Procalcitonin, Interleukin-6 and C-reactive Protein Levels Predict Renal Adverse Outcomes and Mortality in Patients with Acute Type A Aortic Dissection
- 1Division of Nephrology, Department of Medicine, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Ultrasound, West China Hospital of Sichuan University, Chengdu, China
- 3Department of Orthopedics, Second People’s Hospital of Chengdu, Chengdu, China
- 4Department of Pediatric Nephrology, West China Second University Hospital, Sichuan University, Chengdu, China
Background: Acute type A aortic coarctation (AAAD) is a highly deadly and serious life-threatening disease. The purpose of this study was to estimate the predictive value of peak procalcitonin, interleukin-6, and C-reactive protein levels on adverse renal outcomes and mortality in patients undergoing surgery for AAAD.
Methods: Perioperative peak PCT, CRP, and IL-6 levels were retrospectively collected in 331 patients hospitalized with AAAD from 2009 to 2021. The primary endpoints were AKI stage 2–3 and mortality. The receiver operating characteristic (ROC) curves were used to compare the predictive values of peak PCT, CRP, and IL-6 for different clinical outcomes. Multivariable logistic regression analysis was used to find risk factors for AKI and 30-day mortality.
Results: The incidence of AKI stage 2–3 following AAAD was 50.8% (168/331). The 30-day and overall mortality were significantly greater in the AKI 2–3 group than in the AKI 0–1 group (P = 0.000). ROC curve analysis showed that peak PCT, with an area under the ROC curve (AUC) of 0.712, was a more accurate predictor of adverse renal outcomes than peak IL-6 and CRP. Multivariable logistic regression analysis revealed that PCT > 0.39 ng/mL was an independent risk factor for AKI stage 2–3. Peak IL-6 > 259 pg/mL was found to be an independent risk factor for 30-day mortality.
Conclusion: In patients with surgery for AAAD, peak PCT provides a well-predictive indicator of AKI stage 2–3 and peak IL-6 indicates a favorable predictor of 30-day mortality.
Introduction
Acute kidney injury (AKI) is a common complication in hospitalized patients and in intensive care unit (ICU) admissions of critically ill patients (1–3), associated with high morbidity and mortality (4). AKI develops in 30% of patients undergoing cardiac surgery, with approximately 1% of patients requiring dialysis (5). AKI is associated with increased mortality after cardiac surgery (6, 7). In contrast to other heart surgeries, aortic surgery results in a higher incidence of dialysis-requiring acute kidney injury (D-AKI) (8).
Gender, age, body mass index (BMI), obesity, hypertension, renal malperfusion, preoperative serum creatinine (Scr), prolonged cardiopulmonary bypass (CPB) time, postoperative blood transfusion, and sepsis are risk factors for the development of AKI in cardiac surgery (9–12). The pathophysiological mechanisms of AKI associated with cardiac surgery are complex, including the direct inflammatory injury following renal hypoperfusion and ischemia-reperfusion injury (13), with the inflammatory response being central (14).
In addition, the pathogenesis and prognosis of acute type A aortic dissection (AAAD) are also relevant to the inflammatory response (15, 16). A number of inflammatory markers such as interleukin-6 (IL-6), interleukin-10 (IL-10), and C-reactive protein (CRP) have been revealed to be independent risk factors for AKI and mortality in patients after cardiac surgery (17, 18). Inflammatory biomarkers are receiving increasing attention as prognostic indicators in patients after cardiac surgery. The impact of perioperative inflammatory biomarkers on clinical outcomes has been understudied in patients undergoing surgery for AAAD.
Inflammatory biomarker levels during the perioperative period vary continuously with factors such as time, extent of disease, and medical intervention. Peak inflammatory markers may be more predictive than those measured at admission (19). In this study, we postulated that higher peak inflammatory biomarkers would have a predictive value for prognosis in patients with AAAD. The aim of this study was to evaluate the role of peak procalcitonin (PCT), CRP, and IL-6 in predicting renal adverse outcomes and mortality in patients undergoing surgery for AAAD.
Materials and Methods
Patients
This retrospective study was approved by the ethics committee of West China Hospital, Sichuan University, and registered at the Chinese Clinical Trial Registry (ChiCTR1900021290). Informed consent was waived given that this was a retrospective study. We reviewed the electronic medical records of adult patients undergoing surgery for AAAD at West China Hospital, Sichuan University, between 2009 and 2021. All participants were diagnosed as AAAD by enhanced computed tomography (CT) or echocardiography. Patients were excluded with the following factors: end-stage renal disease (ESRD) or requiring renal replacement therapy (RRT); post kidney transplantation; death within 24 h after admission to hospital; incomplete data. Finally, the study included 331 patients.
Data Collection
From the electronic medical record system, we obtained baseline characteristics and perioperative data of the patients. Laboratory data were available from the clinical laboratory of the investigator’s hospital by analysis of venous blood specimens collected on admission. Peak CRP, PCT and IL-6 were defined as the highest levels of CRP, PCT and IL-6 in the perioperative period.
