- Department of Experimental Psychology, Ghent University, Ghent, Belgium
Why can’t we keep as many items as we want in working memory? It has long been debated whether this resource limitation is a bug (a downside of our fallible biological system) or instead a feature (an optimal response to a computational problem). We propose that the resource limitation is a consequence of a useful feature. Specifically, we propose that flexible cognition requires time-based binding, and time-based binding necessarily limits the number of (bound) memoranda that can be stored simultaneously. Time-based binding is most naturally instantiated via neural oscillations, for which there exists ample experimental evidence. We report simulations that illustrate this theory and that relate it to empirical data. We also compare the theory to several other (feature and bug) resource theories.
Introduction
The existence of resource constraints on cognition is undebated: Just consider listening to a long list of grocery items to be fetched, and heading off to the supermarket without a piece of paper (or smartphone) to support your memory. What is debated, however, is the nature of these resource constraints. Of course, resource is a broad term that has been applied throughout psychology and neuroscience (e.g., Barlow, 1961). However, we will restrict our attention to theories with immediate implications for working memory (e.g., as in the supermarket example). With this delineation out of the way, we note that a long research tradition has empirically investigated the nature of resource constraints (Miller, 1956; Cowan, 2001; Bays and Husain, 2008; Oberauer and Lin, 2017) by positing a limited quantity of some sort, and then deriving predictions (perhaps supported by a formal model) with respect to behavioral data in the working memory domain. This is the “bug” approach mentioned in the abstract. However, in line with David Marr and the “feature” approach, we first consider what a computational perspective would stipulate for flexible cognition (Holroyd and Verguts, 2021). To be clear from the start, “computational” is often used as in “instantiated in a formal model”; this is not what we mean here. By computational, we refer to the computations that are required in tasks relying on flexible cognition (such as getting one’s groceries, in the upcoming example). Our detour into flexible cognition lays the groundwork for our main thesis: The resource constraint is a consequence of the computational requirements to implement flexible cognition. Then, we consider the implementational perspective, and present some simulations to illustrate our theory, based on a recent oscillatory model of working memory (Pina et al., 2018). Finally, we relate our theory to other (similar and different) proposals in the General Discussion section.
Role-Filler Binding
Cognition requires the flexible binding and unbinding of two or more elements. For example, an experimenter may instruct a subject to detect the red squares in a stream of stimuli, but ignore the blue squares and red triangles (Treisman and Gelade, 1980). More mundanely, a mother may ask her son to go to the store to buy a pack of gluten free pasta and 1 kg of apples. If he comes home with 1 kg of regular pasta and a pack of gluten free apples, he is likely to be sent back. As another example, acting appropriately in a restaurant requires binding the waiter role to the person running around with the drinks; this binding allows one to know how, what, and when to order. In a sense, cognitive life is built on binding.
A particularly important type of binding is that between roles to be filled and fillers of those roles (role-filler binding; Hummel, 2011). For example, suppose one wants to memorize that the fruit aisle is on the left of the dry food department in the supermarket. The roles are here “Left” and “Right”; the fillers are “fruit aisle” and “dry food department”; and the relevant role-filler bindings are (Left, fruit aisle) and (Right, dry food department). As an aside, these roles can be implemented via different types of representational codes, including verbal or spatial (Gevers et al., 2010; van Dijck and Fias, 2011); we currently remain agnostic about their nature. Consider as another example of role-filler binding, syntactic constructions such as the Subject—Verb—Object (SVO) type sentence. For example, in a sentence like “Tom buys pasta,” the relevant role-filler bindings are (Subject, Tom), (Verb, buys), and (Object, pasta). Other syntactic constructions are possible to represent this information [e.g., (Buyer, Tom), (Object-bought, pasta)], but the syntactic structure doesn’t matter for our argument, and we will stick to SVO constructions to explain our argument. We will discuss a few constraints on role-filler bindings in cognition, and how these constraints impose processing bounds on cognition.
Some sentences (such as “I love you”) occur sufficiently frequently to be stored as a separate chunk in memory, independent from other information. There is indeed evidence that such (high-frequency) chunks are important in language (McCauley and Christiansen, 2014), and perhaps in cognition more generally. However, chunking is not a realistic possibility for coding SVO sentences in general. For example, if there are N possible fillers (Tom, buy, book, …) and three possible roles (Subject, Verb, Object), a systematic chunking approach confronts a combinatorial problem, as it would require storage of 3N2 chunks of knowledge. More importantly, a chunking approach does not easily lend itself to generalization (Marcus, 2001, 2018). If one learns something about books, generalization requires that this novel information generalizes to the statement “Tom buys a book” (Fodor and Pylyshyn, 1988). For example, even a rudimentary knowledge about books is enough to conclude that buying a book entails a very different process than buying a house. But if the proposition that “Tom buys a book” is stored as a separate chunk in memory, such generalization between propositions is not possible.
The solution to this generalization problem involves compositionality (Fodor and Pylyshyn, 1988): Storing all components (or building blocks, here, roles, and fillers) separately, in such a way that they can later enter into novel relations with other components. Applied to roles and fillers, this principle is also called role-filler independence (Hummel, 2011). Indeed, if one stores “book” information separately, the concept can later be independently enriched; and the novel information (e.g., that a book can be bought in bookstores, without the hassle and administration involved in buying a house) can thus be applied to instances like “Tom buys a house.”
With role-filler independence, the memory requirements are much lighter than in a chunking approach. Consider Figure 1: Here, N fillers and 3 roles are represented, with a much lighter memory requirement of just 3 + N elements. Any specific sentence (“Tom buys a book”) involves a combination of the corresponding roles and fillers.
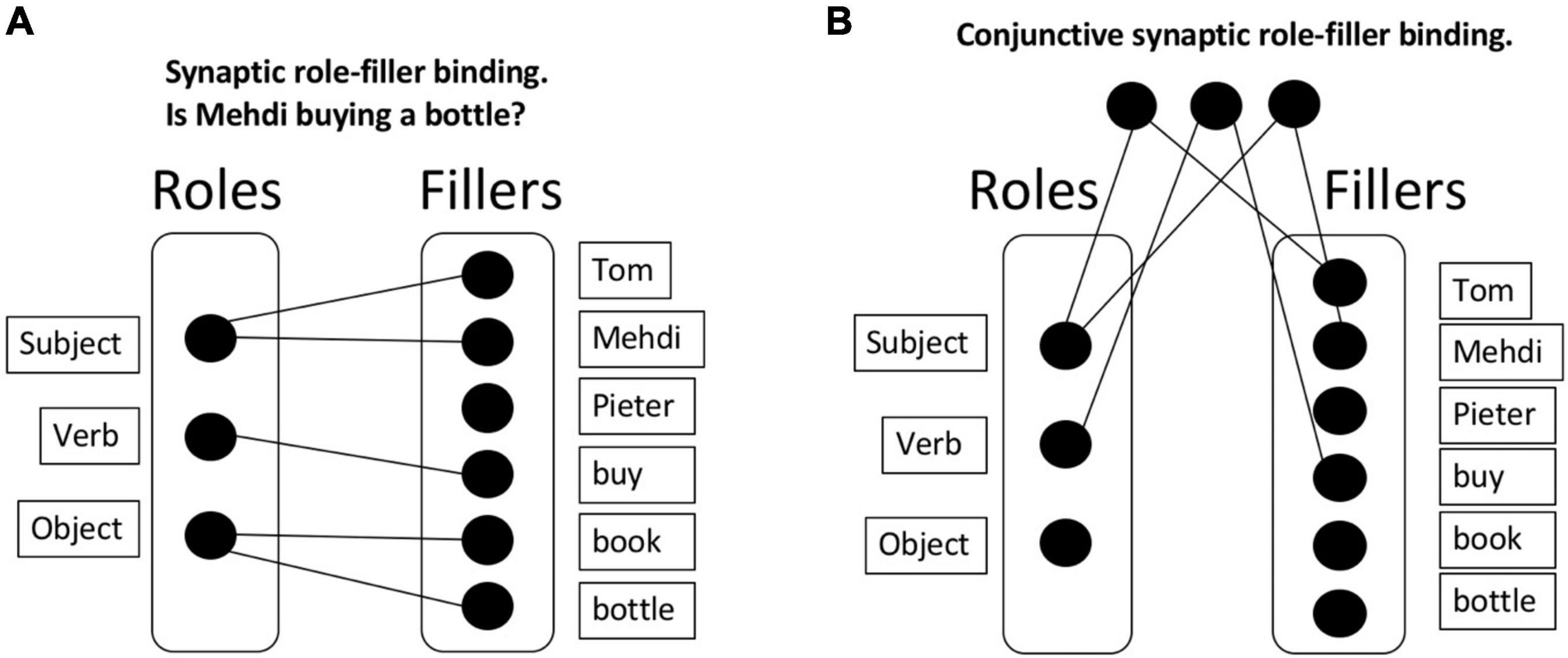
Figure 1. Synaptic binding. (A) Synaptic role-filler binding. This approach allows to bind roles and fillers but has an important downside: After the presentation of multiple sentences, the roles and fillers are soon saturated, connecting all roles with all fillers. If synapses are not immediately removed, this approach will lead to interference; which we refer to as the unbinding problem. (B) Conjunctive synaptic role-filler binding. An alternative solution is to implement a set of “gates” that would, for instance, activate specific roles and fillers. Once activated, a gate would activate the corresponding (role or filler) elements. This approach has the same unbinding problem, and additionally presents a combinatorial problem: 3N2 gates must be created to store all possible bindings.
