- Plasticity and Regeneration Lab, School of Biosciences, University of Birmingham, Birmingham, United Kingdom
The human brain can change throughout life as we learn, adapt and age. A balance between structural brain plasticity and homeostasis characterizes the healthy brain, and the breakdown of this balance accompanies brain tumors, psychiatric disorders, and neurodegenerative diseases. However, the link between circuit modifications, brain function, and behavior remains unclear. Importantly, the underlying molecular mechanisms are starting to be uncovered. The fruit-fly Drosophila is a very powerful model organism to discover molecular mechanisms and test them in vivo. There is abundant evidence that the Drosophila brain is plastic, and here we travel from the pioneering discoveries to recent findings and progress on molecular mechanisms. We pause on the recent discovery that, in the Drosophila central nervous system, Toll receptors—which bind neurotrophin ligands—regulate structural plasticity during development and in the adult brain. Through their topographic distribution across distinct brain modules and their ability to switch between alternative signaling outcomes, Tolls can enable the brain to translate experience into structural change. Intriguing similarities between Toll and mammalian Toll-like receptor function could reveal a further involvement in structural plasticity, degeneration, and disease in the human brain.
Introduction
The brain can change throughout life. Cells, neurites (axons and dendrites), and synapses are generated with experience and learning as we adapt to the environment. They are also eliminated, maintaining neural circuit stability and normal behavior. The balance between the former, known as structural plasticity, and the latter, known as structural homeostasis, characterizes brain health, and its breakdown accompanies a brain disease—such as brain tumors, psychiatric disorders, and neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s diseases). Structural plasticity and homeostasis may reveal how the brain works, as brain function relies on structural changes to cells.
Structural plasticity is often understood to be “hebbian,” whereby co-active synapses are stabilized, reinforcing connections. Structural homeostasis is also known as “non-hebbian plasticity” and includes elimination and “compensatory plasticity,” such as increases in synapse number as arborizations decrease, to deliver normal function. Synapses can also manifest physiological plasticity such as synaptic potentiation, depression, or homeostatic plasticity (e.g., involving regulatory adjustments in neurotransmitter release or post-synaptic excitability to maintain a normal function). Here we will deal only with structural changes.
Mammalian cortical plasticity has long been known (Feldman and Brecht, 2005). Classical experiments by Wiesel and Hubel showed that synapses and neurites are modified in response to neuronal activity and experience (Wiesel and Hubel, 1965). When kittens were deprived of light in one eye, arborizations from active neurons originating from the open eye outcompeted the silent ones from the closed eye, altering the pattern of innervation in the cortex. Importantly, recurrent cycles of light deprivation and exposure did not cause spine elimination to restore original spine profiles but instead maintained and built on newly formed synapses (Hofer et al., 2009). This suggests that structural changes in neurons may store information from the past, enabling the brain to adapt and respond in the future. However, plasticity may not necessarily be adaptive nor beneficial, but just a consequence of available connectivity opportunities for neurons (Feldman and Brecht, 2005).
Structural plasticity can also involve neurogenesis. Enriched environments, exercise, voluntary running, and learning can induce neurogenesis in the adult mammalian brain (Gage, 2004, 2019; Deng et al., 2010). In mammals, neurogenesis occurs in restricted brain areas, including the dentate gyrus of the hippocampus and the subventricular zone generating olfactory bulb neurons (Eriksson et al., 1998; Spalding et al., 2013; Goncalves et al., 2016; Simoes and Rhiner, 2017). Hippocampal neurogenesis may be required for spatial navigation and learning and memory to encode time into memory and prevent interference with old memories (Deng et al., 2010). However, this remains to be established.
The molecular mechanisms underlying structural brain plasticity and homeostasis remain virtually unknown. The neurotrophin BDNF and its receptor TrkB are currently the key factors known to be involved, and there is evidence that Toll-like receptors (TLRs) could also be involved (Lu et al., 2005, 2013; Okun et al., 2011; Park and Poo, 2013). Progress in understanding the link between molecular mechanisms, circuits, structural brain plasticity, homeostasis, and brain health in mammals has long been challenging.
The fruit-fly Drosophila is a powerful model organism to discover molecular mechanisms and link genes to neurons, circuits, and behavior. Here we review pioneering work on structural brain plasticity and homeostasis in Drosophila, current evidence of underlying molecular mechanisms, and how they could relate to the mammalian brain.
