- Natural Sciences and Science Education, National Institute of Education, Nanyang Technological University, Singapore, Singapore
It is well established that oxidative stress is an important cause of cellular damage. During stress conditions, plants have evolved regulatory mechanisms to adapt to various environmental stresses. One of the consequences of stress is an increase in the cellular concentration of reactive oxygen species, which is subsequently converted to H2O2. H2O2 is continuously produced as the byproduct of oxidative plant aerobic metabolism. Organelles with a high oxidizing metabolic activity or with an intense rate of electron flow, such as chloroplasts, mitochondria, or peroxisomes are major sources of H2O2 production. H2O2 acts as a versatile molecule because of its dual role in cells. Under normal conditions, H2O2 immerges as an important factor during many biological processes. It has been established that it acts as a secondary messenger in signal transduction networks. In this review, we discuss potential roles of H2O2 and other signaling molecules during various stress responses.
Introduction
In plants, reactive oxygen species (ROS) are continuously produced in different cellular compartments as byproducts of various metabolic pathways such as respiration and photosynthesis. All aerobic organisms use molecular oxygen as terminal oxidant during respiration. Oxygen is neither very reactive nor harmful, but it has the potential to be only partially reduced, leading to the formation of very reactive and therefore toxic intermediates like singlet oxygen (1O2), superoxide radical (), hydrogen peroxide (H2O2) and hydroxyl radical (∙OH). All ROS are extremely reactive, causing damage to membranes and other cellular components. ROS also have strong affinities toward membrane, DNA, proteins, carbohydrates and lipids in plant cells (Anjum et al., 2015; Jajic et al., 2015). Hence, these molecules are constantly scavenged by different non-enzymatic and enzymatic detoxification mechanisms that are often confined to particular compartments (Alscher et al., 1997). It is important to remove ROS from the plant system in order to abate stress response, taking also into account that the final consequence of an eventual disequilibrium due to adverse environmental factors is the rapid increase of intracellular ROS levels, the so-called “oxidative burst" (Sharma et al., 2012). However, the balance between frequent production and scavenging of ROS may be disturbed by a number of adverse environmental factors such as light, temperature, drought, salinity, cold, heavy metal ions, UV exposure and water (Boyer, 1982). The usual external stress factors that affect ROS production in plants can be biotic (executed by other organisms) or abiotic (arising from changes in chemical of physical environment). However, in plants the constant regulation of the ROS concentration, including H2O2, is controlled by the enzymes such as catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutathione S-transferases (GSTs), glutathione reductase (GR), and peroxyredoxin; and non-enzymatic compounds, like ascorbate, glutathione (GSH), α-tocopherol and flavonoids (Kapoor et al., 2015).
Recent studies have elucidated that under different stress conditions, plants react in a very complex manner which includes various physiological and cellular changes (Atkinson and Urwin, 2012). In order to combat stress response, plants use various signaling mechanisms derived from hormonal regulations. Nevertheless, recent studies indicate that plants also make use of ROS as signaling molecules for regulating development and various physiological responses. There is also increasing focus on ROS production and its integration with various hormonal signaling pathway in regulation of plant growth and stress tolerance (Xia et al., 2015).
Amongst all, superoxide and H2O2 are two ROS that have been given more importance in recent studies. The main focus of this review is on H2O2. H2O2 is freely diffusible across membranes, which enables it to diffuse the damage. It is relatively long lived and it acts as a central player in stress signal transduction pathways (Møller et al., 2007). Thenard was the first in 1818 to isolate H2O2 which later came across as a cell damaging molecule when accumulated at higher concentrations in the cell (Plaine, 1955). In early 90 s Ievinsh and Tiberg also predicted the role of H2O2 as a signaling molecule (Ievinsh and Tiberg, 1995). Based on earlier studies, it is certain that H2O2 is part of oxidative metabolism and is involved in various metabolism and signaling cascades which are essential for plant growth and development, such as development of root hair, reinforcement of plant cell wall, xylem differentiation, resistance enhancement, cell wall structural cross linking and cell wall loosening in stomatal control (Dempsey and Klessig, 1995).
H2O2 being a versatile molecule acts as an important signal at normal levels, whereas under abiotic or biotic stress conditions it induces oxidative stress. Its unique property of stability and less reactivity differentiates H2O2 from the rest of the ROS molecules (Yang and Poovaiah, 2002; Quan et al., 2008), thus making it a good signaling molecule. In plants, H2O2 works as a key factor in non-toxic level concentration. As a signaling molecule, it shows tolerance to biotic and abiotic stress by getting involved in various pathways (Mittler et al., 2004; Reczek and Chandel, 2015). At toxic concentration levels H2O2 showed involvement in programmed cell death (PCD; Dat et al., 2003). In a recent article, the dual nature of H2O2 has been studied where 600 μM H2O2 treatment caused an increase in the vase life of hybrid lily “Manissa,” while an increase in concentrations resulted in negative effects (Liao et al., 2012).
Several studies have indicated that H2O2 interplays with other signaling molecules such as abscisic acid (ABA) and ethylene which are important for plant development and senescence (Jubany-Mari et al., 2009; Chen et al., 2012). Table 1 shows recent studies unveiling the mechanism by which H2O2 is involved in various biological processes. A recent study indicated the involvement of nitric oxide (NO) and H2O2 in salicylic acid (SA)-induced salvianolic acid B production in Salvia miltiorrhiza cell culture. Increase in H2O2 production has been observed by SA despite being inhibited by IMD (an inhibitor of NADPH oxidase) or DMTU (a quencher of H2O2) which further increases NO production and Sal B accumulation (Guo et al., 2014). In mung bean seedlings SA also plays roles in adventitious root formation and its effect on H2O2 accumulation has been observed. It has been concluded from the study that H2O2 accumulation acts as a downstream process in regulation with SA induced adventitious root formation (Yang et al., 2013).
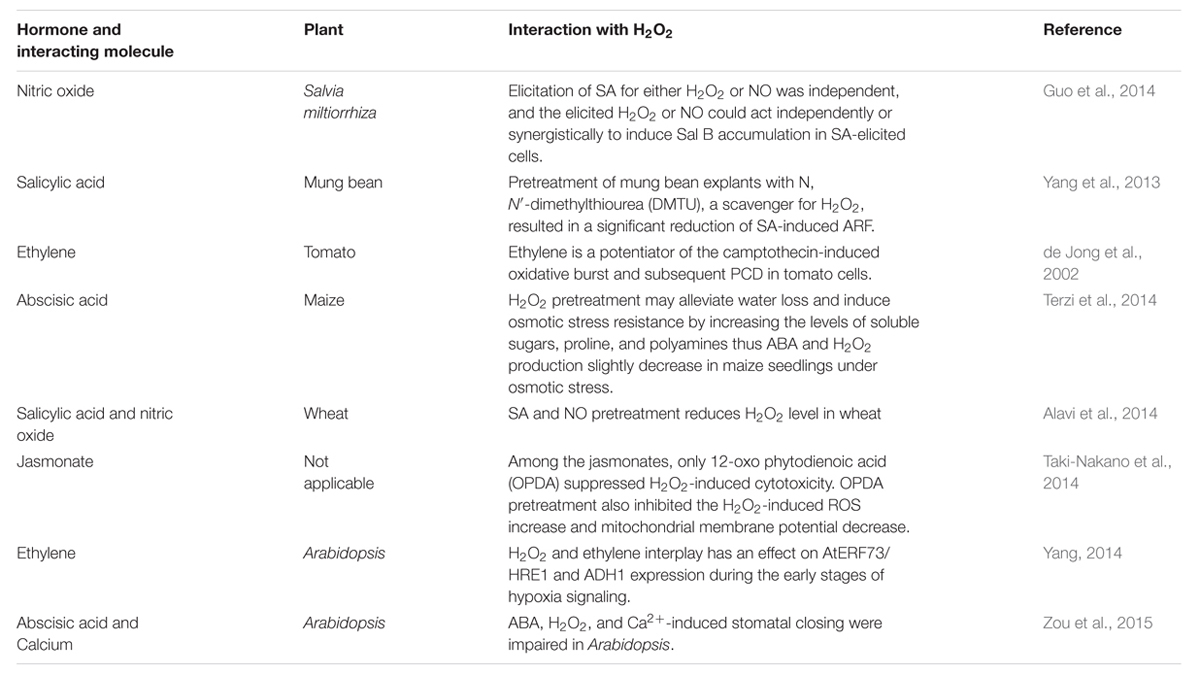
TABLE 1. Recent studies showing the interrelation between H2O2 and its interaction with signaling molecules.
H2O2 pretreatment in maize leaves significantly increased ABA content (Terzi et al., 2014). Pre-treatment of sodium nitroprusside (SNP) and SA in wheat seedlings decreased the effect of osmotic stress. It was also observed that pre-treatment of seedlings with methylene blue (a guanylatecyclase inhibitor) diminished the protective effects of both SA and SNP. Therefore, it is concluded that protective effect may only be limited to NO (Alavi et al., 2014). Another study on jasmonate revealed that 12-oxo phytodienoic acid is involved in reduced H2O2 accumulation (Taki-Nakano et al., 2014). Yang (2014), proposed a model describing three pathways in modulating the transcription factor AtERF73/HRE1 which includes ethylene dependent, ethylene-independent/H2O2-dependent pathway, and an ethylene and H2O2-independent pathway. This study also proposes involvement of H2O2 and ethylene in AtERF73/HRE1 and ADH1 gene expression under stress. There is another study stating the involvement of ethylene in H2O2 accumulation during PCD (de Jong et al., 2002). In drought stress, calcium-dependent protein kinase (CPK8) has been involved in ABA-mediated stomatal regulation via CAT3 (CPK8-interacting protein) activity. It also has been observed that cpk8 and cat3 plants showed reduced CAT activity and higher H2O2 accumulation (Zou et al., 2015).
Guo et al. (2014) studied the possibility of SA involvement in H2O2 and NO induced salvianolic acid B accumulation; where the main function of NO is to downstream SA signaling which results in reduced oxidative stress (Alavi et al., 2014). Exogenous application of H2O2 and its induction in high light showed different effects on gene expression (Olemiec et al., 2014). It was shown that H2O2 could be involved in the signaling of plant growth regulators such as ethephon (Chen et al., 2012). The application of ethephon results in an elevation in H2O2 levels, which is accompanied by the increased expression of sweet potato CAT.
H2O2 Production in Plant Cells and its Reactivity in Different Cellular Compartments
H2O2 is produced in photosynthesizing cells, majorly in peroxisomes (photosynthetic carbon oxidation cycle) and minorly in choroloplast, mitochondria (respiratory electron transport chain) (Figure 1), the endoplasmic reticulum (ER), nucleus and plasma membranes (del Río et al., 2006; Bhattacharjee, 2012). Peroxisomes and chloroplasts may accumulate 30–100 times higher H2O2 as compared to mitochondria (Hossain et al., 2015). H2O2 production occurs during the oxidative burst when reduction of molecular oxygen (O2) into superoxide anion () occurs (Sutherland, 1991). At the cell wall this undergoes spontaneous dismutation at a higher rate and at an acidic pH. Nicotinamide adenine dinucleotide (NADH) undergoes oxidation to form NAD+ by cell wall peroxidase with further reduction of O2 to resulting in the production of O2 and H2O2 (Bhattacharjee, 2005). In apoplast, amine oxidase and germin-like oxidase have been proposed to generate H2O2 (Bolwell and Wojtaszek, 1997). Cell membrane NADPH-dependent oxidase serves as a H2O2 source. H2O2 production occurs in the cell via reaction between the oxygen molecules (O2), forming superoxide anion (). During the stress response O2 is reduced to which undergoes spontaneous dismutation (Sutherland, 1991). is also catalyzed and reduced by superoxide dismutase (SOD) and protein kinase C (PKC) to form H2O2 (Scandalios, 1993). SOD enzyme catalyzes which mainly occurs in cytosol, chloroplast and mitochondria (Scandalios, 1993). Rather than superoxide disproportionation, H2O2 is also produced by reduction by reductants such as ferredoxins, thiols, ascorbate (Asada and Takahashi, 1987). PKC also shows involvement in H2O2 production. OH is produced in the reaction of H2O2 with Fe2+ (Arora et al., 2002). To maintain the balance between H2O2-scavenging enzymes and SODs, equilibrium for H2O2 concentration in cells should be attained (Mittler et al., 2004).