Outcomes
The primary outcomes were the development of AKI stage 2–3 and mortality. According to the kidney disease: Improving Global Outcomes criteria (KDIGO) (20), patients with ≥200% Scr rise from baseline within 7 days, urine output <0.5 mL/kg/h for more than 12 h or requiring for RRT were assigned to group of AKI stage 2–3. 30-day mortality was defined as death within 30 days after surgery for AAAD. Overall mortality referred to the total number of deaths during the follow-up period.
The second outcomes were acute kidney disease (AKD), requiring for continuous renal replacement therapy (CRRT), Ventilator time, and length of stay (LOS) in hospital.
Statistical Methods
Continuous variables were presented as means ± standard deviations (SD) or medians (25th, 75th percentile). Categorical variables were presented as percentages. Student’s t-test, chi-square test, or Mann-Whitney U-test were performed to compare differences between groups. The area under the receiver operating characteristic (ROC) curve (AUC) was used to compare the predictive power of PCT, IL-6, and CRP for adverse outcomes. The z-test was applied to verify the difference between the different ROC curves. Multivariable logistic regression analysis was used to identify independent risk factors for the occurrence of AKI stage 2–3 and 30-day mortality. The Hosmer-Lemeshow test was applied to test the goodness of fit of these logistic regression models. A P value of <0.05 was considered statistically significant.
All statistical analysis and statistical plots were performed with the SPSS software package, version 26.0 (IBM Corp., Chicago, USA), GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA) and Medcalc software.
