The validity and applications of the analgesia nociception index: a narrative review
- 1Department of Anesthesiology, Stony Brook University Health Science Center, Stony Brook, NY, United States
- 2Medical Scientist Training Program, Renaissance School of Medicine, Stony Brook University, Stony Brook, NY, United States
Pain refers to the subjective, unpleasant experience that is related to illness or injury. In contrast to pain, nociception refers to the physiological neural processing of noxious stimuli, such as intra-operative surgical stimuli. One novel device, the Analgesia Nociception Index (ANI), aims to objectively measure intra-operative nociception by analyzing the heart rate variability in patients undergoing surgery. Through this method of nociceptive monitoring, the ANI device aims to provide an objective, continuous evaluation of patient comfort levels and allow anesthesiologists to better manage surgical stress and patient analgesia, perhaps with even better efficacy than current practices used to assess nociception. Additionally, ANI may have clinical application in settings outside of the operating room, such as in the intensive care unit. In this narrative review, we compiled and summarized the findings of many studies that have investigated ANI's validity and applications in different clinical settings. Currently, the literature appears mostly supportive of ANI's ability to detect nociception in both surgical and non-surgical settings. However, the ability for ANI to provide clinical benefits, such as decreased intra-operative opioid use, post-operative opioid use, and post-operative pain compared to standard practices appear controversial. Because of the wide variety of methodology, clinical settings, patient populations, and limitations in these studies, more investigation of ANI is needed before any firm conclusions can be drawn on its clinical benefits.
1. Introduction
Nociception is the body's neural process of encoding noxious stimuli. Numerous methods were developed to help clinicians assess nociception, such as monitoring hemodynamic parameters. Assessing changes in hemodynamic parameters can be especially useful intra-operatively, as it allows anesthesiologists to monitor their patients’ nociceptive response to surgical stimuli, optimize opioid administration, reduce pain, and improve post-operative outcomes.
Parameters such as blood pressure (BP) and heart rate (HR) may provide insight in a patient's nociception, but may be subject to confounding factors and not always accurately reflect nociception. To address this, many new devices have been developed to monitor nociception more accurately, such as the Analgesia Nociception Index (ANI) developed by MDoloris. ANI obtains electrocardiogram (ECG) data through two electrodes placed on the patient's chest and analyzes the interval between the R-R waves of the ECG. The changes in the R-R intervals, also known as the heart rate variability (HRV), are influenced by changes in sympathetic and parasympathetic tone (1). Changes in HRV in the high frequency (HF) range (0.15–0.40 Hz) are influenced by parasympathetic activity, whereas changes in HRV in the low frequency (LF) range (0.04–0.15 Hz) are influenced by both sympathetic and parasympathetic activities (2). A decrease in HF HRV or an increase in LF HRV suggests a decrease in parasympathetic tone and subsequently was shown to be indicative of pain or unpleasant stimuli (3, 4). With this information, ANI creates a value from 0 to 100, where a value greater than 50 indicates adequate analgesia (high parasympathetic tone) and a value less than 50 indicates nociception (a high sympathetic tone) and therefore inadequate analgesia and a likely chance of a hemodynamic response occurring within a few minutes.
Given ANI's potential in managing anesthetized patients and ease of use, many studies assessed for its clinical benefits in the intra-operative setting, the post-anesthesia care unit (PACU), and the intensive care unit (ICU). Additionally, many studies also assessed ANI's clinical relevance in various patient populations, such as anesthetized, pediatric, critically ill, maternal, and septic patients. PubMed search terms utilized for study selection included “nociception,” “analgesia,” “analgesia nociception index,” “ANI,” and “pain monitoring.” Following the search, two co-authors chose studies independently for inclusion by considering their study design, clinical importance, and journal characteristics. In this narrative review, we summarized the selected studies’ results on ANI's validity and other applications.
2. Validation of ANI's ability to detect intra-operative nociception and predict hemodynamic responses
2.1. Validation of ANI using artificial noxious stimuli
To validate ANI's ability to detect nociceptive surgical stimuli, four studies used an artificial noxious stimulus and monitored fluctuations in ANI values and hemodynamic responses. In theory, if a change in ANI preceded a hemodynamic response (such as a significant increase in BP or HR), this would support ANI's ability to predict whether a hemodynamic response will occur and provide anesthesiologists better insight in managing anesthetized patients.
In Gruenewald et al. and Susano et al., tetanic noxious stimulation was applied to patients with simultaneous monitoring of their ANI values (5, 6). Both studies had similar findings: ANI significantly decreased after the introduction of a noxious stimulus (i.e., ANI reflected nociception); however, there were no significant changes in hemodynamic responses (HR and BP). In Susano et al., one possible suggestion for the lack of change in hemodynamics was that ANI could be better than traditional hemodynamics at reflecting noxious stimulation (6). Alternatively, it is possible that noxious stimulus applied in the study was simply not strong enough to lead to changes in hemodynamic parameters.
Funcke et al. had both a similar and different conclusion compared to the previous two studies: ANI's prediction probability (PK) for detecting tetanic noxious stimuli was 0.98 (where PK values range from 0.5 to 1; 1 representing a perfect prediction and 0.5 representing mere chance), with a sensitivity and specificity of 87.9% and 98.5% respectively (7). Contrary to the previous two studies, Funcke et al. found that ANI's prediction probability for a hemodynamic response (defined in their study as “increase in heart rate or blood pressure by >5 beats/min or >5 mmHg, respectively, or >10%”) was 0.70 with a sensitivity and specificity of 20.6% and 46.8%, respectively. Funcke et al. concluded as ANI having limited predictive value for hemodynamic responses.
Jozefowicz et al. had a contradictory finding compared to the previous three studies (8). In patients who received a tetanic noxious stimulation prior to tracheal intubation, there was no significant difference in ANI between patients who had a hemodynamic response and those who did not. Additionally, during intubation, the ability for ANI to predict a hemodynamic response was found to not be reliable given that the area under a receiver operating characteristic curve (AUC ROC) was 0.61 (Table 1).

Table 1. Summary of ANI's validity in detecting experimental and intra-operative nociception and predicting hemodynamic responses.