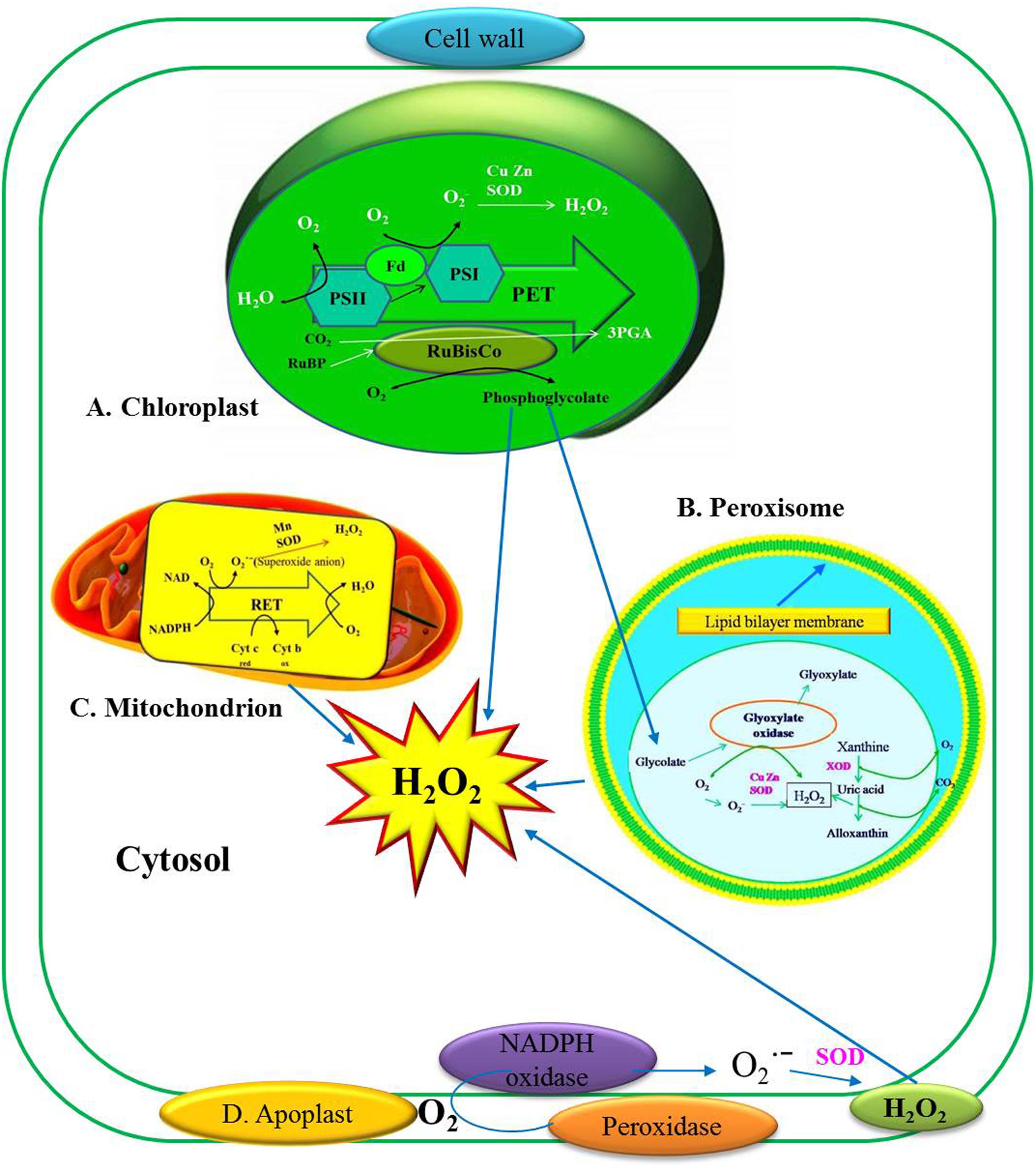
FIGURE 1. Production of hydrogen peroxide (H2O2) in plant cells and their reactivity in various cellular components. (A) H2O2 production in Chloroplast at the site of PSI and PSII. (B) H2O2 production in Peroxisomes. XOD (Xanthine oxidase), GOX (glycolate oxidase). (C) H2O2 production in Mitochondria. (D) H2O2 production in Apoplast (Figure modified from Jajic et al., 2015).
H2O2 production in chloroplasts originates from several locations and occurs mainly in Chl associated with the photosynthetic electron transport chain (PET) which is the primary source of O2. In chloroplast, molecular oxygen (O2) uptake is associated with photoreduction of O2 to superoxide radical (). Singlet oxygen (1O2) is produced by energy transfer to triplet oxygen (3O2) in photosystem II (PSII). Photosystem II excitation results in the oxidation of water (H2O) to O2 (Figure 1A). The reductant formed by this process donates electrons (e–) to plastoquinone (PQ). These e– eventually passes through the cytochrome f (Cyt f) complex, plastocyanin (PC), and then to photosystem I (PSI). The PET chain includes a number of enzymes on the reducing (acceptor) side of photo system I (PSI): Fe–S centers, reduced thioredoxin (TRX), and ferredoxin. When ferredoxin (Fd) is over reduced during photosynthesis electron transfer, electron may be transferred from photosystem I (PSI) to O2 to form by the process called Mehler reaction. The formed then undergoes dismutation to hydrogen peroxide (H2O2) either spontaneously or facilitated by SOD (Figure 1A).
In plants, the main function of peroxisome is photorespiration which involves O2 uptake (light-mediated) and the emission of CO2 in simulation with H2O2 formation (Dat et al., 2000). H2O2 production in peroxisome results from the oxygenase activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) (Noctor et al., 1999). It is being stated that plants exposed to favorable conditions for oxygenation are more subjected to produce H2O2 (Foyer and Noctor, 2000). H2O2 is generated during the oxidation of glycolate in the C2 cycle of peroxisomes (Figure 1B). Production of H2O2 is attributed to the matrix-localized enzyme, xanthine oxidase (XOD), which catalyses the oxidation of xanthine or hypoxanthine to uric acid, and is a well-known producer of in the process. This is then converted into H2O2 by Cu, Zn-SOD (Figure 1B) (Corpas et al., 2001). An NAD(P)H-dependent production site which uses as an electron acceptor is present in the peroxisomal membrane and it releases the into the cytosol. This site appears to be formed by a small ETC which is composed of a flavoprotein NADH and Cyt b (del Río et al., 2006).
Two major mitochondrial sites for superoxide radical production in electron transport chain are cytochrome bc1 complex and NAD(P)H dehydrogenases (Moller, 2001). The mitochondrial respiratory electron transport (RET) harbors electrons with sufficient free energy to directly reduce O2, which is considered as a primary source of H2O2 generation (Figure 1C). This direct reduction of O2 to takes place in the flavoprotein region of the NADH dehydrogenase segment, which is responsible for the O2 consumption. During RET, the oxygen radical is markedly enhanced in the presence of antimycin A, which blocks the electron flow after ubiquinone (UQ). Autooxidation of reduced UQ results in the production of which is a major precursor of H2O2 production in UQ location of the RET. Completely reduced UQ donates e– to cytochrome c (Cyt c) and leaves an unstable, highly reducing semiquinone species, which would normally reduce cytochrome b (Cyt b), which instead reduces the O2 to (Figure 1C). This is further reduced by manganese SOD (Mn-SOD) dismutation to H2O2 (Moller, 2001).
Some other sources of H2O2 production are plasma membrane, cytoplasm and the extracellular matrix (ECM). There are various enzymes responsible for H2O2 production in plasma membrane (Vianello and Macri, 1991). H2O2 production in cytoplasm occurs by the electron transport chain which is associated with the ER. This oxidation and hydroxylation process involve cytochrome P450 and cytochrome P450 reductase whereas, fatty acid desaturation involves cytochrome b5 and cytochrome b5 reductase, which donate electrons for H2O2 formation (Bartosz, 1997; Mittler et al., 2004).
Studies have shown that NADPH oxidase at the plasma membrane in the plant cell is involved in plant defense reactions to pathogen attack (Torres et al., 2002) and in response to various abiotic stresses (Kwak et al., 2003). The NADPH-dependent oxidase system sometimes referred to as rboh (for respiratory burst oxidase homolog), similar to that present in mammalian neutrophils, has received the most attention. It catalyzes the production of by one-electron reduction of oxygen using NADPH as the electron donor (Desikan et al., 2003; Mahalingam and Federoff, 2003; Apel and Hirt, 2004). The superoxide anion radical is most likely located in the apoplastic space and is converted to H2O2 either spontaneously or by extracellular SOD (Karpinska et al., 2001; Bolwell et al., 2002) (Figure 1D). There are other plant species which NADPH oxidase or rboh genes have been cloned (Desikan et al., 2003).
Abscisic Acid and Interaction with H2O2
Abscisic acid is one of the crucial phytohormones which play important roles under various environmental cues. It accumulates as a response to stressful environmental conditions such as dehydration, cold temperature or shortened day length. The application of ABA plays fundamental role in different physiological processes and biological pathways such as seed dormancy and delay in germination, development of seeds, promotion of stomatal closure, embryo morphogenesis, synthesis of storage proteins and lipids, leaf senescence and also defense against pathogen (Swamy and Smith, 1999).
It has been reported that the ABA signaling pathway is identified as a central regulator of abiotic stress response in plants, triggering major changes in gene expression and adaptive physiological responses (Figure 2). Recently, MAPK (mitogen activated protein kinase) cascades have also been shown to be implicated in ABA signaling. External ABA treated plants induced the transcriptional regulations, protein accumulation and stability, and kinase activity of several components of distinct MAPK signaling cascades in many plant species. These existing evidences suggest that MAPK cascades are actively involved in several ABA responses, including abiotic stress defense mechanisms and guard cell signaling (Zhang et al., 2007; Jammes et al., 2009; Zong et al., 2009). Rodriguez et al. (2010) reported that the MAPK cascade is activated by the exogenous H2O2 which in turn is mediated by the hormones like ABA, Jasmonic acid (JA) and SA.