Results
Demographic Characteristics of Patients
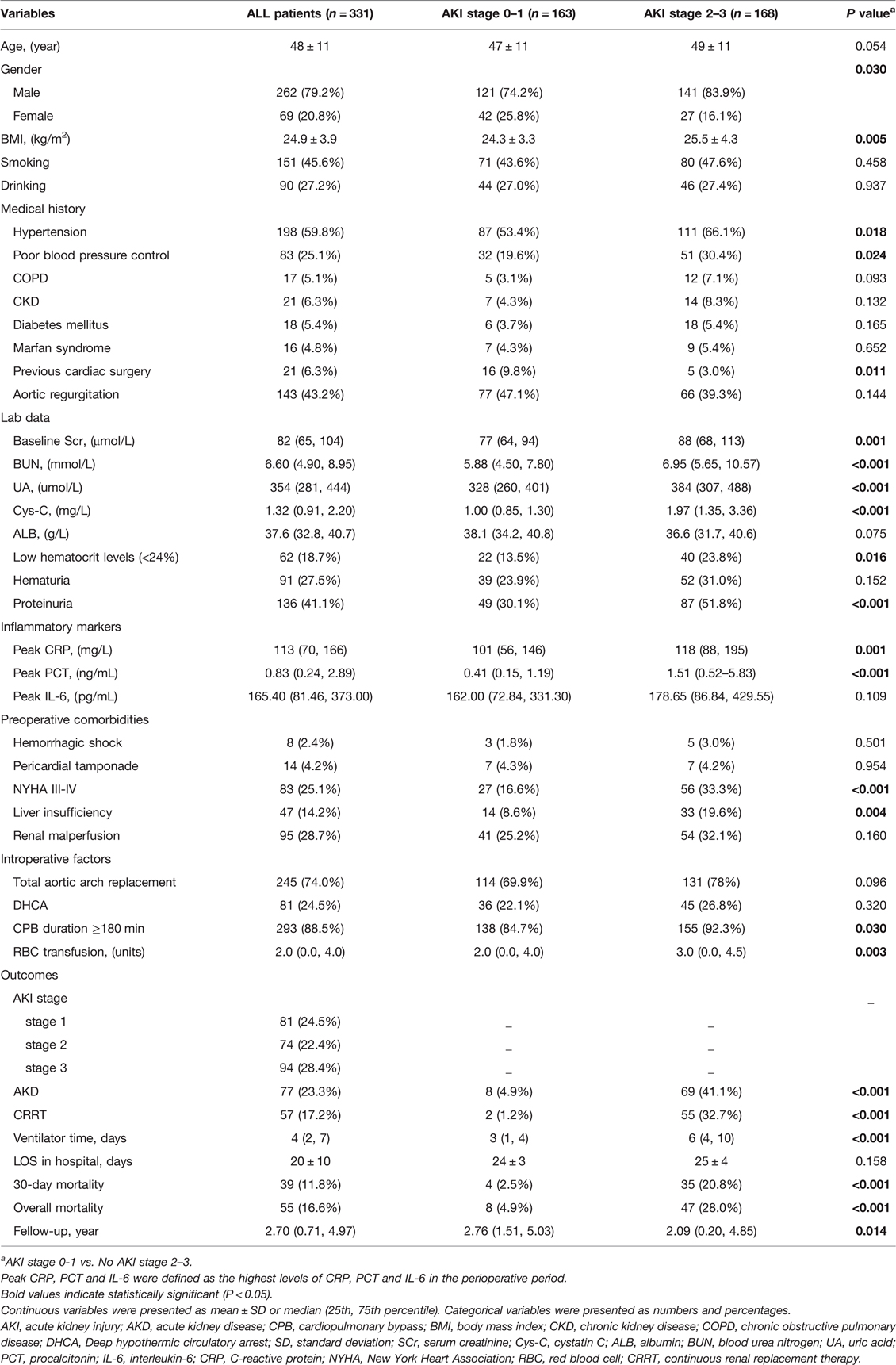
According to Table 1, the prevalence of AKI stage 0–1 and AKI stage 2–3 after surgery in this study was 49.2% (163/331) and 50.8% (168/331), respectively. Preoperative variables such as BMI, hypertension, poor blood pressure control, New York Heart Association (NYHA) III–IV, and liver insufficiency were statistically different between the two groups (P < 0.05). Based on laboratory findings, two groups showed statistically significant differences in baseline Scr, urea nitrogen (BUN), uric acid (UA), cystatin C (Cys-C) proteinuria, and low hematocrit levels (<24%). Compared to the AKI stage 0–1 group, the AKI stage 2–3 group had a higher incidence of CPB duration ≥180 min and red blood cell (RBC) transfusion during the procedure for AAAD (P < 0.05). Additionally, those with AKI stage 2–3 were more likely to require CRRT, had a higher incidence of AKD, delayed recovery of renal function, and a longer duration of mechanical ventilation than patients with AKI stages 0–1 (P < 0.05). Finally, there was a significant difference in 30-day and overall mortality between patients with AKI stage 0–1 and 2–3 (P < 0.05).
Peak CRP, PCT and IL-6 Levels and ROC Analysis
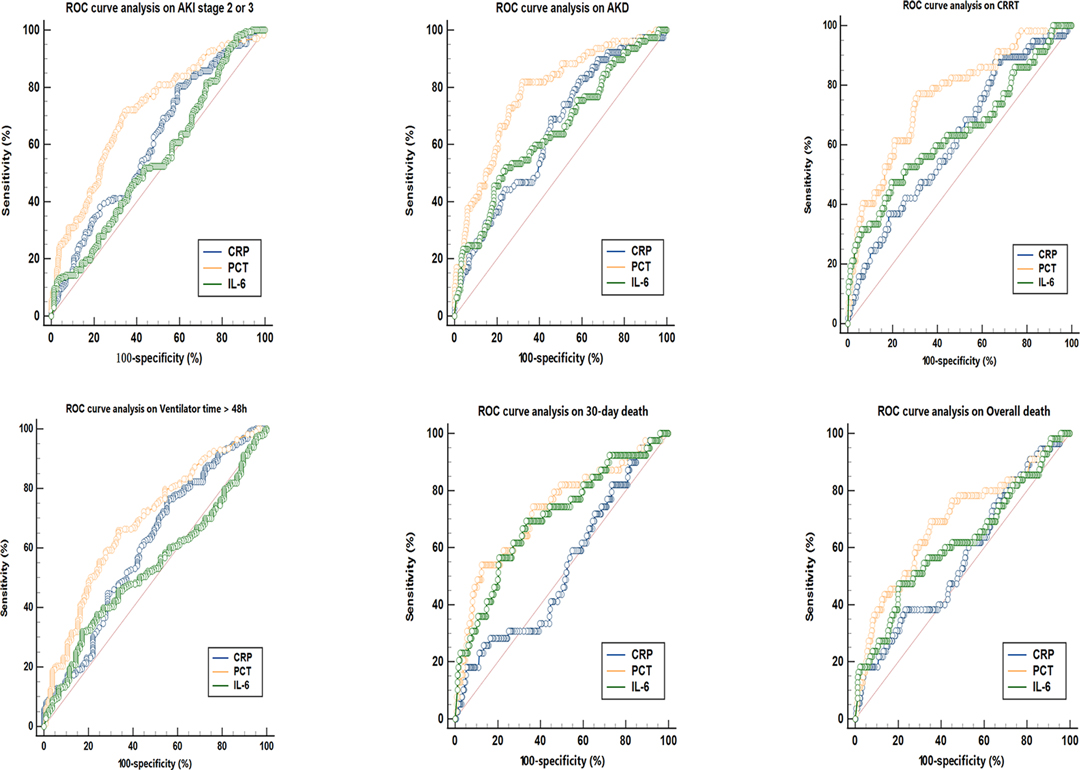
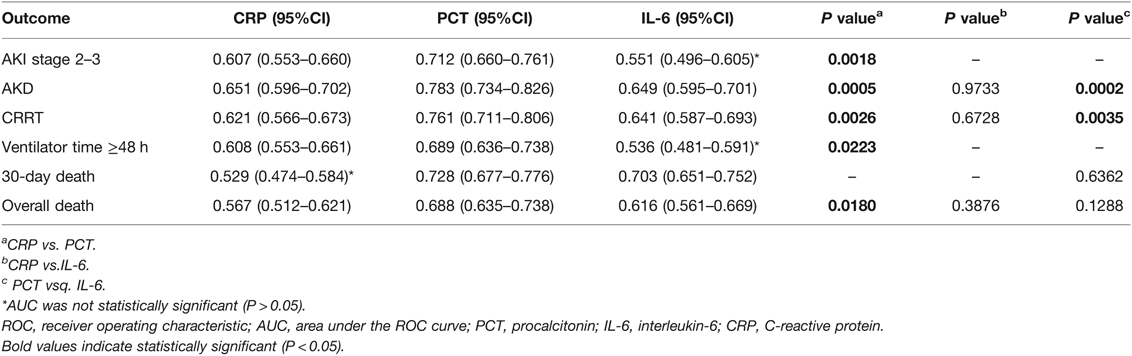
