- 1Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China
- 2School of Public Health, Nanjing Medical University, Nanjing, China
- 3Department of STD Epidemiology, National Center for STD Control, Nanjing, China
- 4Department of HIV/STD Control and Prevention, Guizhou Provincial Center for Disease Control and Prevention, Guiyang, China
- 5Department of HIV/STD Control and Prevention, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
Background: People living with HIV (PLWH) are at an increased risk of syphilis infection. The objectives of this study were to assess the overall prevalence of syphilis among PLWH in China and identify factors associated with syphilis infection among PLWH.
Methods: We searched Medline, Embase, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals Database (VIP), Wan-fang Data, and Chinese Biomedical Literature Database (CBM) to identify studies that reported the prevalence of syphilis among PLWH in China and were published in English or Chinese from January 1, 1990, to May 31, 2022. The reference lists of retrieved articles and relevant reviews were also checked to identify additional studies. A random-effect model was fitted to calculate the pooled syphilis prevalence among PLWH. Subgroup analyses, meta-regression analyses and sensitivity analyses were conducted to determine the potential source of heterogeneity.
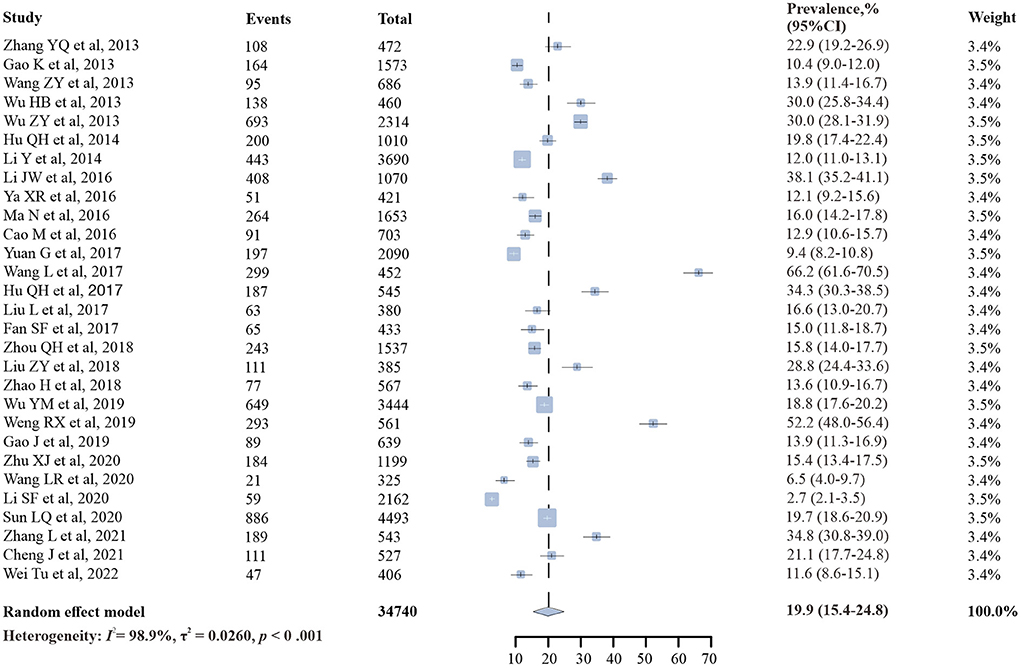
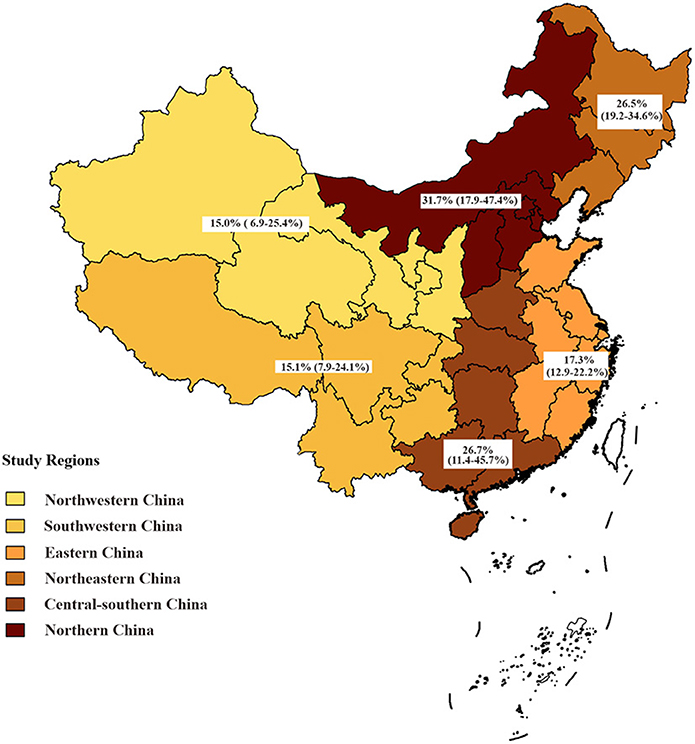
Results: Of the 1,599 articles screened, 29 studies involving 34,740 participants were eligible for inclusion in this meta-analysis. The overall prevalence of syphilis among PLWH in China was 19.9% [95% confidence interval (CI): 15.4–24.8%, I2 = 98.9%]. Subgroup analysis showed that the pooled prevalence of syphilis among men who have sex with men (MSM) with HIV (21.9%, 95% CI: 17.2–26.9%) was much higher than that among heterosexuals (10.3%, 95% CI: 5.2–16.8%); there was regional diversity in the prevalence of syphilis, the highest in northern China (31.7%, 95% CI: 17.9–47.4%), followed by central-southern China (26.7%, 95% CI: 11.4–45.7%), and the lowest in northwestern China (15.0%, 95% CI: 6.9–25.4%); the syphilis prevalence among PLWH decreased as CD4 + T cell count increased (19.6% in CD4 + T cell < 200 vs. 8.7% in ≥ 500) and was higher among non-antiretroviral therapy (non-ART) HIV-infected patients (21.0%, 95% CI: 9.9–35.0%) than that among ART ones (16.1%, 95% CI: 3.9–34.3%).
Conclusions: Our study showed a significantly high prevalence of syphilis among PLWH in China, particularly among MSM with HIV. Developing national guidelines for the integrated screening, monitoring, and management of HIV and syphilis as well as syphilis diagnosis and treatment training programs for physicians at designated HIV treatment hospitals is urgent and crucial to combat HIV and syphilis coinfection in China.