If roles and fillers are stored independently, a next crucial property for flexible cognition is dynamic role-filler binding (Hummel, 2011). Specifically, it must be possible to rapidly bind and unbind roles and fillers in order to understand complex events in the real world. Consider hearing the story that Tom buys a book, then that Mehdi buys a bottle, and finally that Tom gives his book to Mehdi. In order to understand the three events and their logical relations, and in order to answer questions about the situation (Who currently owns two items?), it is important to initially bind Tom to Subject and book to Object; and then bind Mehdi to Subject (and unbind Tom and Subject) and bind bottle and Object (and unbind book and Object); and so on.
Synaptic Binding
It then remains to be explained how dynamic role-filler bindings are formed. One approach is to construct a synaptic connection between each (role, filler) pair for each sentence that is currently of relevance. We will call this a synaptic binding approach; presumably, a configuration of synapses stores the relevant information. Note that the term “synapse” can be interpreted either literally in the biological sense, or more metaphorically; the only functional requirement of a synapse for our purposes is that two memory elements are connected. It is the approach applied, for example, in neural network training algorithms (e.g., backpropagation; Rumelhart et al., 1986). Whereas originally thought to contribute mainly to long-term memory, recent work suggests that synaptic binding also supports working memory (the synaptic model of working memory; Mongillo et al., 2008; Stokes, 2015). However, this synaptic binding approach has its downsides. In particular, if synapses are not immediately removed after a sentence, interference will quickly occur. Consider again first representing that Tom buys a book, then that Mehdi buys a bottle, perhaps followed by some other purchases and exchanges of goods. In this case, roles and fillers will soon be saturated, connecting all roles with all fillers, and thus abolishing any meaning (see Figure 1A as an example). We will call this the unbinding problem of synaptic binding. Computationally, the problem manifests itself in catastrophic interference between partially overlapping tasks, which is a huge problem in neural networks (French, 1999), with several solutions being proposed to overcome it (McClelland et al., 1995; Kirkpatrick et al., 2017; Verbeke and Verguts, 2019). Also biologically, it’s not clear that the construction and destruction of biological synapses can occur at the time scale required for cognitive processing (Kasai et al., 2003; Caporale and Dan, 2008).
One could argue that the problem in the previous scenario derives from the direct synapses between roles and fillers. Thus, an alternative synaptic binding solution could be to implement a set of “gates” that filter out or activate specific roles and fillers. For example, there could be one gate for Tom, one for Mehdi, another for Subject, and so on. When the corresponding gates are activated, they in turn activate their corresponding (role or filler) elements. This approach would obviate the requirement of direct bindings between roles and fillers. However, in this approach, suppose each gate is selective for a specific role or filler; then appropriate (role, filler) pairs cannot be kept apart. Consider for example representing that Tom buys a book and Mehdi buys a bottle; in such a system, the interpreter of the system has no way to know whether the activated book belongs with Tom or with Mehdi. To solve this problem, one could suppose, instead, that there is a separate gate for each (role, filler) pair (Figure 1B). This approach could solve the problem of disambiguating different meanings. Because neurons are here dedicated to (role, filler) conjunctions, this falls under the more general conjunctive coding approach (Bouchacourt and Buschman, 2019). However, here the combinatorial problem (3N2 gates must be created) and the unbinding problems appear again; furthermore, it’s not clear how one can generalize information about (say) a filler to other (role, filler) pairs (Hummel, 2011). We conclude that a pure synaptic binding approach is likely insufficient to implement dynamic role-filler bindings at the time scale required in systematic cognition.
Time-Based Binding
Instead of synapses, one could consider using the time dimension to bind roles and fillers; we will call this a time-based binding approach. In particular, suppose that at time t, the role-filler pair (Subject, Tom) is active. However, a single time point doesn’t leave enough time for processing; moreover, the system doesn’t necessarily know when exactly the information will be of use in further task processing. It is thus useful to repeat the information for some period of time. Let’s suppose that the binding is repeated at intervals of length d. Hence, at all times A1 = {t, t + d, t + 2d, …} both elements of the (role, filler) pair (Subject, Tom) are active. Note that Subject and Tom are indeed joined by time only; there is no synaptic connection between them.
Besides representing (Subject, Tom), we also need to represent the pair (Verb, Buy). However, if the pair (Verb, Buy) were active at the same time as (Subject, Tom) (say, at time t) we run the risk of interference, as explained in the previous paragraph. We must thus represent it at some other time, say t + e. Just like for (Subject, Tom), we repeat the (Verb, Buy) pair at the same distance d so the two pairs maintain their temporal separation. Thus, we conclude that at times A2 = {t + e, t + d+ e, t + 2d + e,..} the pair (Verb, Buy) is active.1 With a similar logic, at times A3 = {t + 2e, t + d+ 2e, t + 2d + 2e} the pair (Object, Book) will be active.
Synaptic Learning on Time-Based Bindings
It is well known that a neural network training rule (such as backpropagation) can learn complex tasks via synaptic binding, especially if it has available appropriate (here, compositional) representations of the input space. We postulate that this allows an efficient combining of the synaptic and time-based approaches. Specifically, once a time-based binding system as sketched above is constructed, a synaptic learning rule operating on its representations can subsequently learn various tasks. For example, in the book-buying context, the training rule could learn to answer questions such as “Who bought a book?”; “Who owns a book?”; and so on. Or in an experiment context, relevant mappings to be learned could be “Press the f key if you see a red square, the j key if you see a blue circle, and nothing otherwise.”
Importantly, such a representational system with independent and dynamic role-filler pairs, allows for generalization. For example, if novel information is learned about, say, Tom, this novel information can be attached (by the learning rule) to the representation of Tom, and thus be immediately generalized to other contexts in which Tom may appear.
Moreover, during both learning and performance, it’s very easy to delete old, no longer relevant information without leaving any trace to be erased: No synapses were created for binding, so none need be erased. It’s straightforward to represent the fact that Tom buys a book, followed by the fact that Mehdi buys a bottle. Finally, it’s relatively easy to construct new (role, filler) pairs via synchronizing “bursts” (Verguts, 2017; and see section “Discussion”).
In summary, we propose that synaptic and time-based binding ideally complement each other for the purpose of flexible cognition. Time-based binding allows quickly constructing and destructing connections. In contrast, synaptic binding allows application of very powerful learning rules. In this way, advantages of both synaptic and time-based binding are exploited, and their respective disadvantages are mitigated.
A Resource Bound to Time-Based Binding
Despite its several advantages, there is a constraint to the time-based approach. Specifically, this system of representing information will only work if the elements of sets A1, A2, and A3 [i.e., the timings of the different (role, filler) pairs] remain sufficiently separate (where “sufficient” depends on the level of precision required to robustly pass a message to a downstream neural area). Hence, such a system of representations can efficiently represent information [via dynamic (role, filler) bindings], and forget old, no longer relevant information. But it has an inherent constraint: It can only represent a limited number of elements at the same time.
Can we characterize this constraint more precisely? Note first that all distinct elements (or groups of bounded elements), referred to as items, should be activated once before any of them gets reactivated, otherwise, there is an ambiguity which set an item belongs to. For instance, if three items are presented in a specific order (item 1, item 2, item 3), and are activated at t, t + e, and t + 2e, then item 3 must be activated before item 1 gets reactivated. In other words, we require that t + ne < t + d, that is, n < d / e. Storage capacity n has thus an upper bound d/e, determined by the period (d) of each set, and the time (e) between elements. This bound cannot be made arbitrarily high: If d is too high,2 the time between different elements [(role, filler) bindings] is too long, and the items cannot be simultaneously processed by a downstream neural area that must interpret the bindings. Imagine having to remember a grocery list with several minutes between the different items. Similarly, if e is too small, the separate elements cannot be disentangled from each other, either because of noise or because of the time scale of the downstream neural area. We propose that these factors together impose a bound on how many novel items agents can store simultaneously.
Note that the argument is purely computational: Any agent (biological or artificial) who is confronted with a task with the described requirements (simultaneous but systematic storing of possibly rapidly changing facts, using a single representational space), should use time-based binding; and as a result, he or she is subject to the constraints. The argument also clarifies when the bound applies and when it does not. It is perfectly possible to store thousands of facts via synaptic binding, as long as they do not require on-the-fly constructions and destructions of conjunctions of information. In other words, the resource bound applies to (non-synaptic) working memory, not to long-term memory.