As presented in Figure 1, patients were classified into separate groups based on clinical outcomes in order to assess differences in inflammatory biomarker levels. The study’s study revealed that there was a significant difference in peak CRP and PCT levels, but no difference in IL-6 levels, between the AKI stage 2–3 and 0–1 groups. In comparison to surviving patients, dead patients (both those at 30 days and overall death) had significantly different peak PCT and IL-6 levels, while CRP levels were not statistically different. The peak CRP, PCT, and IL-6 levels were higher in the groups of AKD and CRRT (P < 0.05). The ROC curves were used to verify the predictive power of CRP, PCT, and IL-6 on renal outcomes and mortality, and the results were presented in Table 2. PCT (AUC, 0.712) showed a better predictive value for AKI stage 2–3 compared to CRP (AUC, 0.607). The AUC of IL-6 for predicting AKI stage 2–3 was 0.551 (0.496–0.605, P > 0.05), which was not statistically significant. Among the three inflammatory markers, PCT demonstrated the greatest predictive value for AKD and CRRT, while IL-6 and CRP had similar predictive effects. In predicting 30-day mortality, PCT had a similar predictive value to IL-6, while CRP had no predictive value. In Figure 2, the sensitivity and specificity of peak CRP, PCT, and IL-6 in predicting clinical endpoints were shown.
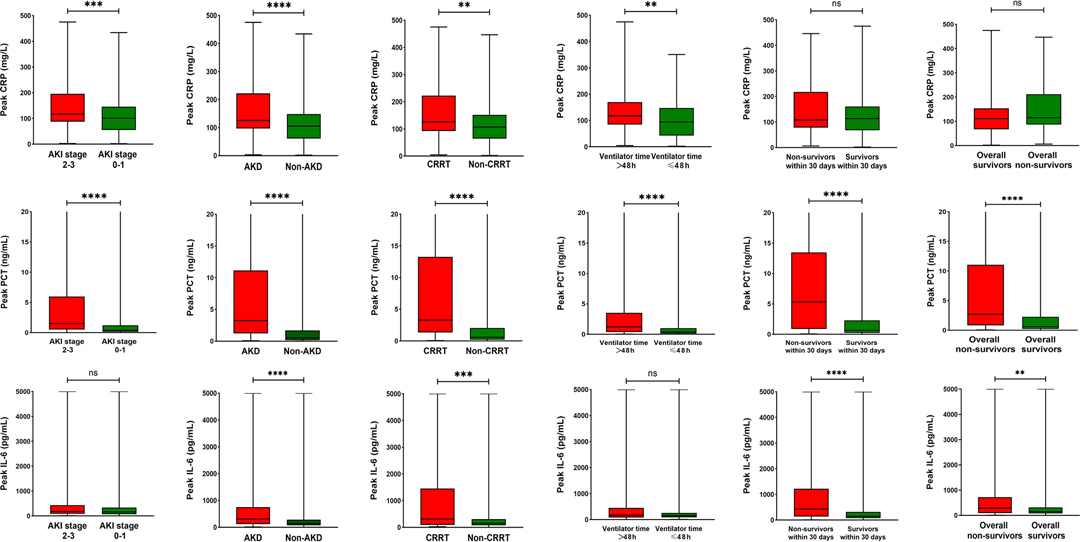
Figure 1. Peak CRP, PCT and IL-6 levels in the different groups according to major clinical outcomes. **P < 0.05, ***P < 0.001, ****P < 0.0001, “ns” represents no statistically significant differences (P > 0.05). AKI, acute kidney injury; AKD, acute kidney disease; CRRT, continuous renal replacement therapy; PCT, procalcitonin; IL-6, interleukin-6; CRP, C-reactive protein.