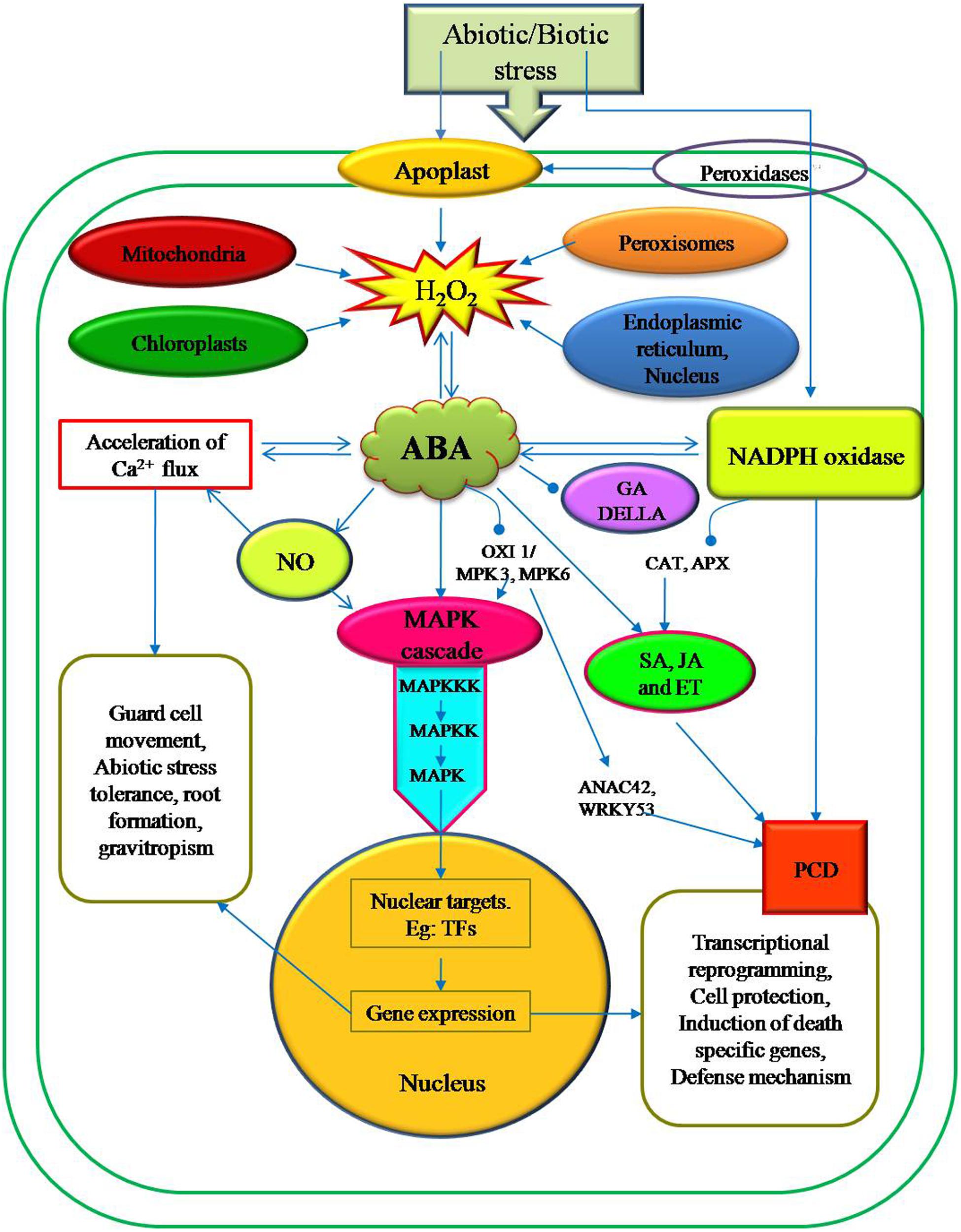
FIGURE 2. Schematic representation of H2O2 generation in various intra-and extra cellular sites and its involvement in the various signaling pathways associated with the regulation of defense gene expression in plant cells. ABA is extensively associated with wide range of abiotic stress signals and administers with growth and development processes in plants. In contrast to ABA, other phytohormones such as SA, GA, JA, and ethylene have significant role during biotic stress tolerance in plants. Nevertheless, ABA is an important signaling molecule in H2O2 production and activating the MAPK cascade by interacting with other plant hormones.
In plants, MAPK cascades are generally organized pathways in which the upstream activated MAPK kinase kinase (MAPKKK) undergoes the sequential phosphorylation and subsequent activation of downstream MAPK kinases (MAPKKs) and MAPKs. This MAPK cascades translate incoming environmental signals into post-translational modifications of target proteins that ultimately reorganize gene expression and stress adaptation. In Arabidopsis, H2O2 activates the MAPKs (MPK3 and MPK6) via the MAPK kinase kinase (MAPKKK) ANP1 (Kovtun et al., 2000). MKP2 is a key regulator of the MPK3 and MPK6 networks that are involved in controlling both abiotic and biotic stress responses (Zhou et al. (2012). Treatment with ABA in Arabidopsis plants induced the transcriptional regulation of MPK3, MPK5, MPK7, MPK18, MPK20, MKK9, MAPKKK1 (ANP1), MAPKKK10 (MEKK3), MAPKKK14, MAPKKK15, MAPKKK16, MAPKKK17, MAPKKK18, MAPKKK19, Raf6, Raf12, and Raf35 (Menges et al., 2008; Wang et al., 2011) signifying a promising role in ABA signaling. Recently, Jammes et al. (2009) used a cell type-specific functional genomics approach and identified that the MAPKs, MPK9 and MPK12 were preferentially expressed in guard cells. It was also reported that MPK12 were activated by ABA and H2O2. MPK9 and MPK12 mediate ABA signals in guard cells (Montillet et al., 2013). In Arabidopsis, a T-DNA oxi1 null mutant is impaired in the activation of the two MAPKs, MPK3 and MPK6, upon oxidative stress (Rentel et al., 2004), suggesting that serine/threonine kinase OXI1 functions downstream of ROS but upstream of the MAPK module. H2O2 also increases the expression of the Arabidopsis nucleoside diphosphate (NDP) kinase 2 (Moon et al., 2003). H2O2 accumulation was reduced by the over expression of At-NDPK2 and enhanced tolerance to multiple stresses, including cold, salt, and oxidative stress. Moreover, the MAPKs, MPK3 and MPK6 are activated by H2O2 induced NDPK2. Gechev et al. (2005) identified a transcription factor ANAC42 and reported that it was highly regulated by H2O2 which in turn is achieved through OXI1/MPK3 and MPK6 activation (Figure 2). Interestingly, ANAC42 plays a role in senescence and H2O2 induced PCD (Fujii, 2014). Also, the MAPKKK can interact directly with WRKY53, a transcription factor that involved in senescence induced PCD (Figure 2), thus bypassing the downstream kinases (Miao et al., 2006). Nevertheless, it is clear from the past reports that MPK3 and MPK6 are two important integrating points of signals from cellular developmental programs and the external environment changes (Wang et al., 2008). Even though most of the connections between ABA and MAPKs are poorly understood, it is evident that these pathways are part of the complex cellular signaling network of plants to integrate various environmental signals (Danquah et al., 2014).
One of the most important functions of ABA is to induce stomatal closure by reducing the turgor of guard cells under water deficit. There is evidence that H2O2 may function as an intermediate in ABA signaling in Vicia faba guard cells (Pei et al., 2000). There are reports which suggest that H2O2 is one of the major ROS and plays an important role as a second messenger in ABA induced stomatal closure (Pei et al., 2000; Miao et al., 2006). While ABA induced the synthesis of H2O2 and superoxide under stressful condition and caused oxidative stress (Guan et al., 2000). However, ABA is a natural growth regulator which accumulates in plants under plant stress conditions (Zheng et al., 2010). Results of (Zhang et al., 2001) provide evidence for H2O2 involvement in ABA induced stomatal movement in V. faba. Major findings of this study showed stomatal closure by exogenous application of H2O2 with ABA whereas, reverse action of H2O2 generation by scavenging its activity results in reduced stomatal closure (Guan et al., 2000). Overproduction of ABA induces H2O2 accumulation which enhances the stomatal closure by activating plasma membrane calcium channels (Pei et al., 2000). Plasma membrane-located NADPH oxidases Atrboh D and Atrboh F were found to be required for ABA-induced stomatal ROS production (Kwak et al., 2003). It has been reported that during oxidative burst, NADPH oxidase can trigger Ca2+, and mitogen-activated protein kinase (MAPK) signaling pathways as well as suppress the hormone signal transfer routes such as SA, JA, and ethylene (Overmyer et al., 2003; Evans et al., 2005) (Figure 2). Abiotic stress condition enhances ABA/gibberellic acid (GA) ratio supporting DELLA protein accumulation which in turn lowers H2O2 levels (Considine and Foyer, 2014). In rice seeds, ABA decreased ROS production, which leads to a repression of ascorbate and GA accumulation (Ye et al., 2012). It was also reported that H2O2 is involved in GA/ABA signaling in barley aleurone cells (Ishibashi et al., 2012). Calcium and calmodulin (CaM)-dependent protein kinase (CCaMK) belongs to calcium/CaM-dependent protein kinase superfamily (Harmon et al., 2000); activation by free Ca2+ and Ca2+ bound to CaM has been indicated to be involved in ABA signaling during abiotic stress responses (Yang et al., 2011) and ABA-induced antioxidant defense by functioning upstream of ABA-activated MAPK (Shi et al., 2014).
These available pieces of evidence clearly indicate that the ABA is a key hormone in inducing abiotic stress responses. Downstream events mediated by MAPK cascade, alterations in Ca2+ fluxes and the activation of ion channels changes the redox state of the cell. All these actions lead to transcriptional reprogramming, which results in target gene expression such as cell death specific nucleases and proteases, and eventually PCD (Figure 2). Most of the genes (NADPH oxidases, SOD and extracellular peroxidases) expressed in the early signals are involved in the ROS generation, essential for triggering PCD. While other genes are responsible for maintaining ROS levels (CAT and APXs) (Gadjev et al., 2008) (Figure 2).
Calcium and Interaction with H2O2
Calcium (Ca2+) is important for robust cell wall formation. It is also crucial for the root system and in young, growing cells. Alteration in Ca2+ level is observed under shifting environmental conditions, including changes in light, temperature, and drought (Mahajan and Tuteja, 2005). Ca2+ is significant for cross-linking acidic pectin residues and permeability of the plasma membrane. As a secondary messenger, Ca2+ concentration is balanced by storing it in vacuoles and is released whenever necessary (Mahajan and Tuteja, 2005). It is indispensable for all important signaling pathways. Plant signaling network has the capability to alter its functioning under various environmental challenges. The plant cell primary response under stress condition is a modification in plasma membrane permeability leading to calcium and proton influx that appears to be necessary and sufficient for the induction of H2O2 (Pei et al., 2000). Plant metabolism involves various Ca2+/CaM proteins having different functions out of which some are involved in H2O2 signaling such as NAD kinase (Harding et al., 1997). CAT is also important for H2O2 regulation and its deficiency can lead to H2O2 accumulation (Gechev et al., 2004). H2O2 and Ca2+ interrelation study has been shown by Yang and Poovaiah (2002) in Arabidopsis. Another study on Arabidopsis seedlings suggests the role of H2O2 in biphasic Ca2+ elevation, with the first peak located in cotyledons and the second in the root (Rentel and Knight, 2004). The antioxidant system may also be considered as a target of Ca2+ influence, for example, the efficiency of H2O2 scavenging in Arabidopsis plants depends on the peroxisomal Ca2+ concentration (Costa et al., 2010).
Continuous Ca2+ invasion is most importantly required for H2O2 accumulation which also activates NADPH oxidase located in the plasma membrane (Lamb and Dixon, 1997). There is another study suggesting the role of biphasic (Ca2+) and H2O2 in aequorin tobacco cell culture’s expression (Lecourieux et al., 2002). Pollen tube growth has been enhanced by H2O2 regulated spermidine oxidase, which also induces Ca2+ channel (Wu et al., 2010). H2O2 involvements in Ca2+ influx via Ca2+ permeable channel and partial stomatal closure were observed in the study (Pei et al., 2000). Significant induction in nuclear and cytosolic Ca2+ level by free sphingoid Long Chain Base (LCB) sphinganine has been observed in simulation with decreased accumulation of H2O2 in tobacco cells (Lachaud et al., 2011). Later studies have revealed that CAT can scavenge H2O2 production which is likely mediated by Ca2+ homeostasis in Arabidopsis (Suzuki et al., 2011). In this case, cytoplasmic Ca2+ was shown to bind to rboh at the N-terminal region and thus to promote the activation of rboh and produce H2O2 (Takeda et al., 2008). H2O2 mediated rapid gene expression (LeCDPK1) in tomato leaves has been observed (Chico et al., 2002) whereas, H2O2 treatment in wheat plant also leads to enhanced responsiveness in eight out of 20 studied calcium-dependent protein kinase (CDPKs) (Li et al., 2008). Induction in gene expression (GST1) by Ca2+response in association with H2O2 may be due to changes in redox balance (Rentel and Knight, 2004).