Simulation
We next consider how a time-based binding system may be neurally implemented. To construct such a system for representing novel, on-the-fly constructions, one needs a periodic or oscillatory function [i.e., f(X + c) = f(X) for some c and for all X)]. The simplest choice is perhaps a sinusoidal (sine or cosine) function, but this is not necessary.
Consistently, a long research tradition has suggested an important role of oscillatory functions (oscillations in short) for cognition in general, and for binding elements in memory in particular (Gray and Singer, 1989). It is well known that coupled excitatory and inhibitory neurons can easily be employed to generate oscillations (Wilson and Cowan, 1972; Gielen et al., 2010). The coupling parameters (synaptic weights) between excitatory and inhibitory neurons determine the characteristics of the oscillations, such as their phase, amplitude, and frequency.
To illustrate our argument, we used a recently proposed architecture of binding through oscillations to model working memory (Pina et al., 2018). This oscillatory neural network can bind and maintain elementary features (each represented by one node of the network) over time, while keeping different bindings apart. Each node of the network is composed of three components (a neural triplet, see Figure 2A). The three components are fast-excitatory (u, emulating AMPA synapses), slow-excitatory (n, emulating NMDA synapses), and inhibitory (v, emulating GABA synapses), respectively. The fast-excitatory—inhibitory pair constitutes a Wilson-Cowan type system (Wilson and Cowan, 1972). This pair exhibits limit cycle behavior (i.e., oscillations) and, as stated above, the characteristics of these oscillations (e.g., amplitude, frequency) can be controlled by changing the coupling weights between these components. Additionally, the slow excitatory component provides excitatory input to the inhibitory and fast excitatory components, thereby allowing bistability of the neural triplet (Lisman et al., 1998): an inactive state with low amplitude fluctuations, and an active state with persistent high amplitude oscillations. The left part of Figure 2A shows the connectivity between each component of a neural triplet; see Appendix for a full description of the differential equations defining each component’s activity, and the value of each parameter including the weights between nodes of the network. We kept all parameter values equal to the main simulations in Pina et al. (2018), and only varied the inhibitory component’s time scale (τi, Equation 2 in Appendix). This parameter defines the speed at which the inhibitory component’s activity is updated. Varying τi allows to manipulate the oscillatory frequency of the nodes’ active state. At the neurophysiological level, this parameter can be considered to reflect temporal aspects of the response function of GABA receptors. This response function, which can be thought of as a band-pass temporal filter on incoming inputs to the synapse (Robinson et al., 2004), has been shown to be affected by neuromodulators (e.g., noradrenaline Sillar et al., 2002), which provide a mechanism to control neural populations’ dynamics (Berger et al., 2003; see also Shine et al., 2021 for a review on computational models of neuromodulatory systems). Moreover, in this model, nodes form a network (upper-right part of Figure 2A) in which all fast-excitatory components excite each other, and all inhibitory components inhibit each other (see bottom-right part of Figure 2A). This connectivity allows these nodes to form a network that exhibits binding by phase; that is, when the peak of two nodes coincide, within a certain temporal range that we will call a “binding window,” they attract each other and align their peaks, forming a bound state. This network is further also characterized by competition between active nodes (or between groups of nodes that are bound together); i.e., when the peak of two nodes are separated by an interval outside of the binding window range, they will repel each other and remain active in an out-of-phase state. Due to the intrinsic attracting and repelling dynamics of this architecture, it can thus bind and maintain information to form distinct memories (a single active node, or bindings between nodes), while avoiding mixing them, through time-based binding. Each memory consists of one or multiple bound elementary features, each represented by a node. In line with the theory postulated above, a memory is activated only periodically (see Figure 2B).
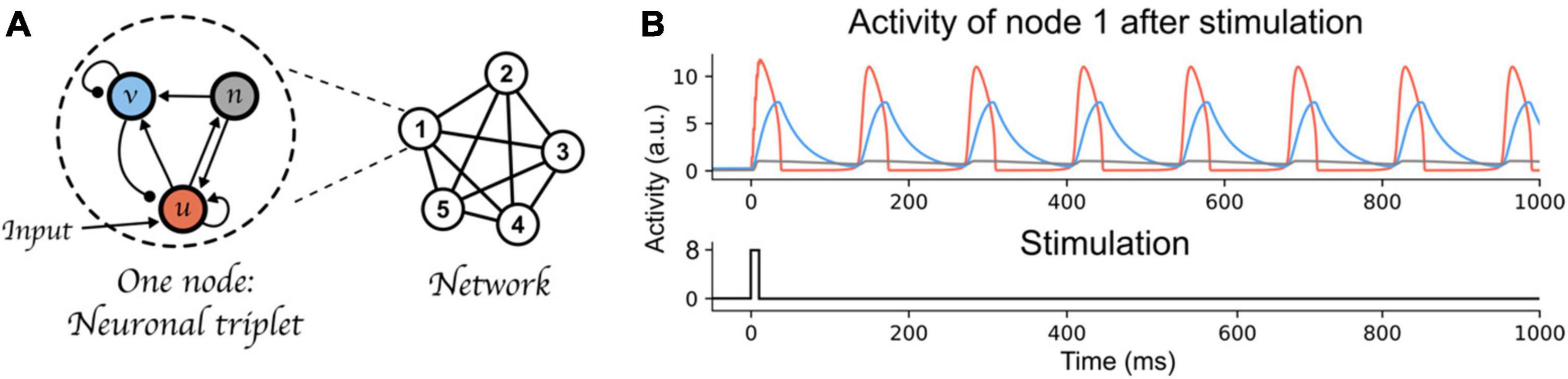
Figure 2. Time-based binding network. (A) Each node of the network is a “neural triplet” composed of three components: A fast excitatory (u) neuron, an inhibitory (v) neuron, and a slow excitatory (n) neuron. Stimulation to a node affects unit u (as represented by the “Input” arrow). This architecture allows each node to start oscillating thanks to the excitatory-inhibitory pair of neurons (u and v, respectively), and maintain this oscillation through time (i.e., very slow decay) due to the slow excitatory neuron (n). When interconnected through excitatory-to-excitatory, and inhibitory-to-inhibitory connections, these nodes form a network that exhibits binding by phase and competition between active nodes or bound group of nodes. Synaptic weights are represented as lines between components or nodes: arrow ended lines represent excitatory connections, circle-ended lines represent inhibitory connections. (B) Top: Example of node 1 being activated by an input stimulation. The red curve represents the activity of the fast-excitatory neuron (u), the blue curve represents the activity of the inhibitory neuron (v), and the gray curve represents the activity of the slow-excitatory neuron (n). Bottom: stimulation time course.
The ability to concurrently store multiple items of information in this manner, relies on two important features. First, the elements of each item must be bound together. For instance, nodes representing the role “Subject” and the filler “Tom” are in synchrony. Second, to concurrently maintain multiple memories (e.g., Subject-Tom and Verb-Buy), the two nodes representing the Subject-Tom pair must remain out of synchrony with those representing the Verb-Buy pair (see units 1–2 and 3–4 in Figure 3). This mechanism entails that the number of distinct memories that can be maintained simultaneously without interference, is limited by the frequency of the oscillation. In doing so, this model exemplifies how capacity limits emerge as a property of a system using oscillatory mechanisms for binding, i.e., the time interval between two peaks of activated nodes (or groups of nodes).