Structural Plasticity and Homeostasis in the Adult Drosophila Brain
Structural Plasticity: Brain Size Is Variable and Regulated by Experience
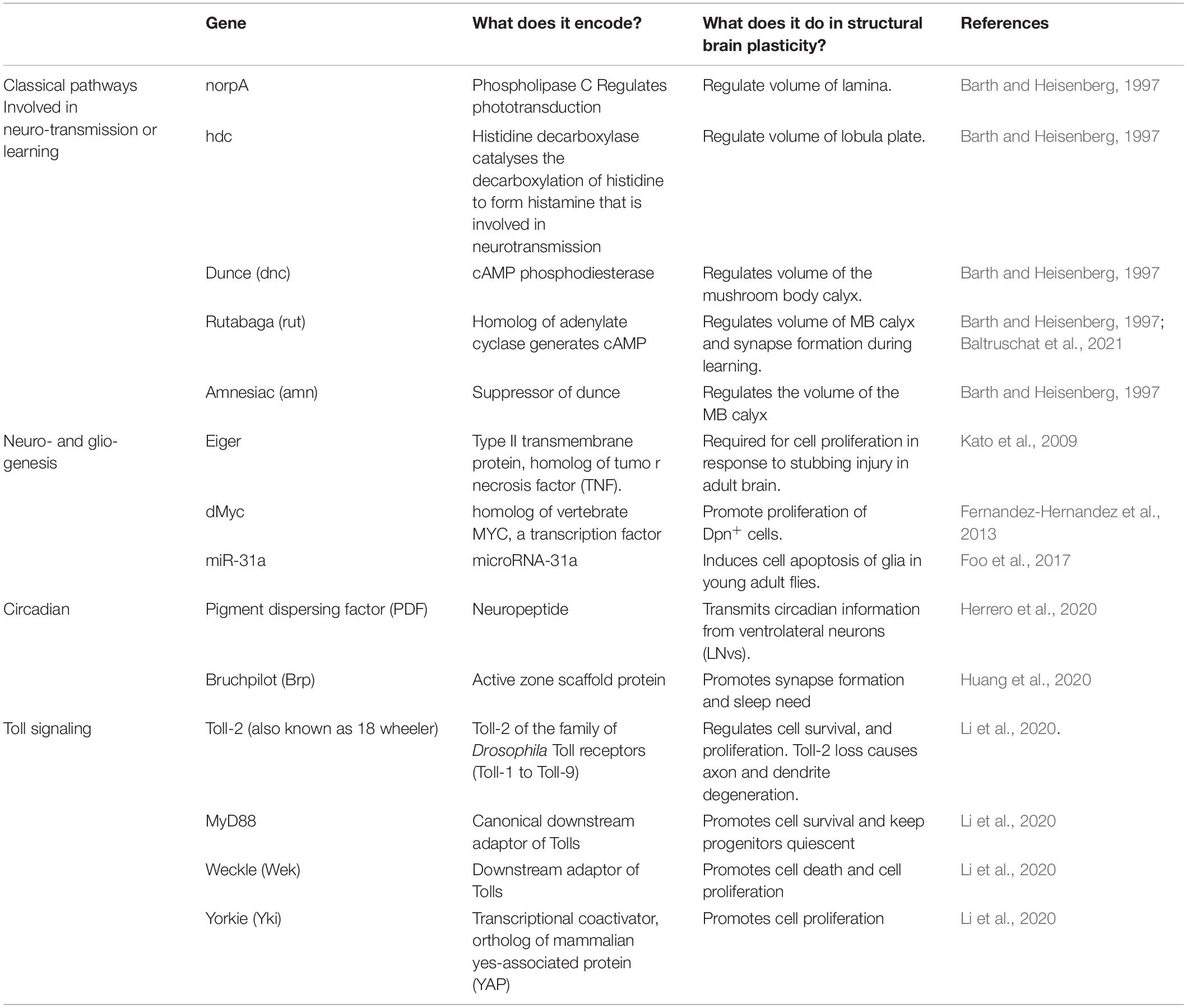
Pioneering work by Martin Heisenberg’s lab started the structural plasticity journey through the Drosophila brain. Gerhard Technau showed that, in Drosophila, fiber number in the adult mushroom body peduncle can increase by 15% at 1 week after adult fly eclosion, and this was influenced by environmental stimuli (Technau, 1984). Flies reared in isolation, constant darkness, or olfactory deprivation had a significantly reduced fiber number (Technau, 1984). In fact, Kenyon cell number in wild-type flies is variable and can change depending on larval growth conditions and experience in the first week of adult fly life (Balling et al., 1987; Heisenberg et al., 1995). Brain size also changed with experience. Eclosed adult flies were kept either in isolation or single sex populations (i.e., deprived environment) or in large population cages or mixed sexes (i.e., enriched) for 19 days, and then the brain volume was measured. The volume of various brain structures increased in enriched vs. deprived flies, including the mushroom bodies, calyces, and the visual system lamina, medulla, and lobula (Heisenberg et al., 1995). Furthermore, compared to flies reared in constant darkness, stimulation of the visual system by breeding flies in constant light during a critical period spanning the first 5 days after eclosion increased the volume of the lamina and lobula plate, the size of photoreceptors and glial cells, the mushroom body calyces, and the central complex (Barth and Heisenberg, 1997; Barth et al., 1997). These pioneering studies did not resolve the cellular bases of structural brain plasticity. Some limited proliferation was detected in adult brains, but it was deemed to be insufficient to explain the extent of structural brain change (Technau, 2007). This work altogether showed that brain size can be modified by experience and that different experiences can affect brain regions differentially.
Findings from the olfactory system provided independent evidence for structural plasticity during the critical period in other brain regions (Devaud et al., 2003). The olfactory glomeruli continue to grow between days 1 and 12 of adult fly life, and the volume of particular glomeruli was influenced by experience—i.e., exposure to odorant—concomitantly with the maturation of odorant-induced behavior (Devaud et al., 2003). Similarly, when flies were exposed to a high concentration of carbon dioxide (CO2) during the critical period, the volume of the CO2-specific V glomerulus in the antennal lobe significantly increased (Sachse et al., 2007). This structural change was reversible after recovering at normal air exposure for another 5 days. By contrast, CO2 exposure after the critical period did not cause a significant structural alteration in the V glomerulus (Sachse et al., 2007). The microglomeruli that form as projection neurons from the antennal lobe connecting to the Kenyon cell dendrites at the calyx are also modified by experience. After conditioning young flies with an odorant (11-cis-vaccenylacetate) to form long-term memory, the size of the microglomeruli responding to this odorant decreased, but the number of such microglomeruli increased (Baltruschat et al., 2021). This demonstrated that new synaptic boutons are formed during learning and for long-term memory and that structural change is linked to brain function.
To conclude, multiple regions within the adult brain—visual and olfactory systems and central complex—can be modified by experience (Figure 1). Whether this is restricted to a critical period within the first week of adult fly life or continues throughout the life course remains to be tested further and established. Most studies show that plasticity takes place within a critical period in the first week of adult life but do not carry out test beyond. Heisenberg showed that, at least under some conditions, the brain remains plastic beyond this period and into at least the second week (Heisenberg et al., 1995). This varies across brain regions, suggesting that different mechanisms with distinct dynamics regulate plasticity in the different brain domains (Heisenberg et al., 1995).
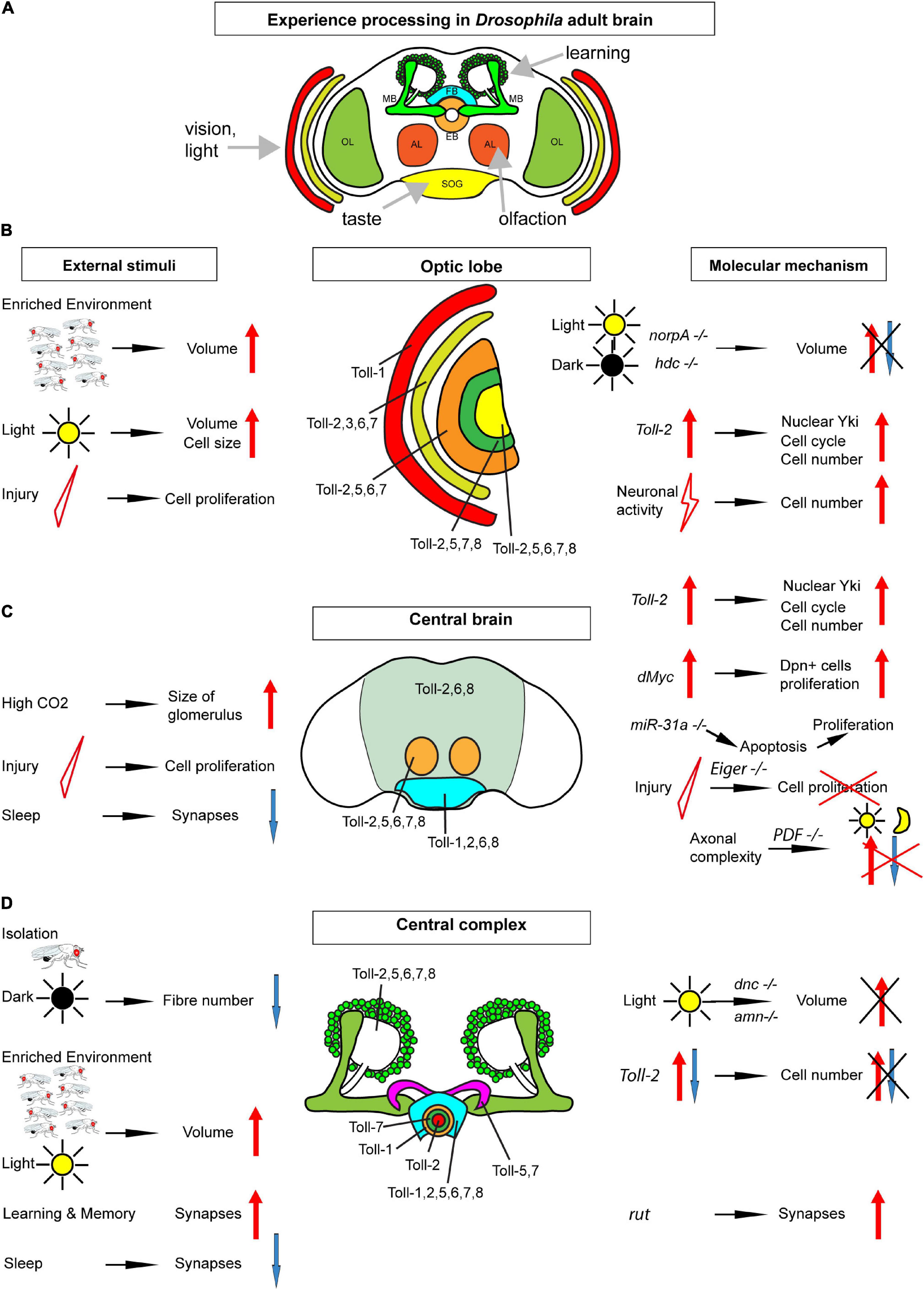
Figure 1. Map of Toll receptors in a Drosophila adult brain. (A) Drosophila adult brain illustrating some modules receiving sensory input and involved in learning. (B) Optic lobe (including retina, lamina, medulla, lobula, and lobula plate from outer to inner layers). (C) Central brain [including neuropiles (pale green), antennal lobes (orange), and sub-esophageal ganglion (bright blue)]. (D) Central complex: mushroom bodies (green) ellipsoid body in rings, fan-shaped body (bright blue), and protocerebral bridge (purple). Each brain module expresses different Tolls or a combination of Tolls; thus, they could be regulated differentially in distinct brain modules. From (B–D), the key findings involved in structural brain plasticity are summarized with external stimuli on the left and molecular manipulations and mechanisms on the right. OL, optic lobe; MB, mushroom body; FB, fan-shaped body; EB, ellipsoid body; AL, antennal lobe; SOG, sub-esophageal ganglion.
