in cell culture and whose full genome sequence is available in the GenBank Database, many of the others are just molecular isolates derived from arthropods with very limited sequence data for comparison. Functional and structural analysis of R. asembonensis is needed to ascertain differences and/or similarities between it and other rickettsial species. Moreover, research concerning the known/potential hosts of R. asembonensis, its current/potential arthropod vectors (both common and non-common), and its potential for interference with other rickettisal flea-borne pathogens (R. felis and R. typhi), as well as non-rickettsial pathogens such as Yersinia pestis, will be crucial to fully defining its pathogenicity and probability as a public health concern/nuisance across the world.
Author Contributions
All authors contributed to the conception and design of the review. AM wrote the first draft of the manuscript. JJ, AL-F, HS, CF, and AR wrote revisions of the manuscript. All authors contributed to the manuscript's final version, and read and approved the submitted version.
Funding
Funding for this project was provided by the Global Emerging Infections Surveillance section of the Armed Forces Health Surveillance Branch of the Military Health System work unit A1402.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The views expressed herein are those of the authors and do not necessarily represent the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. AR is an employee of the U.S. Government and his work was prepared as part of his official duties. Title 17 U.S.C. §105 provides that Copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
References
1. Maina AN, Luce-Fedrow A, Omulo S, Hang J, Chan T-C, Ade F, et al. Isolation and characterization of a novel Rickettsia species (Rickettsia asembonensis sp. nov) obtained from cat fleas (Ctenocephalides felis). Int J Syst Evol Microbiol. (2016) 66:4512–7. doi: 10.1099/ijsem.0.001382
2. Ogata H, Renesto P, Audic S, Robert C, Blanc G, Fournier PE, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. (2005) 3:e248. doi: 10.1371/journal.pbio.0030248
3. Mediannikov O, Aubadie-Ladrix M, Raoult D. Candidatus ‘Rickettsia senegalensis' in cat fleas in Senegal. New Microbes New Infect. (2014) 3:24–8. doi: 10.1016/j.nmni.2014.10.005
4. Parola P, Sanogo O, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez J, et al. Identification of Rickettsia spp. and Bartonella spp in fleas from the Thai-Myanmar Border Annals New York Academy Sci. (2003) 990:173–81. doi: 10.1111/j.1749-6632.2003.tb07359.x
5. Reeves WK, Nelder MP, Korecki JA. Bartonella and Rickettsia in fleas and lice from mammals in South Carolina, USA. J Vector Ecol. (2005) 30:310.
6. Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, et al. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am J Trop Med Hyg. (2006) 75:41–8. doi: 10.4269/ajtmh.2006.75.41
7. Reeves WK, Loftis AD, Szumlas DE, Abbassy MM, Helmy IM, Hanafi HA, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. (2007) 41:101. doi: 10.1007/s10493-006-9040-3
8. Gilles J, Silaghi C, Just F, Pradel I, Pfister K. Polymerase chain reaction detection of Rickettsia felis-like organism in Archaeopsylla erinacei (Siphonaptera: Pulicidae) from Bavaria, Germany. J Med Entomol. (2009) 46:703–7. doi: 10.1603/033.046.0338
9. Forshey BM, Stewart A, Morrison AC, Gálvez H, Rocha C, Astete H, et al. Epidemiology of spotted fever group and typhus group rickettsial infection in the Amazon Basin of Peru. Am J Trop Med Hyg. (2010) 82:683–90. doi: 10.4269/ajtmh.2010.09-0355
10. Hornok S, Meli ML, Perreten A, Farkas R, Willi B, Beugnet F, et al. Molecular investigation of hard ticks (Acari: Ixodidae) and fleas (Siphonaptera: Pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet Microbiol. (2010) 140:98–104. doi: 10.1016/j.vetmic.2009.07.013
11. Foongladda S, Inthawong D, Kositanont U, Gaywee J. Rickettsia, Ehrlichia, Anaplasma, and Bartonella in ticks and fleas from dogs and cats in Bangkok. Vector Borne Zoonotic Dis. (2011) 11:1335–41. doi: 10.1089/vbz.2010.0174
12. Troyo A, Álvarez D, Taylor L, Gabriela A, Ólger C-A, Maria LZ, et al. Rickettsia felis in Ctenocephalides felis from Guatemala and Costa Rica. Am J Trop Med Hyg. (2012) 86:1054–6. doi: 10.4269/ajtmh.2012.11-0742