Risk Factors of AKI stage 2–3 and 30-day mortality
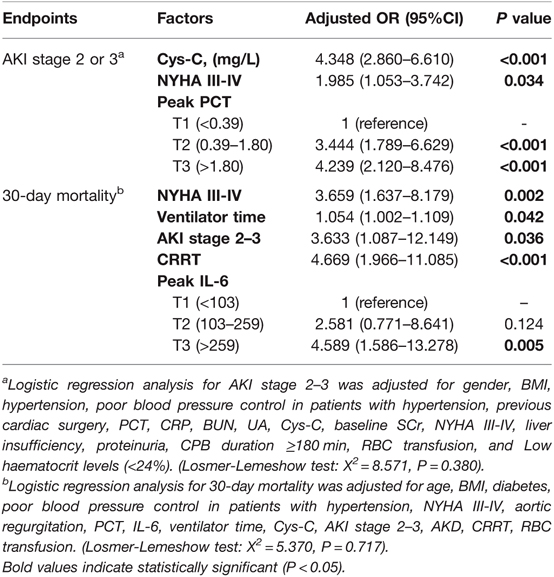
In Table 3, multivariable binary logistic regression analysis suggested that Cys-C (adjusted odds ratio [OR], 4.348, P < 0.001) and NYHA III–IV (adjusted OR, 1.985, P = 0.034) were independent risk factors for AKI stage 2–3. The second tertile (adjusted OR, 3.444, P < 0.001) and the third tertile (adjusted OR, 4.239, P < 0.001) of peak PCT were independently associated with AKI stage 2–3 when compared to the lowest tertile. Peak CRP and IL-6 were not found to be independently associated with AKI stage 2–3.
Kaplan-Meier analysis indicated that patients with higher peak IL-6 and PCT had lower survival rates during 30 days after surgery for AAAD (Figure 3). Another logistic regression analysis showed that NYHA III–IV (adjusted OR, 3.659, P = 0.002), requiring CRRT (adjusted OR, 4.669, P < 0.001), ventilator time (adjusted OR, 1.054, P = 0.042), and AKI stage 2–3 (adjusted OR, 3.633, P = 0.036) were independently associated with 30-day mortality. While the highest tertile (adjusted OR, 3.633, P = 0.036) of peak IL-6 was associated with a higher risk of 30-day mortality compared with the lowest tertile (Table 3).
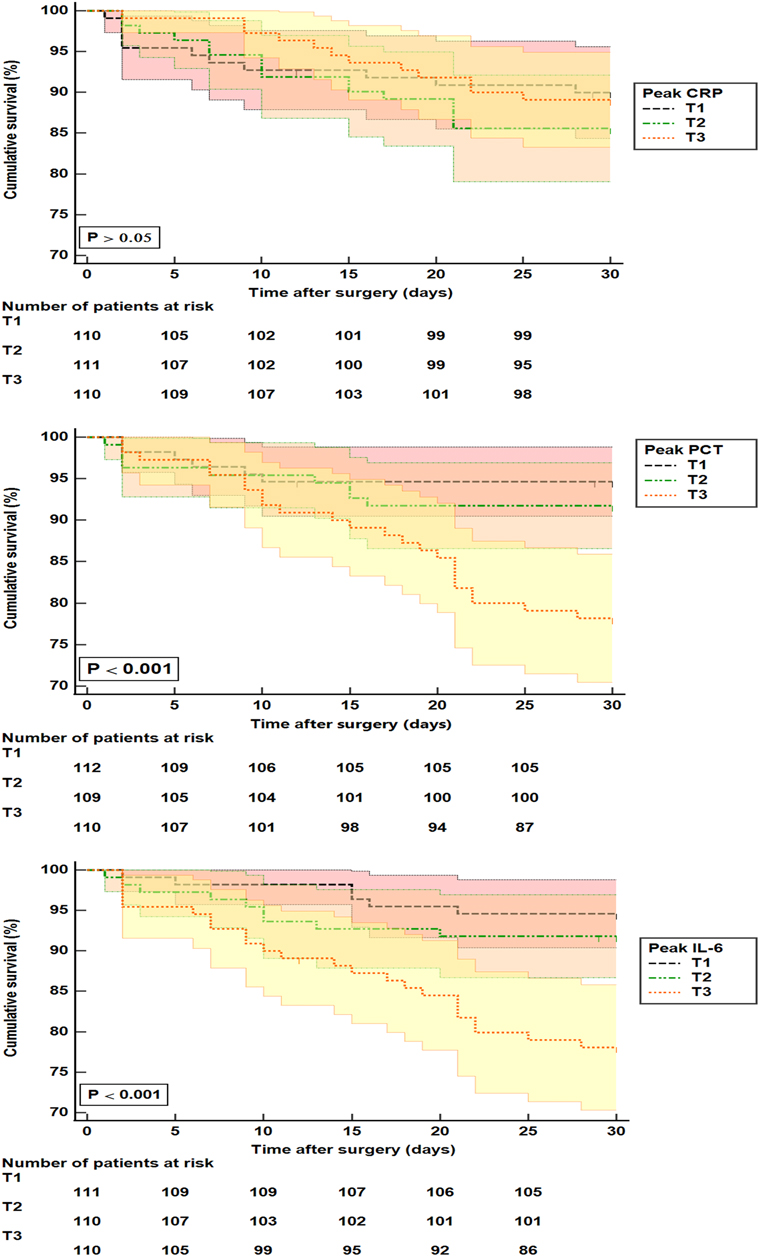
Figure 3. Kaplan-Meier analysis for 30-day mortality in patients with surgery for AAAD. CRP: T1 (<88 mg/L); T2 (88–145 mg/L); T3 (>145 mg/L). PCT: T1 (<0.39 ng/mL); T2 (0.39 –1.80 ng/mL); T3 (>1.80 ng/mL). IL-6: T1 (<103 pg/mL); T2 (103–259 pg/mL); T3 (>259 pg/mL). T, tertile; PCT, procalcitonin; IL-6, interleukin-6; CRP, C-reactive protein.