Structural Homeostasis: Elimination and Compensation
Structural homeostasis was initially reported in the adult brain of Musca domestica (Kral and Meinertzhagen, 1989). Eclosed adult flies were subjected for 2 days to two regimens: they were either kept in constant darkness or stimulated by flickering green light (Kral and Meinertzhagen, 1989). As a result, the synapse number in lamina neurons that connect to photoreceptors decreased with light exposure and increased in flies reared in the dark (Kral and Meinertzhagen, 1989). Thus, neuronal stimulation provoked synapse elimination, and the lack of stimulation increased synapse formation. Subjecting adult flies to cold-shock also decreased the synapse number, and synapses could be recovered by transferring the flies to warmer temperatures (Brandstatter and Meinertzhagen, 1995). These findings provided evidence for structural homeostasis in the lamina of the visual system of the house fly. Structural homeostasis was also revealed in the context of olfaction in Drosophila. Exposure of adult fruit-flies to a high concentration of an odorant for 4 days resulted in behavioral adaptation to the odorant, normal olfactory transduction, and a reduction in olfactory glomeruli size in the antennal lobes (Devaud et al., 2001).
A subcellular evidence of structural homeostasis in the Drosophila brain was found in the mushroom body calyx and photoreceptor terminals in the lamina (Kremer et al., 2010; Sugie et al., 2015). Inhibiting action potential firing in antennal lobe projection neurons that connect to mushroom body Kenyon cells increased the size of microglomeruli in the mushroom body calyx (Kremer et al., 2010). Pre-synaptic active zone density also increased, which means that, upon a reduction in neuronal activity, the neurons increase in synapse number to restore function (Kremer et al., 2010). This is evidence of compensatory plasticity. On the other hand, exposing flies to constant light for the first 1–3 days after eclosion decreased the number of synaptic active zones and T-bars from photoreceptors in the lamina (Sugie et al., 2015). Thus, neuronal stimulation induced the disassembly of presynaptic active zones.
The adult fly brain altogether manifests activity-dependent structural homeostasis, as neuronal activity decreased the olfactory glomeruli size and caused synapse elimination, and compensatory plasticity, as the microglomeruli size and synapse number increased in the absence of stimulation (Figure 1).
Circadian Plasticity and Sleep
Structural changes in the brain have long been known to occur with circadian rhythms. Neurotransmitters were first shown to affect cell size with circadian periodicity (Pyza and Meinertzhagen, 1996). The size of the nuclei of lamina L2 neurons changes with the circadian rhythm, larger in the morning and smaller at night (Gorska-Andrzejak et al., 2005). L2 neuron dendrites are also largest in the morning and smallest in the middle of the night (Weber et al., 2009). However, whether this reflects changes directly controlled by the circadian clock or changes in response to the environment is less clear. For instance, lamina neuron dendritic changes were eliminated when flies were kept in constant light (Weber et al., 2009). Axonal arborizations of clock neurons, such as pigment-dispensing factor (PDF) neurons, are also more complex during the day than at night (Herrero et al., 2020), but activating PDF neurons with TrpA1 increased axonal complexity in the night to the same level as in the morning (Herrero et al., 2020). Thus, changes induced in daytime, by light, could reflect activity-dependent plasticity in clock neurons as well as other neuron types.
Sleep is linked to the circadian clock, and fruit-flies also sleep during night-time. The synapse number and size increase in the brain during waking time, synapse accumulation during the day drives sleep need, and synapse number decreases with sleep (Bushey et al., 2011; Huang et al., 2020). Thus, sleep is required for the homeostatic re-normalization in synapse number (Bushey et al., 2011; Huang et al., 2020). Whether sleep or the lack of it can have further structural consequences in circuits is an interesting question.
Cell Number Plasticity in the Adult Brain
Cell number plasticity reflects changes in cell proliferation, survival, or death. Apoptosis in the adult fruit-fly brain is limited to the critical period. Dying cells were detected in multiple brain areas, but by the fifth day of adult life, apoptosis was no longer detected (Ito and Hotta, 1992; Kato et al., 2009). In the intact, normal, young brain, glial proliferation was observed adjacently to apoptotic cells, suggesting that apoptosis in the adult brain induces compensatory proliferation in the nearby glia (Kato et al., 2009).
Evidence of adult neurogenesis in adult Drosophila was recently reviewed in Li and Hidalgo (2020) and will not be dealt with in detail here. In brief, [3H]-thymidine and BrdU experiments labeling cells going through S-phase had suggested that cell proliferation might take place (Technau, 1984, see also Li and Hidalgo, 2020), but this could reflect polyploidy instead (Nandakumar et al., 2020). Importantly, the developmental neuroblasts that make the adult brain are eliminated through either apoptosis or cell cycle exit in the larva or pupa, before the adult ecloses (Siegrist et al., 2010; Li and Hidalgo, 2020). Thus, in the absence of neuroblasts, it was unclear how neurogenesis could proceed. A careful temporal profile of BrdU pulses applied to the adult brain showed that not only there are cells in S-phase in the adult brain but also the number of labeled cells critically increased over time (Kato et al., 2009). This was robust evidence of cell division in the adult brain. Cell proliferation was also reported using clonal analyses (Kato et al., 2009; Fernandez-Hernandez et al., 2013; Foo et al., 2017) and cell cycle markers (Li et al., 2020). The incidence of cell proliferation increased with genetic manipulations and injury (Kato et al., 2009; Fernandez-Hernandez et al., 2013). Injury in the optic lobe or central brain by stabbing with a needle and antennal ablation induced the proliferation of glial cells (Kato et al., 2009; Fernandez-Hernandez et al., 2013). Injury-induced cell proliferation seemed to be restricted to a critical period in the first 10 days of young flies, although this remains to be confirmed. The dividing cells were shown to be, in some cases, glia but could also include progenitor cells, as in the normal, intact, adult brain, there are cells that express the common neural stem cell marker deadpan (Fernandez-Hernandez et al., 2013; Li et al., 2020).
These findings altogether mean that cell number plasticity in the adult Drosophila brain includes both loss and generation of new cells (Figure 1). There is suggestive evidence for a critical period in cell number plasticity in the young fly, but this remains to be confirmed.