2.2. Validation of ANI in clinical settings with adult patients
The following studies assessed both ANI's ability to detect nociceptive surgical and predict hemodynamic responses in various clinical settings. In these five studies with different noxious stimuli, the conclusions were similar: ANI significant decreased after a nociceptive stimulus and was followed by a significant change in hemodynamic parameters (defined as a minimum of 10% increase in BP or HR in Ledowski et al. and a minimum of 20% increase in BP or HR in Jeanne et al. and Boselli et al.) (9–13). As such, each study concluded that ANI reflected nociception and could predict hemodynamic changes following nociceptive stimuli. More notably, Ledowski et al. also demonstrated a significant increase in ANI after fentanyl administration, indicating adequate analgesia and decreased nociception after analgesic use (9) (Table 1).
2.3. Validation of ANI in clinical settings with pediatric patients
Many studies have also evaluated the validity of ANI to detect nociception in pediatric patients as well. In fact, four different studies all found evidence that supports ANI's ability to detect nociception in pediatric patients (18–21). These studies had similar methodology: measuring and comparing ANI values before and after noxious surgical stimuli. In addition to these findings, two studies also suggested that ANI may be better predictors of nociception than hemodynamic parameters. In Weber et al., when ANI was <50 (indicative of nociception) at the time of analgesic administration, ANI values would increase above 60 within 2 min, whereas there was no observed change in HR (20). From this observation, Weber et al. suggested that ANI may be better than HR at predicting nociception. Julien-Marsollier et al.'s AUROC analysis (predictive value) for ANI to detect nociceptive surgical stimuli was >0.75, whereas the AUROC values for HR, systolic BP, diastolic BP, and mean arterial pressure were 0.51, 0.60, 0.57, and 0.58 respectively (21). These authors concluded that hemodynamic parameters had little predictive value for noxious surgical stimuli compared to ANI (21) (Table 1).
2.4. ANI and intra-operative opioid use
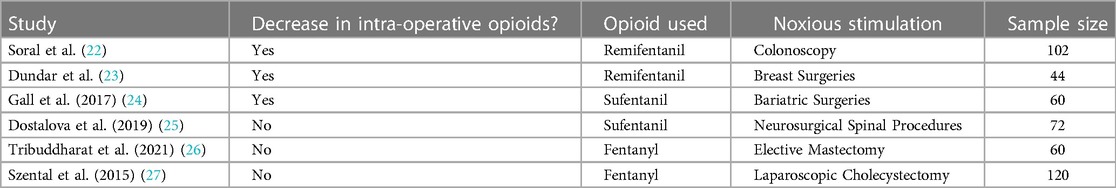
Another potential application of ANI is optimizing intra-operative opioid consumption. Many studies have assessed this potential to determine if ANI-guided opioid administration alongside standard practice would lead to decreased intra-operative opioid use compared to standard practice. Three studies had a similar methodology: comparing the intra-operative opioid use in an ANI-guided group and a standard practice (control) group during surgery. Of the three studies, Soral et al. and Dundar et al. assessed remifentanil consumption, whereas Gall et al. assessed sufentanil consumption (22–24). All three studies, which involved three different types of surgeries, found a significantly lower opioid use in patients who underwent surgery with ANI monitoring compared to patients who were assessed via conventional means (Table 2).
While those studies found that ANI guidance decreased intra-operative opioid consumption, some studies did not find a significant difference in opioid use between ANI-guided protocols and conventional methods (25–27). However, one notable conclusion mentioned by Dostolova et al. was that while ANI use did not lead to intra-operative opioid sparing, it still has potential clinical utility as there was also no significant difference in post-operative cortisol levels, pain scores, or complication rates (25) (Table 2).
3. ANI in the post-operative setting
3.1. Validation of ANI in detecting post-operative nociception
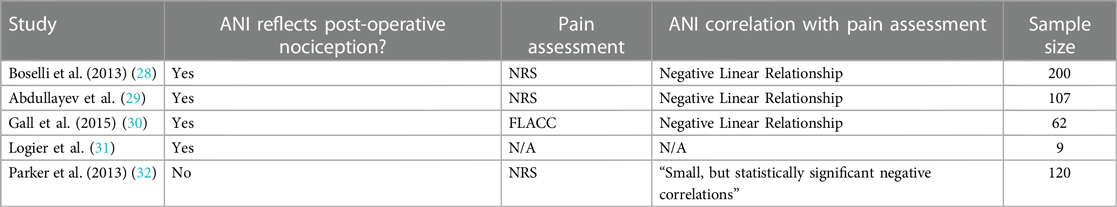
In addition to minimizing intra-operative opioid consumption, many studies were conducted on whether nociception could be detected reliably post-operatively. In these studies, ANI values were measured and compared post-operatively with a subjective pain assessment scale, such as the Numeric Rating Scale (NRS) or the Face, Legs, Activity, Cry, Consolability scale for pediatric patients (FLACC). In Boselli et al., there was a significantly negative linear relationship between the NRS and ANI values, with a sensitivity and specificity for ANI values <57 to detect an NRS >3 was 78% and 80% respectively, with an AUC ROC of 0.86 within 10 min of PACU arrival (28). Additionally, the sensitivity and specificity for ANI <48 to detect an NRS >7 was 92% and 82% respectively, with an AUC ROC of 0.91. Abdullayev et al. also found a significantly negative linear relationship between ANI and NRS scores (r2 = −0.312, p = 0.001) (29). Both studies' findings are consistent with ANI's intended clinical use: a higher ANI value (indicative of less nociception/sympathetic tone) should inversely correlate with a lower self-reported pain score. Similarly, Gall et al. (2015) findings support ANI's ability to predict post-operative pain in pediatric patients: children who had a surgical procedure had significantly lower ANI values (indicative of nociception) upon arrival to the PACU compared to children who underwent imaging; ANI and FLACC values were found to have a significantly negative linear relationship as well (30). Lastly, while Logier et al. did not compare post-operative ANI values to a pain scale, they found that post-operative ANI values significantly increased (indicative of less nociception) upon administration of truncal analgesia (31) (Table 3).