Discussion
Here, we evaluated the predictive value of peak inflammatory biomarkers for renal adverse outcomes and mortality in patients with AAAD. The findings were as follows: (1) Peak PCT was a better predictor of renal outcomes, including AKI stage 2–3, requiring CRRT, and AKD, compared to CRP and IL-6. A high level of PCT (>0.39 ng/mL) was shown to be an independent risk factor for AKI stage 2–3. (2) Peak PCT and IL-6 were better predictors of mortality than CRP. Peak IL-6 (>259 pg/mL) was an independent risk factor for 30-day mortality. Our study confirmed the association between high levels of PCT and AKI 2–3. In addition, we also noted that PCT levels were higher in patients requiring CRRT and in those with a longer duration of renal injury. A prospective study by Kurtul et al. (21) demonstrated that serum PCT levels at admission were independently associated with the development of contrast-associated acute kidney injury. Clementi et al. (22) and Brocca A et al. (14) both concluded that PCT levels within 48 h after cardiac surgery were predictive of AKI. Liu et al. (23) also suggested that PCT levels were predictive of the development of AKI stage 3. The relationship between cardiac surgery and post-operative AKI and PCT levels is complicated. Trauma, tissue damage, infection, and inflammation are known to cause PCT to remain elevated (24). Because of the systemic inflammatory response syndrome (SIRS) (25), cardiac surgery can lead to elevated serum PCT levels and acute kidney injury (26).Furthermore, the direct cytotoxicity of PCT can exacerbate kidney damage (27). Renal dysfunction could contribute to a decrease in renal clearance of PCT, indirectly causing an increase in PCT levels (28), leading to a vicious cycle. In our analysis, this may be the reason why PCT levels at different time periods can be used as a biomarker for AKI.
CRP represents an independently risk factor for various cardiovascular diseases (29, 30). CRP levels are significantly elevated in patients with AAAD and are related to poor prognosis in patients with AAAD. Schillingeret al. and Wen et al. also concluded that patients with high levels of CRP at admission were at high risk of short-term mortality (18, 31). In the present study, peak CPR levels were higher in patients with adverse renal outcomes, while surprisingly, no differences were found in CRP levels between 30-day and overall survivors and non-survivors. This inconsistency may be due to the fact that the survival of patients with AAAD is also affected by a variety of factors, including age, medical conditions, surgery-related factors, and postoperative complications (32, 33).
Our findings indicated that IL-6 (>259 pg/mL) would be an independent risk factor for 30-day mortality postoperatively. The predictive value of IL-6 and PCT for 30-day mortality and overall mortality after surgery for AAAD was greater in comparison to CRP. Moreover, the risk of 30-day mortality was in a dose-dependent manner related to the PCT and IL-6 levels. Studies reported that IL-6 was a good biomarker to predict mortality after cardiac surgery (14, 22). IL-6 is a pro-inflammatory cytokine that plays a critical role in the initiation and resolution of a variety of inflammation and immunological responses, as well as inducing the synthesis of significant levels of C-reactive protein (34). SIRS after cardiac surgery can be exacerbated by prolonged CPB, cytokinemia, and high IL-6 levels (35, 36). Furthermore, SIRS and IL-6 levels were found to be associated with an increased risk of pneumonia, multiple organ failure, and mortality (37). Remarkably, no direct connection between IL-6 and AKI stage 2–3 was observed in the current investigation. This finding contradicts prior research indicating that increased IL-6 levels following cardiac surgery are significantly associated with an increased risk of AKI (17, 38). It has been claimed that IL-6 expression is associated with the development and severity of AKI and that IL-6 plays a dual function in its induction, aggravating renal injury via a cell-mediated immune response and reducing renal injury by activation of a protective response in the tubular epithelium (39). Since this study relies on retrospective research, a relatively small sample size, and the possibility of bias, these findings should be interpreted with caution. A greater IL-6 level was shown to be associated with AKD and CRRT patients. Brocca et al. also reported a significant increase in IL-6 in patients requiring RRT (14).
Study Limitations
This study has several limitations. First, this is a retrospective study conducted in a single center with a limited number of patients studied. Clinical data were collected by different people at different times and may be subject to selection bias. Therefore, a prospective large-scale multicenter study is needed to confirm our results. Second, we recorded only peak PCT, IL-6, and CRP levels, not variations in these inflammatory markers. As a result, the prognostic significance of inflammatory marker levels at certain time points (e.g., admission, one day preoperatively, one day postoperatively, seven days postoperatively, etc.) could not be determined. Despite these limitations, it is reasonable to conclude that peak PCT, IL-6, and CRP levels are useful for predicting renal outcomes and mortality.