Structural Brain Plasticity Is Common in Insects
Structural brain plasticity occurs in other insects and could be widespread. In moths, pre-exposure to bat sound increased the volume of some glomeruli in antennal lobes and mushroom body calyces (Anton et al., 2016). In bumblebees kept in complete darkness for 7 days, lack of visual stimulation increased the relative volume of antennal lobes, mushroom body calyces, and neuropils (Jones et al., 2013). In honeybees, the synaptic bouton number in the calyx of the lip—one of the mushroom body compartments—increased when the bees were reared in impoverished conditions with reduced social interactions and olfactory and visual stimuli compared to bees reared in the hive (Cabirol et al., 2017). The structure of both the lip and dense collar (also mushroom body compartments) were also affected by foraging behavior. The volume and bouton number of the lip and dense collar correlated positively with the time spent foraging (Cabirol et al., 2018). These and further findings (Hourcade et al., 2009) showed that, in honeybees, the volume and synapse number of both antennal lobes and mushroom bodies can be modified by environmental stimuli, which, in turn, modified the behavior.
Thus, like in the mammalian brain, environmental stimuli induce structural brain plasticity in multiple insect species, including fruit-flies, bumblebees, honeybees, and moths. This suggests that structural brain plasticity is a fundamental property of how brains work and are modified through experience. In both insects and humans, the brain modules involved in olfaction and learning and memory were found to be plastic. Remarkably, contrary to the limited neurogenic sites of the human brain but similar to its cortical brain plasticity, plasticity appears to be widespread throughout the insect brain.
Molecular Mechanisms of Structural Brain Plasticity
The molecular mechanisms underlying structural brain plasticity are beginning to be uncovered (Table 1 and Figure 1). Pioneering work first addressed this question by testing genes involved in phototransduction and learning and memory. Mutations in genes required for phototransduction such as norpA (causing loss of phospholipase-C) and hdcjk910 (deficient in histamine, the main neurotransmitter in the retina) obliterated the differences in brain volume observed when rearing fruit-flies in constant light vs. darkness (Barth and Heisenberg, 1997). This meant that visual experience is required for light-induced structural brain plasticity in the optic lobes. By contrast, mutations in genes involved in cAMP signaling dunce (dnc), rutabaga (rut), and amnesiac (amn), involved in learning, did not cause volume changes in the optic lobe with light rearing, but dnc and amn did cause volume changes in the mushroom body calyx (Barth and Heisenberg, 1997). Furthermore, when kept in the dark, wild-type flies in large social groups had larger calyces than flies housed in smaller groups, but this effect was lost in all three mutants (Barth and Heisenberg, 1997). It was recently shown that rut, which encodes Ca2+/CaM-dependent adenyl cyclase, is also required for structural plasticity at the calyx. In fact, long-term memory modifies the size and number of microglomeruli in the calyx, but this effect was abolished in rut mutants or by blocking protein synthesis (Baltruschat et al., 2021). Thus, cAMP regulates plasticity in calyx but not the visual system, which means that distinct molecular pathways may underlie structural plasticity in distinct brain regions (Barth and Heisenberg, 1997).
Several genes promoting cell proliferation in the adult Drosophila brain were identified. The TNF homolog Eiger is required for cell proliferation in response to injury in the adult brain, as cell proliferation was not induced in injured eiger mutants (Kato et al., 2009). Over-expression of the oncogene dMyc in the adult brain increased the proliferation of Dpn+ progenitor cells (Fernandez-Hernandez et al., 2013). Mutant microRNA miR-31a induced the apoptosis of glia in young adult flies, which was followed by compensatory proliferation (Foo et al., 2017). So, these data altogether suggested that, in the adult brain, common fundamental cellular processes can also drive cell number adjustments, at least upon cell death or injury.
In clock neurons, the expression of PDF increases during the day, positively correlating with an increase in axonal complexity of PDF neurons (Herrero et al., 2020). Conversely, conditional PDF knock-down in the adult prevents structural plasticity. These findings altogether demonstrate that PDF regulates structural plasticity in clock neurons (Herrero et al., 2020).
Drosophila neurotrophins are encoded by the spätzle (spz) paralog gene family, which includes Drosophila neurotrophin-1 (DNT1, also called spz-2), DNT2/spz-5, and Spz-1 (Zhu et al., 2008; Sutcliffe et al., 2013; Foldi et al., 2017). During nervous system development, DNTs promote neuronal survival, cell death, connectivity, structural synaptic plasticity, and compensatory plasticity (Zhu et al., 2008; Sutcliffe et al., 2013; Foldi et al., 2017; Ulian-Benitez et al., 2017). Mammalian neurotrophins bind Trk and p75 receptors, but DNTs bind kinase-free Trk homolog encoded by the kekkon genes and Toll receptors (Mandai et al., 2009; Ulian-Benitez et al., 2017). Toll-1 in Drosophila is responsible for dorso-ventral body axis in embryogenesis and for innate immunity (Hashimoto et al., 1988; Lemaitre et al., 1996), and it led to the discovery of TLRs throughout most organisms. Tolls and TLRs not only have universal, evolutionarily conserved functions controlling innate immunity, but they also have non-immune functions (Anthoney et al., 2018). There are nine Tolls in the Drosophila genome, and during embryonic and larval nervous system development, at least some Tolls have been shown to be involved in connectivity, cell number plasticity, and structural synaptic plasticity (Zhu et al., 2008; McIlroy et al., 2013; Ballard et al., 2014; Ward et al., 2015; McLaughlin et al., 2016; Foldi et al., 2017; Ulian-Benitez et al., 2017; Li et al., 2020). At least seven Tolls are expressed in the adult brain (Toll-1, −2, −3, −5, −6, −7, and −8), in overlapping but distinct expression patterns that coincide with brain anatomical domains (Li et al., 2020; Figure 1).
Tolls can promote either cell quiescence, proliferation, neuronal survival, or death, depending on time, cell type, and cell context, in the CNS (McIlroy et al., 2013; Foldi et al., 2017; Li et al., 2020; Figure 2). Canonical Toll signaling proceeds via the MyD88 adaptor, leading to the activation of NF-κB homologs Dorsal and Dif downstream, which in the CNS promote cell survival (Foldi et al., 2017; Anthoney et al., 2018). Tolls can also function via Weckle (Wek) and Sarm to promote cell death (Foldi et al., 2017). Sarm is an evolutionarily conserved inhibitor of MyD88, and it induces apoptosis via JNK downstream (Foldi et al., 2017). Tolls bind Wek, which binds Sarm facilitating its inhibition of MyD88 (Foldi et al., 2017). Sarm also has NAD-ase activity which drives axonal degeneration (Carty and Bowie, 2019). Toll-2 also regulates cell proliferation during development, via Wek and Yorkie (Yki) (Li et al., 2020). Concerted knock-down of multiple Tolls throughout development causes dramatic reductions in brain size, most likely due to combined increased cell death and reduced cell proliferation (Li et al., 2020). Although Tolls can have redundant functions, they are not equal, and they can lead to distinct cellular outcomes. For example, in the pupal CNS, Toll-1 and Toll-6 can induce both cell survival and cell death, but Toll-1 has a stronger pro-apoptotic effect, whereas Toll-2 does not induce cell death (Foldi et al., 2017; Li et al., 2020).