13. Roucher C, Mediannikov O, Diatta G, Trape J-F, Raoult D. A new Rickettsia species found in fleas collected from human dwellings and from domestic cats and dogs in Senegal. Vector Borne Zoonotic Dis. (2012) 12:360–5. doi: 10.1089/vbz.2011.0734
14. Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K, et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia asemboensis in fleas from human habitats, Asembo, Kenya. Vector Borne Zoonotic Dis. (2013) 13:550–8. doi: 10.1089/vbz.2012.1123
15. Tay S, Mokhtar A, Low K, Mohd Zain S, Jeffery J, Abdul Aziz N, et al. Identification of rickettsiae from wild rats and cat fleas in Malaysia. Med Vet Entomol. (2014) 28:104–8. doi: 10.1111/mve.12075
16. Odhiambo AM, Maina AN, Taylor ML, Jiang J, Richards AL. Development and validation of a quantitative real-time polymerase chain reaction assay specific for the detection of Rickettsia felis and not Rickettsia felis-like organisms. Vector Borne Zoonotic Dis. (2014) 14:476–81. doi: 10.1089/vbz.2013.1518
17. Oteo JA, Portillo A, Portero F, Zavala-Castro J, Venzal JM, Labruna MB. Candidatus Rickettsia asemboensis' and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasites Vectors (2014) 7:455. doi: 10.1186/s13071-014-0455-0
18. Rzotkiewicz S, Gutiérrez R, Krasnov BR, Morick D, Khokhlova IS, Nachum-Biala Y, et al. Novel evidence suggests that a ‘Rickettsia felis-like'organism is an endosymbiont of the desert flea, Xenopsylla ramesis. Mol Ecol. (2015) 24:1364–73. doi: 10.1111/mec.1310
19. Faccini-Martínez ÁA, Ramírez-Hernández A, Forero-Becerra E, Cortés-Vecino JA, Escandón P, Rodas JD, et al. Molecular evidence of different Rickettsia species in Villeta, Colombia. Vector Borne Zoonotic Dis. (2016) 16:85–7. doi: 10.1089/vbz.2015.1841
20. Tay ST, Koh FX, Kho KL, Sitam FT. Rickettsial infections in monkeys, Malaysia. Emerg Infect Dis. (2015) 21:545–7. doi: 10.3201/eid2103.141457
21. Kho KL, Koh FX, Singh HKL, Zan HAM, Kukreja A, Ponnampalavanar S, et al. Spotted fever group rickettsioses and murine typhus in a Malaysian teaching hospital. Am J Trop Med Hyg. (2016) 95:765–8. doi: 10.4269/ajtmh.16-0199
22. Kolo AO, Sibeko-Matjila KP, Maina AN, Richards AL, Knobel DL, Matjila PT. Molecular detection of zoonotic rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector Borne Zoonotic Dis. (2016) 16:245–52. doi: 10.1089/vbz.2015.1849
23. Troyo A, Moreira-Soto RD, Calderon-Arguedas O, Mata-Somarribas C, Ortiz-Tello J, Barbieri AR, et al. Detection of rickettsiae in fleas and ticks from areas of Costa Rica with history of spotted fever group rickettsioses. Ticks Tick Borne Dis. (2016) 7:1128–34. doi: 10.1016/j.ttbdis.2016.08.009
24. Krueger L, Bai Y, Bennett S, Fogarty C, Kosoy M, Maina A, et al. Identification of zoonotic and vector-borne infectious agents associated with opossums (Didelphis virginiana) in residential neighborhoods of Orange County, California. Proc Vertebr Pest Conf. (2016) 27:268–79. Available online at: https://escholarship.org/uc/item/88p773zr
25. Dall'Agnol B, Souza U, Webster A, Weck B, Stenzel B, Labruna M, et al. “Candidatus Rickettsia asemboensis” in Rhipicephalus sanguineus ticks, Brazil. Acta Tropica. (2017) 167:18–20. doi: 10.1016/j.actatropica.2016.12.008
26. Kho KL, Koh FX, Hasan LIM, Wong LP, Kisomi MG, Bulgiba A, et al. Rickettsial seropositivity in the indigenous community and animal farm workers, and vector surveillance in Peninsular Malaysia. Emerg Microbes Infections. (2017) 6:e18. doi: 10.1038/emi.2017.4
27. Low VL, Prakash BK, Tan TK, Sofian-Azirun M, Anwar FHK, Vinnie-Siow WY, et al. Pathogens in ectoparasites from free-ranging animals: Infection with Rickettsia asembonensis in ticks, and a potentially new species of Dipylidium in fleas and lice. Veterinary Parasitol. (2017) 245:102–5. doi: 10.1016/j.vetpar.2017.08.015
28. Silva AB, Vizzoni VF, Costa AP, Costa FB, Moraes-Filho J, Labruna MB, et al. First report of a Rickettsia asembonensis related infecting fleas in Brazil. Acta Trop. (2017) 171:240. doi: 10.1016/j.actatropica.2017.04.004
29. Palomar AM, Cevidanes A, Portillo A, Kalema-Zikusoka G, Chirife AD, Romero L, et al. High prevalence of Rickettsia spp. in dog fleas (Siphonaptera: Pulicidae) in rural Uganda. J Med Entomol. (2017) 54:1076–9. doi: 10.1093/jme/tjx048