One study by Parker et al., however, concluded that ANI was not able to reflect post-operative pain as measured by the NRS scale (32). This study also compared NRS and ANI values upon arrival to the PACU. While there was a statistically significant negative correlation between NRS and ANI, the correlation was not strong (spearman's ro coefficient = −0.075) and that ANI's ability to distinguish NRS scores of 0 from NRS scores of 6–10 had low sensitivity and specificity (Table 3).
3.2. Intra-operative ANI use and post-operative pain
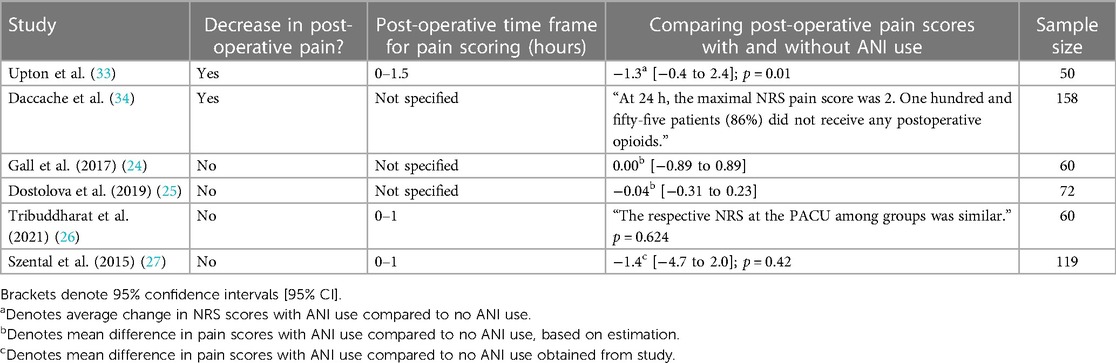
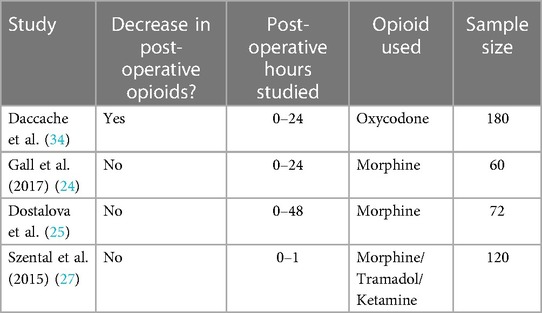
Given the studies validating the use of ANI to assess post-operative nociception, the next steps in exploring the use of ANI would be to evaluate whether intra-operative ANI use led to decreased post-operative pain. Upton et al. and Daccache et al. both had results that supported this idea. Specifically, Upton et al. observed that patients who underwent ANI-guided fentanyl administration for lumbar discectomies or laminectomies had significantly lower NRS scores, lower post-operative fentanyl administration, lower nausea scores, and lower incidence of shivering compared to the control group (standard practice) (33). In Daccache et al., 155 out of 180 patients that received intra-operative ANI-guided fentanyl for elective surgeries did not receive any post-operative opioids for pain; additionally, the cohort's maximal pain NRS score was 2 at 24 h post-surgery (34) (Tables 4, 5).
Other studies had contradictory findings. In Gall et al. and Dostalova et al., the studies previously discussed that found ANI-guidance decreased intra-operative opioid use, compared post-operative pain between patients with and without ANI-guided analgesia using the NRS scale and visual analog scale (VAS), respectively (24, 25). Despite both studies primary outcomes supporting less opioid use through ANI guidance, there was no significant difference in post-operative pain scores between the ANI groups and the control groups. Tribuddharat et al. and Szental et al., the studies discussed in the previous section that did not find a significant difference in intra-operative opioid use between ANI-treated and control groups, also did not see a significant difference in pain scores between ANI-treated and control groups (using NRS and VAS to assess pain, respectively) (26, 27) (Tables 4, 5).
3.3. Intra-operative ANI use and post-operative outcomes
There are a few studies that evaluated post-operative outcomes in patients that had ANI-guided analgesia. An observational study by Ramos-Luengo et al. found that patients whose ANI values were higher than 50 for at least 60% of the time under anesthesia had a significantly lower length of stay post-operatively (35). While this study didn't test whether an ANI-treated group had a lower length of stay compared to a control group, this study could suggest that ensuring ANI values stay above 50 for a significant amount of time intra-operatively can reduce a patient's length of stay. This isn't generalizable to all surgeries, however, as this was studied in patients undergoing varicose vein intervention. In Yang et al., elderly patients that had ANI guidance during spinal surgeries were found to have a higher post-operative neurocognition (36).
3.4. Intra-operative ANI use and post-operative outcomes in pediatric patients
In pediatric patients, some of the post-operative outcomes of ANI that were assessed include post-operative pain and agitation. As mentioned previously, Gall et al. found that children admitted in the PACU after surgery had significantly lower ANI scores (indicative of nociception) compared to children admitted in the PACU after medical imaging, where no painful stimulus occurred. Additionally, they observed a statistically significant negative linear relationship between ANI values and FLACC scores, further supporting ANI's ability to evaluate post-operative pain in pediatric children (30). Larsen et al. assessed whether ANI-guided analgesia would affect post-operative agitation based on the Richmond Agitation-Sedation scale in pediatric patients (37). ANI-guided analgesia led to a decrease in the number of children who had emergence agitation (9 out of 30 children) compared to the control group (15 out of 31 children). However, it is important to note that this was not statistically significant (p = 0.070). Furthermore, it is also important to note that the ANI-treated group of children had a statistically significantly higher average dose of fentanyl. However, this can also be interpreted as ANI-guidance allowed anesthesiologists to administer the most appropriate amount of opiates, which is reflected in the lower emergence agitation frequency.