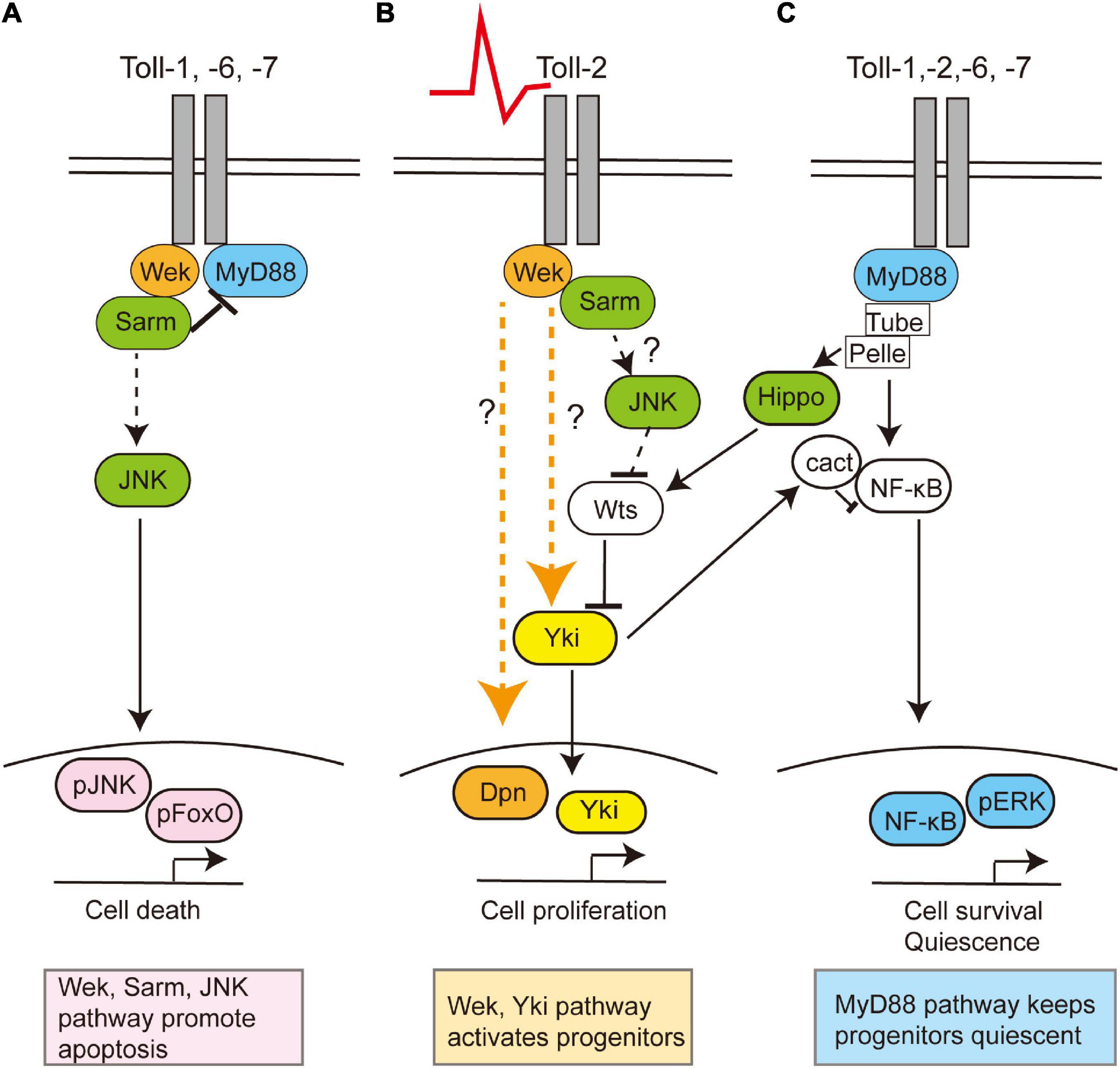
Figure 2. Toll receptors can regulate cell survival, death, quiescence, or proliferation via alternative signaling pathways. Toll receptors share common functions, but they can also each elicit distinct cellular outcomes. This is a summary of data evidence on signaling by Toll-1, −2, −6, and −7 in the central nervous system in embryonic, larval, and/or pupal development and Toll-2 in the adult brain. (A) During development, Toll receptors can promote cell apoptosis via Wek, Sarm, and JNK, (B) but in the adult brain, Toll-2 receptors can also function via Wek and Yki to promote cell proliferation. Question marks denote how or whether Wek signals remain unknown. (C) Toll receptors can promote cell survival via MyD88-NFkB signaling both throughout development and in the adult. Moreover, adult progenitor cells are normally kept quiescent via MyD88. In other contexts, the activation of Toll by gram-positive bacteria prevents proliferation by activating the Hippo pathway, thus inhibiting Yki, although whether this is also the case in the brain is unknown.
Toll-2, at least, is responsible for generative processes underlying structural brain plasticity in the adult (Li et al., 2020). It is neuroprotective, and Toll-2 knock-down causes axonal misrouting, loss of axons and dendrites, and loss of neurons and impairs behavior (Li et al., 2020). Toll-2 promotes neurogenesis in the adult brain, as the conditional over-expression of Toll-2 restricted to the adult promotes cell proliferation via Yki, and increases cell number and brain size (Li et al., 2020). Neuronal activation with TrpA1 also increases cell number, and this depends on Toll-2 (42). There are Dpn+ progenitors in the adult Drosophila brain (Li et al., 2020), which express MyD88. Toll signaling via MyD88 inhibits the proliferation of progenitor cells, whereas signaling via wek promotes cell proliferation (Li et al., 2020). Similarly, in immunity, Toll signaling via MyD88 activates Hippo signaling, inhibiting Yki and cell proliferation (Liu et al., 2016). Thus, Toll signaling via MyD88 keeps progenitor cells quiescent, and neuronal activity or Toll-2 signaling can activate an alternative Wek downstream pathway that promotes cell proliferation via Yki (Li et al., 2020; Figure 2). Thus, depending on the availability of downstream adaptors, Toll receptors can function via alternative signaling pathways, with distinct cellular outcomes (Foldi et al., 2017). What enables Tolls to switch between signaling via MyD88, Sarm, or Wek to promote cell survival and quiescence, apoptosis, or cell proliferation, respectively, is not known.
The topographic expression of Tolls together with the multiple possible outcomes downstream (Figures 1, 2) contributes to the formation of the distinct brain modules in development and could have driven the formation and diversification of distinct brain types in the course of evolution. Importantly, it enables them to regulate structural plasticity independently in different brain domains. In fact, the cell number in the optic lobe medulla and central brain was found to be more plastic than mushroom body Kenyon cells (Li et al., 2020). By regulating the cell number independently in distinct brain domains (e.g., visual, olfactory), Tolls can match a sensory experience (e.g., vision and olfaction) to a structural change. In contrast to the limited neurogenic niches of the mammalian brain, cell cycling markers and changes in cell number were found spread out through the Drosophila brain, reminiscent of widespread adult neurogenesis in zebrafish. These findings meant that the cell number in the adult fruit-fly brain is plastic, and experience can elicit structural changes in the brain via Toll receptor signaling.
Sleep need can be driven by increasing the levels of the active zone scaffold protein Bruchpilot (Brp) (Huang et al., 2020). Increasing the levels of Brp led to a dose-dependent increase in synapse formation, and this was sufficient to quantitatively tune sleep patterns reminiscent of sleep deprivation (Huang et al., 2020). When knocking down sleep-regulating genes, such as wide awake, insomniac, or fumin, the expression level of the active zone scaffold protein Bruchpilot significantly increased in a brain-wide manner (Huang et al., 2020). Moreover, knocking down brp expression in R2 neurons in flies that otherwise over-expressed Brp was sufficient to partially rescue a sleepless behavior and a deficient short-term memory, thus demonstrating the importance of these neurons and Brp in the regulation of sleep (Huang et al., 2020).