Nitric Oxide and Interaction with H2O2
Increasing evidence based on experiments has shown a vital role of NO in protecting plants against stress conditions (Wink et al., 1993). It is generated in higher plants through oxidative (arginine or hydroxylamine-dependent) and reductive (nitrate-dependent) pathways (Gupta et al., 2011). NO being part of various physiological processes in plant system makes it one of the major signaling molecules. Initially NO was considered to be a toxic gas. However, this idea changed after the discovery of the signaling role of NO in regulating the cardiovascular system (Skovgaarda et al., 2005). One of the major areas in the study of NO is its involvement in coordinating several defense responses during both biotic and abiotic stress conditions in the plants. In the past 2 decades, much focus was given to NO related studies toward its role in plant defense system under oxidative stress. Studies on adaptive mechanisms of plants have shown an increased basal level of NO in water and heat stressed plants, suggesting its importance in abating stress (Leshem and Haramaty, 1996; Leshem et al., 1998). The defensive mechanism of NO in plants under oxidative stress is associated with its ability to function as an antioxidant by directly scavenging the ROS, thus reducing cellular damage (Romero-puertas and Sandalio, 2016) and acting as a signaling molecule which eventually results in changes in gene expression (Lamattina et al., 2003).
A study focused on H2O2 generation in simultaneous correlation with NO production was shown during the hypersensitive response (HR) in which both cooperates to trigger hypersensitive cell death (Delledonne et al., 2001). The function of NO is tightly linked to ROS and it has a half-life of only a few seconds, once produced, interacts rapidly with ROS, giving rise to a number of reactive nitrogen species, such as nitrogen dioxide (NO2), which degrades to nitrite and nitrate in aqueous solutions (Neill et al., 2008; Bellin et al., 2013). There were studies showing involvement of both NO and H2O2 in bacterially induced PCD in soybean where both signals work synergistically (Delledonne et al., 1998) and in Arabidopsis they work as additive (Clarke et al., 2000). H2O2 formation may occur via superoxide radical (). There is a probability that NO reacts with to form highly reactive peroxynitrite anion ONOO- and subsequent cellular effects may then be induced by peroxynitrite (Bellin et al., 2013).
In mammals, NO has been shown to react with glutathione to form S-nitrosoglutathione (GSNO) which serves as a systemic source of NO and a similar situation has been suggested for plants (Durner and Klessig, 1999). It is clear that both H2O2 and NO can mediate transcription, but the involved processes remain unclear. There is a possibility of both H2O2 and NO having a direct effect on transcription factors by S-nitrosylation and oxidation of cysteine. A recent study suggests characterization of redox-sensitive factor in yeast where H2O2 oxidation alters the activity of this protein (Delaunay et al., 2000). Phosphorylation of cascade such as the mitogen-activated protein kinases (MAPK) is suggested to play another important role on H2O2 and NO. There is another study on tobacco Bright Yellow-2 (TBY-2) cells, suggesting an involvement of both H2O2 and NO in the activation of PCD, and treatment of scavenger for both the signaling molecules restores the cell viability (De Pinto et al., 2006).
In a new study, the cloning of rice NOE1, a gene whose knockout enhances NO production, revealed that this is indeed the rice CAT OsCATC (Lin et al., 2011). Increase in leaf H2O2 content leads to a characterization of mutant NOE1 which in turn leads to nitrate reductase (NR) dependent NO production. Increased H2O2 concentrations provoked by ABA may in turn trigger NO generation by NR and nitrogen oxide synthase (NOS)-like enzymes (Neill et al., 2008). NO accumulation under abiotic stress is similar to the events seen in H2O2 production (Wang et al., 2014). In Arabidopsis, both H2O2 and NO showed similar function which influences the induction and reduction in root growth stimulated by various concentrations of nucleotides (Clark et al., 2010). He et al. (2013) reported that NO and H2O2 are also involved in the stimulation of stomatal closure in Arabidopsis in response to ultraviolet-B exposure. Exclusion of H2O2 with antioxidants or inhibition of its synthesis by inhibiting NADPH oxidase activity prevents NO generation and stomatal closure. Wang et al. indicated the idea that H2O2-induced synthesis of NO might be mediated by MPK6 in Arabidopsis (Wang et al., 2010).
Salicylic Acid and Interaction with H2O2
Salicylic acid is one of the key phytohormones involved in both abiotic (Kunihiro et al., 2011; Drzewiecka et al., 2012; Liu et al., 2012) and biotic (Vlot et al., 2008; Dempsey et al., 2011) stress adaptation. The discovery of the salicylate role in thermotolerance during potato tissue culture research was mere coincidence. Inclusion of the artificial SA analog acetyl salicylic acid (ASA) in the culture medium of microplants of potato (Solanum tuberosum L.) causes potentially useful effects such as tuberization. It has been shown to play a central role as a signaling molecule involved in both local defense reactions and in the induction of systemic resistance (Durner and Klessig, 1999; Herrera-Vásquez et al., 2015). Another important aspect is gene regulation of antifungal hydrolases by SA, such as pathogenesis-related (PR) encoding PR1 and PR proteins which target to the plant cell wall (Durner and Klessig, 1999). Reduced synthesis of SA in transgenic plants due to disruption of SA pathways results in vulnerability toward fungal (Phytophtora parasitica, Cercospora nicotianae), bacterial (Pseudomonas syringae), and viral (tobacco mosaic virus) pathogens (Delaney et al., 1994).
There are various reports suggesting involvement of SA in various biotic and abiotic stresses (Wan et al., 2012; Miura and Tada, 2014; Herrera-Vásquez et al., 2015). Various environmental factors such as temperature, salinity, drought and high light exposure are responsible for ROS generation in cell organelles (peroxisomes, chloroplast) (Apel and Hirt, 2004; Holuigue et al., 2007). SA can be directly or indirectly involved in signaling pathways and interplays with ROS and GSH in stressed plants (Mateo et al., 2006; Lee and Park, 2010; Herrera-Vásquez et al., 2015). Under drought stress, increased level of SA has been observed in oat plants (Sánchez-Martín et al., 2015), whereas another study also stated the same condition in peroxisome and chloroplast for the gene cat2 knockout (Chaouch et al., 2010) and thylakoidal ascorbate PRX gene silencing (Maruta et al., 2012; Noshi et al., 2012), respectively. H2O2 stimulated PCD, SA accumulation and sesquiterpene production in cultured cell suspensions of Aquilaria sinensis (Liu et al., 2014). H2O2 production induced SA up-regulated the mRNA transcription of heat shock protein (Hsp) genes through AtHsfA2, a key component of acquiring thermotolerance in Arabidopsis (Nie et al., 2015).
Salicylic acid and H2O2 interrelation is suggested by Leon, since the pathway involved benzoic acid (the immediate precursor of SA) leads to activation of benzoic acid 2-hydroxylase which is H2O2 dependent (León et al., 1995). Relative pathway of this case suggest that H2O2 production in cell organelles (peroxisome and chloroplast) induces SA synthesis, and leads to protective mechanism such as stomatal closure and cell death. Salicylate can increase H2O2 levels in plant tissues (Rao et al., 1997; Dat et al., 1998), on the contrary SA accumulation can be induced by elevated H2O2 levels (Chamnongpol et al., 1998). The germination of sid2 seeds under high salinity is hypersensitive to H2O2, but the physiological concentrations of SA modulate antioxidant activity to prevent oxidative damage (Lee et al., 2010). There is another study suggesting exogenous application of SA relieves Cd toxicity by reducing the H2O2 accumulation in root apoplasts of the legumes Phaseolus aureus and Vicia sativa (Zhang et al., 2011).
The HR to pathogens exhibits an early ‘oxidative burst’ of superoxide which rapidly dismutates to H2O2. This mechanism involves key interactive roles for SA and H2O2, as the HR was impaired in tobacco plants with an H2O2-inducible SA-hydroxylase transgene (Mur et al., 1997). It is considered that mammalian plasma membrane NADPH oxidase is a homolog of oxidative stress enzyme (Keller et al., 1998) and it may be that this enzyme is potentiated by SA (Kauss and Jeblick, 1996). Increased accumulation of SA and enhancement in H2O2 concentration in simultaneous pathogenesis gene induction were observed in GR1 dependent glutathione (Mhamdi et al., 2010).
Ascorbic Acid and Interaction with H2O2
Ascorbic acid (AsA) is a critical water soluble phytohormone found in plant and animals (Levine, 1986; Sies and Stahl, 1995). It acts as a signal for plant growth and development, and regulates cell division, growth and signal transduction (Kerk and Feldman, 1995; Smirnoff and Wheeler, 2000). In the mitochondria plants synthesize AsA which is then transported to other parts of the plants (Shao et al., 2008). There can be a direct or indirect reaction of H2O2 with AsA, which is catalyzed by APX. APX is responsible for scavenging H2O2 hyperaccumulation found in higher plants (cytosol, chloroplast and mitochondria) (Mittler and Zilinskas, 1991).
H2O2 detoxification can be done by various antioxidants in peroxisomes such as CAT in the matrix, APX and monodehydroascorbate reductase (MDAR) in association with AsA, resulting in a decrease in the accumulation of H2O2 (Yamaguchi et al., 1995; Karyotou and Donaldson, 2005). In the chloroplast stroma, where the pH is higher during the day time, there is a consequence of AsA consumption during H2O2 reduction. A rate limiting amount of dehydroascorbate reductase (DHAR) efficiently catalyzes the recycling of AsA. The signaling function of H2O2 in guard cells is controlled by the rate of its production and the rate of its removal, in which AsA and DHAR play a critical role. The slower responsiveness of guard cells of DHAR over expressing tobacco allows more ozone to diffuse into the leaf interior (Chen and Gallie, 2004). However, the increase of AsA content in all cells and consequent increase in their ability to detoxify entered ozone, reduce the oxidative load of the leaf (i.e., lower levels of foliar and apoplectic H2O2). From past reports, it is clear that the oxidative stress induced ROS level increases monodehydroascorbate (MDA) accumulation which is being converted into L-ascorbate (AsA) and dehydroascorbate (DHA) (Figure 3A). The accumulated H2O2 is reduced to H2O by oxidation of AsA to MDA radical, which is catalyzed by APX. The MDHA is subsequently reduced back to AsA by either ferredoxin reduction or NAD(P)H catalyzed monodehydroascorbate reductase (MDHAR) (Sano et al., 2005). In GPX cycle, similar to APX, GPX uses GSH as a reducing agent to detoxify H2O2 to H2O. In addition to GPX, the organellar redox state is regulated by different enzymatic antioxidants like GR, MDHAR in addition to GPX. Disproportionation in L-ascorbate and MDHA is maintained by GSH (Venkatesh and Park, 2014) (Figure 3A).