4. ANI in the ICU and COVID-19
4.1. Validation of ANI to detect nociception in ICU patients
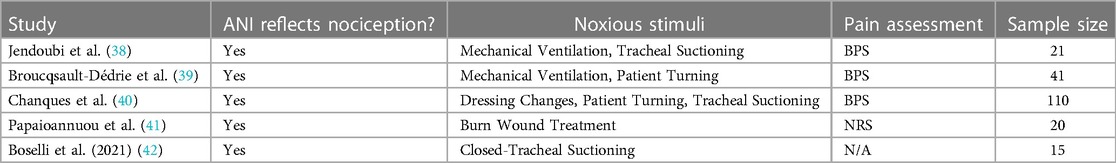
In the ICU setting, there are numerous studies that validates ANI ability to measure nociception (Table 6). Jendoubi et al. and Broucqsault-Dédrie et al. assessed ANI changes in response to painful stimuli in deeply sedated patients on mechanical ventilators and found that ANI significantly decreased (indicative of nociception) during painful stimuli compared to ANI values at rest (38, 39). Interestingly, Jendoubi et al. found a significantly negative correlation between ANI values and the behavioral pain scale (BPS) measurements (r2 = −0.469, p < 0.001), whereas Broucqsault-Dédrie et al. did not. However, it is worth noting that one major difference between the two studies is that the patients in Jendoubi et al. all had traumatic brain injuries, whereas the patients in Broucqsault-Dédrie et al.'s were from two different medical ICUs.
In contrast to the previous studies examining deeply sedated patients on mechanical ventilators, Chanques et al. and Papaioannuou et al. validated ANI's effectiveness in detecting nociception in non-comatose, communicative ICU patients (40, 41). For Chanques et al. specifically, they found that “ANIi,” which they defined as the ANI average “calculated over a shorter period of time (64 s)” had a significantly negative correlation with the BPS scale (r = −0.30; p < 0.001) during routine care procedures (dressing change for a central venous/arterial catheter, turning a patient over, tracheal suctioning in intubated patients) (40). Interestingly, “ANIm,” defined as “an average calculated over the previous 4 min,” had no significant change during these procedures nor correlation to the BPS scale.
4.2. Application of ANI with COVID-19 ICU patients
In more recent times, ANI had application in COVID-19 studies as well (Table 6). Boselli et al. (2021) retrospectively analyzed how the ANI values would change during closed-tracheal suction of 15 ICU patients who had severe COVID-19 pneumonia (42). Through ANI, the authors found that ANI significantly decreased during tracheal suctioning, leading them to conclude that ANI can be a useful tool in detecting nociception during this procedure in COVID-19 ICU patients. In another study, Aragón-Benedí et al. assessed whether ANI monitoring could predict the outcomes of 14 critically ill patients with COVID-19 in the surgical ICU (43). These patients had their “ANIm” values (defined as “the mean ANI of the last 240 s”) measured for 240 s in a morning before their daily washing, and 30 days after this measurement the researchers examined whether each patient was still alive. From this, they categorized the patients into a “survivor” and “non-survivor” group. This study concluded that in critically ill COVID-19 patients, there was higher parasympathetic tone (as indicated by higher ANI values), potentially due to low sympathetic activity. In these patients with low sympathetic activity, there were higher mortality rates. However, it is important to note that this study has a very small sample size (n = 14), and thus this finding should not be generalized about COVID-19 related mortalities.
5. ANI application in maternity patients
There are a few studies that evaluated ANI's application in maternity patients as well. Le Guen et al. evaluated both the subjective VAS and the objective ANI values of patients in labor in four instances at 5 min intervals, regardless whether or not the patient was experiencing uterine contractions (44). This study found that the pain scores from the VAS model were significantly higher (signifying nociception) when patients experienced uterine contractions and that ANI values were significantly lower (signifying nociception) during uterine contractions. Furthermore, there was a statistically significant inverse correlation between the VAS and ANI values recorded, supporting ANI's application in this setting.
ANI was also applied in a study that assessed whether mother-infant skin-to-skin contact immediately after birth had an effect on maternal comfort (45). By using ANI, Vamour et al. found that the median ANI values at the end of skin-to-skin contact was significantly higher than before skin-to-skin contact, suggesting the importance of skin contact for mothers immediately after giving birth and further support for ANI's use in evaluating discomfort and pain.
6. ANI and septic patient outcomes
One potential application of ANI could be in predicting the outcomes of septic patients. In one study by Pontet et al. (2003), HRV was measured using an electrocardiogram in 47 septic patients starting from the beginning of their ICU admission (46). This study found that septic patients who developed multiple organ dysfunction syndrome (MODS) had a significantly decreased HRV at the time of ICU admission compared to patients who did not develop MODS. In another prospective study by Chen et al., 132 septic patients had their HRV measured using ECG shortly after admission into the emergency department and the study. In this study, patients who did not survive were found to have significantly lower HRV values compared to those that did survive (survival was defined as patients who were discharged in less than 28 days or remained alive for more than 28 days) (47). In another study, Annane et al. (1999) also found that septic shock patients had a significant reduction in HRV (48).
7. Discussion
7.1. ANI in the intra-operative setting
ANI has excellent potential in the intra-operative setting. In an artificial intra-operative setting, ANI is sensitive to the introduction of a noxious stimulus; however, the changes in ANI after an artificial stimulus cannot predict a significant hemodynamic response (5–8). It is possible that in these studies did not provide a stimulus strong enough to convert changes in sympathetic nervous system signals to a change in hemodynamic responses. In the future, studies will need to change the nature of the stimulus (such as duration, amplitude, and type) to see if ANI is context dependent.
In a real-life intra-operative setting, ANI was sensitive (i.e., decreased) during nociceptive stimuli during surgery in adult and pediatric patients (14–17). ANI's sensitivity to the presence of a noxious stimulus is not dependent on the method of intubation and returns to baseline by the end of the procedure (14, 21). However, the latency period between a change in ANI and a change in a patient's hemodynamic state is not known. Furthermore, the correlation between latency period and the amplitude of the hemodynamic change as not been studied. It is possible that longer latency periods could result in smaller amplitudes and could explain situations where a patient does not have a hemodynamic change after a nociceptive stimulus. Interestingly, the studies that utilized artificial stimuli found evidence that did not support ANI's ability to predict hemodynamic changes, whereas the adult studies in clinical settings did support ANI's predictive capabilities. It is also important to note that each study presented with various limitations: for instance, many of them mentioned a small sample size for their study. Among the studies mentioned, only the study by Ledowski et al. showed that the decreased ANI values from noxious stimulation is reversed by opioid administration (9). There is also no evidence that ANI changes are independent of patient fluid status. Overall, it appears that ANI may have clinical benefit in detecting nociception; however, hemodynamic predictive capabilities still appear controversial. As such, further studies should be conducted in a wider variety of clinical settings to further validate its use.