Toll and Spz are also involved in regulating sleep homeostasis. Astroglial calcium increases with sleep need and is required for sleep rebound after sleep deprivation (Blum et al., 2021). It has been proposed that astrocytes secrete Spz, which signals via Toll-1 in R5 neurons to promote a sleep rebound (Blum et al., 2021). The intracellular Ca2+ level in R5 neurons significantly increased after sleep deprivation, and knocking down Toll suppressed this increase (Blum et al., 2021). Moreover, knocking down Spz in astroglia or Toll in R5 neurons reduced the homeostatic sleep rebound (Blum et al., 2021). However, whether Spz and Toll-1 can also cause structural modifications in R5 neurons and whether this is linked to sleep homeostasis was not investigated.
To conclude, evidence indicates that these and other yet-to-be-discovered molecular mechanisms underlie structural changes in the fly brain, modifying neural circuits in response to experience to deliver appropriate behavior, learning, and sleep and to adapt to the environment.
Similarities Between Tolls and Mammalian TLRs in Structural Brain Plasticity
There are 10 TLRs in the human brain. Investigation of TLR function in the mammalian brain has mostly focused on their immune-like functions in microglia and astrocytes (Okun et al., 2009, #76; Okun et al., 2011, #87; Shmueli et al., 2018, #308; Anthoney et al., 2018, #35; Chen et al., 2019, #312; Donnelly et al., 2020, #311), but TLRs are also expressed in neural stem cells, NG2 glia, and neurons throughout development and in the adult. TLRs also have non-immune functions in the mammalian nervous system (Rolls et al., 2007, #78; Okun et al., 2010, #221; Chen et al., 2019, #312; Donnelly et al., 2020, #311). They are required to either promote or prevent neural stem cell proliferation and differentiation into neurons or glia and to regulate cell survival or death, neurite growth or retraction, synaptogenesis, and the compensation of spine density and size. In neurons, they can regulate slow cellular processes through the canonical MyD88 and ERK signaling pathways and gene expression (Rolls et al., 2007; Okun et al., 2010) and also fast neuronal action (e.g., < 1 min) in interaction with channels (e.g., TrpA1 and TrpV1), enabling fast responses to sensory stimuli, such as heat, pain, and itch (Donnelly et al., 2020, #311). This repertoire of functions reveals that TLRs could be involved in regulating brain function and plasticity independently of immunity.
Drosophila Tolls can carry out overlapping yet distinct functions (Foldi et al., 2017). Similarly, in mammals, TLR-2, −3, and -4 regulate neurogenesis and cell differentiation; TLR-7, −8, and -9 can induce apoptosis; and TLR-3, −7, and -8 regulate arborizations through neurite growth and retraction, spine density, and size (Ma et al., 2006; Cameron et al., 2007; Rolls et al., 2007; Okun et al., 2010; Chen et al., 2019). Thus, not all Tolls and TLRs are equal, and they may each elicit distinct cellular outcomes.
Whether TLRs are expressed topographically in the brain, like Drosophila Tolls, is not yet known. There is evidence that TLRs are expressed with distinct temporal profiles from brain development to the adult brain (Ma et al., 2006; Kaul et al., 2012; Barak et al., 2014; Arnaboldi et al., 2020). They might be expressed in partly overlapping and at least partly distinct cell types in the brain, as their loss of function causes region-specific behavioral phenotypes. For instance, TLR4 regulates the levels of neuromodulators in the frontal cortex and hippocampus (Femenia et al., 2018). Mice with altered TLR-3 or -4 function have altered spatial navigation, learning and memory, anxiety, and social interactions (Okun et al., 2012). However, these observations were made in mutant mice, and it is compelling to find out whether conditional alterations in TLR function restricted to the adult would also influence behavior and in a spatially dependent manner. Finding out whether TLRs are distributed topographically like in Drosophila would explain the experience-dependent regulation of brain plasticity and the consequences in behavior.
The endogenous ligands that could regulate non-immune neuronal TLR functions in the sterile, undamaged brain are unknown. Neurotrophins are ligands for Tolls in Drosophila, but whether they can also bind mammalian TLRs remains unexplored. Interestingly, TLR4 knock-out mice with anxiety behavior also had an altered expression of BDNF in the frontal cortex and hippocampus (Femenia et al., 2018). Intriguingly, in cell culture, human neurotrophins BDNF and NGF can modify human TLR signaling in response to their endogenous ligands and induce TLR-4 signaling in the absence of any other ligand (McIlroy et al., 2013; Foldi et al., 2017; Li et al., 2020). This means either that binding of NGF and BDNF to Trk or p75 receptors modifies TLR signaling or that NGF and BDNF can directly bind at least TLR4 (Foldi et al., 2017). It is compelling to find out whether TLRs could also respond to NGF or BDNF in vivo in the mammalian brain.
Understanding TLR function in the brain is important, as alterations result in brain diseases, including anxiety, neuropsychiatric disorders, schizophrenia, autism, Alzheimer’s disease, autoimmune diseases (e.g., multiple sclerosis), and stroke (Okun et al., 2011).
Conclusion
There is abundant evidence of structural brain plasticity in the Drosophila brain, involving modifications to regional volumes, cell number, cell shape, and synapses, altogether modifying neural circuits. In Drosophila, Toll receptors regulate structural brain plasticity topographically, activating alternative signaling pathways downstream that can promote cell proliferation, quiescence, survival, or death. In this way, Tolls enable the link between sensory experience and structural brain change. There are striking similarities in the way Drosophila Tolls and mammalian TLRs function in the brain, including through distinct Toll-specific functions, cellular outcomes, and consequences in behavior. Although mammalian TLRs have been investigated mostly in the context of immunity, they also have non-immune functions in the sterile, undamaged brain, and these functions could be crucial to understanding brain diseases. Communication between Drosophila and mammalian findings can expedite the understanding of structural change in the human brain—in health and disease.
Author Contributions
GL and AH wrote and revised the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work was funded by the BBSRC project grants BB/R017034/1 and BB/R00871X/1.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our lab members for the discussions and comments on the manuscript.
References
Anthoney, N., Foldi, I., and Hidalgo, A. (2018). Toll and Toll-like receptor signalling in development. Development 145:dev156018.
Anton, S., Chabaud, M. A., Schmidt-Busser, D., Gadenne, B., Iqbal, J., Juchaux, M., et al. (2016). Brief sensory experience differentially affects the volume of olfactory brain centres in a moth. Cell Tissue Res. 364, 59–65. doi: 10.1007/s00441-015-2299-0
Arnaboldi, F., Sommariva, M., Opizzi, E., Rasile, M., Camelliti, S., Busnelli, M., et al. (2020). Expression of Toll-like receptors 4 and 7 in murine peripheral nervous system development. Ann Anat. 231:151526. doi: 10.1016/j.aanat.2020.151526
Ballard, S. L., Miller, D. L., and Ganetzky, B. (2014). Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol. 204, 1157–1172. doi: 10.1083/jcb.201308115
Balling, A., Technau, G. M., and Heisenberg, M. (1987). Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J. Neurogenet. 4, 65–73. doi: 10.3109/01677068709102334
Baltruschat, L., Prisco, L., Ranft, P., Lauritzen, J. S., Fiala, A., Bock, D. D., et al. (2021). Circuit reorganization in the Drosophila mushroom body calyx accompanies memory consolidation. Cell Rep. 34:08871.