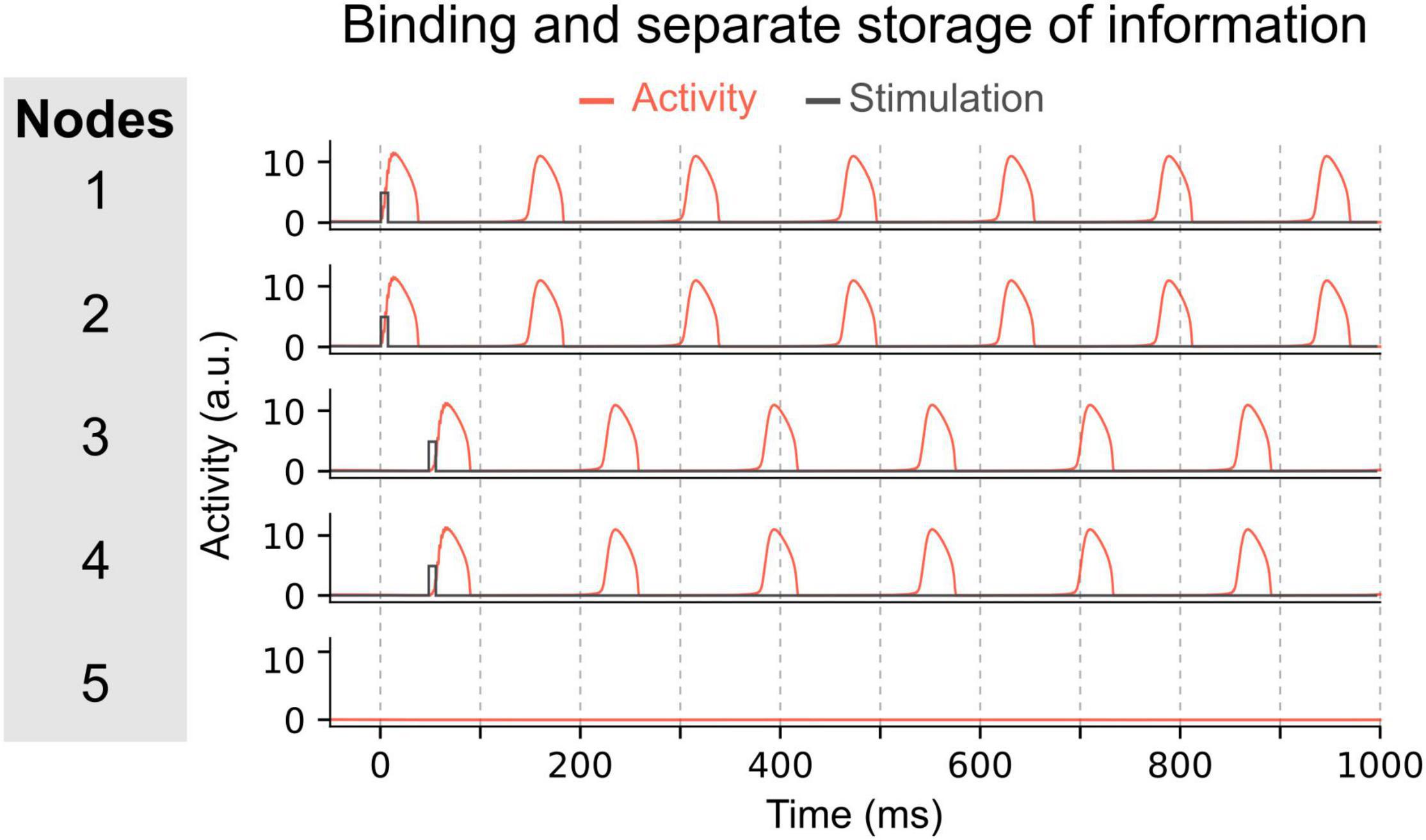
Figure 3. Maintenance of two pairs of items in the network. In this network, pairs of nodes are activated simultaneously, i.e. nodes 1 and 2 at time 0 ms, and nodes 3 and 4 at time 50 ms. Due to the inter-node coupling, each node of a pair will oscillate in synchrony and can thus represent a bound multi-element memory. The other pair will oscillate out-of-phase with the first pair allowing to store each binding separately and to permit read-out of each multi-element memory by downstream areas.
One of the parameters that determines the memory capacity, is the oscillatory frequency of the network, which itself is determined by the τ parameters. To illustrate the effect of frequency, we changed the temporal scale of the inhibitory component (τi) of all nodes. In a first simulation (using τi = 32), each node oscillates at ∼6 Hz once activated. The network can maintain up to 3 memories out-of-phase (i.e., their activity periodically peaks but never at the same time, allowing a downstream area to read-out each memory independently, see Figure 4A). In this network, the memory capacity is thus 3. In a second simulation we decreased the temporal scale of inhibitory components to obtain a network in which nodes oscillate at a slower frequency (τi = 20). In this network, activated nodes will oscillate at a faster frequency of ∼10 Hz (see Figure 4B). When activating the first two nodes, they start oscillating out-of-phase, thereby maintaining 2 memories in the network. But when activating a third node, it will start competing with the first or second active node (depending on the exact timing of the stimulation of the third node). This competition will lead to one of three possible states: (1) the third node may not be able to sustain activation and this third memory will be lost; (2) the third node inhibits one of the other two nodes and the network will thus loose one of the previously stored memories; or (3) the third node synchronizes (or binds) with one of the activated nodes, thus creating a new bound memory. This last state is illustrated in Figure 4B. Each of these options shows that in this faster network, three distinct memories cannot be maintained concurrently, and therefore that a faster oscillatory frequency leads to a lower memory capacity.
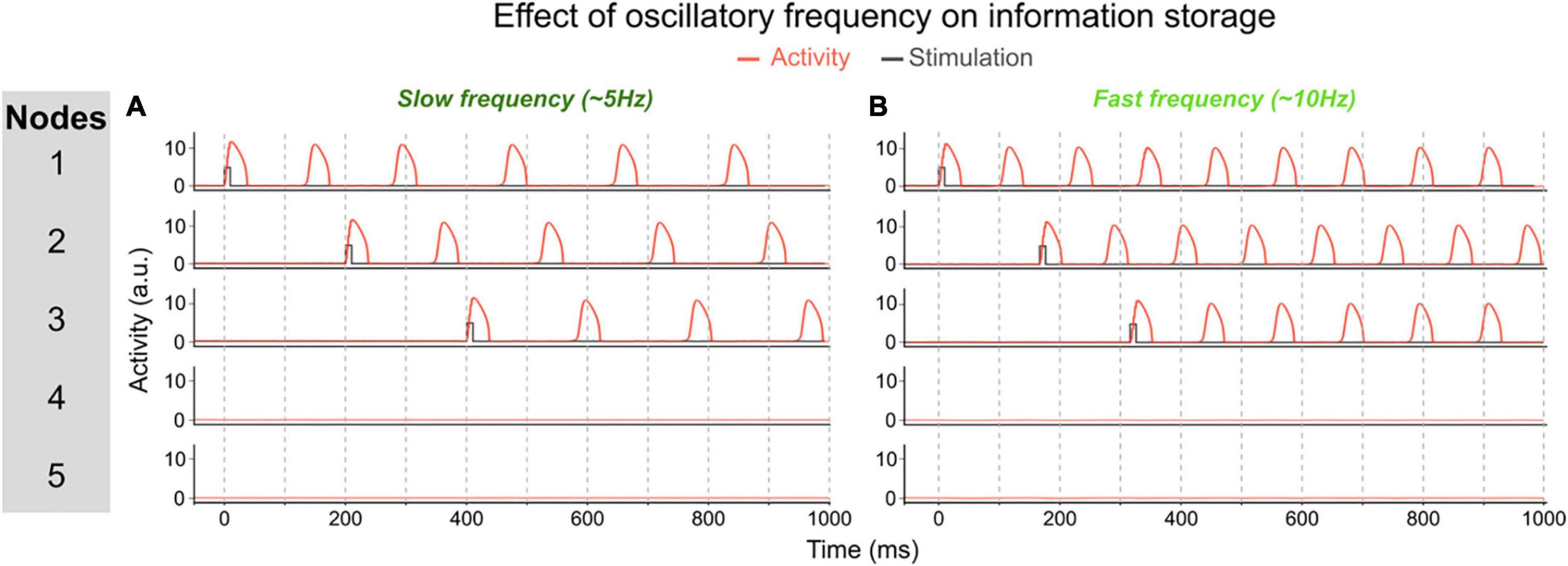
Figure 4. Effect of oscillatory frequency on maximum number of maintained items. (A) In this network each activated node will oscillate at ~6 Hz (τi = 32). This allows to activate up to three units out-of-phase, i.e., three separately maintained memories. In this example, each node is activated sequentially and stays activated. (B) In this network, the inhibitory time scale (τi) has been decreased to 20 which increases the frequency of activated nodes to ~10 Hz. This prevents three nodes to be activated out-of-phase because of the short time interval between the activity peaks of activated nodes to “store” a third memory. In this example, node 3 is activated after nodes 1 and 2. Node 3 then shifts its phase and aligns with node 1, thereby losing the distinct information represented by each of the three nodes. This faster network thus has a lower memory capacity.
General Discussion
To sum up, we have argued that synaptic and time-based binding have complementary advantages for the implementation of flexible cognition. Time-based binding can be quick and literally leaves no traces behind; but it leads to a natural processing (or resource) bound. Instead, synaptic binding is slower, prone to interference, but it does not suffer from this processing bound. We illustrated this theory with simulations of a recent oscillatory model. In the General Discussion, we relate our proposal to resource (or “bug”) theories, computational (or “feature”) theories, and to earlier oscillation theories. We end by pointing out some empirical predictions.
Resource Theories
In the current section, we discuss three influential theories on the nature of working memory constraints and resources, key data that earlier literature interpreted as supporting the respective theories, and how our own perspective accounts for those data.