Introduction
Syphilis is a sexually transmitted disease (STD) caused by Treponema pallidum, a subspecies pallidum. It affects various systems of the human body, leading to clinical chronic manifestations such as neurosyphilis, ocular syphilis, otosyphilis, and cardiovascular syphilis (1, 2). Syphilis is known as a serious public health threat worldwide, with an estimate of 7.1 million new cases in 2020 (3). In China, the number of reported syphilis cases has been increasing since the resurgence of syphilis in the late 1970s and remained the third among class B national notifiable infectious diseases since 2009 (4, 5), which has become a major disease burden. In recent years, the incidence of syphilis kept rising, the case rate increased from 31.85 per 100,000 to 38.37 per 100,000 during 2015 and 2019 (6).
The epidemic of HIV is also a serious public health concern in China. HIV has spread from drug users to men who have sex with men (MSM), and to the general urban population, the transmission pattern has changed from intravenous injection through shared syringes to sexual transmission, as a real sexually transmitted infection (7). The high-risk and vulnerable groups of HIV are the same as syphilis, such as MSM, female sex workers (FSWs), college students, and the elderly (8). The national HIV/AIDS sentinel surveillance showed that the HIV prevalence among MSM in China has been on the rise, from 3.0 to 8.0% during 2006 and 2015 (9), and the number of newly diagnosed college students has seen an annual growth rate ranging from 30 to 50% over the past several years (7). The number of reported HIV cases in China increased from 50,330 in 2015 to 71,204 in 2019 (10), with an increase of 41.5%. The Chinese Center for Disease Control and Prevention (Chinese CDC) estimated that by the end of 2020, there were 1.05 million people living with HIV (PLWH), and 351,000 AIDS-related deaths, representing a severe disease burden in the country (8).
The bidirectional interaction between syphilis and HIV has been referred to as “epidemiological synergy” (11). Studies confirmed that syphilis could facilitate the transmission and acquisition of HIV infection, increasing infectiousness through effects on HIV shedding, HIV replication, and increases in viral diversity, and increasing susceptibility by mucosal disruption, immune changes in the genital tract and effects on the genital tract microenvironment (12–14). In turn, HIV could alter the syphilis manifestations and blur the distinction of the stages (12). HIV and syphilis coinfection patients also face a higher risk of treatment failure and the emergence of neurosyphilis (15).
Due to the reciprocal synergistic interaction between syphilis and HIV, the rising epidemic of this concomitant syphilis and HIV infection remains hard to manage. At present, China faces the double challenge of syphilis and HIV epidemic. In order to better control the prevalence of syphilis and HIV, it is an important option to take their coinfection as the entry point for prevention and treatment, and there is of great significance to strengthen the monitoring and treatment of syphilis in HIV infections. However, the coinfection of syphilis and HIV has not received enough attention in China, indicating a gap in the prevention and control of syphilis and HIV. To fill the gap and to address HIV and syphilis coinfection, primarily we need to determine the prevalence of syphilis among PLWH. The objectives of this meta-analysis were to determine the overall prevalence of syphilis among PLWH in China and identify factors associated with syphilis among PLWH.
Methods
Search strategy
We searched eligible studies that reported syphilis prevalence among PLWH published in English or Chinese within each of the following databases: Medline (Ovid & PubMed interface), Embase, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals Database (VIP), Wan-fang Data, and Chinese Biomedical Literature Database (CBM) from January 1, 1990, to May 31, 2022. The search was conducted by using free-text terms and Medical Subject Headings (MeSH) terms that combined “human immunodeficiency virus,” “syphilis,” “co-infection,” and “China” in the international databases, and equivalent terms in Chinese in domestic databases. The search strategies are given in details in Supplementary Table 1. Reference lists of the included articles and relevant reviews were also checked to identify additional publications. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to report the results of this meta-analysis (16).
Inclusion and exclusion criteria
Publications were included if they met the following criteria: (1) study was conducted in China, (2) the prevalence of syphilis among PLWH was reported, (3) study participants aged 15 years or older or age-specific syphilis prevalence was available, (4) HIV and syphilis infections were confirmed by standard laboratory serologic testing, and (5) the number of HIV-infected participants was more than 300, for getting the stable and reliable results based on sample size calculation (17). If multiple publications were reported from the same study, only one with the most comprehensive reporting or the largest sample size was included.
Study selection and quality assessment
For studies returned by the search strategy, we created an Endnote library (version 20.1) to catalog the search results and de-duplicate references. After removing duplicates, two reviewers (YW and WZ) independently screened the title and abstract of all the records. If a study could not be definitively excluded based on its title and abstract, its full text was obtained for thorough screening. We then screened the full text for inclusion and assessed the quality of the included studies. For excluded studies, we recorded the explicit reason of exclusion for each study (Supplementary Table 2). We also included dissertations and conference abstracts if eligible. Disagreements were resolved through discussion between the two reviewers, and, if necessary, consultation with the third reviewer (XG). Study quality was assessed by Loney's 8-item scale (each item was assigned a score of 1 point, totaling 8 points): study design and sampling method, sampling frame, sample size, appropriate measurement, unbiased measurement, response rate, estimates of prevalence, and description of study subjects (17) (Supplementary Table 3). A score of 7–8 is considered high quality, 4–6 moderate quality, and 0–3 low quality.
Data extraction
A standardized data extraction form was developed and piloted specifically for this study. Information was extracted by the two reviewers (YW and WZ) in five aspects: (1) basic information (e.g., first author, publication year, publication language, study location, study period, recruitment site, sampling method, and sample size), (2) the prevalence of syphilis among PLWH and the most likely transmission route of HIV, (3) HIV and syphilis testing methods, (4) sociodemographic characteristics of PLWH, e.g., age and sex, and (5) history of diagnosis and treatment of HIV.
Statistical analysis
After Freeman-Tukey double arcsine transformation to stabilize the variances (18), a random-effects model meta-analysis was performed to estimate the pooled prevalence of syphilis and its 95% confidence interval (CI), because there was substantial heterogeneity across studies. The I2 statistic (values of 25, 50, and 75% are considered to represent low, medium, and high heterogeneity, respectively) was calculated to assess the heterogeneity of the studies (19).
Subgroup analyses were conducted to assess the associations of sample size, publication language, study quality, recruitment site, study region, study period, transmission category, sex, age, CD4 + T cell count, and antiretroviral therapy (ART) with the prevalence of syphilis among PLWH. Differences between subgroups were analyzed using the Q test based on the fixed-effects model (20). We also performed random-effects multivariable meta-regression analyses to examine the possible sources of heterogeneity with the following covariates: sample size, publication language, study quality, recruitment site, and study region.
Potential publication bias was assessed by visual inspection of the funnel plot and statistical Egger's test (a p-value < 0.10 was considered statistically significant) (21). Sensitivity analyses were conducted with the leave-one-out method or finding outliers of syphilis prevalence and omitting the corresponding study.
All statistical analyses were performed using R studio (version 1.4.1717) with the package meta (version 4.19-0).