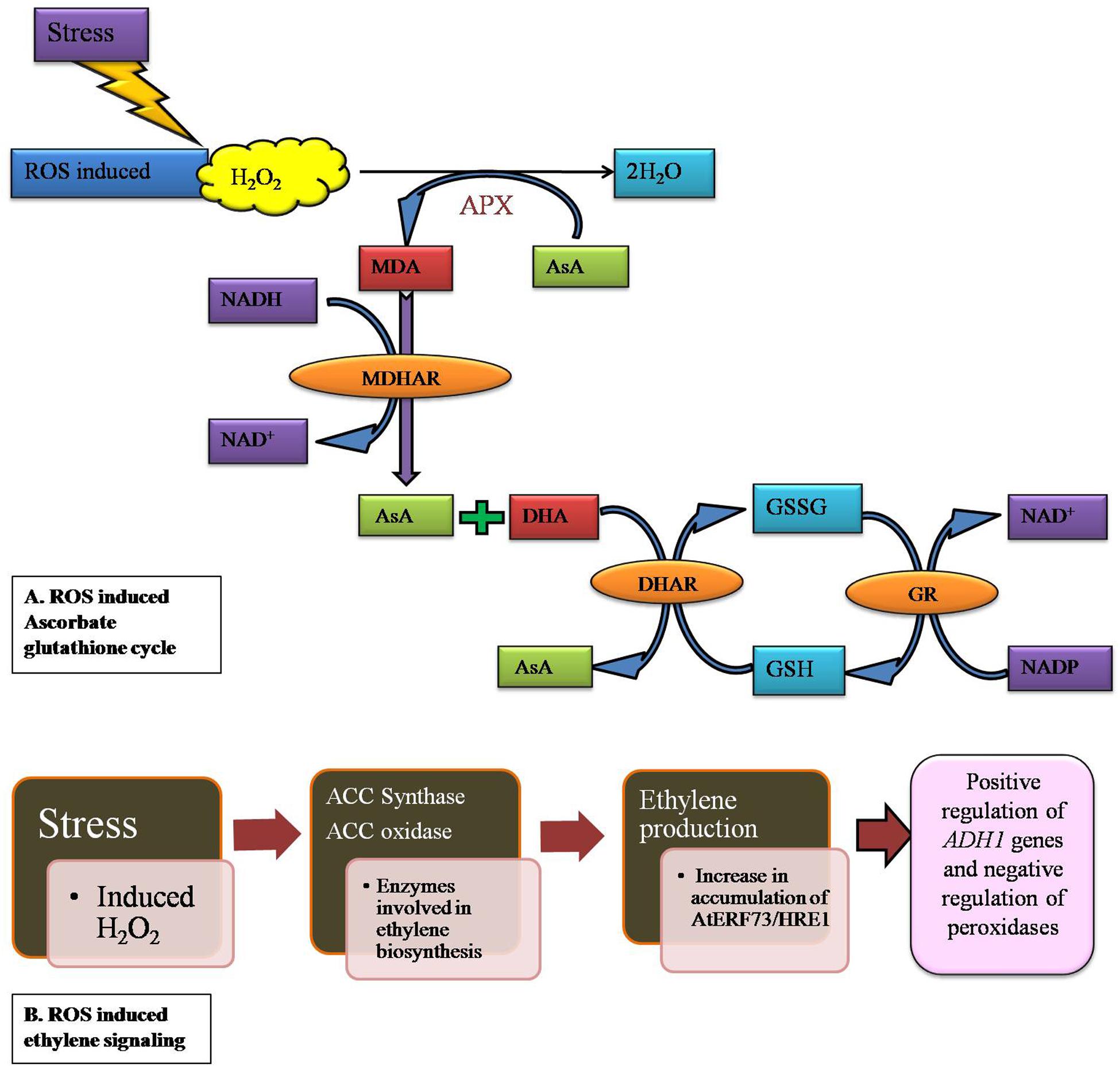
FIGURE 3. H2O2 induced antioxidant mechanism in plants which involved in detoxification process. (A) Plants synthesize ascorbic acid in the mitochondria and transported to other parts of the plants. Ascorbate peroxidase (APX) uses AsA as a substrate to reduce H2O2 to H2O in the ascorbate-glutathione cycle and generate mono dehydroascorbate (MHA), which further dissociate to AsA and dehydroascorbate (DHA). (B) Ethylene responsiveness in gene induction upon H2O2 accumulation in plant cells. Enzymes which involved in ethylene production activate an ethylene responsive group (AtERF73/HRE1). These genes are involved in negative regulation of peroxidases which in turn reduces H2O2 accumulation.
Jasmonate and Interaction with H2O2
Phytohormones act as a major factor responsible for plant growth and development. Oxylipin is considered to be one of the most important signaling molecules, i.e., plant hormone JA. Due to the unique physiological properties and abundance, jasmonate and its derivate (methyl jasmonate) came into the limelight as bioactive in nature. Chloroplast membrane is considered to be the initial site for JA synthesis where membrane phospholipids acts as a source for alfa-linolenic acid (C18:3) and hexadecatrienoic acid (C16:3) production (Ishiguro et al., 2001). Major pathway for JA synthesis in plants is supposed to be an octadecanoid pathway with the involvement of alfa-linolenic acid as a substrate (Mueller et al., 1993).
The defensive mechanism of JA has been observed in tomato against tobacco hornworm larvae (Howe et al., 1996) whereas in Arabidopsis against the fly Bradysia (McConn et al., 1997). There are studies confirming the role of JA as protective agent against pathogen (Pythium mastophorum) attack in Arabidopsis (Vijayan et al., 1998). Hu et al. (2003), came across with the result that both H2O2 and JA are primary signaling molecules during the cellular response involved in saponin biosynthesis mediated by oligogalacturonic acid (OGA) which also leads to the H2O2 mediated upregulation of JA. JA derivate (methyl jasmonate) has also been studied for its involvement in the induction of H2O2 accumulation in parsley suspension-cultured cells (Kauss et al., 1994), whereas another study suggested its role in inducing defensive genes of tomato (Orozco-Cardenas et al., 2001).
Jasmonic acid induces glutathione, an important antioxidant for redox balance. Increased expression of nuclear factor erythroid 2-related factor 2 (NrF2) has also been observed, which reduces the ROS level induced by H2O2 (Taki-Nakano et al., 2014). In association with this study, increased expression of glutamyl cysteine ligase with an increase in NrF2 helps in regulating enzymes reducing oxidative stress (Bea et al., 2003).
Ethylene and Interaction with H2O2
Ethylene has long been regarded as a stress hormone (Morgan and Drew, 1997). It is not only involved in plant growth and development, but also involved in plant responses to biotic stress, such as pathogen attack; and abiotic stress, such as wounding, ozone, and salinity (Abeles et al., 1992; Wang et al., 2009). Ethylene regulates many different processes in plants and has shown response in defense mechanism as well (Ecker, 1995). In order to evaluate the defensive role of ethylene against various environmental conditions signal transduction pathways for ethylene has been studied with mutants.
The roles of ethylene have been established in damage control caused by virulent bacteria or fungal pathogens when it is being inoculated (Bent et al., 1992; Lund et al., 1998) but its importance against avirulent bacteria infected plants has yet to be proven (Bent et al., 1992). The most important signaling molecules in the ethylene pathway are ETR1 and EIN2 (Buer et al., 2006). Change in gene expression of the ethylene receptor (ETR1) results in reduced ethylene response (O’Malley et al., 2005).
Environmental stress affects many signaling pathways in plants which also includes an alternative pathway (AP). Despite slight evidence about H2O2 and ethylene playing roles in inducing AP, there is no clear picture of how these signaling molecules are inducing the AP under various environmental conditions. Results of Wang et al. (2010) showed the possibility of involvement of H2O2 and ethylene mediated induction of AP under salt stress as it shows activity in wild-type callus whereas no activity was observed with ETR1-3 callus. In recent years, an increasing number of positive results on ethylene toward mutants in Arabidopsis have confirmed its role in signaling pathways (Guo and Ecker, 2004). In another study H2O2 accumulation in simultaneous production of ethylene has been observed in tobacco plant stressed with ozone (Schraudner et al., 1998). In plants, oxygen-deficient conditions shifts energy metabolism from aerobic to anaerobic, which in turn adversely affects nutrient and water uptake. Eventually, hypoxiasignalling triggers the production of both hydrogen peroxide (H2O2) and ethylene. H2O2 and ethylene interplay has an effect on AtERF73/HRE1 and ADH1 expression during the early stages of hypoxia signaling in Arabidopsis. Hypoxia signaling induces the ethylene biosynthesis enzymes such as ACC synthase (ACS) and ACC oxidase (ACO) (Peng et al., 2005) (Figure 3B). Arabidopsis AtERF73/HRE1 is very similar to the rice Sub1A and SNORKEL genes, which belongs to the group VII ERF (ethylene responsive factor) subfamily. They play major roles in the submergence tolerance of lowland and deepwater rice (Hattori et al., 2009). According to Yang (2014), AtERF73/HRE1 positively regulates ADH1 genes as well as negatively regulates peroxidase and cytochrome P450 genes in hypoxia signaling (Figure 3B).
Conclusion
Increasing urge to identify the role of hydrogen peroxide as a signaling molecule has gathered the interest of researchers to focus their work on the mechanisms regulating the generation of hydrogen peroxide, and this is certainly an important growing area of research. Significant scientific effort in the last 10 years has determined the position of H2O2 in signal transduction networks in plants, demonstrating that it is essential for both the communication between external biotic and abiotic stimuli, and the control of developmentally regulated processes. There are many signaling pathways for H2O2 mediated stress and defense responses that have been studied, but it remains a large scope of additional research unexplored, which can further clarify the mechanism involved in these pathways. The focus should be imposed on a clear description of roles of endogenous compounds which modify the plant responses. It has been reported that the phytohormones like ABA, SA, JA, GA, and ethylene regulates the protective responses in plants under abiotic stress by involving in different H2O2 induced signaling. Despite of its regular activities in plant growth and development, ABA plays crucial role in H2O2 mediated stress cues. Zhang et al. (2007) indicated that ABA-induced H2O2 production mediates NO generation, which in turn, activates MAPK cascade and results in the over expression and up regulation in of antioxidant enzyme activities in ABA signaling. However, there are some contradictory roles of NO. According to Orozco-Cárdenas and Ryan (2002), NO has been shown to negatively modulate wound signaling in tomato plant blocking H2O2 production and proteinase inhibitor synthesis by JA, contradicting with previous study in which NO has been considered to show positive response in abiotic stress. Nevertheless, there are many studies suggesting H2O2 response in association with NO generation under biotic/abiotic stress (Delledonne et al., 2001; Romero-puertas and Sandalio, 2016).
Due to different results suggesting various roles of H2O2, it is important to focus future studies in getting a clear picture of signaling pathways during stress response in various conditions. Interactions between different signaling molecules and their biological functioning with the involvement in various pathways still needs to be cataloged. Another important aspect that should be focused on is the role and localization of enzymes, which are involved in signaling pathways. Some important factors for future research should be the identification of the site for H2O2 production in the cell and the major factors influencing its interaction with other signaling molecules.
Author Contributions
ZC initiated the project. SS produced the figures. IS, SS, and ZC wrote the manuscript. SS and ZC revised the manuscript.
Funding
MOE NTU Tier 1 Research Grant (RP 1/14 CZ) and NIE AcRF Research Grant (RI 3/13 CZ).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abeles, F. B., Morgan, P. W., and Saltveit, M. E. Jr. (1992). Ethylene in Plant Biology, 2Edn. San Diego, CA: Academic Press.
Alavi, S. M. N., Arvin, M. J., and Kalantari, K. M. (2014). Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings. J. Plant Interact. 9, 683–688. doi: 10.1080/17429145.2014.90012
Alscher, R. G., Donahue, J. H., and Cramer, C. L. (1997). Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 100, 224–233. doi: 10.1034/j.1399-3054.1997.1000203.x
Anjum, N. A., Sofo, A., Scopa, A., Roychoudhury, A., Gill, S. S., Iqbal, M., et al. (2015). Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 22, 4099–4121. doi: 10.1007/s11356-014-3917-1
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arora, A., Sairam, R. K., and Srivastava, G. C. (2002). Oxidative stress and antioxidative system in plants. Curr. Sci. 82, 1227–1234.