A long-standing slots theory holds that working memory consists of a fixed number of slots (with the proposed number varying from 1 to 7) (Miller, 1956; Cowan, 2001; Zhang and Luck, 2008); one can consider slots as a discrete resource. A key behavioral signature interpreted as favoring slots theory, is the observation of a fixed precision for memoranda held in working memory beyond the slots upper bound. To be more specific, Zhang and Luck asked their subjects to retain from 1 up to 6 colors presented on different locations on the screen in working memory. In the test phase, they subsequently queried which colors were accurately remembered (subjects indicated the colors by clicking on a color wheel). Fitting a mixture model on the behavioral data in the test phase, the authors observed that the precision (inverse variance) of memory was statistically similar for 3 and for 6 items. They interpreted this as meaning that the number of available slots was equal to around 3. Instead, the precision in working memory did increase from 1 to 3; the authors interpreted this as meaning that more than 1 slot can be devoted to the same object (e.g., an object represented by two slots will be represented more precisely than one represented by just a single slot). However, fine-grained experimental paradigms with continuous manipulations of the relevant features (e.g., color or physical location), have since then demonstrated that there is no abrupt non-linearity around 3 items (Ma et al., 2014). From the lowest set sizes on, when more items must be held in working memory, the representational precision of the remembered items gradually decreases. This data pattern was interpreted in terms of a continuous resource theory (often called resource theory in short), which holds there is a continuous but finite resource to be divided among the memoranda (Bays and Husain, 2008; Ma et al., 2014).
How to account for this data in a time-based binding perspective? We propose the following tentative theory. Suppose each neuron has a specific receptive field across some feature space (e.g., color space or Euclidean space). In the example, color- and location-sensitive neurons must bind to one another in order to represent the stimuli correctly. Suppose that there is a pool of neurons responding to specific colors and locations, in the color and Euclidean spaces, respectively. Suppose further that each neuron in one pool (e.g., responding to an active color) must be bound to at least one neuron from the other pool (responding to an active location) in order to influence downstream processing (i.e., be in working memory). Then, the precision will gradually decrease as more items must be retained: Indeed, more items retained means that less neuron pairs can be devoted to any specific item, given a finite period length of the oscillation. At first sight, it would seem that this theory predicts a hard bound at the maximal number of bindings that fit in a cycle, as in slots theory. However, given that several variables (including pool sizes, average receptive field, item location, period length, etc.), may vary from trial to trial, precision will also gradually decrease when additional items are in memory.
Another important behavioral signature is the occurrence of misbinding errors. This means that when (say) colors need to be remembered at specific locations, colors and locations may swap places in the participant’s memory. The existence of misbinding errors is naturally in line with a binding account; if two features (say, location and color) are incorrectly bound in memory, a binding error at behavioral level automatically follows.
The existence of misbinding errors was originally interpreted in terms of a third influential perspective on the nature of cognitive constraints on working memory, namely interference theory (Oberauer and Lin, 2017). Interference theory is in line with a long tradition of computational modeling via synaptic binding, specifically in neural networks. Interference theory holds that the postulation of a (discrete or continuous) resource is not required.3 Cognitive processing in a neural network already leads to massive processing limitations due to (catastrophic) interference, and the latter is sufficient to explain the behavioral-level processing impairments that arise in tasks requiring the maintenance of several items at the same time.
At the risk of being overly reconciliatory, it’s worth pointing out that our time-based binding theory shares commonalities with each of the classical (discrete and continuous resources, interference) theories of working memory constraints. Like slots theory, it holds that just a fixed number of elements (here, bindings) can be maintained simultaneously. However, because the memoranda are bindings between neurons with variable parameters (cf. above), our perspective can predict, just like continuous resource theory, that there is no fixed bound at any number of items, and representational precision instead gradually decreases with more remembered items. Finally, binding elements together is rarely sufficient to solve actual tasks. The bindings must be read out by downstream task-specific processing modules. Such processing modules can most naturally be composed of standard neural networks, which implement synaptic binding, and are trained with gradient-based algorithms. The latter naturally also leads to similarities with interference theory.
Computational-Level Theories
Generally speaking, computational-level theories consider that humans and other agents act in such a way as to achieve some goal (Lieder and Griffiths, 2020). As applied to working memory constraints, it holds that working memory may not be bound by the scarcity of a discrete or continuous resource, but that its boundedness is an optimal response to its environment. The early selection-for-action theory, for example, held that working memory must subserve action in the world (discussed in Hommel et al., 2001); and because action must be integrated (one cannot, for example, prepare a gratin dauphinois and play a video game at the same time), some environmental features must be selected, and others ignored. A generalization of the selection-for-action theory is the selection-for-procedures theory proposed by Ansorge et al. (2021). These authors argued that the bottleneck for actions (i.e., very few and typically just one action can be carried out at the same time), also applies to the execution of procedures. Using their own example, drawing the correct conclusion from the premises “All bees are insects” and “All bumblebees are bees,” requires that the relevant information is active at specific times, not all at the same time; thus to avoid drawing conclusions such as that all insects are bumblebees. The current theory is very much in line with this generalization of selection-for-action theory, and proposes a specific computational reason why time-sharing is beneficial (it keeps the number of synapses low), but, because time is one-dimensional, it introduces a processing bottleneck.
More recently, Musslick and Cohen (2021) have combined computational-level and interference theory (see previous paragraph) to explain why cognitive control appears to be limited. Their starting point is the dilemma between learning and processing efficiency in standard neural networks: Efficient learning requires overlapping (shared) representations between tasks, but at the same time such overlap impairs multitasking (i.e., simultaneously performing two or more tasks). When tasks share input or output features, multitasking is almost impossible in such neural networks. Their simulations demonstrate that, in standard neural networks, basically just one task can be performed at any time. Thus, the optimal agent chooses to carry out just one task at a time. In their perspective, the bound is an optimal choice, given the computations one has to do and given the architecture that is available for doing so. In principle, all tasks could be carried out at the same time; but because of the massive interference this would engender, an optimal agent chooses to limit the number of simultaneously processed items. Alternatively, time-based bindings could allow to perform multiple tasks at the same time by attributing processing for different tasks at alternating oscillatory cycles. However, like bindings for working memory, also the number of tasks that one can alternate between is bounded by n < d/e. Moreover, such alternating task processing might decrease robustness of task representations when e is small or when noise enters the system. Hence, it would introduce a trade-off between cognitive stability (robust task processing supporting high performance) and cognitive flexibility (rapidly switching between different tasks).
Furthermore, it has been proposed that highly specialized separate representations might develop for high frequency tasks or stimuli (see also Musslick et al., 2020; and our discussion of high-frequency sentences in the Introduction). For instance, features of familiar faces are stored independently from other facial features (Landi et al., 2021). Hence, with more extensive learning synaptic bindings become more separated, decreasing the importance of time-based bindings.
Oscillation Theories
The interest in neural oscillations dates back at least to the theoretical work of von der Malsburg (von der Malsburg and Singer, 1988). Around the same time, Gray and Singer (1989) detected that neural spiking in cells in the visual cortex phase-lock to the gamma rhythm (50–90 Hz); especially for features that are perceived as belonging to the same object. This led to the “binding by synchrony” and later the “communication through coherence” (CTC) theory. According to CTC, neurons in different brain areas can be bound together by firing in the same gamma phase (or more generally, by firing in a consistent and appropriate gamma phase difference; Fries, 2015). In particular, neurons in distant areas with a consistent gamma phase difference would share information more efficiently. For example, suppose the peak of the gamma wave is the phase where information can be most efficiently sent to other neural areas; if two neurons in distant areas always fire at this phase of the wave (i.e., the peak), this coincidence can be read out by downstream areas, and thus the two neurons are functionally (but not physically) bound. The binding of these different-area gamma waves could be orchestrated by a slower theta (4–7 Hz) wave (Voloh and Womelsdorf, 2016; Verguts, 2017). Originally, this theory was proposed for the visual cortex, but has later been extended to cortical processing more generally. At this time, a massive amount of electrophysiological data supports (aspects of) the CTC theory (Womelsdorf et al., 2010), also in human cognitive control (Cavanagh and Frank, 2014), and in particular its relation with the slower theta wave (see also “Empirical predictions” section below).
In a second broad oscillation theory, Lisman and Jensen (2013) proposed that neural spikes that are locked to different gamma waves represent different pieces of information, where each gamma wave itself is locked to a different phase of the slower theta waves. This theory originates from findings observed in the hippocampus. In particular, the phenomenon of theta phase precession (O’Keefe and Recce, 1993) entails that as an animal proceeds in a cell’s preferred location, the cell’s spike firing time relative to theta phase moves earlier (processes) in time. From this observation, it was proposed that the time of spike firing relative to theta phase is informative for downstream areas. This is what we will call the theta-phase binding (TPB) theory. Lisman and Jensen (2013) generalized this theta phase precession theory by proposing that items in working memory (and, potentially, their presentation order) are stored by locking consecutive items in the list (each of them represented as neurons locked to gamma waves), to consecutive phases of theta (see also “Empirical predictions” section below). Regarding ordered sets, during an individual theta cycle, neurons representing each item of the sequence fire in a fixed temporal order (e.g., the order of presentation), thereby conserving the ordinal information of the sequence.
Clearly, CTC and TPB theories have some commonalities; and they can be combined in the same framework, as was already demonstrated by McLelland and VanRullen (2016). Specifically, in a two-layer neural network model, they demonstrated that inhibition in the higher layer only, would cause patterns similar to what CTC would predict; whereas inhibition in both lower and higher layers, would instead cause patterns more similar to TPB. Also, our own time-based binding theory combines elements of CTC and TPB. Like CTC, it proposes that binding elements together is crucial for cross-area communication. Also, like CTC, it proposes that such binding is efficiently implemented via time. Like TPB, it holds that different packages of bindings can each be locked to a phase of a slower wave.