The effect of ANI on intra-operative opioid use is controversial. The use of different opioids (e.g., remifentanil, fentanyl, sufentanil), different surgical procedures that have varying length and nociceptive stimuli, and sample sizes could explain the different results (22–24). The results of studies that assess intra-opioid consumption using ANI could vary depending on the length of the surgery and the pharmacological properties of the different anesthetics used. Another important consideration is that while objective evaluation of intraoperative nociception may lead to changes in opioid dosing on individual patients, it may not result in an overall reduction in opioid dosing. Additionally, while no change in the average opioid dose may suggest that patients were dosed appropriately without ANI, ANI could allow more optimal titration of opioids for each patient, and potentially with better timing as well. It is also important to note that many studies assessed whether intra-operative ANI use can lead to decreased intra-operative opioid use; however, intra-operative opioid sparing could lead to undesirable outcomes such as increased post-operative pain and opioid use (49). As such, significant reduction in intra-operative opioid consumption may not be the most reflective endpoint (nor should it be the only endpoint) to assess ANI's ability to improve patient outcomes and presents a potential limitation to the applicability of these studies. After all, intra-operative opioid use is only one of many aspects in peri-operative pain management. Nevertheless, among the studies that utilized this endpoint to assess ANI's clinical relevance and found a significant decrease in opioid consumption, there was not a significant change in factors post-operation, such as side effects, complications, or recovery time (22–24). Other clinically relevant outcomes that could be assessed in future studies to improve our understanding in ANI's clinical relevance could be opioid-related adverse events and post-operative complications secondary to pain or sympathetic overactivation. However, given that these events are rare, many existing ANI studies are currently not powered for these outcomes.
7.2. ANI in the post-operative setting
ANI corelates well with subjective pain measures in the post-operative setting in both adult and pediatric patients (32–36). Furthermore, ANI-guided opioid administration can decrease post-operative opioid use (28, 29, 37, 38). However, there is contradictory evidence on whether intra-operative use of ANI-guided opioid administration decreases post-operative pain (measured by subjective pain scores) (28–31). In pediatric patients, one study also demonstrated a trend (although insignificant) between ANI-guided analgesia and lower post-operative agitation (41). The contradiction between decreased post-operative opioid use and unchanged subjective pain could be due to subjective nature of NRS and VAS. In the future, studies should replace subjective pain measures with more objective measures such as hemodynamic factors, ANI, and EEG signals.
Furthermore, there is a need to determine how ANI-guided opioid administration impacts patient outcomes. While Ramos-Luengo et al. found that ANI values could be related to longer hospital stays, the study's findings—that ensuring ANI values staying above 50 can reduce a patient's length of stay—are limited due lack of comparison to a control group and the fact that the study was conducted only on varicose vein interventions. For elderly patients that underwent ANI guidance during spinal surgery, it is possible that these patients have higher cognitive outcomes; however, the effect of ANI by itself is not clear (40). We believe that there needs to be significant more research to clearly understand the effect of ANI-guided anesthesia administrate on patient outcomes.
In pediatric patients, the current literature has varied findings on ANI's post-operative use and these applications of ANI should be studied more extensively before any conclusions could be made (34, 41). Additionally, it would be beneficial to explore the long-term effects of ANI-guided analgesia on post-operative outcomes in pediatric patients. The present narrative review mainly focused on short-term outcomes such as immediate post-operative pain and agitation. Investigating the impact of ANI on factors such as recovery time, length of hospital stay, and overall patient satisfaction could provide a more comprehensive understanding of the clinical benefits associated with ANI-guided analgesia in pediatric populations.
7.3. ANI in the ICU and COVID-19
The literature reviewed in this study provides evidence supporting the effectiveness of ANI in measuring nociception in the ICU setting. Studies on deeply sedated patients on mechanical ventilators demonstrated a significant decrease in ANI values during painful stimuli, indicating its ability to assess pain (42, 43). The correlation between ANI values and pain scales varied across studies, potentially influenced by patient population differences. ANI also showed promise in detecting nociception in non-comatose, communicative ICU patients (44, 45). In the context of COVID-19, ANI was found to be useful in detecting nociception during tracheal suctioning and showed potential in predicting outcomes in critically ill patients (17, 49). However, it is important to note that these COVID-19 studies had low sample sizes. Nevertheless, the current literature regarding ANI shows promising evidence for future application in research conducted in the ICU.
To enhance the utilization of ANI in the ICU, future research should focus on standardizing ANI measurement and interpretation, exploring correlations with pain scales across different patient populations and procedures, and conducting larger studies to validate its applicability, particularly in COVID-19 patients. In conclusion, ANI shows promise as a valuable tool for assessing nociception in the ICU setting. By addressing the identified areas for improvement, ANI can be optimized for pain management and improve outcomes in critically ill patients.
7.4. ANI application in under-studied patient populations
There are not many studies on the use of ANI in the maternity field. These studies have demonstrated ANI's potential in assessing pain and discomfort during labor and postpartum (14, 48). However, further studies should be conducted to evaluate ANI's validity in this unique patient setting given ANI's potential in this setting, such as evaluating how ANI changes because of epidural anesthesia or delivery, or if ANI could have some predictive power in maternity patient outcomes. In the future, standardization of ANI protocols and guidelines specific to this patient population would enhance its utility. Furthermore, ANI's relationship with other validated pain assessment tools in the maternity field should be explored to fully characterize its strengths and weaknesses in this patient population.
In septic patients, the studies consistently support the idea that a lower HRV in septic patients could lead to poorer outcomes (e.g., MODS or mortality). Given that ANI also measures HRV to assess the autonomic nervous system, there is potential for ANI use to have future application in the treatment of septic patients, and perhaps early evaluation of HRV through ANI in these patients could open opportunities to improve outcomes (15–17). Again, for a stronger conclusion of ANI's clinical use in septic patients, more studies are needed. In the future, larger-scale studies with diverse patient populations and ANI's correlation with other factors other than HRV are important to better understand ANI's place in sepsis management.