Asada, K., and Takahashi, M. (1987). “Photoinhibition,” in Production and Scavenging Active Oxygen in Photosynthesis, eds D. J. Kyle, C. B. Osmond, and C. J. Amtzen (Amsterdam: Elsevier), 227–287.
Atkinson, N. J., and Urwin, P. E. (2012). The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3543. doi: 10.1093/jxb/ers100
Bartosz, G. (1997). Oxidative stress in plants. Acta. Physiol. Plant. 19, 47–64. doi: 10.1007/s11738-997-0022-9
Bea, F., Hudson, F. N., Chait, A., Kavanagh, T. J., and Rosenfeld, M. E. (2003). Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circ. Res. 92, 386–393. doi: 10.1161/01.RES.0000059561.65545.16
Bellin, D., Asai, S., Delledonne, M., and Yoshioka, H. (2013). Nitric oxide as a mediator for defense responses. Mol. Plant. Microbe Interact. 26, 271–277. doi: 10.1094/MPMI-09-12-0214-CR
Bent, A. F., Innes, R. W., Ecker, J. R., and Staskawicz, B. J. (1992). Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol. Plant-Microbe Interact. 5, 372–378. doi: 10.1094/MPMI-5-372
Bhattacharjee, S. (2005). Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plant. Curr. Sci. 89, 1113–1121. doi: 10.1007/s10661-015-4939-y
Bhattacharjee, S. (2012). The language of reactive oxygen species signaling in plants. J. Bot. 2012:22. doi: 10.1155/2012/985298
Bolwell, G. P., Bindschedler, L. V., Blee, K. A., Butt, V. S., Davies, D. R., Gardner, S. L., et al. (2002). The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 53, 1367–1376. doi: 10.1093/jexbot/53.372.1367
Bolwell, G. P., and Wojtaszek, P. (1997). Mechanisms for the generation of reactive oxygen species in plant defense broad perspective. Physiol. Mol. Plant Pathol. 51, 347–366. doi: 10.1006/pmpp.1997.0129
Boyer, J. S. (1982). Plant productivity and environment. Science 218, 443–448. doi: 10.1126/science.218.4571.443
Buer, C. S., Sukumar, P., and Muday, G. K. (2006). Ethylene induced flavonoid synthesis modulates root gravitropism. Plant Physiol. 140, 1384–1396. doi: 10.1104/pp.105.075671
Chamnongpol, S., Willekens, H., Moeder, W., Langebartels, C., Sandermann, H., Van Montagu, M., et al. (1998). Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. U.S.A. 95, 5818–5823. doi: 10.1073/pnas.95.10.5818
Chaouch, S., Queval, G., Vanderauwera, S., Mhamdi, A., Vandorpe, M., Langlois-Meurinne, M., et al. (2010). Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 153, 1692–1705. doi: 10.1104/pp.110.153957
Chen, H. J., Wu, S. D., Huang, G. J., Shen, C. Y., Afiyanti, M., Li, W. J., et al. (2012). Expression of a cloned sweet potato catalase SPCAT1 alleviates ethephon-mediated leaf senescence and H2O2 elevation. J. Plant Pysiol. 169, 86–97. doi: 10.1016/j.jplph.2011.08.002
Chen, Z., and Gallie, D. R. (2004). The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16, 1143–1162. doi: 10.1105/tpc.021584
Chico, J. M., Raíces, M., Téllez-Iñón, M. T., and Ulloa, R. M. (2002). A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol. 128, 256–270. doi: 10.1104/pp.010649
Clark, G., Wu, M., Wat, N., Onyirimba, J., Pham, T., Herz, N., et al. (2010). Both the stimulation and inhibition of root hair growth induced by extracellular nucleotides in Arabidopsis are mediated by nitric oxide and reactive oxygen species. Plant Mol. Biol. 74, 423–435. doi: 10.1007/s11103-010-9683-7
Clarke, A., Desikan, R., Hurst, R. D., Hancock, J. T., and Neill, S. J. (2000). NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 24, 667–677. doi: 10.1046/j.1365-313x.2000.00911.x
Considine, M. J., and Foyer, C. H. (2014). Redox regulation of plant development. Antioxid. Redox Signal. 21, 1305–1326. doi: 10.1089/ars.2013.5665
Corpas, F. J., Barroso, J. B., and Del Rio, L. A. (2001). Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150. doi: 10.1016/S1360-1385(01)01898-2
Costa, A., Drago, I., Behera, S., Zottini, M., Pizzo, P., Schroeder, J. I., et al. (2010). H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J. 62, 760–772. doi: 10.1111/j.1365-313X.2010.04190.x
Danquah, A., de Zelicourt, A., Colcombet, J., and Hirt, H. (2014). The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 32, 40–52. doi: 10.1016/j.biotechadv.2013.09.006
Dat, J., Vandenabeele, S., Vranova, E., van Montagu, M., Inze, D., and van Breusegem, F. (2000). Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 57, 779–795. doi: 10.1007/s000180050041
Dat, J. F., Lopez-Delago, H., Foyer, C. H., and Scott, I. M. (1998). Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 116, 1351–1357. doi: 10.1104/pp.116.4.1351
Dat, J. F., Pellinen, R., Beeckman, T., Van De Cotte, B., Langebartels, C., Kangasjärvi, J., et al. (2003). Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 33, 621–632. doi: 10.1046/j.1365-313X.2003.01655.x
de Jong, A. J., Yakimova, E. T., Kapchina, V. M., and Woltering, E. J. (2002). A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214, 537–545. doi: 10.1007/s004250100654
De Pinto, M. C., Paradiso, A., Leonetti, P., and De Gara, L. (2006). Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 48, 784–795. doi: 10.1111/j.1365-313X.2006.02919.x
del Río, L. A., Sandalio, L. M., Corpas, F. J., Palma, J. M., and Barroso, J. B. (2006). Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 141, 330–335. doi: 10.1104/pp.106.078204
Delaney, T. P., Uknes, S., Bernooij, B., Friedrich, L., Weymann, K., Negrotto, D., et al. (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250. doi: 10.1126/science.266.5188.1247
Delaunay, A., Isnard, A. D., and Toledano, M. B. (2000). H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19, 5157–5166. doi: 10.1093/emboj/19.19.5157
Delledonne, M., Xia, Y., Dixon, R. A., and Lamb, C. (1998). Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588. doi: 10.1038/29087
Delledonne, M., Zeier, J., Marocco, A., and Lamb, C. (2001). Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc. Natl. Acad. Sci. U.S.A. 98, 13454–13459. doi: 10.1073/pnas.231178298
Dempsey, D. A., and Klessig, D. F. (1995). Signals in plant disease resistance. Bull. Inst. Pasteur. 93, 167–186. doi: 10.1016/0020-2452(96)81488-6
Dempsey, D. A., Vlot, A. C., Wildermuth, M. C., and Klessig, D. F. (2011). Salicylic acid biosynthesis and metabolism. Arabidopsis Book 9, e0156. doi: 10.1199/tab.0156
Desikan, R., Hancock, J. T., and Neill, S. J. (2003). “Oxidative stress signaling,” in Plant Responses to Abiotic Stress: Topic in Current Genetics, eds H. Hirt and K. Shinozaki (Berlin: Springer-Verlag), 121–148.
Drzewiecka, K., Borowiak, K., Bandurska, H., and Golinski, P. (2012). Salicylic acid – a potential bio- marker of tobacco Bel-W3 cell death developed as a response to ground level ozone under ambient conditions. Acta Biol. Hung. 63, 231–249. doi: 10.1556/ABiol.63.2012.2.6
Durner, J., and Klessig, D. F. (1999). Nitric oxide as a signal in plants. Curr. Opin. Plant Biol. 2, 369–374. doi: 10.1016/j.niox.2014.06.008
Ecker, J. R. (1995). The ethylene signal transduction pathway in plants. Science 268, 667–675. doi: 10.1126/science.7732375
Evans, N. H., McAinsh, M. R., Hetherington, A. M., and Knight, M. R. (2005). ROS perception in Arabidopsis thaliana: the ozone-induced calcium response. Plant. J. 41, 615–626. doi: 10.1111/j.1365-313X.2004.02325.x
Foyer, C. H., and Noctor, G. (2000). Oxygen processing in photosynthesis: regulation and signaling. New Phytol. 146, 359–389. doi: 10.1046/j.1469-8137.2000.00667.x
Fujii, H. (2014). “Abscisic acid implication in plant growth and stress responses,” in Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications, eds L. S. Tran and S. Pal (New York, NY: Springer), 37–54.
Gadjev, I., Stone, J. M., and Gechev, T. (2008). Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 270, 87–144. doi: 10.1016/S1937-6448(08)01403-2
Gechev, T., Gadjev, I., and Hille, J. (2004). An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell. Mol. Life Sci. 61, 1185–1197. doi: 10.1007/s00018-004-4067-2
Gechev, T. S., Minkov, I. N., and Hille, J. (2005) Hydrogen peroxide-induced cell death in Arabidopsis: transcriptional and mutant analysis reveals a role of an oxoglutarate-dependent dioxygenase gene in the cell death process. IUBMB Life 57, 181–188. doi: 10.1080/15216540500090793
Guan, L., Zhao, J., and Scandalios, J. G. (2000). Cis-elements and trans-factors that regulate expression of the maize cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22, 87–95. doi: 10.1046/j.1365-313x.2000.00723.x
Guo, H., and Ecker, J. R. (2004). The ethylene signaling pathway: new insights. Curr. Opin. Plant Biol. 7, 40–49. doi: 10.1016/j.pbi.2003.11.011
Guo, H., Xiaolin, D., and Juane, D. (2014). Hydrogen peroxide and nitric oxide are involved in salicylic acid-induced salvianolic acid B production in Salvia miltiorrhiza cell cultures. Molecules 19, 5913–5924. doi: 10.3390/molecules19055913
Gupta, K. J., Fernie, A. R., Kaiser, W. M., and van Dongen, J. T. (2011). On the origins of nitric oxide. Trend. Plant Sci. 16, 160–168. doi: 10.1016/j.tplants.2010.11.007
Harding, S. A., Oh, S. H., and Roberts, D. M. (1997). Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO J. 16, 1137–1144. doi: 10.1093/emboj/16.6.1137
Harmon, A. C., Gribskov, M., and Harper, J. F. (2000). CDPKs–a kinase for every Ca2+ signal? Trends Plant. Sci. 5, 154–159.
Hattori, Y., Nagai, K., Furukawa, S., Song, X. J., Kawano, R., Sakakibara, H., et al. (2009). The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460, 1026–1030. doi: 10.1038/nature08258
He, J. M., Ma, X. G., Zhang, Y., Sun, T. F., Xu, F. F., Chen, Y. P., et al. (2013). Role and interrelationship of Ga protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol. 161, 1570–1583. doi: 10.1104/pp.112.211623
Herrera-Vásquez, A., Salinas, P., and Holuigue, L. (2015). Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 6:171. doi: 10.3389/fpls.2015.00171
Holuigue, L., Salinas, P., Blanco, F., and Garreton, V. (2007). “Salicylic acid and reactive oxygen species in the activation of stress defense genes,” in Salicylic Acid – A Plant Hormone, eds S. Hayat and A. Ahmad (Dordrecht: Springer Science & Business Media), 197–246.