We emphasized that role-filler independence is a crucial property of an efficient (learning) cognitive system; role-filler independence itself can be considered a special case of factorization or disentanglement, a major aspect of modern Artificial Intelligence systems (Higgins et al., 2017; Steenbrugge et al., 2018). Role-filler independence implies that the two constituents must be bound somehow, and we mentioned both synaptic and time-based approaches for achieving this. One synaptic approach to achieve role-filler independence was described by Kriete et al. (2013). These authors propose that the “address” of a filler would be gated into a “slot” relevant for a specific role that is encoded in the prefrontal cortex. In this way, roles and fillers remain separate (disentangled), but can still be combined when a filler is queried (e.g., who owns the book?). Finally, we note that, besides synapses and time, other binding schemes can be devised. For example, Akam and Kullmann (2010, 2014) proposed that also frequency could be used to bind elements together (as is also implemented in telecommunication systems). In general, any “labeling” of two or more elements would in principle be usable. Time and frequency happen to be the ones that are most naturally implemented via oscillations.
Empirical Predictions
Our perspective also leads to several empirical directions for future research. Ideally, empirical predictions are derived from a computational model (e.g., Senoussi et al., 2020b). However, whereas the current model can be used to illustrate theoretical concepts (as we did here), it is not ideal for deriving specific empirical predictions, because it lacks critical components to derive such predictions, such as a response interface. While the full model awaits further development, we already point out more general predictions in the remainder of this section.
One interesting direction is to look at evidence for oscillations in behavioral measures. Recent literature has started to do just that by using dense temporal sampling paradigms in which the time interval between two events (e.g., a cue and a target) is varied across trials, allowing to estimate a time course of behavioral performance. For instance, Landau and Fries (2012) asked their subjects to pay attention to two horizontally lateralized gratings and notify the appearance of a brief contrast decrease. Spectral analysis (e.g., using Fast-Fourier Transform) of time-course of the accuracy data, obtained via a dense temporal sampling paradigm, revealed that attention fluctuated at theta frequency between the two gratings. Several other studies have replicated this finding (e.g., Fiebelkorn et al., 2013; Dugué et al., 2016; Senoussi et al., 2019; Michel et al., 2021; see Kienitz et al., 2021 for a review), and additionally expanded the study of oscillations in behavioral performance to the field of working memory (Peters et al., 2020; Pomper and Ansorge, 2021). Of importance, although the findings on fluctuations in attentional sampling might be interpreted as reflecting a different role of oscillations than the one that is proposed in this article, we believe that these potential roles share a core function supported by oscillations, namely to avoid interference. Fiebelkorn and Kastner (2019) proposed that across one cycle of a theta oscillation, there is an alternation between sensory information sampling (e.g., at a spatial location) and motor processes associated with shifting the focus of attention (e.g., to another location or object). They argue that this temporal isolation of distinct processes (i.e., sensory and motor) allows to resolve potential conflicts between sampling and shifting functions by organizing them temporally. This is in line with the role of oscillation we consider in the current article which is to avoid the massive interference that parallel processing in neural networks may naturally yield. Altogether, and irrespective of the specific role attributed to oscillations, this rapidly growing body of literature provides converging evidence that oscillatory processes are central to behavioral performance in a wide range of cognitive functions, in which they provide both a mechanism to sample or bind information, as well as a capacity limit of these functions.
In the field of working memory, predictions from the TPB theory have received support from several studies. According to the TPB theory, theta oscillations originating from medial temporal lobe and basal forebrain structures (e.g., hippocampus, septum) are hypothesized to support the maintenance of the ordinal information in an item sequence in working memory (Lisman and Jensen, 2013): the phase of theta oscillations structures the activation of distinct neural populations oscillating at gamma frequency, each representing an item of the maintained sequence. This theory thus predicts that a lower frequency of theta oscillations, leading to longer periods in which items could be nested, would lead to higher working memory capacity. Some studies have confirmed this prediction empirically by showing that higher working memory loads led to a reduction of theta frequency (Axmacher et al., 2010; Kosciessa et al., 2020). Moreover, a recent study causally tested this prediction using transcranial alternating current stimulation (tACS; Wolinski et al., 2018) and showed that stimulating a fronto-parietal network at a slow theta frequency (i.e., 4 Hz) led to higher working memory capacity than stimulating at a faster theta frequency (i.e., 7 Hz). These studies confirm some predictions from the TPB theory as applied to working memory; and thus, they strengthen the view proposed in this article that oscillatory frequency modulates capacity limits in working memory, thereby constituting a factor limiting cognitive resources. Finally, we note that TPB naturally accounts for contiguity effects (i.e., if item n from a sequence in a free recall paradigm is recalled, then contiguous items at locations n - 1 and n + 1 are likely to be recalled next; Healey et al., 2019); and particularly the asymmetric nature of contiguity effects (the item at location n + 1 is more likely to be recalled than the item at location n - 1). Indeed, in TPB, items are preferentially “replayed” in the order in which they appeared, and this has been observed empirically (Reddy et al., 2015, 2021; Kok et al., 2017; Blom et al., 2020; Senoussi et al., 2020a). However, a long list of benchmark phenomena relating to the contiguity effect have been reported (e.g., 34 phenomena by Healey et al., 2019), and we do not claim that we can explain them all based on time-based binding; nor, indeed, that time-based binding is responsible for all of them. In fact, the reader will recall that we propose that time-based and synaptic processes necessarily co-exist for the construction of (episodic) memories. It will remain a future challenge to disentangle which of the two processes accounts for which phenomenon.
A related body of work has investigated the role of theta oscillations generated by the anterior cingulate cortex (ACC) in cognitive control processes (Cavanagh and Frank, 2014). Several studies have shown that these frontal theta oscillations are elicited when control is needed, i.e., during conflict or in preparation of a difficult task (Cavanagh and Frank, 2014), and allow to coordinate distant neural populations to create task-relevant functional networks through synchronization (Bressler et al., 1993; Varela et al., 2001; Palva et al., 2005; Canolty et al., 2006; Voloh and Womelsdorf, 2016). This theta-rhythmic process has been shown to support successful task performance (Voloh et al., 2015) and to support the instantiation of task rules (Womelsdorf et al., 2010). Critically, the frequency of these oscillations has recently been proposed to shift in response to task demands. Indeed, a recent study proposed that theta frequency balances reliable instantiation of task rules and the rapid gating of sensory and motor information relevant for the task at hand (Senoussi et al., 2020b). They showed that this shift is observable both in oscillation of behavioral performance (using a dense behavioral sampling paradigm) and electrophysiological data, and that the magnitude of this shift correlates with inter-individual differences in task performance (Senoussi et al., 2020b). Other studies have also reported the involvement of different low-frequency bands during top-down control processes, especially in hierarchical task implementation (Cooper et al., 2019; de Vries et al., 2020; Riddle et al., 2020; Formica et al., 2021). Together, these results open interesting avenue for future research on the conventional frequency limits of oscillations supporting cognitive control (usually attributed to the theta band) and more generally on the nature of the constraints controlling and limiting frequency shifts in neural oscillations. Future studies investigating the causes and consequences of frequency shifts in neural oscillations supporting cognitive control, for instance through neuromodulatory systems (Sara, 2015; Silvetti et al., 2018), will undoubtedly provide valuable insights on the neural bases of cognitive resources and their limitations.
Conclusion
We proposed that neural oscillations are both a solution to and a problem for flexible cognition. They are a solution because they allow items to be bound “on the spot,” leaving no synaptic traces that need to be erased afterward, thus causing minimal interference (a notorious problem in standard artificial intelligence). They are also a problem because of the natural bound this system imposes; in this sense (only), the theory could be considered a resource theory.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary material, and the scripts to run the model and reproduce the simulations are available on this GitHub repository: https://github.com/mehdisenoussi/resources. Further inquiries can be directed to the corresponding author.
Author Contributions
MS, PV, and TV developed the model. MS and PV analyzed model simulations. All authors wrote the manuscript.
Funding
MS and TV were supported by grant G012816 from Research Foundation Flanders. TV was supported by grant BOF17-GOA-004 from the Research Council of Ghent University. PV was supported by grant 1102519N from Research Foundation Flanders. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Without loss of generalization, we can assume e < d.
- ^ Note that the timescale of an upstream neural area may also impose an upper bound on d (there is a lower limit in frequencies produced by neural ensembles).
- ^ For completeness, it must also be mentioned that, in line with earlier work of Oberauer (2003) and Oberauer and Lin (2017) also included a focus of attention in their model; an extra storage component that can hold just a single item. One could think of this storage component as a single slot (discrete resource).