30. Loyola S, Flores C, Torre A, Kocher C, Melendrez M, Luce-Fedrow A, et al. Rickettsia asembonensis characterization by multi-locus sequence typing of complete genes, Peru. Emerg Infect Dis. (2018) 24:931–3. doi: 10.3201/eid2405.170323
31. Mediannikov O, Audoly G, Diatta G, Trape J-F, Raoult D. New Rickettsia sp. in tsetse flies from Senegal. Comp Immunol Microbiol Infect Dis. (2012) 35:145–50. doi: 10.1016/j.cimid.2011.12.011
32. Luce-Fedrow A, Maina AN, Otiang E, Ade F, Omulo S, Ogola E, et al. Isolation of Candidatus Rickettsia asemboensis from Ctenocephalides fleas. Vector Borne Zoonotic Dis. (2015) 15:268–77. doi: 10.1089/vbz.2014.1744
33. Azad A. Epidemiology of murine typhus. Annual Rev Entomol. (1990) 35:553–70. doi: 10.1146/annurev.en.35.010190.003005
34. Eisen RJ, Gage KL. Transmission of flea-borne zoonotic agents. Annual Rev Entomol. (2012) 57:61–82. doi: 10.1146/annurev-ento-120710-100717
35. Adams WH, Emmons RW, Brooks JE. The changing ecology of murine (endemic) typhus in Southern California. Am J Trop Med Hyg. (1970) 19:311–8. doi: 10.4269/ajtmh.1970.19.311
36. Rennoll SA, Rennoll-Bankert KE, Guillotte ML, Lehman SS, Driscoll TP, Beier-Sexton M, et al. The cat flea (Ctenocephalides felis) immune deficiency signaling pathway regulates Rickettsia typhi infection. Infect Immun. (2018) 86:e00562–17. doi: 10.1128/IAI.00562-17
37. Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, et al. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS ONE (2007) 2:e266. doi: 10.1371/journal.pone.0000266
38. Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. (2009) 46:723–36. doi: 10.1603/033.046.0402
39. Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. (2011) 17:996–1000. doi: 10.1111/j.1469-0691.2011.03516.x
40. Maina AN, Fogarty C, Krueger L, Macaluso KR, Odhiambo A, Nguyen K, et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County California. (2016). PLoS ONE 11:e0160604. doi: 10.1371/journal.pone.0160604
41. Mullins K, Maina A, Krueger L, Jiang J, Cummings R, Drusys A, et al. Rickettisal infections among cats and cat fleas in Riverside County, California. Am J Trop Med Hyg. (2018) 99:291–6. doi: 10.4269/ajtmh.17-0706
42. Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. (2012) 18:328. doi: 10.3201/eid1802.111372
43. Fournier P-E, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov J Clin Microbiol. (2003) 41:5456–65. doi: 10.1128/JCM.41.12.5456-5465.2003
44. Jima DD, Luce-Fedrow A, Yang Y, Maina AN, Snesrud EC, Otiang E, et al. Whole-genome sequence of “Candidatus Rickettsia asemboensis” strain NMRCii, isolated from fleas of western Kenya. Genome Announcements. (2015) 3:e00018–e00015. doi: 10.1128/genomeA.00018-15
45. Whiting MF, Whiting AS, Hastriter MW, Dittmar K. A molecular phylogeny of fleas (Insecta: Siphonaptera): origins and host associations. Cladistics (2008) 24:677–707. doi: 10.1111/j.1096-0031.2008.00211.x
46. Sansyzbayev Y, Nurmakhanov T, Berdibekov A, Vilkova A, Yeskhodzhayev O St. John HK et al. Survey for rickettsiae within fleas of Great Gerbils, Almaty Oblast, Kazakhstan. Vector Borne Zoonotic Dis. (2017) 17:172–8. doi: 10.1089/vbz.2016.2049
Keywords: Rickettsia, Rickettsia asembonensis, flea-borne, worldwide distribution, arthropod hosts, Rickettsia felis-like organisms
Citation: Maina AN, Jiang J, Luce-Fedrow A, St. John HK, Farris CM and Richards AL (2019) Worldwide Presence and Features of Flea-Borne Rickettsia asembonensis. Front. Vet. Sci. 5:334. doi: 10.3389/fvets.2018.00334
Received: 19 September 2018; Accepted: 14 December 2018;
Published: 08 January 2019.
Edited by:
Michael Kosoy, Centers for Disease Control and Prevention (CDC), United StatesReviewed by:
Sandor Karpathy, Centers for Disease Control and Prevention (CDC), United StatesMarcos Rogério André, Universidade Estadual Paulista Júlio de Mesquita Filho, Brazil
Marina Eremeeva, Georgia Southern University, United States
Copyright © 2019 Maina, Jiang, Luce-Fedrow, St. John, Farris and Richards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allen L. Richards, allen.richards@comcast.net
 Alice N. Maina1
Alice N. Maina1  Ju Jiang
Ju Jiang Alison Luce-Fedrow
Alison Luce-Fedrow Christina M. Farris
Christina M. Farris Allen L. Richards
Allen L. Richards