7.5. The existence of alternative available nociceptive monitoring technology
In addition to ANI, there exists numerous other novel alternative devices that propose to also monitor intraoperative nociception. These devices include, but are not limited to, the surgical plethysmographic index, nociception level index, the pupillary pain index, and the qNOX index (50–53). These devices offer both similar and different ways of interpreting nociception, particularly through modalities such as the plethysmograph amplitude, HRV, skin conductivity, pupillary diameter, and EEG physiology. Furthermore, they contrast from ANI in how they are set up as well; for instance, the surgical plethysmograph index utilizes only a pulse oximeter. Given the growing variety in which nociception can be assessed, it is important to note that ANI is not the sole device on the market that claims this purpose, and that each and every pain monitor should be well analyzed in their own studies and in different clinical settings to assess for their individual validities, applicability, and limitations in clinical medicine.
8. Conclusion
The literature regarding ANI's validity in reflecting nociception is mostly supportive both intra-operatively and in other clinical settings. In terms of whether intra-operative ANI use makes a significant difference in intra-operative opioid consumption, the literature is mixed in its clinical benefits. For post-operative pain and opioid use, many studies have not found a clinical benefit using ANI intra-operatively. Additionally, applications of ANI have also been found to be useful in other settings and patient populations, such as in COVID-19, maternal, and septic patients; however, it is important to note that these studies are limited in number and cannot be generalized.
The potential for the various novel nociceptive monitoring devices to improve patient care is an exciting advancement in the field of surgery and anesthesia. Many studies were conducted on the validity and applications of those alternative devices as well; in this narrative review, we focused primarily on a critical analysis of the ANI device. Despite all the findings presented in this review, each study presents with their own limitations, such as different patient populations, clinical settings, nociceptive stimuli, opioid choices, and small sample sizes that makes generalizing ANI's clinical benefits difficult. There appears to be support for ANI's ability to reflect nociception, but further research on ANI's benefits and applications are necessary before larger conclusions can be made.
Author contributions
BH, AC, and SB contributed to the literature search for the manuscript. BH contributed to writing of the initial manuscript. BH, AC, ZJ, ME, KT, and SB contributed to revisions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
KT is a student in the Medical Scientist Training Program at the Renaissance School of Medicine at Stony Brook University. He is supported by the National Institute of Health T32 Training Grant (5T32GM008444-31). All other authors have no relevant funding sources to report.
Conflict of interest
The authors ZJ and SB declared that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yoshida K, Obara S, Inoue S. Analgesia nociception index and high frequency variability index: promising indicators of relative parasympathetic tone. J Anesth. (2022) 37:130–7. doi: 10.1007/s00540-022-03126-8
2. Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. (2006) 44:1031–51. doi: 10.1007/s11517-006-0119-0
3. Forte G, Troisi G, Pazzaglia M, De Pascalis V, Casagrande M. Heart rate variability and pain: a systematic review. Brain Sci. (2022) 12:153. doi: 10.3390/brainsci12020153
4. Miu AC, Heilman RM, Miclea M. Reduced heart rate variability and vagal tone in anxiety: trait versus state, and the effects of autogenic training. Auton Neurosci Basic Clin. (2009) 145(1–2):99–103. doi: 10.1016/j.autneu.2008.11.010
5. Gruenewald M, Ilies C, Herz J, Schoenherr T, Fudickar A, Höcker J, et al. Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol–remifentanil anaesthesia. Br J Anaesth. (2013) 110(6):1024–30. doi: 10.1093/bja/aet019
6. Susano MJ, Vide S, Ferreira AD, Amorim P. Effects of varying remifentanil concentrations on analgesia nociception index® under Propofol: an observational study. J Clin Monit Comput. (2021) 35(1):199–205. doi: 10.1007/s10877-020-00457-3
7. Funcke S, Sauerlaender S, Pinnschmidt HO, Saugel B, Bremer K, Reuter DA, et al. Validation of innovative techniques for monitoring nociception during general anesthesiaa clinical study using tetanic and intracutaneous electrical stimulation. Anesthesiology. (2017) 127(2):272–83. doi: 10.1097/ALN.0000000000001670
8. Jozefowicz E, Sabourdin N, Fontaine V, Lambelin V, Lejeune V, Menu H, et al. Prediction of reactivity during tracheal intubation by pre-laryngoscopy tetanus-induced ANI variation. J Clin Monit Comput. (2022) 36(1):93–101. doi: 10.1007/s10877-020-00624-6
9. Ledowski T, Averhoff L, Tiong WS, Lee C. Analgesia nociception index (ANI) to predict intraoperative haemodynamic changes: results of a pilot investigation. Acta Anaesthesiol Scand. (2014) 58(1):74–9. doi: 10.1111/aas.12216
10. Jeanne M, Delecroix M, De Jonckheere J, Keribedj A, Logier R, Tavernier B. Variations of the analgesia nociception index during propofol anesthesia for total knee replacement. Clin J Pain. (2014) 30(12):1084–8. doi: 10.1097/AJP.0000000000000083
11. Jeanne M, Clément C, De Jonckheere J, Logier R, Tavernier B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. (2012) 26(4):289–94. doi: 10.1007/s10877-012-9354-0
12. Boselli E, Bouvet L, Bégou G, Torkmani S, Allaouchiche B. Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using analgesia/nociception index (ANI): a prospective observational study. Minerva Anestesiol. (2015) 81(3):288–97. 25014481.25014481