References
Akam, T., and Kullmann, D. M. (2010). Oscillations and filtering networks support flexible routing of information. Neuron 67, 308–320. doi: 10.1016/j.neuron.2010.06.019
Akam, T., and Kullmann, D. M. (2014). Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat. Rev. Neurosci. 15, 111–122. doi: 10.1038/nrn3668
Ansorge, U., Büsel, C., Forstinger, M., Gugerell, D., Grüner, M., Pomper, U., et al. (2021). Procedural control versus resources as potential origins of human hyper selectivity. Front. Psychol. 12:718141. doi: 10.3389/fpsyg.2021.718141
Axmacher, N., Henseler, M. M., Jensen, O., Weinreich, I., Elger, C. E., and Fell, J. (2010). Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107, 3228–3233. doi: 10.1073/pnas.0911531107
Barlow, H. B. (1961). “Possible principles underlying the transformation of sensory messages,” in Sensory Communication, ed. W. A. Rosenblith (Cambridge, MA: MIT Press), 217–734.
Bays, P. M., and Husain, M. (2008). Dynamic shifts of limited working memory resources in human vision. Science 321, 851–854. doi: 10.1126/science.1158023
Berger, T., Senn, W., and Lüscher, H. R. (2003). Hyperpolarization-activated current I h disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J. Neurophysiol. 90, 2428–2437.
Blom, T., Feuerriegel, D., Johnson, P., Bode, S., and Hogendoorn, H. (2020). Predictions drive neural representations of visual events ahead of incoming sensory information. Proc. Natl. Acad. Sci. U.S.A. 117, 7510–7515. doi: 10.1073/pnas.1917777117
Bouchacourt, F., and Buschman, T. J. (2019). A flexible model of working memory. Neuron 103, 147–160.
Bressler, S. L., Coppola, R., and Nakamura, R. (1993). Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature 366, 153–156. doi: 10.1038/366153a0
Canolty, R. T., Edwards, E., Dalal, S. S., Soltani, M., Nagarajan, S. S., Kirsch, H. E., et al. (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628. doi: 10.1126/science.1128115
Caporale, N., and Dan, Y. (2008). Spike timing–dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46. doi: 10.1146/annurev.neuro.31.060407.125639
Cavanagh, J. F., and Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 18, 414–421. doi: 10.1016/j.tics.2014.04.012
Cooper, P. S., Karayanidis, F., McKewen, M., McLellan-Hall, S., Wong, A. S. W., Skippen, P., et al. (2019). Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. NeuroImage 189, 130–140. doi: 10.1016/j.neuroimage.2019.01.022
Cowan, N. (2001). The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav. Brain Sci. 24, 87–114. doi: 10.1017/S0140525X01003922
de Vries, I. E. J., Slagter, H. A., and Olivers, C. N. L. (2020). Oscillatory control over representational states in working memory. Trends Cogn. Sci. 24, 150–162. doi: 10.1016/j.tics.2019.11.006
Dugué, L., Roberts, M., and Carrasco, M. (2016). Attention reorients periodically. Curr. Biol. 26, 1595–1601. doi: 10.1016/j.cub.2016.04.046
Fiebelkorn, I. C., and Kastner, S. (2019). A rhythmic theory of attention. Trends Cogn. Sci. 23, 87–101.
Fiebelkorn, I. C., Saalmann, Y. B., and Kastner, S. (2013). Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr. Biol. 23, 2553–2558. doi: 10.1016/j.cub.2013.10.063
Fodor, J. A., and Pylyshyn, Z. W. (1988). Connectionism and cognitive architecture: a critical analysis. Cognition 28, 3–71. doi: 10.1016/0010-0277(88)90031-5
Formica, S., González-García, C., Senoussi, M., and Brass, M. (2021). Neural oscillations track the maintenance and proceduralization of novel instructions. NeuroImage 232:117870. doi: 10.1016/j.neuroimage.2021.117870
French, R. M. (1999). Catastrophic forgetting in connectionist networks. Trends Cogn. Sci. 3, 128–135. doi: 10.1016/S1364-6613(99)01294-2
Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220–235. doi: 10.1016/j.neuron.2015.09.034
Gevers, W., Santens, S., Dhooge, E., Chen, Q., Van den Bossche, L., Fias, W., et al. (2010). Verbal-spatial and visuospatial coding of number–space interactions. J. Exp. Psychol. 139, 180–190. doi: 10.1037/a0017688
Gielen, S., Krupa, M., and Zeitler, M. (2010). Gamma oscillations as a mechanism for selective information transmission. Biol. Cybern. 103, 151–165. doi: 10.1007/s00422-010-0390-x
Gray, C. M., and Singer, W. (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 86, 1698–1702. doi: 10.1073/pnas.86.5.1698
Healey, M. K., Long, N. M., and Kahana, M. J. (2019). Contiguity in episodic memory. Psychon. Bull. Rev. 26, 699–720.
Higgins, I., Matthey, L., Pal, A., Burgess, C., Glorot, X., Botvinick, M., et al. (2017). β-VAE: learning basic visual concepts with a con-strained variational framework. Int. Conf. Learn. Represent. 2:6.
Holroyd, C. B., and Verguts, T. (2021). The best laid plans: computational principles of anterior cingulate cortex. Trends Cogn. Sci. 25, 316–329. doi: 10.1016/j.tics.2021.01.008
Hommel, B., Müsseler, J., Aschersleben, G., and Prinz, W. (2001). The theory of event coding (TEC): a framework for perception and action planning. Behav. Brain Sci. 24, 849–878. doi: 10.1017/S0140525X01000103
Hummel, J. E. (2011). Getting symbols out of a neural architecture. Conn. Sci. 23, 109–118. doi: 10.1080/09540091.2011.569880
Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N., and Nakahara, H. (2003). Structure–stability–function relationships of dendritic spines. Trends Neurosci. 26, 360–368. doi: 10.1016/S0166-2236(03)00162-0
Kienitz, R., Schmid, M. C., and Dugué, L. (2021). Rhythmic sampling revisited: experimental paradigms and neural mechanisms. Eur. J. Neurosci. Available online at: https://onlinelibrary.wiley.com/doi/full/10.1111/ejn.15489
Kirkpatrick, J., Pascanu, R., Rabinowitz, N., Veness, J., Desjardins, G., Rusu, A. A., et al. (2017). Overcoming catastrophic forgetting in neural networks. Proc. Natl. Acad. Sci. U.S.A. 114, 3521–3526. doi: 10.1073/pnas.1611835114
Kok, P., Mostert, P., and de Lange, F. P. (2017). Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci. U.S.A. 114, 10473–10478. doi: 10.1073/pnas.1705652114
Kosciessa, J. Q., Grandy, T. H., Garrett, D. D., and Werkle-Bergner, M. (2020). Single-trial characterization of neural rhythms: potential and challenges. NeuroImage 206:116331. doi: 10.1016/j.neuroimage.2019.116331
Kriete, T., Noelle, D. C., Cohen, J. D., and O’Reilly, R. C. (2013). Indirection and symbol-like processing in the prefrontal cortex and basal ganglia. Proc. Natl. Acad. Sci. U.S.A. 110, 16390–16395.
Landau, A. N., and Fries, P. (2012). Attention samples stimuli rhythmically. Curr. Biol. 22, 1000–1004. doi: 10.1016/j.cub.2012.03.054
Landi, S. M., Viswanathan, P., Serene, S., and Freiwald, W. A. (2021). A fast link between face perception and memory in the temporal pole. Science, 373, 581–585. doi: 10.1126/science.abi6671
Lieder, F., and Griffiths, T. L. (2020). Resource-rational analysis: understanding human cognition as the optimal use of limited computational resources. Behav. Brain Sci. 43:e1. doi: 10.1017/S0140525X1900061X
Lisman, J. E., and Jensen, O. (2013). The theta-gamma neural code. Neuron 77, 1002–1016. doi: 10.1016/j.neuron.2013.03.007
Lisman, J. E., Fellous, J.-M., and Wang, X.-J. (1998). A role for NMDA-receptor channels in working memory. Nat. Neurosci. 1, 273–275. doi: 10.1038/1086
Ma, W. J., Husain, M., and Bays, P. M. (2014). Changing concepts of working memory. Nat. Neurosci. 17, 347–356. doi: 10.1038/nn.3655
Marcus, G. F. (2001). The Algebraic Mind: Integrating Connectionism and Cognitive Science. Bradford, PA: A Bradford Book.
McCauley, S. M., and Christiansen, M. H. (2014). Acquiring formulaic language: a computational model. Ment. Lex. 9, 419–436. doi: 10.1075/ml.9.3.03mcc
McClelland, J. L., McNaughton, B. L., and O’Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457. doi: 10.1037/0033-295X.102.3.419
McLelland, D., and VanRullen, R. (2016). Theta-gamma coding meets communication-through-coherence: neuronal oscillatory multiplexing theories reconciled. PLoS Comput. Biol. 12:e1005162. doi: 10.1371/journal.pcbi.1005162
Michel, R., Dugué, L., and Busch, N. A. (2021). Distinct contributions of alpha and theta rhythms to perceptual and attentional sampling. Eur. J. Neurosci. doi: 10.1111/ejn.15154
Miller, G. A. (1956). The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol. Rev. 63, 81–97. doi: 10.1037/h0043158
Mongillo, G., Barak, O., and Tsodyks, M. (2008). Synaptic theory of working memory. Science 319, 1543–1546.