Results
Study selection
A total of 1,599 studies were identified by the initial search. After removing 630 duplicates, we screened the remaining 969 studies by reviewing their title and abstract. A total of 205 full-text articles were assessed for eligibility, of which 176 were excluded (description of excluded studies is recorded in Supplementary Table 2). Finally, 29 studies were included in this meta-analysis (22–50). The flow chart of our systematic literature search is presented in Figure 1.
Study characteristics
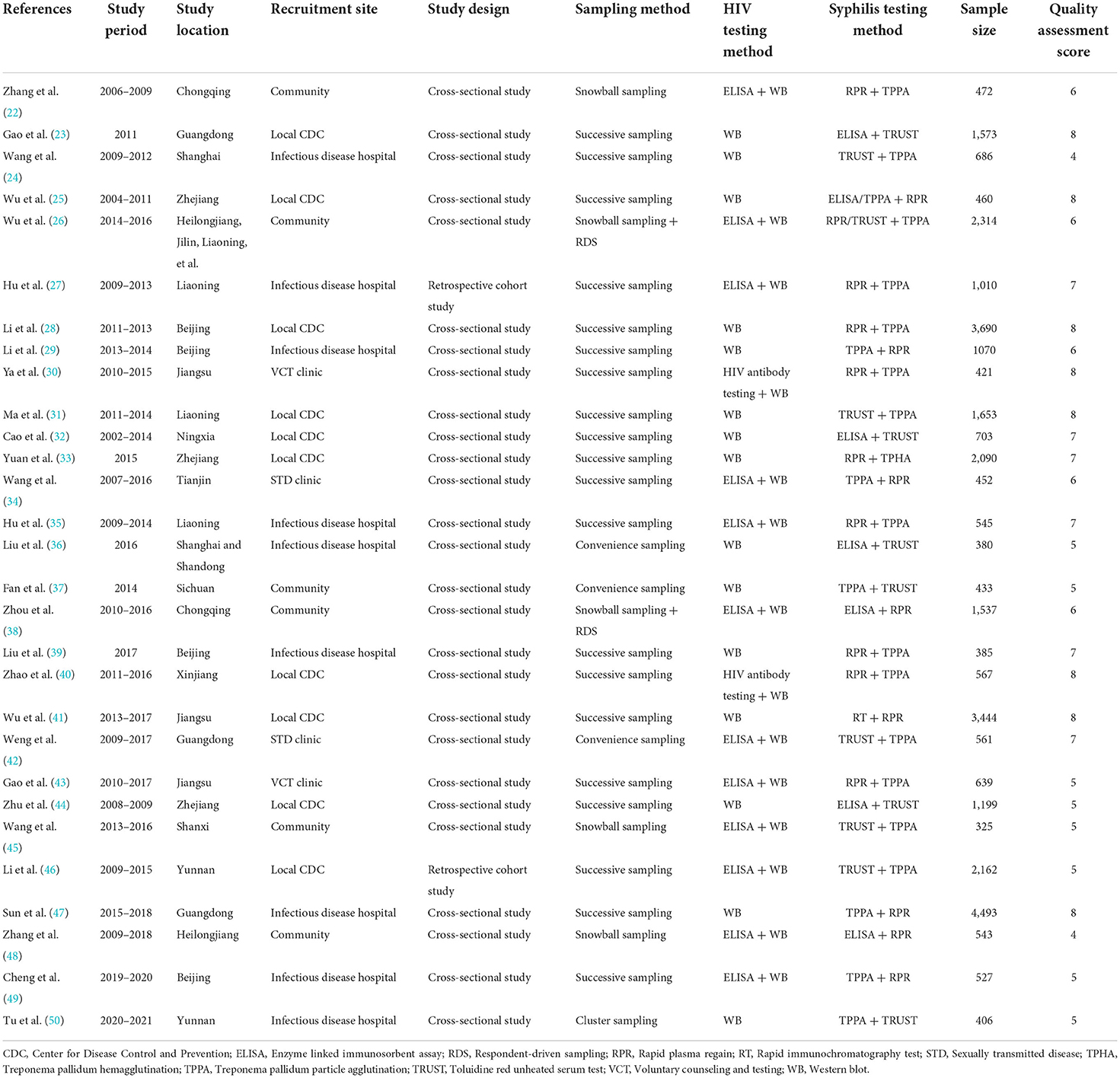
The characteristics of the included studies are presented in Table 1. Of these studies, eight studies (28%) were published in English and 21 studies (72%) in Chinese, covering 67 cities from 30 provinces and municipalities in mainland China (no studies were identified in Tibet). There were no studies of low quality (Table 1; Supplementary Table 4). The total number of PLWH study participants was 34,740.
Pooled prevalence of syphilis among PLWH
The prevalence of syphilis among PLWH in China ranged from 2.7 to 66.2%, and the overall random-effects pooled prevalence was 19.9% (95% CI: 15.4–24.8%), with substantial heterogeneity (I2 = 98.9%, p < 0.001) (Figure 2).
Subgroup analysis
Subgroup analysis showed that syphilis prevalence among PLWH attending STD clinics was significantly higher (59.2%, 95% CI: 45.4–72.4%) than that of other PLWH. The pooled prevalence of syphilis among PLWH was the highest in northern China (31.7%, 95% CI: 17.9–47.4%), followed by central-southern China (26.7%, 95% CI: 11.4–45.7%), and the lowest in northwestern China (15.0%, 95% CI: 6.9–25.4%) (Table 2; Figure 3). Despite some fluctuations over time, the prevalence of syphilis among PLWH in China remained high (Table 2).