13. Boselli E, Logier R, Bouvet L, Allaouchiche B. Prediction of hemodynamic reactivity using dynamic variations of analgesia/nociception index (ΔANI). J Clin Monit Comput. (2016) 30(6):977–84. doi: 10.1007/s10877-015-9802-8
14. Sriganesh K, Theerth KA, Reddy M, Chakrabarti D, Rao GSU. Analgesia nociception index and systemic haemodynamics during anaesthetic induction and tracheal intubation: a secondary analysis of a randomised controlled trial. Indian J Anaesth. (2019) 63(2):100. doi: 10.4103/ija.IJA_656_18
15. Kommula LK, Bansal S, Umamaheswara Rao GS. Analgesia nociception index monitoring during supratentorial craniotomy. J Neurosurg Anesthesiol. (2019) 31(1):57–61. doi: 10.1097/ANA.0000000000000464
16. Anderson TA, Segaran JR, Toda C, Sabouri AS, De Jonckheere J. High-frequency heart rate variability index: a prospective, observational trial assessing utility as a marker for the balance between analgesia and nociception under general anesthesia. Anesth Analg. (2020) 130(4):1045–53. doi: 10.1213/ANE.0000000000004180
17. Xie H, Chen W, Liu J, Li J, Li S. Changes of analgesia/nociception index under different surgical steps in abortion under general anesthesia: a prospective clinical study. J Clin Anesth. (2020) 66:109898. doi: 10.1016/j.jclinane.2020.109898
18. Migeon A, Desgranges FP, Chassard D, Blaise BJ, De Queiroz M, Stewart A, et al. Pupillary reflex dilatation and analgesia nociception index monitoring to assess the effectiveness of regional anesthesia in children anesthetised with sevoflurane. Paediatr Anaesth. (2013) 23(12):1160–5. doi: 10.1111/pan.12243
19. Avez-Couturier J, De Jonckheere J, Jeanne M, Valleé L, Cuisset JM, Logier R. Assessment of procedural pain in children using analgesia nociception index a pilot study. Clin J Pain. (2016) 32(12):1100–4. doi: 10.1097/AJP.0000000000000360
20. Weber F, Geerts NJE, Roeleveld HG, Warmenhoven AT, Liebrand CA. The predictive value of the heart rate variability-derived analgesia nociception index in children anaesthetized with sevoflurane: an observational pilot study. Eur J Pain. (2018) 22(9):1597–05. doi: 10.1002/ejp.1242
21. Julien-Marsollier F, Rachdi K, Caballero MJ, Ayanmanesh F, Vacher T, Horlin AL, et al. Evaluation of the analgesia nociception index for monitoring intraoperative analgesia in children. Br J Anaesth. (2018) 121(2):462–8. doi: 10.1016/j.bja.2018.03.034
22. Soral M, Altun GT, Dinçer PÇ, Arslantaş MK, Aykaç Z. Effectiveness of the analgesia nociception index monitoring in patients who undergo colonoscopy with sedo-analgesia. Turkish J Anaesthesiol Reanim. (2020) 48(1):50. doi: 10.5152/TJAR.2019.45077
23. Dundar N, Kus A, Gurkan Y, Toker K, Solak M. Analgesia nociception index (ani) monitoring in patients with thoracic paravertebral block: a randomized controlled study. J Clin Monit Comput. (2018) 32(3):481–6. doi: 10.1007/s10877-017-0036-9
24. Le Gall L, Lle David A, Carles P, Bastien Leuillet S, Chastel B, Fleureau C, et al. Benefits of intraoperative analgesia guided by the analgesia nociception index (ANI) in bariatric surgery: an unmatched case-control study. Anaesth Crit Care Pain Med. (2019) 38(1):35–9. doi: 10.1016/j.accpm.2017.09.004
25. Dostalova V, Schreiberova J, Bartos M, Kukralova L, Dostal P. Surgical pleth index and analgesia nociception index for intraoperative analgesia in patients undergoing neurosurgical spinal procedures: a comparative randomized study. Minerva Anestesiol. (2019) 85(12):1265–72. doi: 10.23736/S0375-9393.19.13765-0
26. Tribuddharat S, Sathitkarnmanee T, Sukhong P, Thananun M, Promkhote P, Nonlhaopol D. Comparative study of analgesia nociception index (ANI) vs. standard pharmacokinetic pattern for guiding intraoperative fentanyl administration among mastectomy patients. BMC Anesthesiol. (2021) 21(1):50. doi: 10.1186/s12871-021-01272-2
27. Szental JA, Webb A, Weeraratne C, Campbell A, Sivakumar H, Leong S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI®) monitoring: a randomized clinical trial. Br J Anaesth. (2015) 114(4):640–5. doi: 10.1093/bja/aeu411
28. Boselli E, Daniela-Ionescu M, Bégou G, Bouvet L, Dabouz R, Magnin C, et al. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI). Br J Anaesth. (2013) 111(3):453–9. doi: 10.1093/bja/aet110
29. Abdullayev R, Uludag O, Celik B. Analgesia nociception index: assessment of acute postoperative pain. Brazilian J Anesthesiol. (2019) 69(4):396–402. doi: 10.1016/j.bjan.2019.01.003
30. Gall O, Champigneulle B, Schweitzer B, Deram T, Maupain O, Montmayeur Verchere J, et al. Postoperative pain assessment in children: a pilot study of the usefulness of the analgesia nociception index † †this study was presented, in part, during the 2012 annual meeting of the French society of anaesthesia and intensive care. Br J Anaesth. (2015) 115(6):890–5. doi: 10.1093/bja/aev361
31. Logier R, Jeanne M, De Jonckheere J, Dassonneville A, Delecroix M, Tavernier B. Physiodoloris: a monitoring device for analgesia/nociception balance evaluation using heart rate variability analysis. Annu Int Conf IEEE Eng Med Biol Soc. (2010) 2010:1194–7. doi: 10.1109/IEMBS.2010.5625971
32. Parker N, Ledowski T, Tiong WS, Lee C, Wong B, Fiori T. Analgesia nociception index: evaluation as a new parameter for acute postoperative pain. Br J Anaesth. (2013) 111(4):627–9. doi: 10.1093/bja/aet111
33. Upton HD, Ludbrook GL, Wing A, Sleigh JW. Intraoperative “analgesia nociception index”-guided fentanyl administration during sevoflurane anesthesia in lumbar discectomy and laminectomy: a randomized clinical trial. Anesth Analg. (2017) 125(1):81–90. doi: 10.1213/ANE.0000000000001984