Conclusion
This study suggested that elevated perioperative inflammatory biomarkers were associated with postoperative clinical outcomes in patients with AAAD. Peak PCT could be a useful inflammatory biomarker for predicting AKI stages 2–3 in patients with AAAD surgery, while peak IL-6 could be a reliable predictive indicator of 30-day mortality.
Data Availability Statement
The datasets were obtained from the database of West China Hospital. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by The Biomedical Ethics Committee of West China Hospital of Sichuan University (Approved No. of the ethic committee: No. 47, 2019). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Research idea and study design: LY, XC; data acquisition: XC, JZ, MF; statistical analysis: XC, MF, JY, XW, SW; supervision or mentorship: LY. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China [NO.82102067] and 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University [NO.2020HXFH049].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394(10212):1949–64. doi: 10.1016/S0140-6736(19)32563-2
2. Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. (2018) 14(10):607–25. doi: 10.1038/s41581-018-0052-0
3. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41(8):1411–23. doi: 10.1007/s00134-015-3934-7
4. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. (2021) 7(1):52. doi: 10.1038/s41572-021-00284-z
5. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. (2006) 1(1):19–32. doi: 10.2215/CJN.00240605
6. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119(18):2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011
7. Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. (1998) 104(4):343–8. doi: 10.1016/S0002-9343(98)00058-8
8. Chen JJ, Chang CH, Wu VC, Chang SH, Hung KC, Chu PH, et al. Long-term outcomes of acute kidney injury after different types of cardiac surgeries: a population-based study. J Am Heart Assoc. (2021) 10(9):e019718. doi: 10.1161/JAHA.120.019718
9. Roh GU, Lee JW, Nam SB, Lee J, Choi JR, Shim YH. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg. (2012) 94(3):766–71. doi: 10.1016/j.athoracsur.2012.04.057
10. Neugarten J, Sandilya S, Singh B, Golestaneh L. Sex and the risk of AKI following cardio-thoracic surgery: a meta-analysis. Clin J Am Soc Nephrol. (2016) 11(12):2113–22. doi: 10.2215/CJN.03340316
11. Freeland K, Hamidian Jahromi A, Duvall LM, Mancini MC. Postoperative blood transfusion is an independent predictor of acute kidney injury in cardiac surgery patients. J Nephropathol. (2015) 4(4):121–6. doi: 10.12860/jnp.2015.23
12. Rydén L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation. (2014) 130(23):2005–11. doi: 10.1161/CIRCULATIONAHA.114.010622
13. Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. (2011) 121(11):4210–21. doi: 10.1172/JCI45161
14. Brocca A, Virzì GM, de Cal M, Giavarina D, Carta M, Ronco C. Elevated levels of procalcitonin and interleukin-6 are linked with postoperative complications in cardiac surgery. Scand J Surg. (2017) 106(4):318–24. doi: 10.1177/1457496916683096
15. Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. (2009) 8(1):31–5. doi: 10.1016/j.arr.2008.08.001
16. Nomura F, Tamura K, Yoshitatsu M, Katayama A, Katayama K, Ihara K. Changes in coagulation condition, cytokine, adhesion molecule after repair of type A aortic dissection. Eur J Cardiothorac Surg. (2004) 26(2):348–50. doi: 10.1016/j.ejcts.2004.05.001
17. Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al.. Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol. (2015) 26(12):3123–32. doi: 10.1681/ASN.2014080764
18. Schillinger M, Domanovits H, Bayegan K, Hölzenbein T, Grabenwöger M, Thoenissen J, et al. C-reactive protein and mortality in patients with acute aortic disease. Intensive Care Med. (2002) 28(6):740–5. doi: 10.1007/s00134-002-1299-1
19. Takahashi T, Anzai T, Yoshikawa T, Maekawa Y, Asakura Y, Satoh T, et al. Serum C-reactive protein elevation in left ventricular remodeling after acute myocardial infarction–role of neurohormones and cytokines. Int J Cardiol. (2003) 88(2-3):257–65. doi: 10.1016/S0167-5273(02)00416-3
20. Section 2: AKI Definition. Kidney Int Suppl. (2011), 2012. 2(1): 19–36. doi: 10.1038/kisup.2011.32
21. Kurtul A, Murat SN, Yarlioglues M, Duran M, Ocek AH, Celik IE, et al. Procalcitonin as an early predictor of contrast-induced acute kidney injury in patients with acute coronary syndromes who underwent percutaneous coronary intervention. Angiology. (2015) 66(10):957–63. doi: 10.1177/0003319715572218
22. Clementi A, Brocca A, Virzì GM, de Cal M, Giavarina D, Carta M, et al. Procalcitonin and interleukin-6 levels: are they useful biomarkers in cardiac surgery patients? Blood Purif. (2017) 43(4):290–7. doi: 10.1159/000454672
23. Liu H, Luo Z, Liu L, Yang X, Zhuang Y, Tu G, et al. Inflammatory biomarkers to predict adverse outcomes in postoperative patients with acute type A aortic dissection. Scand Cardiovasc J. (2020) 54(1):37–46. doi: 10.1080/14017431.2019.1689289
24. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. (2000) 49(Suppl 1):S57–S61.