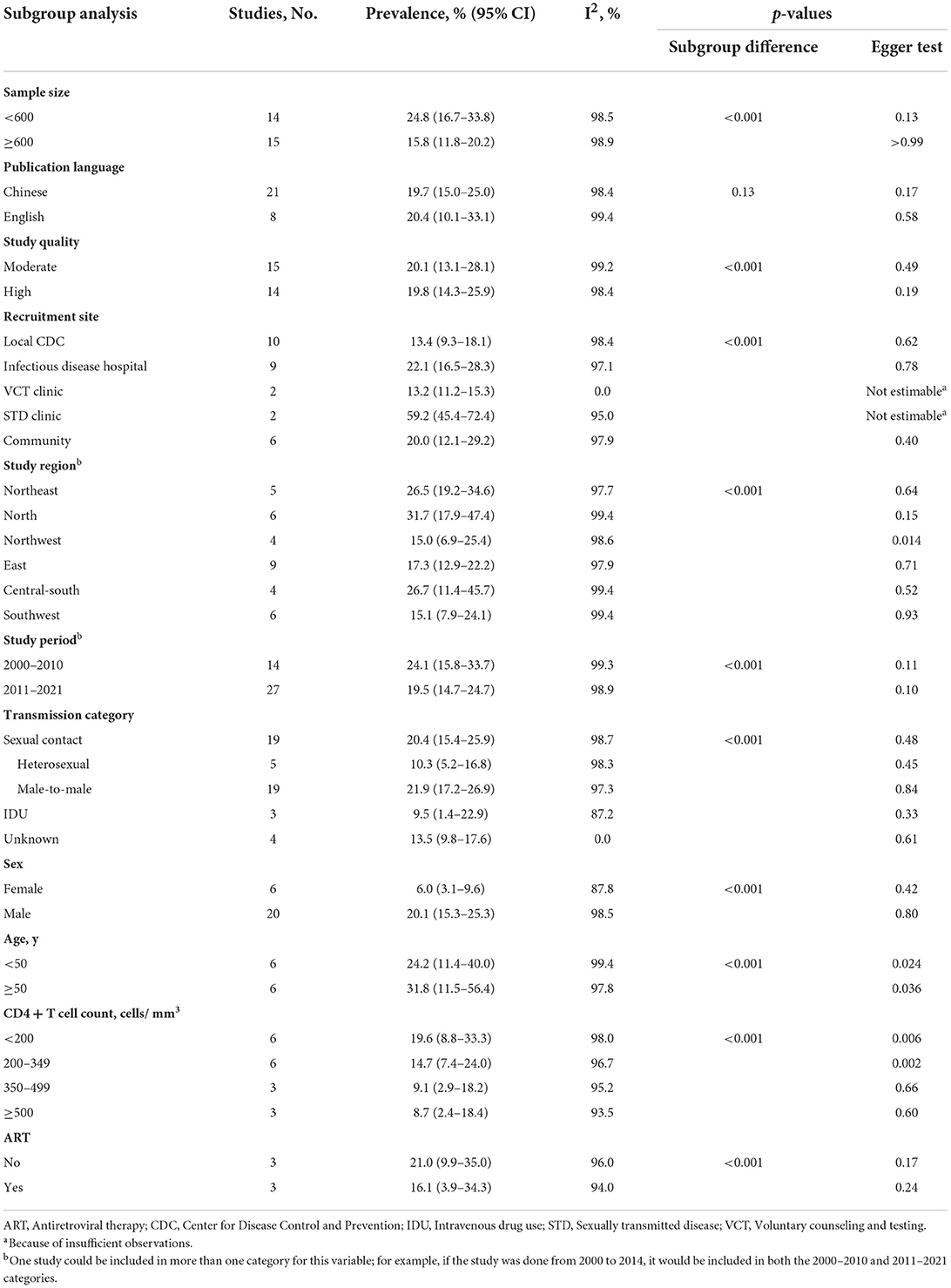
Table 2. Subgroup analysis of factors associated with syphilis prevalence among people living with HIV.
Nineteen studies (22, 25–27, 30, 31, 35–39, 42–49) reported the prevalence of syphilis among PLWH whose most likely transmission route was male-to-male sexual contact, five studies among PLWH with heterosexual transmission (27, 44, 46, 47, 49), and three studies (44, 46, 49) among PLWH who reported intravenous drug use (IDU). The prevalence of syphilis was found to be the highest among PLWH who reported male-to-male sexual contact (21.9%, 95% CI: 17.2–26.9%). We included 20 studies (22, 25–27, 30, 31, 33, 35–39, 42–49) that reported the prevalence of syphilis among PLWH by sex. The prevalence of syphilis among males living with HIV (20.1%, 95% CI: 15.3–25.3%) was significantly higher than that among females (6.0%, 95% CI: 3.1–9.6%). The analysis by age showed that the prevalence of syphilis among PLWH was significantly higher in those aged ≥ 50 years (31.8%, 95% CI: 11.5–56.4%) than in those aged < 50 years (24.2%, 95% CI: 11.4–40.0%). When stratified by CD4 + T cell count, we found that syphilis prevalence among PLWH decreased as CD4 + T cell count increased. Subgroup analysis by ART status showed higher syphilis prevalence among non-treated HIV-infected patients (21.0%, 95% CI: 9.9–35.0%) than that among treated patients (16.1%, 95% CI: 3.9–34.3%). The differences were statistically significant for all subgroups except publication language (p = 0.13). Substantial heterogeneity was present within all subgroups except for HIV voluntary counseling and testing (VCT) clinics, and unknown HIV transmission route subgroups (Table 2; Supplementary Figure 1).
Meta regression analysis
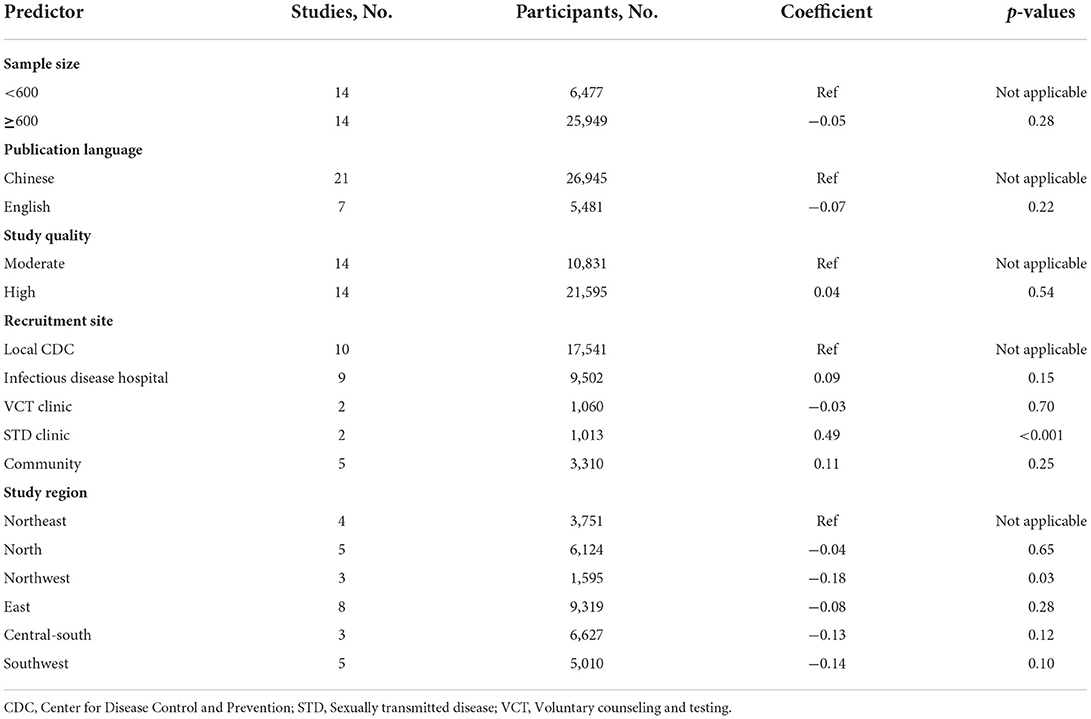
The results from our multivariable meta-regression indicated that recruitment site (STD clinic: coefficient = 0.49, p < 0.001) and study region (northwestern China: coefficient = −0.18, p = 0.03) contributed to the highest heterogeneity across studies (Table 3).