Hossain, M. A., Bhattacharjee, S., Armin, S. M., Qian, P., Wang, X., Li, H. Y., et al. (2015). Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 6:420. doi: 10.3389/fpls.2015.00420
Howe, G. A., Lightner, J., Browse, J., and Ryan, C. A. (1996). An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8, 2067–2077. doi: 10.1105/tpc.8.11.2067
Hu, X., Bidney, D. L., Yalpani, N., Duvick, J. P., Crasta, O., Folkerts, O., et al. (2003). Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol. 133, 170–181. doi: 10.1104/pp.103.024026
Ievinsh, G., and Tiberg, E. (1995). Stress-induced ethylene biosynthesis in pine needles: a search for the putative 1-aminocy-clopropane-1-carboxylic-independent pathway. J. Plant Physiol. 145, 308–314. doi: 10.1016/S0176-1617(11)81895-X
Ishibashi, Y., Tawaratsumida, T., Kondo, K., Kasa, S., Sakamoto, M., Aoki, N., et al. (2012). Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 158, 1705–1714. doi: 10.1104/pp.111.192740
Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I., and Okada, K. (2001). The defective in anther dehiscence1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191–2209. doi: 10.1105/tpc.010192
Jajic, I., Sarna, T., and Strzalka, K. (2015). Senescence, stress, and reactive oxygen species. Plants 4, 393–411. doi: 10.3390/plants4030393
Jammes, F., Song, C., Shin, D., Munemasa, S., Takeda, K., Gu, D., et al. (2009). MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 20520–20525. doi: 10.1073/pnas.0907205106
Jubany-Mari, T., Munne-Bosch, S., Lopez-Carbonell, M., and Alegre, L. (2009). Hydrogen peroxide is involved in the acclimation of the mediterranean shrub, cistus albidus l., to summer drought. J. Exp. Bot. 60, 107–120. doi: 10.1093/jxb/ern274
Kapoor, D., Sharma, R., Handa, N., Kaur, H., Rattan, A., Yadav, P., et al. (2015). Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 3:13. doi: 10.3389/fenvs.2015.00013
Karpinska, B., Karlsson, M., Schinkel, H., Streller, S., Süss, K. H., Melzer, M., et al. (2001). A novel superoxide dismutase with a high isoelectric point in higher plants. Expression, regulation, and protein localization. Plant Physiol. 126, 1668–1677. doi: 10.1104/pp.126.4.1668
Karyotou, K., and Donaldson, R. P. (2005). Ascorbate peroxidase, a scavenger of hydrogen peroxide in glyoxysomal membranes. Arch. Biochem. Biophys. 434, 248–257. doi: 10.1016/j.abb.2004.11.003
Kauss, H., and Jeblick, W. (1996). Influence of salicylic acid on the induction of competence for H2O2 elicitation. Plant Physiol. 111, 755–763.
Kauss, H., Jeblick, W., Ziegler, J., and Krabler, W. (1994). Pretreatment of parsley (Petroselinum crispum) suspension cultures with methyl jasmonate enhanced elicitation of activated oxygen species. Plant Physiol. 105, 89–94.
Keller, T., Damude, H. G., Werner, D., Doerner, P., Dixon, R. A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes an intrinsic plasma membrane protein with Ca2+-binding and RanGAP1 domains. Plant Cell 10, 255–266. doi: 10.1105/tpc.10.2.255
Kerk, N. M., and Feldman, N. J. (1995). A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development 121, 2825–2833.
Kovtun, Y., Chiu, W. L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. U.S.A. 97, 2940–2945. doi: 10.1073/pnas.97.6.2940
Kunihiro, S., Hiramatsu, T., and Kawano, T. (2011). Involvement of salicylic acid signal transduction in aluminum-responsive oxidative burst in Arabidopsis thaliana cell suspension culture. Plant Signal. Behav. 6, 611–616. doi: 10.4161/psb.6.5.14895
Kwak, J. M., Mori, I. C., Pei, Z. M., Leonhardt, N., Torres, M. A., Dangl, J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. doi: 10.1093/emboj/cdg277
Lachaud, C., Da Silva, D., Amelot, N., Beziat, C., Briere, C., Cotelle, V., et al. (2011). Dihydrosphingosine-induced programmed cell death in tobacco BY-2 cells is independent of H2O2 production. Mol. Plant 4, 310–318. doi: 10.1093/mp/ssq077
Lamattina, L., Garcia-Mata, C., Graziano, M., and Pagnussat, G. (2003). Nitric oxide: the versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 54, 109–136. doi: 10.1146/annurev.arplant.54.031902.134752
Lamb, C., and Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. doi: 10.1146/annurev.arplant.48.1.251
Lecourieux, D., Marzars, C., Pauly, N., Ranjeva, R., and Pugin, A. (2002). Analysis and effects of cytosolic free calcium increase in response to elictous Nicotiana plumbaginigolia cells. Plant Cell 14, 2627–2641. doi: 10.1105/tpc.005579
Lee, S., Kim, S. G., and Park, C. M. (2010). Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 188, 626–637. doi: 10.1111/j.1469-8137.2010.03378.x
Lee, S., and Park, C. M. (2010). Modulation of reactive oxygen species by salicylic acid in Arabidopsis seed germination under high salinity. Plant Signal. Behav. 5, 1534–1536. doi: 10.4161/psb.5.12.13159
León, J., Lawton, M. A., and Raskin, I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol. 108, 1673–1678.
Leshem, Y. Y., and Haramaty, E. (1996). The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. foliage. J. Plant Physiol. 148, 258–263. doi: 10.1016/S0176-1617(96)80251-3
Leshem, Y. Y., Wills, R. B. H., and Venga-Ku, V. (1998). Evidence for the function of free radical gas-nitric oxide (NO) – as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol. Biochem. 36, 825–833. doi: 10.1016/S0981-9428(99)80020-5
Levine, M. (1986). New concepts in the biology and biochemisty of ascorbic acid. New Engl. J. Med. 314, 892–902. doi: 10.1056/NEJM198604033141407
Li, A., Wang, X., Leseberg, C. H., Jia, J., and Mao, L. (2008). Biotic and abiotic stress responses through calcium-dependent protein kinase (CDPK) signalling in wheat (Triticum aestivum L.). Plant Signal. Behav. 3, 654–656. doi: 10.4161/psb.3.9.5757
Liao, W. B., Zhang, M. L., Huang, G. B., and Yu, J. H. (2012). Hydrogen peroxide in the vase solution increases vase life and keeping quality of cut Oriental × Trumpet hybrid lily “manissa.” Sci. Hortic. 139, 32–38. doi: 10.4161/psb.3.9.5757
Lin, A., Wang, Y., Tang, J., Xue, P., Li, C., Liu, L., et al. (2011). Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 158, 451–464. doi: 10.1104/pp.111.184531
Liu, J., Xu, Y., Zheng, Z., and Wei, J. (2014). Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Funct. Plant Biol. 42, 337–346. doi: 10.1071/FP14189
Liu, N., You, J., Shi, W., Liu, W., and Yang, Z. (2012). Salicylic acid involved in the process of aluminum induced citrate exudation in Glycine max L. Plant Soil 352, 85–97. doi: 10.1007/s11104-011-0981-x
Lund, S. T., Stall, R. E., and Klee, H. J. (1998). Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10, 371–382. doi: 10.1105/tpc.10.3.371
Mahajan, S., and Tuteja, N. (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444, 139–158. doi: 10.1016/j.abb.2005.10.018
Mahalingam, R., and Federoff, N. (2003). Stress response, cell death and signalling: the many faces of reactive oxygen species. Physiol. Plant 119, 56–68. doi: 10.1034/j.1399-3054.2003.00156.x
Maruta, T., Noshi, M., Tanouchi, A., Tamoi, M., Yabuta, Y., Yoshimura, K., et al. (2012). H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 287, 11717–11729. doi: 10.1074/jbc.M111.292847
Mateo, A., Funck, D., Muhlenbock, P., Kular, B., Mullineaux, P. M., and Karpinski, S. (2006). Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 57, 1795–1807. doi: 10.1093/jxb/erj196
McConn, M., Creelman, R. A., Bell, E., Mullet, J. E., and Browse, J. (1997). Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 94, 5473–5477. doi: 10.1073/pnas.94.10.5473
Menges, M., Dóczi, R., Okrész, L., Morandini, P., Mizzi, L., Soloviev, M., et al. (2008). Comprehensive gene expression atlas for the Arabidopsis MAP kinase signalling pathways. New. Phytol. 179, 643–662. doi: 10.1111/j.1469-8137.2008.02552.x
Mhamdi, A., Hager, J., Chaouch, S., Queval, G., Han, Y., Taconnat, L., et al. (2010). Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signalling pathways. Plant Physiol. 153, 1144–1160. doi: 10.1104/pp.110.153767
Miao, Y., Lv, D., Wang, P., Wang, X. C., Chen, J., Miao, C., et al. (2006). An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18, 2749–2766. doi: 10.1105/tpc.106.044230
Mittler, R., Vanderauwera, S., Gollery, M., and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trend. Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Mittler, R., and Zilinskas, B. A. (1991). Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol. 97, 962–968. doi: 10.1104/pp.97.3.962
Miura, K., and Tada, Y. (2014). Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 5:4. doi: 10.3389/fpls.2014.00004
Moller, I. M. (2001). Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 561–591. doi: 10.1146/annurev.arplant.52.1.561
Møller, I. M., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant. Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
Montillet, J. L., Leonhardt, N., Mondy, S., Tranchimand, S., Rumeau, D., Boudsocq, M., et al. (2013). An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 11:3. doi: 10.1371/journal.pbio.1001513
Moon, H., Lee, B., Choi, G., Shin, D., Prasad, D. T., Lee, O., et al. (2003). NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. U.S.A. 100, 358–363. doi: 10.1073/pnas.252641899
Morgan, P. W., and Drew, M. C. (1997). Ethylene and plant responses to stress. Physiol. Plant 100, 620–630. doi: 10.1111/j.1399-3054.1997.tb03068.x
Mueller, M. J., Brodschelm, W., Spannagl, E., and Zenk, M. H. (1993). Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. U.S.A. 90, 7490–7494. doi: 10.1073/pnas.90.16.7490
Mur, L. A. J., Bi, Y. M., Darby, R. M., Firek, S., and Draper, J. (1997). Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersion during lesion establishment in TMV-infected tobacco. Plant J. 12, 1113–1126. doi: 10.1046/j.1365-313X.1997.12051113.x
Neill, S., Barros, R., Bright, J., Desikan, R., Hancock, J., Harrison, J., et al. (2008). Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 59, 165–176. doi: 10.1093/jxb/erm293
Nie, S., Yue, H., and Da, X. (2015). A potential role for mitochondrial produced reactive oxygen species in salicylic acid-mediated plant acquired thermotolerance. Plant Physiol. doi: 10.1104/pp.15.00719 [Epub ahead of print].