Musslick, S., and Cohen, J. D. (2021). Rationalizing constraints on the capacity for cognitive control. Trends Cogn. Sci. 25, 757–775. doi: 10.1016/j.tics.2021.06.001
Musslick, S., Saxe, A., Novick, A., Reichmann, D., and Cohen, J. D. (2020). On the rational boundedness of cognitive control: shared versus separated representations. Psyarxiv [Preprint] doi: 10.31234/osf.io/jkhdf
O’Keefe, J., and Recce, M. L. (1993). Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. doi: 10.1002/hipo.450030307
Oberauer, K. (2003). Selective attention to elements in working memory. Exp. Psychol. 50, 257–269. doi: 10.1026/1618-3169.50.4.257
Oberauer, K., and Lin, H.-Y. (2017). An interference model of visual working memory. Psychol. Rev. 124:21.
Palva, J. M., Palva, S., and Kaila, K. (2005). Phase synchrony among neuronal oscillations in the human cortex. J. Neurosci. 25, 3962–3972. doi: 10.1523/JNEUROSCI.4250-04.2005
Peters, B., Kaiser, J., Rahm, B., and Bledowski, C. (2020). Object-based attention prioritizes working memory contents at a theta rhythm. J. Exp. Psychol. 150, 1250–1256. doi: 10.1037/xge0000994
Pina, J. E., Bodner, M., and Ermentrout, B. (2018). Oscillations in working memory and neural binding: a mechanism for multiple memories and their interactions. PLoS Comput. Biol. 14:e1006517. doi: 10.1371/journal.pcbi.1006517
Pomper, U., and Ansorge, U. (2021). Theta-rhythmic oscillation of working memory performance. Psychol. Sci. 32, 1801–1810. doi: 10.1177/09567976211013045
Reddy, L., Poncet, M., Self, M. W., Peters, J. C., Douw, L., Van Dellen, E., et al. (2015). Learning of anticipatory responses in single neurons of the human medial temporal lobe. Nat. Commun. 6:8556.
Reddy, L., Self, M. W., Zoefel, B., Poncet, M., Possel, J. K., Peters, J. C., et al. (2021). Theta-phase dependent neuronal coding during sequence learning in human single neurons. Nat. Commun. 12:4839.
Riddle, J., Vogelsang, D. A., Hwang, K., Cellier, D., and D’Esposito, M. (2020). Distinct oscillatory dynamics underlie different components of hierarchical cognitive control. J. Neurosci. 40, 4945–4953. doi: 10.1523/JNEUROSCI.0617-20.2020
Robinson, P. A., Rennie, C. J., Rowe, D. L., and O’connor, S. C. (2004). Estimation of multiscale neurophysiologic parameters by electroencephalographic means. Hum. Brain Mapp. 23, 53–72.
Rumelhart, D. E., Hinton, G. E., and Williams, R. J. (1986). Learning representations by back-propagating errors. Nature 323, 533–536. doi: 10.1038/323533a0
Sara, S. J. (2015). Locus Coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 35, 87–94. doi: 10.1016/j.conb.2015.07.004
Senoussi, M., Moreland, J. C., Busch, N. A., and Dugué, L. (2019). Attention explores space periodically at the theta frequency. J. Vis. 19, 22–22. doi: 10.1167/19.5.22
Senoussi, M., VanRullen, R., and Reddy, L. (2020a). Anticipatory reinstatement of expected perceptual events during visual sequence learning. BioRxiv [Preprint] doi: 10.1101/2020.11.28.402123
Senoussi, M., Verbeke, P., Desender, K., Loof, E. D., Talsma, D., and Verguts, T. (2020b). Theta oscillations shift towards optimal frequency for cognitive control. BioRxiv [Preprint] doi: 10.1101/2020.08.30.273706
Shine, J. M., Müller, E. J., Munn, B., Cabral, J., Moran, R. J., and Breakspear, M. (2021). Computational models link cellular mechanisms of neuromodulation to large-scale neural dynamics. Nat. Neurosci. 24, 765–776.
Sillar, K. T., McLean, D. L., Fischer, H., and Merrywest, S. D. (2002). Fast inhibitory synapses: targets for neuromodulation and development of vertebrate motor behaviour. Brain Res. Rev. 40, 130–140.
Silvetti, M., Vassena, E., Abrahamse, E., and Verguts, T. (2018). Dorsal anterior cingulate-brainstem ensemble as a reinforcement meta-learner. PLoS Comput. Biol. 14:e1006370. doi: 10.1371/journal.pcbi.1006370
Steenbrugge, X., Leroux, S., Verbelen, T., and Dhoedt, B. (2018). Improving generalization for abstract reasoning tasks using disentangled feature representations. arXiv [Preprint] arXiv:1811.04784,
Stokes, M. G. (2015). ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn. Sci. 19, 394–405. doi: 10.1016/j.tics.2015.05.004
Treisman, A. M., and Gelade, G. (1980). A feature-integration theory of attention. Cogn. Psychol. 12, 97–136. doi: 10.1016/0010-0285(80)90005-5
van Dijck, J.-P., and Fias, W. (2011). A working memory account for spatial–numerical associations. Cognition 119, 114–119. doi: 10.1016/j.cognition.2010.12.013
Varela, F., Lachaux, J.-P., Rodriguez, E., and Martinerie, J. (2001). The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
Verbeke, P., and Verguts, T. (2019). Learning to synchronize: how biological agents can couple neural task modules for dealing with the stability-plasticity dilemma. PLoS Comput. Biol. 15:e1006604. doi: 10.1371/journal.pcbi.1006604
Verguts, T. (2017). Binding by random bursts: a computational model of cognitive control. J. Cogn. Neurosci. 29, 1103–1118. doi: 10.1162/jocn_a_01117
Voloh, B., and Womelsdorf, T. (2016). A role of phase-resetting in coordinating large scale neural networks during attention and goal-directed behavior. Front. Syst. Neurosci. 10:18. doi: 10.3389/fnsys.2016.00018
Voloh, B., Valiante, T. A., Everling, S., and Womelsdorf, T. (2015). Theta–gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc. Natl. Acad. Sci. U.S.A. 112, 8457–8462. doi: 10.1073/pnas.1500438112
von der Malsburg, C., and Singer, W. (1988). Principles of cortical network organization. Neurobiol. Neocortex 69:99.
Wilson, H. R., and Cowan, J. D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophys. J. 12, 1–24. doi: 10.1016/S0006-3495(72)86068-5
Wolinski, N., Cooper, N. R., Sauseng, P., and Romei, V. (2018). The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biol. 16:e2005348. doi: 10.1371/journal.pbio.2005348
Womelsdorf, T., Johnston, K., Vinck, M., and Everling, S. (2010). Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. U.S.A. 107, 5248–5253. doi: 10.1073/pnas.0906194107
Zhang, W., and Luck, S. J. (2008). Discrete fixed-resolution representations in visual working memory. Nature 453, 233–235. doi: 10.1038/nature06860
Appendix
The activity of each neuron of a neural triplet (u, v, and n) are defined by this system of differential equations:
Where uj, vj, and nj are the activity of the fast-excitatory, inhibitory and slow-excitatory components of node j, respectively. sj(t)is the input signal (i.e., stimulation). Intratriplet coupling strengths are denoted by a parameters. Temporal constants are denoted by τ, note that there is no temporal constant the fast-excitatory component (i.e., τe=1). The parameters’ index e refers to the fast excitatory component, the index i to the inhibitory component, and the index n to the slow-excitatory component. The parameters’ values are given in the table below:

The function f(x) represents the firing rate (approximating a noisy-integrate-and-fire spiking neuron): .
The interaction between nodes (i.e., neural triplets) is defined by this equation:
Where αj, denotes one of the three components of a triplet (i.e., uj, vj, or nj). Intertriplet coupling strengths are denoted by c parameters. The parameters’ values are given in the table below:

Keywords: resources, binding, working memory, oscillations, modeling, simulations, cognitive flexibility
Citation: Senoussi M, Verbeke P and Verguts T (2022) Time-Based Binding as a Solution to and a Limitation for Flexible Cognition. Front. Psychol. 12:798061. doi: 10.3389/fpsyg.2021.798061
Received: 19 October 2021; Accepted: 15 December 2021;
Published: 24 January 2022.
Edited by:
Gesine Dreisbach, University of Regensburg, GermanyReviewed by:
Ulrich Ansorge, University of Vienna, AustriaSebastian Musslick, Brown University, United States
Copyright © 2022 Senoussi, Verbeke and Verguts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Senoussi, mehdi.senoussi@ugent.be
†These authors have contributed equally to this work and share first authorship
 Mehdi Senoussi
Mehdi Senoussi Pieter Verbeke
Pieter Verbeke Tom Verguts
Tom Verguts