Publication bias
The funnel plot (Figure 4) and Egger's test were similarly not suggestive of publication bias. When subgroup analyses were conducted, publication bias was found for the following grouping variables: study region, age, and CD4 + T cell count (Table 2).
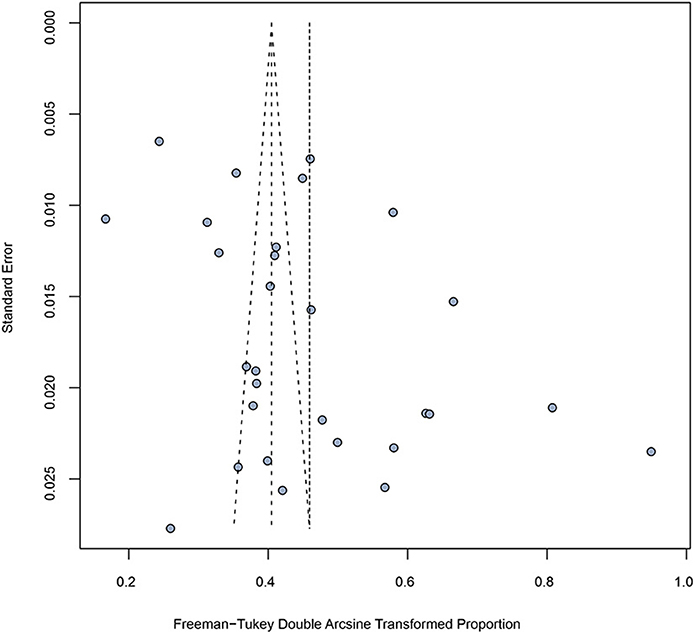
Figure 4. Funnel plot of included studies showing estimate of syphilis prevalence among people living with HIV.
Sensitivity analysis
In the sensitivity analysis (Supplementary Table 5), when removing a study (34) that focused on middle-aged and elderly people from an STD clinic whose prevalence was an outlier, the pooled prevalence of syphilis among PLWH remained high (18.6%, 95% CI: 14.8–22.6%). The results of the leave-one-out method showed that none of the studies had a disproportionate effect on the pooled estimate (Supplementary Table 6).
Discussion
This meta-analysis from 29 eligible studies involving 34,740 individuals found that the overall prevalence of syphilis among PLWH in China reached 19.9% (95% CI: 15.4–24.8%), which was higher than the prevalence of syphilis among other populations, such as MSM (10.9%) (51) and drug users (7.8%) (52). The findings demonstrated that PLWH could be as an important high-risk group for syphilis and the urgent need for enhanced syphilis monitoring and management programs in this population.
The estimated syphilis prevalence among PLWH in China was similar to Brazil (20.5%) (53), and higher than Singapore (12.3%) (54) and Turkey (8%) (55). Our estimate was approximately twice as high as that reported in a systematic review published by Kalichman et al. in 2011 (9.5%) (56), and there may be two potential reasons for the difference. First, differences in study time, Kalichman et al. conducted their systematic review ~10 years ago. Second, differences in study regions and populations, of the 37 studies included in this article, 34 (92%) were conducted in non-Asia regions, mainly in North America and Latin America/Caribbean, however, our study only focused on the Chinese mainland population, thus the difference may be attributable to variation of geographical and social environment as well as ethnicity.
The results of subgroup analyses showed higher prevalence of syphilis in the following groups: MSM, STD clinic attendees, and males. In this study, more than half of the STD clinic attendees and male participants were MSM. MSM are at a higher risk of HIV and syphilis infection because of risk behaviors such as multiple sexual partners and unprotected anal intercourse (57). A meta-analysis reported that the prevalence of HIV and syphilis among MSM in China was 7.7 and 10.9% in 2010–2013, respectively (51). This study showed that the prevalence of syphilis among MSM living with HIV in China was 21.9%. Public Health England reported that the prevalence of syphilis among MSM living with HIV in England was 14.1 to 17.0% from 2014 to 2019, lower than our estimate, and MSM who were HIV-positive had over 5 times the rate of syphilis than those who were HIV-negative or of unknown HIV status (58). In China, the national guidelines for HIV screening require HIV testing among all patients with syphilis and other sexually transmitted infections (STIs) (59), but syphilis screening among PLWH has not been clearly included in any guidelines. To efficiently control and prevent both HIV and syphilis, it is necessary to consider PLWH be one of the high priority populations for syphilis monitoring and management, and strengthen syphilis screening among PLWH, especially among sex active MSM living with HIV, to whom syphilis screening every 3 months should be recommended (58). Studies from other countries have reported that “chemsex” (60) and “seroadaptive” (61) behaviors in this group accelerated syphilis transmission among MSM, but we are lacking such data in China, suggesting that future research needs to be carried out in this area.
In the subgroup analysis of CD4 + T cell count, we found that the lower the CD4 + T cell count, the higher the prevalence of syphilis among PLWH. This is consistent with the finding from a study conducted in Canada that coinfection with syphilis can exacerbate the progression of HIV (62). On the other hand, HIV infection also has an effect on syphilis that immunocompromised individuals are less able to mount a protective response against T. pallidum (56), increasing the chance of progressing to neurosyphilis, ocular syphilis, and otosyphilis. Subgroup analysis by ART status showed lower syphilis prevalence among treated HIV-infected patients than among non-treated patients, suggesting that ART may reduce susceptibility to syphilis among PLWH. China has implemented pre-exposure prophylaxis (PrEP) as an intervention for HIV infection (63), but its impact on syphilis and other STIs is unclear.
The present study revealed significant heterogeneity across the included 29 studies, we found that the recruitment site and study region were the sources of study heterogeneity through multivariable meta-regression analyses. The pooled syphilis prevalence among PLWH attending STD clinics (59.2%) was significantly higher than that of participants in other recruitment sites, and the prevalence of syphilis in northern China was twice as high as that in northwestern China. It is easy to understand that there are differences in some characteristics of the included participants in different recruitment sites and regions, such as variations in sexual behaviors and social culture in the large country (64, 65).
In summary, we think that it is urgent to develop national guidelines on the screening, monitoring and management of syphilis among PLWH, furthermore implementing protocols suitable to variations for sexual behaviors and social culture in different regions and subgroups. The China's Plan for Syphilis Prevention and Control (2010–2020) called for syphilis prevalence monitoring to be combined with local AIDS monitoring efforts (66). This requires the close collaboration between the National Center for HIV/AIDS Control and Prevention (NCAIDS) and the National Center for STD Control and Prevention (NCSTD) to develop integrated national guidelines for screening, monitoring, and management of syphilis and HIV, under the new national action plans for syphilis/HIV control in future, in order to find out the potential syphilis and HIV coinfection and combat the double epidemics.