Noctor, G., Arisi, A. C. M., Jouanin, L., and Foyer, C. H. (1999). Photorespiratory glycine enhances glutathione accumulation in both the chloroplastic and cytosolic compartments. J. Exp. Bot. 50, 1157–1167. doi: 10.1093/jxb/50.336.1157
Noshi, M., Maruta, T., and Shigeoka, S. (2012). Relationship between chloroplastic H2O2 and the salicylic acid response. Plant Signal. Behav. 7, 944–946. doi: 10.4161/psb.20906
Olemiec, E., Tokarz, K., Wielanek, M., and Niewiadomska, E. (2014). A dissection of the effects of ethylene, H2O2 and high irradiance on antioxidants and several genes associated with stress and senescence in tobacco leaves. J. Plant Pysiol. 171, 269–275. doi: 10.1016/j.jplph.2013.08.007
O’Malley, R. C., Rodriguez, F. I., Esch, J. J., Binder, B. M., O’Donnell, P., Klee, H. J., et al. (2005). Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J. 41, 651–659. doi: 10.1111/j.1365-313X.2004.02331.x
Orozco-Cardenas, M. L., Narvaez-Vasquez, J., and Ryan, C. A. (2001). Hydrogen per-oxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell 13, 179–191. doi: 10.1105/tpc.13.1.179
Orozco-Cárdenas, M. L., and Ryan, C. A. (2002). Nitric oxide negatively modulates wound signaling in tomato Plants. Plant Physiol. 130, 487–493. doi: 10.1104/pp.008375
Overmyer, K., Brosché, M., Kangaskärvi, J. (2003). Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 8, 335–342. doi: 10.1016/S1360-1385(03)00135-3
Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cell. Nature 406, 731–734. doi: 10.1038/35021067
Peng, H. P., Lin, T. Y., Wang, N. N., and Shih, M. C. (2005). Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant. Mol. Biol. 58, 15–25. doi: 10.1007/s11103-005-3573-4
Plaine, H. L. (1955). The effect of oxygen and hydrogen peroxide on the action of a specific gene and on tumour induction in drosophila melanogaster. Genetics 40, 268–280.
Quan, L. J., Zhang, B., Shi, W. W., and Li, H. Y. (2008). Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 50, 2–18. doi: 10.1111/j.1744-7909.2007.00599.x
Rao, M. V., Paliyath, G., Ormrod, D. P., Murr, D. P., and Watkins, C. B. (1997). Influence of salicylic acid on H2O2 production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol. 115, 137–149. doi: 10.1104/pp.115.1.137
Reczek, C. R., and Chandel, N. S. (2015). ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13. doi: 10.1016/j.ceb.2014.09.010
Rentel, M. C., and Knight, M. R. (2004). Oxidative stress-induced calcium signalling in Arabidopsis. Plant Physiol. 135, 1471–1479. doi: 10.1104/pp.104.042663
Rentel, M. C., Lecourieux, D., Ouaked, F., Usher, S. L., Petersen, L., Okamoto, H., et al. (2004). OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427, 858–861. doi: 10.1038/nature02353
Rodriguez, M. C., Petersen, M., and Mundy, J. (2010). Mitogen-activated protein kinase signalling in plants. Annu. Rev. Plant Biol. 61, 621–649. doi: 10.1146/annurev-arplant-042809-112252
Romero-puertas, M. C., and Sandalio, L. M. (2016). Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 7:316. doi: 10.3389/fpls.2016.00316
Sánchez-Martín, J., Heald, J. I. M., Kingston-Smith, A., Winters, A. N. A., Rubiales, D., Sanz, M., et al. (2015). A metabolomic study in oats (Avena sativa) highlights a drought tolerance mechanism based on salicylate signalling pathways and the modulation of carbon, antioxidant and photo-oxidative metabolism. Plant Cell Environ. 38, 1434–1452. doi: 10.1111/pce.12501
Sano, S., Tao, S., Endo, Y., Inaba, T., Hossain, M. A., Miyake, C., et al. (2005). Purification and cDNA cloning of chloroplastic monodehydroascorbate reductase from spinach. Biosci. Biotechnol. Biochem. 69, 762–772. doi: 10.1271/bbb.69.762
Scandalios, J. G. (1993). Oxygen stress and superoxide dismutases. Plant Physiol. 101, 7–12. doi: 10.1104/pp.101.1.7
Schraudner, M., Moeder, W., Wiese, C., Camp, W. V., Inzé, D., Langebartels, C., et al. (1998). Ozone-induced oxidative burst in the ozone biomonitor plant tobacco Bel W3. Plant J. 16, 235–245. doi: 10.1046/j.1365-313x.1998.00294.x
Shao, H. B., Chu, L. Y., Lu, Z. H., and Kang, C. M. (2008). Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 4, 8–14. doi: 10.7150/ijbs.4.8
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 8:e23681. doi: 10.1155/2012/217037
Shi, H., Ye, T., Zhu, J.-K., and Chan, Z. (2014). Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 65, 4119–4131. doi: 10.1093/jxb/eru184
Sies, H., and Stahl, W. (1995). Vitamins E and C, β-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 62, 1315–1321.
Skovgaarda, N., Gallia, G., Abea, A., Taylora, E. W., and Wanga, T. (2005). The role of nitric oxide in regulation of the cardiovascular system in reptiles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 142, 205–214. doi: 10.1016/j.cbpb.2005.05.049
Smirnoff, N., and Wheeler, G. L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 35, 291–314. doi: 10.1080/10409230008984166
Sutherland, M. W. (1991). The generation of oxygen radicals during host plant responses to infection. Physiol. Mol. Plant Pathol. 39, 79–93. doi: 10.1016/0885-5765(91)90020-I
Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M. A., and Mittler, R. (2011). Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 14, 691–699. doi: 10.1016/j.pbi.2011.07.014
Swamy, P. M., and Smith, B. (1999). Role of abscisic acid in plant stress tolerance. Curr. Sci. 76, 1220–1227.
Takeda, S., Gapper, C., Kaya, H., Bell, E., Kuchitsu, K., and Dolan, L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244. doi: 10.1126/science.1152505
Taki-Nakano, N., Ohzeki, H., Kotera, J., and Ohta, H. (2014). Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochem. Biophys. Acta 1840, 3413–3422. doi: 10.1016/j.bbagen.2014.09.003
Terzi, R., Kadioglu, A., Kalaycioglu, E., and Saglam, A. (2014). Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J. Plant Interact. 9, 559–565. doi: 10.1080/17429145.2013.871077
Torres, M. A., Dangl, J. L., and Jones, J. D. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. U.S.A. 99, 517–522. doi: 10.1073/pnas.012452499
Venkatesh, J., and Park, S. W. (2014). Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 55:38.
Vianello, A., and Macri, F. G. (1991). Generation of superoxide anion and hydrogen peroxide at the surface of plant cell. J. Bioenerg. Biomembr. 23, 409–423. doi: 10.1007/BF00771012
Vijayan, P., Shockey, J., Levesque, C. A., Cook, R. J., and Browse, J. (1998). A role for jasmonate in pathogen defense of Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 95, 7209–7214. doi: 10.1073/pnas.95.12.7209
Vlot, A. C., Klessig, D. F., and Park, S. W. (2008). Systemic acquired resistance: the elusive signal(s). Curr. Opin. Plant Biol. 11, 436–442. doi: 10.1016/j.pbi.2008.05.003
Wan, D., Li, R., Zou, B., Zhang, X., Cong, J., Wang, R., et al. (2012). Calmodulin-binding protein CBP60g is a positive regulator of both disease resistance and drought tolerance in Arabidopsis. Plant Cell Rep. 31, 1269–1281. doi: 10.1007/s00299-012-1247-7
Wang, H., Liang, X., Huang, J., Zhang, D., Lu, H., Liu, Z., et al. (2010). Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiol. 51, 1754–1765. doi: 10.1093/pcp/pcq134
Wang, H., Liang, X., Wan, Q., Wang, X., and Bi, Y. (2009). Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230, 293–307. doi: 10.1007/s00425-009-0946-y
Wang, H., Liu, Y., Bruffett, K., Lee, J., Hause, G., Walker, J. C., et al. (2008). Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 20, 602–613. doi: 10.1105/tpc.108.058032
Wang, L., Guo, Y., Jia, L., Chu, H., Zhou, S., Chen, K., et al. (2014). Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiol. 164, 2184–2196. doi: 10.1104/pp.113.229369
Wang, R. S., Pandey, S., Li, S., Gookin, T. E., Zhao, Z., Albert, R., et al. (2011). Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC Genomics 12:216. doi: 10.1186/1471-2164-12-216
Wink, D. A., Hanbauer, L., Krishna, M. C., and DeGraff, W. (1993). Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 90, 9813–9817. doi: 10.1073/pnas.90.21.9813
Wu, J., Shang, Z., Jiang, X., Moschou, P. N., Sun, W., Roubelakis-Angelakis, K. A., et al. (2010). Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 63, 1042–1053. doi: 10.1111/j.1365-313X.2010.04301.x
Xia, X. J., Zhou, Y. H., Shi, K., Zhou, J., Foyer, C. H., and Yu, J. Q. (2015). Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Expt. Bot. 66, 2839–2856. doi: 10.1093/jxb/erv089
Yamaguchi, K., Mori, H., and Nishimura, M. (1995). A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol. 36, 1157–1162.
Yang, C. Y. (2014). Ethylene and hydrogen peroxide are involved in hypoxia signaling that modulates AtERF73/HRE1 expression. Plant Signal. Behav. 9:e28583. doi: 10.4161/psb.28583
Yang, T., and Poovaiah, B. W. (2002). Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc. Natl. Acad. Sci. U.S.A. 99, 4097–4102. doi: 10.1073/pnas.052564899
Yang, X., Yang, Y. N., Xue, L. J., Zou, M. J., Liu, J. Y., Chen, F., et al. (2011). Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 5, 1397–1409. doi: 10.1104/pp.111.173427
Yang, W., Zhu, C., Ma, X., Li, G., Gan, L., Ng, D., et al. (2013). Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung bean seedlings. PLoS ONE 8:e84580. doi: 10.1371/journal.pone.0084580
Ye, N., Zhu, G., Liu, Y., Zhang, A., Li, Y., Liu, R., et al. (2012). Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 63, 1809–1822. doi: 10.1093/jxb/err336
Zhang, A., Jiang, M., Zhang, J., Ding, H., Xu, S., Hu, X., et al. (2007). Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense inmaize leaves. New Phytol. 175, 36–50. doi: 10.1111/j.1469-8137.2007.02071.x
Zhang, F., Zhang, H., Xia, Y., Wang, G., Xu, L., and Shen, Z. (2011). Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 30, 1475–1483. doi: 10.1007/s00299-011-1056-4
Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D. W., and Song, C. P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. doi: 10.1104/pp.126.4.1438
Zheng, L., Xin, Z., Amandeep, K. S., and Liwei, G. (2010). Effects of exogenous abscisic acid on yield, antioxidant capacities, and phytochemical contents of greenhouse grown lettuces. J. Agric. Food Chem. 58, 6503–6509. doi: 10.1021/jf1006962
Zhou, J., Wang, J., Shi, K., Xia, X. J., Zhou, Y. H., and Yu, J. Q. (2012). Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 60, 141–149. doi: 10.1016/j.plaphy.2012.07.010
Zong, X. J., Li, D. P., Gu, L. K., Li, D. Q., Liu, L. X., and Hu, X. L. (2009). Abscisic acid and hydrogen peroxide induce a novelmaize group CMAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229, 485–495. doi: 10.1007/s00425-008-0848-4
Zou, J. J., Li, X. D., Ratnasekera, D., Wang, C., Liu, W. X., Song, L. F., et al. (2015). Arabidopsis calicium-dependent protein kinases8 and catalases3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27, 1445–1460. doi: 10.1105/tpc.15.00144
Keywords: H2O2, ROS, abscisic acid, nitric oxide, biotic/abiotic stress, phytohormones
Citation: Saxena I, Srikanth S and Chen Z (2016) Cross Talk between H2O2 and Interacting Signal Molecules under Plant Stress Response. Front. Plant Sci. 7:570. doi: 10.3389/fpls.2016.00570
Received: 30 November 2015; Accepted: 13 April 2016;
Published: 28 April 2016.
Edited by:
Mirza Hasanuzzaman, Sher-e-Bangla Agricultural University, BangladeshCopyright © 2016 Saxena, Srikanth and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Chen, zhong.chen@nie.edu.sg
 Ina Saxena
Ina Saxena Sandhya Srikanth
Sandhya Srikanth Zhong Chen
Zhong Chen