34. Daccache G, Caspersen E, Pegoix M, Monthé-Sagan K, Berger L, Fletcher D, et al. A targeted remifentanil administration protocol based on the analgesia nociception index during vascular surgery. Anaesth Crit Care Pain Med. (2017) 36(4):229–32. doi: 10.1016/j.accpm.2016.08.006
35. Ramos-Luengo A, Gardeta Pallarés A, Asensio Merino F. Usefulness of ANI (analgesia nociception index) monitoring for outpatient saphenectomy surgery outcomes: an observational study. J Clin Monit Comput. (2021) 35(3):491–7. doi: 10.1007/s10877-020-00491-1
36. Yang S, Xiao W, Wu H, Liu Y, Feng S, Lu J, et al. Management based on multimodal brain monitoring may improve functional connectivity and post-operative neurocognition in elderly patients undergoing spinal surgery. Front Aging Neurosci. (2021) 13. doi: 10.3389/fnagi.2021.705287
37. Larsen LG, Wegger M, Lé Greves S, Erngaard L, Hansen TG. Emergence agitation in paediatric day case surgery: a randomised, single-blinded study comparing narcotrend and heart rate variability with standard monitoring. Eur J Anaesthesiol. (2022) 39(3):261–8. doi: 10.1097/EJA.0000000000001649
38. Jendoubi A, Abbes A, Ghedira S, Houissa M. Pain measurement in mechanically ventilated patients with traumatic brain injury: behavioral pain tools versus analgesia nociception index. Indian J Crit Care Med. (2017) 21(9):585–8. doi: 10.4103/ijccm.IJCCM_419_16
39. Broucqsault-Dédrie C, De Jonckheere J, Jeanne M, Nseir S. Measurement of heart rate variability to assess pain in sedated critically ill patients: a prospective observational study. PLoS One. (2016) 11(1):e0147720. doi: 10.1371/journal.pone.0147720
40. Chanques G, Tarri T, Ride A, Prades A, De Jong A, Carr J, et al. Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study. Br J Anaesth. (2017) 119(4):812–20. doi: 10.1093/bja/aex210
41. Papaioannou V, Chouvarda I, Gaertner E, Benyamina M, Ferry A, Maurel V, et al. Heart rate variability and cardiac baroreflex inhibition-derived index predicts pain perception in burn patients. Burns. (2016) 42(7):1445–54. doi: 10.1016/j.burns.2016.04.017
42. Boselli E, Fatah A, Ledochowski S, Allaouchiche B. ANI and BIS variations in supine and prone position during closed-tracheal suction in sedated and myorelaxed ICU patients with severe COVID-19: a retrospective study. J Clin Monit Comput. (2021) 35(6):1403–9. doi: 10.1007/s10877-020-00612-w
43. Aragón-Benedí C, Oliver-Forniés P, Galluccio F, Altinpulluk EY, Ergonenc T, El Sayed Allam A, et al. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS One. (2021) 16:e0249128. doi: 10.1371/journal.pone.0249128
44. Le Guen M, Jeanne M, Sievert K, Al Moubarik M, Chazot T, Laloë PA, et al. The analgesia nociception index: a pilot study to evaluation of a new pain parameter during labor. Int J Obstet Anesth. (2012) 21(2):146–51. doi: 10.1016/j.ijoa.2012.01.001
45. Vamour C, De Jonckheere J, Mestdagh B, Storme L, Richart P, Garabedian C, et al. Impact of skin-to-skin contact on maternal comfort in patients with elective caesarean section: a pilot study. J Gynecol Obstet Hum Reprod. (2019) 48(8):663–8. doi: 10.1016/j.jogoh.2019.07.011
46. Pontet J, Contreras P, Curbelo A, Medina J, Noveri S, Bentancourt S, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. (2003) 18(3):156–63. doi: 10.1016/j.jcrc.2003.08.005
47. Chen WL, Chen JH, Huang CC, Kuo CD, Huang CI, Lee LS. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med. (2008) 26(4):395–401. doi: 10.1016/j.ajem.2007.06.016
48. Annane D, Trabold F, Sharshar T, Jarrin I, Blanc AS, Raphael JC, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. (1999) 160(2):458–65. doi: 10.1164/ajrccm.160.2.9810073
49. Santa Cruz Mercado LA, Liu R, Bharadwaj KM, Johnson JJ, Gutierrez R, Das P, et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. (2023) 02129:1–11. doi: 10.1001/jamasurg.2023.2009
50. Ledowski T, Schneider M, Gruenewald M, Goyal RK, Teo SR, Hruby J. Surgical pleth index: prospective validation of the score to predict moderate-to-severe postoperative pain. Br J Anaesth. (2019) 123(2):328–32. doi: 10.1016/j.bja.2018.10.066
51. Sabourdin N, Giral T, Wolk R, Louvet N, Constant I. Pupillary reflex dilation in response to incremental nociceptive stimuli in patients receiving intravenous ketamine. J Clin Monit Comput. (2018) 32(5):921–8. doi: 10.1007/s10877-017-0072-5
52. StÖcKle PA, Julien M, Issa R, DÉcary E, Brulotte V, Drolet P, et al. Validation of the PMD100 and its nol index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. (2018) 84:1160–8. doi: 10.23736/S0375-9393.18.12720-9
Keywords: analgesia, nociception, analgesia nociception index, monitoring, pain
Citation: Hum B, Christophides A, Jin Z, Elias M, Taneja K and Bergese SD (2023) The validity and applications of the analgesia nociception index: a narrative review. Front. Surg. 10:1234246. doi: 10.3389/fsurg.2023.1234246
Received: 3 June 2023; Accepted: 31 July 2023;
Published: 10 August 2023.
Edited by:
Hong Liu, UC Davis Health, United StatesReviewed by:
Etrusca Brogi, University of Pisa, ItalyDavid R. Drover, Stanford University, United States
© 2023 Hum, Christophides, Jin, Elias, Taneja and Bergese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bill Hum bill.hum@stonybrookmedicine.edu
Abbreviations BP, blood pressure; HR, heart rate; ANI, analgesia nociception index; ECG, electrocardiogram; HRV, heart rate variability; HF, high frequency; LF, low frequency; PACU, post-anesthesia care unit; ICU, intensive care unit; PK, prediction probability; AUC ROC, area under a receiver operating characteristic curve; NRS, numeric rating scale; FLACC, face, legs, activity, cry, consolability scale; VAS, visual analog scale; BPS, behavioral pain scale; MODS, multiple organ dysfunction syndrome.
 Bill Hum
Bill Hum Alexa Christophides1
Alexa Christophides1  Zhaosheng Jin
Zhaosheng Jin Kamil Taneja
Kamil Taneja Sergio D. Bergese
Sergio D. Bergese