In addition, the PLWH are required to receive treatment at designated hospitals in China. Based on the current estimate of 1.05 million PLWH by the Chinese CDC (8) and the 19.9% overall prevalence of syphilis among PLWH from this meta-analysis, we estimated that about 209,005 PLWH are co-infected with syphilis and need medical care for syphilis. Because at those designated HIV treatment hospitals, doctors are not sufficiently trained in diagnosis and treatment of syphilis (67), we recommend developing syphilis training programs tailored to the needs of these HIV physicians, reducing the burden of syphilis and preventing syphilis patients with HIV from progressing to malignant syphilis.
Strengths and limitations
To our knowledge, this is the first study to systematically review the prevalence of syphilis among PLWH in China. We conducted meta-analysis to summarize prevalence estimates and identify factors associated with syphilis infection among PLWH. We tried to include high-quality articles to get more accurate and reliable results.
However, there are some limitations to the present meta-analysis. Firstly, there was significant heterogeneity across the included studies. We conducted subgroup analyses and meta-regression analyses to investigate the source of heterogeneity, 11 relevant variables can be used for subgroup analyses, but only five of them can be employed for multivariable meta-regression analyses, and found that recruitment site and study region induced substantial heterogeneity, other six variables that were not included in the meta-regression analyses due to the variation in study design and data collection across these studies, may not be still excluded as the source of heterogeneity. Secondly, although FSWs have been identified as a target population for syphilis and HIV control and prevention (3), none of the published literatures searched include study on syphilis prevalence in FSWs living with HIV, we cannot estimate the pooled syphilis prevalence among this subpopulation, this may be due to very low HIV prevalence in FSWs in China (0.19%) (68). Thirdly, publication bias was present in three subgroup analyses, including study region, age, and CD4 + T cell count, and the results of such subgroup analyses should be interpreted with caution.
Conclusion
In conclusion, we found that the prevalence of syphilis among PLWH was markedly high in China, especially among MSM living with HIV. Findings from this meta-analysis suggested that PLWH should be one of the priority populations for syphilis control and prevention, and the strategies include regular syphilis testing and consistent condom use. Furthermore, developing national guidelines for the integrated screening, monitoring, and management of HIV and syphilis as well as syphilis diagnosis and treatment training programs for physicians at designated HIV treatment hospitals is urgent and crucial to combat HIV and syphilis coinfection in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XG conceived and designed the study. YW, WZ, and XG carried out the literature searches, extracted the data, and assessed the study quality. YW, WZ, XY, CS, and MZ performed the statistical analysis. YW and WZ wrote the manuscript. XG and GF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Guizhou Provincial Project of Science and Technology (Grant Number: 2021-026). The funder had no role in study design, data collection, analysis and interpretation of data, writing of the manuscript, and the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LS declared a shared affiliation with one of the authors GF to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1002342/full#supplementary-material
References
2. Peeling RW, Hook EW 3rd. The pathogenesis of syphilis: the Great Mimicker, revisited. J Pathol. (2006) 208:224–32. doi: 10.1002/path.1903
3. World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016-2021: Actions for Impact. (2021). Available online at: https://www.who.int/publications/i/item/9789240027077 (accessed May 10, 2022).
4. Gong XD, Jiang WH, Wang QP, Zhang JY. Epidemiological analysis of syphilis in China from 1979 to 1998. Zhongguo Gong Gong Wei Sheng. (2000) 16:1020–2. doi: 10.3321/j.issn:1001-0580.2000.11.025
5. Gong XD, Yue XL, Teng F, Jiang N, Men PX. Syphilis in China from 2000 to 2013: epidemiological trends and characteristics. Zhonghua Pi Fu Ke Za Zhi. (2014) 47:310–5.
6. Yue XL, Gong XD, Li J, Zhang JH. Epidemiological trends and features of syphilis in China, 2014-2019. Zhonghua Pi Fu Ke Za Zhi. (2021) 54:668–72. doi: 10.35541/cjd.20210098
7. Li G, Jiang Y, Zhang L. HIV upsurge in China's students. Science. (2019) 364:711. doi: 10.1126/science.aay0799
8. He N. New progress of AIDS epidemiology in China. Zhonghua Ji Bing Kong Zhi Za Zhi. (2021) 25:1365–8 + 480. doi: 10.16462/j.cnki.zhjbkz.2021.12.001
9. National Center for AIDS/STD Control Prevention, China CDC. Guidelines for HIV Interventions for Men Who Have Sex With Men. (2016). Available online at: https://www.chinaaids.cn/xwgy/jswj3/201609/W020160913346815395954.pdf (accessed September 1, 2022)
10. National Health Commission of the People's Republic of China. An Overview of the Epidemic Situation of Notifiable Infectious Diseases in China in 2020. (2021). Available online at: http://www.nhc.gov.cn/jkj/s3578/202103/f1a448b7df7d4760976fea6d55834966.shtml (accessed May 15, 2022).
11. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. (1999) 75:3–17. doi: 10.1136/sti.75.1.3
12. Wu MY, Gong HZ, Hu KR, Zheng HY, Wan X, Li J. Effect of syphilis infection on HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect. (2021) 97:525–33. doi: 10.1136/sextrans-2020-054706
13. Palacios R, Jiménez-Oñate F, Aguilar M, Galindo MJ, Rivas P, Ocampo A, et al. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV-infected patients. J Acquir Immune Defic Syndr. (2007) 44:356–9. doi: 10.1097/QAI.0b013e31802ea4c6
14. Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. (2004) 2:33–42. doi: 10.1038/nrmicro794
15. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. (1990) 113:872–81. doi: 10.7326/0003-4819-113-11-872
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. (1998) 19:170–6.
18. Miller JJ. The inverse of the Freeman – Tukey double arcsine transformation. Am Stat. (1978) 32:138. doi: 10.2307/2682942
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
20. Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. (2013) 14:134–43. doi: 10.1007/s11121-013-0377-7
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Zhang Y, Chen P, Lu R, Liu L, Wu Y, Liu X, et al. Prevalence of HIV among men who have sex with men in Chongqing, China, 2006-2009: cross-sectional biological and behavioural surveys. Sex Transm Infect. (2012) 88:444–50. doi: 10.1136/sextrans-2011-050295
23. Gao K, Wang C, Chen L, Xu HF, Han ZG. Analysis of syphilis infection among HIV/AIDS patients in Guangzhou in 2011. Hua Nan Yu Fang Yi Xue. (2013) 39:32–5.