Barak, B., Feldman, N., and Okun, E. (2014). Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front. Neurosci. 8:272. doi: 10.3389/fnins.2014.00272
Barth, M., and Heisenberg, M. (1997). Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem. 4, 219–229. doi: 10.1101/lm.4.2.219
Barth, M., Hirsch, H. V., Meinertzhagen, I. A., and Heisenberg, M. (1997). Experience-dependent developmental plasticity in the optic lobe of Drosophila melanogaster. J. Neurosci. 17, 1493–1504. doi: 10.1523/jneurosci.17-04-01493.1997
Blum, I. D., Keles, M. F., Baz, E. S., Han, E., Park, K., Luu, S., et al. (2021). Astroglial Calcium Signaling Encodes Sleep Need in Drosophila. Curr. Biol. 31, 150–162.e7.
Brandstatter, J. H., and Meinertzhagen, I. A. (1995). The rapid assembly of synaptic sites in photoreceptor terminals of the fly’s optic lobe recovering from cold shock. Proc. Natl. Acad. Sci. U. S. A. 92, 2677–2681. doi: 10.1073/pnas.92.7.2677
Bushey, D., Tononi, G., and Cirelli, C. (2011). Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332, 1576–1581. doi: 10.1126/science.1202839
Cabirol, A., Brooks, R., Groh, C., Barron, A. B., and Devaud, J. M. (2017). Experience during early adulthood shapes the learning capacities and the number of synaptic boutons in the mushroom bodies of honey bees (Apis mellifera). Learn. Mem. 24, 557–562. doi: 10.1101/lm.045492.117
Cabirol, A., Cope, A. J., Barron, A. B., and Devaud, J. M. (2018). Relationship between brain plasticity, learning and foraging performance in honey bees. PLoS One. 13:e0196749. doi: 10.1371/journal.pone.0196749
Cameron, J. S., Alexopoulou, L., Sloane, J. A., DiBernardo, A. B., Ma, Y., Kosaras, B., et al. (2007). Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J. Neurosci. 27, 13033–13041. doi: 10.1523/jneurosci.4290-06.2007
Carty, M., and Bowie, A. G. S. A. R. M. (2019). From immune regulator to cell executioner. Biochem. Pharmacol. 161, 52–62. doi: 10.1016/j.bcp.2019.01.005
Chen, C. Y., Shih, Y. C., Hung, Y. F., and Hsueh, Y. P. (2019). Beyond defense: regulation of neuronal morphogenesis and brain functions via Toll-like receptors. J. Biomed. Sci. 26:90.
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350. doi: 10.1038/nrn2822
Devaud, J. M., Acebes, A., and Ferrus, A. (2001). Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J. Neurosci. 21, 6274–6282. doi: 10.1523/jneurosci.21-16-06274.2001
Devaud, J. M., Acebes, A., Ramaswami, M., and Ferrus, A. (2003). Structural and functional changes in the olfactory pathway of adult Drosophila take place at a critical age. J. Neurobiol. 56, 13–23. doi: 10.1002/neu.10215
Donnelly, C. R., Chen, O., and Ji, R. R. (2020). How do sensory neurons sense danger signals? Trends Neurosci. 43, 822–838. doi: 10.1016/j.tins.2020.07.008
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317.
Feldman, D. E., and Brecht, M. (2005). Map plasticity in somatosensory cortex. Science 310, 810–815. doi: 10.1126/science.1115807
Femenia, T., Qian, Y., Arentsen, T., Forssberg, H., and Diaz Heijtz, R. (2018). Toll-like receptor-4 regulates anxiety-like behavior and DARPP-32 phosphorylation. Brain Behav. Immun. 69, 273–282. doi: 10.1016/j.bbi.2017.11.022
Fernandez-Hernandez, I., Rhiner, C., and Moreno, E. (2013). Adult neurogenesis in Drosophila. Cell Rep. 3, 1857–1865. doi: 10.1016/j.celrep.2013.05.034
Foldi, I., Anthoney, N., Harrison, N., Gangloff, M., Verstak, B., Nallasivan, M. P., et al. (2017). Three-tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J. Cell Biol. 216, 1421–1438. doi: 10.1083/jcb.201607098
Foo, L. C., Song, S., and Cohen, S. M. (2017). miR-31 mutants reveal continuous glial homeostasis in the adult Drosophila brain. EMBO J. 36, 1215–1226. doi: 10.15252/embj.201695861
Gage, F. H. (2004). Structural plasticity of the adult brain. Dialogues Clin. Neurosci. 6, 135–141. doi: 10.31887/dcns.2004.6.2/fgage
Gage, F. H. (2019). Adult neurogenesis in mammals. Science 364, 827–828. doi: 10.1126/science.aav6885
Goncalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult Neurogenesis in the Hippocampus: from Stem Cells to Behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Gorska-Andrzejak, J., Keller, A., Raabe, T., Kilianek, L., and Pyza, E. (2005). Structural daily rhythms in GFP-labelled neurons in the visual system of Drosophila melanogaster. Photochem. Photobiol. Sci. 4, 721–726. doi: 10.1039/b417023g
Hashimoto, C., Hudson, K. L., and Anderson, K. V. (1988). The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52, 269–279. doi: 10.1016/0092-8674(88)90516-8
Heisenberg, M., Heusipp, M., and Wanke, C. (1995). Structural plasticity in the Drosophila brain. J. Neurosci. 15, 1951–1960.
Herrero, A., Yoshii, T., Ispizua, J. I., Colque, C., Veenstra, J. A., Muraro, N. I., et al. (2020). Coupling Neuropeptide Levels to Structural Plasticity in Drosophila Clock Neurons. Curr. Biol. 30, 3154–3166.e4.
Hofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T., and Hubener, M. (2009). Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317. doi: 10.1038/nature07487
Hourcade, B., Perisse, E., Devaud, J. M., and Sandoz, J. C. (2009). Long-term memory shapes the primary olfactory center of an insect brain. Learn. Mem. 16, 607–615. doi: 10.1101/lm.1445609
Huang, S., Piao, C., Beuschel, C. B., Gotz, T., and Sigrist, S. J. (2020). Presynaptic Active Zone Plasticity Encodes Sleep Need in Drosophila. Curr. Biol. 30, 1077–1091.e5.
Ito, K., and Hotta, Y. (1992). Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 149, 134–148. doi: 10.1016/0012-1606(92)90270-q
Jones, B. M., Leonard, A. S., Papaj, D. R., and Gronenberg, W. (2013). Plasticity of the worker bumblebee brain in relation to age and rearing environment. Brain Behav. Evol. 82, 250–261. doi: 10.1159/000355845
Kato, K., Awasaki, T., and Ito, K. (2009). Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development 136, 51–59. doi: 10.1242/dev.023366
Kaul, D., Habbel, P., Derkow, K., Kruger, C., Franzoni, E., Wulczyn, F. G., et al. (2012). Expression of Toll-like receptors in the developing brain. PLoS One. 7:e37767. doi: 10.1371/journal.pone.0037767
Kral, K., and Meinertzhagen, I. A. (1989). Anatomical plasticity of synapses in the lamina of the optic lobe of the fly. Philos. Trans. R. Soc. Lond. B Biol. Sci. 323, 155–183. doi: 10.1098/rstb.1989.0004
Kremer, M. C., Christiansen, F., Leiss, F., Paehler, M., Knapek, S., Andlauer, T. F., et al. (2010). Structural long-term changes at mushroom body input synapses. Curr. Biol. 20, 1938–1944. doi: 10.1016/j.cub.2010.09.060
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M., and Hoffmann, J. A. (1996). The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. doi: 10.1016/s0092-8674(00)80172-5
Li, G., Forero, M. G., Wentzell, J. S., Durmus, I., Wolf, R., Anthoney, N. C., et al. (2020). A Toll-receptor map underlies structural brain plasticity. Elife 9:e52743.