25. Taylor KM. SIRS–the systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. (1996) 61(6):1607–8. doi: 10.1016/0003-4975(96)00225-1
26. Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care. (2006) 10(5):R145. doi: 10.1186/cc5067
27. Araujo M, Doi SQ, Palant CE, Nylen ES, Becker KL. Procalcitonin induced cytotoxicity and apoptosis in mesangial cells: implications for septic renal injury. Inflamm Res. (2013) 62(10):887–94. doi: 10.1007/s00011-013-0646-8
28. Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. (2001) 18(2):79–87. doi: 10.1097/00003643-200102000-00004
29. Li JJ, Zhu CG, Yu B, Liu YX, Yu MY. The role of inflammation in coronary artery calcification. Ageing Res Rev. (2007) 6(4):263–70. doi: 10.1016/j.arr.2007.09.001
30. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. (2016) 67(6):712–23. doi: 10.1016/j.jacc.2015.11.037
31. Wen D, Wu HY, Jiang XJ, Zhang HM, Zhou XL, Li JJ, et al. Role of plasma C-reactive protein and white blood cell count in predicting in-hospital clinical events of acute type A aortic dissection. Chin Med J (Engl). (2011) 124(17):2678–82.
32. Helgason D, Helgadottir S, Ahlsson A, Gunn J, Hjortdal V, Hansson EC, et al. Acute kidney injury after acute repair of type A aortic dissection. Ann Thorac Surg. (2021) 111(4):1292–8. doi: 10.1016/j.athoracsur.2020.07.019
33. Sasabuchi Y, Kimura N, Shiotsuka J, Komuro T, Mouri H, Ohnuma T, et al. Long-term survival in patients with acute kidney injury after acute type A aortic dissection repair. Ann Thorac Surg. (2016) 102(6):2003–9. doi: 10.1016/j.athoracsur.2016.05.006
34. Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month: interleukin 6 (IL-6). J Clin Pathol. (2014) 67(11):932–7. doi: 10.1136/jclinpath-2014-202493
35. Takenaka K, Ogawa E, Wada H, Hirata T. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J Crit Care. (2006) 21(1):48–55. doi: 10.1016/j.jcrc.2005.07.001
36. Hirai S. Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. (2003) 9(6):365–70.
37. Giannoudis PV, Harwood PJ, Loughenbury P, Van Griensven M, Krettek C, Pape HC. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff predict a SIRS state? J Trauma. (2008) 65(3):646–52. doi: 10.1097/TA.0b013e3181820d48
38. Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. (2015) 30(9):1519–27. doi: 10.1007/s00467-015-3088-4
Keywords: acute type A aortic coarctation, inflammatory biomarkers, interleukin-6, C-reactive protein, procalcitonin, adverse renal outcomes, mortality
Citation: Chen X, Zhou J, Fang M, Yang J, Wang X, Wang S and Yang L (2022) Procalcitonin, Interleukin-6 and C-reactive Protein Levels Predict Renal Adverse Outcomes and Mortality in Patients with Acute Type A Aortic Dissection. Front. Surg. 9:902108. doi: 10.3389/fsurg.2022.902108
Received: 22 March 2022; Accepted: 11 April 2022;
Published: 28 April 2022.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Ismail Necati Hakyemez, University of Health Scienses, Bursa Yuksek Ihtisas Training & Research Hospital, TurkeyMagdalena Mierzchała-Pasierb, Wroclaw Medical University, Poland
Copyright © 2022 Chen, Zhou, Fang, Yang, Wang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lichuan Yang ylcgh@163.com
†These authors have contributed equally to this work and share first authorship
Speciality section: This article was submitted to Heart Surgery a section of the journal Frontiers in Surgery
Abbreviations: AAAD, acute type A aortic dissection; AKI, acute kidney injury; AKD, acute kidney disease; PCT, procalcitonin; IL-6, interleukin-6; CRP, C-reactive protein; IL-6, interleukin-10; ROC, the receiver operating characteristic curve; D-AKI, dialysis-requiring acute kidney injury; AUC, area under the ROC curve; BMI, body mass index; CPB, cardiopulmonary bypass; CKD, chronic kidney disease; ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; DHCA, Deep hypothermic circulatory arrest; LOS, length of stay; ICU, intensive care unit; CRRT, continuous renal replacement therapy; SD, standard deviations; 95% CI, 95% confidence interval; OR, odds ratio; T, tertile; SCr, serum creatinine; Cys-C, cystatin C; ALB, albumin; BUN, blood urea nitrogen; UA, uric acid; NYHA, New York Heart Association; SIRS, systemic inflammatory response syndrome; RBC, red blood cell.
 Xuelian Chen
Xuelian Chen Jiaojiao Zhou2†
Jiaojiao Zhou2†  Lichuan Yang
Lichuan Yang