24. Wang ZY, Zheng YF, Liu L, Zhang RF, Shen YZ, Lu HZ. Infection rate of syphilis and hepatitis virus in 686 AIDS patients diagnosed for the first time. Zhonghua Chuan Ran Bing Za Zhi. (2013) 31:492–5. doi: 10.3760/cma.j.issn.1000-6680.2013.08.012
25. Wu HB, Luo Y, Cheng J, Ding JM, Jin DD. An analysis of infection status of syphilis and its risk factors among HIV positive MSM in Hangzhou. Zhejiang Yu Fang Yi Xue. (2013) 25:25–8. doi: 10.3969/j.issn.1007-0931.2013.07.008
26. Wu ZY, Xu J, Liu E, Mao YR, Xiao Y, Sun XH, et al. HIV and syphilis prevalence among men who have sex with men: a cross-sectional survey of 61 cities in China. Clin Infect Dis. (2013) 57:298–309. doi: 10.1093/cid/cit210
27. Hu QH, Xu JJ, Zou HC, Liu J, Zhang J, Ding HB, et al. Risk factors associated with prevalent and incident syphilis among an HIV-infected cohort in Northeast China. BMC Infect Dis. (2014) 14:658. doi: 10.1186/s12879-014-0658-1
28. Li Y, Li J, Xin RL, Zhang Q, Sun WD, Ma XY, et al. An analysis of syphilis infection and related factors in HIV/AIDS patients in Beijing. Zhongguo Ai Zi Bing Xing Bing. (2014) 20:519–23. doi: 10.13419/j.cnki.aids.2014.07.018
29. Li JW, Zhang XF, Li AP, Shao Y, Ye JZ, He L, et al. Analysis of HIV, HBV, HCV and TP co-infection among patients in Beijing Youan Hospital. Zhongguo Ai Zi Bing Xing Bing. (2016) 22:608–10. doi: 10.13419/j.cnki.aids.2016.08.08
30. Ya XR, Tian RF, Cao XP, Fu ZH. Serological test results of men who have sex with men in the voluntary HIV testing clinics in Suzhou from 2010 to 2015. Jiangsu Yu Fang Yi Xue. (2016) 27:454–5. doi: 10.13668/j.issn.1006-9070.2016.04.030
31. Ma N, Yan H, Zhou D, Zhang Y, Wang L, Yao WQ. Syphilis prevalence in HIV positive men who have sex with men in Liaoning. Ji Bing Jian Ce. (2016) 31:659–62. doi: 10.3784/j.issn.1003-9961.2016.08.011
32. Cao M, Wu ZL, Yang DZ, Ma XM, Chen XY, Wang XM. Analysis of infection status of syphilis in patients infected with HIV in Ningxia. Zhongguo Wei Sheng Jian Yan Za Zhi. (2016) 26:403–5.
33. Yuan G, Hu YR, Sun FY, Zheng NH, Li HL. Prevalence and influence factors of syphilis among people infected with HIV in Ningbo municipality. Zhongguo Gong Gong Wei Sheng. (2017) 33:377–9. doi: 10.11847/zgggws2017-33-03-09
34. Wang L, Li WY, Ji B, Gu YG, Liu CL, Li HF, et al. Analysis of co-infection of HIV and syphilis among middle-aged and elder people in STD clinic from 2007 to 2016. Zhongguo Xing Ke Xue. (2017) 26:64–7. doi: 10.3969/j.issn.1672-1993.2017.12.022
35. Hu QH, Xu JJ, Chu ZX, Zhang J, Yu YQ, Yu H, et al. Prevalence and determinants of herpes simplex virus type 2 (Hsv-2)/ syphilis co-infection and hsv-2 mono-infection among human immunodeficiency virus positive men who have sex with men: a cross-sectional study in Northeast China. Jpn J Infect Dis. (2017) 70:284–9. doi: 10.7883/yoken.JJID.2016.177
36. Liu L, Zheng YJ, Yin WY, Wang XC. Prevalence and risk factors of sexually transmitted infections among MSM infected with HIV. Zhongguo Ai Zi Bing Xing Bing. (2017) 23:616–9. doi: 10.13419/j.cnki.aids.2017.07.11
37. Fan SF, Shi YY, Liu F, Wu XQ. Study on HIV related high risk factors among MSM infected with HIV/AIDS in Chengdu. Zhongguo Ai Zi Bing Xing Bing. (2017) 23:126–8. doi: 10.13419/j.cnki.aids.2017.02.10
38. Zhou QH, He JC, Lu RR, Wang Y, Ling H, Zhang M. Serological test results analysis of men who have sex with men in Chongqing from 2010 to 2016. Jian Yan Yi Xue Yu Lin Chuang. (2018) 15:2033–5. doi: 10.3969/j.issn.1672-9455.2018.14.002
39. Liu ZY, Wang XX, Hong JJ, Duan Y, Shi WY, Li YQ, et al. Syphilis infection and its influencing factors among HIV positive MSM population. Xian Dai Yu Fang Yi Xue. (2018) 45:520–3 + 8.
40. Zhao H, Ma L, Wusiman YSF, Liu WS. Analysis of related factors of HIV complicated with syphilis infection in Urumqi from 2011 to 2016. Zhongguo Ai Zi Bing Xing Bing. (2018) 24:420–1. doi: 10.13419/j.cnki.aids.2018.04.30
41. Wu YM, Xu WJ, Wang Y, Dong XX, Dong XQ, Zhang HY. Analysis of syphilis infection in HIV antibody positive cases in Nanjing from 2013 to 2017. Xian Dai Yu Fang Yi Xue. (2019) 46:1490–3.