Li, G., and Hidalgo, A. (2020). Adult Neurogenesis in the Drosophila Brain: the Evidence and the Void. Int. J. Mol. Sci. 21:6653. doi: 10.3390/ijms21186653
Liu, B., Zheng, Y., Yin, F., Yu, J., Silverman, N., and Pan, D. (2016). Toll Receptor-Mediated Hippo Signaling Controls Innate Immunity in Drosophila. Cell 164, 406–419. doi: 10.1016/j.cell.2015.12.029
Lu, B., Nagappan, G., Guan, X., Nathan, P. J., and Wren, P. (2013). BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 401–416. doi: 10.1038/nrn3505
Lu, B., Pang, P. T., and Woo, N. H. (2005). The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614. doi: 10.1038/nrn1726
Ma, Y., Li, J., Chiu, I., Wang, Y., Sloane, J. A., Lu, J., et al. (2006). Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 175, 209–215. doi: 10.1083/jcb.200606016
Mandai, K., Guo, T., St Hillaire, C., Meabon, J. S., Kanning, K. C., Bothwell, M., et al. (2009). LIG family receptor tyrosine kinase-associated proteins modulate growth factor signals during neural development. Neuron 63, 614–627. doi: 10.1016/j.neuron.2009.07.031
McIlroy, G., Foldi, I., Aurikko, J., Wentzell, J. S., Lim, M. A., Fenton, J. C., et al. (2013). Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 16, 1248–1256. doi: 10.1038/nn.3474
McLaughlin, C. N., Nechipurenko, I. V., Liu, N., and Broihier, H. T. A. (2016). Toll receptor-FoxO pathway represses Pavarotti/MKLP1 to promote microtubule dynamics in motoneurons. J. Cell Biol. 214, 459–474. doi: 10.1083/jcb.201601014
Nandakumar, S., Grushko, O., and Buttitta, L. A. (2020). Polyploidy in the adult Drosophila brain. Elife 9:e54385.
Okun, E., Barak, B., Saada-Madar, R., Rothman, S. M., Griffioen, K. J., Roberts, N., et al. (2012). Evidence for a developmental role for TLR4 in learning and memory. PLoS One. 7:e47522. doi: 10.1371/journal.pone.0047522
Okun, E., Griffioen, K., Barak, B., Roberts, N. J., Castro, K., Pita, M. A., et al. (2010). Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 107, 15625–15630. doi: 10.1073/pnas.1005807107
Okun, E., Griffioen, K. J., Lathia, J. D., Tang, S.-C., Mattson, M. P., and Arumugam, T. V. (2009). Toll-like receptors in neurodegeneration. Brain Res. Rev. 59, 278–292. doi: 10.1016/j.brainresrev.2008.09.001
Okun, E., Griffioen, K. J., and Mattson, M. P. (2011). Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 34, 269–281. doi: 10.1016/j.tins.2011.02.005
Park, H., and Poo, M. M. (2013). Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23. doi: 10.1038/nrn3379
Pyza, E., and Meinertzhagen, I. A. (1996). Neurotransmitters regulate rhythmic size changes amongst cells in the fly’s optic lobe. J. Comp. Physiol. A 178, 33–45.
Rolls, A., Shechter, R., London, A., Ziv, Y., Ronen, A., Levy, R., et al. (2007). Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 9, 1081–1088. doi: 10.1038/ncb1629
Sachse, S., Rueckert, E., Keller, A., Okada, R., Tanaka, N. K., Ito, K., et al. (2007). Activity-dependent plasticity in an olfactory circuit. Neuron 56, 838–850. doi: 10.1016/j.neuron.2007.10.035
Shmueli, A., Shalit, T., Okun, E., and Shohat-Ophir, G. (2018). The toll pathway in the central nervous system of flies and mammals. Neuromolecular Med. 20, 419–436. doi: 10.1007/s12017-018-8515-9
Siegrist, S. E., Haque, N. S., Chen, C. H., Hay, B. A., and Hariharan, I. K. (2010). Inactivation of both Foxo and reaper promotes long-term adult neurogenesis in Drosophila. Curr. Biol. 20, 643–648. doi: 10.1016/j.cub.2010.01.060
Simoes, A. R., and Rhiner, C. A. (2017). Cold-Blooded View on Adult Neurogenesis. Front. Neurosci. 11:327. doi: 10.3389/fnins.2017.00327
Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227.
Sugie, A., Hakeda-Suzuki, S., Suzuki, E., Silies, M., Shimozono, M., Mohl, C., et al. (2015). Molecular Remodeling of the Presynaptic Active Zone of Drosophila Photoreceptors via Activity-Dependent Feedback. Neuron 86, 711–725. doi: 10.1016/j.neuron.2015.03.046
Sutcliffe, B., Forero, M. G., Zhu, B., Robinson, I. M., and Hidalgo, A. (2013). Neuron-type specific functions of DNT1, DNT2 and Spz at the Drosophila neuromuscular junction. PLoS One. 8:e75902. doi: 10.1371/journal.pone.0075902
Technau, G. M. (1984). Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J. Neurogenet. 1, 113–126. doi: 10.3109/01677068409107077
Technau, G. M. (2007). Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. J. Neurogenet. 21, 183–196. doi: 10.1080/01677060701695359
Ulian-Benitez, S., Bishop, S., Foldi, I., Wentzell, J., Okenwa, C., Forero, M. G., et al. (2017). Kek-6: a truncated-Trk-like receptor for Drosophila neurotrophin 2 regulates structural synaptic plasticity. PLoS Genet. 13:e1006968. doi: 10.1371/journal.pgen.1006968
Ward, A., Hong, W., Favaloro, V., and Luo, L. (2015). Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron 85, 1013–1028. doi: 10.1016/j.neuron.2015.02.003
Weber, P., Kula-Eversole, E., and Pyza, E. (2009). Circadian control of dendrite morphology in the visual system of Drosophila melanogaster. PLoS One. 4:e4290. doi: 10.1371/journal.pone.0004290
Wiesel, T. N., and Hubel, D. H. (1965). Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 28, 1029–1040. doi: 10.1152/jn.1965.28.6.1029
Keywords: Drosophila, structural brain plasticity, neurodegeneration, adult neurogenesis, neurotrophin, Toll, TLR, homeostasis
Citation: Li G and Hidalgo A (2021) The Toll Route to Structural Brain Plasticity. Front. Physiol. 12:679766. doi: 10.3389/fphys.2021.679766
Received: 12 March 2021; Accepted: 02 June 2021;
Published: 05 July 2021.
Edited by:
Gaia Tavosanis, German Center for Neurodegeneratives, Helmholtz Association of German Research Centers (HZ), GermanyReviewed by:
Daewoo Lee, Ohio University, United StatesBruno Van Swinderen, The University of Queensland, Australia
Copyright © 2021 Li and Hidalgo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicia Hidalgo, a.hidalgo@bham.ac.uk
 Guiyi Li
Guiyi Li Alicia Hidalgo
Alicia Hidalgo