42. Weng RX, Hong FC, Yu WY, Cai YM. Compare HIV/syphilis infections between age groups and explore associated factors of HIV/ syphilis co-infections among men who have sex with men in Shenzhen, China, from 2009 to 2017. PLoS ONE. (2019) 14:e0223377. doi: 10.1371/journal.pone.0223377
43. Gao J, Fu ZH, Zhao XP, Cao XP. Syphilis infection and related factors among men who have sex with men in Suzhou. Zhongguo Ai Zi Bing Xing Bing. (2019) 4:392–4. doi: 10.13419/j.cnki.aids.2019.04.017
44. Zhu XJ, Wu XF, Xu SD, Shen YH, Zha YF. Infection status and CD4 + T lymphocyte results of syphilis and HIV co-infection. Zhongguo Ai Zi Bing Xing Bing. (2020) 26:1066–8. doi: 10.13419/j.cnki.aids.2020.10.09
45. Wang L, Santella AJ, Wei X, Zhuang G, Li H, Zhang H, et al. Prevalence and protective factors of HIV and syphilis infection among men who have sex with men in Northwest China. J Med Virol. (2020) 92:1141–7. doi: 10.1002/jmv.25622
46. Li S, Dong W, Chen L, Li S, Su S. Syphilis seroprevalence and seroconversion among people newly diagnosed with HIV during the pre-antiretroviral therapy period in rural China. Int J STD AIDS. (2020) 31:876–85. doi: 10.1177/0956462420923550
47. Sun LQ, Liu JY, Liu XN, Song Y, Cao YZ, Tian YM, et al. Rate and influencing factors of patients with human immunodeficiency virus co-infected with syphilis in Shenzhen. Zhonghua Shi Yan He Lin Chuang Gan Ran Bing Za Zhi. (2020) 14:284–90. doi: 10.3877/cma.j.issn.1674-1358.2020.03.004
48. Zhang L, Shen ZJ, Yang YS, Li CW, Luo C, Wang SB, et al. Status of HIV infection among men who have sex with men in Harbin, 2009-2018. Zhonghua Liu Xing Bing Xue Za Zhi. (2021) 42:538–43. doi: 10.3760/cma.j.cn112338-20200328-00462
49. Cheng J, Suo XH, Song B, Huang HH, Li AX, Jiang TJ. Clinical characteristics and anti-syphilis therapeutic effect of human immunodeficiency virus patients with syphilis infection. Lin Chuang Yao Wu Zhi Liao Za Zhi. (2021) 19:9–13. doi: 10.3969/j.issn.1672-3384.2021.03.002
50. Tu W, Li YY, Kuang YQ, Xie RH, Dong XQ, Zhang D, et al. High prevalence of sexually transmitted infections and risk factors among HIV-positive individuals in Yunnan, China. Eur J Med Res. (2022) 27:9. doi: 10.1186/s40001-022-00635-w
51. Huang Q, Li QQ, Li Y, Zeng G, Cui XY, Yan PJ, et al. Prevalence of HIV infection and syphilis, sexual behaviors and awareness of HIV/AIDS related knowledge among men who have sex with men in China: a meta-analysis of data collected from 2010 to 2013. Zhonghua Liu Xing Bing Xue Za Zhi. (2015) 36:1297–304. doi: 10.3760/cma.j.issn.0254-6450.2015.11.023
52. Wang BX, Zhang L, Wang YJ, Yan JW, Wan YN, Peng WJ, et al. Epidemiology of syphilis infection among drug users at methadone maintenance treatment clinics in China: systematic review and meta-analysis. Int J STD AIDS. (2014) 25:550–8. doi: 10.1177/0956462413515444
53. Adolf R, Bercht F, Aronis ML, Lunardi LW, Schechter M, Sprinz E. Prevalence and risk factors associated with syphilis in a cohort of HIV positive individuals in Brazil. AIDS Care. (2012) 24:252–8. doi: 10.1080/09540121.2011.597706
54. Lim RB, Tan MT, Young B, Lee CC, Leo YS, Chua A, et al. Risk factors and time-trends of cytomegalovirus (CMV), syphilis, toxoplasmosis and viral hepatitis infection and seroprevalence in human immunodeficiency virus (HIV) infected patients. Ann Acad Med Singap. (2013) 42:667–73.
55. Sarigül F, Sayan M, Inan D, Deveci A, Ceran N, Çelen MK, et al. Current status of HIV/AIDS-syphilis co-infections: a retrospective multicentre study. Cent Eur J Public Health. (2019) 27:223–8. doi: 10.21101/cejph.a5467
56. Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. (2011) 87:183–90. doi: 10.1136/sti.2010.047514
57. Liu H, Yang H, Li X, Wang N, Liu H, Wang B, et al. Men who have sex with men and human immunodeficiency virus/sexually transmitted disease control in China. Sex Transm Dis. (2006) 33:68–76. doi: 10.1097/01.olq.0000187266.29927.11
58. Prochazka M, Evans J, Thorn L, Sinka K. Tracking the Syphilis Epidemic in England: 2010 to 2019. (2021). Available online at: https://www.gov.uk/government/publications/tracking-the-syphilis-epidemic-in-england (accessed June 18, 2022).
59. National Health Commission of the People's Republic of China. Implementation plan to curb the spread of AIDS (2019-2022). Zhongguo Bing Du Za Zhi. (2020) 10:47–50. doi: 10.16505/j.2095-0136.2020.0001
60. Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings ('chemsex') and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect. (2015) 91:564–8. doi: 10.1136/sextrans-2015-052052
61. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis. (2019) 12:1178633719883282. doi: 10.1177/1178633719883282
62. Burchell AN, Allen VG, Gardner SL, Moravan V, Tan DH, Grewal R, et al. High incidence of diagnosis with syphilis co-infection among men who have sex with men in an HIV cohort in Ontario, Canada. BMC Infect Dis. (2015) 15:356. doi: 10.1186/s12879-015-1098-2
63. AIDS and Hepatitis C Professional Group. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018). Xin Fa Chuan Ran Bing Dian Zi Za Zhi. (2019) 4:65–84. doi: 10.3969/j.issn.1674-9081.2019.01.006
64. Hu LH, Zhao JM, Lin F. A study of HIV knowledge behavior and syphilis infection risk factors among male STD clinic attendees. Zhong Guo Ma Feng Pi Fu Bing Za Zhi. (2012) 28:443–4. doi: 10.3969/j.issn.1009-1157.2012.06.037
65. Liu H, Feng T, Ha T, Liu H, Cai Y, Liu X, et al. Chinese culture, homosexuality stigma, social support and condom use: a path analytic model. Stigma Res Action. (2011) 1:27–35. doi: 10.5463/sra.v1i1.16
66. Ministry of Health, People's Republic of China. Notice of the Ministry of Health on Issuing National Plan for Prevention and Control of Syphilis in China (2010–2020). (2010). Available online at: http://www.gov.cn/gzdt/2010-06/21/content_1632301.htm (accessed June 10, 2022).
67. Yue XL, Gong XD, Jiang N. Preliminary analysis on the evaluation of STD case-reporting system in China. Zhongguo Ai Zi Bing Xing Bing. (2015) 21:875–8. doi: 10.13419/j.cnki.aids.2015.10.14
Keywords: HIV, syphilis, prevalence, coinfection, meta-analysis
Citation: Wu Y, Zhu W, Sun C, Yue X, Zheng M, Fu G and Gong X (2022) Prevalence of syphilis among people living with HIV and its implication for enhanced coinfection monitoring and management in China: A meta-analysis. Front. Public Health 10:1002342. doi: 10.3389/fpubh.2022.1002342
Received: 25 July 2022; Accepted: 29 September 2022;
Published: 17 October 2022.
Edited by:
Weiming Tang, University of North Carolina at Chapel Hill, United StatesReviewed by:
Peizhen Zhao, Southern Medical University, ChinaLingen Shi, Jiangsu Center for Disease Control and Prevention, China
Gifty Marley, University of North Carolina China-Project (SESH Global), China
Copyright © 2022 Wu, Zhu, Sun, Yue, Zheng, Fu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangdong Gong, gxdstd@pumcderm.cams.cn
†These authors have contributed equally to this work and share first authorship
 Yuelin Wu
Yuelin Wu Wenqian Zhu1,2†
Wenqian Zhu1,2† Min Zheng
Min Zheng Gengfeng Fu
Gengfeng Fu Xiangdong Gong
Xiangdong Gong